Abstract
Regenerative medicine is a new branch of medicine based on tissue engineering technology. This rapidly developing field of science offers revolutionary treatment strategy aimed at urinary bladder regeneration. Despite many promising announcements of experimental urinary bladder reconstruction, there has been a lack in commercialization of therapies based on current investigations. This is due to numerous obstacles that are slowly being identified and precisely overcome. The goal of this review is to present the current status of research on urinary bladder regeneration and highlight further challenges that need to be gradually addressed. We put an emphasis on expectations of urologists that are awaiting tissue engineering based solutions in clinical practice. This review also presents a detailed characteristic of obstacles on the road to successful urinary bladder regeneration from urological clinician perspective. A defined interdisciplinary approach might help to accelerate planning transitional research tissue engineering focused on urinary tracts. Stem Cells Translational Medicine 2017;6:2033–2043
Keywords: Stem cells, Urinary bladder, Regeneration, Translational medicine, Urology
Significance Statement.
The goal of this review is to present the current status of research on urinary bladder regeneration and highlight further challenges that need to be gradually addressed. We put an emphasis on expectations of urologists that are awaiting tissue engineering based solutions in clinical practice. This review presents a detailed characteristic of obstacles on the road to successful urinary bladder regeneration from a urological clinician perspective.
Introduction
Tissue engineering of the urinary bladder underwent progress during the last two decades. The constant development of stem cell biotechnology and material science provides new opportunities to translate experimental methods of bladder regeneration into clinical applications.
Urinary diversions based on urinary bladder wall replacement with bowel remain the gold standard despite associated complications, including stricture and fistula formation, and the development of metabolic disorders.
This proposed strategy has been routinely applied for over a century since it first was proposed. Due to the appropriate mechanical endurance, accessibility and anatomical vascularization of the intestinal wall, it is a justified choice for bladder reconstruction. Nevertheless, established surgical techniques are reaching their limits in terms of a functional result that might be improved upon by gradual implementation of tissue engineering solutions. Ability to restore complex histological structures of the bladder wall with integrated epithelial, neural, and muscle components offers a superior treatment over currently available solutions. In this context, regenerative medicine should be a natural developmental path of urinary bladder reconstruction and as such, should gain attention of, and support from the urological community.
Despite the promising announcement of in vivo urinary bladder reconstruction by Atala et al., there has been a lack in commercialization of therapies based on current investigations 1. This is due to numerous obstacles that are slowly being identified and precisely overcome. A goal of regenerative medicine is to develop new concepts suitable for bladder regeneration that use biomaterials and stem cells. Our review provides a brief presentation of data regarding experimental bladder regeneration. We also highlight the obstacles that delay the introduction of regenerative medicine into clinical practice.
Tissue Engineering of the Urinary Bladder, for Whom and When?
As the field of tissue engineering advances, the reality of replacing a diseased urinary bladder is becoming a possibility. Before the introduction of this technology into the clinic, a need to identify objectives and specific contra indications justifying the tissue engineering approach is required.
Dividing urinary bladder disease into malignant and benign may be an indicator for replacement. Primary patient populations potentially suitable for tissue engineered urinary diversion have been identified by the authors (Fig. 1). These include the management of bladder cancer, neurogenic bladder conditions that threaten renal function, severe injury to the bladder due to radiation, intractable incontinence in females and chronic pain syndromes, and young patients with high‐pressure‐low‐compliant bladders due to congenital anomalies such as bladder exstrophy and myelomeningocele, which usually requires augmentation cystoplasty at the early stage of treatment.
Figure 1.

Indications for cystectomy with subsequently performed urine diversion comprise malignant and nonmalignant conditions. Invasive bladder cancer is the most common of them. Tissue engineering strategies included noncellular (A) and cellular (B) grafts, developed for bladder replacement.
Worldwide, urothelial bladder cancer is the ninth most common malignancy and 13th most common cause of cancer death 2. During 2017, an estimated 75,000 patients will be diagnosed with bladder cancer, corresponding to 5% of the annual cancer cases 3. Thus, bladder cancer is a major public health concern, hence, available therapies require continuous improvement. This is clearly demonstrated in the treatment of invasive bladder cancer, where the urologists must reconstruct the urinary tract after cystectomy and account for risk factors as well as the quality of life (QoL) after surgery in the patients. Worldwide, 95% of cystectomies performed are due to the diagnosis of invasive bladder cancer 4.
