Abstract
Polybrominated diphenyl ethers (PBDEs) were used extensively as flame retardants in furniture containing polyurethane foam until they were phased out of use, beginning in 2004. We examined temporal changes in PBDE concentrations from 1998 to 2013 and characterized patterns of exposure over the early lifecourse among 334 children (903 samples) between birth and 9 years. We examined time trends by regressing PBDE concentration on year of sample collection in age-adjusted models and characterized developmental trajectories using latent class growth analysis (LCGA). Controlling for age, BDE-47 concentrations decreased 5% (95% confidence interval (CI): −9, −2) per year between 1998 and 2013. When considering only postnatal samples, this reduction strengthened to 13% (95% CI: −19, −9). Findings for BDEs-99, 100 and 153 were similar, except that BDE-153 decreased to a lesser extent when both prenatal and postnatal samples were considered (−2%, 95% CI: −7, 0). These findings suggest that, on average, pentaBDE body burdens have decreased since the 2004 phase-out of these chemicals. When examining developmental period, PBDE concentrations peaked during toddler years for the majority of children, however, our observation of several unique trajectories suggests that a single measure may not accurately reflect exposure to PBDEs throughout early life.
Keywords: PBDE, flame retardant, exposure, prenatal, childhood
Introduction
In 1970, approximately 37% of adults smoked and household fires attributable to ignition of upholstered furniture from improperly extinguished cigarettes was the leading cause of fire-related deaths in the United States1. In response to these statistics, the state of California initiated legislation requiring that companies manufacture fire-safe furniture and in 1975, Technical Bulletin 117 (Cal-117) was ratified, which required that all components of upholstered furniture pass an ‘open flame’ test before entering state commerce2. Between 1975 and 2004, polybrominated diphenyl ethers (PBDEs) were the primary flame retardant chemical used to comply with Cal-117. Commercially, PBDEs were used as components of three technical mixtures known as pentaBDE, octaBDE and decaBDE3. Beginning in 2004, PBDEs were phased out of use owing to their persistence in the environment and potential for human toxicity4–7. The present study focuses on BDEs-47, −99, −100, and −153, which are the predominant congeners in the pentaBDE formulation and are estimated to make up 90% of the human body burden8.
The United Nations Environmental Program (UNEP) estimates that 100 000 tons of pentaBDE was manufactured globally between 1975 and 20109, with approximately 85% used in North America10, where exposure is ubiquitous and body burdens are the highest in the world11. PentaBDE was primarily used in couches, mattresses, carpet padding and other upholstered products3 and typically comprised approximately 3% (by weight) of the polyurethane foam used in these products12. Peak pentaBDE use occurred in 2004 (17 000 tons); considering a product’s first lifespan (~15 years), it is estimated that the majority of pentaBDE-containing products will enter end of life waste streams by 202013.
During production, PBDEs are not chemically bonded to base polymers, thus they have a propensity to migrate away from consumer products and accumulate in the indoor environment14. In the United States, human exposure occurs primarily through incidental ingestion of dust, with consumption of meat, fish and dairy products considered secondary sources15, 16. Owing to their lipophilic properties, PBDEs accumulate in adipose tissue17, readily cross the placenta18, and partition into breast milk19, placing fetuses and infants at risk for elevated exposure. Estimated half-lives of these congeners in adults range from 1.4 – 2.4 years for BDE-47 and 3.6 – 12.4 years for BDE-15320, however, little is known about how the unique exposure pathways (i.e. increased mouthing behaviors), metabolic differences, and other characteristics specific to children influence PBDE body burden.
Previous research has documented higher exposure to pentaBDE congeners among children compared to adults21, likely owing to the increased amount of time infants and toddlers spend in close proximity to the floor and the frequency with which young children mouth fingers, toys and other objects22. While several studies have investigated temporal trends in PBDE exposure, the majority of existing research has been conducted in adults and/or has been cross-sectional by design23–26. In the present analysis, we aimed to address several of these limitations by investigating both time and age-specific changes in PBDE concentrations over the early lifecourse among a cohort of children that were born in the years spanning the pentaBDE phase-out.
Materials and Methods
Study participants
The study sample includes 334 of the 727 children enrolled in the Columbia Center for Children’s Environmental Health (CCCEH) Mothers and Newborns birth cohort. As previously described27, healthy, non-smoking women living in Northern Manhattan or the South Bronx were enrolled during pregnancy between 1998 and 2006 and followed prospectively. Data analyzed in the present paper were collected between 1998 and 2013 at birth and at age 2, 3, 5, 7 and 9-year follow-up visits, resulting in a total of 903 data points. At each visit, a bilingual (English/Spanish) research worker conducted a structured interview with the mother to ascertain information related sociodemographic and lifestyle factors. Details related to housekeeping behaviors were collected by asking the mother about the frequency with which the home was cleaned with a vacuum, dust mop, damp mop or wet mop. Household material hardship was assessed based on the mothers self-reported ability to afford adequate food, clothing, or housing28. Before each visit, mothers were informed about all study procedures and provided written informed consent to participate; after age 7 years, children additionally provided informed assent. Study protocols were approved by the Institutional Review Board of Columbia University; it was determined at the Centers for Disease Control and Prevention (CDC) that the agency was not engaged in human subjects’ research.
Sample collection and laboratory analysis
At the child’s birth, umbilical cord blood was collected by study staff, and at age 2, 3, 5, 7 and 9-year visits child venous blood was collected by a pediatric phlebotomist. Following collection, blood was separated and stored at −70°C at the CCCEH laboratory. Aliquots of all available stored samples from each age period (Ncord=327, N2-years=56, N3-years=115, N5-years=42, N7-years=203, and N9-years=160) were shipped to the CDC for measurement of 11 PBDE congeners (BDEs: 17, 28, 47, 66, 85, 99, 100, 153, 154, 183, and 209). The present study examines BDEs 47, 99, 100 and 153, which were the most frequently detected congeners across study visits (Table 2). Details of the analytic method have been previously published29, 30. Briefly, samples were processed using automatic fortification with internal standards and extracted by automated liquid liquid extraction (Gilson Inc.; Middleton, WI). Analytic determinations were made by gas chromatography isotope dilution high resolution mass spectrometry. Final data were corrected for the median concentration detected in blank samples included in each analytic run (3 blanks per 30 samples). Lipids were co-extracted and removed on a silica: silica/sulfuric acid column using the Rapid Trace equipment (Biotage; Uppsala, Sweden). Total cholesterol and triglyceride levels were determined by standard enzymatic methods using commercially available test kids (Roche Diagnostics; Indianapolis, IN). Child total plasma lipids were estimated from these measured components using the short formula described by Phillips et al.31 and cord plasma lipids were estimated using a recently-developed cord blood specific formula [total cord blood lipids = 2.66 × total cord blood cholesterol + cord blood triglycerides + 0.268, in g lipids/L plasma] (Sjödin A, personal communication).
