Abstract
Objective
To compare and contrast illustrative examples of the adoption of high value practices and the de-adoption of low value practices.
Design
(1) Retrospective, population-based audit of low molecular weight heparin (LMWH) for venous thromboembolism (VTE) prophylaxis (high value practice) and albumin for fluid resuscitation (low value practice) and (2) cross-sectional survey of healthcare providers.
Setting
Data were collected from nine adult medical-surgical intensive care units (ICUs) in two large Canadian cities. Patients are managed in these ICUs by a group of multiprofessional and multidisciplinary healthcare providers.
Participants
Participants included 6946 ICU admissions and 309 healthcare providers from the same ICUs.
Main outcome measures
(1) The use of LMWH for VTE prophylaxis (per cent ICU days) and albumin for fluid resuscitation (per cent of patients); and (2) provider knowledge of evidence underpinning these practices, and barriers and facilitators to adopt and de-adopt these practices.
Results
LMWH was administered on 38.7% of ICU days, and 20.0% of patients received albumin.
Most participants had knowledge of evidence underpinning VTE prophylaxis and fluid resuscitation (59.1% and 84.2%, respectively). Providers perceived these practices to be followed. The most commonly reported barrier to adoption was insufficient knowledge/understanding (32.8%), and to de-adoption was clinical leader preferences (33.2%). On-site education was the most commonly identified facilitator for adoption and de-adoption (67.8% and 68.6%, respectively).
Conclusions
Despite knowledge of and self-reported adherence to best practices, the audit demonstrated opportunity to improve. Provider-reported barriers and facilitators to adoption and de-adoption are broadly similar.
Keywords: quality improvement, healthcare system, under-use and over-use, appropriateness, intensive care
Strengths and limitations of this study.
A strength of this study is the use of mixed methods to comprehensively compare adoption of high value practices and de-adoption of low value practices in the intensive care unit.
Another strength is the use of population-based data to capture current clinical practices.
The survey used to assess barriers and facilitators of the two illustrative practices was derived from a validated survey instrument.
The survey used was simple and designed to garner a representative perspective from all provider professions and therefore captured key concepts, but not granular data.
Introduction
Optimising the quality of care1 is of particular importance in the intensive care unit (ICU) due to the acuity of patient illness and substantial resources required to care for these patients. However, practice change (adopting high value practices or de-adopting low value practices) can lag behind the publication of evidence hindering delivery of evidence-based practices and may be different when adopting or de-adopting practices.2 3 To minimise the latency for change, it is important to find ways to improve the implementation of evidence-based practices.
A growing body of evidence has evaluated barriers and facilitators for adopting high value practices (effective at improving outcomes).4–7 Substantially less is known about the barriers and facilitators for de-adopting low value practices (ineffective at improving outcomes or harmful), and how they compare to those for adopting high value practices.8 9 De-adoption, also known by several other terms such as disinvestment and de-implementation,8 is the discontinuation of a practice that has been previously adopted.10 Some have suggested that the adoption of high value practices and de-adoption of low value practices involves similar processes and common facilitators and barriers11 12; however, others suggest that the two are clearly distinct.9 13 There has been limited comparative evaluation of adoption and de-adoption and this is an important knowledge gap given the growing number of initiatives aimed at de-adopting low value practices.13–16
The objective of this study was to describe illustrative example practices of the adoption of a high value practice (use of low molecular weight heparin (LMWH) instead of unfractionated heparin (UFH) for venous thromboembolism prophylaxis (VTE) and the de-adoption of a low value practice (albumin for fluid resuscitation) in the ICU. The results of this study prompted a subsequent implementation study to improve these two practices. The audit data identified important opportunities to improve clinical care, and the perceived barriers and facilitators identified in the survey were used to inform the development of interventions.
Methods
Study design
This multimethod observational study included: (1) a retrospective cohort study of patients admitted to ICUs to describe current VTE prophylaxis and fluid resuscitation practices, and (2) a cross-sectional survey of ICU healthcare providers to examine: knowledge of evidence underpinning these two practices, and perceived barriers and facilitators to adopt LMWH for VTE prophylaxis and de-adopt albumin for fluid resuscitation.
Setting
All data were collected from nine adult medical-surgical ICUs in the two largest cities in a Canadian province (population of 4.1 million). A single health services provider is responsible for the provision of all hospital-based care in the province and uses a single formulary across all ICUs (clinical practices may differ between cities and sites). ICU patients are managed by a multidisciplinary and multiprofessional group of healthcare providers, including (but not limited to): physicians, medical trainees (clinical fellows and residents), nurse practitioners (NPs with prescribing privileges), pharmacists and nurses (managers, educators, bedside).
Audit of current practices
Participants
We included patients admitted to nine adult medical-surgical ICUs between 1 January, 2014 and 31 December, 2014. For analyses, patients were grouped into two cohorts. (1) The adoption cohort consisted of patients without a contraindication for pharmacological VTE prophylaxis where according to international and local guidelines LMWH should be prescribed.17–21 Contraindications to pharmacological prophylaxis included a diagnosis potentially associated with a high risk of bleeding (online supplementary content 1), daily assessed platelet count <50×109/L, International Normalized Ratio (INR) ≥2, Partial Thromboplastin Time (PTT) ≥55 s or receipt of therapeutic anticoagulation.
bmjopen-2018-024159supp001.pdf (62.1KB, pdf)
(2)The de-adoption cohort consisted of patients without an indication for use of albumin for fluid resuscitation and where according to the current evidence, albumin should not be used for fluid resuscitation.22–25 Potential indications for albumin included documented liver disease (cirrhosis or hepatic failure), or receipt of plasma exchange.26–29 The two study cohorts were drawn from the same patient population and patients satisfying both sets of clinical indications were included in both cohorts.