The choice of strategy for the treatment of bladder cancer depends on the presence of muscle wall infiltration. Approximately 70%–80% of the most common lesions are identified in the urothelium (pCIS), mucosa (pTa), or lamina propria (pT1), which are managed with bladder‐sparing treatments and rigorous surveillance due to recurrence and being at risk of progression to higher‐stage disease 5. In contrast, tumors invading the muscularis propia (pT2 or higher disease), require complex oncological management, that is, radical cystectomy (RC) 6. A particular group of pT1 patients with recognized high‐risk features (i.e., multifocality, recurrence after intravesical therapy, extensive lamina propria invasion, and concomitant carcinoma in situ [CIS]) are at considerable risk for disease progression and benefit in the short‐term from early radical management 7.
RC carries a significant risk of short‐ and long‐term complications. Despite improved patient selection, anesthetic, surgical refinements, and perioperative care, RC remains a technically challenging procedure 8. Routinely offered to oncological patients is the replacement of resected urinary bladder with orthotopic neobladder or conduit. These are the most frequently performed urinary diversions 9. Robot assisted surgery can perform total intracorporeal urinary diversion with lower risk of complications but with comparable functional outcomes to other open techniques 10. Notwithstanding this invasiveness reduction, explosion opening and detubularization of the bowel segment is a requirement of the procedure. This is recognized to be a major factor in determining high complication rates, especially in older patients 11. To date, the usual solutions involve using segments of intestine. Although these sophisticated surgical techniques restore the proper function of reconstructed urinary tracts, they may increase the risk of stricture and fistula formation, and the development of metabolic disorders 12.
From the early clinical attempts at the beginning of the century, the bowel wall has been routinely used to restore urinary tract continuity after cystectomy. This solution is a compromise between the intestine's biomechanical characteristics; suitable for urine storage and the risk for metabolic disorders related to a different histological structure. Chronic diarrhea, malabsorption syndromes, that is, such as vitamin B12 deficiency, electrolyte abnormalities including hyperchloremic metabolic acidosis, or hypokalemia, hypocalcemia, and hypomagnesemia are one of the main reasons for diminished QoL after urinary diversion 13. Depending on the bowel segment used, the length of the bowel segment and the type of diversion, these metabolic consequences will be more or less pronounced. In older patients with reduced body compensation, the ability of these disorders might even endanger patient lives. Thus, side effects of known urinary diversion techniques are the major argument for pushing forward tissue engineering research focused on urinary tract regeneration.
Reconstructive urologic surgeons consider continent orthotopic neobladder replacement as the most favorable in terms of QoL for patients. This is because of the ability to void naturally via the native urethra and the undisturbed body image 14. Nonetheless, based on literature, it remains unclear if this approach does in fact guarantee a better QoL for patients. Interestingly, even though using urostoma massively reduces the patient's QoL, having a neobladder was not proven to display better QoL 15. Studer et al., who developed and popularized orthotopic ileal neobladder, reliably demonstrated positive results for the long‐term follow‐up functional aspects for the procedure 16, 17. Ten years after the initial surgery, 96% of their patients could void spontaneously. Studer et al. explained their outcomes through rigorously developed proactive postoperative management that was difficult to comply with in other centers 18. Tissue engineering of the urinary bladder aims to provide a treatment method based on research and then commercialized bioengineered grafts. This strategy holds promise to provide replicative, highly standardized and characterized therapy worldwide. Hence, the clinical endpoint of each future tissue engineered solution should guarantee to have a lasting functional and sustainable result, of which will be less dependent on highly specialized academic departments. This approach is also believed to propagate tissue engineering therapies in countries with an underfunded healthcare system.
Tissue engineering in reconstructive urology is considered to be an experimental field without any successful translational research. In this situation, it is crucial to highlight the benefits of tissue engineered solutions and their potential superiority over currently used methods. Off‐the‐shelf tissue engineered implants serving as urinary diversion would revolutionize reconstructive urology by removing the necessity of using bowel as a replacement for the urinary bladder wall (Fig. 1).
Geriatric patients would greatly benefit from tissue engineered urinary diversion modalities. With advances in medical care, life expectancy continues to increase; a recent study showed that the mean life expectancy in the U.S. for men and women was 80 ± 7.0 and 80 ± 9.1 years, respectively. Bladder cancer is a disease primarily effecting the elderly, with a peak incidence occurring at 80 years of age 19. Maintenance of an active lifestyle for this group of patients will also gradually become more important and decisive during therapeutic planning. Moreover, the management of invasive bladder cancer in the elderly is particularly challenging due to age‐related complications and behavioral profile determining decreased compliance and adaptation to live with urinary diversion 20. Urinary diversions made from biomaterial might solve this problem. This approach would convert demanding reconstructive surgeries with many nonstandardized steps into a one‐stage procedure where resected bladder would be replaced with a commercial graft only needing ureter and urethra anastomosis. Nevertheless, creation of tissue engineering based on continent urinary diversion is still a visionary project. Thus, a more plausible option might be the introduction of an artificial urinary conduit as a first available product in urology obtained from the tissue engineering industry 21. This solution offers the ability to largely reduce the severity of urinary tract reconstruction after cystectomy and would certainly present a favorable treatment option for a number of patients from different age groups.
