Abstract
The American Diabetes Association recommends annual assessment of glomerular filtration rate (GFR) to screen for diabetic nephropathy. GFR is measured indirectly using markers that, ideally, are eliminated only by glomerular filtration. Measured GFR, although the gold standard, remains cumbersome and expensive. GFR is therefore routinely estimated using creatinine and/or cystatin C and clinical variables. In pediatrics, the Schwartz creatinine-based equation is most frequently used even though combined creatinine and cystatin C-based equations demonstrate stronger agreement with measured GFR. In adults, the CKD Epidemiology Collaboration (CKD-EPI) equations with creatinine and/or cystatin C are the most accurate and precise estimating equations. Despite recent advances, current estimates of GFR lack precision and accuracy before chronic kidney disease stage 3 (GFR<60mL/min/1.73m2). There is therefore an urgent need to improve the methods for estimating and measuring GFR. In this review we examine the current literature and data addressing measurement and estimation of GFR in diabetes.
Keywords: Glomerular filtration rate, cystatin C, creatinine, iohexol, diabetic kidney disease, renal hyperfiltration, rapid GFR decline, albuminuria, impaired GFR
Introduction
Assessment of renal function through the measurement of glomerular filtration rate (GFR) is an essential tool for nephrologists and diabetologists caring for patients with type 1 and 2 diabetes. GFR evaluation is crucial for the diagnosis of early (renal hyperfiltration [GFR greater than 120–150mL/min/1.73m2) and rapid GFR decline [annual GFR loss greater than 3mL/min/1.73m2 or > 3.3%/year] as well as late phenotypes of diabetic kidney disease (impaired GFR [<60mL/min/1.73m2]). Furthermore, individual GFR trajectories over time are strongly associated with incident chronic kidney disease (1, 2). Accordingly, the American Diabetes Association recommends routine screening of GFR in adults with diabetes, and recently expanded screening recommendations to include adolescents with diabetes (3, 4).
The first widely used GFR estimating equations were developed four decades ago to estimate GFR in adults from serum creatinine concentrations. In recent years, a number of new equations have been developed based on serum creatinine and cystatin C assays. Despite advances in GFR measurement, current estimates of GFR lack precision (i.e. too much random error) and accuracy (i.e. too much systematic error) before chronic kidney disease stage 3 (GFR <60 mL/min/1.73m2) (5). A recent DCCT-EDIC paper also reported that changes in eGFR over a 3 year period may not reflect changes in measured GFR (6, 7). This is of particular concern in adolescents and young adults with diabetes, in whom renal hyperfiltration is present in approximately 50% of individuals and which may promote renal injury (8, 9). The dissociation between changes in eGFR vs. measured GFR is of further concern since rapid changes in GFR may be missed due to a lack of acceptable screening methods for subtle changes in renal function (10).
Diabetic nephropathy is the leading cause of end-stage renal disease and dialysis in the US, and is characterized by a long clinically silent period without signs or symptoms of disease (10, 11). In 2009, overall Medicare expenditure for people with chronic kidney disease and diabetes accounted for $18 billion (11). There is therefore an urgent need for improved methods of estimating and measuring GFR. Accordingly, in this review we examine the current literature and data addressing measurement and estimation of GFR in diabetes. We also focus on the early phenotypes of diabetic kidney disease and their potential treatment, including renal hyperfiltration and rapid GFR decline, review of current methods to estimate and measure GFR, challenges that are specific to diabetes, and current and potential treatments to prevent diabetic kidney disease.
Early Diabetic Kidney Disease
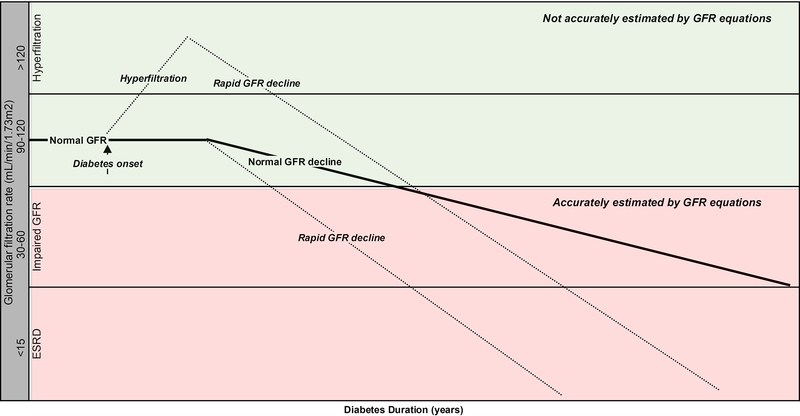
Diabetic nephropathy is the leading cause of end-stage renal disease (ESRD) in the western world (10, 12–14). In fact, the 2011 US Renal Data System showed that diabetic nephropathy accounted for 44.5% of all cases of ESRD in the United States in 2009 (11). Diabetic nephropathy is also an important risk factor for coronary artery disease (CAD) (15–17) and overall mortality (15, 18). The natural history of diabetic nephropathy is characterized by a long silent period without overt clinical signs and symptoms of nephropathy (Figure 1). For that reason, early detection of diabetic nephropathy may have a pivotal role in the prevention of ESRD in diabetes (19). While the appearance of microalbuminuria is often the earliest clinical sign of diabetic nephropathy, this classical paradigm has been questioned over the past few years after the demonstration that microalbuminuria does not necessarily imply progressive nephropathy, and may in fact regress to normoalbuminuria (20, 21), and that CKD stage 3 can develop in the absence of microalbuminuria (7, 22). Albuminuria is still an important risk factor in diabetes as it is strongly associated with dyslipidemia and cardiovascular disease (23, 24). Renal hyperfiltration is typically defined by a glomerular filtration rate (GFR) of between 120 mL/min to 150 mL/min/1.73m2, or greater than 2 standard deviations above the mean GFR in normal, healthy individuals (9), and is thought to represent the earliest hemodynamic abnormality seen in diabetes (25). Phenotypes of early diabetic nephropathy prior to the loss of renal function, such as renal hyperfiltration and rapid GFR decline are considered stronger predictors of nephropathy progression in type 1 diabetes than albuminuria (10, 26–30). For that reason, GFR is the most clinically relevant measure of kidney function in diabetes. The American Diabetes Association, National Kidney Foundation and International Society of Nephrology recommend annual measurement of estimated glomerular filtration rate to identify and monitor diabetic nephropathy (31–33). However, as we will review, current methods to measure or estimate GFR early in the pathogenesis of diabetic nephropathy in the normal to elevated GFR range present a particular challenge for physicians managing patients with diabetes.
Figure 1. Stages of Diabetic Nephropathy and Challenges of Determining GFR.
The area of the figure colored green represents glomerular filtration rates (GFR) not accurately estimated by creatinine and cystatin-C based equations. In contrast, the red area is typically accurately estimated by the equations.
