Abstract
Background
This study investigates the frequency of near-miss events and compares correlates in the world’s newest nation.
Methods
A cross-sectional study was carried out to audit near-miss events and their causes. A total of 1,041 mothers were sampled. Data were gathered using World Health Organization near-miss evaluation tools according to morbidity and organ failure-based criteria. Intensive care unit admission criteria were not used (as there is no functional intensive care unit in Juba Teaching Hospital). Descriptive statistics and bivariate and multivariable logistic regression were used to analyze the data. The study adhered to the Declaration of Helsinki.
Results
Nearly half (49.7%) of the clients were young pregnant women (aged 15–24 years), with a mean age of 25.07±5.65 years. During the study period, there were 994 deliveries, 94 near-misses, and 10 maternal deaths. This resulted in maternal near-miss and mortality rates of 94.1 per 1,000 and 1,007 per 100,000 live births, respectively. Severe maternal outcome and maternal near-miss rates were 10.47 per 1,000 (morbidity-based criteria) and 41.3 per 1,000 (organ failure-based criteria), respectively. The likelihood of mortality was 25% (95% CI 10%–51%) for a ruptured uterus, 9% (95% CI 4%–17%) for severe postpartum hemorrhage, and 11% (95% CI 3%–30%) for eclampsia. Anemia, hemorrhage, and dystocia were the highest contributory factors in the occurrence of maternal near-misses.
Conclusion
The near-miss rate was high. Contributing factors were lack of resources, low quality of primary health care, and delays in care. All near-misses should be regarded as opportunities to improve the quality of maternity care. Health institutes should address delays in conducting interventions, referral barriers, and personnel gaps. Fully functional intensive-care units must be created in all facilities, including Juba Teaching Hospital and other hospitals. Notification policies for all near-miss cases should be in place in all health care units, with a “no shame, no blame” approach.
Keywords: severe maternal morbidity, near-miss, Juba Teaching Hospital, near-miss audit, severe acute morbidity, South Sudan
Introduction
An obstetric near-miss (NM) is a situation in which a woman would have died had she not received quality medical care.1 Women who endure severe morbidities in the course of pregnancy, childbirth, and the postpartum period can help us to better understand the set of circumstances and avoidable factors that contribute to maternal deaths.2 Maternal NM (MNM) was recently defined by the World Health Organization (WHO) as “the near death of a woman from a complication during pregnancy, childbirth, or within 42 days after the termination of pregnancy”.3 NM cases and maternal deaths are together referred to as severe maternal outcomes (SMOs). Data on severe morbidity are vital for policymakers to recognize essential obstetric care. NM findings are also better indicators than maternal mortality alone for planning, monitoring, follow-up, and evaluating safe-motherhood programs.1,4,5
The WHO sums up the identification of NM cases in three main classes of criteria: clinical criteria, intervention based or the process of management of NMs, and organ system-dysfunction criteria, such as cardiovascular or respiratory failure.3,6 In sub-Saharan Africa, the unacceptably high number of maternal deaths overshadows severe maternal morbidity. For this reason, comprehensive studies on MNMs are of great value to clinical audit and quality improvement.7–10
In a WHO survey of maternal and prenatal health in Latin America, for eight countries the mean proportional ratio of NMs was 34 per 1,000 deliveries. Age >35 years, being single, primipara/parity above three, and delivery at public institutions were associated with the occurrence of an NM.11
A study conducted in Sudan’s Kassala found that among 9,578 deliveries, the MNM and maternal mortality rates were 22.1 per 1,000 live births and 432 per 100,000 live births, respectively. Hemorrhage accounted for the most common event (40.8%), followed by infection (21.5%). Mortality rates were 22.2%, 10.0%, 10.0%, 8.8%, and 2.4% for infection, dystocia, anemia, hemorrhage, and hypertensive disorders, respectively.12
Reproductive health indicators in South Sudan are among the poorest in the world: only about 25% of the population in South Sudan have access to health care. Maternal mortality is 2,054 per 100,000 live births. Since women in South Sudan have an average of six to seven births in their lifetime, this corresponds to approximately 20% of women dying in the process of giving birth.13 The major causes of maternal death in South Sudan are similar to most developing countries: infection, hemorrhage, obstructed labor, abortion, and hypertension in pregnancy.14,15
In 2015, South Sudan, a mainly rural country, had an estimated population of around 12,152,000, projected from the 2008 census.16 After five decades of civil war, the Comprehensive Peace Agreement provided for a referendum in January 2011 and full independence in July 2011. South Sudan is among the least developed countries in the world. The society’s infrastructure has been devastated by decades of civil war, and infectious and malnutrition-related diseases run rife, with unacceptably low provisions for health care.17
Nearly 46.7% of pregnant women attend at least one antenatal-care session. However, only 19.4% of deliveries are institutional deliveries by skilled professionals.18,19 Access to comprehensive emergency obstetric and neonatal care is limited. For instance, only 0.5% of pregnancies were delivered by cesarean section at the three teaching hospitals in Juba, Malakal, and Wau.20
Documentation and reporting at health institutions in South Sudan is often poor, and statistics on morbidity and mortality are scarce, with poor reliability, even in large teaching hospitals like Juba Teaching Hospital (JTH).21 No data are available on severe maternal morbidity or NM events in South Sudan. This study investigates the proportion of NM events and their causes, analyzes the mortality index of each event, and compares correlates of NM cases with maternal deaths. These data will be beneficial for program planners in developing countries, specifically South Sudan.
Methods
A cross-sectional study was conducted to audit NM cases from March 20 to June 12, 2016 in JTH. Juba is the nation’s capital, and the hospital itself serves >3 million people. It has more than 524 beds and provides all types of services, including obstetric and gynecologic services. Deliveries take place in the maternity ward, where the quantitative data were collected. JTH’s maternity unit provides an average of 500 deliveries per month. All women with obstetric and gynecology complications are referred to JTH.