Data source
All nine ICUs employ a shared integrated, prospective, clinical information system that captures and delivers multimodal patient data (demographic, clinical, outcome) in real time to the bedside (eCritical MetaVision, iMDsoft, MetaVision), and is also a repository and clinical analytics system that stores these data (eCritical TRACER) to support quality improvement and clinical research. eCritical TRACER was used to extract all data.
Variables
Patient and ICU demographic variables included age, sex, comorbidities, admission type, disease severity (Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score), ICU and hospital length of stay, ICU and hospital mortality. Data abstracted included: (1) type of VTE prophylaxis (mechanical included antiembolic stockings and sequential compression devices, and pharmacological included UFH and LMWH), (2) ICU day that VTE prophylaxis was administered, (3) if the patient received albumin, (4) quantity (units) of albumin and (5) ICU day that albumin was administered. An ICU day was defined as any portion of a day between 07:00 and 06:59, recognising that follow-up time on admission day and discharge day may be less than 24 hours.
Data analysis
Descriptive statistics (means with SD, medians with IQR, frequencies with proportions) were used to describe the two cohorts. The proportion of admissions and ICU days with LMWH, UFH and mechanical VTE prophylaxis by ICU and ICU day; and with any albumin administration by ICU and patient were calculated to describe current clinical practices. The unit of analysis for our outcome for the adoption cohort (LMWH use) was patient days because VTE prophylaxis is a routine clinical practice that should be performed on a daily basis. Conversely, the unit of analysis for our outcome for the de-adoption cohort (albumin use) was per patient because fluid resuscitation is a sporadic event that is not part of routine daily patient care.
To examine potential associations between patient demographic and sites, and the use of the high value practice (LMWH) a multivariable generalised estimating equations (GEEs) logistic regression model with exchangeable correlation structure given daily measurements (clustering by patient) was used. To examine potential associations between demographic and site-level factors, and the use of the low value practice (albumin) a multivariable logistic regression model given a single measurement per patient was used.
Barriers and facilitators to adopting low molecular weight heparin for venous thromboembolism prophylaxis and de-adopting albumin for fluid resuscitation
Survey development
The survey was modelled after previous work on adoption of LMWH for VTE prophylaxis,30 and refined to include questions regarding fluid resuscitation. Because research around barriers and facilitators of de-adopting low value practices is in its infancy31 the evidence of barriers and facilitators for adopting high value practices was employed.
The survey was divided into four sections: participant demographic information, knowledge of the current evidence underpinning the best practices and perceptions of barriers and facilitators to the use of the two illustrative examples of best practices (online supplementary content 2).
bmjopen-2018-024159supp002.pdf (473.1KB, pdf)
The survey was pilot tested in two phases: phase (1) seven providers completed the survey and identified unnecessary, missing or poorly worded items. The survey was modified and pilot tested with 12 additional ICU providers (one attending physician, two residents, one clinical fellow, one NP, one nurse manager/charge nurse, one nurse educator, two bedside nurses and three pharmacists). Phase (2) providers completed the survey twice (7–10 days apart) and an additional brief questionnaire to rate the clinical sensibility of the survey. Test-retest reliability of the survey demonstrated a mean intraclass correlation coefficient (ICC) of 0.66 (SD 0.47) for continuous responses and a mean proportion of agreement of 0.86 (SD 0.10) for categorical responses. The low ICC for continuous responses is due to low variability in responses for questions relating to knowledge of best practices. The participants agreed that the survey had face validity (100%), content validity (92%), clarity (92%), utility (100%), discriminability (75%) and minimal redundancy (100%).
Participants
Healthcare providers (as described in setting) that cared for patients in the nine ICUs were invited by email to participate in the study. Invitations to participate were sent to healthcare providers by the principal investigators or by a local clinical leader and included a link to the electronic survey (Fluid Survey) or were provided a paper copy if requested. Weekly reminders were sent for 3 weeks. Providers that responded to the survey were offered entry into a draw for one of three $20 coffee gift cards.
Data analysis
We used descriptive statistics to describe demographic features of participants, knowledge of best practices, perceived barriers to adopting high value practices and de-adopting low value practices, perceived facilitators to encourage adopting high value practices and de-adopting low value practices. Barriers and facilitators to the use of best practices were described overall, and by professional group. Professions were categorised into three groups for analysis: (1) physicians/NPs (those who prescribe), (2) nurses (those who administer) and (3) pharmacists (those who advise prescribers). χ2 tests were used to test for statistical significance between groups.
Patient and public involvement
Patient and family representatives were members of a committee that identified and prioritised research questions for improving the care of critically ill patients.32 LMWH for VTE prophylaxis and de-adopting albumin for fluid resuscitation were two of the research questions identified by this committee. Patients were not involved in the design, the recruitment and conduct of this study. The results of this study have been disseminated to patient and family advisors through oral presentations.
Results
Audit of current practices
There were 6946 ICU admissions during the study period, from 6299 unique patients. Patient characteristics are presented in online supplementary content 3.
bmjopen-2018-024159supp003.pdf (87.1KB, pdf)
The adoption cohort consisted of 4931 admissions (71.0% of all admissions) without a contraindication to pharmacological VTE prophylaxis, and the de-adoption cohort consisted of 6467 admissions (93.1%) without a potential indication for albumin (online supplementary content 4).
bmjopen-2018-024159supp004.pdf (46.3KB, pdf)
During the ICU stay LMWH was given on 38.7% of ICU days, UFH on 45.3% of ICU days and mechanical prophylaxis (exclusive of pharmacological prophylaxis) on 7.7% of ICU days. The type of VTE prophylaxis administered varied throughout patients’ ICU stay; administration of mechanical devices and UFH decreased over the course of the ICU stay while administration of LMWH increased (online supplementary content 5).
bmjopen-2018-024159supp005.pdf (38.2KB, pdf)
A total of 6804 units of albumin were administered to 20.0% of the 6467 admissions without documented liver disease or receipt of plasma exchange. Among those receiving at least 1 unit of albumin, the median number of units per patient was 3 (IQR=1.0–6.0). Albumin was administered on 6.5% of ICU days.