Measurement of Glomerular Filtration Rate
GFR is measured indirectly as the clearance of exogenous filtration markers that are eliminated exclusively by glomerular filtration (Table 1). Such markers include inulin (which is considered the gold standard), iohexol, iothalamate, technetium 99m diethylenetriamine pentaacetic acid (99mTc-DTPA) and chromium 51-ethylenediaminetetraacetic acid (51Cr-EDTA). As an alternative, urinary clearance techniques are another direct method that can be used to measure GFR, but this remains inconvenient and associated with errors around the timing of urine collections. For these reasons, it is more common to measure GFR by plasma clearance. The major disadvantage of plasma clearance is the duration of testing needed to calculate the clearance curve accurately, which can be up to 8 hours. GFR measurements, by either urinary or plasma clearance techniques, therefore remain impractical and expensive and as a result are not routinely performed in clinical practice. For that reason, GFR is typically estimated through the use of serum concentrations of endogenous filtration markers (i.e. serum creatinine and/or cystatin C, as described below). Although estimated GFR is sufficient for clinical decision making in many circumstances, particularly when GFR is <60 ml/min/1.73m2 (34), patients with diabetes would likely benefit from having their GFR measured using more accurate and precise techniques, due to their increased risk of kidney disease. Furthermore, GFR measurements are estimates of renal function and are affected by excessive intake of drinks containing caffeine (35), protein load (36), exercise (37) and certain medications (e.g. diuretics, antibiotics) (38). Another major challenge specific to diabetes when estimating and measuring GFR is hyperglycemia which is known to influence GFR, as reviewed in detail below (39). The early diagnosis of declining renal function may be important due to the potential for early interventions aimed at delaying the progression to ESRD. Accordingly, to diagnose early diabetic nephropathy, accurate and precise diagnostic tools are necessary.
Table 1.
Methods to measure GFR in pediatrics and adults
| Exogenous filtration markers | Comparison with inulin | Reference | Pros | Cons |
|---|---|---|---|---|
|
Inulin - Plasma - Urinary |
(96) | - Classically considered gold-standard | - Expensive – the cost of Inutest (Fresenius Kabi Austria GmbH) has increased more than tenfold during recent years - Rigorous and time consuming - Inulin is viscous and slowly reaches its volume of distribution, requiring constant infusion rate with multiple repeat blood and urine collections and careful timing of blood sampling. |
|
|
51Cr-EDTA - Plasma - Urinary |
- Correlation: 0.94 - Difference: +2.2 to 2.8mL/min/1.72m2 |
(97) | - Isotopic marker freely filtered in glomeruli - Widely available in Europe |
- Radioactive - Time consuming - 10–12% is bound to plasma protein |
|
99m Tc-DTPA - Plasma - Urinary |
- Correlation: 0.97 - Difference: −15 to +21 mL/min/1.72m2 |
(98) | - Isotopic marker freely filtered in glomeruli - Widely available in US |
- Radioactive - Time consuming - 10–12% is bound to plasma protein |
|
125I-Iothalamate - Plasma |
- Correlation: N/A - Difference: +38% |
(99) | - Ionic contrast product - Long half-life |
- Cannot be used in patients with allergies to iodine - Radioactive - Non-radioactive iothalamate assay is expensive - 10–12% is bound to plasma protein |
|
Iohexol - Plasma - Dried blood spots |
- Correlation: 0.92 - Difference: +2.0 to 2.7mL/min/1.72m2 |
(100) | - Non-ionic contrast - Inexpensive - Not radioactive - Available on DBS - Used in doses 10–50x in neonates for radiographs |
- Cannot be used in patients with allergies to iodine - Time consuming - 2% is bound to plasma protein |
Estimation of Glomerular Filtration Rate
i. Estimation of GFR in adults
There are several equations available to estimate GFR in adults using endogenous filtration markers (serum creatinine and/or cystatin C) (Table 2). The most state-of-the-art equations are the three CKD-EPI equations: CKD-EPI Creatinine, CKD-EPI Cystatin C and CKD-EPI Creatinine and Cystatin C (5). The number of available equations is in part due to the nonequivalent results obtained from using different creatinine and cystatin C assays (40). More recently the accuracy of creatinine measurements has improved with the availability of higher-order reference methods (isotope dilution-mass spectrometry (IDMS) reference methods) (41, 42). Similarly, the number of equations using cystatin C is due to previous lack of an international cystatin C calibrator (40). In 2010, the first certified reference material for serum cystatin C was published (ERM-DA471/IFCC (43, 44). Assay reproducibility over time and between laboratories is important with GFR as it is a longitudinal measure of renal function in research and clinical care.
Table 2.
Adult GFR estimating equations
| Names | References | Equations |
|---|---|---|
| Serum creatinine-based | ||
| CKD-EPI Creatinine | (5) | eGFR = 141 × min(serum creatinine /κ) * max(serum creatinine/κ) * 0.993age * 1.018 [if female] * 1.159 [if African American] |
| MDRD | (101) | eGFR = 186 × [serum creatinine (umol/l)/88.4]−1.154 * [age (years)]−0.203 × (0.742 if female) × (1.21 if black) |
| Mayo quadratic equation | (102) | eGFR = (1.911 + (5.249/screat)- (2.114/(screat*screat))- (0.00686*age) – (0.205 if female)) |
| Cystatin C-based | ||
| CKD-EPI Cystatin C | (5) | eGFR = 133 × min(cystatin C/0.8, 1)−0.499 * max(cystatin C /0.8, 1)−1.328 * 0.996age [ × 0.932 if female] |
| Tan equation | (103) | eGFR = 87.1/ plasma cystatin C (mg/L) − 6.87 |
| Perkins equation | (104) | eGFR = 100/(cystatin C) |
| Arnal equation | (105) | eGFR = 74.835/(cystatin C1.333) |
| MacIsaac equation | (106) | eGFR = (84.6/cystatin) – 3.2 |
| Stevens equation | (107) | eGFR = 127.7/(cystatin C1.17) * [age (years)] −0.13 * (0.91 if female) * (1.06 if black) |
| CAPA | (40) | eGFR = 130 * cystatin C−1.069 * age−0.117 – 7 |
| Combined creatinine and cystatin C | ||
| CKD-EPI Creatinine and Cystatin C | (5) | eGFR = 135 × min(serum creatinine/k)−a× max(serum creatinine /k)−0.601* min(cystatin C/0.8)−0.375* max( cystatin C/0.8)−0.711* 0.995age[ * 0.969 if female ] [ * 1.08 if black ] |
| Stevens equation | (108) | eGFR = 177.6 * [serum creatinine (umol/l/88.4)] −0.65 * (cystatin C−0.57) * [age (years)] −0.20 * (0.82 if female) * (1.11 if black) |
Both serum creatinine and cystatin C are affected by factors other than GFR, i.e. non-GFR determinants, (Table 3), but cystatin C is considered to be less biased by age and weight compared to creatinine-based measurements, and correlates more closely with direct measures of GFR over a wide spectrum of plasma glucose levels compared to creatinine based measures in experimental studies (45, 46). These data suggest that cystatin C more accurately reflects measured GFR in subjects with type 1 diabetes, favoring its use as an estimate of GFR in this population. Cystatin C has also been shown to be associated with fat mass rather than lean mass in some but not all studies, which may impair the accuracy of GFR estimates by cystatin C in obese patients and in those with significant changes in adiposity (5, 6). The Prevention of Renal and Vascular End-Stage Disease (PREVEND) study also demonstrated an association between cystatin C and c-reactive protein (47), which may explain the association between fat mass and cystatin C, since higher body mass being associated with inflammation and insulin resistance (48, 49). In contrast to cystatin C based equations, creatinine-based GFR estimates are influenced by other confounders, including filtration fraction (FF = GFR/effective renal plasma flow) (50). This implies that creatinine is affected by hyperfiltration and therefore weakens its diagnostic performance as a GFR marker in the presence of hyperfiltration (50), an interaction that has been reported by our group (46).
