Abstract
Anxiety disorders, depression and pain are highly prevalent pathologies. Their pharmacotherapy is associated with unwanted side effects; hence there is a clinical need to develop more effective drugs with fewer adverse reactions.
Chalcones are one of the major classes of naturally occurring compounds. Chalcones and their derivatives have a huge importance in medicinal chemistry, displaying a wide range of pharmacological activities including anti-inflammatory, antimicrobial, antioxidant, cytotoxic and antitumor actions.
The aim of this work was to evaluate chalcone effects on different targets involved in these pathologies. We have synthesized a series of simple chalcone derivatives taking common structural requirements described in literature related to their anxiolytic-like, antidepressant-like and/or antinociceptive properties into account.
Furthermore, their potential in vitro effects towards different targets involved in these pathologies were evaluated. We have obtained twenty chalcones with moderate to high yields and assessed their ability to bind distinctive receptors, from rat brain homogenates, by displacement of labelled specific ligands: [3H] FNZ (binding site of benzodiazepines/GABAA), [3H] 8-OH-DPAT (serotonin 5-HT1A) and [3H] DAMGO (μ-opioid). Those compounds that showed the better in vitro activities were evaluated in mice using different behavioural tasks. In vivo results showed that 5′-methyl-2′-hydroxychalcone (9) exerted anxiolytic-like effects in mice in the plus maze test. While chalcone nuclei (1) revealed antidepressant-like activities in the tail suspension test. In addition, the novel 5′-methyl-2′-hydroxy-3′-nitrochalcone (12) exhibited antinociceptive activity in acute chemical and thermal nociception tests (writhing and hot plate tests). In conclusion, chalcones are thus promising compounds for the development of novel drugs with central nervous system (CNS) actions.
Keywords: Biochemistry, Neuroscience, Pharmaceutical chemistry, Pharmaceutical science
1. Introduction
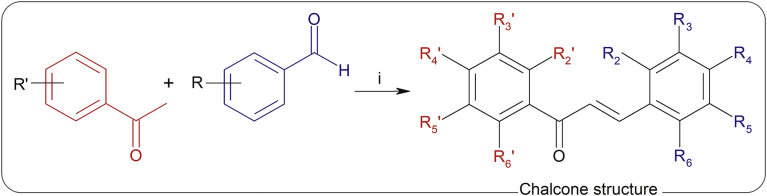
Natural sources are still one of the most important inspirations for the discovery and design of new chemical entities as potential drugs [1]. Chalcones (1,3-diaryl-2-propen-1-ones) (Fig. 1) belong to the flavonoid family. Chemically they consist of two aromatic rings joined by a three-carbon α,β-unsaturated carbonyl system [2]. Natural and synthetic chalcone derivatives have shown promising biological activity, safety profiles and potential as lead compounds for the discovery of antioxidant, anti-inflammatory, anticancer, anti-infective agents, among others [3]. It was reported that simple and synthetically produced chalcones represent a new challenge for the medicinal chemistry field with the possibility of extending the scientific importance of these compounds [1].
Fig. 1.
Synthesis of compounds 2–21. Reagents and conditions: i) MeOH, NaOH (50%), 70 °C, 3–5 h. For fluorinated derivatives NaOH (25%) was used.
Anxiety disorders, depression and pain represent some of the most common and proliferating health problems that affect a large percentage of the population worldwide. Individuals suffering from these pathologies are faced with considerable disruption of their quality of life, psychological well-being and greater risk for various somatic conditions [4, 5, 6]. Despite the existence of anxiolytic, antidepressant and pain relief drugs, there is a need to develop more effective pharmacotherapies with fewer side effects than the existing drugs [7, 8]. The endogenous gamma aminobutyric acid (GABA), serotonergic and opioid systems are critical for the regulation of many physiological and behavioural functions. Specially for these diseases, GABA type A receptors (GABAA) can be allosterically modulated by different drugs such as benzodiazepines (BDZs) causing anxiolytic effects [9]. Within the seven main classes of 5-HT (serotonin) receptors, 5-HT1A subtype plays an important role in the regulation of mental disorders such as depression, anxiety or schizophrenia [10]. On the other hand, of three known ‘classical’ types of opioid receptors (μ, δ and k), μ-opioid receptor is thought to be primarily responsible for the mediation of opioid antinociception [11]. Thus, in vivo activities of anxiolytic, antidepressant and antinociceptive drugs could be related to these biological targets.
In this work, we have made a survey of bibliographic reports of simple chalcones with anxiolytic, antidepressant and antinociceptive activities to recognize common structural determinants (see Table 1). Only few natural and synthetic chalcones have been reported in relation to these pathologies, therefore revealing chalcones as promising scaffold for the development of new derivatives. Most of the reported compounds have a hydroxyl group substitution in position 2′. Also, methoxy, methyl, dimethylamine, halogens and nitro groups substitutions are present in both rings of these derivatives. Additionally, the natural compound isoliquiritigenin (2′,4′,4-trihydroxychalcone) was described as a ligand for the benzodiazepine binding site (BDZ-bs) of the GABAA receptor, with a Ki value of 0.45 μM, being a positive allosteric modulator [12]. Also, we have already reported that the chalcone nucleus itself exhibits moderate affinity for the μ-opioid receptor [13].
Table 1.
Reported simple chalcones with anxiolytic-like, antidepressant-like and antinociceptive activities in rodents.
| Name | R2’ | R3’ | R4’ | R5’ | R6’ | R2 | R3 | R4 | R5 | R6 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chalcones with anxiolytic-like effect | |||||||||||
| Isoliquiritigenin | OH | H | OH | H | H | H | H | OH | H | H | [14] |
| Butein | OH | H | OH | H | H | H | OH | OH | H | H | [14] |
| Chalcones with antidepressant-like effect | |||||||||||
| Isoliquiritin | OH | H | OH | H | H | H | H | O-β-Glc | H | H | [15] |
| Isoliquiritigenin | OH | H | OH | H | H | H | H | OH | H | H | [16] |
| Butein | OH | H | OH | H | H | H | OH | OH | H | H | [17] |
| 4,2′,4′,6′- tetrahydroxychalcone | OH | H | OH | H | OH | H | H | OH | H | H | [18] |
| 3,4,2′,4′,6′-pentahydroxychalcone | OH | H | OH | H | OH | H | OH | OH | H | H | [18] |
| 3-methoxy-4,2′,4′,6′-tetrahydroxychalcone | OH | H | OH | H | OH | H | OCH3 | OH | H | H | [18] |
| 2-bromo-2′,4′,6′-trihydroxychalcone | OH | H | OH | H | OH | Br | H | H | H | H | [18] |
| 3-bromo-2′,4′,6′-trihydroxychalcone | OH | H | OH | H | OH | H | Br | H | H | H | [18] |
| 3-bromo-2′,4′-dihydroxychalcone | OH | H | OH | H | H | H | Br | H | H | H | [16] |
| 2-fluoro-2′,4′,6′-trihydroxychalcone | OH | H | OH | H | OH | F | H | H | H | H | [18] |
| 3-chloro-2′,4′,6′-trihydroxychalcone | OH | H | OH | H | OH | H | Cl | H | H | H | [18] |
| 4-chloro-2′,4′,6′-trihydroxychalcone | OH | H | OH | H | OH | H | H | Cl | H | H | [18] |
| 2,4-dichloro-2′,4′,6′-trihydroxychalcone | OH | H | OH | H | OH | Cl | H | Cl | H | H | [18] |
| 2,6-dichloro-2′,4′-dihydroxychalcone | OH | H | OH | H | H | Cl | H | H | H | Cl | [16] |
| Chalcones with peripheral antinociceptive effect | |||||||||||
| Chalcone | H | H | H | H | H | H | H | H | H | H | [19] |
| 4′-chlorochalcone | H | H | Cl | H | H | H | H | H | H | H | [19] |
| 4′-bromochalcone | H | H | Br | H | H | H | H | H | H | H | [19] |
| 3′,4′-dichlorochalcone | H | Cl | Cl | H | H | H | H | H | H | H | [19] |
| 4,3′,4′-trichlorochalcone | H | Cl | Cl | H | H | H | H | Cl | H | H | [19] |
| 3′,4′-dichloro-4-dimethylaminechalcone | H | Cl | Cl | H | H | H | H | (-N(CH3)2) | H | H | [19] |
| 4′,6′-dimethoxy-2′-hydroxychalcone | OH | H | OCH3 | H | OCH3 | H | H | H | H | H | [20] |
| 4,4′,6′-trimethoxy-2′-hydroxychalcone | OH | H | OCH3 | H | OCH3 | H | H | OCH3 | H | H | [20] |
| 4′,6′-dimethoxy-4-methyl-2′-hydroxychalcone | OH | H | OCH3 | H | OCH3 | H | H | CH3 | H | H | [20] |
| 6-chloro-4′,6′-dimethoxy-2′-hydroxychalcone | OH | H | OCH3 | H | OCH3 | H | H | H | H | Cl | [20] |
| 3,4-dichloro-4′,6′-dimethoxy-2′-hydroxychalcone | OH | H | OCH3 | H | OCH3 | H | Cl | Cl | H | H | [20] |
| 4′,6′-dimethoxy-2′-hydroxy-3-nitrochalcone | OH | H | OCH3 | H | OCH3 | H | NO2 | H | H | H | [20] |
| 4′,6′-dimethoxy-2′-hydroxy-4-nitrochalcone | OH | H | OCH3 | H | OCH3 | H | H | NO2 | H | H | [20] |
| 3′-bromo-4′,6′-dimethoxy-2′-hydroxychalcone | OH | Br | OCH3 | H | OCH3 | H | H | H | H | H | [20, 21] |
| 3′-bromo-4′,6′-dimethoxy-2′-hydroxy-3-nitrochalcone | OH | Br | OCH3 | H | OCH3 | H | NO2 | H | H | H | [20] |
| 6-chloro-3′-bromo-4′,6′-dimethoxy-2′-hydroxychalcone | OH | H | OCH3 | H | OCH3 | H | H | H | H | Cl | [20] |
| 2-hydroxychalcone | H | H | H | H | H | OH | H | H | H | H | [22] |
| 4′-methyl-2-hydroxychalcone | H | H | CH3 | H | H | OH | H | H | H | H | [22] |
| 4′-methoxy-2-hydroxychalcone | H | H | OCH3 | H | H | OH | H | H | H | H | [22] |
| 4′-chloro-2-hydroxychalcone | H | H | Cl | H | H | OH | H | H | H | H | [22] |
| 4′-bromo-2-hydroxychalcone | H | H | Br | H | H | OH | H | H | H | H | [22] |
| 3′,4′-dichloro-2-hydroxychalcone | H | Cl | Cl | H | H | OH | H | H | H | H | [22] |
| 2-hydroxy-4′-nitrochalcone | H | H | NO2 | H | H | OH | H | H | H | H | [22] |
| Chalcones with central antinociceptive effect | |||||||||||
| 2′,4′,4,5-tetrahydroxychalcone | OH | H | OH | H | H | H | H | OH | OH | H | [23] |
| 2′,4′-dimethoxy-6′-hydroxychalcone | OCH3 | H | OCH3 | H | OH | H | H | H | H | H | [24] |
| 3,4-methylenedioxy-4′,6′-dimethoxy-2′-hydroxychalcone | OH | H | OCH3 | H | OCH3 | H | M | M | H | H | [21] |
| 4′,6′-dihydroxy-3′,5′-dimethyl-2′-methoxychalcone | OCH3 | CH3 | OH | CH3 | OH | H | H | H | H | H | [25] |
R2’, R3’, R4’, R5’, R6’, R2, R3, R4, R5, R6 represent the substituent as indicated in chalcone structure from Fig. 1.
Sugar moieties: glucose (Glc), M: methylenedioxy.
Thus, the aim of this work was to synthesize a series of simple chalcones and assess their potential effect on different biological receptors involved in anxiety disorders, depression and pain. Afterwards, those compounds of the series that showed the best affinity for each receptor were tested in different behavioural paradigms related to these pathologies.