When controlling for demographic and site-level factors, the odds of receiving LMWH for VTE prophylaxis and not receiving albumin for fluid resuscitation were significantly lower for those patients with higher severity of illness (APACHE II score). The odds of receiving LMWH for VTE prophylaxis were significantly higher for patients with non-surgical admissions compared with those with elective surgical admissions (OR=1.34 [95% CI 1.08 to1.66]; table 1). There were significant differences in the odds of using LMWH for VTE prophylaxis, and not using albumin for fluid resuscitation across ICUs (online supplementary content 6), and when controlling for patient-level factors some of these differences persisted especially with regard to the use of LMWH for VTE prophylaxis (table 1).
Table 1.
Association between patient demographic and sites, and the use of low molecular weight heparin (LMWH) for venous thromboembolism (VTE) prophylaxis and not using albumin for fluid resuscitation
| Appropriate VTE prophylaxis OR (95% CI)* |
Appropriate fluid resuscitation OR (95% CI)† |
|
| Age | NS‡ | 0.999 (0.999 to 1.00) |
| Female | NS‡ | NS‡ |
| Any comorbidity | NS‡ | NS‡ |
| Admission type | ||
| Elective surgery | 1.00 (reference group) | 1.00 (reference group) |
| Emergent surgery | 1.19 (0.92 to 1.53) | 0.92 (0.88 to 0.95) |
| No surgery | 1.34 (1.08 to 1.66) | 1.02 (0.98 to 1.05) |
| APACHE II score (ICU admission) | 0.958 (0.951 to 0.965) | 0.989 (0.988 to 0.990) |
| Site | ||
| C1 | 1.00 (reference group) | 1.00 (reference group) |
| C2 | 1.32 (1.07 to 1.64) | 0.96 (0.92 to 1.00) |
| C3 | 1.13 (0.89 to 1.46) | 0.98 (0.94 to 1.03) |
| C4 | 1.48 (1.15 to 1.90) | 0.98 (0.93 to 1.02) |
| E1 | 2.12 (1.66 to 2.73) | 0.90 (0.86 to 0.95) |
| E2 | 0.86 (0.71 to 1.05) | 0.90 (0.87 to 0.92) |
| E3 | 7.26 (5.46 to 9.65) | 0.92 (0.87 to 0.97) |
| E4 | 0.76 (0.63 to 0.92) | 0.88 (0.85 to 0.91) |
| E5 | 1.61 (1.23 to 2.10) | 0.75 (0.72 to 0.79) |
All ‘C’ sites indicate intensive care unit (ICU) in Calgary and all ‘E’ sites indicate ICU in Edmonton.
*Multivariable generalised estimating equations logistic regression model with exchangeable correlation structure given daily measurements (clustering by patient); ‘appropriate’ considered use of LMWH.
†Standard multivariable logistic regression model given single measurement per patient; ‘appropriate’ considered not using albumin.
‡NS=non significant, removed from model.
bmjopen-2018-024159supp006.pdf (26.5KB, pdf)
Barriers and facilitators to adopting low molecular weight heparin for venous thromboembolism prophylaxis and de-adopting albumin for fluid resuscitation
Participants
83.8% (259 of 309) of participants responded; physicians/NPs (48.3%), nurses (42.5%) and pharmacists (9.3%). Participants worked in healthcare for a median of 13 years (IQR=7.1–20.0) and in critical care for a median of 8 years (IQR=3.0–15.0; online supplementary content 7).
bmjopen-2018-024159supp007.pdf (41KB, pdf)
Knowledge of evidence
Most participants reported that LMWH was most effective at preventing deep vein thrombosis and pulmonary embolism; and that crystalloids were most effective for fluid resuscitation (table 2). Perceptions regarding the effectiveness of VTE prophylaxis varied by professional group, as did perceptions regarding the risks of harm (table 2). Perceptions regarding effectiveness of albumin for fluid resuscitation and risks of harm associated with each form of fluid resuscitation did not vary by professional group but perceptions regarding the risk of fluid overload did (table 2).
Table 2.
