Abstract
Background/objective
Although regular exercise plays a role in achieving healthy aging, a specific mode of exercise may be required for elderly individuals with hypertension (HT). Therefore, this study aimed to assess the effects of combined endurance and strength training (CBT) on blood pressure (BP) and antioxidant capacity in elderly individuals with HT.
Methods
In a single-blinded, randomized controlled trial, 54 older men and women aged 67 5.8 years completed endurance training (ET, n = 13), strength training (ST, n = 13), combined endurance and strength training (CBT, n = 16) or served as controls (CON, n = 12). The intervention was a supervised exercise training (1-h sessions, three per week for 12 weeks), followed by a self-supervised exercise training for 12 weeks. Measurements of BP, glutathione peroxidase (GPx), total nitrite/nitrate (NOx-), malondialdehyde (MDA), and high-sensitive C-reactive protein (hs-CRP) were obtained before and after the supervised and the self-supervised periods.
Results
After the supervised period, systolic BP (SBP) decreased by 7.9% in the ET (p 0.05) and 8.2% in the CBT (p 0.01); GPx activity increased by 41.3% in the ET (p 0.01), 19.1% in the ST (p 0.05), and 49.2% in the CBT (p 0.01); NOx-concentrations increased by 66.2% in the ET and 71.9% in the CBT (both p 0.01), MDA concentrations decreased by 65.1% in the ST (p 0.05) and 61% in the CBT (p 0.01); hs-CRP concentrations decreased by 49.2% in only the CBT (p 0.05). After the self-supervised period, SBP decreases by 7.5% in only the CBT (p 0.01); NOx-concentrations increased by 68.5% in the ET and 92.4% in the CBT (both p 0.01). However, there was no significant difference in SBP, GPx activity, NOx-, MDA and hs-CRP concentrations between the training groups.
Conclusion
The hypotensive and antioxidant effects of the CBT seem to be similar to the ET after the supervised training period. However, after the self-supervised training period, the CBT program might affect better due to greater exercise adherence and attendance in elderly individuals with HT.
Keywords: Aging, Antioxidant defense, Combined exercise training, Inflammation, Lipid peroxidation
Introduction
Oxidative stress is characterized by an imbalance between free radical production and antioxidant defenses and is implicated in the aging process.1 The age-associated accumulation of molecular and cellular damage is attributed to an increasing assault by reactive oxygen species (ROS) and/or a decline in antioxidant enzymes, especially, glutathione peroxidase (GPx).1,2 Oxidative stress increases with advancing age and is associated with the development of age-related diseases.3,4
Hypertension (HT) represents one of the most potentially modifiable diseases and the incidence of HT is the greatest among older adults.3 Growing evidence indicates that the link between inflammation/oxidative stress and HT appears to be endothelial dysfunction.3 Prolonged elevations in inflammatory mediators during aging contribute to chronic overproduction of ROS.5,6 Together with aged-related declines in nitric oxide (NO) production and bioavailability,7 these increase ROS production. The overproduction of ROS and the decreased efficiency of antioxidant defenses8 contribute to oxidative stress. Chronic inflammation and oxidative stress have been consistently documented as contributors to endothelial dysfunction,9,10 contributing directly to increased systemic vascular resistance, and therefore increased blood pressure (BP).
Regular physical activity plays a role in achieving healthy aging and contributes significantly to a longer life and well-being.11 Exercise and regular physical activity prevent the deleterious effects of aging, not only by inhibiting oxidative stress and inflammation, but also by exerting additional antioxidant and anti-inflammatory actions.12,13 Previous studies have reported that although exercise increases ROS generation, it is also able to upregulate the expression of antioxidant defense systems.14,15 However, considering that aging and being HT are already risk factors for inflammation and oxidative stress by themselves,1,3,16 adaptability of the organism gradually attenuates and this increases susceptibility to oxidative stress.16,17 It remains to be elucidated whether exercise can compensate for its ROS production in elderly individuals with HT.
Endurance training (ET) has been shown to be effective in a decrease of the occurrence of ROS-associated HT18 and exerts a protective effect on oxidative damage independently of age.19 However, older adults can have a perception that exercise is a tiring activity and can cause health and safety concerns.20 They may prefer an exercise session with moderate-intensity and short duration, which is more enjoyable than a prolonged approach.20,21 Consequently, although ET is of benefit for elderly individuals with HT,18 some reports have shown that it is quite boring to complete the entire session, causing eventually an abolishment.20,21
On the other hand, other studies have indicated that strength training (ST) provides a protective effect similar to ET.22, 23, 24 Significant decreases in systolic blood pressure (SBP)25 and in ROS26 thanks to ST in elderly individuals with HT have been reported. However, prescribing a ST program depends on demographic and health-related variables. Elderly individuals may have their own preferences as well.27 Moreover, the higher number of repetitions, the higher rating of perceived exertion.28,29
We hypothesized that combined endurance and strength training (CBT) may be suitable for elderly individuals with HT and has benefit of reducing BP and improving antioxidant capacity and oxidative stress. The CBT may also improve exercise adherence and reduce perceptual exercise barrier especially during self-supervised exercise. To our knowledge, a limited number have compared the effect of ET, ST, and CBT on antioxidant defenses and oxidative stress. An investigation in young adults has reported that the three training types induced the same changes in antioxidant capacity and oxidative stress, but at different rates.30 Nevertheless, another investigation in middle-aged adults with type II diabetes has reported that only the ET improves antioxidant capacity, NO bioavailability and oxidative stress.31
Therefore, we investigated the effects of a CBT program, compared with an ET program and a ST program, on BP, circulating antioxidants, oxidative stress and inflammatory marker in elderly individuals with HT.
Methods
Subjects and study design
Subjects recruited through advertisements were aged over 60 years, had community-dwelling status and underwent their annual routine health checkup at the 19th Somdet Pha Sangkharat Hospital in Thailand, during 2016–2017. Sixty-seven subjects aged 67.6 6.2 years (12 male and 55 female) with HT (SBP 130 mmHg or diastolic blood pressure (DBP) 80 mmHg) were eligible32 and presented physically independent functional status determined by responses to the 12-item of the Composite Physical Functioning Scale.33 They also presented sedentary behavior with sedentary time of 176.6 87.7 min per week, moderate physical activity of 41.4 21.3 min per week and no high physical activity, determined using Thai Version of the General Practice Assessment Questionnaire.34 In all subjects, general health status and resting electrocardiogram were examined by a physician.
Subjects were excluded if they had 1) unstable and/or uncontrolled cardiovascular diseases (CVD); 2) musculoskeletal limitations to physical exercise; 3) known cancer or limited life expectancy; 4) any psychiatric problems; 5) cognitive impairment with a dementia diagnosis; or 6) severe visual impairment. The use of statins, antihypertensive, and diabetes medications was permitted. The size of the sample was based on the ability to detect a medium effect (0.5) according to the previous report of Schaun et al.35 It was decided to require 80% power at a significance level of 0.05. Thus, having at least 12 subjects was required to finish this study. The study was conducted in accordance with the principles of the Declaration of Helsinki and a local hospital research ethics committee provided ethical approval (REC NO.1/59).