Table 3.
Non-GFR determinants of creatinine and cystatin C
| Creatinine | Cystatin C |
|---|---|
| Increasing age ↑ | Increasing age ↑ |
| Male gender ↑ | Male gender ↑ |
| Lean muscle mass ↑ | Thyroid dysfunction (inconclusive) |
| Malnutrition | Fat mass (inconclusive) |
| Protein diet ↑ | Inflammation (inconclusive) |
| Inflammation ↑ | Very large doses of glucocorticoids ↑ |
| Certain medications (cimetidine, trimethoprim, dronedarone, cobicistat) |
GFR estimated by cystatin C also appears to better predict micro- and macrovascular complications in subjects with type 1 diabetes compared to creatinine-based equations (17, 26, 49, 51). Cystatin C more accurately detects rapid GFR decline than creatinine-based measurements in type 1 diabetes subjects with normal renal function (51). Rapid GFR decline estimated by cystatin C is also associated with a higher risk for cardiovascular complications and mortality than creatinine based GFR estimated (52, 53). Furthermore, Skupien et al demonstrated that GFR staging with cystatin C is superior for predicting ESRD and mortality compared to GFR with creatinine and cystatin C, which suggests that serum creatinine counters the predictive ability of serum cystatin C in adults with type 1 diabetes (54). Finally, Shlipak et al demonstrated that the use of cystatin C compared to creatinine strengthens the association between eGFR and risk of death and ESRD in 11 diverse general-population studies that included both diabetic and non-diabetic participants (27). One limitation to the use of cystatin C has been the cost (approximately USD $5–6 vs. $1–3 for serum creatinine), although this difference is much less than in the past.
Despite the possible superiority of cystatin C compared to creatinine, estimates of GFR by both serum creatinine and cystatin remain imperfect (17, 55, 56). The creatinine and cystatin-C based eGFR equations are associated with greater variability when eGFR >60 mL/min/1.73m2 (5). However, by the time eGFR is ≤60 mL/min/1.73m2 almost half of renal function has already been lost (32). A study by Rognant et al. demonstrated that the mean absolute bias of CKD-EPI creatinine was −12.7 ± 12 mL/min/1.73 m2 compared to measured GFR, with an interquartile range of 16 mL/min/1.73m2, and 10% (P10) and 30% (P30) accuracies were, respectively 28.0 and 80.1% (57). In other words, 28% of study participants had GFR estimates with less than a 10% bias, and 80.1% had GFR estimates with a bias up to 30% compared to measured GFR (this would be an eGFR of 70–130 mL/min/1.73 m2 for a ‘true’ GFR of 100 mL/min/1.73 m2). Furthermore, when eGFR is >90mL/min/1.73m2, agreement (concordance) between eGFR calculated by CKD-EPI cystatin C and eGFR calculated by CKD-EPI creatinine in the same individual has been reported to be as low as 56% (53, 58). For that reason, improved methods to easily and accurately measure GFR as well as changes in renal function in the normal and hyperfiltration range are needed (10, 59).
ii. Estimation of GFR in pediatrics
The American Diabetes Association recently recommended routine screening of glomerular filtration rate (GFR) in adolescents with type 1 diabetes in the Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association from 2014 and in the 2015 Standards of Care, although this is not yet routinely performed clinically (3, 4). There are developmental changes during childhood and adolescence that affect measurement of GFR, including growth spurts due to the rapid increase in muscle mass and extracellular volume (60). Numerous equations have been formulated to estimate GFR in pediatric and adolescent patients (61, 62) (Table 4). These equations are derived from different populations with a variety of underlying nephropathies and with significant variability in GFR (Table 4). To our knowledge, no single equation has been specifically developed or validated in adolescents with type 1 or type 2 diabetes. Furthermore, the majority of GFR equations have been based on non-standardized creatinine and cystatin C assays (63). The Schwartz creatinine based equation from 2009, adjusted to be traceable to isotope dilution mass spectrometry, is the most widely used in clinical practice, but has been demonstrated to be most accurate in the range of 25–75 mL/min/1.73m2 (64). Stronger agreement with measured GFR is observed with cystatin C and combined creatinine and cystatin C equations (e.g. CKiD, Schwartz, Bouvet combined creatinine and cystatin C equations) compared to creatinine equations (62, 64). Berg et al. recently reported unstable performance of standardized creatinine based equations across levels of measured GFR in comparison with standardized cystatin C equations in children (63). The diagnostic accuracy of the various cystatin C and combined creatinine cystatin C equations varies with GFR (65), which is in part a function of the GFR levels in the cohorts of patients used to derive the equations (Table 4). For instance, the CKiD and Zappitelli equations demonstrate higher accuracy in GFR <90mL/min/1.73m2, whereas the Bouvet, Bokenkamp and Filler equations have greater accuracy in GFR categories ≥135mL/min/1.73m2 (65). For that reason, applying these equations in clinical practice and research requires knowledge of the expected GFR, which is not always possible. The lack of a single equation that performs well across the span of GFR in pediatrics has limited the use of estimating equations to measure longitudinal changes in GFR. Recently, Berg et al. demonstrated accuracy (P30, percentage of GFR estimates within 30% of measured GFR) of 75%, 85%, 88%, 83% and 95% for GFR estimated by Berg cystatin C equation in children with inulin measured GFR of <30, 30–59, 60–89, 90–119 and ≥120 mL/min/1.73m2 respectively (63). Caucasian and Asian pediatric and adult subjects (CAPA) equations also performed well across the span of GFR with accuracy of 80%, 85%, 98%, 84% and 90% for inulin measured GFR of <30, 30–59, 60–89, 90–119 and ≥120 mL/min/1.73m2 respectively (63).
Table 4.