2. Material and methods
2.1. Chemistry
2.1.1. General
2′-hydroxyacetophenone, 2′-hydroxy-4′-methoxyacetophenone, 2′-hydroxy-5′-methoxyacetophenone, 2′-hydroxy-6′-methoxyacetophenone, 5′-chloro-2′-hydroxyacetophenone, 5′-fluoro-2′-hydroxyacetophenone, 5′-bromo-2′-hydroxyacetophenone, 2′-hydroxy-5′-methylacetophenone, 2′-hydroxy -3′,5′-dibromoacetophenone, 2′-hydroxy- 4′,5′-dimethylacetophenone, 2′-hydroxy-5′-methyl-3′- nitroacetophenone, 4′-hydroxy-3′-methoxyacetophenone, 4′-aminoacetophenone, 4′-methylacetophenone and 4-dimethylaminobenzaldehyde were obtained from Sigma-Aldrich Chemical Company. Benzaldehyde was purchased from Mallinckrodt Pharmaceuticals. Acetophenone, 3-chlorobenzaldehyde and 4-chlorobenzaldehyde were obtained from Fluka and 3-nitrobenzaldehyde and 4-nitrobenzaldehyde from Acros Organics. All solvents were obtained commercially from Sigma-Aldrich and Fluka.
The reaction progress and product were analyzed by TLC on silica gel on polyester sheets, with 254 nm fluorescent indicator (Sigma, USA) and by HPLC performed using C18 reversed phase Vydac columns (The Separation Group, Hesperia, CAL, USA). Purity and structures of the obtained compounds were confirmed by chromatographic (TLC, HPLC) and spectroscopic (NMR, MS) methods. 1H NMR and 13C RMN spectra were recorded in a Criomagneto Bruker-Oxford BZH 300/89 of 7.05 Tesla apparatus. Electrospray ionization ion trap mass spectra were recorded on a LCQ-Duo (ESI-IT) spectrometer ThermoFisher. Elemental (C, H, N) analysis was carried out on Carlo Erba 1108 analyzer and Exeter CE 440. Analysis indicated by the symbols of the elements or functions were within ±0.4 % of the theoretical values. cLog P values of the compounds were calculated using ChemDraw Ultra 12.0.
[3H]-flunitrazepam (FNZ), [3H]-8-hydroxy-2-(dipropylamine)tetralin ([3H]-8-OH-DPAT) and [3H]-DAMGO ([D-Ala2, N-Me-Phe4, Gly-ol5] enkephalin) were obtained from Perkin Elmer Life and Analytical Sciences, Boston, MA, USA. Diazepam (DZ) was obtained from Roche Diagnostics. Naltrexone hydrochloride, imipramine hydrochloride (IMP) and serotonin hydrochloride were obtained from Sigma-Aldrich Chemical Company, USA. Morphine hydrochloride (MOR) was obtained from Gramon Millet (Argentina).
2.1.2. General methods for the synthesis of 2-21
The synthesis of the chalcone derivatives was performed following [26] with some modifications (Fig. 1): To a stirred solution of each acetophenone (1 mmol) and benzaldehyde (1 mmol) in MeOH (10 mL), 1 mL of a 50% aqueous solution of NaOH was added. The solution was heated at 70 °C for 3–5 h. After cooling, the reaction mixture was neutralized with HCl 10% and extracted with CH2Cl2. The organic layer was dried over anhydrous Na2SO4, filtered, concentrated under reduced pressure and recrystallized from MeOH/H2O. In the case of fluorinated derivatives NaOH (25%) was used. HPLC purity of all the chalcone derivatives were >98%.
2.1.2.1. (2E)-1-(2-hydroxyphenyl)-3-phenyl-2-propen-1-one (2)
Yield 85 %, yellow crystals. 1H NMR (300 MHz, CDCl3) δ 12.85 (s, 1H), 7.98–7.93(m, 2H), 7.72–7.67 (m, 3H), 7.56–7.45 (m, 4H), 7.07–6.95 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 193.76, 163.62, 145.50, 136.43, 134.62, 130.95, 129.66, 129.06, 128.68, 120.03, 120.03, 118.87, 118.67. MS: m/z 225.0 [M+1H]+ (C15H12O2).
2.1.2.2. (2E)-1-(2-hydroxy-4-methoxyphenyl)-3-phenyl-2-propen-1-one (3)
Yield 34 %, orange crystals. 1H NMR (300 MHz, CDCl3) δ 13.46 (s, 1H), 7.90 (d, J = 15.58 Hz, 1H), 7.85 (d, J = 9 Hz, 1H), 7.67–7.62 (m, 2H), 7.59 (d, J = 15.48 Hz, 1H), 7.45–7.44 (m, 3H), 6.52–6.49 (m, 2H), 3.88 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 191.85, 166.72, 166.24, 144.41, 134.79, 131.24, 130.66, 128.99, 128.53, 120.33, 114.09, 107.78, 101.08, 55.61. MS: m/z 255.0 [M+1H]+ (C16H14O3).
2.1.2.3. (2E)-1-(2-hydroxy-5-methoxyphenyl)-3-phenyl-2-propen-1-one (4)
Yield 76 %, orange oil. 1H NMR (300 MHz, CDCl3) δ 12.39 (s,1H), 7.95 (d, J = 15.45 Hz, 1H), 7.71–7.66 (m, 2H), 7.63 (d, J = 15.48 Hz, 1H), 7.48–7.42(m, 3H), 7.39–7.38(m, 1H), 7.19–7.12 (m, 2H), 3.87 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 193.40, 157.97, 151.75, 145.61, 134.61, 130.96, 129.06, 128.67, 124.14, 120.20, 119.65, 113.56, 56.02. MS: m/z 255.0 [M+1H]+ (C16H14O3).
2.1.2.4. (2E)-1-(2-hydroxy-6-methoxyphenyl)-3-phenyl-2-propen-1-one (5)
Yield 70 %, orange oil. 1H NMR (300 MHz, CDCl3) δ 13.15 (s, 1H), 7.87–7.85 (m, 2H), 7.65–7.62 (m, 2H), 7.45–7.35 (m, 4H), 6.64 (d, J = 8.37 Hz,1H), 6.45 (d, J = 8.29 Hz,1H), 3.97 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 194.49, 164.86, 160.99, 142.94, 135.92, 135.34, 130.30,128.92, 128.47, 127.60, 112.00, 110.97, 101.54, 55.96. MS: m/z 255.0 [M+1H]+ (C16H14O3).
2.1.2.5. (2E)-1-(5-chloro-2-hydroxyphenyl)-3-phenyl-2-propen-1-one (6)
Yield 86 %, yellow crystals. 1H NMR (300 MHz, CDCl3) δ 12.75 (s, 1H), 7.97 (d, J = 15.46 Hz, 1H), 7.89 (d, J = 1.6Hz, 1H), 7.72–7.70 (m, 2H), 7.60 (d, J = 15.49 Hz, 1H), 7.48–7.46 (m, 4H), 7.01 (d, J = 8.88 Hz,1H). 13C NMR (75 MHz, CDCl3) δ 192.77, 161.73, 146.52, 136.15, 134.32, 131.27, 129.11, 128.85, 123,60, 120.62, 120.25, 120.15, 119.50. MS: m/z 260.1/262.3 (rel. 3/1) [M+1H]+ (C15H11ClO2).
2.1.2.6. (2E)-1-(5-fluoro-2-hydroxyphenyl)-3-phenyl-2-propen-1-one (7)
Yield 85 %, yellow crystals. 1H NMR (300 MHz, CDCl3) δ 12.56 (s, 1H), 7.98 (d, J = 15.43 Hz, 1H), 7.71–7.68 (m, 2H), 7.63–7.59 (m, 1H), 7.57 (d, J = 15.54 Hz, 1H), 7.49–7.46 (m, 3H), 7.30–7.24 (m, 1H), 7.03 (dd, 1H). 13C NMR (75 MHz, CDCl3) δ 192.90, 159.76, 156.45, 146.38, 134.35, 131.22, 129.11, 128.79, 124.09, 119.93, 119.83, 119.56, 114.39. MS: m/z 243.1 [M+1H]+ (C15H11FO2).
2.1.2.7. (2E)-1-(5-bromo-2-hydroxyphenyl)-3-phenyl-2-propen-1-one (8)
Yield 62 %, yellow crystals. 1H NMR (300 MHz, CDCl3) δ 12.77 (s, 1H), 8.03(d, J = 2.1Hz 1H), 7.97 (d, J = 15.40 Hz, 1H), 7.72–7.71 (m, 2H), 7.61–7.56 (m, 2H), 7.49–7.48 (m, 3H), 6.96 (d, J = 8.90 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 192.75, 162.51, 146.60, 138.97, 134.31, 131.85, 131.29, 129.12, 128.87, 121.26, 120.67, 119.42, 110.46. MS: m/z 303.9/305.9 (rel. 1/1) [M+1H]+ (C15H11BrO2).
2.1.2.8. (2E)-1-(2-hydroxy-5-methylphenyl)-3-phenyl-2-propen-1-one (9)
Yield 88 %, orange crystals. 1H NMR (300 MHz, CDCl3) δ 12.66 (s, 1H), 7.94 (d, J = 15.49 Hz, 1H), 7.71–7.66 (m, 2H), 7.69 (d, J = 15.51 Hz, 1H), 7.49–7.45 (m, 4H), 7.35 (dd, J = 8.49 Hz, 1.77 Hz, 1H), 6.96 (d, J = 8.46 Hz,1H), 2.38 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 193.66, 161.54, 145.26, 137.51, 134.69, 130.87, 129.35, 129.03 (C5), 128.67, 127.93, 120.27, 119.68, 118.39, 20.63. MS: m/z 239.0 [M+1H]+ (C16H14O2).
2.1.2.9. (2E)-1-(3,5-dibromo-2-hydroxyphenyl)-3-phenyl-2-propen-1-one (10)
Yield 81 %, orange crystals. 1H NMR (300 MHz, CDCl3) δ 13.56 (s, 1H), 8.02 (d, J = 15.36 Hz, 1H), 8.01 (d, J = 2.10 Hz 1H), 7.91(d, J = 2.00 Hz, 1H), 7.72–7.70 (m, 2H), 7.68 (d, J = 15.36 Hz, 1H), 7.50–7.48 (m, 3H). 13C NMR (75 MHz, CDCl3) δ 192.45, 159.24, 147.70, 141.33, 134.08, 131.62, 131.13, 129.18, 129.02, 118.83, 110.34, 109.50. MS: m/z 381.8/383.8/385.8 (rel.1/2/1) [M+1H]+ (C15H10Br2O2).
2.1.2.10. (2E)-1-(4,5-dimethyl-2-hydroxyphenyl)-3-phenyl-2-propen-1-one (11)
Yield 45 %, orange crystals. 1H NMR (300 MHz, CDCl3) δ 12.71 (s, 1H), 7.92 (d, J = 15.55 Hz, 1H), 7.71–7.69 (m, 4H), 7.47–7.45 (m, 3H), 6.85 (s, 1H), 2.31 (s, 3H), 2.29 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 193.08, 161.99, 147.11, 144.76, 134.80, 130.72, 129.72, 129.00, 128.61, 127.13, 120.45, 119.16, 117.97, 20.56, 19.04. MS: m/z 253.0 [M+1H]+ (C17H16O2).
2.1.2.11. (2E)-1-(5-methyl-3-nitro-2-hydroxyphenyl)-3-phenyl-2-propen-1-one (12)
Yield 83 %, yellow solid. 1H NMR (300 MHz, CDCl3) δ 13.08 (s, 1H), 8.07 (br. s, 1H), 7.96–7.91 (m, 2H), 7.70–7.68 (m, 2H), 7.60 (d, J = 15.54 Hz, 1H), 7.49–7.47 (m, 3H), 2.45 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 192.19, 154.38, 146.68, 136.92, 136.60, 134.28, 131.35, 130.94, 129.12, 128.88, 128.38, 124.93, 121.37, 20.35. MS: m/z 283.9 [M+1H]+. Anal. Calc. (%) for C16H13NO4: C 67.8, H 4.6, N 4.9, O 22.6; found: C 67.4, H 4.9, N 4.8, O 22.9.