Knowledge of best practices for VTE prophylaxis and fluid resuscitation
| Survey question | % (N) | |||
| Overall n=259 |
Physicians/NPs 48.3% (n=125) |
Nurses 42.5% (n=110) |
Pharmacists 9.3% (n=24) |
|
| What form(s) of prophylaxis is/are most effective at preventing deep vein thrombosis?* | ||||
| LMWH only | 59.1 (153) | 63.2 (79) | 51.8 (57) | 70.8 (17) |
| UFH only | 4.3 (11) | 2.4 (3) | 7.3 (8) | 0.0 (0) |
| LMWH & UFH | 16.2 (42) | 24.0 (30) | 5.5 (6) | 25.0 (6) |
| Mechanical only | 1.9 (5) | 0.0 (0) | 4.6 (5) | 0.0 (0) |
| (LMWH or UFH) and Mechanical | 15.1 (39) | 8.0 (10) | 25.5 (28) | 4.2 (1) |
| Unsure only | 3.5 (9) | 2.4 (3) | 5.5 (6) | 0.0 (0) |
| What form(s) of prophylaxis is/are most effective at preventing pulmonary embolism?* | ||||
| LMWH only | 56.8 (147) | 72.0 (90) | 33.6 (37) | 83.3 (20) |
| UFH only | 18.2 (47) | 1.6 (2) | 40.9 (45) | 0.0 (0) |
| LMWH & UFH | 12.7 (33) | 20.8 (26) | 3.6 (4) | 12.5 (3) |
| Mechanical only | 0.4 (1) | 0.0 (0) | 0.9 (1) | 0.0 (0) |
| (LMWH or UFH) & mechanical | 8.5 (22) | 3.2 (4) | 15.5 (17) | 4.2 (1) |
| Unsure only | 3.5 (9) | 2.4 (3) | 5.5 (6) | 0.0 (0) |
| Which form(s) of prophylaxis is/are most cost effective?* | ||||
| LMWH only | 51.0 (132) | 70.4 (88) | 22.7 (25) | 79.2 (19) |
| UFH only | 15.4 (40) | 12.8 (16) | 20.0 (22) | 8.3 (2) |
| LMWH & UFH | 4.3 (11) | 5.6 (7) | 0.9 (1) | 12.5 (3) |
| Mechanical only | 10.0 (26) | 4.8 (6) | 18.2 (20) | 0.0 (0) |
| (LMWH or UFH) & Mechanical | 2.7 (7) | 0.0 (0) | 6.4 (7) | 0.0 (0) |
| Unsure only | 16.6 (43) | 6.4 (8) | 31.8 (35) | 0.0 (0) |
| Which form(s) of pharmacological prophylaxis has/have the lowest risk of bleeding?† | ||||
| LMWH only | 57.5 (149) | 47.2 (59) | 69.1 (76) | 58.3 (14) |
| UFH only | 24.7 (64) | 32.8 (41) | 18.2 (20) | 12.5 (3) |
| LMWH & UFH | 5.0 (13) | 6.4 (8) | 0.0 (0) | 20.8 (5) |
| Unsure only | 12.7 (33) | 13.6 (17) | 12.7 (14) | 8.3 (2) |
| Which form(s) of pharmacological prophylaxis has/have the lowest risk of heparin induced thrombocytopenia?* | ||||
| LMWH only | 86.1 (223) | 94.4 (118) | 74.6 (82) | 95.8 (23) |
| UFH only | 6.6 (17) | 3.2 (4) | 11.8 (13) | 0.0 (0) |
| LMWH & UFH | 0.4 (1) | 0.0 (0) | 0.0 (0) | 4.2 (1) |
| Unsure only | 7.0 (18) | 2.4 (3) | 13.6 (15) | 0.0 (0) |
| To what extent do you think best practices are followed for preventing DVT/PE in your ICU? 0=never and 7=always, median (IQR) | ||||
| 6 (5–6) | 6 (5–6) | 6 (6–7) | 6 (5–6) | |
| Survey question | Overall n=259 |
Physicians/NPs 48.3% (n=125) |
Nurses 42.5% (n=110) |
Pharmacists 9.3% (n=24) |
| What form(s) of intravenous fluids is/are most effective for fluid resuscitation?‡ | ||||
| Albumin only | 3.5 (9) | 2.4 (3) | 5.5 (6) | 0.0 (0) |
| Crystalloids only | 84.2 (218) | 83.2 (104) | 82.7 (91) | 95.8 (23) |
| Albumin & crystalloids | 8.5 (22) | 9.6 (12) | 9.1 (10) | 0.0 (0) |
| Unsure only | 3.9 (10) | 4.8 (6) | 2.7 (3) | 4.2 (1) |
| Which form(s) of intravenous resuscitation fluids are most cost effective?‡ | ||||
| Albumin only | 0.4 (1) | 0.0 (0) | 0.9 (1) | 0.0 (0) |
| Crystalloids only | 94.6 (245) | 94.4 (118) | 95.5 (105) | 91.7 (22) |
| Albumin & crystalloids | 0.4 (1) | 0.8 (1) | 0.0 (0) | 0.0 (0) |
| Unsure only | 4.6 (12) | 4.8 (6) | 3.6 (4) | 8.3 (2) |
| Which form(s) of intravenous resuscitation fluids has the lowest risk of fluid overload?* | ||||
| Albumin only | 47.1 (122) | 32.8 (41) | 69.1 (76) | 20.8 (5) |
| Crystalloids only | 29.7 (77) | 36.8 (46) | 23.6 (26) | 20.8 (5) |
| Albumin & crystalloids | 1.9 (5) | 3.2 (4) | 0.0 (0) | 4.2 (1) |
| Unsure only | 21.2 (55) | 27.2 (34) | 7.3 (8) | 54.2 (13) |
| Which form(s) of intravenous resuscitation fluids has the lowest risk of infectious disease?‡ | ||||
| Albumin only | 2.7 (7) | 1.6 (2) | 4.6 (5) | 0.0 (0) |
| Crystalloids only | 86.5 (224) | 87.2 (109) | 87.3 (96) | 79.2 (19) |
| Albumin & crystalloids | 0.8 (2) | 0.8 (1) | 0.9 (1) | 0.0 (0) |
| Unsure only | 10.0 (26) | 10.4 (13) | 7.3 (8) | 20.8 (5) |
| To what extent do you think best practices are followed for prescribing fluid boluses in your ICU? 0=never and 7=always; median (IQR) | ||||
| 6 (5–6) | 5 (5–6) | 6 (5–6) | 5 (5–6) | |
1The order of the survey items are as presented in this table.
2Evidence suggests the efficacy of LMWH for deep vein thrombosis is similar to or better than UFH.18 19 45 46 Evidence suggests that LMWH is more efficacious than UFH for preventing pulmonary embolism, has a lower incidence of heparin induced thrombocytopenia, and a similar or lower risk of bleeding.18 19 45 46
3Evidence suggests that LMWH is more cost effective than UFH.18
4Evidence suggests that albumin and crystalloids are similarly effective for fluid resuscitation.21 24–26 Evidence suggests that albumin has a higher risk of infectious disease transmission than crystalloids and is less cost effective than crystalloids.
*Responses varied by professional group (P<0.001),
†Responses varied by professional group (P=0.01),
‡Responses did not vary by professional group (P>0.05)
DVT, deep vein thrombosis; ICU, intensive care unit; IQR, interquartile range (p25 - p75); LMWH, low molecular weight heparin; n, number; NP, nurse practitioner; PE, pulmonary embolism; UFH, unfractionated heparin.