Table 2.
Summary of PBDE concentrations measured in umbilical cord and child plasma between birth and age 9 years (n=903 samples from 334 children).
| Cord (n=327) | Age 2 (n=56) | Age 3 (n=115) | Age 5 (n=42) | Age 7 (n=203) | Age 9 (n=160) | |
|---|---|---|---|---|---|---|
| BDE-47 | ||||||
| GM±GSD (pg/g serum) | 30.8±1.9 | 139.4±21.0 | 133.1±13.4 | 98.6±15.0 | 90.2±6.8 | 77.8±6.4 |
| GM±GSD (ng/g lipid) | 14.1±0.9 | 37.8±5.8 | 32.1±3.1 | 25.6±3.8 | 23.2±1.7 | 18.1±1.4 |
| <LOD (n, %) | 66 (20) | 0 (0) | 1 (1) | 1 (2) | 5 (2) | 1 (1) |
| Non-reportable (n, %) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| BDE-99 | ||||||
| GM±GSD (pg/g serum) | 8.7±0.4 | 42.8±7.8 | 34.6±3.6 | 23.9±3.9 | 23.1±1.8 | 19.5±1.6 |
| GM±GSD (ng/g lipid) | 3.7±0.2 | 18.1±11.7 | 8.2±0.9 | 6.1±1.0 | 5.8±0.5 | 4.4±0.4 |
| <LOD (n, %) | 161 (49) | 1 (2) | 9 (8) | 7 (17) | 40 (20) | 30 (19) |
| Non-reportable (n, %) | 1 (0.3) | 13 (23) | 9 (8) | 0 (0) | 0 (0) | 0 (0) |
| BDE-100 | ||||||
| GM±GSD (pg/g serum) | 6.7±0.3 | 26.6±3.9 | 27.5±2.5 | 23.3±3.5 | 20.7±1.4 | 17.6±1.4 |
| GM±GSD (ng/g lipid) | 2.9±0.2 | 7.2±1.1 | 6.6±0.6 | 6.0±0.9 | 5.3±0.4 | 4.0±0.3 |
| <LOD (n, %) | 191 (58) | 0 (0) | 5 (4) | 4 (10) | 17 (8) | 12 (8) |
| Non-reportable (n, %) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.6) |
| BDE-153 | ||||||
| GM±GSD (pg/g serum) | 5.7±0.2 | 18.0±2.5 | 20.4±1.8 | 23.4±3.7 | 25.1±1.6 | 23.7±1.8 |
| GM±GSD (ng/g lipid) | 2.6±0.1 | 4.8±0.7 | 4.9±0.4 | 6.1±1.0 | 6.4±0.4 | 5.5±0.4 |
| <LOD (n, %) | 204 (62) | 1 (2) | 7 (6) | 4 (10) | 12 (6) | 9(6) |
| Non-reportable (n, %) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1) |
| Total lipids (mg/dL) | 236±11a | 385±70b | 433±77b | 398±62b | 406±79b | 450±87b |
Abbreviations: GM: geometric mean, GSD: geometric standard deviation, LOD: limit of detection; PBDE: polybrominated diphenyl ether.
Estimated using: total cord blood lipids = 2.66 × cord blood total cholesterol + cord blood triglycerides + 0.268, in g lipid/L plasma
Estimated using: total blood lipids = 2.27 × total cholesterol + triglycerides + 0.623, in g lipid/L plasma
Statistical analysis
We examined descriptive statistics and visualized age-specific distributions of BDEs-47, −99, 100, and −153 using histograms and boxplots. We calculated within congener correlations over time, as well as correlations between congeners at each time point. As previously described32, we imputed concentrations below the limit of detection (LOD) using a distribution-based multiple imputation method that accounts for sample-specific LOD. Distribution-based methods for imputing non-detected concentrations have been shown to produce unbiased results, even in the presence of a large number of samples (50–70%) with non-detectable concentrations of certain analytes33.
We examined temporal trends in exposure by regressing lipid-standardized, log10-transformed PBDE concentration on year of sample collection. We built separate models for each congener and used the generalized estimating equations approach with an exchangeable working correlation to account for repeated measures within a child over time. We isolated effects driven by time, rather than age, by adjusting these models for exact age at blood collection, which we included as a time-varying covariate. In addition to examining time as a continuous variable, we investigated the annual percent change in pentaBDE concentration in age-adjusted models stratified by whether samples were collected from 1998 to 2005 (n=457) or 2006 to 2012 (n=446).
To examine trajectories of PBDE exposure over early life, we used latent class growth analysis (LCGA) to empirically estimate discrete groups of children with shared patterns of measured PBDE concentrations (ng/g lipid) from birth through age 9 years34. This approach models PBDE concentration as a continuous function of age at the time of blood collection and estimates the probability of trajectory membership for each child. It is well-suited for complicated data structures as it allows for inclusion of all children with PBDE concentrations measured at a minimum of one time point. We log10-transformed PBDE concentrations to better approximate a normal distribution and estimated models with varying numbers of groups (1–6) and shapes (linear, quadratic, cubic). We evaluated model fit using the Bayesian Information Criterion (BIC), as well as the magnitude of group membership posterior probabilities.
