Abstract
The free-living amoebae are thermophilic organisms that may play an increasing role among diseases of a warming world. They are uncommon, accidental, yet high consequence pathogens, with differing pathologic syndromes. New prospects for diagnosis and life-saving treatment make early disease recognition imperative. We review the three most commonly diagnosed species that infect humans: Naegleria fowleri, Acanthamoeba species, and Balamuthia mandrillaris.
Keywords: Amoeba, Ameba, Naegleria, Acanthamoeba, Balamuthia, Meningitis, Encephalitis
Introduction
The free-living amoebae (FLA, spelled “amebae” in the U.S.) are accidental but high consequence pathogens. Amoebic meningoencephalitis can be divided into two categories, primary amoebic encephalitis (PAM) and granulomatous amoebic encephalitis (GAE). PAM is caused by (and is largely synonymous with) Naegleria fowleri. Newer molecular assays have uncovered cases of presumed viral meningitis to have been caused by a newly-identified species, Paravahlkamfia francinae. Similarly, cases previously attributed to Acanthamoeba GAE by morphology have been identified as Balamuthia mandrillaris. Most GAE to date is attributed to Acanthamoeba species and B. mandrillaris, but rare cases have identified Sappinia species. Up to 60% encephalitis cases go undiagnosed [1], thus advances and expanded use of rapid molecular diagnostics remain unmet needs in reducing morbidity and mortality of these central nervous system (CNS) infections. These are critical gaps, given that climate change and an aging public water infrastructure may promote growth and escalate the human risks from thermophilic, water-borne pathogens, including FLA. This overview covers the three commonest pathogens associated with PAM and GAE in humans: Naegleria fowleri, Balamuthia mandrillaris, and Acanthamoeba species.
Naegleria fowleri
History
PAM due to Naegleria fowleri was first diagnosed at the Adelaide Children’s Hospital in Australia in 1965 [2]. Four cases were described, three children and an adult, with autopsy findings suggesting that the organism had reached the CNS along the olfactory nerve filaments [2]. Although Fowler and Carter were first to publish their cases of N. fowleri, the Pathological Museum in London may have housed an earlier example of amoebic meningoencephalitis; the specimen, dated April 1909, was histologically indistinguishable from those described in 1969 [3].
Organism
Naegleria fowleri is the only known human pathogen of the 30 Naegleria species that have been identified. Molecular sequencing suggests that N. fowleri evolved from the non-pathogenic Naegleria lovianienesis [4]. The life cycle of N. fowleri involves 3 stages, including trophozoites, flagellates, and cysts. Invasive human disease is caused by the reproductive and active stage of the organism, the trophozoite. The trophozoite is thermophilic, requiring a temperature of 45 °C to multiply [5]. The trophozoites and flagellated forms of N. fowleri can revert from one to the other depending on environmental conditions; this is a unique feature of N. fowleri. The cyst stage is resilient and can survive dormant for up to 8 months in 4 °C, although it becomes nonviable within 5 min of drying [6,7]. The fulminance of clinical illness distinguishes PAM caused by N. fowleri from GAE. N. fowleri invades the CNS directly and causes rapidly progressive meningoencephalitis, rather than disseminating secondarily from skin, lung, or gastrointestinal tract.
Features that may contribute to virulence include rapid motility of trophozoites, strong chemotaxis toward acetylcholine and other olfactory nerve products, surface proteins homologous to epithelial cell receptors that promote adhesion, resistance to complement-mediated immunity, a variety of enzymatic breakdown products, and intense stimulus of proinflammatory cytokines [[8], [9], [10]]. Most cases of PAM have been reported in individuals without known abnormal immunity. Those who have been tested demonstrated normal levels of mucosal IgA and IgG, including Naegleria-specific antibody, which does not appear protective [9]. Complement-mediated and cell-mediated defenses appear much more relevant in experimental infection of mice, although this may serve to cause damage rather than benefit the host. N. fowleri is able to resist complement by enveloping attached membrane attack complexes into vesicles and discarding them. In the midst of the host’s attack, trophozoites carry on with destruction, phagocytizing cellular breakdown products, as well as directly “biting into” them via feeding cups [[10], [11], [12], [13]].
Epidemiology
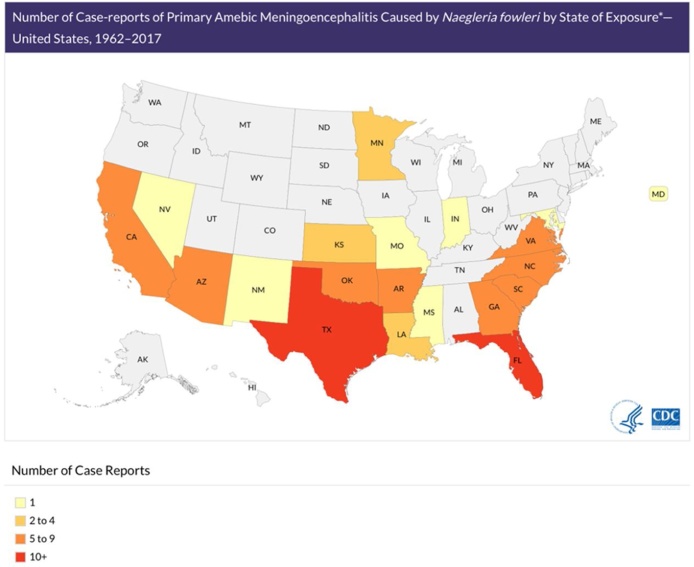
N. fowleri is found worldwide in soil and freshwater, such as rivers, lakes, and thermal springs. N. fowleri is not found in salt water, and infection has not been associated with swallowed water. To date, the only continent in which N. fowleri has not been discovered is Antarctica. In the United States from 1962 to 2017, over half of 143 reported cases originated in Florida or Texas (Fig. 1). Recent cases occurred as far North as Minnesota and Maryland. Infection has predominantly been seen in males, the median age was 12 years, and of these cases, only 4 have survived [8]. N. fowleri has been found in inadequately chlorinated manmade recreational waters, as well as residential plumbing and water heaters. In the last two decades, N. fowleri in diverse water systems has been associated with PAM: a manmade whitewater rafting park in North Carolina, bathwater from a community well water system in Arizona, municipal water in Louisiana used for sinus irrigation and used for a lawn water slide, and tap water in Pakistan used for religious nasal ablution [[14], [15], [16], [17]].
Fig. 1.
Case-reports of Primary Amebic Meningoencephalitis by State of Exposure.
Swabs from the nose, mouth and pharynx of healthy individuals have isolated N. fowleri. Antibody surveys suggest that N. fowleri exposure without apparent sequelae is exceedingly common, with over 98% seropositivity in a survey of youth in Northwestern Mexico and many other areas where N. fowleri is endemic.
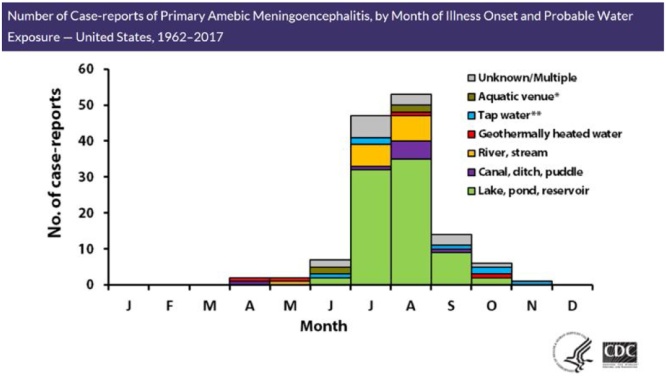
Over the last 4 decades in the U.S., the number of PAM cases reports that have been reported has been fairly stable (Fig. 2) with a trend towards the summer months of the year. In recent years, however, whether from increased awareness or expansion of warm temperatures from the equator, PAM has been reported in the northernmost U.S. states. As with other infectious diseases endemic to the tropics, the incidence of amoebic meningoencephalitis may become more geographically widespread, or rise along with temperature trends.