In context of this speculative paragraph, the expected constitution of regenerative medicine in urology may contribute to the recognition of new indications for total cystectomies. In the light of new abilities to regenerate or replace urinary bladders with a great functional result, decisions on treatment for benign bladder conditions will prioritize this approach. The concept of “preventing cystectomy” in patients with noninvasive bladder cancer could also be hypothetically established and gain importance.
Urinary Conduit Construction
The first experimental attempt to create a conduit urinary diversion with a tissue engineering approach was performed in 2007 on a rat model. In this study, SIS was used as scaffold, and seeded fibroblasts were intended to enhance the graft's biocompatibility 22. Five years later, a research team from The Netherlands published a similar strategy with a different scaffold material (Collagen type I with VyproII mesh) on porcine model 23. In both studies, no differences between seeded and unseeded scaffold were observed. Studies performed later showed superiority of scaffolds (Bladder acellular matrix [BAM]; polyglycoic acid [PGA]/poly(lactic‐co‐glycolic acid) [PLGA]) seeded with cells over the acellular ones 24, 25. The major leverage of tissue engineered conduit over neobladder is its less complex structure that can be obtained with available techniques.
Despite initial reports which failed to demonstrate the necessity of using a cell based approach, results from studies on induced regeneration of tubular organs like ureter, urethra, or blood vessels indicated better regeneration outcomes within cell seeded constructs 26. Soon, there will be a chance to receive the final answer which strategy is more efficient and should be translated into clinics. The research group who tried to reproduce results published by Atala et al. in 2006, registered a study entitled “Incontinent urinary diversion using and autologous neo‐urinary conduit” (NCT01087697) 27. Artificial urinary conduit was designed to be created form biodegradable a PGA/PLGA mesh seeded with fatty tissue derived smooth muscle cells. The most interesting part of this study included the evaluation of these artificial conduits during the clinical trial. The study's status is “completed,” and we are now awaiting results that may open up a new clinical application perspective for tissue engineering.
Tissue Engineering for Urinary Bladder; Current Status
Two major approaches of tissue or organ regeneration by tissue engineering include the use of acellular and cellular scaffolds. The acellular scaffold approach involves either natural or synthetic biomaterials that stimulate the in vivo spontaneous regenerative process by serving as a solid support for the in‐growth of the patient's native cells. The cellular scaffold approach involves biomaterials and patient's autologous cells to create neo‐tissue in vitro. Generated neotissue is implanted back into the patient in order to complete the regeneration process in vivo.
To date, urinary bladder reconstruction utilizing tissue engineering techniques, have been used in 141 patients (Fig. 2). Both acellular (n = 124) and cellular (n = 17) scaffolds were used. The most common indication for urinary bladder reconstruction was cancer and neurogenic dysfunctions (Fig. 3).
Figure 2.

Type of biomaterials used for urinary bladder regeneration in 141 patients. Indications for bladder reconstruction and applied material for augmentation among 141 cases does not indicate any variability of data. Abbreviations: BAM, bladder acellular matrix; BP, bovine pericardium; FPDB, formalin preserved dog bladder; GS, gelatin sponge; JP, Japanese paper; LHD, lyophilized human dura; PGA, polyglycoic acid; PLA, polylactic acid; PM, plastic mold; SIS, small intestinal submucosa.
Figure 3.
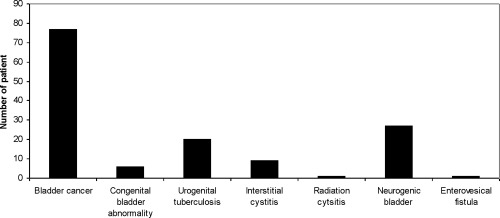
Indications for urinary bladder reconstruction in 141 patients treated by tissue engineering methods. Indications for bladder reconstruction and applied material for augmentation among 141 cases does not indicate any variability of data.