We performed multinomial logistic regression within the LCGA modeling framework to identify sociodemographic and lifestyle characteristics that predict a child’s trajectory assignment. In these models, trajectory membership is treated as the outcome variable and for each covariate the probability of belonging to a given trajectory (versus the persistent low trajectory) is estimated. In addition to date of birth, which we evaluated as a continuous variable in 3-year increments (1998–2000, 2001–2003, 2004–2006), we explored the following variables in bivariate models: ethnicity (African American vs. Dominican), gender (male vs. female), parity (nulliparous vs. multiparous), maternal age at delivery (>24 years vs. ≤24 years), maternal level of education (high school vs. less than high school), household material hardship (inability to afford food, housing, or clothing vs. access to all), breastfeeding duration ( ≥12 weeks vs. <12 weeks), presence of a smoker in the home (yes vs. no), and frequency of vacuuming (ever vs. never), dust mopping (ever vs. never), damp mopping (ever vs. never) and wet mopping (ever vs. never). We included variables in congener-specific multivariable models if the p-value from bivariate associations was less than 0.10 for any one of the trajectories across the four congeners. We conducted linear regression analyses using SAS v9.4 (SAS Institute Inc., Cary, North Carolina) and performed LCGA and multinomial logistic regression using the SAS Proc Traj procedure35.
Results
We measured PBDE concentrations in 903 samples collected repeatedly from birth to age 9 years among 334 children born between 1998 and 2006. All children were African American or Dominican. Table 1 presents sociodemographic and lifestyle characteristics of maternal-child pairs included in the analysis. These 334 children did not significantly differ at the p=0.05 level from the fully enrolled cohort (n=727) on any sociodemographic or lifestyle factor examined in this analysis with the following exceptions: children with a measure of PBDEs were more likely to be born to a nulliparous mother (50% vs. 40%), were more likely to live in a household that used a dust mop at the prenatal period (12% vs. 7%), and were less likely to live in a household that used a dust mop at the 7-year period (20% vs. 27%) or a damp mop at the 3-year period (63% vs. 70%). To allow time to age into the later study visits, children with PBDE measures were more likely to be born in 1998–2000 (n=183) compared to 2001–2003 (n=88) and 2004–2006 (n=63).
Table 1.
Characteristics of maternal-child pairs (n=334).
| n (%) | |
|---|---|
| Child birth: 1998–2000 | 183 (55) |
| Child birth: 2001–2003 | 88 (26) |
| Child birth: 2004–2006 | 63 (19) |
| African American | 124 (37) |
| Dominican | 210 (63) |
| Maternal age ≤24 yearsa | 161 (48) |
| Maternal <H.S. educationa | 117 (35) |
| Nulliparousa | 168 (50) |
| Child sex (female) | 182 (54) |
| Breastfed < 12 weeks | 217 (66) |
| Smoker in home | |
| Prenatal | 114 (34) |
| 3 years | 71 (21) |
| 7 years | 49 (15) |
| Material hardship | |
| Prenatal | 129 (39) |
| 3 years | 96 (31) |
| 7 years | 104 (36) |
| Ever vacuum home | |
| Prenatal | 57 (17) |
| 3 years | 51 (17) |
| 7 years | 70 (22) |
| Ever dust mop home | |
| Prenatal | 41 (12) |
| 3 years | 52 (16) |
| 7 years | 67 (20) |
| Ever damp mop home | |
| Prenatal | 190 (57) |
| 3 years | 209 (63) |
| 7 years | 202 (60) |
| Ever wet mop home | |
| Prenatal | 174 (58) |
| 3 years | 194 (61) |
| 7 years | 194 (64) |
At delivery
Abbreviations: high school (H.S.)
PBDE concentrations
Across samples and congeners (BDE-47, −99, −100, −153), LODs for cord and child plasma PBDE concentrations ranged from 0.29 to 11.59 ng/g and 0.45 to 20.20 ng/g lipid, respectively. PBDE concentrations were more frequently detected in child compared to cord plasma samples and at all ages BDE-47 was the most frequently detected congener (Table 2). Geometric mean BDE-47 concentrations were highest in samples collected at age 2 years (38±6 ng/g lipid) and lowest in cord plasma samples (14±1 ng/g lipid); we observed a similar pattern for BDEs-99 and −100, however, while BDE-153 concentrations were also lowest in cord plasma, they peaked at age 7 years (25.1±1.6 ng/g lipid vs. 5.7±0.2 in cord blood). Within congeners, PBDE concentrations measured in cord plasma were poorly correlated with concentrations measured in child plasma, however, concentrations measured between ages 2 and 9 years were moderately to highly correlated (see Supplemental Material, Table S1). Within age periods, BDEs-47, −99 and −100 were moderately to highly correlated (minimum RSpearman: 0.76 in cord plasma to maximum RSpearman 0.96 in 3-year plasma) (see Supplemental Material, Table S2).
Changes over time
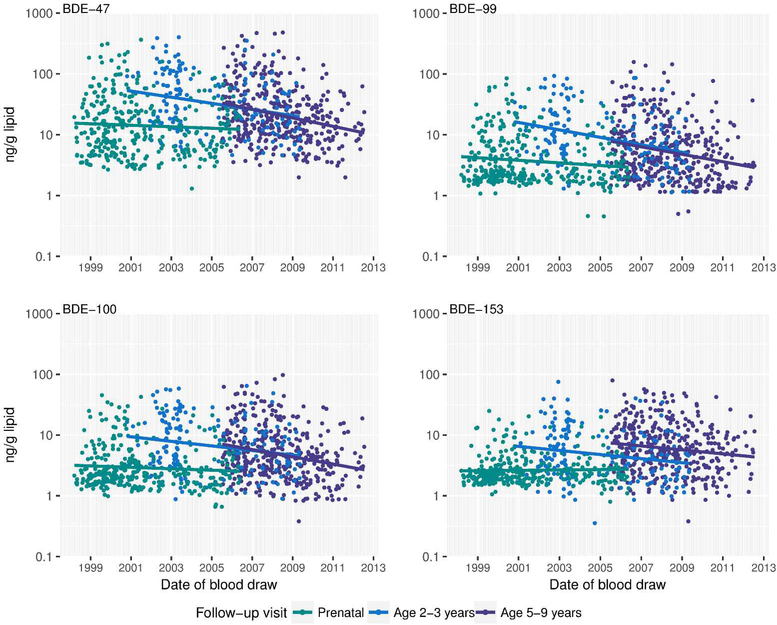
Controlling for child age at blood draw, BDEs-47, −99, −100 and −153 decreased by approximately 5% (95% CI: −9, −2), 7% (−9, −2), 5% (−7, −1), and 2% (−7, 0) per year between 1998 and 2013, respectively (Table 3 and Figure 1). When considering only samples collected during the postnatal period, which likely reflect direct exposure to PBDEs from the environment rather than from maternal transfer, concentrations decreased by 13% (−19, −9), 13% (−19, −9), 11% (−15, −7), and 11% (−15, −7) per year between 2000 and 2013 for BDEs-47, −99, −100 and −153, respectively. In date-stratified models (1998–2005 vs. 2006–2012), the annual percent decrease in plasma BDE-47 concentration was approximately 5% (−9, 1) for samples collected in 1998–2005, compared to 16% (−21, −10) for samples collected in 2006–2012. Further, plasma BDE-47 concentrations were significantly higher among toddlers who turned 2–3 years old before (GM±SE: 40.2±3.9, n=127) versus after (GM±SE: 20.4±2.9, n=44) 2005. We observed a similar pattern at older ages, such that children who were 7–9 years old in 2005 had plasma BDE-47 concentrations (GM±SD: 29.7±5.1, n=20) that were 42% higher compared to children who were 7–9 year olds in 2011–2012 (12.4±1.9, n=30).