Fig. 2.
Case-reports of PAM by Month of Illness Onset and Probable Water Exposure.
Unlike other FLA, N. fowleri has not been confirmed to cause infection via organ or tissue transplantation to date [18].
Clinical illness
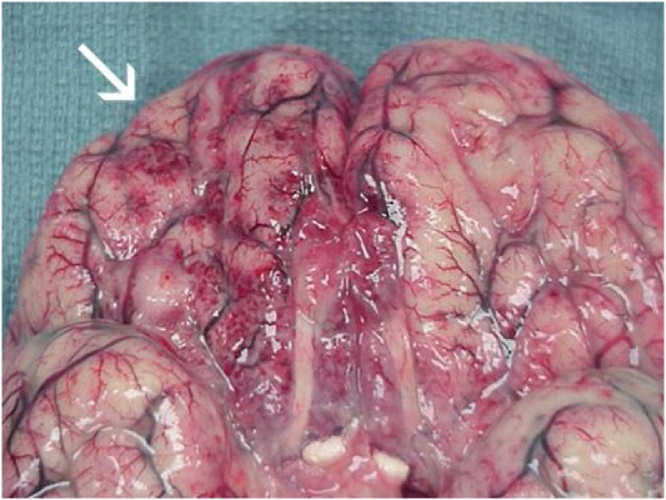
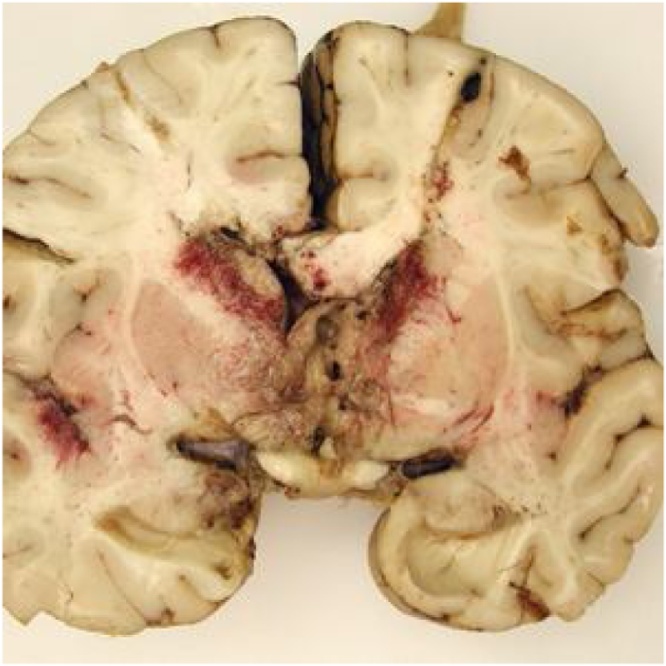
N. fowleri invades by migrating along the olfactory nerve across the cribriform plate [8,19]. On reaching the CNS, it produces a rapidly progressive, hemorrhagic, and necrotizing meningoencephalitis. Onset of symptoms occurs within one to nine days (median of five days) after exposure. Early symptoms include changes to taste or smell, however loss of olfaction may not be readily noticed. Closely mimicking bacterial meningitis, PAM presents with fevers, severe frontal headache, photophobia, meningismus, nausea and vomiting. As disease rapidly progresses and cerebral edema ensues, confusion, visual hallucinations, focal or generalized seizures, and progression to coma are common. Death occurs a median of 5 days from symptom onset. Upon autopsy, edema, hemorrhage and necrosis of the olfactory bulbs are seen (Fig. 3). Trophozoites are found on olfactory nerves, in the purulent exudate of the meninges and brain parenchyma, and within the perivascular spaces of small to medium sized arterial vessels. Trophozoites may be found in other organs as well, including the heart, lungs, and spleen. Myocarditis has been described, as well as focal demyelination of the spinal cord without obvious amoebae; the cause of these findings remains unknown. The predominance of pathology involves the brain as the point of entry. Cause of death is usually related to cerebral edema with brainstem herniation, systemic inflammatory response syndrome, or multiorgan system failure [8,19,20].
Fig. 3.
Primary amoebic meningoencephalitis: Extensive exudate and hemorrhage of the frontal cerebral cortex [21]. CDC, public domain image.
https://www.cdc.gov/parasites/naegleria/naegleria-fowleri-images.html#photos
Unfortunately, less than 30% of cases from 1937 to 2013 were diagnosed premortem in the U.S., possibly due to late consideration of the diagnosis, difficulty detecting the organism in cerebrospinal fluid (CSF), or rapid death. As PAM cannot be distinguished from acute bacterial or pyogenic meningoencephalitis by physical or CSF findings, solicitating a history of freshwater exposure is critical to diagnosis, outcomes, and epidemiologic reporting.
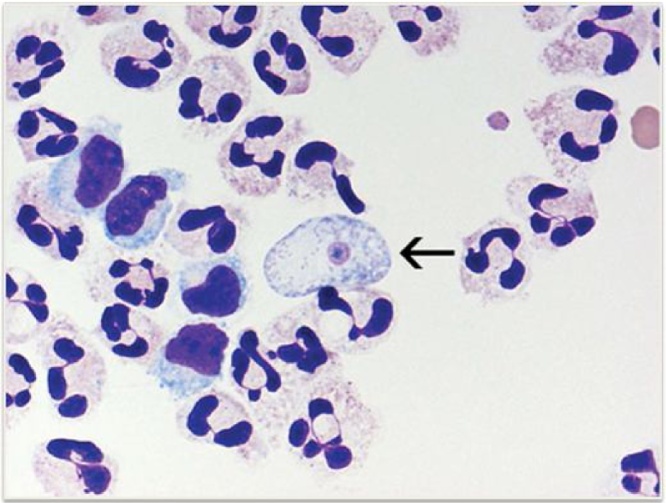
Once a history of freshwater exposure is elicited, CSF must be promptly examined via lumbar puncture. Opening pressure should always be measured and is usually high. The triad of raised CSF white blood cells, negative Gram stain, and history of freshwater exposure should raise suspicion of PAM. Either neutrophils or lymphocytes may predominate. The CSF Gram stain is usually negative because it does not stain the large, round nucleolus that distinguishes N. fowleri from inflammatory cells and macrophages. Wright or Giemsa stain, however, is routinely performed for the CSF WBC differential and has been very useful as a rapid detection method within hours in recent cases of early diagnosis and successful treatment9,22,2323. Wright and Giemsa stains readily distinguish the pale blue cytoplasm and small pink nuclei of Naegleria from surrounding inflammatory cells (Fig. 4). Trophozoites may also be visually observed during manual hematocytometry for the WBC differential, although automated hematocytometry may misidentify amoebae as inflammatory cells; in 1969, Duma et al noted over 100 motile amoebae per μL by hematocytometry in a case report of amoebic meningoencephalitis [9,20,23].
Fig. 4.
Naegleria fowleri trophozoite: CSF cytospin sediment stained with Wright-Giemsa, 1000x magnification [20]. CDC, public domain image.