This study had a single-blinded, randomized controlled trial in which the subjects were blinded from the test results to reduce experimental bias. Following the initial assessments, the subjects were randomly divided into four groups: 1) an endurance training group (ET; n = 16, 93.8% females), 2) a strength training group (ST; n = 14, 100% females), 3) a combined endurance and strength training group (CBT; n = 17, 70.6% females), or 4) a control group (CON; n = 20, 70% females). Subject characteristics are presented in Table 1. They were matched for age, body mass index (BMI), and underlying diseases.
Table 1.
Characteristics of subjects.
| CON |
ET |
ST |
CBT |
|
|---|---|---|---|---|
| (n = 12) | (n = 13) | (n = 13) | (n = 16) | |
| Age (years) | 66.7 5.8 | 65.6 4.5 | 68 7.4 | 67.3 5.9 |
| Gender (male/female) | 5/7 | 1/12 | 0/13† | 5/11 |
| Height (cm) | 157.6 6.1 | 156.6 7.8 | 151.8 3.3 | 157.6 9.3 |
| Weight (kg) | 63.4 9.2 | 58.5 7.7 | 52.3 7.6∗ | 60.2 9.1 |
| BMI (kgm−2) | 25.6 4.3 | 23.8 2.4 | 22.6 2.6 | 24.1 2 |
| MMSE | 28.8 1.1 | 28.4 1.2 | 28.6 1.3 | 28.4 1.3 |
| Medication | ||||
| Antihypertensive‡ | 7 (58.3%) | 6 (46.2%) | 6 (46.2%) | 8 (50%) |
| Antihyperglycemia | 2 (16.7%) | 1 (7.7%) | 2 (15.4%) | 2 (12.5%) |
| Lipid-lowering drug therapy | 5 (41.7%) | 5 (38.5%) | 6 (46.2%) | 5 (31.3%) |
Data are means SD and n(%). CON, control group; ET, endurance training group; ST, strength training group; CBT, combined endurance and strength training group; BMI, body mass index; MMSE, Mini-Mental State Examination.
*p 0.05 vs. control group. for categorical variables.
†p 0.05 to between-group comparison.
‡Comprises hydrochlorothiazide (25 mg), propranolol (40 mg), captopril (25 mg), enalapril (10 mg), losartan (50 mg), and amlodipine (5 mg).
The intervention composed of 2 training periods: a supervised training period and a self-supervised training period. All subjects were asked to avoid commercial vitamins and other dietary supplements throughout the study. They were also asked to maintain their habitual activities with no exercise for one week before the intervention and at the end of each training period.
The measurements took place three times: 1) at the beginning before the intervention; 2) after the supervised training period; and 3) after the self-supervised training period. In order to avoid any potential acute effects of exercise on the outcome variables, all measurements were performed at least 48 h after the last exercise session. Blood samples were collected after a 12-h fast to measure plasma GPx, total nitrite/nitrate (NOx-), malondialdehyde (MDA), serum high-sensitive C-reactive protein (hs-CRP), lipid profiles, and blood glucose concentrations. Measurements for the body composition and BP were performed as well.
During the supervised training period, the training groups trained for 12 weeks (three sessions per week) at the 19th Somdet Pha Sangkharat Hospital Health Center. The exercise programs in this study were prescribed according to guidelines.36,37 All training sessions were supervised by experienced trainers. During the self-supervised training period, the training groups performed by themselves at the center and received weekly calls for 12 weeks to prompt and reinforce regular exercise. The calls involved assessment and feedback on exercise adherence, reinforcement, and motivation by positive role modeling regarding the benefits of exercise. If exercise adherence was under 80%, the subject would be advised on how to overcome their specific barriers. The CON subjects were asked to maintain their activity levels throughout the experimental period.
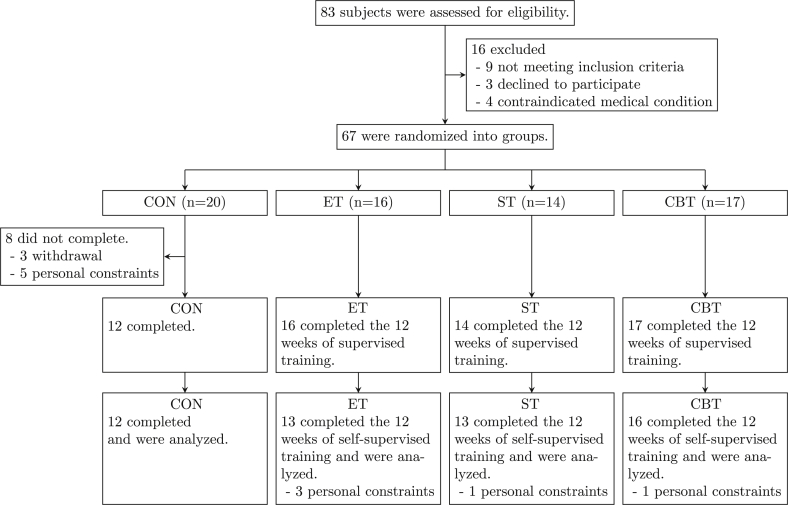
After the intervention, the trial was completed by 81.3% for the ET (n = 13, 92.3% females), 92.9% for the ST (n = 13; 100% females), 94.1% for the CBT (n = 16; 68.8% females) and 60% for the CON (n = 12; 58.3% females). Fig. 1 outlines the subject flow from initial contact through to study completion.
Fig. 1.
Flowchart of the study.
Data collection
Body composition
Body composition was measured after a 12-h fast using a bioimpedance analysis device (Inbody 720, Biospace Inc., Seoul, Korea) with light clothing and without shoes. Height was measured using a spring-coil measuring tape, which was placed on a flat surface with the backboard supported by a wall. BMI was calculated as the body mass divided by the squared value of the height. The measurements were performed by the same investigator throughout the study period.
Blood pressure
BP was measured 3 times at 2-min intervals at a fixed time in the morning (between 8:00 and 10:00 a.m.) after a 10-min seated rest and using an oscillometric semiautomatic device (Omron model HEM 705-CP, Omron Corporation, Tokyo, Japan). The measurements were taken by the same person following a validated protocol.38 The subjects were asked to take antihypertensive and other drugs after the measurements. Mean arterial pressure (MAP) was calculated using the standard equation: MAP = (SBP + 2 DBP)/3.
Blood samples
Blood samples were obtained from an antecubital vein after a 12-h fast before and after the supervised and the self-supervised training periods. Blood samples were collected into tubes containing ethylenediaminetetraacetic acid (K3EDTA; 8.4 mg/Vacutainer) or into serum collection tubes and then centrifuged at 4 °C at 1,500 g for 5 min. The samples were stored immediately at 80 °C.
Glutathione peroxidase assay
The antioxidant enzymatic activity of GPx was measured using a commercial GPx assay kit (catalog no. 703102, Cayman Chemical®, Ann Arbor, MI, USA). The assay was performed according to the manufacturer's instructions. In the assay system for total GPx activity, the oxidation of glutathione (GSH) was coupled to nicotinamide adenine dinucleotide phosphate (NADPH) oxidation by GSH reductase. The decrease in absorbance at 340 nm was recorded every 1 min for 6 min. The reduction rate of the absorbance is directly proportional to the GPx activity in the sample. Each assay was assessed in duplicate, and the plasma GPx activity unit was expressed as nmolmin−1mL−1.