Table 3.
Change in cord or child plasma PBDE concentrations (ng/g lipid) over time in GEE models adjusting for age at sample collection.
| Percent change/year (95% CI)a | N сhildrenb | N observationsb | |
|---|---|---|---|
| Cord & child samples (1998–2013) | |||
| BDE-47 | −4.5 (−8.8, −2.3) | 334 | 903 |
| BDE-99 | −6.7 (−8.8, −2.3) | 334 | 880 |
| BDE-100 | −4.5 (−6.7, −0.9) | 334 | 902 |
| BDE-153 | −2.3 (−6.7, 0.0) | 334 | 901 |
| Child samples only (2000–2013) | |||
| BDE-47 | −12.9 (−18.7, −8.8) | 288 | 576 |
| BDE-99 | −12.9 (−18.7, −8.8) | 285 | 554 |
| BDE-100 | −10.9 (−14.9, −6.7) | 288 | 575 |
| BDE-153 | −10.9 (−14.9, −6.7) | 281 | 574 |
Percent change calculated using: ((1–10β) × 100), where the β coefficient is estimated by regressing log10-transformed PBDE concentration on year of sample collection.
Sample size varies due to non-reportable PBDE results (see Table 2)
Figure 1.
Changes in age-adjusted plasma PBDE concentrations (ng/g lipid) between 1998 and 2013 (n=903 samples from 334 children). Prenatal concentrations were measured in cord plasma.
Early life trajectories
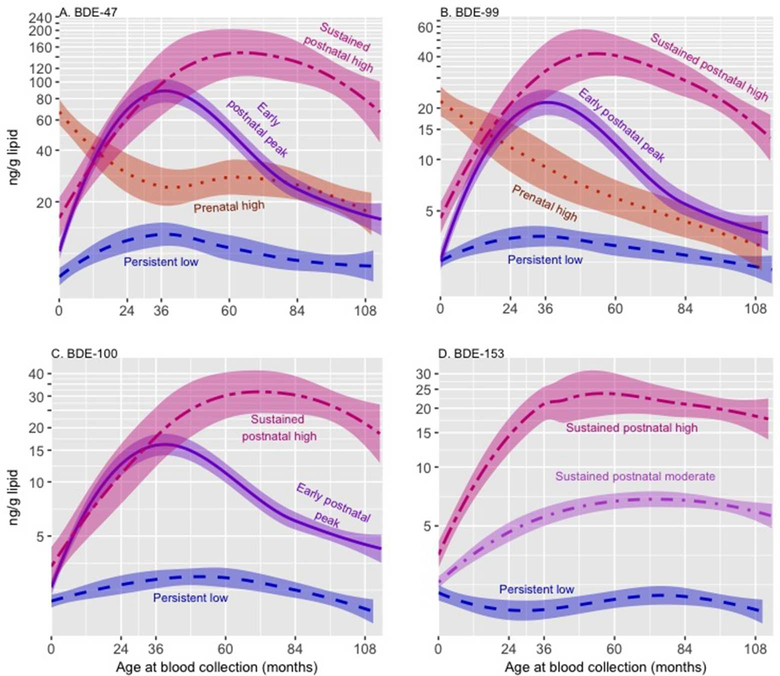
As illustrated by Figure 2, the best fitting LCGA model revealed four trajectories of BDEs-47, 99, and 100. One trajectory was characterized by low PBDE concentrations at all ages (‘persistent low’). Two trajectories were defined by high concentrations during childhood, one of which showed a decrease after age 2–3 years (‘early postnatal peak’) and a second that remained elevated throughout childhood (‘sustained postnatal high’). The fourth trajectory was characterized by high prenatal concentrations that decreased after birth (‘prenatal high’). Across these three congeners, the majority of children were assigned to the ‘persistent low’ (34–51%) or ‘early postnatal peak’ (24–38%) trajectories. We identified three relatively age-invariant trajectories of BDE-153, which we refer to as ‘persistent low’, ‘sustained postnatal moderate’, and ‘sustained postnatal high’. Congener-specific sample sizes and frequencies for each trajectory are presented in Table 4. Owing to its small size (<10% of the sample), we do not plot the BDE-100 ‘prenatal high’ trajectory, nor do we examine it in regression models; however, we retained the trajectory as it improved LCGA model fit. Across congeners, the mean posterior probability of trajectory membership (0.7–0.9) met or exceeded the widely-accepted threshold for satisfactory group assignment (mean of 0.7), indicating a high likelihood that a child’s exposure pattern fit well within his or her assigned trajectory36 (see Supplemental Material, Table S3).
Figure 2.
Trajectories of plasma PBDE concentrations (ng/g lipid) from birth through 9 years (n=334) estimated using latent class growth analysis; bands represent 95% confidence intervals. The ‘persistent low’ trajectory serves as the reference category.
Table 4.