Pediatric GFR estimating equations
| Name | Equation | Reference Method | Cohort GFR Median or Range Used for Data Generation | References |
|---|---|---|---|---|
| Serum creatinine-based | ||||
| Schwartz | eGFR = [length (cm) * k] / Scr k = 0.45 for infants 1 to 52 weeks old k = 0.55 for children 1 to 13 years old k = 0.55 for adolescent females 13–18 years old k = 0.7 for adolescent males 13–18 years old |
Inulin | 3–220 mL/min/1.73m2 ¥ | (109) |
| Schwartz 2009 | eGFR = 0.413 * Ht / Scr | Iohexol | 41 mL/min/1.73m2 | (62) |
| Cystatin C-based | ||||
| Filler | eGFR = 10(1.962 + (1.123 * log(1/cystatin C)) | 99mTc-DTPA | 103 mL/min/1.73m2 | (65) |
| Bökenkamp | eGFR = 137/cystatin C – 20.4 | Inulin | 77 mL/min/1.73m2 | (65) |
| Berg | eGFR = 91 * cystatin C−1.213 | Inulin | 84 mL/min/1.73m2 | (63) |
| CAPA | eGFR = 130 * cystatin C−1.069 * age−0.117 − 7 | Inulin and iohexol | 103 mL/min/1.73m2 | (40) |
| Combined creatinine and cystatin C | ||||
| Zappitelli | eGFR = 25.38[1/CysC]0.331[1/Scr]0.602[1.88height] | Iothalamate | 74 mL/min/1.73m2 | (65) |
| Bouvet | eGFR = 46.1[1.2/CysC]0.33[(96/88.4)/(Scr)]0.55[weight/45]0.77[age/14]0.17 | 51Cr-EDTA | 92 mL/min/1.73m2 | (110) |
| Schwartz | eGFR = 39.1[height (m)/Scr (mg/dl)](0.516) x [1.8/cystatin C (mg/L)](0.294)[30/BUN (mg/dl)](0.169)[1.099](male)[height (m)/1.4](0.188) | Iohexol | 41 mL/min/1.73m2 | (62) |
Schwartz et al. measured GFR on 186 subjects between ages of 6 month to 20 years, and the GFR distribution had a bimodal appearance, with peaks in the 10–30mL/min/1.73m2 and 110–140mL/min/1.73m2. The 10–30 are those with advanced renal disease, and 110–140mL/min/1.73m2 are those children with normal kidney function (109).
While these newer standardized equations hold promise, there are no currently published equations that have been validated against measured GFR in youth with diabetes. An additional challenge in measuring GFR in diabetes is the acute effect of blood glucose, as discussed below. This is of particular concern in adolescents and young adults with type 1 diabetes, in whom renal hyperfiltration is common and may promote renal injury, or rapid change in GFR may be missed due to lack of accurate screening method for GFR.
Acute hyperglycemia increases GFR
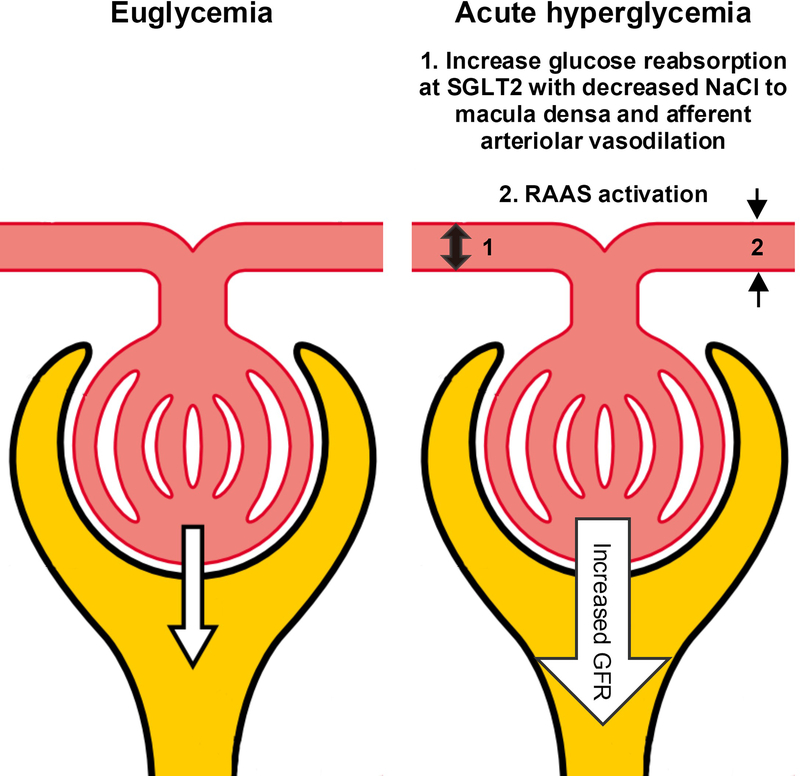
Multiple factors can influence GFR measurements, but in people with diabetes, an additional challenge in measuring GFR is the acute effect of blood glucose, which was established in studies dating back to the 1970s, although generally not accounted for in current clinical or research assessments of GFR in people with diabetes. Hyperglycemia is known to affect renal hemodynamic function and increase GFR by up to 20 mL/min/1.73 m2 (46, 66–69). The mechanism responsible for the increase GFR in the setting of acute glycemia is incompletely understood, but has been in part attributed to the effect of hyperglycemia on renin–angiotensin–aldosterone system (RAAS) (70, 71). We have previously demonstrated that RAAS blockade by aliskiren (a direct renin inhibitor) blunts the increased GFR as measured by inulin clearance provoked by hyperglycemia (72). Moreover, hyperglycemia has been proposed to increase proximal tubular glucose delivery causing a maladaptive increase in glucose reabsorption along with sodium via sodium-glucose cotransporter 2 (SGLT-2) in the proximal tube. Distal sodium chloride delivery to the macula densa is subsequently decreased, and perceived as low effective circulating volume by the juxtaglomerular apparatus, which causes vasodilation of the afferent renal arteriole, renal hyperperfusion and an increase in GFR (73). Figure 2 illustrates these proposed mechanisms.
Figure 2. Acute Hyperglycemia Increases GFR.
The figure illustrates the effect of hyperglycemia on glomerular filtration. Acute glycemia is associated with (1) increase glucose reabsorption at SGLT2 with decreased delivery of NaCl to macula densa and subsequent afferent arteriolar vasodilation, and (2) RAAS activation and efferent arteriolar vasoconstriction.
Despite these observations, the effect of blood glucose on renal function is generally not accounted for when measuring GFR in people with type 1 diabetes (65, 74–76). Therefore, failure to maintain euglycemia, or possibly account for hyperglycemia, could potentially result in differential misclassification and bias in measurement (and estimation) of GFR, thereby hindering the ability to determine early changes in GFR within an individual (46, 58, 77). Accounting for ambient blood glucose could improve intra-individual precision in GFR measurement, as well as changes in renal function over time in people diabetes.
New methods to evaluate GFR
Similar to clinical practice, a significant barrier in diabetic kidney research is a clinically easy and accurate method to measure GFR in adolescents and adults with diabetes (78). Improved methods should be less cumbersome and time consuming than current methods to measure GFR and provide more accurate and precise assessment of GFR than estimates from equations.
i. Beta-trace protein
Beta-trace protein (BTP), a low molecular weight protein, is a promising endogenous marker used to estimate GFR. It shares many of the features of cystatin C as a marker for GFR (79, 80). In contrast to cystatin C which is strongly positively charged, BTP is more isoelectric (85). Recent data support the use of BTP to estimate GFR in newborns (81), in children who have had a renal transplant and in pregnancy (82, 83). Equations have been developed and validated in children and adults. For example, Witzel et al recently presented sex-specific equations to estimate GFR with BTP in children, which demonstrated better accuracy estimating GFR than previous equations at GFR levels ≥60mL/min/1.73m2 (84). To our knowledge these equations have not been validated in children or adults with diabetes.