Blood transfusion unfortunately is not a common practice in JTH, because of the scarcity of blood and poor performance of blood banks. Voluntary blood donation is almost nonexistent, and patients’ relatives (if they are present) will donate when a need for blood arises. This process usually takes >2 hours. Drugs, including essential drugs, anesthetics, antiseptic solution, and basic consumables, such as gloves, are often unavailable in the hospital. Access to JTH is sometimes difficult, especially at night from some neighborhoods, such as Gumbo and Sherikat, where the bridge is closed every day from 2 pm until morning.
Overall, there is a lack of agreed criteria for documentation of cases of severe maternal morbidity (NMs). Identification of cases varies across studies and is often complex to apply in a developing-country setting.22,23 The sample-size formula to estimate a single population proportion is based on the following assumptions: level of confidence 95%, 3% margin of error, and 8% prevalence of NM cases for developing countries.3 Therefore, after adding a 5% nonresponse rate (for refusal to consent and erratum data sheets), the final sample size calculated is 1,041. The sample size was calculated using OpenEpi (http://www.openepi.com/SampleSize/SSCohort.htm).
MNM cases
MNM cases were operationalized as any event of obstetric complication that threatened life, near death but not resulting in death, either by chance or because of medical care received during pregnancy, labor, and delivery or within the puerperium period.24 Disease-specific criteria employed by Filippi et al9 were applied. Complications considered were: hemorrhage leading to shock; emergency hysterectomy, coagulation defects and/or blood transfusion ≥2 L, hypertensive disorders in pregnancy, including eclampsia, severe preeclampsia, and dystocia, uterine rupture; and impending rupture as a result of prolonged obstructed labor, infection, and severe anemia (hemoglobin level <7 g/dL).9,25
The WHO tool for evaluating maternal health was adopted to collect the data. The validity and reliability of this tool has been tested elsewhere.26 Data were gathered using the operational definition of NM and morbidity-based and organ failure-based criteria. As there is no functional intensive-care unit (ICU) in JTH, ICU-admission criteria were not used. For each case, data were also collected on sociodemographic characteristics, nature of obstetric complication(s), data on organ and/or system dysfunction, and source of referral. Six senior midwives and house officers were involved in the data collection.
Data entry was carried out using Epi Info version 2000 and analysis using SPSS for Windows version 21. For each obstetric event, the mortality index was calculated to determine the standard of care delivered for each complication. The association of maternal characters with outcome was established using χ2 tests. P<0.05 indicated statistical significance. A logistic regression model was fitted to the data to determine the effects of predictor variables on the main outcome, which was NM event. The likelihood-ratio statistic was calculated to determine the effect of each cause of NM events to maternal death.
MNM results were calculated: MNM-incidence ratio as number of MNM cases per 1,000 live births, MNM mortality rat as MNMs divided by maternal deaths (higher ratios indicate better care), and mortality index as number of maternal deaths to number of women with NM conditions (higher index values show more women with life-threatening conditions are dying [low quality of care], while lower values suggest good-quality health care).
The study was approved by the Ethical Review Board at the Ministry of Health, Republic of South Sudan, and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Each participant was given a consent form to sign after the purpose and objective of the study had been explained (Figure S1). If the pregnant individual was found to be aged <18 years, the guardian was given a parent/guardian consent form (Figure S2). Pregnant girls were also given informed-consent forms to ensure their right to decline.
Results
Sociodemographic characteristics
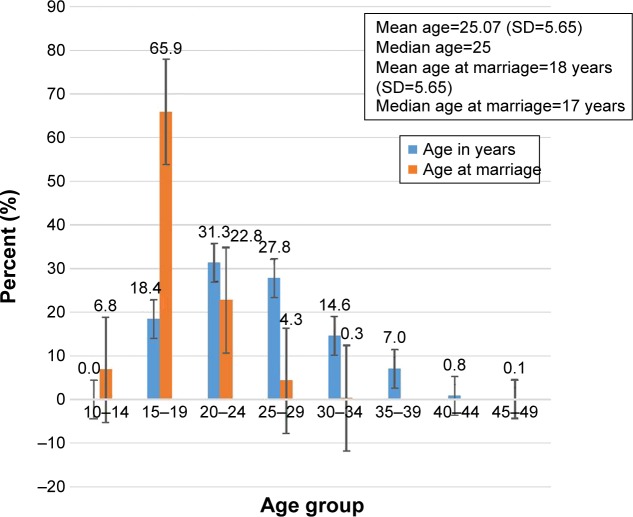
A total of 1,010 mothers at JTH participated in the study (from a total of 1,041 sampled), making the response rate 97.5%. Nearly half (49.7%) the clients visiting JTH’s Maternity and Gynecology Unit were young pregnant women (15–24 years of age) at the time of the visit, with a mean age of 25.07 (SD 5.65) years. The majority of respondents (96.6%) were married, with mean and median ages at marriage of 18 and 17 years, respectively (Figure 1). Nearly a third (32.6%) of the mothers did not have formal education. Almost all ethnic groups were represented in the study. The median distance clients traveled was 17.8 km (IQR 799 km; Table 1).
Figure 1.
Age at marriage and age distribution of others visiting Juba Teaching Hospital, May–August, 2016.
Table 1.
Sociodemographic characteristics of clients admitted to JTH, May–August, 2016
| n (%) | |
|---|---|
| Residence | |
| Urban | 828 (82.0) |
| Rural | 182 (18.0) |
| Total | 1,010 (100) |
| Ethnicity | |
| Dinka | 146 (14.5) |
| Nuer | 55 (5.4) |
| Bari | 261 (25.8) |
| Zande | 25 (2.5) |
| Other | 523 (51.8) |
| Total | 1,010 (100) |
| Educational status | |
| Unable to read and write | 244 (24.2) |
| Able to read and/or write only | 85 (8.4) |
| Primary education | 368 (36.4) |
| Senior level | 227 (22.5) |
| Some certificate training | 20 (2.0) |
| College-level education | 66 (6.5) |
| Total | 1,010 (100) |
| Religion | |
| Catholic | 469 (46.4) |
| Protestant | 400 (39.6) |
| Muslim | 89 (8.8) |
| Others (traditional) | 52 (5.1) |
| Total | 1,010 (100) |
| Marital status | |
| Single | 55 (5.4) |
| Married | 955 (96.6) |
| Total | 1,010 (100) |
Abbreviation: JTH, Juba Teaching Hospital.