Total nitrite/nitrate assay
The plasma NOx-concentration was determined as (nitrite/nitrate) using a commercial colorimetric assay kit (catalog no. 760871, Cayman Chemical®, Ann Arbor, MI, USA) according to the manufacturer's instructions. The absorbance was then detected at 540 nm using a PowerWave microplate spectrophotometer (BioTek Instruments, Winooski, VT, USA). Each assay was assessed in duplicate, and the plasma NOx-concentration was expressed as μmol.
Lipid peroxidation assay
The plasma MDA concentration was estimated using the method of Draper et al. (1993)39 using 1,1,3,3-tetra-ethoxypropane as the standard. The absorbance of the supernatant was measured at 532 nm using a spectrophotometer (Genesys 20, SN: 35 gk 130009; Thermo Fisher Scientific, Waltham, MA, USA). The plasma MDA concentration was expressed as μmolL−1.
High-sensitivity C-reactive protein assay
The serum hs-CRP concentration was assessed using a high-sensitivity immunoturbidimetric assay on a Hitachi autoanalyzer (Roche Diagnostics Corporation, Indianapolis, IN, USA). The hs-CRP was analyzed by the Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand. The coefficient of variation obtained from blinded simultaneously analyzed quality controls was 3%. The hs-CRP concentration was expressed as mgL−1.
Blood glucose and lipid profiles assessment
Fasting blood glucose (FBG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) concentrations (mgdL−1) were assessed using an automated colorimetric assay (BS-200, Shenzhen Mindray Bio-medical Electronics Co®, Nanshan, China) using specific commercially available kits (Bioclin®, Quibasa, MG, Brazil). Low-density lipoprotein cholesterol (LDL-C) data were calculated using the Friedewald equation.40
Training program
Endurance training
The endurance training was carried out three times a week for 60 min per session. During the supervised period (12 weeks), all sessions were supervised by an experienced trainer using heart rate (HR) monitoring. Prior to the exercise session, there was a 10-min period of warm-up and stretching exercises. During the exercise session, subjects performed 40 min of walking at an intensity of 50–60% of their maximum heart rate (HRmax) (weeks 1–6). The walk program consisted of walk with arms up, heel hit behind, tiptoe, arms adduction/abduction and knee lift. They were continuously encouraged to exercise at their set training HR and to increase their speed of walking after 6 weeks (weeks 7–12) to an intensity of 60–70% HRmax whenever the HR monitor gave a signal. There was also a 10-min cool-down period following the exercise session. During the self-supervised period, subjects were asked to complete three unsupervised exercise sessions each week for 12 weeks and they were asked to attend once-monthly supervised exercise sessions at the site.
Strength training
The strength training was carried out three times a week for 60 min per session. During the supervised period (12 weeks), the training program was a progressive resistance exercise program for the lower and upper extremities and trunk muscles. The standardized exercise sessions were led by an experienced trainer. Prior to the exercise session, there was a 10-min period of warm-up and stretching exercises the same as endurance exercise. During the first 6 weeks of the training (weeks 1–6), the subjects trained with loads of 50–70% of the one-repetition maximum (1RM). The subjects performed 15 repetitions per set and performed 3 sets of each exercise. During the last 6 weeks of the training (weeks 7–12), the subjects performed with higher training loads (60–80% of 1RM), 10 repetitions per set and performed 3 sets of each exercise. Each training session included squat, legs raise, knee extension, unilateral knee flexion exercise, leg adduction/abduction exercise, leg kick back, shoulder press, bench press, bicep curl, triceps dip, lateral flexion exercise, sit-up exercise and back extension. There was also a 10-min cool-down period following the exercise session. During the self-supervised period, the subjects were asked to complete three unsupervised exercise sessions each week for 12 weeks and they were asked to attend once-monthly supervised exercise sessions at the site.
Combined endurance and strength training
The CBT subjects performed both endurance training and strength training at the same intensity as the other training groups. They also performed three sessions a week for 60 min per session. Subjects performed 20 min of walking, at an intensity of 50–60% of their HRmax during the first 6 weeks. They were continuously encouraged to exercise at their set training HR and to increase their speed of walking after 6 weeks to an intensity of 60–70% HRmax. For strength exercise, the subjects performed 12 repetitions per set, 2 sets for each exercise with the same load as the ST program (50–70% of 1RM) during the week 1–6. During the week 7–12, they performed 8 repetitions per set, 2 sets for each exercise (60–80% of 1RM). Prior to the endurance exercise, there was a 10-min period of warm-up and flexibility exercises. Following the endurance exercise session, subjects trained using resistance exercise. There was also a 10-min cool-down period following exercise session.
Statistical analysis
The results were expressed as mean standard deviation (SD). Normality was assessed using the Shapiro-Wilk test. Baseline comparisons between groups were performed using one-way analysis of variance (ANOVA) or as appropriate. Baseline values were used as covariates. If necessary, the data were transformed logarithmically before analysis of covariance (ANCOVA) to fulfill the criterion of normal distribution. The measurement outcomes were tested to determine whether they met the assumptions of normality, linearity, and homogeneity of variance. Training effects and differences between the groups were performed using repeated measures ANCOVA. Within group analyses were performed with repeated measures ANOVA. Pairwise comparisons were performed using the Tukey test or Bonferroni test. The effect size (ES) was calculated between each pair of measurements according to Hedges et al. (1999)41 and Cohen (1988).42 The magnitude of the ES was classified as trivial ( 0.2), small ( 0.2–0.6), moderate ( 0.6–1.2), large ( 1.2–2.0), and very large ( 2.0).43 Pearson's correlation was use to assess the relationships between the changes in antioxidant and oxidative stress variables and BP after adjustment for gender as a confounding factor. The statistical significance was set at p 0.05. The statistical analyses were conducted using the SPSS software for Windows (SPSS Inc., Chicago, IL, USA).
Results
The characteristics of subjects were not different among the groups, except for body weight and gender (both p 0.05). All groups had similar profiles for all clinical parameters examined (Table 1). Use of medication did not change during the study. In the supervised training period, 67 subjects were randomized and 59 completed the study, yielding a total drop-out rate of 11.9%. Eight subjects in the CON withdrew prior to completion of the intervention, while no subject withdrew in the training groups. Adherence data in the supervised training period were available as 16, 14 and 17 completers in the ET, the ST and the CBT, respectively. Average attendances were 88.7%, 93.6% and 88.7% for the ET, the ST and the CBT, respectively. The adherence differences between group did not reach statistical significance (p = 0.19). In the self-supervised training period, 54 completed the study, yielding a total drop-out rate of 19.4%. Attrition rates from the ET, the ST, the CBT and the CON were 18.8%, 7.1%, 5.9% and 40%, respectively. Adherence data in the self-supervised training period were available from 13 of the 16 completes in the ET, from 13 of the 14 completes in the ST and from 16 of the 17 completes in the CBT. Average attendances were 76.5%, 85.7% and 80.7% for the ET, the ST and the CBT, respectively. The percentage of attendance in the ST was significantly greater than in the ET (p 0.01) and the CBT (p 0.05), while there was a trend significantly greater in the CBT than in the ET (p = 0.06).