It was perceived that both best practices were being followed in the ICUs where the participants practiced (table 2).
Barriers to adopting low molecular weight heparin for venous thromboembolism prophylaxis and de-adopting albumin for fluid resuscitation
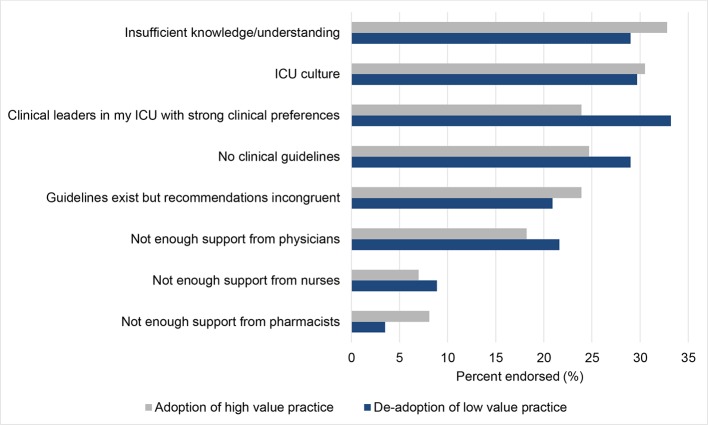
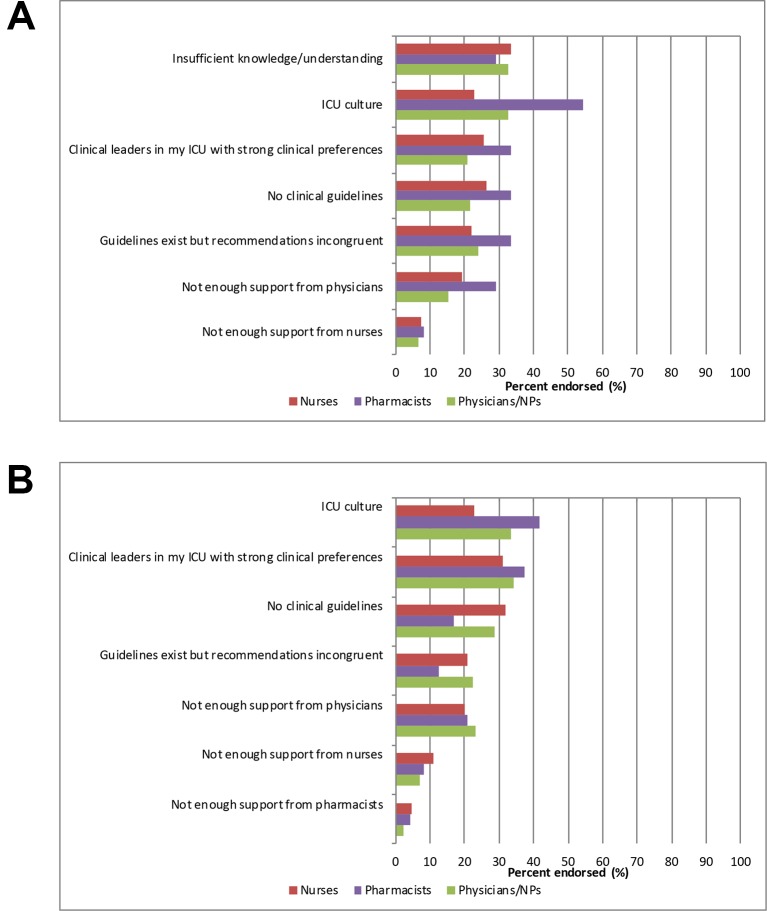
Barriers to adoption and de-adoption were reported by 65.2% and 64.9% of respondents, respectively. The most commonly reported perceived barriers to adopting LMWH for VTE prophylaxis were insufficient knowledge or understanding, ICU culture and no clinical guidelines (figure 1). The most commonly reported barriers to de-adopting albumin for fluid resuscitation were a strong clinical preference of the local clinical leaders in the ICUs, ICU culture and insufficient knowledge or understanding (figure 1). Reported barriers differed between professional groups for both adoption (figure 2A) and de-adoption (figure 2B).
Figure 1.
Barriers to the adoption of high value practices (low molecular weight heparin for venous thromboembolism prophylaxis) and de-adoption of low value practices (albumin for fluid resuscitation). Abbreviations: ICU, intensive care unit.
Figure 2.
(A) Barriers to the adoption of high value practices (low molecular weight heparin for venous thromboembolism prophylaxis) by professional group. (B) Barriers to the de-adoption of low value practices (albumin for fluid resuscitation) by professional group. ICU, intensive care unit; NP, nurse practitioner.
Facilitators to adopting low molecular weight heparin for venous thromboembolism prophylaxis and de-adopting albumin for fluid resuscitation
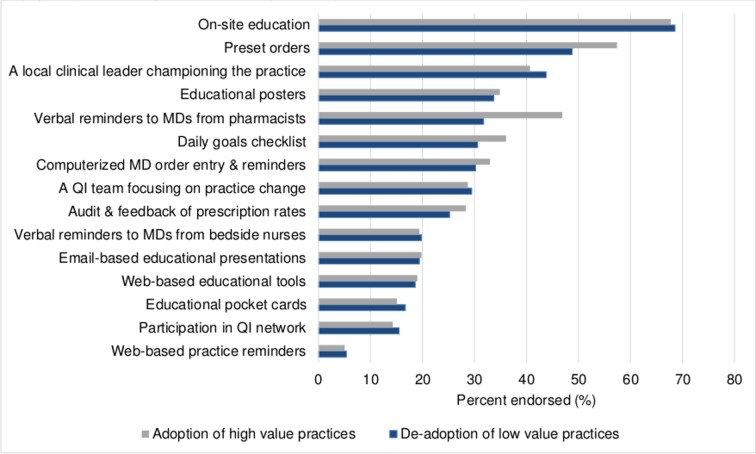
On site education and preset orders were perceived to be the most commonly reported facilitator of both adoption and de-adoption (figure 3). Verbal reminders from pharmacists to physicians were commonly reported as a perceived facilitator for adopting LWMH for VTE prophylaxis. A local leader championing the practice was commonly reported as a perceived facilitator for de-adopting albumin for fluid resuscitation (figure 3). There was no variability by professional group.