Sample size of each PBDE exposure trajectory, N (%)
| Persistent low | Prenatal high | Early postnatal peak | Sustained postnatal moderate | Sustained postnatal high | |
|---|---|---|---|---|---|
| BDE-47 | 113 (34) | 68 (20) | 116 (35) | NA | 37 (11) |
| BDE-99 | 148 (44) | 49 (15) | 81 (24) | NA | 56 (17) |
| BDE-100 | 155 (46) | NA | 117 (35) | NA | 32 (10) |
| BDE-153 | 88 (26) | NA | NA | 185 (55) | 61 (18) |
Predictors of trajectory assignment
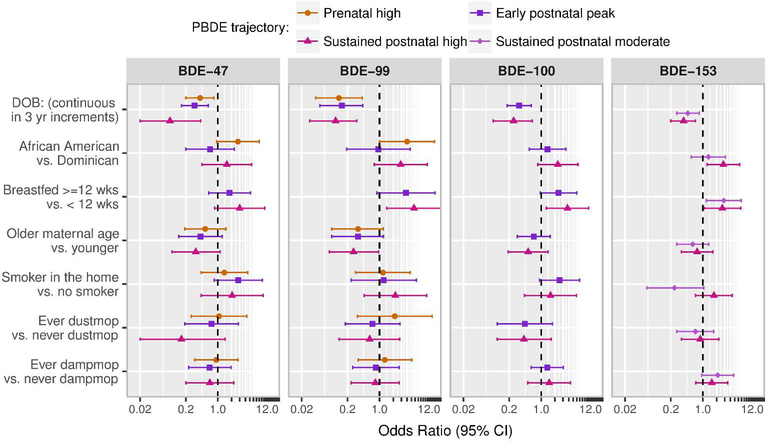
Figure 3 presents odds ratio (OR) estimates from multivariable multinomial models examining determinants of PBDE trajectory membership, which were fit within the LCGA modeling framework. In all models, the ‘persistent low’ trajectory serves as the reference category. Four children are excluded from these models due to missing information on breastfeeding history. Consistent with changes in concentration over time, year of birth was the most important determinant of trajectory assignment; across congeners, children born later in the cohort were significantly less likely to be assigned to the ‘prenatal high’ (ORBDE-47=0.41, 95% CI: 0.20, 0.82; ORBDE-99=0.16, 95% CI: 0.07, 0.37), ‘early postnatal peak’ (ORBDE-47=0.31, 95% CI: 0.16, 0.62; ORBDE-99=0.28, 95% CI: 0.13, 0.59, ORBDE-100=0.33, 95% CI: 0.18, 0.61), or ‘sustained postnatal high’ (ORBDE-47=0.09, 95% CI: 0.02, 0.42; ORBDE-99=0.27, 95% CI: 0.13, 0.58, ORBDE-100=0.25, 95% CI: 0.09, 0.64, ORBDE-153=0.38, 95% CI: 0.20, 0.69) versus the ‘persistent low’ trajectory. In addition to year of birth, the following variables met our criteria (bivariate p-value <0.10) for inclusion in multivariable models: ethnicity, maternal age at delivery, breastfeeding duration, presence of a cigarette smoker residing in the home, dust mopping the home, and damp mopping the home. For time-varying covariates (smoker in the home and household cleaning behaviors), we modeled predictors collected at the prenatal, 3-year and 7-year study visits for the ‘prenatal high’, ‘early postnatal peak’ and ‘sustained postnatal high’ trajectories, respectively.
Figure 3.
Odds ratios (ORs) from multivariable multinomial models examining determinants of PBDE trajectories over early life. The ‘persistent low’ trajectory serves as the reference category.
OR from models examining breastfeeding as a predictor of the prenatal high trajectory are not plotted due to the small number of breastfed children that were assigned to this trajectory and resulting wide confidence intervals.
In general, across trajectories and congeners, African American (versus Dominican) ethnicity, younger maternal age at delivery, longer breastfeeding duration, and living in a household with an active smoker were associated with higher odds of assignment to the ‘prenatal high’, ‘early postnatal peak’ or ‘sustained postnatal high’ trajectories versus the ‘persistent low’ trajectory (see Figure 3). With regard to cleaning behaviors, dust mopping was associated with lower odds of assignment to the ‘sustained postnatal high’ BDE-47 trajectory; however, this association was imprecisely estimated given the relatively low prevalence of dust mopping in the cohort (20% at the 7-year visit). In contrast, while dust mopping was not associated with the ‘sustained postnatal moderate’ or ‘postnatal high’ trajectories of BDE-153, children in households that used a damp mop were more likely to be assigned to these groups.
Discussion
In the present analysis, we measured plasma PBDE concentrations over a 15-year period. Given our relatively large sample size and the frequency of repeated measures, this study provides one of the most comprehensive PBDE exposure assessments that has been conducted among children to date. Controlling for age, we found that concentrations of congeners in the pentaBDE technical mixture, which was phased out of U.S. commerce in 2004, significantly decreased between 1998 and 2013.
Several previous studies have examined temporal trends in PBDE concentrations with inconsistent findings. For example, while Zota et al. found geometric mean pentaBDE concentrations decreased by 65% between 2008/2009 and 2011/2012 among two small samples of pregnant women living in California26, Hurley et al. found that serum pentaBDE concentrations increased marginally among 1253 older women (40–94 years) living in California between 2011 to 201523. Notably, it is difficult to compare our findings to previous research investigating temporal trends, as the majority of studies have examined adult populations and/or have been limited to relatively short time frames that did not span the pentaBDE phase-out. When examining changes in concentration across age, we found that average plasma pentaBDE concentrations were consistent with other U.S.-based longitudinal37–41 and cross-sectional21, 24, 42–50 studies of children, except that we detected slightly lower concentrations of BDEs-47, −99 and −100 at older ages and lower concentrations of BDE-153 at all ages (Supplemental Material, Table S4 and Figure S5).
In addition to examining average changes over time and age, we used LCGA to identify children with similar developmental patterns of PBDE exposure over early life. While this method is used extensively in psychology and the social sciences (i.e. criminology, econometrics, sociology), it has rarely been used in the field of epidemiology. When it has been applied, it has typically been used to model exposure to social risk factors (i.e. violence51, socioeconomic status52) or health outcomes (i.e. obesity53, wheeze54) over time. Despite its applicability to the field of exposure science, we know of no studies that have used LCGA to model changes in biomarker concentrations over time. Our finding of peak PBDE concentrations during toddler years is consistent with results from cross-sectional studies that indicate exposure peaks at approximately 2–3 years among a majority of children25. Other studies have found that PBDE concentrations peak between 4–6 years24, which is consistent with our identification of a ‘sustained postnatal high’ trajectory. The different trajectory patterns we observed for BDEs-47, −99, and −100 versus BDE-153 may be attributable to both differential exposure sources and toxicokinetics. Specifically, BDE-153 is more readily stored in lipid compartments compared to the other three congeners, which may reflect its slower rate of enzymatic metabolism55. Given its longer half-life20, BDE-153 concentrations within the body are expected to increase with age relative to the other congeners56; this is consistent with our finding of no decreasing BDE-153 trajectory. Further, given its high lipophilicity, dietary sources including breast milk, may contribute more to BDE-153 exposure compared to the other three congeners investigated. A limitation of this analysis is the lack of information on maternal and child diet.