Table 2 shows the results of body composition and physiological outcomes before and after the supervised and the self-supervised training periods. No significant differences were observed in body weight, BMI, FM, %BF, SMM, or resting HR after the supervised and the self-supervised training periods in any of the groups. After the supervised training period, SBP decreased by 11.1 mmHg (7.9%) (ES = 0.7, p 0.05) in the ET and 11.6 mmHg (8.2%) (ES = 1.1, p 0.01) in the CBT, but was unchanged in the ST or the CON. Only the CBT produced a reduction in SBP of 10.6 mmHg (7.5%) (ES = 0.7, p 0.05) after the self-supervised training period. The reductions in SBP in the CBT were significantly greater than in the CON after the supervised and the self-supervised training periods (ES = 1, p 0.05 and ES = 1, p 0.01, respectively). DBP decreased by 8.1 mmHg (9.9%) (ES = 1.1, p 0.05) in only the CBT after the self-supervised training period, but with no significant differences among groups. MAP decreased by 7 mmHg (6.9%) (ES = 1, p 0.01) and 8.9 mmHg (8.8%) (ES = 1.1, p 0.05) in only the CBT after the supervised and the self-supervised training periods, respectively, but with no significant differences among groups (Table 2).
Table 2.
Body composition and physiological outcomes before and after the supervised and the self-supervised training periods.
| CON |
ET |
ST |
CBT |
|
|---|---|---|---|---|
| (n = 12) | (n = 13) | (n = 13) | (n = 16) | |
| Weight (kg) | ||||
| Baseline | 63.4 9.2 | 58.5 7.7 | 52.3 7.6 | 60.2 9.1 |
| After supervised period | 61.5 6.9 | 58.2 7.6 | 51.9 3.3 | 59.8 9.4 |
| After self-supervised period | 63.4 10 | 58.1 7.5 | 51.9 7.6 | 59.3 9.2 |
| BMI (kgm−2) | ||||
| Baseline | 25.6 4.3 | 23.8 2.4 | 22.6 2.6 | 24.1 2.0 |
| After supervised period | 25 2.6 | 23.7 2.4 | 22.4 2.5 | 24 2.1 |
| After self-supervised period | 25.6 4.3 | 23.7 2.6 | 22.5 2.6 | 23.9 2.3 |
| FM (kg) | ||||
| Baseline | 22.6 7.2 | 18.8 4.9 | 17.7 4.9 | 19.4 4.3 |
| After supervised period | 20.8 4.4 | 18.4 4.4 | 17.1 5 | 18.7 4.2 |
| After self-supervised period | 23.1 7.6 | 17.9 4.9 | 17 5.1 | 18.3 4.9 |
| %BF | ||||
| Baseline | 35.3 7 | 32.3 7.6 | 33.4 5 | 32.3 6.3 |
| After supervised period | 33.8 5.7 | 31.7 6.7 | 32.3 5.3 | 31.4 5.9 |
| After self-supervised period | 35.8 7 | 30.8 7.2 | 32.3 5.7 | 31.8 6.3 |
| SMM (kg) | ||||
| Baseline | 22 3.7 | 21.3 4.8 | 18.1 2.2 | 21.9 5.1 |
| After supervised period | 22 3.5 | 21.4 4.4 | 18.2 2.1 | 22.1 5.0 |
| After self-supervised period | 21.9 3.6 | 21.5 4.2 | 18.3 2.3 | 21.5 4.6 |
| HR (beatsmin−1) | ||||
| Baseline | 71.9 9 | 77.5 12.2 | 73 10.8 | 75.6 7.2 |
| After supervised period | 73.4 11.5 | 79.4 10.2 | 72.9 9.3 | 74.3 9.3 |
| After self-supervised period | 74.9 11.6 | 82 15.1 | 72.3 10.7 | 74.2 9.9 |
| SBP (mmHg) | ||||
| Baseline | 140.6 18.2 | 141 15.9 | 146.8 23.6 | 142 12.2 |
| After supervised period | 142.1 13.1 | 128.3 15.4∗ | 141.8 17 | 130.4 9.4∗∗,† |
| After self-supervised period | 147.3 20 | 133.3 15.7 | 144.8 21.7 | 131.4 15.9∗,† |
| DBP (mmHg) | ||||
| Baseline | 82.5 10.1 | 84.1 10 | 80.5 7.8 | 81.7 5.6 |
| After supervised period | 82.1 12.6 | 76.6 7.5 | 74.4 8.7 | 76.9 7.4 |
| After self-supervised period | 81.5 12.1 | 80.1 7.3 | 76.6 9.9 | 73.6 9.3∗ |
| MAP (mmHg) | ||||
| Baseline | 93.4 31.4 | 95.1 30.1 | 102.6 11.8 | 101.8 6.6 |
| After supervised period | 96.6 47.2 | 93.8 9.4 | 89.4 28.1 | 94.8 7.2∗∗ |
| After self-supervised period | 94.8 32.4 | 97.8 9.3 | 99.4 12.2 | 92.9 9.9∗ |
Data are means SD. CON, control group; ET, endurance training group; ST, strength training group; CBT, combined endurance and strength training group; BMI, body mass index; FM, fat mass; %BF, body fat percentage; SMM, skeletal muscle mass; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
∗p 0.05 and ∗∗p 0.01 to within-group comparison (baseline vs. after supervised period vs. after self-supervised period).
†p 0.05 and ††p 0.01 to between-group comparison (vs. control group).
Table 3 shows the results of biochemical outcomes before and after the supervised and the self-supervised training periods. No significant differences were observed in FBG, TC, TG, or LDL-C concentrations after the supervised and the self-supervised training periods in any of the groups. Only in the ST, HDL-C concentrations increased by 7.2 mgdL−1 (12.2%) (ES = 0.7, p 0.01), and the TC/HDL-C and the LDL-C/HDL-C ratios decreased by 0.5 (15%) (ES = 0.8, p 0.05) and 0.4 (20.9%) (ES = 0.7, p 0.05), respectively, after the self-supervised training period, but with no significant differences among groups (Table 3).
Table 3.
Biochemical outcomes before and after the supervised and the self-supervised training periods.