2.1.2.12. (2E)-1-(4-hydroxy-3-methoxyphenyl)-3-phenyl-2-propen-1-one (13)
Yield 68 %, yellow crystals. 1H NMR (300 MHz, CDCl3) δ 7.84 (d, J = 15.57 Hz, 1H), 7.69–7.66 (m, 4H), 7.57 (d, J = 15.63 Hz, 1H), 7.45–7.43 (m, 3H), 7.0.2 (d, J = 8.00 Hz, 1H), 4.02 (s, 3H).13C NMR (75 MHz, CDCl3) δ 188.55, 150.37, 146.88, 143.96, 135.10, 130.34, 129.27, 128.36, 123.70, 121.70, 113.79, 110.50, 56.16. MS: m/z 255.0 [M+1H]+ (C16H14O3).
2.1.2.13. (2E)-1-(4-aminophenyl)-3-phenyl-2-propen-1-one (14)
Yield 70 %, yellow crystals. 1H NMR (300 MHz, CDCl3) δ 7.96 (d, J = 8.49 Hz, 2H), 7.81 (d, J = 15.66 Hz, 1H), 7.67–7.65 (m, 2H), 7.57(d, J = 15.63 Hz, 1H), 7.43–7.42 (m, 3H), 7.73 (d, 2H), 4.18 (s, 2H). 13C NMR (75 MHz, CDCl3) δ 188.10, 151.08, 143.13, 135.35, 131.09, 131.09, 130.07, 128.87, 128.60, 128.27, 122.07, 113.94, 113.94. MS: m/z 224.1 [M+1H]+ (C15H13NO).
2.1.2.14. (2E)-1-(4-methylphenyl)-3-phenyl-2-propen-1-one (15)
Yield 35%, white crystals. 1H NMR (300 MHz, CDCl3) δ 7.97 (d, J = 8.10 Hz, 2H), 7.83 (d, J = 15.66 Hz, 1H), 7.68–7.66 (m, 2H), 7.56 (d, J = 15.72 Hz, 1H), 7.45–7.43 (m, 3H), 7.33 (d, J = 7.95Hz, 2H), 2.44 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 190.04, 144.39, 143.64, 135.67, 135.04, 130.42, 129.25, 128.94, 128.66, 128.40, 122.15, 21.67. MS: m/z 223.1 [M+1H]+ (C16H14O).
2.1.2.15. (2E)-3-(3-nitrophenyl)-1-phenyl-2-propen-1-one (16)
Yield 35%, white crystals. 1H NMR (300 MHz, dimethyl sulfoxide (DMSO)-d6) δ 8.80 (s ancho, 1H), 8.34–8.26 (m, 1H), 8.23–8.20 (m, 2H), 7.78–7.68 (m, 4H), 7.44–7.41 (m, 3H); 13C NMR (75 MHz, DMSO-d6) δ 189.54, 148.91, 141.92, 137.69, 137.06, 135.57, 133.91, 130.82, 129.34, 129.20, 125.28, 125.15, 123.54. MS: m/z 254.0 [M+1H]+ (C15H11NO3).
2.1.2.16. (2E)-3-(4-nitrophenyl)-1-phenyl-2-propen-1-one (17)
Yield 35%, whitish crystals. 1H NMR (300 MHz, DMSO-d6) δ 8.31–8.13 (m, 7H), 7.84 (d, J = 15.50 Hz, 1H), 7.73–7.71 (m, 1H), 7.63–7.58 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ 189.52, 148.58, 141.60, 137.61, 134.00, 130.36, 129.35, 129.18, 126.55), 124.40, 123.33. MS: m/z 254.0 [M+1H]+ (C15H11NO3).
2.1.2.17. (2E)-3-(3-chlorophenyl)-1-phenyl-2-propen-1-one (18)
Yield 76 %, reddish oil. 1H NMR (300 MHz, DMSO-d6) δ 8.06 (d, J = 15.80 Hz, 1H), 8.00–7.96 (m, 2H), 7.59–7.49 (m, 4H), 7.19–7.00 (m, 4H); 13C NMR (75 MHz, DMSO-d6) δ 189.55, 142.75, 137.41, 137.24, 133.81, 133.10, 131.16, 129.29, 129.17, 129.12, 128.47, 128.39, 128.29. MS: m/z 243.0/245.0 (rel. 3/1) [M+1H]+ (C15H11ClO2).
2.1.2.18. (2E)-3-(4-chlorophenyl)-1-phenyl-2-propen-1-one (19)
Yield 73%, white crystals. 1H NMR (300 MHz, DMSO-d6) δ 8.17 (d, J = 7.3 Hz, 2H), 7.99 (d, J = 15.40 Hz, 1H), 7.95 (d, J = 7.80 Hz, 2H), 7.75 (d, J = 15.80 Hz, 1H), 7.70–7.40 (m, 5H); 13C NMR (75 MHz, DMSO-d6) δ 189.58, 143.00, 137.90, 135.59, 134.12, 133.72, 131.08, 129.43, 129.28, 129.03, 123.28. MS: m/z 243.1/245.0 (rel. 3/1) [M+1H]+ (C15H11ClO2).
2.1.2.19. (2E)-3-[4-(dimethylamino)phenyl]-1-phenyl-2-propen-1-one (20)
Yield 62 %, yellow crystals. 1H NMR (300 MHz, CDCl3) δ 7.78–7.74 (m, 2H), 7.57–7.56 (m, 2H), 7.55–7.54 (m, 3H), 7.48–7.47 (m, 1H), 7.33 (d, J = 15.51 Hz, 1H), 6.73 (d, J = 8.79 Hz, 2H), 3.1 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 190.29, 154.36, 145.76, 139.09,131.98, 130.40, 129.28, 128.32, 125.24, 112.06, 111.02, 40.02. MS: m/z 252.1 [M+1H]+ (C17H17NO).
2.1.2.20. (2E)-1-(4-aminophenyl)-3-(3-nitrophenyl)-2-propen-1-one (21)
Yield 62 %, brown crystals. 1H NMR (300 MHz, CDCl3) δ 8.53 (s, 1H), 8.26 (d, J = 8.19 Hz, 1H), 7.98 (d, J = 8.58 Hz, 2H), 7.92(d, J = 7.92 Hz, 1H), 7.82 (d, J = 15.75 Hz, 1H), 7.68 (d, J = 15.82 Hz, 1H), 7.60 (d, J = 7.90 Hz, 1H), 6.74 (d, J = 8.58 Hz, 2H), 4.24 (s, 2H,). 13C NMR (75 MHz, CDCl3) δ 186.10, 154.62, 148.91, 139.40, 137.57, 135.23, 131.88, 130.76, 125.80, 125.52, 124.57, 123.07, 113.24. MS: m/z 269.1 [M+1H]+ (C15H12N2O3).
2.2. Animals, administrations and procedures
2.2.1. Animals
Adult male Swiss mice and adult male Wistar rats (Central Animal House of the School of Pharmacy and Biochemistry, University of Buenos Aires.), weighing 25–30 g (mice used for the pharmacological tests) and 200–300 g (rats used for binding assays) were used throughout the study. For behavioural assays mice were housed in plastic cages, in groups of five, with free access to standard laboratory food and water, and kept in a regulated environment (20–23 °C) under a 12-h light/dark cycle (light on at 7:00 AM). Experiments were carried out between 10:00 AM and 2:00 PM and tested by experimenters who were kept unaware of the treatment administered. Behavioural sessions were recorded using a video camera SONY HDR-AS30/AS30V.
Housing, handling, and experimental procedures complied with the recommendations and regulations set forth by the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1996) and the Institutional Committees for the Care and Use of Laboratory Animals of the Faculty of Pharmacy and Biochemistry, University of Buenos Aires, Argentina (CICUAL, protocol's approved code numbers: EXP-UBA N°: 0031682/2014, Res. N°: 3295). Accordingly, to the ARRIVE guidelines, all possible efforts were made to minimize animal suffering and discomfort and to reduce the number of experimental subjects. The number of animals used was the minimum number, consistent with obtaining significant data. Mice were randomly assigned to any treatment groups and were used only once.
2.2.2. Administrations and procedures
Compounds 1, 9, 12 and DZ were diluted by using the sequential addition of dimethylsulfoxide, a solution of 0.25% Tween 80 and saline; up to final concentrations of 5%, 20% and 75%, respectively. MOR and IMP were dissolved in saline solution. For each assay, a control group receiving only vehicle (VEH) was tested in parallel with those animals receiving drug treatment. Neither of the tests performed showed significant differences between VEH control mice vs. mice treated with saline (data not shown). The volume of intraperitoneally (i.p.) injections was 0.15–0.30 ml/30 g of body weight. Compounds tested in behavioural assays were evaluated 30 minutes after i.p. administrations. Doses of the chalcones used for in vivo assays in this study were chosen based on pilot experiments and previous reports on similar derivatives (see Table 1).
Protein concentration was determined by the Bradford's method using bovine serum albumin as standard [27].
2.3. Biological activity
2.3.1. In vitro studies
2.3.1.1. Radioligand binding assays
For tissue preparations rats were humanely killed by decapitation and the brains were removed, washed and rapidly dissected out on ice. Homogenizations were performed by using a PRO Scientific Inc homogenizer at 30,000 rpm for 1 min. Synaptosomal membranes were prepared from different brain regions according to literature as follows: rat cerebral cortex for [3H]-FNZ [28] and 5HT1A [29], rat forebrains for [3H]-DAMGO [13]. In all cases membranes were stored at -80 °C until use.
For the [3H]-FNZ (81.8 Ci/mmol) binding assay the compounds were added to 0.2 mg membrane protein thawed and suspended in 1 ml of 25 mM Tris–HCl buffer in the presence of [3H]-FNZ (81.8 Ci/mmol) 0.3 nM. DZ (0.01 nM-600 nM) was used as positive control. Nonspecific binding was measured in the presence of 10 μM FNZ. The incubations were carried out at 4 °C for 1 h [28].
For the [3H]-8-OH-DPAT binding assay, membranes were suspended in 50 mM Tris–HCl pH 7.4, with 1mM MnCl2 to a final protein concentration of 0.5 mg/ml. The incubation was performed at 25 °C for 1 h in a final volume of 1 ml of membrane suspension in the presence of the compounds and with 0.2 nM of [3H]-8-OH-DPAT (170.2 Ci/mmol). Serotonin (0.1 nM-300 nM) was used as positive control. Nonspecific binding was determined in parallel incubations in the presence of serotonin (10 μM) [29].
For the [3H]-DAMGO binding assay, membranes were suspended in 50 mM Tris–HCl pH 7.4 to 0.35 mg/mL final protein concentration. The incubation was done for 1 h at 25 °C in a final volume of 1 mL of membrane suspension in the presence of the compound and 1 nM of [3H]-DAMGO (56.8 Ci/mmol) [13]. Naltrexone (0.03 nM-100 nM) was used as positive control. Nonspecific binding was determined in parallel incubations in the presence of 10 μM naltrexone.
Following incubations, assays were terminated by filtration under vacuum through Whatman GF/A glass-fiber filters. Then, two or three washing steps with buffer were performed as follows: 3 ml each wash for the [3H]-FNZ assay (three times), 3 ml each wash for the [3H]-DAMGO assay (two times), 3.5 ml for the [3H]-8-OH-DPAT assay (three times). Individual filters were incubated overnight with scintillation cocktail (OptiPhase ‘HiSafe’ 3) before measuring radioactivity in a Wallac Rackbeta 1214 liquid scintillation counter.
For screening assays each compound was tested at 300 μM in duplicate. For competition assays the incubations were done with a range of concentrations (0.1 μM-600 μM) of the compounds.