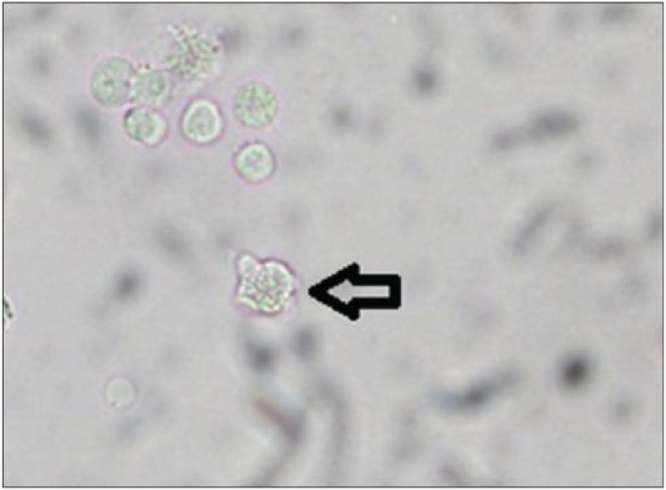
Trophozoites may also be sought by phase contrast microscopy of a “wet mount” of fresh (CSF); slowly moving cells may be difficult to distinguish from inflammatory white blood cells (WBC) (Fig. 5). Even with a high index of suspicion and patience, trophozoites may be difficult to detect. Warming the slide may enhance movement. If distilled water is added, trophozoites may flagellate over time and become motile [9,20], Personal communication, Francine Marciano-Cabral.
Fig. 5.
Naegleria fowleri trophozoite: A wet mount of (arrow) in CSF. Image used with permission of the author, Francine Marciano-Cabral [24].
Other CSF findings do not exclude the diagnosis. The WBC count and differential, glucose, and total protein in CSF may mimic benign viral meningitis in early PAM, only to suggest fulminant bacterial meningitis several hours later.
Most clinical laboratories outside of research centers do not perform specific diagnostic testing for free-living amoebae. Some commercial reference laboratories offer culture of human specimens for “amoebae” or “Acanthamoeba/Naegleria”. These typically several days to complete, and only detect whether stains of the culture are positive for amoebae. Definitive diagnosis of the organism requires molecular confirmation in CSF or tissue and is rapidly diagnostic, typically by polymerase chain reaction (PCR) or immunofluorescence staining. Naegleria fowleri PCR on human specimens is available through reference laboratories such as the U.S. Centers for Disease Control and Prevention (CDC) [9,20].
Radiographic findings in PAM are nonspecific, and unlike GAE, do not typically reveal focal lesions. Computed tomography (CT) or magnetic resonance imaging (MRI) in PAM may reveal nonspecific cerebral and meningeal inflammation and increased intracranial pressure [25].
Expert guidance is critical for prompt diagnostic and treatment assistance, regardless of preliminary findings. Consultation is available 24 h a day, 7 days a week, from the CDC Emergency Operations Center at 770-488−7100 [9,20].
Treatment
While successful treatment is possible, delay correlates with neurologic impairment in those who survive. Based on recent case histories, the CDC recommends a regimen of intravenous and intra-thecal amphotericin, fluconazole, rifampin, azithromycin, and miltefosine. Induced hypothermia may help limit damage and edema. Intracranial pressure must be monitored and may be reduced via ventricular drainage, hyperosmolar therapy, hyperventilation, and intravenous dexamethasone. Three of the five successful cases, all diagnosed very early, received combination antimicrobials that included miltefosine, intracranial pressure-reducing therapies, and therapeutic hypothermia [22,26].
Unfortunately, despite the small numbers reported, fatality rates associated with PAM remain above 95% despite advances in antimicrobial therapies, supportive care measures and increasing educational and preventative practices [18]. The most effective treatment for PAM is prevention. Public awareness of the low-but-present possibility of PAM during warmer months throughout the world (at home or while traveling) may help reduce the impact of this high-consequence infection. Recommendations include proper chlorination of man-made recreational waters as outlined by the CDC Model Aquatic Code [27], as well as monitoring the chlorination of residential and municipal water supplies. During activities in unchlorinated freshwater, measures should be taken to prevent forceful entry of water into the upper nasal cavity: keeping the head above water, holding the nose when jumping into water, and the use of well-fitting nose clips or other means of sealing the nostrils from forced water entry. Water in residential plumbing and water heaters may lose chlorine to chemical consumption and evaporation over time, and PAM has been acquired during bathing and showering. Keeping the head upright during showers and above water during baths may limit water entry. Nasal or sinus rinsing should only be performed with water labelled as distilled or sterile; if tap water is used, it should be boiled for at least 1 min at sea level (3 min above 6500 feet) and allowed to cool. Filtration is only effective with filters labeled for “cyst removal”, having an absolute pore size of 1 μm or smaller. Water may be chemically disinfected using household bleach according to methods outlined by CDC to achieve chlorination of at least 1 ppm; because Naegleria fowleri is chlorine-tolerant, the concentration needed is higher than that recommended for disinfecting water to drink [20,28].
Acanthamoeba species
History
The genus Acanthamoeba was discovered by Sir Aldo Castellani as an incidental finding in a Cryptococcus culture in 1930 [29]. The first human case of GAE attributed to Acanthamoeba was described in the 1960s [30]. Acanthamoeba species most commonly cause keratitis in those with normal immunity. GAE and disseminated systemic disease are much less frequent. Disseminated disease due to Acanthamoebae is characterized by hematogenous spread, usually from respiratory system to the skin and multiple organ systems, with or without GAE.
Unike N. fowleri, Acanthamoeba cause infection in both immunocompetent and immunocompromised hosts. GAE, and especially disseminated disease, are more likely to occur in the latter. Acanthamoeba feed on bacteria, fungi, and algae in the environment, a number of which have adapted resistance to digestion and are able to multiply in trophozoites. The trophozoites rupture and release organisms with upregulated virulence and defense mechanisms. Viable bacteria have been observed in trophozoites or cysts. For this reason, Acanthamoeba has been described as a “Trojan horse”, acting as vector and virulence inducer for a diverse number of pathogens, including bacteria such as Legionella, yeasts, viruses and protozoa. Naegleria may also host pathogenic bacteria experimentally, but is much less likely to do so in natural environments [31].
Organism
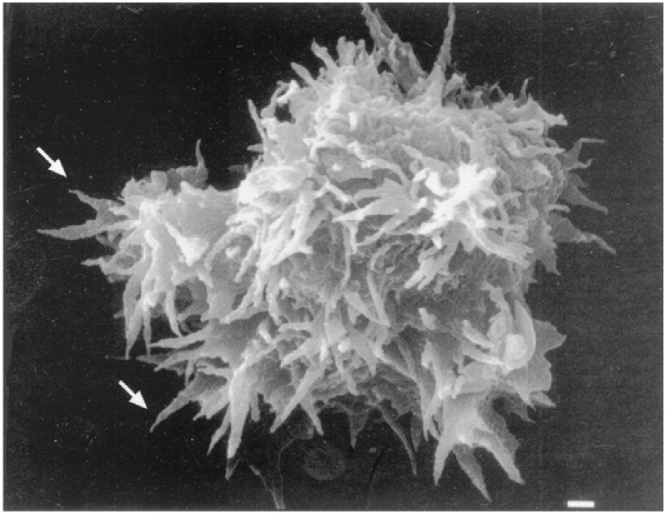
The Acanthamoebae have 2 life stages, the cyst and trophozoite. Acanthamoeba were originally identified by size and structural morphology. For example, the presence of thin, tapered, spike-like pseudopodia (as suggested by the Greek prefix, acanth, Fig. 6) is a distinguishing feature of the trophozoites in tissue or culture. It is difficult to distinguish each species morphologically, however. Furthermore, it is very similar to Balamuthia species, which was discovered more recently and identified as a separate genus of the family Acanthamoebidae by molecular testing. Acanthamoeba species are more reliably grouped into genotypes by 16S ribosomal DNA (rDNA) sequence [24,32]. At the time of writing, seventeen genotypes have been identified, with more likely to follow, and an increasing number have been associated with human disease. The most commonly identified pathogen is the Acanthamoeba castellani complex, corresponding to genotype T4, which can cause encephalitis and keratitis [24,33].