Obstetric characteristics of participants
Table 2 shows obstetric characteristics of the women. Two-thirds (62.8%) of the pregnancies were planned and wanted, which is typical of a country where there is high demand for fertility. Only 128 (12.6%) women were referred from primary health care centers, while the majority of referrals (88.6%) were initiated by either their neighbors or themselves, indicating a poor referral system.
Table 2.
Distribution of respondents by obstetric characteristics among clients admitted to JTH, May–August, 2016
| Severe maternal outcomes | P-value | Remarks | |||
|---|---|---|---|---|---|
| No | Yes | Total | |||
| Current pregnancy wanted or unwanted | |||||
| Wanted and planned | 571 (90.1%) | 63 (9.9%) | 634 (100%) | 0.228 | |
| Wanted but unplanned | 285 (90.2%) | 31 (9.8%) | 316 (100%) | ||
| Unwanted and unplanned | 50 (83.3%) | 10 (16.7%) | 60 (100%) | ||
| Total | 906 (89.7%) | 104 (10.3%) | 1,010 (100%) | ||
| History of obstetric morbidity* (eg, PPH, abortion, dystocia, eclampsia) | |||||
| No | 444 (91.7%) | 40 (8.3%) | 484 (100%) | <0.0001** | |
| Yes | 200 (85.5%) | 34 (14.5%) | 234 (100%) | ||
| Total | 644 (89.7%) | 74 (10.3%) | 718* (100%) | ||
| Parity | |||||
| Primipara | 266 (89.9%) | 30 (10.1%) | 296 (100%) | 0.038* | Mean gravidity 3.1 (SD 2.1) Mean parity 2.9 (SD 2.1) |
| Multipara | 640 (89.6%) | 74 (10.4%) | 714 (100%) | ||
| Total | 906 (89.7%) | 104 (10.3%) | 1,010 (100%) | ||
| Gestational age | |||||
| First trimester (1–12 weeks) | 80 (90.9%) | 8 (9.1%) | 88 (100%) | 0.61 | Mean 33.6 (SD 12.1) |
| Second trimester (13–28 weeks) | 102 (87.2%) | 15 (12.8%) | 117 (100%) | ||
| Third trimester (>28 weeks) | 724 (89.9%) | 81 (10.1%) | 805 (100%) | ||
| Total | 906 (89.7%) | 104 (10.3%) | 1,010 (100%) | ||
| Duration of labor | |||||
| <24 hours | 797 (89.3%) | 95 (10.7%) | 892 (100%) | 0.069 | Median 9.5 hours Range 95 hours |
| >24 hours | 27 (79.4%) | 7 (20.6%) | 34 (100%) | ||
| Total | 824 (89.0%) | 102 (11.0%) | 926 (100%) | ||
| Source of referral | |||||
| Health institute | 95 (74.2%) | 33 (25.8%) | 128 (100%) | <0.0001 | |
| Self-referral | 648 (91.8%) | 58 (8.2%) | 706 (100%) | ||
| Neighbor/family | 32 (86.5%) | 5 (13.5%) | 37 (100%) | ||
| Other | 131 (94.2%) | 8 (5.8%) | 139 (100%) | ||
| Total | 906 (89.7%) | 104 (10.3%) | 1,010 (100%) | ||
Notes:
Associated;
strongly associated.
Abbreviations: JTH, Juba Teaching Hospital; PPH, postpartum hemorrhage.
The mean duration of labor among clients was 9.5 hours, with a range of 95 hours. Factors associated with SMOs (MNMs and maternal death) included history of obstetric morbidity (P=0), being primigravida (P=0.03), and being referred from other health institutes. The evidence of association of the latter was expected, since a referral entails observing signs of some abnormal pregnancy indicators. However, the status of pregnancy (planned vs unplanned), gestational age, and duration of labor were not predictors of SMOs (Table 2).
MNM indicators
Table 3 shows NM indicators of women who received care at JTH during the study period. The total number of live births that took place at JTH during the data-collection period (source population) was documented and used for calculations to produce consistency with maternal mortality-rate results. The severe maternal outcome ratio (SMOR) and MNM ratio were outcome indicators of maternal morbidity events occurring in the population. Accordingly, during the study period there were 994 deliveries, 993 live births, 94 NM cases, ten maternal deaths, and 165 NM events, giving a mean of 1.7 severe life-threatening morbidities per case. This resulted in a total MNM and maternal mortality rate of 94.1 per 1,000 live births and 1,007/100,000 live births, respectively, based on morbidity-based criteria.
Table 3.
MNM indices among clients admitted to JTH, May–August, 2016
| Morbidity- based | Organ failure- based* | Maternal death | Live births | IUFD | Stillbirths | |
|---|---|---|---|---|---|---|
| Total NM cases (n) | 94 | 31 | 10 | 993 | 22 | 23 |
| MNMR (MNMs/LBs) (%) | 9.47% | 3.12% | ||||
| WLTCs (MNMs + MDs) (ratio) | 10.4 | 41 | ||||
| SMOR** (n) | 10.47 | 41.3 | ||||
| Mortality index (MDs/MNMs + MDs)# (n) | 9.62 | 24.4 | ||||
| MNM mortality ratio (MNMs:MDs)## (n) | 10.04 | 4.1 | ||||
| MMR (MD/LBs) (n) | 1,007.05 | |||||
| Stillbirth rate | 45.3 per 1,000 LBs | |||||
Notes:
Only clinical criteria were used;
only laparotomy and fistula combined were considered;
the higher the index, the more women with life-threatening conditions die (low quality of care), whereas the lower the index, the fewer women with life-threatening conditions die (better quality of care);
higher ratios indicate better care.
Abbreviations: MNM, maternal near-miss; JTH, Juba Teaching Hospital; MNMR, MNM ratio; LBs, live births; WLTCs, women with life-threatening conditions; MD, maternal death; SMOR, severe maternal outcome ratio; MMR, maternal mortality ratio; IUFD, intrauterine fetal death; NM, near-miss.