2.3.1.2. Cell viability assay
SH-SY5Y cells (2 × 104/well in 100 μl) were plated in 96 well microplates and maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum. The cells were grown in humidified 5% CO2/air on standard plastic culture dishes. 24–48 h later the medium was removed and the cells were washed and incubated in complete medium with increasing concentrations (up to 200 μM) of chalcone (1), 5′-methyl-2′-hydroxychalcone (9) or 5′-methyl-2′-hydroxy-3′-nitrochalcone (12) (dissolved in DMSO). The viability assay was performed 48 h later by measuring the activity of the lysosome enzyme β-hexosaminidase as described previously [30].
2.3.1.3. PAMPA assay
The in vitro permeability through lipid membrane was determined for 5 commercial drugs and compounds 1, 9 and 12. The compounds were dissolved in DMSO (10 mM stock solution) and diluted with Prisma HT buffer (5 μ L/1 mL at pH = 7.4; bought from Pion Inc.). Then, the donor 96-well microplate (Millipore filter plate) was filled with solution of the tested compounds in buffer (200 μ L/well). The filter membrane in acceptor 96-well microplate (Millipore filter plate) was impregnated with lipid solution (4 μ L/well, hexane/dodecane = 1/3; Polar brain lipid extract 20 mg/mL) and the plate was prepared with Brain Sink Buffer (200 μ L/well; bought from Pion Inc.). The acceptor and donor plates were then configured in a »sandwich« configuration in order to provide contact from lipid filter to the acceptor solution volume and were left undisturbed for 16 h at 37 °C. After incubation, the plates were separated, well solutions transferred to a UV-plate (Millipore) and concentrations measured using Biotek Synergy HT microplate reader (λ = 230–500 nm in 4 nm steps). Negative logarithm of the effective permeability (-logPe) was calculated using the permeability equation under sink conditions published by Avdeef and co-workers [31] implemented in-house using Python programming language and Python SciPy library. Assay validation was performed by using the experimental permeability of the five commercial drugs used as reference standards and binning as follows: CNS+, -logPe < 5.6, high permeability; CNS–, -logPe > 6.3, low permeability; intermediate was labelled as uncertain blood-brain barrier (BBB) permeability.
2.3.2. In vivo studies
2.3.2.1. Elevated plus-maze test
This test is a model to assess anxiety-like responses in rodents based on the general aversion of rodents to open elevated spaces of the maze.
The apparatus consists of two open arms (25 cm × 5 cm) positioned opposite to one another, separated by a crossing point, and two arms of the same dimension, but enclosed by walls (15 cm high) forming a cross, with free access to all arms from the crossing point. The maze was elevated 50 cm from the room floor.
Mice were placed on the crossing point from the maze facing an open arm. The number of arm entries and the time spent going into open arms were recorded during 5 min under red dim light. The total exploratory activity (number of entries in both arms) was also determined [32]. Mice were i.p. injected with 10 mg/kg of compounds 1, 9, 14, 15, 16, and 1 mg/kg of DZ (reference compound) or VEH. Compound 9 was further tested at the doses of 3 mg/kg and 30 mg/kg.
2.3.2.2. Tail suspension test
The total duration of immobility induced by tail suspension was measured according to the method of Steru and co-workers [33]. It is a useful mouse behavioral test for the screening of potential antidepressant drugs [7]. Mice were individually suspended by their tails to a metal hook (distance from floor: 18 cm) with adhesive tape (distance from tip of tail: 2 cm) for 6 min. Mice, especially at the beginning of the session, manifest several escape oriented behaviours (which constitute mobility) interspersed with temporally increasing bouts of immobility. The duration of immobility was recorded during the last 4 min interval of the test. Mice were considered immobile only when they hung passively and completely motionless. Mice were i.p. administered with 10 mg/kg of compounds 1, 5, and 30 mg/kg of IMP (reference compound) or VEH. Compound 1 was also tested at the doses of 3 mg/kg and 30 mg/kg.
2.3.2.3. Antinociceptive assays
2.3.2.3.1. Writhing test
Writhing test is a chemical method used to induce peripheral pain by injection of acetic acid in mice. Analgesic activity of the test compound is inferred from decrease in the frequency of writhings. The test was carried out according to Koster et al [34], with slight modifications. Briefly, mice were i.p. injected with 0.75% acetic acid aqueous solution (0.2 ml/30 g body weight) and placed in a transparent Plexiglas chamber (15 cm × 15 cm×30 cm). Five minutes later the number of writhing responses (abdominal cramps) was counted for 15 min.
The writhing test was performed 30 min after the i.p. injections of compounds 6 and 12 (10 mg/kg), MOR (6 mg/kg, reference compound) or VEH. Compound 12 was subsequently assayed at the doses of 0.3 mg/kg, 3 mg/kg and 30 mg/kg.
Antinociceptive activity after chalcone treatment was expressed as the percentage of inhibition in the number of abdominal writhes normalized to control group.
2.3.2.3.2. Hot plate test
The hot plate test was performed to evaluate sensitivity to acute pain to a thermal stimulus, most commonly used for determining the antinociceptive efficacy of centrally acting test compounds in rodents [35]. Each mouse was placed into a transparent Plexiglass beaker (18 cm height and 10 cm diameter) to avoid the animals escaping from the plate which temperature was set at 52.5 °C ± 0.1 °C. The latency time (in s) to the first response: hind paw licking/fanning or jumping, was recorded with a 45 s cut-off time (to prevent tissue damage). Compounds 6 and 12 at the dose of 30 mg/kg, MOR (reference compound) 6 mg/kg or VEH were i.p. administered. Further, the dose of 10 mg/kg of compound 12 was also evaluated in this test.
2.3.2.4. Locomotor activity
The spontaneous locomotor activity was automatically measured as previously described [36] and was expressed as total light beam counts per 5 min. The test was performed 30 min after the i.p. injection of compounds 1, 9 and 12 (10 mg/kg and 30 mg/kg) or VEH.
2.3.2.5. Rotarod
The Rotarod test is used to assess a possible motor deficit provoked by test compounds. It measures the ability of the mouse to maintain balance on a rotating rod [37].
The equipment consists of a cylindrical shaft 3 cm diameter and 50 cm long, horizontally placed on a support and connected to a motor via a pulley. The axis is separated by discs into five compartments where mice are placed.
Mice were i.p. administered with 30 mg/kg of compound 12 or VEH. Each mouse was placed on the rotating rod and the latency time to fall off is measured (cut-off time: 120 s). Previously, mice were subjected to 3 successive trainings, in order to exclude difference in motivation and motor learning, and only those animals that kept the balance for 2 min in all sessions were used in the trial.
2.4. Statistical analyses
Binding assays data was analyzed by nonlinear regression fit to one site of specific bound vs radioligand concentration. Ki values were calculated using the Cheng-Prusoff/Chou equation (equation 1): Ki = IC50/[1+(L/Kd)], where Ki refers to the unlabeled ligand inhibition constant, IC50 is the unlabeled ligand concentration required to reach 50%-maximal binding, Kd refers to radioactive ligand equilibrium dissociation constant and L refers to the radioactive ligand concentration. Kd values for [3H] FNZ, [3H] 8-OH-DPAT and [3H] DAMGO were obtained from literature and were 2.0 nM, 2.4 nM and 0.5 nM, respectively [38, 39, 40].
The effect of test compounds was evaluated by unpaired t test or one-way analysis of variance (ANOVA). Post-hoc comparisons between treated and VEH groups were made using Dunnett multiple comparison test.
A P value <0.05 was considered statistically significant. All data were expressed as mean ± S.E.M. and analyzed with GraphPad Prism 5.00 software.
3. Results and discussion
3.1. Chemistry
In Table 2 we present the structures of chalcones that were prepared by Claisen-Schimdt condensation of the corresponding acetophenone and aromatic aldehydes with moderate to high yields (34–88%) (Fig. 1). The novel chalcone 12 was characterized by 1H NMR, 13C NMR, mass spectra and elemental analysis.
Table 2.
Molecular structures of the chalcones.
| Compound | R2’ | R3’ | R4’ | R5’ | R6’ | R2 | R3 | R4 | R5 | R6 | cLog P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chalcone | H | H | H | H | H | H | H | H | H | H | 3.59 |
| 2 | 2′-hydroxychalcone | OH | H | H | H | H | H | H | H | H | H | 3.20 |
| 3 | 4′-methoxy-2′-hydroxychalcone | OH | H | OCH3 | H | H | H | H | H | H | H | 3.07 |
| 4 | 5′-methoxy-2′-hydroxychalcone | OH | H | H | OCH3 | H | H | H | H | H | H | 3.07 |
| 5 | 6′-methoxy-2′-hydroxychalcone | OH | H | H | H | OCH3 | H | H | H | H | H | 3.07 |
| 6 | 5′-chloro-2′-hydroxychalcone | OH | H | H | Cl | H | H | H | H | H | H | 3.75 |
| 7 | 5′-fluoro-2′-hydroxychalcone | OH | H | H | F | H | H | H | H | H | H | 3.35 |
| 8 | 5′-bromo-2′-hydroxychalcone | OH | H | H | Br | H | H | H | H | H | H | 4.02 |
| 9 | 5′-methyl-2′-hydroxychalcone | OH | H | H | CH3 | H | H | H | H | H | H | 3.68 |
| 10 | 3′,5′-dibromo-2′-hydroxychalcone | OH | Br | H | Br | H | H | H | H | H | H | 4.85 |
| 11 | 4′,5′-dimethyl-2′-hydroxychalcone | OH | H | CH3 | CH3 | H | H | H | H | H | H | 4.17 |
| 12 | 5′-methyl-2′-hydroxy-3′-nitrochalcone | OH | NO2 | H | CH3 | H | H | H | H | H | H | 3.34 |
| 13 | 3′-methoxy-4′-hydroxychalcone | H | OCH3 | OH | H | H | H | H | H | H | H | 3.07 |
| 14 | 4′-aminochalcone | H | H | NH2 | H | H | H | H | H | H | H | 2.78 |
| 15 | 4′-methylchalcone | H | H | CH3 | H | H | H | H | H | H | H | 4.07 |
| 16 | 3-nitrochalcone | H | H | H | H | H | H | NO2 | H | H | H | 3.31 |
| 17 | 4-nitrochalcone | H | H | H | H | H | H | H | NO2 | H | H | 3.31 |
| 18 | 3-chlorochalcone | H | H | H | H | H | H | Cl | H | H | H | 4.14 |
| 19 | 4-chlorochalcone | H | H | H | H | H | H | H | Cl | H | H | 4.14 |
| 20 | 4-dimethylaminochalcone | H | H | H | H | H | H | H | N(CH3)2 | H | H | 3.87 |
| 21 | 4′-amino-3-nitrochalcone | H | H | NH2 | H | H | H | NO2 | H | H | H | 2.50 |
R2’, R3’, R4’, R5’, R6’, R2, R3, R4, R5, R6 represent the substituent as indicated in chalcone structure from Fig. 1.
3.2. In vitro binding screening
The capacity of the compounds to inhibit the binding of [3H]-FNZ, [3H]-8-OH-DPAT and [3H]-DAMGO to rat BDZ-bs of the GABAA receptor, 5-HT1A and μ-opioid receptors, respectively, is shown in Table 3. Those derivatives that showed more than 60% inhibition of radioligands binding to their respective receptors at the concentration of 300 μM, were selected to determine their inhibitory constant (Ki). In all cases, data obtained were best fitted to one site binding hyperbola.
Table 3.