Fig. 6.
Acanthamoeba trophozoite: Scanning electron micrograph, demonstrating spike-like pseudopodia. Image used with permission from American Society for Microbiology.
Epidemiology
More than 150 cases of GAE due to Acanthamoeba species have been described in the literature. The incidence of has been difficult to determine as imaging and cerebral spinal fluid (CSF) findings are not specific.
Acanthamoebae are universally ubiquitous and contact with them is unavoidable. In natural environments, both trophozoites and cysts may be recovered from vegetation (including vegetables), soil and water (salt or freshwater), warm and cold (Antarctica) locations, and at the surface interface between water and air, and even dust and air [33]. They have been isolated from and man-made water systems, including cooling towers for electrical and nuclear power plants. Other sources cited include bottled mineral and distilled water, humidifiers, air-conditioning units, residential plumbing. Many clinical environments can harbor Acanthamoebae, including medical and surgical equipment, therapeutic pools, and dialysis units. It has been detected in recalled contact lens solutions, as well as contact lenses and storage cases of those with keratitis. Many human specimens in the hospital may harbor these organisms, including nose, throat, sinus, urine and sputum in hospitalized individuals [33]. Serologic surveys have observed serum antibodies in cohorts of healthy persons ranging from 50 to 100%, reflecting the vast ability of this organism to exist in our environment [33,34].
Acanthamoeba GAE or disseminated disease may occur at any time of the year. The trophozoite is considered the infective stage for all clinical presentations, however both trophozoites and cysts have been recovered in biopsied tissue.
There have been several reports of GAE in individuals with normal immunity, but Acanthamoeba GAE is most often observed in individuals with a variety of immunocompromising conditions, especially advanced HIV disease, but also malnutrition, diabetes, renal failure, liver cirrhosis, receipt of steroids or chemotherapy, lymphoproliferative disorders, solid or bone marrow transplants, and agammaglobulinemia. In one case series, 61% were immunocompromised with mortality of 44% [24,[33], [34], [35], [36], [37], [38]]. To date, there has been only one reported case of a solid organ transplant patient who has survived GAE caused by Acanthamoeba [35].
Clinical illness
Following entry, the amoeba invades the blood stream and spreads hematogenously to the brain, lungs, sinuses or the skin. GAE is characterized by indolent, progressive central nervous system infection with features of chronic inflammation of the cerebral cortex, localized granulomata, localized perivascular or vasculitic lesions, hemorrhage, and necrosis. The incubation period of GAE is often difficult to determine, whether due to Acanthamoeba or Balamuthia. CNS symptoms develop several weeks to months from the suspected initial exposure. Acanthamoeba GAE is believed to initiate from a breach in the skin, inhalation via the respiratory tract, or possibly via olfactory nerve tissues similar to N. fowleri.
Clinical findings include fevers, headache, nausea, and vomiting. Neurological symptoms of GAE caused by Acanthamoeba vary depending on the areas of the brain that are involved. GAE may mimic the presentation of space-occupying lesions, cerebrovascular ischemia, and bacterial or viral meningitis. Clinical symptoms include fever, headache, nausea, vomiting, confusion or hallucinations. Meningeal symptoms of nuchal rigidity, Brudzinsky and Kernig signs may occur. More focal signs may include seizures, hemiparesis, cranial nerve palsies. Skin lesions associated with Acanthamoeba GAE are typically firm, nodular, and scattered anatomically, and may ulcerate or drain purulent material. Disseminated skin lesions may precede CNS disease in individuals with advanced immunodeficiency, permitting diagnosis by biopsy of more accessible tissues [24]. Moderate to severe cerebral edema may occur, and increased intracranial pressure often leads to herniation and death.
Imaging findings in GAE are protean. Multiple non-specific ring-enhancing lesions are common and may involve the cerebrum, cerebellum, brain stem, and spinal cord. One case was suggestive of neurocysticercosis, others of toxoplasmosis. Other imaging findings on CT or MRI may suggest brain abscess or tumor [24,39].
CSF analysis may demonstrate a pleocytosis with lymphocytic predominance, elevated protein, and low glucose. Acanthamoeba trophozoites may rarely be found in a wet mount of spinal fluid; they may be mistaken for inflammatory WBC. Wright stain of CSF sediment after centrifuge distinguishes the organism from inflammatory cells, with its small pale blue nucleus occupying approximately one-sixth of the cytoplasm. Trophozoites may also be observed in trichrome stained bronchoalveolar lavage specimens [24].
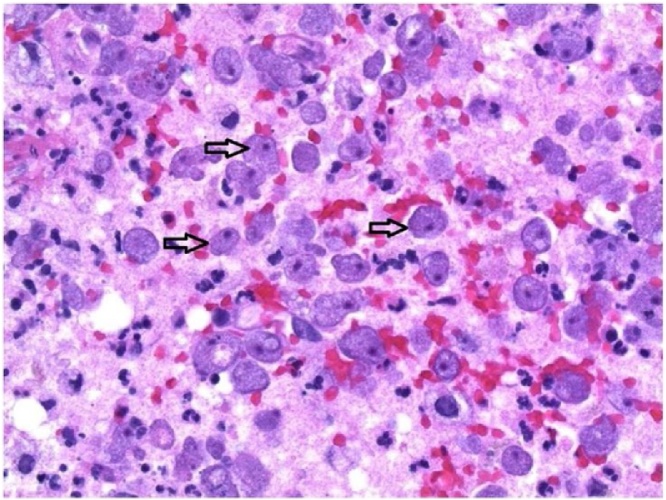
Tissue findings include acute and chronic inflammatory infiltrates, vasculitis and vascular invasion, tissue hemorrhage, and in immunologically intact individuals, characteristic granulomata and multinucleated giant cells. It is important to note the absence of the latter in very immunocompromised hosts such as those with advanced HIV disease or bone marrow suppression. Tissue biopsies stained with hematoxylin and eosin (H&E) or Wright stain may distinguish trophozoites and cysts by characteristic nuclear morphology (a “halo” around the chromatin) from surrounding cells (Fig. 7, Fig. 8). Cysts may be distinguished by staining with Periodic acid-Schiff (red cyst wall), Gomori-methenamine silver (black cyst), and calcofluor white (fluorescent cysts). As with N. fowleri, commercial reference laboratories report only whether cultures of human specimens are positive for amoebae [24].
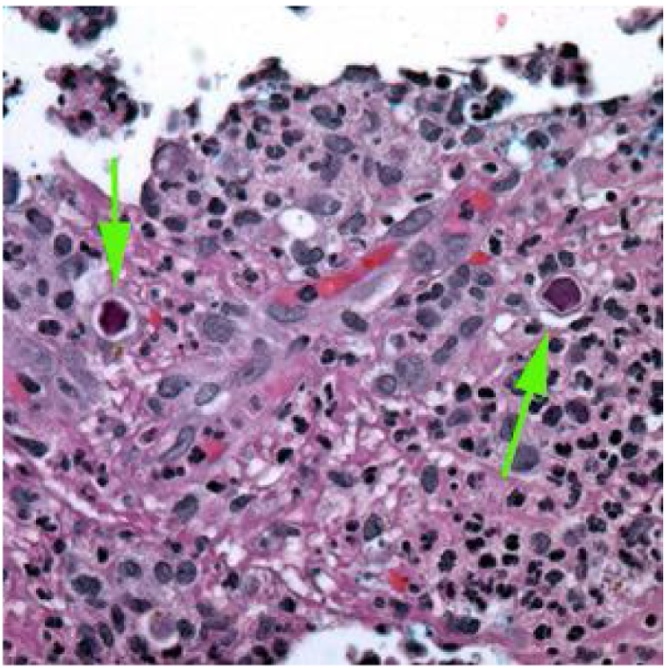
Fig. 7.