| CON |
ET |
ST |
CBT |
|
|---|---|---|---|---|
| (n = 12) | (n = 13) | (n = 13) | (n = 16) | |
| FBG (mgdL−1) | ||||
| Baseline | 97.1 17 | 88.2 6.8 | 89.2 17.1 | 95.7 12.9 |
| After supervised period | 104.6 19.2 | 92 6 | 106 31.4 | 99.1 14.9 |
| After self-supervised period | 95.7 14.7 | 88.4 5.9 | 92.3 14.4 | 92.9 11 |
| TC (mgdL−1) | ||||
| Baseline | 201.4 48 | 210.7 41.1 | 200.8 40.9 | 203.8 33.3 |
| After supervised period | 211.8 43.1 | 211.3 46 | 195.1 41.1 | 209 39.9 |
| After self-supervised period | 202.4 43.3 | 208.3 47.3 | 192 37.8 | 202 30.4 |
| TG (mgdL−1) | ||||
| Baseline | 151.5 64.7 | 122.1 32.9 | 95.6 31.6 | 96.5 27.8 |
| After supervised period | 143.3 57.1 | 134 61.5 | 95.1 31.5 | 109.5 49.4 |
| After self-supervised period | 140.8 67.4 | 125.8 59.7 | 83.2 28.3 | 93.4 24.5 |
| LDL-C (mgdL−1) | ||||
| Baseline | 122.6 46.4 | 136.1 39 | 123.2 38.6 | 128.8 32.9 |
| After supervised period | 133.3 36.8 | 127.8 35.7 | 118.8 38 | 124.3 35.9 |
| After self-supervised period | 124.3 38.5 | 126.8 41.4 | 109.9 34.8 | 125.4 31.5 |
| HDL-C (mgdL−1) | ||||
| Baseline | 48.6 10.8 | 53.8 11.4 | 58.5 9.1 | 55.9 15.1 |
| After supervised period | 49.9 15.5 | 56.8 15.1 | 57.5 7.2 | 57.3 14.5 |
| After self-supervised period | 49.9 8.7 | 56.4 14.6 | 65.7 10.9∗∗ | 57.5 16.7 |
| TC/HDL-C ratio | ||||
| Baseline | 4.2 0.9 | 4 0.9 | 3.5 0.8 | 3.8 1 |
| After supervised period | 4.4 1 | 3.9 1.1 | 3.4 0.7 | 3.8 1 |
| After self-supervised period | 4.1 0.8 | 3.9 1 | 3 0.6∗ | 3.7 1 |
| LDL-C/HDL-C ratio | ||||
| Baseline | 2.5 0.8 | 2.4 1 | 2.1 0.7 | 2.5 0.9 |
| After supervised period | 2.8 0.7 | 2.4 0.8 | 2.1 0.6 | 2.3 0.9 |
| After self-supervised period | 2.5 0.7 | 2.3 0.7 | 1.7 0.5∗ | 2.4 0.9 |
Data are means SD. CON, control group; ET, endurance training group; ST, strength training group; CBT, combined endurance and strength training group; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
∗p 0.05 and ∗∗p 0.01 to within-group comparison (baseline vs. after supervised period vs. after self-supervised period).
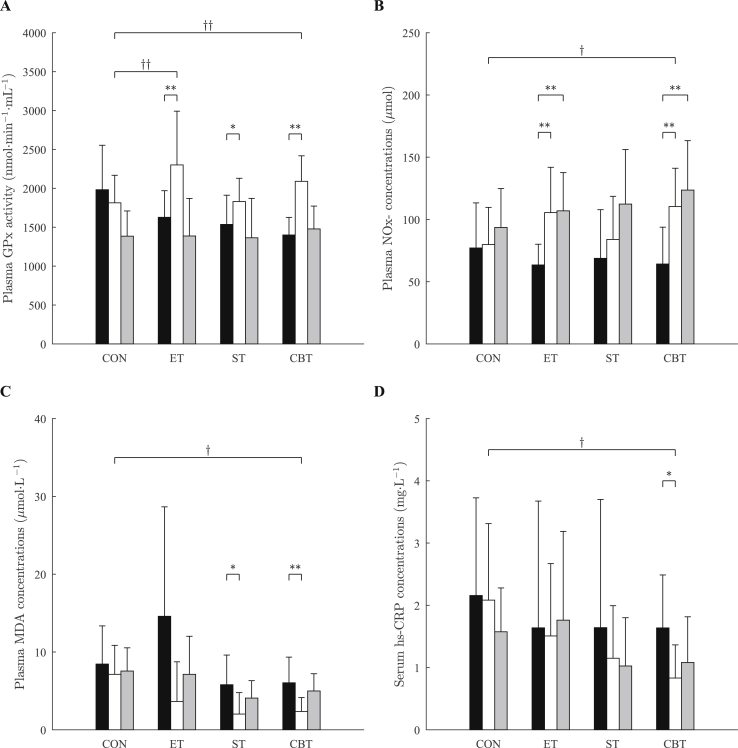
The results regarding the antioxidant, oxidative stress, and inflammatory markers before and after the supervised and the self-supervised training periods are presented in Fig. 2. After the supervised training period, plasma GPx activity increased in all training groups: 41.3% (ES = 1.2, p 0.01) in the ET, 19.1% (ES = 0.9, p 0.05) in the ST, and 49.2% (ES = 2.4, p 0.01) in the CBT, but not in the CON. The GPx activity of the CBT (ES = 0.8, p 0.01) and the ET (ES = 1, p 0.01) increased significantly with respect to the CON after the supervised training period, whereas there were no significant differences among the groups. There were no significant differences compared to baseline for all training groups after the self-supervised training period.
Fig. 2.
Plasma glutathione peroxidase (A), total nitrite/nitrate (B), malondialdehyde (C), and serum high-sensitive C-reactive protein (D) before (black) and after the supervised training period (white) and after the self-supervised training period (gray). Data are means SD. CON, control group; ET, endurance training group; ST, strength training group; CBT, combined endurance and strength training group. ∗p 0.05 and ∗∗p 0.01 to within-group comparison (baseline vs. after supervised period vs. after self-supervised period). †p 0.05 and ††p 0.01 to between-group comparison (vs. control group).
Plasma NOx-concentrations increased by 66.2% and 68.5% (ES = 1.5, p 0.01 and ES = 1.8, p 0.01, respectively) in the ET, and 71.9% and 92.4% (ES = 1.5, p 0.01 and ES = 1.7, p 0.01, respectively) in the CBT after the supervised and the self-supervised training periods, but was unchanged in the ST or the CON. The increment of NOx-concentration seen in only the CBT was significantly greater than in the CON after the supervised training period (ES = 1, p 0.05), whereas there were no significant differences among the groups.
Plasma MDA concentrations decreased by 65.1% (ES = 1.1, p 0.05) in the ST, and 61% (ES = 1.4, p 0.01) in the CBT after the supervised training period, but was unchanged in the ET or the CON. The reduction in MDA concentration seen in only the CBT was significantly greater than in the CON after the supervised training period (ES = 1.7, p 0.01), whereas there were no significant differences among the groups. There were no significant differences compared to baseline for all training groups after the self-supervised training period.
Serum hs-CRP concentrations decreased by 49.2% (ES = 1.1, p 0.05) in only the CBT after the supervised training period, but was unchanged in the ET, the ST or the CON. The hs-CRP concentrations of the CBT decreased significantly with respect to the CON after the supervised training period (ES = 1.4, p 0.05), whereas there were no significant differences compared to baseline for all training groups after the self-supervised training period.
The decrease in SBP in the CBT was positively related to the change in hs-CRP (r = 0.37, p 0.05) and MDA (r = 0.46, p 0.01) concentrations, while the decrease in SBP tended to be negatively related to the change in NOx-concentration (r = −0.31, p = 0.08) after the supervised training period. After the self-supervised training period, the decrease in SBP in the CBT was negatively related to the change in NOx-concentration (r = −0.34, p 0.05). In the ET, the decrease in SBP was negatively related to the change in GPx activity (r = −0.41, p 0.05) and NOx-concentration (r = −0.43, p 0.05) after the supervised training period.