Figure 3.
Facilitators to the adoption of high value practices (low molecular weight heparin for venous thromboembolism prophylaxis) and de-adoption of low value practices (albumin for fluid resuscitation). MD, medical doctor; QI, quality improvement.
Discussion
The present study identified opportunities to improve the use of best practices for VTE prophylaxis (adopting the high value practice of LMWH) and fluid resuscitation (de-adopting the low value practice of albumin). Our audit data demonstrated that current practice does not reflect providers’ understanding of the evidence for these practices. The use of the best practice for these two illustrative examples were less likely for patients with greater severity of illness and varied across institutions. The perceived barriers and facilitators to adoption and de-adoption were broadly similar.
Are de-adoption and adoption just the flip-side of the same coin? There is substantial literature describing the adoption of high value practices, but much less is known about de-adoption of low value practices.8 Science can inform clinical practice through discovery resulting in adoption of a new practice, replacement resulting in a practice update and reversal resulting in de-adoption of an existing practice. It is only recently that the last concept, de-adopting low value practices, has been debated in journals and by professional societies.13 14 16 The practical implication is that there is limited evidence to inform whether the barriers and facilitators for adoption and de-adoption are similar or sufficiently distinct to warrant different approaches.9 11–13 Our study adds to the limited evidence base by suggesting that culture or organisational factors, provider characteristics and patient characteristics are perceived to be important barriers and facilitators that may play broadly similar roles in adoption and de-adoption.11 12
Knowledge translation (KT) interventions are strategies to improve the synthesis, dissemination, exchange and application of evidence to improve health.5 KT interventions tailored to the specific barriers and facilitators of an innovation and the local context are more likely to effect change.5 6 Our study provides insight into the perceived barriers and facilitators of adopting high value practices (LMWH for VTE prophylaxis) and de-adopting low value practices (albumin for fluid resuscitation) within ICUs, which should be taken into consideration when designing KT interventions. Interestingly, despite knowledge of the evidence underlying the illustrative example practices, providers perceived insufficient knowledge or understanding to be a barrier and perceived education to be a facilitator to both adopting high value practices and de-adopting low value practices. These barriers and facilitators are consistent with a systematic review that suggests the most effective KT interventions in the ICU employ a combination of education and protocols.33 While consistent with previous KT studies, this finding is paradoxical. It is possible that while knowledgeable, providers’ confidence in applying their knowledge clinically was low and they believed education to be the intervention needed to improve their confidence in applying their knowledge. Furthermore, confidence in applying new evidence in clinical practice may be particularly challenging in the care of severely ill patients. This hypothesis is supported by two of our findings: (1) the use of LMWH for VTE prophylaxis and not using albumin for fluid resuscitation was inversely associated with severity of patient illness and (2) the use of LMWH and not using albumin increased as the patient became more stable (over ICU stay). Potential hypotheses to explain these observations include that clinicians may employ conservative decision making (use more familiar practices) or unintendedly neglect to use best practices when caring for sicker patients, but this need further exploration. The implications are that KT interventions should consider clinician heuristics that are likely to be influenced by the nature and severity of patient illness.
Our study suggests that factors other than knowledge may contribute to the successful adoption of high value practices and de-adoption of low value practices, which includes culture, providers and the innovation. These factors have previously been identified within the context of the ICU.2 34–39 ICU culture and local clinical leader preferences were among the most commonly endorsed barriers to adopting high value practices and de-adopting low value practices in our study. This is highlighted by the variation in the use of LMWH between ICUs, even when patient level factors were taken into consideration. Interestingly, this finding was less pronounced for de-adoption, which has been previously reported.9 Culture, also referred to as organisational context, is a frequently cited barrier to evidence-based medicine and can have a profound effect on clinical practice.7 40 However, few studies have systematically evaluated the effect of culture on adopting high value practices and de-adopting low value practices, and implementation studies infrequently account for the effect of culture on their practice change interventions.41 Similarly, the professional role of the provider is not often contextualised but may be important (eg, should pharmacists and nurses be targeted in KT interventions designed to change the prescribing patterns of physicians and if so how?).42 This may be especially relevant as healthcare delivery becomes increasingly multi-professional and team-based as illustrated in our setting (ICU).
The characteristics of innovations themselves may influence change in clinical practice. Evidence suggests that if the innovation being adopted is congruent with clinical practice beliefs it can facilitate adoption.7 Furthermore, the quality, quantity and stability of available evidence to support the adoption or de-adoption of an innovation is likely important.43 Although most providers in our study were aware of the evidence to support the adoption of LMWH for VTE prophylaxis and de-adoption of albumin for fluid resuscitation, they may not have perceived the evidence to be sufficient to warrant practice change. A growing awareness of challenges with reproducing scientific evidence and clinician experience with practice reversals2 may result in more conservative provider behaviour and slower practice change in response to new evidence. The suboptimal prescribing practices observed in our study likely represent a combination of all these factors.