The SMOR and MNM ratio were 10.47, based on morbidity-based criteria, and 41.3 per 1,000, based on organ failure-based criteria, respectively. These NM indicators provide a means to estimate the complexity of service that is required by the community served by the health system, and a significant proportion of clients require more multifaceted interventions to endure their complications. The total mortality-index value for NM cases was 9.56% (NM:fatality ratio 1:5.1). The mortality index and MNM mortality ratios provide an estimate of performance. The high mortality-index value highlights that the quality of care provided to severely morbid mothers may need to be reviewed.
Various causes of maternal deaths were identified (Table 4). The likelihood of mortality (posttest odds) was 25% (95% CI 10%–51%) for ruptured uterus (ie, one in four), for severe postpartum hemorrhage 9% (95% CI 4%–17%; ie, around one in eleven), eclampsia 11% (95% CI 3%–30%), sepsis 6% (95% CI 1%–28%), and abortion complications 5% (95% CI 1%–25%). We found eight obstetric fistulae, contributing to 7.7% of the NM cases, giving 8.05 per 1,000 live births. Obstetric fistula cases provide an indicator of the health system’s failure to provide accessible, timely, and appropriate maternal care. Although a cesarean section could have saved many women from death, the overall cesarean section rate at JTH was only 4.49%, which is lower than the WHO recommendation.
Table 4.
Positive likelihood ratio for each near-miss event leading to the probability of presence of maternal death at JTH
| Frequency (n) | Per 100 deliveries | Maternal deaths | Ratio to deaths | Sensitivity | Specificity | Likelihood ratio | Posttest odds (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Severe postpartum hemorrhage | 46 | 46.23 | 4 | 12:1 | 40% | 55.40% | 0.90 (0.41–1.98) | 9% (4%–17%) |
| Eclampsia | 19 | 19.13 | 2 | 10:1 | 20% | 87.10% | 1.11 (0.30–4.11) | 11% (3%–30%) |
| Sepsis | 18 | 18.12 | 1 | 18:1 | 10% | 83.60% | 0.52 (0.08–3.51) | 6% (1%–28%) |
| Ruptured uterus | 9 | 9.06 | 3 | 3:1 | 33% | 93.70% | 3.13 (1.01–9.72) | 25% (10%–51%) |
| Abortion complications | 20 | 20.14 | 1 | 20:1 | 10% | 80.00% | 0.47 (0.07–3.14) | 5% (1%–25%) |
| Given critical care in isolation ward | 40 | 40.28 | 5 | 8:1 | 50% | 62.76% | 1.34 (0.69–2.63) | 12% (7%–22%) |
| Fistula | 8 | 8.05 | 1 | 8:1 | 10% | 92.56% | 1.17 (0.16–8.46) | 11% (2%–47%) |
Abbreviation: JTH, Juba Teaching Hospital.
Process and outcome indicators related to specific conditions
Based on current evidence, use of the first imposed intervention recommended should be 100%. If first-line intervention use is <95%, this should be taken as an opportunity to improve service. Any laparotomy for uterine rupture done after 3 hours of hospital stay indicates a delay in addressing obstructed labor/uterine rupture. Nearly one in three (33.3%) of laparotomies for ruptured uteri were not done within 3 hours. One mother died before she received laparotomy service for a ruptured uterus. A gap was identified in the use of magnesium sulfate in JTH. Among all women that had eclampsia, 84.2% received magnesium sulfate. Only 36.4% of preterm births received corticosteroid therapy (Table 5).
Table 5.
Process and outcome indicators related to specific conditions
| n (%) | |
|---|---|
| Prevention of postpartum hemorrhage | |
| Target population: women giving birth in health care facilities | 1,001 |
| Oxytocin use | 79.5 |
| Use of any uterotonic (including oxytocin) | 29.7 |
| Treatment of severe postpartum hemorrhage | |
| Target population: women with severe postpartum hemorrhage | 46 |
| Oxytocin use | 39 (84.8) |
| Ergometrine | 0 |
| Misoprostol | 11 (10.6) |
| Any of the above uterotonics | 39 (84.6) |
| Tranexamic acid | 0 |
| Removal of retained products | 1 (0.96) |
| Balloon or condom tamponade | 1 (0.96) |
| Artery ligation | 0 |
| Hysterectomy | 12 (11.5) |
| Abdominal packing | 0 |
| Anticonvulsants for eclampsia | |
| Target population: women with eclampsia | 19 |
| Magnesium sulfate | 16 (84.2) |
| Other anticonvulsant | 0 |
| Any anticonvulsant | 16 (84.2) |
| Prevention of cesarean section-related infection | |
| Target population: women undergoing cesarean section | 45 |
| Prophylactic antibiotic during cesarean section | 39 (86.7) |
| Treatment for sepsis | |
| Target population: women with sepsis | 18 |
| Parenteral therapeutic antibiotics | 15 (83.3) |
| Ruptured uterus | |
| Target population: women with ruptured uteri | 9 |
| Laparotomy | 8 (88.9) |
| Laparotomy after 3 hours of hospital stay | 3 (33.3) |
| Preterm birth | |
| Target population: women having a preterm delivery | 22 |
| Corticosteroids for fetal lung maturation | 8 (36.4) |
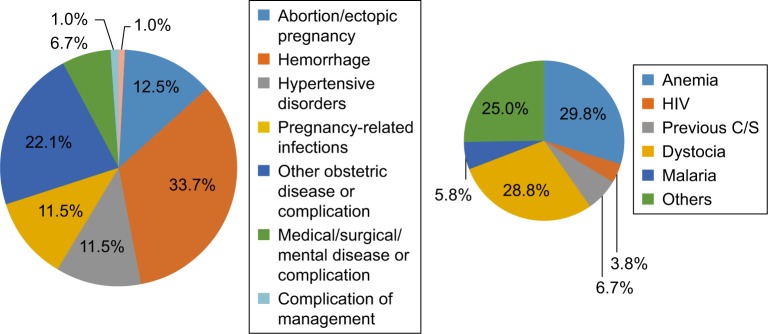
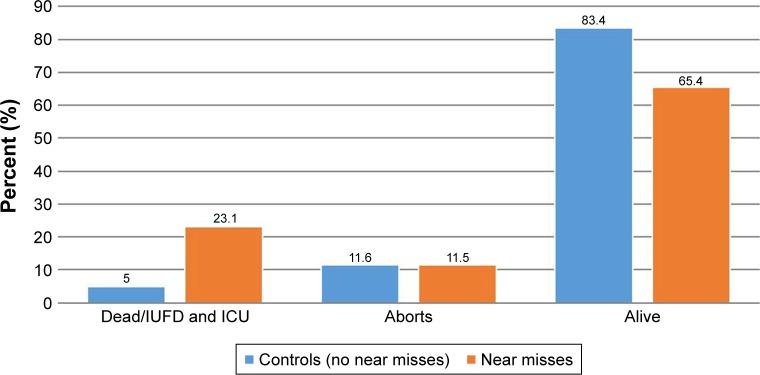
Table 6 compares the characteristics of MNM cases with those women without severe complications. Having no or lower income, age <18 years, and previous cesarean section were independently associated with MNM incidence. The association between parity, past obstetric complications, current pregnancy complications, and maternal morbidity remained statistically significant after adjustment. As depicted in Figure 2, anemia, pregnancy-related hemorrhage, and dystocia were the highest associated and contributory factors for MNM incidents. A higher adverse pregnancy outcome of 23% (death or admitted to isolation unit was recorded in women who suffered NM complications compared to not-NM cases; P=0, Figure 3).