ii. GFR measured by magnetic resonance imaging (MRI)
Blood-oxygen-level dependent functional MRI (BOLD-MRI) is a promising method to non-invasively assess renal function without contrast media, but recent data have been inconsistent. Inoue et al. demonstrated a relationship between BOLD-MRI values and eGFR in non-diabetic nephropathy, but these associations were not significant in diabetic nephropathy (85). Furthermore, recent data demonstrated the failure of BOLD-MRI to distinguish between patients with different stages of chronic kidney disease (86). Additional studies are needed to evaluate the utility of BOLD-MRI in diabetic nephropathy.
iii. GFR measured by iohexol clearance on dried blood spots
Recently, a practical method of measuring GFR by iohexol clearance using dried capillary blood spots (DBS) was developed in non-diabetic patients (87, 88). In 2006 Niculescu-Duvaz et al. demonstrated that iohexol clearance measured on dried blood spots (DBS) on filter paper provided GFR measurements comparable to the iohexol plasma clearance, but at a significantly reduced time and cost (87). This method is ideally suited for patients with type 1 diabetes who routinely prick their fingers to obtain glucose measurements. We recently demonstrated that GFR by iohexol clearance using dried capillary blood spots on filter paper measured GFR accurately in adults with type 1 diabetes (89). This method could be translated to screening for early kidney disease in people with type 1 diabetes (89). This method was piloted for feasibility in adolescents and adults with type 1 diabetes (89, 90). GFR measured in DBS was comparable to the gold standard method of GFR plasma iohexol and more accurate, precise and less biased than GFR estimated by CKD-EPI creatinine, CKD-EPI cystatin C and CKD-EPI both in adults with type 1 diabetes (89). Furthermore, GFR-DBS offers a more convenient approach to quantify GFR, as the bias in the GFR-DBS measurements (2 or 5-spots) compared to GFR plasma iohexol was minimal and very similar to that previously reported in non-diabetic subjects (− 1.17, 95% CI: − 16.12–13.78) (87, 88). In addition, in adolescents with type 1 diabetes, there appeared to be less variability of GFR calculated by iohexol clearance on DBS than the estimated GFR methods (Bouvet and Schwartz) (90), and using 2 spots at 120 and 240 minutes was comparable to using 5 spots (89). All of the adolescent participants also agreed or strongly agreed that the iohexol injection and DBS collection was preferable to an overnight urine sample (90).
Measuring GFR by iohexol clearance on DBS could be incorporated into current clinical research or annual screening tests by placement of a peripheral IV for blood sampling followed by injection of iohexol and removal of the IV prior to a regularly scheduled clinic visit. This would significantly reduce the time required for a standard iohexol GFR study from 4–5 hours. Self-collection of DBS could be performed as an outpatient, since finger-pricks are a common task for patients with type 1 diabetes, and the filter paper could then be mailed back to the lab (88, 90). The relative simplicity of the DBS method and better results compared to eGFR suggest that this or similar methodology may improve upon current practices used to assess GFR, which are either not feasible or effective in ascertaining early renal function loss clinically. Adapting this methodology to an out-patient setting by measuring iohexol clearance, as was also recently reported in a Kenyan population (91), to assess GFR in youth with type 1 diabetes requires further study to determine if it addresses the current need to better screen for early diabetic kidney disease. Given the current variability with estimation of GFR at levels >60 mL/min/1.73m2, which encompasses the majority of patients with diabetes, particularly adolescents and young adults, better methods are required to fulfill the promise of reducing diabetic nephropathy by annual GFR screening in people with diabetes.
Therapies for diabetic nephropathy
While there is solid evidence showing benefit of glycemic and blood pressure control in preventing microvascular complications in type 1 diabetes (12, 92, 93), optimal control does not abolish the risk. Newer therapies, including sodium glucose co-transporter 2 (SGLT2) inhibitors and allopurinol hold promise as therapeutic targets to further prevent progression of diabetic nephropathy. The SGLT2 inhibitor, empagliflozin, was shown to reduce HbA1c and signficantly attenuate renal hyperfiltration in patients with uncomplicated type 1 diabetes and had no significant effect on GFR in those without hyperfiltration (73). This class of agents has also been shown to subacutely lower eGFR in large clinical trials by 5–10 mL/min/1.73m2, a modest effect that likely reflects expected changes in afferent arteriole tone in response to natriuresis caused by this drug class, and which may be responsible for lowering albuminuria (94). An additional hypothesis that lowering serum uric acid with allopurinol will prevent GFR decline in people with type 1 diabetes is being tested in the multi-center double-blind randomized clinical trial “Preventing Early Renal Function Loss – PERL” (95). Improved methods to detect GFR changes would allow us to implement therapies at an earlier stage when renal injury may be reversible or at least more responsive to interventions that either slow or arrest the progression of disease.
Conclusion
A major challenge in preventing diabetic kidney disease relates to the accurate and early identification of high risk patients. The American Diabetes Association now recommends annual assessment of glomerular filtration rate (GFR) in adolescents, in addition to adults, with diabetes to screen for early diabetic nephropathy. Assessment of GFR is essential to accurately diagnose diabetic kidney disease early in the disease process. GFR is, however, difficult and impractical to measure directly with current methodologies. Unfortunately, GFR estimates using serum creatinine and/or cystatin C based equations are only accurate when GFR is <60 ml/min/1.73 m2 (78), a point at which half of renal function may already be lost. Improved methods to measure or estimate GFR will lead to a better ability to accurately identify early changes in GFR and track GFR changes over time. One such method is GFR measured by iohexol clearance on DBS which offers promise as a more convenient approach to accurately quantify GFR in patients with diabetes in clinical practice and research.
Acknowledgements:
D.Z.I.C. was also supported by a Canadian Diabetes Association-KRESCENT Program Joint New Investigator Award.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Petter Bjornstad, David Z. Cherney, and David M. Maahs declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References:
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Padala S, Tighiouart H, Inker LA, Contreras G, Beck GJ, Lewis J, et al. Accuracy of a GFR estimating equation over time in people with a wide range of kidney function. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60(2):217–24. Epub 2012/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isakova T, Craven TE, Lee J, Scialla JJ, Xie H, Wahl P, et al. Fibroblast growth factor 23 and incident CKD in type 2 diabetes. Clinical journal of the American Society of Nephrology : CJASN. 2015;10(1):29–38. Epub 2014/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–54. Epub 2014/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Professional Practice Committee for the Standards of Medical Care in Diabetes-2015. Diabetes Care. 2015;38(Supplement 1):S88–S9. Epub 2014/12/30.•• The most recent recommendations from American Diabetes Association which include annual GFR evaluation in adolescents with diabetes
- 5.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367(1):20–9. Epub 2012/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Zinman B, et al. Longitudinal changes in estimated and measured GFR in type 1 diabetes. Journal of the American Society of Nephrology : JASN. 2014;25(4):810–8. Epub 2013/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer IH. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):24–30. Epub 2013/12/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53(10):2093–104. Epub 2010/05/25. [DOI] [PubMed] [Google Scholar]
- 9.Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes. 2012;3(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Current opinion in endocrinology, diabetes, and obesity. 2014;21(4):279–86. Epub 2014/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2010 Annual Data Report. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(1 Suppl 1):A8, e1–526 Epub 2010/12/28. [DOI] [PubMed] [Google Scholar]
- 12.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus--progress made, more to be done. The Journal of clinical endocrinology and metabolism. 2006;91(10):3757–9. Epub 2006/10/10. [DOI] [PubMed] [Google Scholar]
- 13.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–9. Epub 2010/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. ‘United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59(1 Suppl 1):A7, e1–420 Epub 2011/12/30. [DOI] [PubMed] [Google Scholar]
- 15.Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed). 1987;294(6588):1651–4. Epub 1987/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26(5):1374–9. Epub 2003/04/30. [DOI] [PubMed] [Google Scholar]
- 17.Maahs DM, Jalal D, Chonchol M, Johnson RJ, Rewers M, Snell-Bergeon JK. Impaired Renal Function Further Increases Odds of 6-Year Coronary Artery Calcification Progression in Adults With Type 1 Diabetes: The CACTI study. Diabetes Care. 2013;36(9):2607–14. Epub 2013/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International evaluation of cause-specific mortality and IDDM. Diabetes Epidemiology Research International Mortality Study Group. Diabetes Care. 1991;14(1):55–60. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 19.Twyman S, Rowe D, Mansell P, Schapira D, Betts P, Leatherdale B. Longitudinal study of urinary albumin excretion in young diabetic patients--Wessex Diabetic Nephropathy Project. Diabetic medicine : a journal of the British Diabetic Association. 2001;18(5):402–8. Epub 2001/07/27. [DOI] [PubMed] [Google Scholar]
- 20.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–76. Epub 2004/12/24. [DOI] [PubMed] [Google Scholar]
- 21.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. The New England journal of medicine. 2003;348(23):2285–93. Epub 2003/06/06.•• This study demonstrates regression of microalbuminuria to normoalbuminuria in type 1 diabetes.