Granulomatous meningoencephalitis due to Acanthamoeba: Cysts observed in brain tissue (arrows) stained with hematoxylin and eosin (public domain image, CDC).
Fig. 8.
Granulomatous meningoencephalitis: Autopsy specimen demonstrating extensive necrotizing, mixed inflammatory, vasculitic, and granulomatous encephalitis. CDC, public domain image. Credited to Cook Children’s Hospital, Fort Worth, Texas.
Acanthamoeba immunohistochemical stain and PCR on human specimens is available through the reference laboratory of the U.S. Centers for Disease Control and Prevention (CDC), as well as some research laboratories [40].
Acanthamoeba serum titers are not diagnostically useful, as exposure is nearly universal, most healthy individuals may have titers ranging from 1:20 to 1:60, and severely immunocompromised patients may not manifest high titers. Antibody in mice has only been protective against specific species and after repeated exposure [11,24,34,38,41].
Treatment
Mortality associated with GAE caused by Acanthamoeba has been reported to be greater than 44% on a recent case series to over 85%, with most cases in the past diagnosed postmortem. Currently there is limited experiential data to guide treatment. Of successful cases, antimicrobials used alone or combination have included azoles (ketoconazole, fluconazole, voriconazole, itraconazole), amphotericin B, flucytosine, caspofungin, miltefosine, sulfadiazine, pentamidine isethionate, azithromycin, penicillin, chloramphenicol or rifampicin [[35], [36], [37], [38], [39],42].
Prevention of GAE caused by Acanthamoeba is likely difficult. Periodic inspections of water treatment systems, air filter systems and high contact surfaces/equipment where Acanthamoeba are known to colonize may be reasonable, however, especially as this organism may harbor and trigger the virulence of commoner pathogens such as Legionella. The immunocompromised are at much higher risk of Acanthamoeba GAE, disseminated disease, and mortality than individuals with normal immunity. Practical hygiene measures may include avoidance of activities that may lead to inhalation of dusts, soils, and aerosols, or wearing of dust mask and goggles if unavoidable. Prompt showering after swimming, and washing of skin lacerations with soap and water may prevent a variety of other infections as well. Since Acanthamoeba may colonize contact lens solutions, proper contact lens hygiene and avoidance of overnight wear, may theoretically prevent keratitis and the potential for GAE and disseminated disease by this route [43].
Balamuthia mandrillaris
History
Balamuthia mandrillaris was first identified at the U.S. Centers for Disease Control in 1990, having been isolated in 1986 from the brain of a pregnant mandrill that died of meningoencephalitis at the San Diego Zoo [44]. Initial genome sequencing of this isolate was reported in 2015 [45]. The first human case reports date back to 1975, after initially suspected Acanthamoeba was determined to be B. mandrillaris by later indirect immunoflourescence testing [46]. Approximately 60 of the greater than 125 cases of GAE caused by B. mandrillaris have been diagnosed in the US, mostly on autopsy. B. mandrillaris is increasingly recognized as a cause of cutaneous infections and meningoencephalitis in immunocompetent and immune-impaired individuals. It may cause disseminated disease in immunosuppressed individuals.
Organism
Balamuthia mandrillaris is the only known species of this genus, infecting humans and animals alike. Like Naegleria and Acanthamoeba, it is ubiquitous worldwide in soil and fresh water. Its life cycle consists of two stages, trophozoite and a very hardy cyst, and other than occasionally possessing double nuclei, it is morphologically indistinguishable from Acanthamoeba (and host macrophages) in clinical specimens [46].
Epidemiology
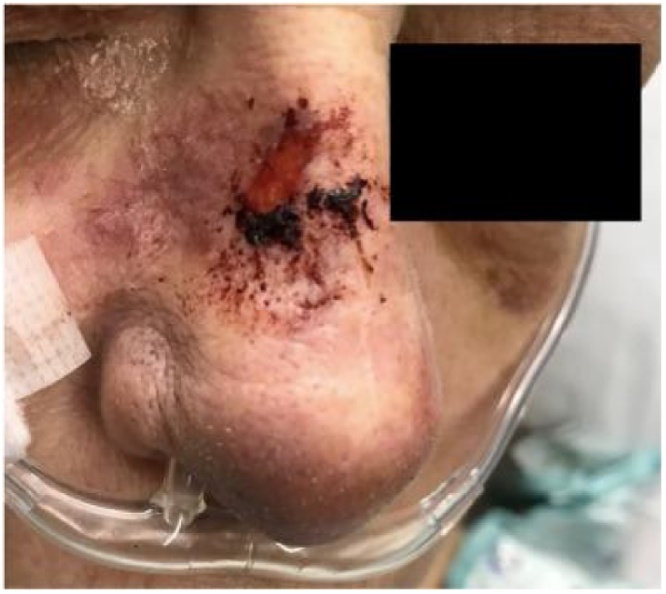
In the U.S., most Balamuthia GAE has been diagnosed in California, followed by Texas and Arizona. To date in the U.S., only 4 patients have survived the disease with a myriad of neurological sequelae [46]. About half of U.S. cases have been diagnosed in persons of Hispanic ethnicity. It is not known whether this may be attributable to geographic exposure, greater likelihood of agricultural employment in an area, a genetic susceptibility, or happenstance [46]. Other amoebic infections have not been linked with specific populations or immune defects. Indeed, with increased awareness, molecular methods, and environmental sampling, this emerging pathogen has been reported in over 200 cases throughout the Americas, especially Peru, Europe, the Middle East, and Asia, including Northern Japan [32,47]. While most infections are diagnosed in warm climates, B. mandrillaris has been isolated in cooler climates as well. Disease has been diagnosed year-round, however, because symptoms manifest over weeks or months, the timing of diagnosis and reporting may obscure a seasonal pattern. While data is limited, most human infections are believed to have been acquired primarily via the respiratory tract (dust, soil), and/or skin breaks (dust, soil, water). Infection via olfactory nerve, as well as the oral and gastrointestinal tract, has been demonstrated experimentally in both immunocompetent and immunodeficient mice. Lack of histopathologic olfactory bulb involvement and the indolent progression of cases over many weeks speaks against invasion of the CNS via the olfactory nerve as a predominant pathway in humans [46,48,49]. One fatal human case in 2018 was been associated with sinus irrigations with tap water filtered only with a household drinking water filter. The encephalitis was preceded for over a year by a persistent, non-specific granulomatous nasal lesion that developed one month into sinus irrigations; biopsies did not demonstrate organisms. Postmortem cerebral pathology was not described [50] (Fig. 9, Fig. 10).
Fig. 9.
Sentinel ulcer of Balamuthia infection: Nasal sore that preceded this patient’s GAE by several months [50]. Image used with permission of Elsevier Ltd.
Fig. 10.
Balamuthia mandrillaris: Hematoxylin and eosin stain of brain tissue demonstrating viable trophozoites (arrows) [50]. Image used with permission of Elsevier Ltd.
Given the frequent association with central facial skin lesions and potential for underreporting, a history of nasal exposure should be elicited during evaluations for GAE.
B. mandrillaris has been diagnosed in humans with both normal and impaired immunity. Transmission of B. mandrillaris by organ transplantation has been documented in five patients from three donors since 2009; four died. In 2014, the Balamuthia-related death of a liver transplant recipient alerted CDC to possible infection from a donor who had died of brain trauma. Four asymptomatic recipients were treated prophylactically with a multidrug regimen that included miltefosine or pentamidine, albendazole, azithromycin, sulfadiazine, and fluconazole; all had survived at 24 months [[51], [52], [53]].