Table 6.
Characteristics of women who experienced a maternal near-miss (crude and adjusted estimates) in JTH, May–August, 2016
| Near-miss | Crude OR (95% CI) | Adjusted OR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| Residence | 0.157 | ||||
| Urban | 748 (90.3%) | 80 (9.7%) | 1 | – | |
| Rural | 158 (86.8%) | 24 (13.2%) | 1.42 (0.90–2.31) | 0.33 (0.12–0.92) | |
| Income | 0.006* | ||||
| Have source of income | 144 (96.0%) | 6 (4.0%) | 1 | 1 | |
| No income/no job | 762 (88.6%) | 98 (11.4%) | 3.09 (1.33–7.17) | 3.01 (1.16–7.84) | |
| Age at marriage, years | |||||
| 10–18 | 385 (89.5%) | 45 (10.5%) | 1 | 1 | |
| 19–30+ | 333 (90.9%) | 35 (9.1%) | 0.85 (0.673–1.08) | – | |
| Estimated distance from home to the JTH | 0.516 | ||||
| <10 km | 554 (89.2%) | 67 (10.8%) | 0.13 (0.78–1.66) | – | |
| >10 km | 352 (90.5%) | 37 (9.5%) | 1 | ||
| Education | 0.053 | ||||
| No formal education | 214 (87.7%) | 30 (12.3%) | 0.985 (0.59–1.64) | – | |
| Primary education | 418 (92.3%) | 35 (7.7%) | 0.59 (0.36–.95) | – | |
| Senior and some college | 274 (87.5%) | 39 (12.5%) | 1 | ||
| Current pregnancy status | 0.228 | ||||
| Wanted and planned | 571 | 63 | 1 | ||
| Wanted but unplanned | 285 | 31 | 1.01 (0.64–1.62) | – | |
| Unwanted and unplanned | 50 | 10 | 1.68 (0.79–3.57) | – | |
| Bad obstetric history* (eg, PPH, abortion, dystocia, eclampsia) | 0.01* | ||||
| Yes | 444 (91.7%) | 40 (8.3%) | 1.89 (1.16–3.07) | 1.81 (1.26–2.89) | |
| No | 200 (85.5%) | 34 (14.5%) | 1 | – | |
| Parity | 0.038* | ||||
| Primipara | 266 (89.9%) | 30 (10.1%) | 1 | – | |
| Multipara | 640 (89.6%) | 74 (10.4%) | 0.24 (0.023–2.45) | – | |
| Medical complications during current pregnancy | 0.263 | ||||
| No | 418 (88.6%) | 54 (11.4%) | 1 | – | |
| Yes | 488 (90.7%) | 50 (9.3%) | 0.79 (0.53–1.19) | – | |
| Current obstetric complications | <0.0001** | ||||
| No | 571 (99.8%) | 1 (0.2%) | 1 | ||
| Yes | 335 (76.5%) | 103 (23.5%) | 75.5 (24.38–126) | 69.5 (22.2–111) | |
Notes:
Associated;
strongly associated.
Abbreviations: JTH, Juba Teaching Hospital; PPH, postpartum hemorrhage.
Figure 2.
Factors associated and contributing to maternal near misses near at Juba Teaching Hospital.
Abbreviation: C/S, cesarean section.
Figure 3.
Pregnancy outcomes among women who experienced maternal near misses and controls (no near misses) at Juba Teaching Hospital, August 2016.
Abbreviations: IUFD, intrauterine fetal death; ICU, intensive-care unit.
Discussion
This study sought to report MNM and its associated factors in the newest nation – South Sudan. Severe maternal morbidity and maternal mortality indices highlight the standard of care provided by health institutions. The finding also reveals facility-level and health care system-level opportunities to improve quality of care. Evidence-based practices in the management of women experiencing severe morbidity are lacking.
The present study identified a total of 165 NM events, with a mean of 1.7 severe morbidities per case. This gives an NM prevalence of 9.41% on morbidity-based criteria and 3.1% on organ failure-based criteria. In a recent review in sub-Saharan Africa, the incidence and case-fatality rates for MNMs ranged 1.1%–10.1% and 3.1%–37.4%, respectively.27 Based on Mantel criteria, the WHO systematic review of articles identified MNM-prevalence rates of 0.6% and 14.98% for disease-specific criteria and 0.14%–0.92% for organ dysfunction-based criteria.28
Our results may not be compared to those of developed countries, as we used different selection criteria for NM cases. For instance, criteria for admission to an ICU may be used to select NMs, yet <10% of NM cases in developing countries have access to an ICU.8 We did not use these criteria in our study, as there is no ICU at JTH. The hospital offers only a small room with two beds assigned as an ICU in the maternity unit, and the equipment necessary for an ICU is almost nonexistent.