Discussion
The present study was conducted to investigate the effects of the CBT program, compared with the ET program and the ST program on BP, antioxidants and oxidative stress among elderly individuals with HT. The present study demonstrated that the reduction in SBP and improvements in antioxidant, oxidative stress and inflammatory markers in the CBT seem to be similar to the ET after the supervised training period. However, after the self-supervised training period, the CBT program might affect better due to greater exercise adherence and attendance in elderly individuals with HT.
The main finding demonstrated that after adjustment for baseline covariates, SBP significantly decreased by 7.9% in the ET and 8.2% in the CBT, but was unchanged in the ST after the supervised training period. Only the CBT produced a reduction in SBP of 10.6 mmHg (7.5%) after the self-supervised training period. The reduction in SBP in the CBT was significantly greater than the CON, but was not significantly different compared to the other training groups. Although SBP increased in parallel with advancing age, slowing the age-related rise in SBP could prevent the associated cardiovascular complications.44 A previous study with a longer term and larger population revealed that a reduction of 10.4 mmHg in SBP in older adults with HT was associated with reduced total mortality by 13%, reduced chronic heart disease death by 18% and reduced strokes by 26%.45 If the present reduction in SBP with the CBT program (10.6 mmHg) were sustained in the long term, it could reduce chronic heart disease death and strokes as well.
Our findings agree with previous studies in which a reduction in BP for older adults with HT have been demonstrated after ET46 and CBT.47 Another study observed a more impressive decrease in BP after CBT compared to ET.48 On the other hand, we observed no change in BP in the ST, which disagrees with another study.25 It is possible that intensity and volume could affect responsiveness. Interestingly, only the CBT produced a reduction in SBP and DBP after the self-supervised training period. However, the DBP was less responsive than the SBP despite its significant reductions. A previous study has also reported a larger decrease in the SBP than in the DBP.47
A possible mechanism responsible for the antihypertensive effect of the ET and the CBT programs in this study may be an improved endothelium-dependent vasodilator. The ET and the CBT may have enhanced NO bioavailability, resulting in improved endothelium-dependent vasorelaxation in the hypertensive subjects. In this study, plasma NOx-concentrations significantly increased by 66.2% and 68.5% in the ET, and 71.9% and 92.4% in the CBT after the supervised and the self-supervised training periods respectively, but was unchanged in the ST. The reductions in SBP in the ET and the CBT were associated with increments in plasma NOx-concentrations. The antihypertensive effect of aerobic exercise in pre-hypertensive and hypertensive individuals has been supported by previous studies.49,50 The endothelial adaptation has been suggested to be a product of exercise-induced changes in shear stress.18,49,50 This was associated with an upregulation of endothelial NO synthase leading to the greater bioavailability of NO.51 Although previous studies have shown reduced bioavailability of NO with aging,52,53 it has been suggested that the exercise-induced endothelial adaptation and the improvement of endothelium-dependent relaxation are mainly mediated by a significant increase in NO production and/or a decrease in NO scavenging by ROS.18 On the other hand, plasma NOx-concentration was unchanged in the ST. It has been demonstrated that ST improves endothelial-dependent vasodilation which only occurs locally in the trained limbs.54 The results of the present study suggested that the effect of aerobic exercise in the ET and the CBT might cause the increase in plasma NOx-concentration.
Several studies in hypertensive individuals suggested that regular exercise, especially aerobic exercise, can improve endothelial function, which relates to restoration of NO availability caused by antioxidant activity.49,50 Our findings demonstrated that circulating GPx activity significantly increased by 41.3% in the ET and 49.2% in the CBT after the supervised training period, but was unchanged in the ST. GPx enzyme reacts with peroxide by converting hydrogen peroxide (H2O2) to H2O. It contributes to low production of hydroxyl radicals and consequently low oxidative stress. It seems that the ET and the CBT increased production of H2O2 enough to stimulate significant increases in circulating GPx activity. This response could be interpreted as a positive feedback mechanism that reflects a response of exposure to oxidative stress in these groups. In contrast, the ST did not significantly increase GPx activity; in all probability, the ST had an insufficient increase in oxidative metabolism and also was less exposed to the production of H2O2 during exercise. These adaptation results were consistent with a previous study in sedentary young individuals.30 There was also no differences in GPx at rest between young and older individuals regardless training status.55
Our results also showed that plasma MDA concentration significantly decreased by 61% in the CBT and by 65.1% in the ST after the supervised training period, but was unchanged in the ET. However, the reduction in plasma MDA concentration seen in only the CBT was significantly greater than in the CON. The reduction in plasma MDA concentration was observed in the CBT, associated with the increase in GPx activity in this study. GPx, which is an antioxidant enzyme responsible for the decomposition of lipid peroxides, protects cellular membranes from peroxidative damage.56 Thus, the reduction in plasma MDA concentration in the CBT probably resulted from the increase in GPx activity.
Previous studies have shown that ET is effective to reduce ROS and enhances the adaptation to oxidative stress by increasing the level of antioxidants in hypertensive individuals.18,51 However, we observed no change in plasma MDA concentration in the ET, which disagrees with the previous studies.18,51 Another previous study has reported that, with the increase of exercise frequency, the oxidative stress markers is improved in hypertensive individuals who participated in walking. This study has suggested that individuals with HT should engage in walking with a frequency of 4 or more days per week.57 Thus, it is possible that the frequency of exercise in the ET program was insufficient to affect responsiveness. The decrease in MDA concentration in the ST was consistent with previous studies.22, 23, 24 These studies have indicated that ST can increase total antioxidant capacity by concomitantly increasing ROS and reactive nitrogen species generation. The intensity and volume of exercise in the ST is possibly sufficient to enhance the adaptation to oxidative stress.
Unlike after the supervised training period, circulating GPx activity and plasma MDA concentration did not present differences compared to baseline for all training groups after the self-supervised training period. The adherence of exercise in the self-supervised training period were decreased by 14.3%, 9.7% and 9.7% for the ET, the ST and the CBT respectively, compared to the supervised training period. Moreover, the exercise intensity is doubtful since it is collected through self-reported adherence. These results suggest that GPx activity and MDA concentration may have no sensitivity to respond to frequency and/or intensity of the programs.
Finally, serum hs-CRP concentration significantly decreased by 49.2% in the CBT after the supervised training period, but was unchanged in the ET and the ST. Our findings indicate that intensity, frequency and duration of the CBT program were sufficient to improve hs-CRP concentration. A systematic review has reported regular exercise reduces hs-CRP concentrations though multiple mechanisms, including a reduction in cytokine production by adipose tissue, skeletal muscles, endothelial and blood mononuclear cells, improved endothelial function and insulin sensitivity, and possibly an antioxidant effect. Moreover, regular exercise has also been demonstrated to normalize the levels and/or expression of pro-inflammatory cytokines,12 supporting the reduction in hs-CRP concentration. However, it is notable that the training programs in this study were progressive as both training intensity and volume during the ET and the ST program. Therefore, it is also possible that the training programs were not intense enough to elicit anti-inflammatory effect.