- 22.Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, de Boer IH, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33(7):1536–43. Epub 2010/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maahs D, Jalal D, Chonchol M, Johnson R, Rewers M, Snell-Bergeon JK. Impaired Renal Function Further Increases Odds of 6 Year Coronary Artery Calcification Progression in Adults with Type 1 Diabetes: The CACTI Study. Diabetes Care (in review). 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjornstad P, Maahs D, Wadwa P, Pyle L, Rewers M, Eckel R, et al. Plasma Triglycerides Predict Incident Albuminuria and Progression of Coronary Artery Calcification in Adults with Type 1 Diabetes: the Coronary Artery Calcification in Type 1 Diabetes Study. Journal of clinical lipidology. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52(4):691–7. Epub 2009/02/10. [DOI] [PubMed] [Google Scholar]
- 26.Maahs DM, Ogden LG, Kretowski A, Snell-Bergeon JK, Kinney GL, Berl T, et al. Serum cystatin C predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes. 2007;56(11):2774–9. Epub 2007/07/31. [DOI] [PubMed] [Google Scholar]
- 27.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. The New England journal of medicine. 2013;369(10):932–43. Epub 2013/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornstad P, Maahs DM, Rivard CJ, Pyle L, Rewers M, Johnson RJ, et al. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta diabetologica. 2014. Epub 2014/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt JH, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care. 2014;37(1):226–34. Epub 2013/08/14.• This study provides evidence that rapid GFR decline may precede the onset of microalbuminuria in type 1 diabetes.
- 30.Bjornstad P, Cherney DZI, Snell-Bergeon J, Pyle L, Rewers M, Johnson RJ, et al. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with type 1 diabetes. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015.• This study demonstrates associations between renal hyperfiltration, rapid GFR decline and impaired GFR in adults with type 1 diabetes.
- 31.Standards of medical care in diabetes−−2013. Diabetes Care. 2013;36 Suppl 1:S11–66. Epub 2013/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;49(2 Suppl 2):S12–154. Epub 2007/02/06. [DOI] [PubMed] [Google Scholar]
- 33.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of internal medicine. 2013;158(11):825–30. Epub 2013/06/05. [DOI] [PubMed] [Google Scholar]
- 34.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. Journal of the American Society of Nephrology : JASN. 2009;20(11):2305–13. Epub 2009/10/17. [DOI] [PubMed] [Google Scholar]
- 35.Clorius JH, Dreikorn K, Zelt J, Raptou E, Weber D, Rubinstein K, et al. Renal graft evaluation with pertechnetate and I-131 Hippuran. A comparative clinical study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1979;20(10):1029–37. Epub 1979/10/01. [PubMed] [Google Scholar]
- 36.Wilkinson J, Fleming JS, Waller DG. Effect of food and activity on the reproducibility of isotopic GFR estimation. Nuclear medicine communications. 1990;11(10):697–700. Epub 1990/10/01. [DOI] [PubMed] [Google Scholar]
- 37.Merrill AJ, Cargill WH, Borders MA, Cavin E. The Effect of Exercise on the Renal Plasma Flow and Filtration Rate of Normal and Cardiac Subjects. The Journal of clinical investigation. 1948;27(2):272–7. Epub 1948/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhathena DB, Bullock WE, Nuttall CE, Luke RG. The effects of amphotericin B therapy on the intrarenal vasculature and renal tubules in man. A study of biopsies by light, electron and immunofluorescence microscopy. Clinical nephrology. 1978;9(3):103–10. Epub 1978/03/01. [PubMed] [Google Scholar]
- 39.Cherney DZ, Scholey JW, Sochett E, Bradley TJ, Reich HN. The acute effect of clamped hyperglycemia on the urinary excretion of inflammatory cytokines/chemokines in uncomplicated type 1 diabetes: a pilot study. Diabetes Care. 2011;34(1):177–80. Epub 2010/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubb A, Horio M, Hansson LO, Bjork J, Nyman U, Flodin M, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clinical chemistry. 2014;60(7):974–86. Epub 2014/05/16. [DOI] [PubMed] [Google Scholar]
- 41.Cattozzo G, Guerra E, Ceriotti F, Franzini C. Commutable calibrator with value assigned by the IFCC reference procedure to harmonize serum lactate dehydrogenase activity results measured by 2 different methods. Clinical chemistry. 2008;54(8):1349–55. Epub 2008/06/10. [DOI] [PubMed] [Google Scholar]
- 42.Ceriotti F, Boyd JC, Klein G, Henny J, Queralto J, Kairisto V, et al. Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clinical chemistry. 2008;54(3):559–66. Epub 2008/01/19. [DOI] [PubMed] [Google Scholar]
- 43.Blirup-Jensen S, Grubb A, Lindstrom V, Schmidt C, Althaus H. Standardization of Cystatin C: development of primary and secondary reference preparations. Scandinavian journal of clinical and laboratory investigation Supplementum. 2008;241:67–70. Epub 2008/08/21. [DOI] [PubMed] [Google Scholar]
- 44.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2010;48(11):1619–21. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 45.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Newman AB, Siscovick DS, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. American journal of nephrology. 2009;30(3):171–8. Epub 2009/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherney DZ, Sochett EB, Dekker MG, Perkins BA. Ability of cystatin C to detect acute changes in glomerular filtration rate provoked by hyperglycaemia in uncomplicated Type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2010;27(12):1358–65. Epub 2010/11/10. [DOI] [PubMed] [Google Scholar]
- 47.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney international. 2004;65(4):1416–21. Epub 2004/04/17. [DOI] [PubMed] [Google Scholar]