Activities of the chalcone derivatives on the BDZ-bs of the GABAA, 5-HT1A and μ-opioid receptors.
| Compound | BDZ-bs |
5-HT1A |
μ-opioid |
|||
|---|---|---|---|---|---|---|
| Binding inhibitiona (%) [300 μM] | Kib (μM) | Binding Inhibitiona (%) [300 μM] |
Kib (μM) | Binding inhibitiona (%) [300 μM] | Kib (μM) | |
| 1 | ++++ | 2.8 ± 1.0 | +++ | 20.8 ± 1.5 | +++ | 28.2 ± 9.9[13] |
| 2 | +++ | 17.1 ± 1.3 | +++ | 71.4 ± 1.4 | + | nd |
| 3 | +++ | 9.0 ± 1.2 | + | nd | + | nd |
| 4 | +++ | 45.7 ± 1.3 | +++ | 303 ± 1.2 | +++ | 54.6 ± 2.9 |
| 5 | - | nd | ++++ | 13.3 ± 0.3 | +++ | 26.7 ± 1.6 |
| 6 | ++ | nd | ++ | nd | +++ | 19.4 ± 3.5 |
| 7 | ++ | nd | +++ | 129 ± 2.7 | +++ | 29.5 ± 6.6 |
| 8 | ++ | nd | ++ | nd | + | nd |
| 9 | +++ | 6.1 ± 1.5 | ++ | nd | +++ | 46.3 ± 1.6 |
| 10 | - | nd | +++ | 1221 ± 469 | +++ | 32.5 ± 9.3 |
| 11 | + | nd | +++ | 164 ± 1.47 | +++ | 29.1 ± 8.5 |
| 12 | + | nd | - | nd | ++++ | 10.8 ± 3.6 |
| 13 | ++ | nd | ++ | 287.8 ± 1.9 | ++ | 101 ± 21 |
| 14 | +++ | 6.1 ± 0.2 | ++ | nd | +++ | 59.4 ± 1.5 |
| 15 | +++ | 6.3 ± 0.8 | ++ | nd | + | nd |
| 16 | ++++ | 0.2 ± 0.05 | ++ | nd | - | nd |
| 17 | - | nd | - | nd | + | nd |
| 18 | ++ | nd | + | nd | + | nd |
| 19 | + | nd | - | nd | + | nd |
| 20 | - | nd | +++ | 59.6 ± 1.5 | +++ | 23.8 ± 3.1 |
| 21 | ++ | nd | +++ | 473 ± 7.3 | ++ | 107 ± 17.6 |
nd: not determined: <60% inhibition at 300 μM.
Capacity of the compounds to inhibit the binding of [3H]-FNZ to the BDZ-bs of the GABAA receptor, [3H]-DAMGO to the μ-opioid receptor and [3H]-8-OH-DPAT to the 5HT1A receptor (at 300 μM) indicated as: inhibition >90% (++++); inhibition 60–90% (+++); inhibition 40–60% (++); inhibition 20–40% (+) and inhibition <20% (-).
Ki ± standard error of the mean values (SEM) are means of 2 independent determinations. Diazepam, serotonin and naltrexone, reference compounds for the BDZ-bs, 5HT1A and μ-opioid receptors, gave Ki values of 3.5 ± 1.2 nM, 2.2 ± 0.01 nM and 0.2 ± 0.01 nM, respectively.
All the synthetic derivatives were able to displace at least one of the radioligands tested. Some of the synthesized compounds showed affinities in the low micromolar range towards the BDZ-bs of the GABAA receptor and moderate to no affinity for μ-opioid and 5-HT1A receptors.
In the [3H]-FNZ binding assay the results showed that compound 16 exhibited the highest affinity with a Ki value of 0.2 ± 0.05 μM, followed by the chalcone nucleus (1) (Ki: 2.8 ± 1.0 μM). Compounds 9, 14 and 15 presented similar Ki values, near 6 μM. The overall rank-order of potencies for inhibition (for the active compounds) was the following: compound 16 > 1 > 9 ≈ 14 ≈ 15 > 3 > 2 > 4.
On the other hand, all the tested compounds exhibited low to medium inhibitory activities on the [3H]-8-OH-DPAT binding to 5HT1A receptors present in rat cortex tissues. The results showed that chalcones 1 and 5 presented the lowest Ki values of this series, with values of 20.8 ± 1.5 μM and 13.3 ± 0.3 μM, respectively.
In the [3H]-DAMGO binding assay the results showed that most of the compounds displayed low to medium activity, though compounds 6 and 12 showed the highest activity in this binding assay, with Ki values of 19.4 ± 3.5 μM 13.5 ± 6.9 μM, respectively (Table 3).
This is the first report describing the potential involvement of the 5-HT1A and μ-opioid receptors on the CNS biological activities of chalcone derivatives.
3.3. Pharmacological studies
Those compounds of the series with the highest affinity for each receptor were selected for further in vivo studies. Then, a cut off value of Ki for each receptor was chosen to reduce the number of animals for the in vivo assays.
3.3.1. Elevated plus-maze test
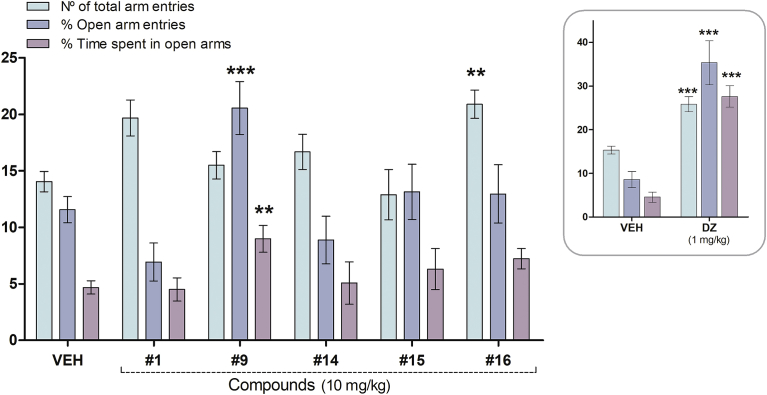
This test is based on the natural aversion of mice to open and elevated areas, as well as on their natural spontaneous exploratory behaviour in novel environments. This assay allows the identification of the anxiolytic-like effects of benzodiazepine-like drugs after their administration in rodents [32]. Chalcones with Ki values less than 6 μM in the BDZ-bs radioligand assay; 1, 9, 14, 15 and 16; were chosen to be tested in this assay. First, mice were tested 30 min after their i.p. injection with 10 mg/kg of the each chalcone (Fig. 2).
Fig. 2.
Effect of the i.p. administration of chalcone (1), 5′-methyl-2′-hydroxychalcone (9), 4′-aminochalcone (14), 4′-methylchalcone (15), 3-nitrochalcone (16) (10 mg/kg) and DZ (1 mg/kg, inset) in mice in the plus maze test. Results are expressed as mean ± S.E.M. of total arm entries, percentage of open arm entries and percentage of time spent in open arms; registered in 5 min sessions. The symbols denote significance levels: **P < 0.01, ***P < 0.001, significantly different from vehicle (VEH); Dunnett's multiple comparison test after one-way ANOVA (n = 6–32 mice per group).
ANOVA indicated a significant effect on the number of total arm entries [F(5,80) = 3.618, P = 0.0050], the percentage of open arm entries [F(5,80) = 5.074, P = 0.0005] and in the time spent in those entries [F(5,80) = 3.113, P = 0.0131]. Comparisons between the VEH control group and experimental groups (Dunnett's test) indicated that compound 9 significantly increased both the percentage of open arm entries and the time spent in open arms (P < 0.001 and P < 0.01, respectively), so this compound presented anxiolytic-like activity at this dose. Meanwhile, compound 16 significantly increased the number of total arm entries (P < 0.001).
Alternatively, mice i.p. injected with DZ (1 mg/kg), a classical benzodiazepine ligand used as a reference compound, showed a significant increase in the three parameters (P < 0.001, Fig. 2, inset).
The results revealed that chalcones 1, 14 and 15 did not exert significant effects at this dose, as neither of the measured parameters were statistically different compared to those of the control group.
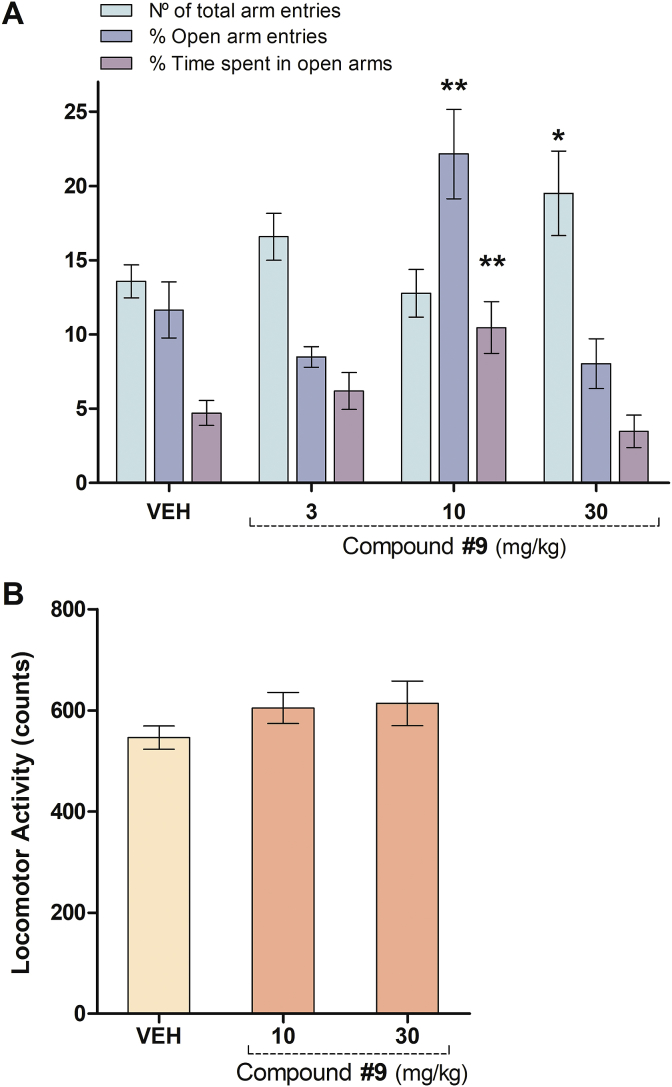
The effect of 5′-methyl-2′-hydroxychalcone (9) in this test was further evaluated at 3, 10 and 30 mg/kg (Fig. 3A). ANOVA indicated a significant effect on the number of total arm entries [F(3,48) = 2.887, P = 0.0459], the percentage of open arm entries [F(3,48) = 6.439, P = 0.0010] and in the time spent in those entries [F(3,48) = 5.688, P = 0.0022]. Mice administered with compound 9 at 10 mg/kg and 30 mg/kg had significantly increased the percentage of open arm entries and the time spent in open arms (P < 0.01, Dunnett's test) and the total arm entries (P < 0.05, Dunnett's procedure) (Fig. 2A), compared to control group, respectively.
Fig. 3.
Effect of the i.p. injection of 5′-methyl-2′-hydroxychalcone (9) in A) the plus-maze (3, 10 and 30 mg/kg) and B) locomotor activity tests (10 and 30 mg/kg) in mice. Results are expressed as mean ± S.E.M. of A) total arm entries, percentage of open arm entries and percentage of time spent in open arms and B) spontaneous locomotor activity counts; registered in 5 min sessions. The symbols denote significance levels: *P < 0.05, **P < 0.01, significantly different from vehicle (VEH); Dunnett's multiple comparison test after one-way ANOVA (n = 7–21 mice per group).
Considering that the parameters measured in the elevated plus-maze test depend on locomotion [41], the effect of the acute administration chalcone 9 in the spontaneous locomotor activity of mice was evaluated in a separate method (Fig. 3B). The results evidenced that animals injected with chalcone 9 at 10 and 30 mg/kg did not show significant differences compared with the control group in the locomotor activity test ([F(2,34) = 1.618, P = 0.2140], Fig. 3B). Therefore, the changes in the parameters measured in the elevated plus-maze test should not be attributed to changes in mice locomotion, but they should be considered an anxiolytic-like effect of compound 9 at 10 mg/kg.
Even though isoliquiritigenin and butein (Table 1), natural chalcones, showed anxiolytic-like effect in the plus-maze test [42], 5′-methyl-2′-hydroxychalcone (9) is the first synthetic chalcone described as anxiolytic with affinity for the BDZ-bs of the GABAA receptor.