Overall, the presence of different developmental trajectories suggests that a single measure may not accurately reflect exposure to PBDEs throughout the early lifecourse. Further, while trajectories were generally similar for BDEs-47, −99 and −100, plasma concentrations of BDE-153 followed a unique pattern, indicating that summed measures of these congeners may reflect different proportional contributions from BDEs-47, −99, and −100 versus BDE-153 depending on the age at sample collection.
We found that maternal age was associated with lower odds of assignment to the BDE-47 and BDE-99 ‘prenatal high’ trajectories, which is consistent with previous research44 and suggests that, unlike other legacy persistent organic pollutants57, PBDE body burdens may not increase with age among adults. Notably, lipophilic chemicals with long half-lives are not expected to differentiate from more rapidly eliminated chemicals until at least 20 years following peak exposure, thus it is plausible that the lack of an association between cord plasma PBDE concentrations and maternal age reflects the relatively limited temporal range of PBDE data, most of which were collected during the transition period following peak PBDE use58. Future research conducted after PBDEs have attained a steady-state in human tissues will be needed to determine whether the age-PBDE concentration trend we observed reflects the timing of study completion (during active PBDE use) or is related to toxicokinetic properties of PBDEs that differ from other persistent organic pollutants.
Our finding that children born to African American (versus Dominican) mothers had higher odds of assignment to the ‘prenatal high’ trajectory likely reflect differences in maternal body burden related to lifetime residential history. Specifically, while all study children were born in New York City, the majority of Dominican mothers (67%) were born in the Dominican Republic, where PBDEs may not have been used as extensively in consumer products. We observed a similar effect of ethnicity on assignment to the ‘sustained postnatal high’ trajectory, which may reflect differences in cleaning behaviors or other cultural differences between African American and Dominican households.
Consistent with previous research demonstrating breastfeeding as a pathway of PBDE exposure15, children who were breastfed 12 weeks or longer were more likely to be assigned to the sustained postnatal high trajectory. Unexpectedly, breastfed children were also more likely to have high prenatal BDE plasma concentrations. In this cohort, breastfeeding (<12 weeks vs. ≥ 12 weeks) was associated with indicators of low socioeconomic status, such as material hardship (OR=1.66, 95% CI: 1.20, 2.82). It is possible that breastfeeding is serving as an indicator of unmeasured cultural or socioeconomic factors associated with PBDEs, such as the use of second-hand or deteriorating household furniture, which may be more likely to contain (due to older age) and leach (due to greater wear and tear) PBDEs. Children born into households with an active smoker were also more likely to be assigned to the ‘prenatal high’ trajectory. The direction of this finding is consistent with the results of a U.S.-based study that detected higher hand wipe PBDE concentrations among young children living in homes with an active smoker59. It is unlikely that cigarettes are a direct source of PBDEs; however, similar to our breastfeeding findings, it is possible that smoking may serve as an indicator of unmeasured socioeconomic factors related to PBDE exposure.
With regard to cleaning behaviors, children in households that used a dust mop were less likely to have high concentrations of BDEs-47, 99 and 100 throughout childhood. In contrast, children in households that reported using a damp mop were more likely to have moderate or high BDE-153 concentrations throughout childhood. While unexpected, this later finding is consistent with results from the Spain-based INMA cohort, which found more frequent housekeeping (>1 times /week, including sweeping, vacuuming, dusting, and mopping) was associated with significantly higher serum concentrations of BDE-153, but not the other congeners, among pregnant women60.
The U.S. Environmental Protection Agency recommends that parents dust, wet mop and use a vacuum with a high efficiency particulate air (HEPA) filter to reduce children’s exposure to flame retardants in dust61; however, given our inconsistent findings related to cleaning behaviors, further research, including household intervention studies, is need to better understand what behavioral modifications are most effective for reducing exposure.
Strengths of our study include the large sample size, variation in both the chronological date and child age of blood collection, and the rich set of prospectively collected covariate data, including information on cleaning behaviors. Specific strengths of LCGA include the ability to retain all children with data at a minimum of one follow-up period, as well as the ability to model variation in the age at blood draw within follow-up periods. Moreover, future research can employ LCGA modeling to investigate the impact of exposure timing on distal health outcomes among children. Importantly, although our data met the generally accepted posterior probability threshold for trajectory assignment, it is possible that some children were misclassified, which may have biased our findings towards the null.
Concurrent with the voluntary 2004 industry phase-out of pentaBDE, New York State passed an environmental law that codified the prohibition of pentaBDE production and use62. Despite these regulatory changes, PBDEs continue to leach from existing consumer products and migrate into house dust. Indeed, in the present study, we detected PBDE concentrations in approximately 80% of cord plasma samples collected between 1998 and 2006, and 100% of child (ages 2–9 years) samples collected between 2000 and 2013. Moreover, our finding of lower plasma BDE-47 concentrations among children who were 7–9 years old in 2011–2012 versus 2005 suggests that while pentaBDE concentrations have been decreasing since their 2004 phase-out, they continue to be detectable in the blood of young children nearly 10 years following their removal from U.S. commerce.
Our findings of several unique PBDE trajectories may inform future research studies as well as interventions designed to target specific windows of peak exposure. Importantly, in the United States, the majority of furniture and other household items containing polyurethane foam are disposed of in landfills. For example, approximately 1.3 million tons of carpet/padding, furniture, and other bulky items were disposed of in California landfills in the year 2004 alone63. With more PBDE-containing items entering end-of-life waste streams in the coming decades, shifts in environmental contamination patterns due to leaching from outdoor reservoirs may trigger a transition in human exposure pathways from dust to dietary sources (fatty fish, seafood, meat, dairy)64. As time since the pentaBDE phase-out elapses, monitoring landfills, surrounding environmental media, and wildlife will be critical for understanding shifts in exposure pathways and reducing human exposure.