One limitation of this study is that the survey used was imperfect. The results of the self-reported survey reflect perceived modifiers of practice among providers rather than factors shown to influence practice patterns as identified in observational studies.44 The survey was purposefully designed to be simple and accessible to garner a representative perspective from all provider professions and therefore captured key concepts, but not granular data. Nevertheless, the survey has been successfully used for a similar purpose by others30; was reliable and reported to have good clinical sensibility. Alternative methodologies such as qualitative analyses of semistructured interviews may have allowed for more in depth exploration of barriers and facilitators to adopting LMWH and de-adopting albumin. Finally, while this study was a provincial and multisite it was constrained to ICUs, which should be taken into consideration when interpreting our findings beyond this setting.
In conclusion, our study provides several insights into similarities and differences between adoption of high value practices and de-adoption of low value practices. Both adoption and de-adoption of the illustrative example practices did not reflect healthcare providers’ knowledge of the evidence. The use of best practices for both illustrative examples practices were less likely for patients with greater severity of illness and varied across institutions. We found that perceived barriers and facilitators are more similar than different between adoption and de-adoption, which suggests existing behaviour change frameworks for adopting high value practices may also be applicable for de-adopting low value practices.
Supplementary Material
Acknowledgments
KS would like to acknowledge salary support from the O’Brien Institute for Public Health & Ward of the 21st Century within the Cumming School of Medicine at the University of Calgary, and the Canadian Institutes of Health Research. SB is supported by a Canada Research Chair in Critical Care Nephrology. DJC holds a Canada Research Chair in Knowledge Translation in the ICU. HTS is supported by a Population Health Investigator Award from Alberta Innovates and an Embedded Clinician Researcher Award from the Canadian Institutes of Health Research.
Footnotes
Contributors: KS contributed to the design and conceptualisation of the study; analysis and interpretation of the data, drafting and revising the manuscript and gave approval of the final version of the manuscript. SMB, DN, JPL, DJC and HTS contributed to the design and conceptualisation of the study, interpretation of the data, providing feedback on the manuscript and gave approval of the final version of the manuscript. AS contributed to the analysis and interpretation of the data, providing feedback on the manuscript and gave approval of the final version of the manuscript. RB-M contributed to the interpretation of the data, providing feedback on the manuscript and gave approval of the final version of the manuscript.
Funding: This work was supported by a Partnership for Research and Innovation in Health Systems grant awarded by Alberta Innovates (Grant #201309 [HTS and SMB]).
Competing interests: None declared.
Ethics approval: This study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB14-0992 and REB15-2147) and the University of Alberta Research Ethics Board (Pro00056709 and Pro00060650).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data will be available if accepted.
Patient consent for publication: Not required.
References
- 1. Institute of Medicine. Crossing the Quality Chiasm. Washington, DC, 2001. [Google Scholar]
- 2. Niven DJ, Rubenfeld GD, Kramer AA, et al. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med 2015;175:801–9. 10.1001/jamainternmed.2015.0157 [DOI] [PubMed] [Google Scholar]
- 3. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med 2003;348:2635–45. 10.1056/NEJMsa022615 [DOI] [PubMed] [Google Scholar]
- 4. Rogers EM. Lessons for guidelines from the diffusion of innovations. Jt Comm J Qual Improv 1995;21:324–8. [DOI] [PubMed] [Google Scholar]
- 5. Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof 2006;26:13–24. 10.1002/chp.47 [DOI] [PubMed] [Google Scholar]
- 6. McCormack B, Kitson A, Harvey G, et al. Getting evidence into practice: the meaning of ’context'. J Adv Nurs 2002;38:94–104. [DOI] [PubMed] [Google Scholar]
- 7. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]
- 8. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med 2015;13:255 10.1186/s12916-015-0488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Bodegom-Vos L, Davidoff F, Marang-van de Mheen PJ. Implementation and de-implementation: two sides of the same coin? BMJ Qual Saf 2017;26:495–501. 10.1136/bmjqs-2016-005473 [DOI] [PubMed] [Google Scholar]
- 10. Rogers EM. The innovation-decision process. Diffusion of Innovations. 5 ed New York, NY: Free Press, 2003. [Google Scholar]
- 11. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci 2014;9:1 10.1186/1748-5908-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montini T, Graham ID. "Entrenched practices and other biases": unpacking the historical, economic, professional, and social resistance to de-implementation. Implement Sci 2015;10:24 10.1186/s13012-015-0211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davidoff F. On the undiffusion of established practices. JAMA Intern Med 2015;175:809–11. 10.1001/jamainternmed.2015.0167 [DOI] [PubMed] [Google Scholar]
- 14. Al-Ani F, Shariff S, Siqueira L, et al. Identifying venous thromboembolism and major bleeding in emergency room discharges using administrative data. Thromb Res 2015;136:1195–8. 10.1016/j.thromres.2015.10.035 [DOI] [PubMed] [Google Scholar]
- 15. Macleod MR, Michie S, Roberts I, et al. Biomedical research: increasing value, reducing waste. Lancet 2014;383:101–4. 10.1016/S0140-6736(13)62329-6 [DOI] [PubMed] [Google Scholar]
- 16. Grady D, Redberg RF. Less is more: how less health care can result in better health. Arch Intern Med 2010;170:749–50. 10.1001/archinternmed.2010.90 [DOI] [PubMed] [Google Scholar]
- 17. Fowler RA, Mittmann N, Geerts W, et al. Cost-effectiveness of dalteparin vs unfractionated heparin for the prevention of venous thromboembolism in critically ill patients. JAMA 2014;312:2135–45. 10.1001/jama.2014.15101 [DOI] [PubMed] [Google Scholar]
- 18. Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3 Suppl):188s–203. 10.1378/chest.126.3_suppl.188S [DOI] [PubMed] [Google Scholar]
- 19. Li G, Cook DJ, Levine MA, et al. Competing risk analysis for evaluation of dalteparin versus unfractionated heparin for venous thromboembolism in medical-surgical critically Ill patients. Medicine 2015;94:e1479 10.1097/MD.0000000000001479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alberta Health Services. Venous thromboembolism prophylaxis (document #PS09-01). 2016. https://extranet.ahsnet.ca/teams/policydocuments/1/clp-venous-thromboembolism-prophylaxis-ps-09-01-guideline.pdf.