Clinical illness
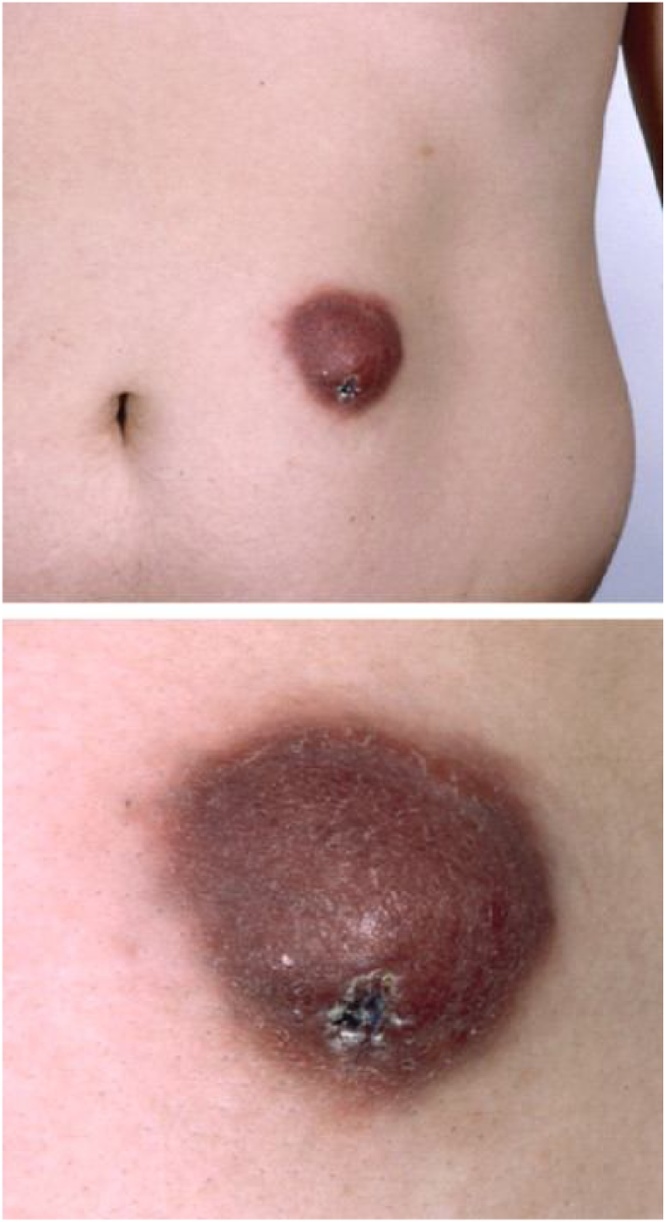
As with Acanthamoeba, GAE due to B. mandrillaris is insidious. Affected individuals often first seek care at the onset of CNS symptoms, at which time infection has been progressing for weeks to months. Cutaneous infection may be diagnosed early by the astute clinician or dermatologist. Unique to Balamuthia GAE, half of cases manifest a nonspecific, indurated plaque or ulceration on the central face or limbs [32,46,[54], [55], [56]] (Fig. 11). Histopathology demonstrates nonspecific granulomata with numerous giant cells, evoking sarcoidosis or lupus, with no identified organism. In very immunosuppressed individuals, granulomata may be absent. When associated with a splinter or thorn prick, findings may be attributed to foreign body reaction. Untreated cutaneous disease spreads, progressing over weeks to months before onset of CNS symptoms. CNS symptoms are nonspecific, but gradually worsening headache, fever, nausea, and fatigue appear in over 30% in one case series. The commonest focal neurologic symptoms in this series were hemiparesis (30.4%), seizures (43.4%), and intracranial hypertension (30.4%) [32]. Encephalitis and mass effect progress to seizures, obtundation, and coma. CNS progression to death may take days or weeks [32,46].
Fig. 11.
Skin lesion of Balamuthia infection: Indurated violaceous or indurated plaque and ulceration seen with Balamuthia mandrillaris infection [56]. Image used with permission of Elsevier Ltd.
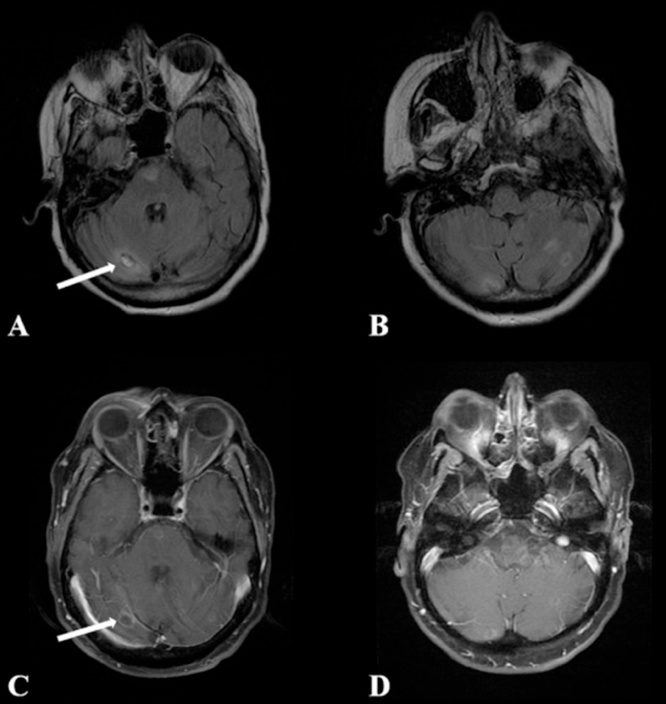
Computed tomography upon onset of neurologic symptoms may reveal low-density lesions in brain parenchyma; magnetic resonance imaging (MRI) may show peripheral ring-enhancing lesions similar to neurocysticercosis and neurotuberculosis. No imaging pattern appears to be specific for GAE due to Acanthamoeba or B. mandrillaris, although progression or increased number of lesions has been observed with corticosteroids and other immunosuppressants [32,46,50,57,58] (Fig. 12).
Fig. 12.
Balamuthia mandrillaris imaging: Ring-enhancing lesions on MRI of the brain [50]. Images used with permission of Elsevier Ltd.
Biopsy of brain tissue may yield nonspecific findings, even suggesting viral or bacterial infection, glioma, or other noninfectious processes. Intense inflammation and hemorrhagic necrosis may be seen surrounding vessels and multinucleated giant cells, suggesting a vasculitis, with negative stains for fungi or mycobacteria [32].
CSF studies are typically un-illuminating. Like Acanthamoeba, B. mandrillaris is not likely to be seen on CSF wet mount. In B. mandrillaris does not feed on bacteria, but on mammalian cells, thus culture on agar seeded with Escherichia coli will not detect it. Growth in axenic culture (sterile, cell-free) media is possible but slow, and thus a suboptimal diagnostic tool. B. mandrillaris antibody titers may be elevated but do not correlate with clinical disease. The California Encephalitis Project, out of 10 cases of GAE caused by B. mandrillaris, Balamuthia DNA was detected by PCR in only 2 CSF specimens [32,46].
Suspicion of GAE should prompt consultation with CDC via the Emergency Operations Center. Definitive diagnosis is made by B. mandrillaris PCR, available at the CDC reference laboratory [46].
Treatment
Treatment is based on scant but evolving clinical experience and literature, and at this time, is best determined in consultation with CDC expertise. At the time of writing, CDC recommendations include a combination of intravenous pentamidine, sulfadiazine, fluconazole, azithromycin or clarithromycin, and miltefosine. Treatment duration has not been established and may depend on underlying immunity, and clinical progress as suggested by laboratory testing and imaging. Case-by-case treatment has ranged from weeks to years. A case of chronic cutaneous and CNS infection in Peru resolved after 7 months of treatment that included miltefosine. After several months of failed regimens, rapid regression of skin lesions & resolution of headache and fever were observed within 4 weeks of initiation. Successful recovery has been reported with neurosurgical resection and extended antimicrobials, while corticosteroids have been observed to hasten progression. Similar to PAM, the prognosis of GAE caused by B. mandrillaris is marginal with a greater than 95% mortality. Case reports of successful treatments add invaluable data to the limited knowledge base and hope of preventing disease and reducing mortality [26,[59], [60], [61]]. It is not known how Balamuthia-associated GAE may be prevented at this time.