Our findings are in the highest margin compared to average sub-Saharan Africa and WHO findings. Similar trends in NM incidence have been reported in Nepal, Syria, and Indonesia.25,27–30 In resource-limited areas, many women’s health condition deteriorates before they reach a higher-level health facility as a result of delays in recognizing the danger signs by these women, their family members, or the first-level health care provider who would then fail to arrange early transfer.9
The SMOR and MNM rate were 10.47 per 1,000 live births on morbidity-based criteria and 41.3 per 1,000 live births on organ failure-based criteria. According to the WHO, higher SMOR (eg, over ten per 1,000 live births) indicates that a considerable proportion of mothers need more complex management to survive complications.26 This is true in the case of JTH. The total mortality index for NM cases was 9.56% (NM:fatality ratio of 1:5.1). This was lower than a report from Sudan (19.5%).12
Nine maternal deaths occurred during the study period, showing a relatively high NM:maternal mortality ratio (10.04). The maternal mortality rate was calculated as 1,007 per 100,000 live births. It has been suggested that maternal mortality of 50–250 per 100,000 live births indicates problems with quality of care, and that >250 per 100,000 live births indicates problems with both quality of care and access.31 Therefore, this finding entails auditing and investigating the quality of care in JTH, as well as strengthening the referral system. According to WE Deming, 15% of errors are worker-related, 85% of errors are system-related, and managers are responsible for systems, which bears the truth considering the process indicators obtained in the present study.
In resource-poor settings, it is important to separate NM events that occurred before arrival at the health facility from those that develop after arrival, as the former show a lack of access to health facilities and/or a defect in the referral system.2,32 More than two-thirds (70.3% for postpartum hemorrhage and 73% for sepsis) of women in this study with complications developed them within the first 12 hours of admission. More than two-thirds of women were admitted without referral, which demonstrates a defect in the referral system and/or failure of the primary health care units to detect a pregnant women with severe morbidities, which demonstrates a delay in either seeking or reaching care.
Matching the degree of care to severity of complications is the key principle in the management of complications related to pregnancy, labor, and delivery. As a rule, a woman usually requires tailored care from basic emergencies to intensive care at a well-equipped ICU.9,33 This study revealed a substantial proportion of women developing organ failure and dying being attributable to the nonexistence of an ICU and dire shortage of essential drug facilities. It can be argued that well-equipped facilities, skilled personnel, and an efficient working system can contribute to averting the toll of severe disability and death.
Our findings indicate an underusage of drugs, such as magnesium sulfate and oxytocin, to treat and/or prevent eclampsia and postpartum hemorrhage, respectively, as well as corticosteroids to induce fetal lung maturation in preterm babies and prophylactic antibiotics during cesarean section. Third delay in the management of obstructed labor was noted.26 Three women with ruptured uteri were operated on after 3 hours, and one woman died before she could be operated on.
In evaluation of the factors associated with the occurrence of severe maternal morbidity/NMs, multiple logistic regression analysis showed only four variables had significant effects: having to undergo a cesarean section in the current pregnancy, presence of comorbidity, having no or lower income, and age <18 years. In addition, associations between parity, past obstetric complications, current pregnancy complications, and maternal morbidity were independently associated with the occurrence of an NM. A higher adverse pregnancy outcome of 23% (death or admitted to isolation unit) was recorded in women who suffered NM complications compared to not-NM cases (P=0). Similar findings have been demonstrated elsewhere.27,34,35 It is important to realize that a cesarean section can be the cause of severe morbidity, but can also represent the result of it, as it is often performed because of compromised maternal condition. The WHO recommendation is that a population-based cesarean rate of 5%–15% is optimal. However, at JTH only 4.3% of pregnant women have access to a cesarean section.
In contrast to our findings, the results of the WHO multi-country survey36 showed that older, less educated, and higher-parity mothers with cesarean deliveries were more likely to have SMOs. These findings may be related to the very low rate of female literacy in South Sudan, which may show the true effect of education. Perinatal outcome was dismal in SMO cases, with a perinatal mortality rate 15 times higher and a proportionate increase in preterm labor and neonatal ICU admissions.
This study tried to assess quality in care at the national referral hospital in South Sudan for the first time. As the primary national tertiary health care center, JTH receives many complicated cases. Therefore, the findings can be useful to the rest of South Sudan. There are some limitations to our study that can be ameliorated by continuous surveillance and establishing auditing systems to improve quality of care.
Recommendations posited based on the findings
To policy makers
There is a high frequency of maternal morbidity and mortality at JTH. Therefore, maternal health policy needs to be concerned not only with averting the loss of life but also with preventing MNM events at all care levels, including the primary level.
Policies for the notification of cases of severe maternal morbidity should be in place in all health care units, with a “no shame, no blame” approach.
Contributing factors are shortage of resources, poor-quality primary health care, and the three delays. Further, factors related to maternal mortality are social, as well as medical, and thus multifaceted strategies are needed to address all these areas if maternal mortality is to be reduced. Distrust and a lack of personal empowerment are integral in modeling health care-seeking behavior. Specific barriers related to cost and distance with strategies that reduce social marginalization need to be addressed. Such strategies may include women-empowerment projects including girls’ education and participation in the economy.
To health care institutes
Quality auditing and organizational change need to be implemented that address delays in intervention, especially in emergency cesarean sections, referral barriers, and manpower problems, in the health system. Fully functional ICUs (structure, supplies, and well-trained providers) need to be available in territorial care units.
Magnesium sulfate, improved blood transfusion, and essential obstetric services need to be consistently available throughout the country.
Performance audits of NMs in particular and essential obstetric care in general should be encouraged at county, state, and teaching-hospital levels, in order to improve the quality of obstetric care.
All maternity units ought to collect and submit complete data on severe maternal morbidity to national audits.