3.3.2. Tail suspension test
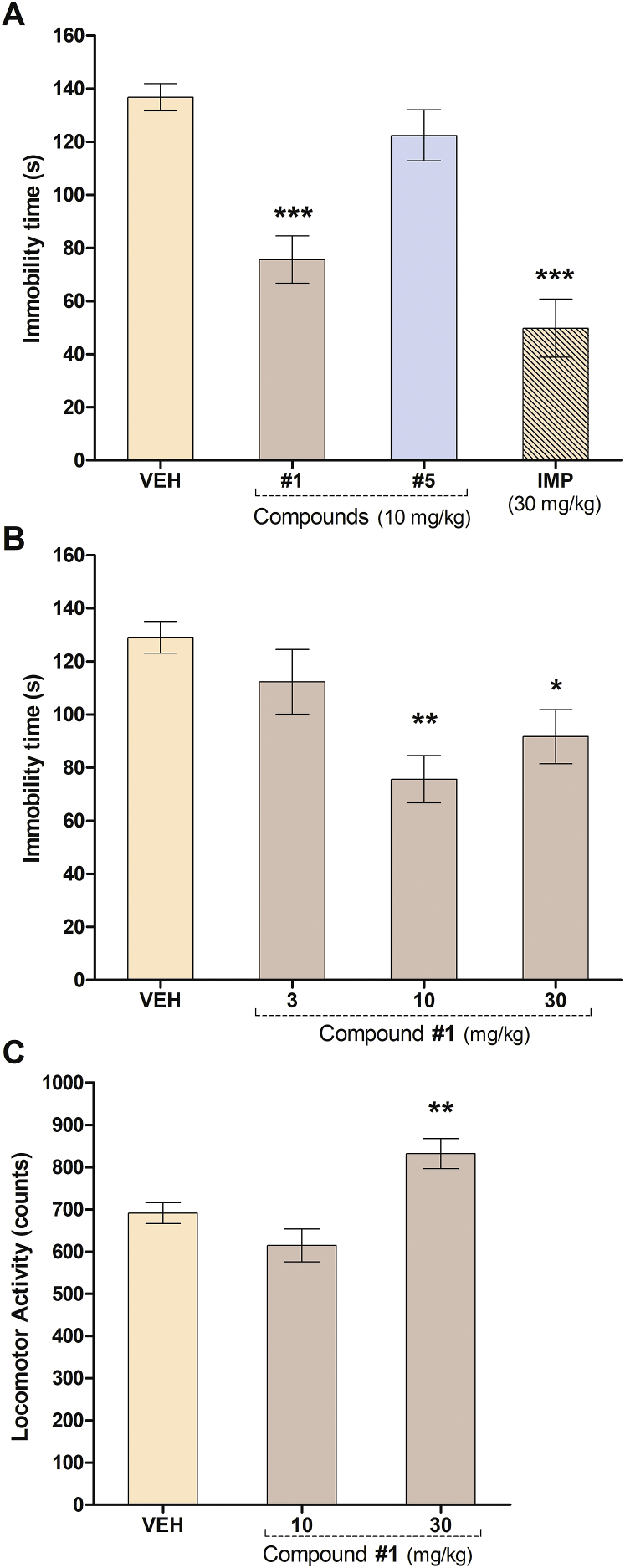
The tail suspension test is one of the most widely used model for assessing antidepressant-like activity in mice. This test is based on the fact that animals subjected to the short-term, inescapable stress of being suspended by their tail, will develop an immobile posture. Several antidepressant medications reverse the immobility time and promote the occurrence of escape-related behaviour [7]. Also, there is accumulating evidence for a critical involvement of serotonergic receptors, particularly 5-HT1A, in the pathophysiology and treatment of depression [10, 43, 44, 45]. Several 5-HT1A agonists decrease the duration of immobility in the in the tail suspension test [46]. Considering the role of the 5-HT1A receptor in the antidepressant-like activity in rodents and humans [47], the potential antidepressant-like effect of chalcones with the higher affinities for the 5-HT1A receptor, chalcones 1 and 5 (Ki values = 20.8 ± 1.5 μM and 13.3 ± 0.3 μM, respectively), were evaluated in mice in the tail suspension test, 30 min after their i.p. injection at the dose of 10 mg/kg (Fig. 4A). ANOVA analysis evidenced statically differences for the immobility time within experimental groups [F(3,45) = 27.21, P < 0.0001, Fig. 4A]. The comparison between the VEH control group and experimental groups by the Dunnett's test indicated that chalcone nuclei (1) at 10 mg/kg (P < 0.001) and IMP (a reference compound, a tricyclic antidepressant mainly acting as a norepinephrine uptake blocker) at 30 mg/kg (P < 0.001) significantly reduced the immobility time of mice. In contrast, compound 5 (10 mg/kg) was not active in this test (Fig. 4A).
Fig. 4.
Effect of acute administration of chalcone (1), 6′-methoxy-2′-hydroxychalcone (5) and IMP (reference drug) in the tail suspension test in mice. A) Effect of i.p. injection of chalcone (1), 6′-methoxy-2′-hydroxychalcone (5) (10 mg/kg) and IMP (30 mg/kg) (n = 8–21 mice per group). B) Effect of chalcone 1 (3, 10 and 30 mg/kg) (n = 8–11 mice per group). C) Effect of chalcone 1 (10 and 30 mg/kg) in the locomotor activity tests in mice (n = 6–13 mice per group). Results are expressed as mean ± S.E.M. of the immobility time (in s) and spontaneous locomotor activity counts; registered in 5 min sessions. Statistical analysis was performed by one-way ANOVA followed by Dunnett's test. ***P < 0.001, *P < 0.05, **P < 0.01, significantly different from vehicle (VEH).
The effect of compound 1 at different i.p. doses (3, 10 and 30 mg/kg) is shown in Fig. 4B. The parameter measured in this test yielded statistically significant differences in ANOVA analysis [F(3,38) = 6.469, P < 0.0013]. The comparison between the VEH control group and experimental groups by the Dunnett's test indicated that compound 1 at 10 mg/kg and 30 mg/kg (P < 0.001, P < 0.05, respectively) significantly reduced the immobility time of mice compared to control group. To avoid false positive results, the spontaneous locomotor activity test was performed at these doses as a control (Fig. 4C). Compound 1 at 30 mg/kg evidenced a significant increase of the locomotor activity of mice [F(2,22) = 9.131, P = 0.0013]. In this case, other factors, such as psychostimulant effects, could have affected mice performance in the tail suspension test at 30 mg/kg. Therefore, compound 1 only revealed antidepressant-like activity at 10 mg/kg.
Even though some authors have used the chalcone scaffold to synthesize novel derivatives with a similar antidepressant-like activity, as the one of fluoxetine (selective serotonin reuptake inhibitor (SSRI) class) in the tail suspension and forced swimming tests (Table 1), the effect of this nucleus per se has not been reported in any of these studies [16, 18].
The study of the mechanism of action of the reported 3-bromo-2′,4′-dihydroxychalcone and 2,6-dichloro -2′,4′-dihydroxychalcone (Table 1) showed that they significantly increased head-twitches in mice and enhanced the mouse lethality in the 5-HT induced head-twitch and yohimbine-induced mortality test. These results indicate that the serotonergic, but not the noradrenergic system, is involved in the antidepressant-like effect of these compounds [16].
On the other hand, some reports also revealed that several flavonoids may exert their antidepressant effect by increasing the bioamine content, thereby restraining the reuptake of bioamines by synaptosomes or by inhibiting monoamine oxidases (MAO) activities [48]. For the chalcone nucleous itsef it was already reported an IC50 value of 1.41 ± 0.070 μM for the hMAO B and no effect for the hMAO A enzymes [49]. So, future experiments should be performed to determine if the mechanism of action of the chalcone nuclei for the antidepressant effect relies just on the binding to 5-HT1A receptors, or whether other mechanisms are needed to develop this effect.
3.3.3. Writhing and hot plate tests
Pain remains a significant clinical problem and μ-opioid receptor agonists, such as hydrocodone and morphine, are still the most widely used treatments for moderate to severe pain. However, the use of opioids to treat pain is limited by unwanted effects, such as constipation, respiratory depression, tolerance, and dependence. These side effects also diminish patient's quality of life, decrease compliance, and are particularly problematic in long-term chronic users. These limitations of chronic opioid therapy have spurred drug discovery efforts to develop new analgesic drugs with an improved therapeutic profile.
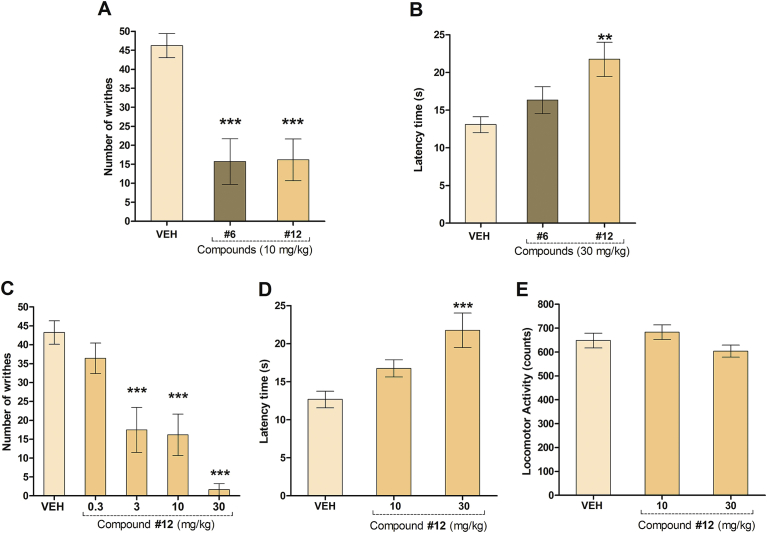
The antinociceptive activity of the synthesized chalcones with Ki values under 20 μM towards μ-opioid receptors, chalcones 6 and 12, was evaluated against chemical and thermal noxious stimuli. First, the acetic acid-induced writhing was performed, as it is a classical model for the assessment of analgesic or anti-inflammatory properties of new agents. This is one of the most sensitive methods, which is able to detect antinociception of nonsteroidal anti-inflammatory drugs (NSAIDs), narcotics and other centrally acting drugs [24]. The effect of compounds 6 and 12 at the dose of 10 mg/kg in this assay is shown in Fig. 5A. ANOVA of the results obtained yielded statistically significant differences in the number of abdominal cramps [F(2,26) = 17.55, P < 0.0001]. The comparison between the VEH control group and experimental groups by the Dunnett's test indicated that both treated groups significantly reduced the number of writhes (P < 0.001).
Fig. 5.
Antinociceptive effects of 5′-chloro-2′-hydroxychalcone (6) and 5′-methyl-2′-hydroxy-3′-nitrochalcone (12) in mice. A) Effect of the i.p. injection of chalcone 6 and 12 (10 mg/kg) on the acetic acid induced writhing (n = 6–14 mice per group). B) Effect of the i.p. injection of chalcone 6 and 12 (30 mg/kg) on the hot plate tests (n = 6–12 mice per group). Effect of acute administration of 5′-methyl-2′-hydroxy-3′-nitrochalcone (12) in C) the acetic acid induced writhing (0.3, 3, 10 and 30 mg/kg) (n = 6–16 mice per group); D) the hot plate (10 and 30 mg/kg) (n = 8–12 mice per group) and E) locomotor activity tests (10 and 30 mg/kg) in mice (n = 7–8 mice per group). Results are expressed as mean ± S.E.M. of the number of writhes, the reaction time of mice in the hot plate test and spontaneous locomotor activity counts in comparison to control animals (injected with vehicle, VEH). Statistical analysis was performed by one-way ANOVA followed by Dunnett's test. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the control group.