Summary
While the incidence of documented infection due to free-living amoebae is low to date, they frequently affect healthy, young, and active individuals. A warming climate may increase exposures to these ubiquitous organisms. Warmer temperatures may increase colonization of plumbing, water distribution, and recreational water systems; temperature changes may further alter effectiveness of chlorination. Man-made beaches, rapids, wave pools, and wakeboarding cable parks offer new challenges for effective prevention of waterborne diseases. Increasingly adventurous travel may increase exposures to low-chlorination pools and hot springs. As with Paravahlkamfia species, evolving molecular diagnostics may disclose more cases and undiscovered pathogens.
Diagnosis and reporting are vital to understanding the epidemiology and public health impact of amoebic meningoencephalitis, and to inform research and policy. The United States Centers for Disease Control and Prevention (CDC) began informally tracking Naegleria fowleri cases voluntarily reported by state health departments in the late 1980s. The Council of State and Territorial Epidemiologists (CSTE) established the first standard case definition for Naegleria fowleri infections in 2011.
Personal preventive recommendations to date have been based primarily on expert guidance. Recreational and municipal water system design must account for the changing climate and increasing incidence of outbreaks of chlorine-tolerant pathogens associated with pools. Tools such as CDC’s Model Aquatic Health Code, developed in concert with public health authorities and the aquatics sector, offer voluntary, evidence-based guidance for public pools and spas. Research is needed on the most effective disinfection methods for novel man-made recreation systems like wave or water rapid parks.
New treatments regimens have yielded unprecedented success in cases of early diagnosis, and clinicians should be aware of these high consequence pathogens and risk factors. As with many other neglected diseases, clinical research and drug discovery are crucial to improving outcomes of these often fatal or disabling infections.
The role of the astute clinician in contributing to the knowledge base on FLA infections cannot be underestimated. Clinical case reports, public health reporting and sharing of experiential data are critical to inform effective both management and public health interventions.
References
- 1.Glaser C.A., Gilliam S., Schnurr D., Forghani B., Honarmand S., Khetsuriani N. In Search of Encephalitis Etiologies: Diagnostic Challenges in the California Encephalitis Project , 1998-2000. Clin Infect Dis. 2003 doi: 10.1086/367841. [DOI] [PubMed] [Google Scholar]
- 2.Blackmore C., Onifade T., Beach M.J. 2012. Case definitions for non-notifiable infections caused by free-living Amebae (Naegleria fowleri, Balamuthia mandrillaris, and Acanthamoeba spp.)https://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/11-ID-15.pdf [Google Scholar]
- 3.Apley J., Clarke S.K.R., Roome A.P.C.H., Sandry S.A., Saygi G., Silk B. Primary Amoebic Meningoencephalitis in Britain. Br Med J. 1970 doi: 10.1136/bmj.1.5696.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jonckheere J.F. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect Genet Evol. 2011 doi: 10.1016/j.meegid.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Schuster F.L. Cultivation of pathogenic and opportunistic free-living amebas. Clin Microbiol Rev. 2002 doi: 10.1128/CMR.15.3.342-354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellings F.M., Amuso P.T., Chang S.L., Lewis A.L. Isolation and identification of pathogenic Naegleria from Florida lakes. Appl Environ Microbiol. 1977 doi: 10.1128/aem.34.6.661-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S.L. Resistance of pathogenic Naegleria to some common physical and chemical agents. Appl Environ Microbiol. 1978 doi: 10.1128/aem.35.2.368-375.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baig A.M. Primary amoebic meningoencephalitis: neurochemotaxis and neurotropic preferences of Naegleria fowleri. ACS Chem Neurosci. 2016 doi: 10.1021/acschemneuro.6b00197. [DOI] [PubMed] [Google Scholar]
- 9.Capewell L.G., Harris A.M., Yoder J.S., Cope J.R., Eddy B., Roy S. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937-2013. J Pediatric Infect Dis Soc. 2015 doi: 10.1093/jpids/piu103. [DOI] [PubMed] [Google Scholar]
- 10.Marciano-Cabral F., Cabral G.A. The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol Med Microbiol. 2007 doi: 10.1111/j.1574-695X.2007.00332.x. [DOI] [PubMed] [Google Scholar]
- 11.Lares-Jiménez L.F., Borquez-Román M.A., Alfaro-Sifuentes R., Meza-Montenegro M.M., Casillas-Hernández R., Lares-Villa F. Detection of serum antibodies in children and adolescents against Balamuthia mandrillaris, Naegleria fowleri and Acanthamoeba T4. Exp Parasitol. 2018 doi: 10.1016/j.exppara.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Rivera F., Rosas I., Castillo M., Chávez M., Gómez R., Chío R. Pathogenic and free-living protozoa cultured from the nasopharyngeal and oral regions of dental patients: II. Environ Res. 1986 doi: 10.1016/s0013-9351(86)80062-7. [DOI] [PubMed] [Google Scholar]
- 13.Abraham S.N., Lawande R.V. Incidence of free-living amoebae in the nasal passages of local population in Zaria, Nigeria. J Trop Med Hyg. 1982 [PubMed] [Google Scholar]
- 14.Lamotte S. CNN Wire; 2016. Brain eating amoeba found at unusually high levels at North Carolina water park. Published July 3. [Google Scholar]
- 15.Yoder J.S., Straif-Bourgeois S., Roy S.L. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis. 2012 doi: 10.1093/cid/cis626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cope J.R., Ratard R.C., Hill V.R., Moore T., Visvesvara G., Ratard R. The first association of a primary amebic meningoencephalitis death with culturable naegleria fowleri in tap water from a US treated public drinking water system. Clin Infect Dis. 2015 doi: 10.1093/cid/civ017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakoor S., Beg M.A., Mahmood S.F., Bandea R., Sriram R., Noman F. Primary amebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg Infect Dis. 2011 doi: 10.3201/eid1702.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy S.L., Metzger R., Chen J.G., Laham F.R., Martin M., Kipper S.W. Risk for transmission of naegleria fowleri from solid organ transplantation. Am J Transplant. 2014 doi: 10.1111/ajt.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.John D.T. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol. 1982;36(1):101–123. doi: 10.1146/annurev.mi.36.100182.000533. [DOI] [PubMed] [Google Scholar]
- 20.Cope J.R., Ali I.K. Primary amebic meningoencephalitis: what have we learned in the last 5 years? Curr Infect Dis Rep. 2016 doi: 10.1007/s11908-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control . 2013. Videos and photos of Naegleria fowleri.https://www.cdc.gov/parasites/naegleria/naegleria-fowleri-images.html#photos [Google Scholar]
- 22.Linam W.M., Ahmed M., Cope J.R., Chu C., Visvesvara G.S., da Silva A.J. Successful Treatment of an Adolescent With Naegleria fowleri Primary Amebic Meningoencephalitis. Pediatrics. 2015 doi: 10.1542/peds.2014-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duma R.J., Ferrell H.W., Nelson E.C., Jones M.M. Primary amebic meningoencephalitis. N Engl J Med. 1969;281(24):1315–1323. doi: 10.1056/NEJM196912112812401. [DOI] [PubMed] [Google Scholar]