- 21. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486–552. 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 22. Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247–56. 10.1056/NEJMoa040232 [DOI] [PubMed] [Google Scholar]
- 23. Lyu PF, Hockenberry JM, Gaydos LM, et al. Impact of a sequential intervention on albumin utilization in critical Care. Crit Care Med 2016;44:1307–13. 10.1097/CCM.0000000000001638 [DOI] [PubMed] [Google Scholar]
- 24. Navickis RJ, Greenhalgh DG, Wilkes MM. Albumin in burn shock resuscitation: a meta-analysis of controlled clinical studies. J Burn Care Res 2016;37:e268–78. 10.1097/BCR.0000000000000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel A, Laffan MA, Waheed U, et al. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ 2014;349:g4561 10.1136/bmj.g4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397–417. 10.1016/j.jhep.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 27. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology 2012;55:1172–81. 10.1002/hep.24786 [DOI] [PubMed] [Google Scholar]
- 28. Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology 2015;62:567–74. 10.1002/hep.27709 [DOI] [PubMed] [Google Scholar]
- 29. Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol 2013;11:123–30. 10.1016/j.cgh.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 30. Cook D, Duffett M, Lauzier F, et al. Barriers and facilitators of thromboprophylaxis for medical-surgical intensive care unit patients: a multicenter survey. J Crit Care 2014;29:471.e1–471.e9. 10.1016/j.jcrc.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 31. Parsons Leigh J, Niven DJ, Boyd JM, et al. Developing a framework to guide the de-adoption of low-value clinical practices in acute care medicine: a study protocol. BMC Health Serv Res 2017;17:54 10.1186/s12913-017-2005-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stelfox HT, Niven DJ, Clement FM, et al. Stakeholder Engagement to Identify Priorities for Improving the Quality and Value of Critical Care. PLoS One 2015;10:e0140141 10.1371/journal.pone.0140141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sinuff T, Muscedere J, Adhikari NK, et al. Knowledge translation interventions for critically ill patients: a systematic review*. Crit Care Med 2013;41:2627–40. 10.1097/CCM.0b013e3182982b03 [DOI] [PubMed] [Google Scholar]
- 34. Gershengorn HB, Wunsch H. Understanding changes in established practice: pulmonary artery catheter use in critically ill patients. Crit Care Med 2013;41:2667–76. 10.1097/CCM.0b013e318298a41e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koo KK, Sun JC, Zhou Q, et al. Pulmonary artery catheters: evolving rates and reasons for use. Crit Care Med 2011;39:1613–8. 10.1097/CCM.0b013e318218a045 [DOI] [PubMed] [Google Scholar]
- 36. Murphy DJ, Needham DM, Netzer G, et al. RBC transfusion practices among critically ill patients: has evidence changed practice?. Crit Care Med 2013;41:2344–53. 10.1097/CCM.0b013e31828e9a49 [DOI] [PubMed] [Google Scholar]
- 37. Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993-2004. JAMA 2007;298:423–9. 10.1001/jama.298.4.423 [DOI] [PubMed] [Google Scholar]
- 38. Munshi L, Gershengorn HB, Fan E, et al. Adjuvants to mechanical ventilation for acute respiratory failure. Adoption, de-adoption, and factors associated with selection. Ann Am Thorac Soc 2017;14:94–102. 10.1513/AnnalsATS.201606-438OC [DOI] [PubMed] [Google Scholar]
- 39. Kahn JM, Le TQ, Tq L. Adoption and de-adoption of drotrecogin alfa for severe sepsis in the United States. J Crit Care 2016;32:114–9. 10.1016/j.jcrc.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 40. Melnyk BM. Culture eats strategy every time: What works in building and sustaining an evidence-based practice culture in healthcare systems. Worldviews Evid Based Nurs 2016;13:99–101. 10.1111/wvn.12161 [DOI] [PubMed] [Google Scholar]
- 41. Dodek P, Cahill NE, Heyland DK. The relationship between organizational culture and implementation of clinical practice guidelines: a narrative review. JPEN J Parenter Enteral Nutr 2010;34:669–74. 10.1177/0148607110361905 [DOI] [PubMed] [Google Scholar]
- 42. Menear M, Grindrod K, Clouston K, et al. Advancing knowledge translation in primary care. Can Fam Physician 2012;58:623e302–7. [PMC free article] [PubMed] [Google Scholar]
- 43. Scott IA, Elshaug AG. Foregoing low-value care: how much evidence is needed to change beliefs? Intern Med J 2013;43:107–9. 10.1111/imj.12065 [DOI] [PubMed] [Google Scholar]
- 44. Lauzier F, Muscedere J, Deland E, et al. Thromboprophylaxis patterns and determinants in critically ill patients: a multicenter audit. Crit Care 2014;18:R82 10.1186/cc13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cook D, Meade M, Guyatt G, et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med 2011;364:1305–14. 10.1056/NEJMoa1014475 [DOI] [PubMed] [Google Scholar]
- 46. Alhazzani W, Lim W, Jaeschke RZ, et al. Heparin thromboprophylaxis in medical-surgical critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care Med 2013;41:2088–98. 10.1097/CCM.0b013e31828cf104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024159supp001.pdf (62.1KB, pdf)
bmjopen-2018-024159supp002.pdf (473.1KB, pdf)
bmjopen-2018-024159supp003.pdf (87.1KB, pdf)
bmjopen-2018-024159supp004.pdf (46.3KB, pdf)
bmjopen-2018-024159supp005.pdf (38.2KB, pdf)
bmjopen-2018-024159supp006.pdf (26.5KB, pdf)
bmjopen-2018-024159supp007.pdf (41KB, pdf)