On the other hand, in the hot plate test, a short thermal stimulus is employed. The behavioural responses measured are considered to be supraspinally integrated responses, so it is suitable for the evaluation of centrally but not of peripherally acting antinociceptive drugs. Compounds exhibiting good antinociceptive effect in this method may be considered as potent analgesics. The effect of compounds 6 and 12 at the dose of 30 mg/kg in this assay is shown in Fig. 5B. Only chalcone 12 at 30 mg/kg caused significant increases in the reaction time of mice against thermal noxious stimulus [F(2,25) = 7.802, P = 0.0026], indicating that only this compound may act by a central mechanism of action. Mice i.p. injected with morphine (MOR) (6 mg/kg), a classical μ opioid receptor and the reference compound, showed no abdominal cramps (data not shown) and displayed a higher latency time in the hot plate assay (latency time = 42.6 s ± 4.2 s).
Anti-inflammatory activities of chalcones remain first in the list of published biological active analogs [50], thus its effects in the writhing test is not unexpected. A number of chalcones and their derivatives have been described as inhibitors of the synthesis of nitric oxide and prostaglandins [2, 3]. Although there is a consistent evidence of chalcones as peripheral analgesic drugs (see Table 1), little is known about their centrally antinociceptive action and the involvement of the opioid system. Only few chalcone derivatives were reported as central analgesic acting drugs in chemical and thermal nociception models in mice (see Table 1 and [23, 24, 51]). Their mechanism of action is not well established as the pretreatment with a non-selective opioid receptor antagonist, naloxone, only reversed the antinociceptive effect of the rigid 3,4-dihydroxychalcone [51] and the natural dimeric chalcones extracted from Myracrodruon urundeuva [23]. In contrast, our study is focused on simple synthetic chalcones as ligands for μ-opioid receptors that evidenced central antinociceptive activity.
The antinociceptive effect of compound 12 was further evaluated at 0.3, 3, 10 and 30 mg/kg in the acetic acid-induced writhing (Fig. 5C) and at 10 and 30 mg/kg in the hot plate test (Fig. 5D). ANOVA evidenced statistically significant differences in the number of abdominal cramps [F(4,44) = 21.48, P < 0.0001, Fig. 5C] and in the reaction time of mice against the thermal stimulus [F(2,27) = 9.628, P = 0.0008, Fig. 5D]. The results revealed that the doses of 3, 10 and 30 mg/kg significantly reduced the number of writhes with 59.7%, 62.6%, 96.1% inhibition of constrictions compared to VEH control group (P < 0.0001, Dunnett's test).
The comparison of the latency time in the hot plate test between the VEH control group and experimental groups by the Dunnett's test indicated that the dose of 30 mg/kg significantly increased the latency time of mice in this assay (P < 0.001).
Lastly, in order to discard any motor abnormalities that could interfere with the parameters measured in the writhing and hot plate tests, the spontaneous locomotor activity (Fig. 5E) and the balance on the Rotarod assay were measured for chalcone 12.
None of the mice treated with chalcone 12 showed any failure in their locomotion (Fig. 5E) or to maintain balance on the Rotarod assay (data not shown), evidencing that this chalcone did not produce neurotoxicity or motor impairment, at the doses tested. Therefore, the experimental results presented in this work correspond to genuine antinociceptive effect, as they were not affected by motor abnormalities.
On the other hand, it is important to stress here that none of the chalcone treated animals tested in this work died during the assays.
According to these results, it can be concluded that the antinociceptive activity of chalcone 12 may occur by central and peripheral mechanisms. Opioids are known to show analgesic activities in both hot plate and writhing tests by acting on central and peripheral nociceptive pathways, respectively [52]. Therefore, chalcones scaffold should be also considered for the development of centrally acting antinociceptive drugs.
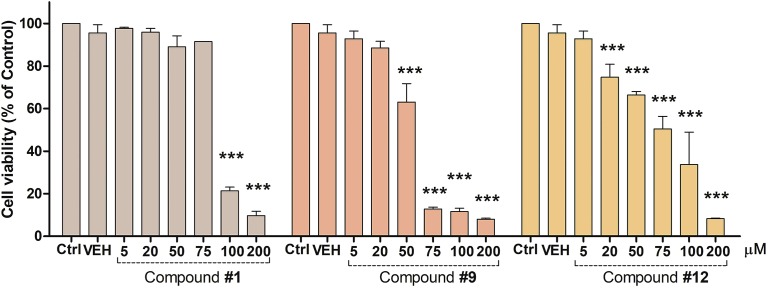
3.3.4. Cell viability assay on the SH-SY5Y cell line
The absence of adverse effects on a cell line of chalcones 1, 9 and 12 was assessed using a cell viability assay on SH-SY5Y human neuroblastoma cell line (Fig. 6).
Fig. 6.
Cell viability of cultured SH-SY5Y cells. Cells were treated with different concentrations of compounds 1, 9 and 12 up to 200 μM for 48 h. Cell viability was quantified by measuring the activity of the endogenous enzyme hexosaminidase. Results are expressed as mean ± S.E.M. of Cell viability (% of Control) performed in triplicate. The control (Ctrl) group was considered as 100% cell viability. ***P < 0.001 versus vehicle (VEH, DMSO).
After treatment for 48 h, chalcone 1 did not affect the cell viability up to 75 μM, compound 9 did not modify the cell viability up to 20 μM meanwhile derivative 12 decreased the cell viability at 20 μM or higher. The active compounds 1, 9 and 12 exhibited 95.9%, 88.5% and 74.8% cell viability at 20 μM, respectively, suggesting that these compounds did not significantly reduce cell viability.
3.3.5. Blood–brain barrier permeability assay
As we are interested in developing compounds with activity towards cerebral receptors, it is crucial that they can reach its targets. Fundamental physiochemical features of CNS drugs are related to their ability to penetrate the BBB and exhibit CNS activity. In the broadest sense, moderately lipophilic drugs cross the BBB by passive diffusion. Polar molecules are generally poor CNS agents unless they undergo active transport across the CNS [53]. As summarized in Table 2, cLog P values for compounds 1–21 were above 2; therefore, it can be estimated that they are capable to cross the BBB and reach its target. Furthermore, BBB permeability of the compounds 1, 9 and 12 was assessed using the parallel artificial membrane permeability assay (PAMPA-BBB) [54]. Five commercial drugs were used as references to validate the experimental system and establish the permeability range (-logPe of 7.2–4.6). Traditional pampa binning was used as follows; maxPe > 2∗10−6 cm/s designated as high permeability, maxPe < 0.5∗10−6 cm/s designated as low permeability and intermediate as uncertain. According to the results summarized in Table 4, all off the tested compounds should be able to cross BBB and reach CNS.
Table 4.
In vitro blood-brain barrier permeability (PAMPA-BBB assay) prediction for commercial drugs and the selected chalcones 1, 9 and 12.
| Compounds | BBB penetration estimation |
|
|---|---|---|
| -logPe (cm/s) Mean ± SEM |
Predictionc | |
| chalcone (1)a | 4.58 ± 0.01 | CNS+ |
| 5′-methyl-2′-hydroxychalcone (9)a | 4.60 ± 0.04 | CNS+ |
| 5′-methyl-2′-hydroxy-3′-nitrochalcone (12)a | 4.47 ± 0.04 | CNS+ |
| sulfasalazinb | 7.13 ± 0.04 | CNS− |
| haloperidolb | 4.67 ± 0.01 | CNS+ |
| propranololb | 4.65 ± 0.01 | CNS+ |
| lidokainb | 5.14 ± 0.03 | CNS+ |
| risperidonb | 4.81 ± 0.005 | CNS+ |
Data are means of four replicates (n = 4).
Data are means of three replicates (n = 3).
CNS+, -logPe < 5.6, high permeability; CNS–, -logPe > 6.3, low permeability; intermediate was labelled as uncertain BBB permeability.
4. Conclusions
In conclusion, a series of 20 chalcone derivatives were synthesized by condensation of the corresponding acetophenone and aromatic aldehydes with moderate to high yields. Some of the synthesized compounds showed affinities in the low micromolar range towards the BDZ-bs of the GABAA receptor and moderate to no affinity for μ-opioid and 5-HT1A receptors. The most active compounds for each receptor were selected for further in vivo studies. Consequently, chalcones 1, 9, 14, 15 and 16 with good affinity for the BDZ-bs of the GABAA receptor, chalcones 1 and 5 with affinity for the 5-HT1A receptor and compounds 6 and 12 for the μ-opioid receptor were chosen to be tested as anxiolytics, antidepressants and antinociceptive drugs in widely used pharmacological tests in mice. The results showed that chalcone 9 (5′-methyl-2′-hydroxychalcone) exerted anxiolytic-like effects in the plus maze assay; chalcone 1 (chalcone nuclei) presented antidepressant-like activity in mice in the tail suspension test; compound 6 (5′-chloro-2′-hydroxychalcone) exhibited antinociceptive action in an acute chemical induce nociception assay, meanwhile the novel 5′-methyl-2′-hydroxy-3′-nitrochalcone (12) revealed peripheral and central antinociceptive activities either in acute chemical and thermal nociception tests. Furthermore, all the pharmacological effects observed were not influenced by motor abnormalities. Additionally, compounds 1, 9 and 12 did not reduce cell viability of SH-SY5Y human neuroblastoma cell line and displayed the potential to cross the BBB as demonstrated by PAMPA-BBB assay.
According to the results summarized simple chalcone derivatives are promising compounds for the development of novel CNS drugs and comprise a promising scaffold in medicinal chemistry for the development of drugs for the treatment of anxiety, depression and pain.
Declarations
Author contribution statement
Josefina Higgs: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Cristina Wasowski: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Carlos Humberto Paván: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Alejandra Marcos, Felicitas de Tezanos Pinto, Marko Jukič, Stanislav Gobec: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Natalia Colettis: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mariel Marder: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP Number 112 201501 00410), Universidad de Buenos Aires (UBA, UBACyT Number 20020150100012BA), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICT Number 2011-0328), Argentina
Competing interest statement
The authors declare no conflict of interest.
Additional information
Data associated with this study has been deposited at PubChem under the accession numbers CID: 637760 and CID: 5708670
Acknowledgements
Travels and stays were carried out through the International Cooperation Program of the Ministerio de Ciencia, Tecnología e Innovación Productiva de la República Argentina (MINCYT) and the Ministry of Higher Education, Science and Technology of Slovenia (MHEST) Number SLO/14/03, Argentina/Slovenia.