Supplementary Material
Acknowledgements:
This research was supported by NIH R01 ES021806. During preparation of this manuscript, WJC was supported by NIH T32 ES023772, NIH T32 ES007322 and EPA FP-91779001. We gratefully acknowledge the contribution of Miss Shenika Christopher who helped to identify potential determinants of interest for investigation in this study.
Footnotes
Competing financial interests: The authors declare they have no actual or potential competing financial interests.
Disclaimer: The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. This publication was developed under STAR Fellowship Assistance Agreement no. FP-91779001 awarded by the U.S. Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this publication are solely those of the authors.
References
- 1.Callahan P, Roe S, Hawthorne M. Playing with Fire. Chicago: Tribune; 2012. [Google Scholar]
- 2.Cal-117. Requirements, Test Procedure and Apparatus for Testing the Flame Retardance of Resilient Filling Materials used in Upholstered Furniture (Technical Bulletin 117). In: Furnishings CBoTIaH, (ed) [Modified 1990], 1975.
- 3.EPA. An Exposure Assessment of Polybrominated Diphenyl Ethers. In: Assessment NCfE, (ed). Washington, DC: Environmental Protection Agency, 2010. [Google Scholar]
- 4.Corportation GLC. Great Lakes Chemical Corporation completes phase-out of two flame retardants. In. Indianapolic, IN: Great Lakes Chemical Corportation, 2005. [Google Scholar]
- 5.EPA. DecaBDE Phase-out Initiative. In. Washington D.C., United States: United States Environmental Protection Agency: Chemical Safety and Pollution Prevention, 2015. [Google Scholar]
- 6.Fromme H, Becher G, Hilger B, Volkel W Brominated flame retardants - Exposure and risk assessment for the general population. Int J Hyg Environ Health 2016; 219: 1–23. [DOI] [PubMed] [Google Scholar]
- 7.Linares V, Belles M, Domingo JL Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol 2015; 89: 335–356. [DOI] [PubMed] [Google Scholar]
- 8.Talsness CE Overview of toxicological aspects of polybrominated diphenyl ethers: a flame-retardant additive in several consumer products. Environmental research 2008; 108: 158–167. [DOI] [PubMed] [Google Scholar]
- 9.UNEP. United Nations Environment Programme Technical review of the implications of reclycling commercial Penta and Octabromodiphenyl ethers, 2010. [Google Scholar]
- 10.Alcock RE, Sweetman AJ, Prevedouros K, Jones KC Understanding levels and trends of BDE-47 in the UK and North America: an assessment of principal reservoirs and source inputs. Environment international 2003; 29: 691–698. [DOI] [PubMed] [Google Scholar]
- 11.Hites RA Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environmental science & technology 2004; 38: 945–956. [DOI] [PubMed] [Google Scholar]
- 12.Cobb D Analysis of FR chemicals added to foams, fabric, batting, loose fill and barriers.. Memorandum to Dale R Ray, Project Manager, Upholstered Furniture, Consumer Products Safety Commission 2005.
- 13.Abbasi G, Buser AM, Soehl A, Murray MW, Diamond ML Stocks and flows of PBDEs in products from use to waste in the U.S. and Canada from 1970 to 2020. Environmental science & technology 2015; 49: 1521–1528. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Diamond ML, Robson M, Harrad S Sources, emissions, and fate of polybrominated diphenyl ethers and polychlorinated biphenyls indoors in Toronto, Canada. Environmental science & technology 2011; 45: 3268–3274. [DOI] [PubMed] [Google Scholar]
- 15.Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE Human internal and external exposure to PBDEs--a review of levels and sources. Int J Hyg Environ Health 2009; 212: 109–134. [DOI] [PubMed] [Google Scholar]
- 16.Lorber M Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol 2008; 18: 2–19. [DOI] [PubMed] [Google Scholar]
- 17.ATSDR. Toxicological profile for polybrominated biphenyls and polybrominated diphenyl ethers (PBDEs). In: Registry AfTSD, (ed). Atlanta, GA, 2017. [PubMed] [Google Scholar]
- 18.Dassanayake RM, Wei H, Chen RC, Li A Optimization of the matrix solid phase dispersion extraction procedure for the analysis of polybrominated diphenyl ethers in human placenta. Analytical chemistry 2009; 81: 9795–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang J, Nyberg E, Winnberg U, Bignert A, Bergman A Spatial and temporal trends of the Stockholm Convention POPs in mothers’ milk -- a global review. Environ Sci Pollut Res Int 2015; 22: 8989–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyer H, Schramm K, Darnerud P, Aune M, Feicht E, Fried K Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Comp 2004; 66: 5. [Google Scholar]
- 21.Lunder S, Hovander L, Athanassiadis I, Bergman A Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environmental science & technology 2010; 44: 5256–5262. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman K, Webster TF, Sjodin A, Stapleton HM Toddler’s behavior and its impacts on exposure to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol 2017; 27: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurley S, Goldberg D, Nelson DO, Guo W, Wang Y, Baek HG et al. Temporal Evaluation of Polybrominated Diphenyl Ether (PBDE) Serum Levels in Middle-Aged and Older California Women, 2011–2015. Environmental science & technology 2017; 51: 4697–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjodin A, Schecter A, Jones R, Wong LY, Colacino JA, Malik-Bass N et al. Polybrominated diphenyl ethers, 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB-153), and p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE) concentrations in sera collected in 2009 from Texas children. Environmental science & technology 2014; 48: 8196–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toms LM, Sjodin A, Harden F, Hobson P, Jones R, Edenfield E et al. Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) than in infants and adults. Environmental health perspectives 2009; 117: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML et al. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environmental science & technology 2013; 47: 11776–11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental health perspectives 2006; 114: 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer S, Jencks C Poverty and the distribition of material hardship. J Hum Resour 1988. 88–112. [Google Scholar]
- 29.Jones R, Edenfield E, Anderson S, Zhang Y, Sjodin A Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Comp 2012; 74: 97–98. [Google Scholar]
- 30.Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE 3rd, Patterson DG Jr. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Analytical chemistry 2004; 76: 1921–1927. [DOI] [PubMed] [Google Scholar]
- 31.Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr., Henderson LO, Needham LL Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol 1989; 18: 495–500. [DOI] [PubMed] [Google Scholar]