Standard comprehensive essential obstetric care in comprehensive emergency obstetric and neonatal care facilities (teaching, state, and county hospitals) and basic emergency obstetric and newborn-care facilities should be in place, accessible, and fully functional. Skilled manpower needs to be provided for these facilities, with regular and progressive assessment.
All maternity units should ensure access to the national/international guidelines on the management of obstetric emergencies.
Fast emergency obstetric responses need to be implemented, such as frequent multidisciplinary training in skills and drill programs (including maternal collapse). These should be prioritized in all maternity units for all professionals at all health care-institute levels.
Strengthen the referral system at all levels by arranging maternity homes for identified complicated maternity cases.
Women need individual birth preparedness and a complication-readiness plan. This should include community birth preparedness and complication-readiness plans, which can be integrated with the Boma Health Initiative.
The referral system needs to be strengthened.
Health institutes in South Sudan need to have a department of quality assurance, which would make periodic quality appraisals and perform continuous clinical audits of staff. Standard-based management and recognition need to be in place in hospitals.
Ensure a continuous supply of consumables and essential drugs. In addition, there should be a workable and efficient system for supply and personnel management.
For researchers
There is a need for research focusing on sociocultural correlates and policy issues of MNMs to validate these findings and improve our knowledge of the extent of the problem. Similar studies should also be extended to state hospitals. Further research is recommended to explore factors leading to the identified higher maternal morbidity rate in South Sudan at large.
Conclusion
The proportion of women requiring comprehensive and basic emergency obstetric care is high. Our estimates of the prevalence of women experiencing severe maternal morbidity (9.41%) and the mortality index (9.2%) are higher than available literature recommendations. The study revealed uterine rupture (sequela of obstructed labor), sepsis, eclampsia, and abortion complications carried a very high risk of death. This suggests poor quality of care. Evidence from this study indicates there exists a gap between practice and WHO-recommended interventions in women experiencing MNMs and mortality.
Notably, laparotomy was performed after 3 hours of hospital stay for three cases of ruptured uterus, and one died before the procedure was performed, suggesting the third delay in managing obstructed labor. The nonexistence of an ICU unit contributed to the higher proportion of women experiencing organ dysfunction or dying due to lack of intensive care. This also entails another third delay. More than two-thirds of women had NM events <12 hours after admission, and the same proportion were admitted bypassing the referral system, attributable to the poor link in the referral system, demonstrating first and second delays (delay in seeking or delay in reaching care). The majority of the causes of maternal mortality and severe morbidities are preventable, even in resource-limited situations.
Ethics approval and consent to participate
The Ethical Review Board, Directorate of Research and Planning at the Ministry of Health, Republic of South Sudan, approved the study (letter dated August 5, 2015). Juba Teaching Hospital administration and the Obstetric Department also signed a consent form and gave a letter of permission. An information sheet was prepared in the local language for each participant. Written consent (signature) was secured from each participant after explaining the procedure and aim of the study. Confidentiality was maintained at each stage.
Supplementary materials
Adult informed consent form.
Abbreviations: JCONAM, Juba College of Nursing and Midwifery; JTH, Juba Teaching Hospital; RMF, Real Medicine Foundation; SDG, Sustainable development goals.
Parent/guardian informed consent form.
Acknowledgments
We acknowledge all participants in the study, who gave us their precious time and honest answers. We are grateful to all data collectors and supervisors. The Ministry of Health, Republic of South Sudan, the Directorate of Research and Planning, Juba Teaching Hospital, and Juba College of Nursing and Midwifery are also greatly acknowledged. This research was funded by the Real Medicine Foundation, South Sudan Country Office.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pattinson R, Say L, Souza JP, Broek NVD, Rooney C. WHO maternal death and near-miss classifications. Bull World Health Organ. 2009;87(10):734. doi: 10.2471/BLT.09.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filippi V, Ronsmans C, Gohou V, et al. Maternity wards or emergency obstetric rooms? Incidence of near-miss events in African hospitals. Acta Obstet Gynecol Scand. 2005;84(1):11–16. doi: 10.1111/j.0001-6349.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 3.Say L, Souza JP, Pattinson RC. Maternal near miss–towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol. 2009;23(3):287–296. doi: 10.1016/j.bpobgyn.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Chhabra P. Maternal near miss: an indicator for maternal health and maternal care. Indian J Community Med. 2014;39(3):132–137. doi: 10.4103/0970-0218.137145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pattinson R, Buchmann E, Mantel G, Schoon M, Rees H. Can enquiries into severe acute maternal morbidity act as a surrogate for maternal death enquiries? BJOG. 2003;110(10):889–893. [PubMed] [Google Scholar]
- 6.WHO . Evaluating the Quality of Care for Severe Pregnancy Complications: The WHO Near-Miss Approach for Maternal Health. Geneva: World health organization; 2011. [Google Scholar]
- 7.Hinton L, Locock L, Knight M, Harris F. Partner experiences of “near-miss” events in pregnancy and childbirth in the UK: a qualitative study. PLoS One. 2014;9(4):e91735. doi: 10.1371/journal.pone.0091735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adisasmita A, Deviany P, Nandiaty F, Stanton C, Ronsmans C. Obstetric near-miss and deaths in public and private hospitals in Indonesia. BMC Pregnancy Childbirth. 2008;8:10. doi: 10.1186/1471-2393-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippi V, Brugha R, Browne E, et al. Obstetric audit in resource-poor settings: lessons from a multi-country project auditing ‘near miss’ obstetrical emergencies. Health Policy Plan. 2004;19(1):57–66. doi: 10.1093/heapol/czh007. [DOI] [PubMed] [Google Scholar]