- 24.Marciano-Cabral F., Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16(2):273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh P., Kochhar R., Vashishta R.K., Khandelwal N., Prabhakar S., Mohindra S. Amebic meningoencephalitis: Spectrum of imaging findings. Am J Neuroradiol. 2006 doi:27/6/1217 [pii] [PMC free article] [PubMed] [Google Scholar]
- 26.Cope J.R., Conrad D.A., Cohen N., Cotilla M., DaSilva A., Jackson J. Use of the Novel Therapeutic Agent Miltefosine for the Treatment of Primary Amebic Meningoencephalitis: Report of 1 Fatal and 1 Surviving Case. Clin Infect Dis. 2015 doi: 10.1093/cid/civ1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control . 2018. Model aquatic health code.https://www.cdc.gov/mahc/editions/current.html [Google Scholar]
- 28.Centers for Disease Control . 2017. Sinus rinsing for health or religious practice.https://www.cdc.gov/parasites/naegleria/sinus-rinsing.htm Published 2017 [Accessed 22 September 2018] [Google Scholar]
- 29.Castellani A. An amoeba found in culture of yeast: preliminary note. J Trop Med Hyg. 1930;33:160. [Google Scholar]
- 30.Jager B.V., Stamm W.P. Brain abscesses caused by free-living amœba probably of the genus Hartmannella in a patient with Hodgkin’s disease. Lancet. 1972;300(7791):1343–1345. doi: 10.1016/s0140-6736(72)92781-x. [DOI] [PubMed] [Google Scholar]
- 31.Guimaraes A.J., Gomes K.X., Cortines J.R., Peralta J.M., Peralta R.H.S. Acanthamoeba spp. as a universal host for pathogenic microorganisms: one bridge from environment to host virulence. Microbiol Res. 2016 doi: 10.1016/j.micres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Khan N., Siddiqui R. Balamuthia mandrillaris: morphology, biology, and virulence. Trop Parasitol. 2015 doi: 10.4103/2229-5070.149888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui R., Khan N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors. 2012 doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brindley N., Matin A., Khan N.A. Acanthamoeba castellanii: high antibody prevalence in racially and ethnically diverse populations. Exp Parasitol. 2009 doi: 10.1016/j.exppara.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Fung K.T.T., Dhillon A.P., McLaughlin J.E., Lucas S., Davidson B., Rolles K. Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transplant. 2008 doi: 10.1002/lt.21409. [DOI] [PubMed] [Google Scholar]
- 36.Fung K.T.T., Dhillon A.P., McLaughlin J.E. Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transplant. 2008 doi: 10.1002/lt.21409. [DOI] [PubMed] [Google Scholar]
- 37.Sison J.P., Kemper C.A., Loveless M., McShane D., Visvesvara G.S., Deresinski S.C. Disseminated acanthamoeba infection in patients with AIDS: case reports and review. Clin Infect Dis. 1995 doi: 10.1093/clinids/20.5.1207. [DOI] [PubMed] [Google Scholar]
- 38.Khurana S., Mewara A., Verma S., Totadri S.K. Central nervous system infection with Acanthamoeba in a malnourished child. BMJ Case Rep. 2012 doi: 10.1136/bcr-2012-007449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena A., Mittal S., Burman P., Garg P. Acanthameba meningitis with successful outcome. Indian J Pediatr. 2009 doi: 10.1007/s12098-009-0205-z. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control . 2019. Acanthamoeba - diagnosis & detection. [Google Scholar]
- 41.Cursons R.T.M., Brown T.J., Keys E.A., Moriarty K.M., Till D. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun. 1980;29(2):401–407. doi: 10.1128/iai.29.2.401-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singhal T., Bajpai A., Kalra V., Kabra S.K., Samantaray J.C., Satphaty G. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr Infect Dis J. 2001 doi: 10.1097/00006454-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control . 2015. Microbial keratitis. [Google Scholar]
- 44.Visvesvara G.S., Martinez A.J., Schuster F.L., Leitch G., Wallace S., Sawyer T. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990 doi: 10.1128/jcm.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Detering H., Aebischer T., Dabrowski P.W., Radonic A., Nitsche A., Renard B. First Draft Genome Sequence of Balamuthia mandrillaris, the Causative Agent of Amoebic Encephalitis. Genome Announc. 2015 doi: 10.1128/genomeA.01013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cope J.R., Landa J., Nethercut H., Collier S.A., Glaser C., Moser M. The Epidemiology and Clinical Features of Balamuthia mandrillaris Disease in the United States, 1974-2016. Clin Infect Dis. 2018:1–30. doi: 10.1093/cid/ciy813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamanouchi K., Arima H., Sakamoto Y., Kanto K., Kasai K., Ito K. First report of the isolation of Balamuthia mandrillaris in the northern region of Japan. Parasitol Res. 2018;117(9):2895–2900. doi: 10.1007/s00436-018-5980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiderlen A.F., Laube U., Radam E., Tata P.S. Oral infection of immunocompetent and immunodeficient mice with Balamuthia mandrillaris amebae. Parasitol Res. 2007 doi: 10.1007/s00436-006-0334-5. [DOI] [PubMed] [Google Scholar]
- 49.Kiderlen A.F., Laube U. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol Res. 2004 doi: 10.1007/s00436-004-1163-z. [DOI] [PubMed] [Google Scholar]
- 50.Piper K.J., Foster H., Susanto D., Maree C.L., Thornton S.D., Cobbs C.S. Fatal Balamuthia mandrillaris brain infection associated with improper nasal lavage. Int J Infect Dis. 2018;77:18–22. doi: 10.1016/j.ijid.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention (CDC) Notes from the field: transplant-transmitted Balamuthia mandrillaris—Arizona, 2010. MMWR Morb Mortal Wkly Rep. 2010 [PubMed] [Google Scholar]
- 52.Balamuthia mandrillaris transmitted through organ transplantation – Mississippi, 2009. Am J Transplant. 2011 [PubMed] [Google Scholar]
- 53.Gupte A.A., Hocevar S.N., Lea A.S. Transmission of Balamuthia mandrillaris through solid organ transplantation: utility of organ recipient serology to guide clinical management. Am J Transplant. 2014 doi: 10.1111/ajt.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martínez D.Y., Seas C., Bravo F., Legua P., Ramos C., Cabello A.M. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis. 2010 doi: 10.1086/653609. [DOI] [PubMed] [Google Scholar]
- 55.Deetz T.R., Sawyer M.H., Billman G., Schuster F.L. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis. 2003 doi: 10.1086/379020. [DOI] [PubMed] [Google Scholar]
- 56.Siddiqui R., Khan N.A. Balamuthia amoebic encephalitis: an emerging disease with fatal consequences. Microb Pathog. 2008 doi: 10.1016/j.micpath.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Doyle J., Campbell E., Fuller A., Spelman D., Cameron R., Malham G. Balamuthia mandrillaris brain abscess successfully treated with complete surgical excision and prolonged combination antimicrobial therapy. J Neurosurg. 2011 doi: 10.3171/2010.10.JNS10677. [DOI] [PubMed] [Google Scholar]
- 58.Healy J.F. Balamuthia amebic encephalitis: radiographic and pathologic findings. Am J Neuroradiol. 2002 [PMC free article] [PubMed] [Google Scholar]
- 59.Schuster F.L., Yagi S., Gavali S., Glaser C., Michelson D., Blomquist I. Under the Radar: Balamuthia amebic encephalitis. Clin Infect Dis. 2009 doi: 10.1086/597260. [DOI] [PubMed] [Google Scholar]
- 60.Jung S., Schelper R.L., Visvesvara G.S., Chang H.T. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med. 2004 doi: 10.5858/2004-128-466-BMMIAI. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control . 2017. Balamuthia mandrillaris - treatment.https://www.cdc.gov/parasites/balamuthia/treatment-hcp.html [Google Scholar]