- 32.Cowell WJ, Sjodin A, Jones R, Wang Y, Wang S, Herbstman JB Determinants of prenatal exposure to polybrominated diphenyl ethers (PBDEs) among urban, minority infants born between 1998–2006. Environmental Pollution 2018; 223: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baccarelli A, Pfeiffer R, Consonni D, Pesatori AC, Bonzini M, Patterson DG Jr. et al. Handling of dioxin measurement data in the presence of non-detectable values: overview of available methods and their application in the Seveso chloracne study. Chemosphere 2005; 60: 898–906. [DOI] [PubMed] [Google Scholar]
- 34.Nagin DS Group-based trajectory modeling: an overview. Ann Nutr Metab 2014; 65: 205–210. [DOI] [PubMed] [Google Scholar]
- 35.Jones B, Nagin D, KA R SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methodology Research 2001; 29: 374–393. [Google Scholar]
- 36.Nagin D Group-based modeling of development. Harvard University Press: Cambridge, Massachusetts,, 2005. [Google Scholar]
- 37.Castorina R, Bradman A, Sjodin A, Fenster L, Jones RS, Harley KG et al. Determinants of serum polybrominated diphenyl ether (PBDE) levels among pregnant women in the CHAMACOS cohort. Environmental science & technology 2011; 45: 6553–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environmental health perspectives 2013; 121: 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagiv SK, Kogut K, Gaspar FW, Gunier RB, Harley KG, Parra K et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicology and teratology 2015; 52: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuong AM, Braun JM, Yolton K, Xie C, Webster GM, Sjodin A et al. Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environmental research 2017; 153: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vuong AM, Webster GM, Romano ME, Braun JM, Zoeller RT, Hoofnagle AN et al. Maternal Polybrominated Diphenyl Ether (PBDE) Exposure and Thyroid Hormones in Maternal and Cord Sera: The HOME Study, Cincinnati, USA. Environmental health perspectives 2015; 123: 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gump BB, Yun S, Kannan K Polybrominated diphenyl ether (PBDE) exposure in children: possible associations with cardiovascular and psychological functions. Environmental research 2014; 132: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU et al. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environmental health perspectives 2007; 115: 1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V et al. Prenatal exposure to PBDEs and neurodevelopment. Environmental health perspectives 2010; 118: 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horton MK, Bousleiman S, Jones R, Sjodin A, Liu X, Whyatt R et al. Predictors of serum concentrations of polybrominated flame retardants among healthy pregnant women in an urban environment: a cross-sectional study. Environmental health : a global access science source 2013; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobson MH, Barr DB, Marcus M, Muir AB, Lyles RH, Howards PP et al. Serum polybrominated diphenyl ether concentrations and thyroid function in young children. Environmental research 2016; 149: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM Polybrominated diphenyl ethers in maternal and fetal blood samples. Environmental health perspectives 2003; 111: 1249–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rose M, Bennett DH, Bergman A, Fangstrom B, Pessah IN, Hertz-Picciotto I PBDEs in 2–5 year-old children from California and associations with diet and indoor environment. Environmental science & technology 2010; 44: 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environmental health perspectives 2011; 119: 1454–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stapleton HM, Eagle S, Sjodin A, Webster TF Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environmental health perspectives 2012; 120: 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baskin D, Sommers I Trajectories of exposure to community violence and mental health symptoms among serious adolescent offenders. Criminal Justice and Behavior 2015; 42: 587–609. [Google Scholar]
- 52.Azad MB, Lissitsyn Y, Miller GE, Becker AB, HayGlass KT, Kozyrskyj AL Influence of socioeconomic status trajectories on innate immune responsiveness in children. PloS one 2012; 7: e38669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziyab AH, Karmaus W, Kurukulaaratchy RJ, Zhang H, Arshad SH Developmental trajectories of Body Mass Index from infancy to 18 years of age: prenatal determinants and health consequences. Journal of epidemiology and community health 2014; 68: 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Q, Just AC, Miller RL, Perzanowski MS, Goldstein IF, Perera FP et al. Using latent class growth analysis to identify childhood wheeze phenotypes in an urban birth cohort. Ann Allergy Asthma Immunol 2012; 108: 311–315 e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lupton SJ, McGarrigle BP, Olson JR, Wood TD, Aga DS Human liver microsomemediated metabolism of brominated diphenyl ethers 47, 99, and 153 and identification of their major metabolites. Chem Res Toxicol 2009; 22: 1802–1809. [DOI] [PubMed] [Google Scholar]
- 56.Bramwell L, Glinianaia SV, Rankin J, Rose M, Fernandes A, Harrad S et al. Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review. Environment international 2016; 92–93: 680–694. [DOI] [PubMed] [Google Scholar]
- 57.Gyalpo T, Toms LM, Mueller JF, Harden FA, Scheringer M, Hungerbuhler K Insights into PBDE Uptake, Body Burden, and Elimination Gained from Australian Age-Concentration Trends Observed Shortly after Peak Exposure. Environmental health perspectives 2015; 123: 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quinn CL, Wania F Understanding differences in the body burden-age relationships of bioaccumulating contaminants based on population cross sections versus individuals. Environmental health perspectives 2012; 120: 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darrow LA, Jacobson MH, Preston EV, Lee GE, Panuwet P, Hunter RE Jr. et al. Predictors of Serum Polybrominated Diphenyl Ether (PBDE) Concentrations among Children Aged 1–5 Years. Environmental science & technology 2017; 51: 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costa O, Lopez-Espinosa MJ, Vizcaino E, Murcia M, Iniguez C, Navarrete-Munoz EM et al. Dietary and Household Sources of Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) in the INMA Birth Cohort (Spain). Environmental science & technology 2016; 50: 5935–5944. [DOI] [PubMed] [Google Scholar]
- 61.EPA . Reducing your child’s exposure to flame retardant chemicals. In, 2016.
- 62.S07621/A10050-A. NY State Senate Bill. In, 2004.
- 63.Petreas M, Oros D Polybrominated diphenyl ethers in California wastestreams. Chemosphere 2009; 74: 996–1001. [DOI] [PubMed] [Google Scholar]
- 64.Shaw SD, Blum A, Weber R, Kannan K, Rich D, Lucas D et al. Halogenated flame retardants: do the fire safety benefits justify the risks? Rev Environ Health 2010; 25: 261–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.