- 48.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, et al. Insulin Sensitivity Is an Important Determinant of Renal Health in Adolescents With Type 2 Diabetes. Diabetes Care. 2014. Epub 2014/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol MB, Johnson RJ, et al. Early Diabetic Nephropathy: A complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care. 2013. Epub 2013/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang SH, Sharma AP, Yasin A, Lindsay RM, Clark WF, Filler G. Hyperfiltration affects accuracy of creatinine eGFR measurement. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(2):274–80. Epub 2010/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care. 2008;31(5):971–3. Epub 2008/03/06. [DOI] [PubMed] [Google Scholar]
- 52.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. Journal of the American Society of Nephrology : JASN. 2009;20(12):2625–30. Epub 2009/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krolewski AS, Warram JH, Forsblom C, Smiles AM, Thorn L, Skupien J, et al. Serum concentration of cystatin C and risk of end-stage renal disease in diabetes. Diabetes Care. 2012;35(11):2311–6. Epub 2012/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skupien J, Warram JH, Groop PH, Krolewski AS. Cystatin-based estimated GFR versus creatinine-based and creatinine- and cystatin-based estimated GFR for ESRD and mortality risk in diabetes. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62(1):184–6. Epub 2013/05/21. [DOI] [PubMed] [Google Scholar]
- 55.Maahs DM, Jalal D, McFann K, Rewers M, Snell-Bergeon JK. Systematic shifts in cystatin C between 2006 and 2010. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(8):1952–5. Epub 2011/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maahs DM, Prentice N, McFann K, Snell-Bergeon JK, Jalal D, Bishop FK, et al. Age and sex influence cystatin C in adolescents with and without type 1 diabetes. Diabetes Care. 2011;34(11):2360–2. Epub 2011/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rognant N, Lemoine S, Laville M, Hadj-Aissa A, Dubourg L. Performance of the chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in diabetic patients. Diabetes Care. 2011;34(6):1320–2. Epub 2011/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjornstad P, McQueen RB, Snell-Bergeon JK, Cherney D, Pyle L, Perkins B, et al. Fasting Blood Glucose-A Missing Variable for GFR-Estimation in Type 1 Diabetes? PloS one. 2014;9(4):e96264 Epub 2014/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maahs DM. Early detection of kidney disease in type 1 diabetes: what do we really know? Diabetes Technol Ther. 2012;14(7):541–4. Epub 2012/05/01. [DOI] [PubMed] [Google Scholar]
- 60.Filler G, Yasin A, Medeiros M. Methods of assessing renal function. Pediatr Nephrol. 2014;29(2):183–92. Epub 2013/02/19. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(11):1832–43. Epub 2009/10/13. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology : JASN. 2009;20(3):629–37. Epub 2009/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berg UB, Nyman U, Back R, Hansson M, Monemi KA, Herthelius M, et al. New standardized cystatin C and creatinine GFR equations in children validated with inulin clearance. Pediatr Nephrol. 2015. Epub 2015/04/24.•• This study describes promising new GFR equations in children and adolescents with standardized cystatin C and creatinine.
- 64.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney international. 2012;82(4):445–53. Epub 2012/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma AP, Yasin A, Garg AX, Filler G. Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(7):1599–608. Epub 2011/06/28.•• This study evaluates the diagnostic accuracy of eGFR equations at different GFR levels in children and adolescents.
- 66.Christiansen JS, Frandsen M, Parving HH. Effect of intravenous glucose infusion on renal function in normal man and in insulin-dependent diabetics. Diabetologia. 1981;21(4):368–73. Epub 1981/10/01. [DOI] [PubMed] [Google Scholar]
- 67.Dullaart RP, Meijer S, Sluiter WJ, Doorenbos H. Renal haemodynamic changes in response to moderate hyperglycaemia in type 1 (insulin-dependent) diabetes mellitus. European journal of clinical investigation. 1990;20(2):208–13. Epub 1990/04/01. [DOI] [PubMed] [Google Scholar]
- 68.Wiseman MJ, Mangili R, Alberetto M, Keen H, Viberti G. Glomerular response mechanisms to glycemic changes in insulin-dependent diabetics. Kidney international. 1987;31(4):1012–8. Epub 1987/04/01. [DOI] [PubMed] [Google Scholar]
- 69.Wiseman MJ, Saunders AJ, Keen H, Viberti G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin-dependent diabetes. The New England journal of medicine. 1985;312(10):617–21. Epub 1985/03/07. [DOI] [PubMed] [Google Scholar]
- 70.Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. Journal of the American Society of Nephrology : JASN. 1999;10(8):1778–85. [DOI] [PubMed] [Google Scholar]
- 71.Miller JA, Floras JS, Zinman B, Skorecki KL, Logan AG. Effect of hyperglycaemia on arterial pressure, plasma renin activity and renal function in early diabetes. Clin Sci (Lond). 1996;90(3):189–95. [DOI] [PubMed] [Google Scholar]
- 72.Cherney DZ, Reich HN, Scholey JW, Daneman D, Mahmud FH, Har RL, et al. The effect of aliskiren on urinary cytokine/chemokine responses to clamped hyperglycaemia in type 1 diabetes. Diabetologia. 2013;56(10):2308–17. Epub 2013/07/31. [DOI] [PubMed] [Google Scholar]
- 73.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. The Renal Hemodynamic Effect of SGLT2 Inhibition in Patients with Type 1 Diabetes. Circulation. 2013. Epub 2013/12/18.•• This study demonstrates the renal hemodynamic effects of SGLT2 inhibitors in adults with type 1 diabetes.