- 10.Hinton L, Locock L, Knight M. Experiences of the quality of care of women with near-miss maternal morbidities in the UK. BJOG. 2014;121(Suppl 4):20–23. doi: 10.1111/1471-0528.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza JP, Cecatti JG, Faundes A, et al. Maternal near miss and maternal death in the World Health Organization’s 2005 global survey on maternal and perinatal health. Bull World Health Organ. 2010;88(2):113–119. doi: 10.2471/BLT.08.057828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali A, Khojali A, Okud A, Adam G, Adam I. Maternal near-miss in a rural hospital in Sudan. BMC Pregnancy Childbirth. 2011;11(1):48. doi: 10.1186/1471-2393-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Government of South Sudan MoH . Basic Package of Health Service and Nutrion service for South Sudan, January 2009. Juba, South Sudan: 2009. [Google Scholar]
- 14.Government of South Sudan Statstics Agency . Sudan household health survey (Southern Sudan Report) Juba, South Sudan: 2006. [Google Scholar]
- 15.Mary’s Hospital St. Report on a Vist to Juba, Southern Sudan by a Team of Health Professional 3rd-7th March. Newport, Isle of Wight, UK: St Mary’s Hospital; 2008. [Google Scholar]
- 16.National Bureau of Statistics (South Sudan) Census Priority Results(June, 2009) Juba, South Sudan: 2015. [Google Scholar]
- 17.Downie R. A report of the CSIS global health policy center The state of Public Health in south Sudan. 2012. [Google Scholar]
- 18.Bucyabahgia J. Nursing and Midwifery in Southern Sudan undersubscribed in high demand environment. South Sudan Med J. 2010;3:3. [Google Scholar]
- 19.Ministry of Health, (NBS) NBoS . The Republic of South Sudan: The Sudan Household Health Survey 2010. II. Juba, South Sudan; 2010. pp. 1–146. [Google Scholar]
- 20.Minstry of Health Republic of South Sudan . Health Sector Development Program 2012–2016. Juba, South Sudan: 2012. [Google Scholar]
- 21.Ayrton J, Attwood D, Kuron LD. A retrospective analysis of mortality distribution in Juba teaching hospital, Southern Sudan. South Sudan Med J. 2009;2(1) Available from: http://www.southsudanmedicaljour-nal.com/archive/2009-02/untitled-resource.html. [Google Scholar]
- 22.Mantel GD, Buchmann E, Rees H, Pattinson RC. Severe acute maternal morbidity: a pilot study of a definition for a near-miss. Br J Obstet Gynaecol. 1998;105(9):985–990. doi: 10.1111/j.1471-0528.1998.tb10262.x. [DOI] [PubMed] [Google Scholar]
- 23.Say L, Pattinson RC, Gülmezoglu AM. WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss) Reprod Health. 2004;1:3. doi: 10.1186/1742-4755-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souza JP, Gulmezoglu AM, Carroli G, Lumbiganon P, Qureshi Z. The world health organization multicountry survey on maternal and newborn health: study protocol. BMC Health Serv Res. 2011;11:286. doi: 10.1186/1472-6963-11-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weeks A, Lavender T, Nazziwa E, Mirembe F. Personal accounts of ‘near-miss’ maternal mortalities in Kampala, Uganda. BJOG. 2005;112(9):1302–1307. doi: 10.1111/j.1471-0528.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization (WHO) Evaluating the quality of care for severe pregnancy complications: the WHO near-miss approach for maternal health. In: Research DoRHa, editor. Avenue Appia 20, CH-1211 Geneva 27. Switzerland: World Health Organization; 2011. [Google Scholar]
- 27.Kaye DK, Kakaire O, Osinde MO. Systematic review of the magnitude and case fatality ratio for severe maternal morbidity in sub-Saharan Africa between 1995 and 2010. BMC Pregnancy Childbirth. 2011;11(65):1–9. doi: 10.1186/1471-2393-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuncalp O, Hindin M, Souza J, Chou D, Sayb L. The prevalence of maternal near miss: a systematic review. BJOG. 2012;119:653–661. doi: 10.1111/j.1471-0528.2012.03294.x. [DOI] [PubMed] [Google Scholar]
- 29.Shrestha NS, Saha R, Karki C. Near miss maternal morbidity and maternal mortality at Kathmandu medical college teaching hospital. Kathmandu Univ Med J (KUMJ) 2010;8(30):222–226. doi: 10.3126/kumj.v8i2.3563. [DOI] [PubMed] [Google Scholar]
- 30.Almerie Y, Almerie M, Matar H, Shahrour Y, Al Chamat A, Abdulsalam A. Obstetric near-miss and maternal mortality in maternity university hospital, Damascus, Syria: a retrospective study. BMC Pregnancy Childbirth. 2010;10(1):65. doi: 10.1186/1471-2393-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell O, Koblinsky M, Marshall T, et al. Lessons learnt: a decade of measuring the effects of safe motherhood programmes. London: London School of Hygiene and Tropical Medicine; 1997. [Google Scholar]
- 32.Jabir M, Abdul-Salam I, Suheil DM, et al. Maternal near miss and quality of maternal health care in Baghdad, Iraq. BMC Pregnancy Childbirth. 2013;13:11. doi: 10.1186/1471-2393-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippi V, Richard F, Lange I, Ouattara F. Identifying barriers from home to the appropriate hospital through near-miss audits in developing countries. Best Pract Res Clin Obstet Gynaecol. 2009;23(3):389–400. doi: 10.1016/j.bpobgyn.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Bashour H, Abdulsalam A, Jabr A, et al. Maternal mortality in Syria: causes, contributing factors, and preventability. Trop Med Int Health. 2009;14(9):1122–1127. doi: 10.1111/j.1365-3156.2009.02343.x. [DOI] [PubMed] [Google Scholar]
- 35.Jabir M, Abdul-Salam I, Suheil MD, et al. Maternal near miss and quality of maternal health care in Baghdad, Iraq. BMC Pregnancy Childbirth. 2013;13:11. doi: 10.1186/1471-2393-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souza JP, Cecatti JG, Haddad SM, et al. The WHO maternal near-miss approach and the Maternal Severity Index model (MSI): tools for assessing the management of severe maternal morbidity. PLoS One. 2012;7(8):e44129. doi: 10.1371/journal.pone.0044129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adult informed consent form.
Abbreviations: JCONAM, Juba College of Nursing and Midwifery; JTH, Juba Teaching Hospital; RMF, Real Medicine Foundation; SDG, Sustainable development goals.
Parent/guardian informed consent form.