After the self-supervised training period, serum hs-CRP concentration did not present differences compared to baseline for all training groups. Due to lower adherence and doubtful intensity of exercise compared to the supervised period, the training programs might not include sufficient frequency and intensity of exercise to elicit anti-inflammatory effect. Moreover, the reduction in hs-CRP concentration seem to be associated with adiposity loss.58
The training programs did not promote any additional benefit in body weight, BMI and %BF or metabolic parameters in this study. Similar results were obtained in a study in which elderly individuals exhibited no changes in body weight or BMI after 12 weeks of CBT.59 In addition, exercise training alone is generally not associated with significant weight loss when diet is not restricted. Body weight loss lower than 2 kg and %BF loss lower than 2% have been reported in subjects who have participated exercise for 6 weeks to 2 years.60
Our findings showed the reduction in SBP and improvements in antioxidant, oxidative stress and inflammatory markers in the ET and the CBT after the supervised training period. This might be a result of the aerobic exercise existing in the both program. However, after the self-supvervised training period, only the CBT produced a reduction in SBP and DBP. This might be due to the adherence and the attendance to the CBT program. The CBT's adherence was the highest (94.1%) and the exercise attendance in the CBT (80.7%) also showed a trend significantly greater than the ET (76.5%). Based on the results of this study, the CBT program might affect better after the self-supervised training period due to greater exercise adherence and attendance in elderly individuals with HT.
The major limitation of the study was the intensity of exercise during the self-supervised exercise, which was self-reported by the subjects. Therefore it was not as reliable as it could have been if measured by a professional. Additionally, subjects who were less self-motivated, less self-condent, and enjoyed exercising in a group would prefer to be supervised by a therapist or a trainer. Finally, the generalizability of the results is limited due to the small sample. The results should therefore be interpreted with caution. Nevertheless, we do believe that our findings can provide insight for future studies to explore adaptation effects of CBT among different populations.
Conclusion
The present study supported the hypothesis that the CBT program has beneficial effects on the improvement of BP and the balance between ROS production, the ability to neutralize it, and the damage in cellular lipid membranes that ROS may produce in the elderly subjects with HT. Moreover, for the self-supervised training, the CBT program has shown its applicability to improve BP and circulating NOx-concentration. This program might affect better due to greater exercise adherence and attendance in the elderly subjects with HT. From a clinical viewpoint, our findings indicated that CBT can be applied as a therapeutic and preventative tool to improve BP and bioavailability of NO and also to attenuate oxidative stress associated with aging.
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgements
This study was supported by the Kasetsart University Research and Development Institute, Kasetsart University (KURDI 8.59). The authors thank the Exercise and Sport Sciences Development and Research Group, Khon Kaen University and the Medical Pathology Group, the 19th Somdet Pha Sangkharat Hospital for the blood chemistry measurement. Finally, the authors thank the participants for their enthusiastic participation in this study.
References
- 1.Stadtman E.R. Role of oxidant species in aging. Curr Med Chem. 2004;11(9):1105–1112. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- 2.Kasapoglu M., zben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001;36(2):209–220. doi: 10.1016/s0531-5565(00)00198-4. [DOI] [PubMed] [Google Scholar]
- 3.Buford T.W. Hypertension and aging. Ageing Res Rev. 2016;26:96–111. doi: 10.1016/j.arr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugamura K., Keaney J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51(5):978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley S.D. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxidants Redox Signal. 2014;20(1):102–120. doi: 10.1089/ars.2013.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinh Q.N., Drummond G.R., Sobey C.G., Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Res Int. 2014;2014 doi: 10.1155/2014/406960. 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torregrossa A.C., Aranke M., Bryan N.S. Nitric oxide and geriatrics: implications in diagnostics and treatment of the elderly. J Geriatr Cardiol: JGC. 2011;8(4):230. doi: 10.3724/SP.J.1263.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji L.L., Leeuwenburgh C., Leichtweis S. Oxidative stress and aging: role of exercise and its influences on antioxidant systems. Ann N Y Acad Sci. 1998;854(1):102–117. doi: 10.1111/j.1749-6632.1998.tb09896.x. [DOI] [PubMed] [Google Scholar]
- 9.Donato A.J., Eskurza I., Silver A.E. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-b. Circ Res. 2007;100(11):1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 10.RodrguezMaas L., ElAssar M., Vallejo S. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8(3):226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 11.Buchner D.M. Physical activity and prevention of cardiovascular disease in older adults. Clin Geriatr Med. 2009;25(4):661–675. doi: 10.1016/j.cger.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Kasapis C., Thompson P.D. The effects of physical activity on serum c-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45(10):1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 13.Sallam N., Laher I. 2016. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases, Oxidative Medicine and Cellular Longevity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radak Z., Chung H.Y., Koltai E., Taylor A.W., Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7(1):34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Rowiski R., Kozakiewicz M., Kdziora-Kornatowska K., Hbner-Woniak E., Kdziora J. Markers of oxidative stress and erythrocyte antioxidant enzyme activity in older men and women with differing physical activity. Exp Gerontol. 2013;48(11):1141–1146. doi: 10.1016/j.exger.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Bailey D.M., McEneny J., Mathieu-Costello O. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol. 2010;109(2):449–456. doi: 10.1152/japplphysiol.00354.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji L.L. Exercise at old age: does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001;928(1):236–247. doi: 10.1111/j.1749-6632.2001.tb05653.x. [DOI] [PubMed] [Google Scholar]
- 18.Larsen M.K., Matchkov V.V. Hypertension and physical exercise: the role of oxidative stress. Medicina. 2016;52(1):19–27. doi: 10.1016/j.medici.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Radak Z., Kaneko T., Tahara S. The effect of exercise training on oxidative damage of lipids, proteins, and dna in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med. 1999;27(1-2):69–74. doi: 10.1016/s0891-5849(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 20.Poon E.T.-C., Sheridan S., Chung A.P.-W., Wong S.H.-S. Age-specific affective responses and self-efficacy to acute high-intensity interval training and continuous exercise in insufficiently active young and middle-aged men. J Exerc Sci Fitness. 2018;16(3):106–111. doi: 10.1016/j.jesf.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartlett J.D., Close G.L., MacLaren D.P., Gregson W., Drust B., Morton J.P. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sport Sci. 2011;29(6):547–553. doi: 10.1080/02640414.2010.545427. [DOI] [PubMed] [Google Scholar]