- 74.Amin R, Turner C, van Aken S, Bahu TK, Watts A, Lindsell DR, et al. The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: The Oxford Regional Prospective Study. Kidney international. 2005;68(4):1740–9. Epub 2005/09/17. [DOI] [PubMed] [Google Scholar]
- 75.Dahlquist G, Stattin EL, Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16(7):1382–6. Epub 2001/06/28. [DOI] [PubMed] [Google Scholar]
- 76.Caramori ML, Gross JL, Pecis M, de Azevedo MJ. Glomerular filtration rate, urinary albumin excretion rate, and blood pressure changes in normoalbuminuric normotensive type 1 diabetic patients: an 8-year follow-up study. Diabetes Care. 1999;22(9):1512–6. Epub 1999/09/10. [DOI] [PubMed] [Google Scholar]
- 77.Cherney D, Maahs DM. Cystatin C versus creatinine for kidney function-based risk. The New England journal of medicine. 2013;369(25):2458 Epub 2013/12/20. [DOI] [PubMed] [Google Scholar]
- 78.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. The New England journal of medicine. 2006;354(23):2473–83. Epub 2006/06/09. [DOI] [PubMed] [Google Scholar]
- 79.Filler G, Priem F, Lepage N, Sinha P, Vollmer I, Clark H, et al. Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clinical chemistry. 2002;48(5):729–36. Epub 2002/04/30. [PubMed] [Google Scholar]
- 80.Benlamri A, Nadarajah R, Yasin A, Lepage N, Sharma AP, Filler G. Development of a beta-trace protein based formula for estimation of glomerular filtration rate. Pediatr Nephrol. 2010;25(3):485–90. Epub 2009/12/02. [DOI] [PubMed] [Google Scholar]
- 81.Harrington MG, Aebersold R, Martin BM, Merril CR, Hood L. Identification of a brain-specific human cerebrospinal fluid glycoprotein, beta-trace protein. Applied and theoretical electrophoresis : the official journal of the International Electrophoresis Society. 1993;3(5):229–34. Epub 1993/01/01. [PubMed] [Google Scholar]
- 82.White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, et al. Estimating GFR using serum beta trace protein: accuracy and validation in kidney transplant and pediatric populations. Kidney international. 2009;76(7):784–91. Epub 2009/07/25. [DOI] [PubMed] [Google Scholar]
- 83.Akbari A, Lepage N, Keely E, Clark HD, Jaffey J, MacKinnon M, et al. Cystatin-C and beta trace protein as markers of renal function in pregnancy. BJOG : an international journal of obstetrics and gynaecology. 2005;112(5):575–8. Epub 2005/04/22. [DOI] [PubMed] [Google Scholar]
- 84.Witzel SH, Huang SH, Braam B, Filler G. Estimation of GFR Using beta-Trace Protein in Children. Clinical journal of the American Society of Nephrology : CJASN. 2015;10(3):401–9. Epub 2014/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. Journal of the American Society of Nephrology : JASN. 2011;22(8):1429–34. Epub 2011/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney international. 2012;81(7):684–9. Epub 2012/01/13. [DOI] [PubMed] [Google Scholar]
- 87.Niculescu-Duvaz I, D’Mello L, Maan Z, Barron JL, Newman DJ, Dockrell ME, et al. Development of an outpatient finger-prick glomerular filtration rate procedure suitable for epidemiological studies. Kidney international. 2006;69(7):1272–5. Epub 2006/04/13. [DOI] [PubMed] [Google Scholar]
- 88.Mafham MM, Niculescu-Duvaz I, Barron J, Emberson JR, Dockrell ME, Landray MJ, et al. A practical method of measuring glomerular filtration rate by iohexol clearance using dried capillary blood spots. Nephron Clinical practice. 2007;106(3):c104–12. Epub 2007/05/25. [DOI] [PubMed] [Google Scholar]
- 89.Maahs DM, Bushman L, Kerr B, Ellis SL, Pyle L, McFann K, et al. A practical method to measure GFR in people with type 1 diabetes. Journal of diabetes and its complications. 2014;28(5):667–73. Epub 2014/07/17. [DOI] [PubMed] [Google Scholar]
- 90.Bjornstad P, Anderson PL, Maahs DM. Measuring glomerular filtration rate by iohexol clearance on filter paper is feasible in adolescents with type 1 diabetes in the ambulatory setting. Acta diabetologica. 2015. Epub 2015/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Margolick JB, Jacobson LP, Schwartz GJ, Abraham AG, Darilay AT, Kingsley LA, et al. Factors affecting glomerular filtration rate, as measured by iohexol disappearance, in men with or at risk for HIV infection. PloS one. 2014;9(2):e86311 Epub 2014/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55(5):1463–9. Epub 2006/04/29. [DOI] [PubMed] [Google Scholar]
- 93.Wood JR, Miller KM, Maahs DM, Beck RW, Dimeglio LA, Libman IM, et al. Most Youth With Type 1 Diabetes in the T1D Exchange Clinic Registry Do Not Meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care. 2013;36(7):2035–7. Epub 2013/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Skrtic M, Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2015;24(1):96–103. [DOI] [PubMed] [Google Scholar]
- 95.Maahs DM, Caramori L, Cherney DZ, Galecki AT, Gao C, Jalal D, et al. Uric Acid Lowering to Prevent Kidney Function Loss in Diabetes: The Preventing Early Renal Function Loss (PERL) Allopurinol Study. Curr Diab Rep. 2013. Epub 2013/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rehling M, Moller ML, Thamdrup B, Lund JO, Trap-Jensen J. Simultaneous measurement of renal clearance and plasma clearance of 99mTc-labelled diethylenetriaminepenta-acetate, 51Cr-labelled ethylenediaminetetra-acetate and inulin in man. Clin Sci (Lond). 1984;66(5):613–9. Epub 1984/05/01. [DOI] [PubMed] [Google Scholar]
- 97.Medeiros FS, Sapienza MT, Prado ES, Agena F, Shimizu MH, Lemos FB, et al. Validation of plasma clearance of 51Cr-EDTA in adult renal transplant recipients: comparison with inulin renal clearance. Transplant international : official journal of the European Society for Organ Transplantation. 2009;22(3):323–31. Epub 2008/12/06. [DOI] [PubMed] [Google Scholar]
- 98.Hernandez Ocampo J, Torres Rosales A, Rodriguez Castellanos F. [Comparison of four methods for measuring glomerular filtration rate by inulin clearance in healthy individuals and patients with renal failure]. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2010;30(3):324–30. Epub 2010/04/24.Comparacion de cuatro metodos de medicion de la tasa de filtracion glomerular con depuracion de inulina en individuos sanos y en pacientes con insuficiencia renal.
- 99.Odlind B, Hallgren R, Sohtell M, Lindstrom B. Is 125I iothalamate an ideal marker for glomerular filtration? Kidney international. 1985;27(1):9–16. Epub 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 100.Berg UB, Back R, Celsi G, Halling SE, Homberg I, Krmar RT, et al. Comparison of plasma clearance of iohexol and urinary clearance of inulin for measurement of GFR in children. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(1):55–61. Epub 2010/09/28. [DOI] [PubMed] [Google Scholar]
- 101.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. 1999;130(6):461–70. Epub 1999/03/13. [DOI] [PubMed] [Google Scholar]
- 102.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Annals of internal medicine. 2004;141(12):929–37. Epub 2004/12/22. [DOI] [PubMed] [Google Scholar]
- 103.Tan GD, Lewis AV, James TJ, Altmann P, Taylor RP, Levy JC. Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care. 2002;25(11):2004–9. Epub 2002/10/29. [DOI] [PubMed] [Google Scholar]
- 104.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. Journal of the American Society of Nephrology : JASN. 2005;16(5):1404–12. Epub 2005/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beauvieux MC, Le Moigne F, Lasseur C, Raffaitin C, Perlemoine C, Barthe N, et al. New predictive equations improve monitoring of kidney function in patients with diabetes. Diabetes Care. 2007;30(8):1988–94. Epub 2007/05/31. [DOI] [PubMed] [Google Scholar]
- 106.Macisaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686–9. Epub 2006/06/06. [DOI] [PubMed] [Google Scholar]
- 107.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;56(3):486–95. Epub 2010/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;51(3):395–406. Epub 2008/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartz GJ, Haycock GB, Edelmann CM Jr., Spitzer A A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58(2):259–63. Epub 1976/08/01. [PubMed] [Google Scholar]
- 110.Bouvet Y, Bouissou F, Coulais Y, Seronie-Vivien S, Tafani M, Decramer S, et al. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol. 2006;21(9):1299–306. Epub 2006/06/24. [DOI] [PubMed] [Google Scholar]