References
- 1.Matos M.J., Vazquez-Rodriguez S., Uriarte E., Santana L. Potential pharmacological uses of chalcones: a patent review (from June 2011 – 2014) Expert Opin. Ther. Pat. 2015;25:351–366. doi: 10.1517/13543776.2014.995627. [DOI] [PubMed] [Google Scholar]
- 2.Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007;42:125–137. doi: 10.1016/j.ejmech.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 3.de Campos-Buzzi F., Padaratz P., Meira A.V., Corrêa R., Nunes R.J., Cechinel-Filho V. 4’-Acetamidochalcone derivatives as potential antinociceptive agents. Molecules. 2007;12:896–906. doi: 10.3390/12040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips C.J. Economic burden of chronic pain. Expert Rev. Pharmacoecon. Outcomes Res. 2006;6:591–601. doi: 10.1586/14737167.6.5.591. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Global Health Estimates; Geneva, Switzerland: 2017. Depression and Other Common Mental Disorders. [Google Scholar]
- 6.Gustorff B., Simoens S., Tourné J., Michelet D., Mikkonen L., Krohn M., Santalahti A., Crespo M., Serrie A., Laroche F., Carton L., Kletzko H., Kohlmann T., Sittl R., Kouvelas D., Vadalouka A., Athanasakis K., Bahill M., Canroy L., Church J., Spandonaro F., Nardi S., Fanelli G., Kuin M., van der Heide R., van de Laar M., Kuijpens D., Haavik P.E., Breivik H., Goncalves S., Castro-Lopes J.M., Romao J.M.S., Macario Paiva M. da L., Darba J., Miquel A., Perucho A., Torralba A., Bagge J., Gustavsson A., Norreflak J.-R., Desmeules J., Alon E., MAcak A., Piguet V., Semmons I., Betteridge N. 2010. Pain Proporsal: Improving the Current and Future Management of Chronic Pain. A European Consensus Report. [Google Scholar]
- 7.Cryan J.F., Mombereau C., Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Milano J., Rossato M.F., Oliveira S.M., Drewes C., Machado P., Beck P., Zanatta N., Martins M.A., Mello C.F., Rubin M.A., Ferreira J., Bonacorso H.G. Antinociceptive action of 4-methyl-5-trifluoromethyl-5-hydroxy-4, 5-dihydro-1H-pyrazole methyl ester in models of inflammatory pain in mice. Life Sci. 2008;83:739–746. doi: 10.1016/j.lfs.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Marder M. Flavonoids as GABAA receptor ligands: the whole story? J. Exp. Pharmacol. 2012;4:9. doi: 10.2147/JEP.S23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celada P., Bortolozzi A., Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs. 2013;27:703–716. doi: 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- 11.Waldhoer M., Bartlett S.E., Whistler J.L. Opioid receptors. Annu. Rev. Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 12.Cho S., Kim S., Jin Z., Yang H., Han D., Baek N.I., Jo J., Cho C.W., Park J.H., Shimizu M., Jin Y.H. Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABA A receptors and shows hypnotic effects. Biochem. Biophys. Res. Commun. 2011;413:637–642. doi: 10.1016/j.bbrc.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Higgs J., Wasowski C., Loscalzo L.M., Marder M. In vitro binding affinities of a series of flavonoids for μ-opioid receptors. Antinociceptive effect of the synthetic flavonoid 3,3-dibromoflavanone in mice. Neuropharmacology. 2013;72:9–19. doi: 10.1016/j.neuropharm.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Jamal H., Ansari W.H., Rizvi S.J. Evaluation of chalcones - a flavonoid subclass, for, their anxiolytic effects in rats using elevated plus maze and open field behaviour tests. Fundam. Clin. Pharmacol. 2008;22:673–681. doi: 10.1111/j.1472-8206.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang W., Hu X., Zhao Z., Liu P., Hu Y., Zhou J., Zhou D., Wang Z., Guo D., Guo H. Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1179–1184. doi: 10.1016/j.pnpbp.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Guan L.P., Zhao D.H., Chang Y., Wen Z.S., Tang L.M., Huang F.F. Synthesis of 2,4-dihydroxychalcone derivatives as potential antidepressant effect. Drug Res. (Stuttg). 2013;63:46–51. doi: 10.1055/s-0032-1333229. [DOI] [PubMed] [Google Scholar]
- 17.Guan L.P., Tang L.M., Pan C.Y., Zhao S.L., Wang S.H. Evaluation of potential antidepressant-like activity of chalcone-1203 in various murine experimental depressant models. Neurochem. Res. 2014;39:313–320. doi: 10.1007/s11064-013-1224-8. [DOI] [PubMed] [Google Scholar]
- 18.Sui X., Quan Y.C., Chang Y., Zhang R.P., Xu Y.F., Guan L.P. Synthesis and studies on antidepressant activity of 2′,4′,6′-trihydroxychalcone derivatives. Med. Chem. Res. 2012;21:1290–1296. [Google Scholar]
- 19.Corrêa R., Pereira M.A., Buffon D., dos Santos L., Cechinell Filho V., Santos A.R., Nunes R.J. Antinociceptive properties of chalcones. Structure-activity relationships. Arch. Der Pharm. 2001;334 doi: 10.1002/1521-4184(200110)334:10<332::aid-ardp332>3.0.co;2-o. 332-324. [DOI] [PubMed] [Google Scholar]
- 20.de Campos-Buzzi F., Pereira de Campos J., Pozza Tonini P., Corrêa R., Augusto Yunes R., Boeck P., Cechinel-Filho V. Antinociceptive effects of synthetic chalcones obtained from xanthoxyline. Arch. der Pharm. 2006;339:361–365. doi: 10.1002/ardp.200600049. [DOI] [PubMed] [Google Scholar]
- 21.Cechinel-Filhol V., Y.R., Vaz Z.R., Zunino L., Calixto J.B. Synthesis of xanthoxyline derivatives with antinociceptive and antioedematogenic activities. Eur. J. Med. Chem. 1996;31:833–839. [Google Scholar]
- 22.Corrêa R., Fenner B.P., Buzzi F.D.C., Cechinel Filho V., Nunes R.J. Antinociceptive activity and preliminary structure-activity relationship of chalcone-like compounds. Zeitschrift für Naturforschung. C, J. Biosci. 2008;63:830–836. doi: 10.1515/znc-2008-11-1208. [DOI] [PubMed] [Google Scholar]
- 23.Viana G.S.B., Bandeira M. a M., a Matos F.J. Analgesic and antiinflammatory effects of chalcones isolated from Myracrodruon urundeuva allemão. Phytomedicine. 2003;10:189–195. doi: 10.1078/094471103321659924. [DOI] [PubMed] [Google Scholar]
- 24.Mohamad A.S., Akhtar M.N., Zakaria Z.A., Perimal E.K., Khalid S., Mohd P.A., Khalid M.H., Israf D.A., Lajis N.H., Sulaiman M.R. Antinociceptive activity of a synthetic chalcone, flavokawin B on chemical and thermal models of nociception in mice. Eur. J. Pharmacol. 2010;647:103–109. doi: 10.1016/j.ejphar.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Angela L., Nesello N., Campos A., Wagner T. Chemical composition and antinociceptive potential of campomanesia reitziana fruits. J. Med. Food. 2016;19:518–520. doi: 10.1089/jmf.2015.0092. [DOI] [PubMed] [Google Scholar]
- 26.Kachadourian R., Day B.J., Pugazhenti S., Franklin C.C., Genoux-Bastide E., Mahaffey G., Gauthier C., Di Pietro A., Boumendjel A. A synthetic chalcone as a potent inducer of glutathione biosynthesis. J. Med. Chem. 2012;55:1382–1388. doi: 10.1021/jm2016073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford M.M. A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Medina J.H., Paladini A.C., Wolfman C., Levi de Stein M., Calvo D., Diaz L.E., Pena C. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem. Pharmacol. 1990;40:2227–2231. doi: 10.1016/0006-2952(90)90716-x. [DOI] [PubMed] [Google Scholar]
- 29.Pastore V., Wasowski C., Enrique A., Higgs J., Luis E., Milesi V., Marder M., Pastore V., Wasowski C., Martin P., Higgs J., Bruno-blanch L.E., Milesi V. N-propyl-2,2-diphenyl-2-hydroxyacetamide, a novel α-hydroxyamide with anticonvulsant, anxiolytic and antidepressant-like effects that inhibits voltage-gated sodium channels. Eur. J. Pharmacol. 2017;819:270–280. doi: 10.1016/j.ejphar.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 30.Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J. lmmunol. Methods. 1984;67:379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 31.Avdeef A. second ed. John Wiley & Sons; 2012. Absorption and Drug Development: Solubility, Permeability, and Charge State. [Google Scholar]
- 32.Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacol. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 33.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 34.Koster R., Anderson M., De Beer E.J. Acetic acid analgesic screening, Fed. Proc. 1959;18:412–417. [Google Scholar]
- 35.Carter R.B. Differentiating analgesic and non-analgesic drug activities on rat hot plate: effect of behavioral endpoint. Pain. 1991;47:211–220. doi: 10.1016/0304-3959(91)90207-E. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez S.P., Wasowski C., Loscalzo L.M., Granger R.E., Johnston G.A., Paladini A.C., Marder M. Central nervous system depressant action of flavonoid glycosides. Eur. J. Pharmacol. 2006;539:168–176. doi: 10.1016/j.ejphar.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Karl T., Pabst R., von Hörsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. Off. J. Gesellschaft Für Toxikologische Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- 38.Regan J.W., Yamamura H.I., Yamada S., Roeske W.R. High affinity renal [3H]flunitrazepam binding: characterization, localization, and alteration in hypertension. Life Sci. 1981;28:991–998. doi: 10.1016/0024-3205(81)90744-x. [DOI] [PubMed] [Google Scholar]
- 39.Arro A., Uustare A., Harro J., Rinken A. Modulation of [3H] 8-OHDPAT binding to rat brain membranes by metal ions. Proc. Est. Acad. Sci. 2001;50:28–38. [Google Scholar]
- 40.Zernig G., Burke T., Lewis J.W., Woods J.H. Mechanism of clocinnamox blockade of opioid receptors: evidence from in vitro and ex vivo binding and behavioral assays. J. Pharmacol. Exp. Ther. 1996;279:23–31. [PubMed] [Google Scholar]
- 41.Dawson G.R., Crawford S.P., Collinson N., Iversen S.D., Tricklebank M.D. Evidence that the anxiolytic-like effects of chlordiazepoxide on the elevated plus maze are confounded by increases in locomotor activity. Psychopharmacol. 1995;118:316–323. doi: 10.1007/BF02245961. [DOI] [PubMed] [Google Scholar]
- 42.Jamal H., Ansari W.H., Rizvi S.J. Evaluation of chalcones-a flavonoid subclass, for, their anxiolytic effects in rats using elevated plus maze and open field behaviour tests. Fundam. Clin. Pharmacol. 2008;22:673–681. doi: 10.1111/j.1472-8206.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 43.Hensler J.G. Differential regulation of 5-HT 1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology. 2002;26:565–573. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- 44.Middlemiss D.N., Price G.W., Watson J.M. Serotonergic targets in depression. Curr. Opin. Pharmacol. 2002;2:18–22. doi: 10.1016/s1471-4892(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 45.Olivier B., Soudijn W., van Wijngaarden I. The 5-HT1A receptor and its ligands: structure and function. In: Jucker E., editor. Prog. Drug Res. Birkhäuser Basel; Basel: 1999. pp. 103–165. [DOI] [PubMed] [Google Scholar]
- 46.Castagné V., Moser P., Roux S., Porsolt R.D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011;55 doi: 10.1002/0471142301.ns0810as55. 8.10A.1-8.10A.14. [DOI] [PubMed] [Google Scholar]
- 47.Millan M.J. Improving the treatment of Schizophrenia : focus on serotonin (5-HT)1A receptors. J. Pharmacol. Exp. Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- 48.Gong J., Huang J., Ge Q., Chen F., Zhang Y. Advanced research on the antidepressant effect of flavonoids. Curr. Opin. Complement. Altern. Med. 2014;1:1–6. [Google Scholar]
- 49.Chimenti F., Fioravanti R., Bolasco A., Chimenti P., Secci D., Rossi F., Yanez M., Orallo F., Ortuso F., Alcaro S. Chalcones: a valid scaffold for monoamine oxidases inhibitors. J. Med. Chem. 2009;52:2818–2824. doi: 10.1021/jm801590u. [DOI] [PubMed] [Google Scholar]
- 50.Katsori A.M., Hadjipavlou-Litina D. Recent progress in therapeutic applications of chalcones. Expert Opin. Ther. Pat. 2011;21:1575–1596. doi: 10.1517/13543776.2011.596529. [DOI] [PubMed] [Google Scholar]
- 51.Heidari M.R., Foroumadi A., Amirabadi A., Samzadeh-Kermani A., Azimzadeh B.S., Eskandarizadeh A. Evaluation of anti-inflammatory and analgesic activity of a novel rigid 3, 4-dihydroxy chalcone in mice. Ann. N. Y. Acad. Sci. 2009;1171:399–406. doi: 10.1111/j.1749-6632.2009.04904.x. [DOI] [PubMed] [Google Scholar]
- 52.Kaplancikli Z.A., Turan-Zitouni G., Zdemir Ö., Can Ö.D., Chevallet P. Synthesis and antinociceptive activities of some pyrazoline derivatives. Eur. J. Med. Chem. 2009;44:2606–2610. doi: 10.1016/j.ejmech.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Pajouhesh H., Lenz G.R. Medicinal chemical properties of successful central nervous system drugs. Neuro Rx J. Am. Soc. Exp. Neurother. 2005;2:541–553. doi: 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di L., Kerns E.H., Fan K., Mcconnell J., Carter G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003;38:223–232. doi: 10.1016/s0223-5234(03)00012-6. [DOI] [PubMed] [Google Scholar]