- 22.de Gonzalo-Calvo D., Fernndez-Garca B., de Luxn-Delgado B. Chronic training increases blood oxidative damage but promotes health in elderly men. Age. 2013;35(2):407–417. doi: 10.1007/s11357-011-9358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padilha C.S., Ribeiro A.S., Fleck S.J. Effect of resistance training with different frequencies and detraining on muscular strength and oxidative stress biomarkers in older women. Age. 2015;37(5):104. doi: 10.1007/s11357-015-9841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent K.R., Vincent H.K., Braith R.W., Lennon S.L., Lowenthal D.T. Resistance exercise training attenuates exercise-induced lipid peroxidation in the elderly. Eur J Appl Physiol. 2002;87(4-5):416–423. doi: 10.1007/s00421-002-0640-2. [DOI] [PubMed] [Google Scholar]
- 25.da Cunha Nascimento D., da Silva C.R., Valduga R. Blood pressure response to resistance training in hypertensive and normotensive older women. Clin Interv Aging. 2018;13:541. doi: 10.2147/CIA.S157479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dantas F.F.O., do Socorro Brasileiro-Santos M., Batista R.M.F. Effect of strength training on oxidative stress and the correlation of the same with forearm vasodilatation and blood pressure of hypertensive elderly women: a randomized clinical trial. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bampton E.A., Johnson S.T., Vallance J.K. Correlates and preferences of resistance training among older adults in alberta, Canada. Can J Public Health. 2016;107(3):272–277. doi: 10.17269/CJPH.107.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley J.P., Borg G.A. Borgs scales in strength training; from theory to practice in young and older adults. Appl Physiol Nutr Metabol. 2011;36(5):682–692. doi: 10.1139/h11-078. [DOI] [PubMed] [Google Scholar]
- 29.Gearhart R.F., Jr., Riechman S.E., Lagally K.M., Andrews R.D., Robertson R.J. Rpe at relative intensities after 12 weeks of resistance-exercise training by older adults. Percept Mot Skills. 2008;106(3):893–903. doi: 10.2466/pms.106.3.893-903. [DOI] [PubMed] [Google Scholar]
- 30.Azizbeigi K., Stannard S.R., Atashak S., Haghighi M.M. Antioxidant enzymes and oxidative stress adaptation to exercise training: comparison of endurance, resistance, and concurrent training in untrained males. J Exerc Sci Fitness. 2014;12(1):1–6. [Google Scholar]
- 31.Oliveira V. N. d., Bessa A., Jorge M.L.M.P. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl Physiol Nutr Metabol. 2012;37(2):334–344. doi: 10.1139/h2012-004. [DOI] [PubMed] [Google Scholar]
- 32.Whelton P.K., Carey R.M., Aronow W.S. acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2017;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. http://www.onlinejacc.org/content/71/19/e127 2018. [DOI] [PubMed] [Google Scholar]
- 33.Rikli R.E., Jones C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontol. 2013;53(2):255–267. doi: 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- 34.Jaturapatporn D., Hathirat S., Manataweewat B. Reliability and validity of a Thai version of the general practice assessment questionnaire (gpaq) J Med Assoc Thail. 2006;89(9):1491–1496. [PubMed] [Google Scholar]
- 35.Schaun M.I., Dipp T., Rossato Jda S. The effects of periodized concurrent and aerobic training on oxidative stress parameters, endothelial function and immune response in sedentary male individuals of middle age. Cell Biochem Funct. 2011;29(7):534–542. doi: 10.1002/cbf.1781. https://www.ncbi.nlm.nih.gov/pubmed/21780310 [DOI] [PubMed] [Google Scholar]
- 36.Ferguson B. Acsms guidelines for exercise testing and prescription 9th ed. 2014. J Can Chiropr Assoc. 2014;58(3):328. [Google Scholar]
- 37.Ratamess N., Alvar B., Evetoch T. Progression models in resistance training for healthy adults. american college of sports medicine. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 38.Iniciativa P.S.L.H. Working meeting on blood pressure measurement: suggestions for measuring blood pressure to use in populations surveys. Revista panamericana de salud publica= Pan Am J Public Health. 2003;14(5):300. doi: 10.1590/s1020-49892003001000003. [DOI] [PubMed] [Google Scholar]
- 39.Draper H., Squires E., Mahmoodi H., Wu J., Agarwal S., Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med. 1993;15(4):353–363. doi: 10.1016/0891-5849(93)90035-s. [DOI] [PubMed] [Google Scholar]
- 40.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 41.Hedges L.V., Gurevitch J., Curtis P.S. The metaanalysis of response ratios in experimental ecology. Ecology. 1999;80(4):1150–1156. [Google Scholar]
- 42.Cohen J. 1988. Statistical Power Analysis for the Behavioral Sciences. 2nd. [Google Scholar]
- 43.Batterham A.M., Hopkins W.G. Making meaningful inferences about magnitudes. Int J Sports Physiol Perform. 2006;1(1):50–57. [PubMed] [Google Scholar]
- 44.Li Y., Staessen J.A., Sheng C.-S., Huang Q.-F., O'Rourke M., Wang J.-G. Age dependency of peripheral and central systolic blood pressures: cross-sectional and longitudinal observations in a Chinese population. Hypertens Res. 2012;35(1):115. doi: 10.1038/hr.2011.160. [DOI] [PubMed] [Google Scholar]
- 45.Beckett N.S., Peters R., Fletcher A.E. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 46.Braz N.F., Carneiro M.V., Oliveira-Ferreira F. Influence of aerobic training on cardiovascular and metabolic parameters in ederly hypertensive women. Int J Prev Med. 2012;3(9):652. [PMC free article] [PubMed] [Google Scholar]
- 47.Lima L.G., Bonardi J., Campos G.O. Combined aerobic and resistance training: are there additional benefits for older hypertensive adults? Clinics. 2017;72(6):363–369. doi: 10.6061/clinics/2017(06)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sousa N., Mendes R., Abrantes C., Sampaio J., Oliveira J. A randomized 9-month study of blood pressure and body fat responses to aerobic training versus combined aerobic and resistance training in older men. Exp Gerontol. 2013;48(8):727–733. doi: 10.1016/j.exger.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson D.W. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive and hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 2000;102(18):e119–e120. doi: 10.1161/01.cir.102.18.e119. [DOI] [PubMed] [Google Scholar]
- 50.Higashi Y., Sasaki S., Kurisu S. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999;100(11):1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 51.Roque F.R., Briones A.M., GarcaRedondo A.B. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol. 2013;168(3):686–703. doi: 10.1111/j.1476-5381.2012.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyberg M., Blackwell J.R., Damsgaard R., Jones A.M., Hellsten Y., Mortensen S.P. Lifelong physical activity prevents an agerelated reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol. 2012;590(21):5361–5370. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taddei S., Virdis A., Ghiadoni L. Age-related reduction of no availability and oxidative stress in humans. Hypertension. 2001;38(2):274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 54.Higashi Y., Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Therapeut. 2004;102(1):87–96. doi: 10.1016/j.pharmthera.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Bouzid M.A., Hammouda O., Matran R., Robin S., Fabre C. Changes in oxidative stress markers and biological markers of muscle injury with aging at rest and in response to an exhaustive exercise. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090420. e90420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.nal M.E., Kanbak G., Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta. 2001;305(1-2):75–80. doi: 10.1016/s0009-8981(00)00422-8. [DOI] [PubMed] [Google Scholar]
- 57.Yu Y., Gao Q., Xia W. Association between physical exercise and biomarkers of oxidative stress among middle-aged and elderly community residents with essential hypertension in China. BioMed Res Int. 2018;2018 doi: 10.1155/2018/4135104. 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martins R.A., Neves A.P., Coelho-Silva M.J., Veríssimo M.T., Teixeira A.M. The effect of aerobic versus strength-based training on high-sensitivity c-reactive protein in older adults. Eur J Appl Physiol. 2010;110(1):161–169. doi: 10.1007/s00421-010-1488-5. [DOI] [PubMed] [Google Scholar]
- 59.Lambert C.P., Wright N.R., Finck B.N., Villareal D.T. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol. 2008;105(2):473. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bacon S.L., Sherwood A., Hinderliter A., Blumenthal J.A. Effects of exercise, diet and weight loss on high blood pressure. Sports Med. 2004;34(5):307–316. doi: 10.2165/00007256-200434050-00003. [DOI] [PubMed] [Google Scholar]