The first clinical studies that can be considered as prototypes for research on urinary bladder regeneration were performed in the 1950s and 1960s 28, 29, 30. Plastic urinary bladder substitutes were implanted orthotopically into 34 patients following cystectomy for several weeks and removed after covering with fibrotic tissue. The generated “pseudo‐bladder” experienced reduced capacity over time. High mortality and postoperative complications associated with the use of the plastic urinary bladder substitutes were a cause of discontinuation for this procedure. A very important fact regarding urinary bladder reconstruction was revealed during these clinical trials: acellular scaffolds allow for spontaneous regeneration of the urothelium but not smooth muscles.
Other biomaterials that have been used for urinary bladder reconstruction were gelatin sponge (n = 9), Japanese paper (n = 17), formalin preserved dog bladder (n = 12), lyophilized human dura (n = 44), bovine pericardium (n = 1), and small intestinal submucosa (n = 5) 31, 32, 33, 34, 35, 36, 37, 38, 39. These biomaterials provided a temporary scaffold for tissue growth, underwent remodeling and degradation over time. A frequently observed complication in these patients was progressive decreasement in urinary bladder capacity. These biomaterials were not used in any future studies despite good clinical outcomes observed in some patients.
Numerous experimental studies have demonstrated that functional bladder tissue can only be created by cell based approaches 40, 41. The first clinical study on urinary bladder reconstruction with a cell seeded scaffold was performed by Atala et al. 1. BAM or collagen‐polyglycoic acid (C/PGA) composite scaffold seeded with autologous urothelial and smooth muscle cells were used for augmentation in seven cystoplasty patients with end‐stage bladder disease due to myelomeningocele. For this purpose, urothelial and smooth muscle cells were isolated from small 1–2 cm2 bladder biopsies, expanded for 7–8 weeks and seeded into 70–150 cm2 of BAM or C/PGA scaffold at a density of 50 × 106 cells per cm3. The grafts were implanted with or without an omental wrapping. The bladders augmented with the composite scaffolds seeded with cells showed improved bladder compliance and increased capacity. Bladder biopsies performed at 31 months postoperatively revealed a proper three‐layered structure of reconstructed bladders. In addition, no adverse events were observed. In contrast, more extensive fibrosis and limited regeneration were observed in bladders reconstructed with BAM. For the BAM group, it should be noted that there was no omental wrapping of reconstructed bladders in three out of four patients. Therefore, it is unknown if the limited regeneration observed in this group were due to the use of a BAM scaffold or lack of omental wrapping. This study demonstrated that bladders tissue engineered with autologous cells seeded on C/PGA scaffolds with omental wrapping can be used for patients requiring cystoplasty 1.
Unfortunately, the recent publication by Joseph et al. was not able confirm these results 42 using an autologous cell seeded polyglycolide/polylactide (PGA/PLA) composite scaffold for augmentation cystoplasty in 10 patients with spina bifida. There was no clinical or statistical improvement in patient bladder capacity at 12 or 36 months. Serious adverse events of bowel obstruction and/or bladder rupture occurred in four patients. At the time of publication, five of the patients underwent or were scheduled for traditional cystoplasty. Although these two clinical studies were comparable in design and used the same clinical background, and cell types, the variability in cell number, biomaterial type, or graft surface area might have influenced the outcomes (Table 1). Therefore, further investigations are required to confirm both safety and efficiency of tissue engineered grafts for augmentation cystoplasty.
Table 1.
Clinical studies using cell seeded grafts for human urinary bladder regeneration
| Atala (2006) | Joseph (2014) | ||
|---|---|---|---|
| Number of patients | 4 | 3 | 10 |
| Patients age (years) | 4–19 | 4–19 | 3–21 |
| Indication for augmentation cystoplasty | Neurogenic bladder myelomeningocele | Neurogenic bladder myelomeningocele | Neurogenic bladder spina bifida |
| Biomaterial | BAM | C/PGA | PGA/PLA |
| Type and number of cells | UCs (50 × 106/cm3) SMCs (50 × 106/cm3) | UCs (50 × 106/cm3) SMCs (50 × 106/cm3) | UCs (no data) SMCs (no data) |
| Omental wrapping, number of patients | 1 | 3 | 10 |
| Bladder cycling technique | Yes | Yes | Yes |
| Follow‐up (months) | 22–61 | 22–61 | 12–36 |
| Postoperatively increased bladder capacity | No | Yes | No |
| Postoperatively improved bladder compliance | No | Yes | No |
| Adverse events | No adverse events | No adverse events |
Bowel obstruction bladder rupture |
| Conversion to traditional ileocystoplasty, number of patients | 0 | 0 | 5 |
Abbreviations: BAM, bladder acellular matrix; PGA, polyglycolic acid; PLA, polylactic acid; SMCs, smooth muscle cells; UCs, urothelial cells.
Urinary Tract Environment and Cellular Aspects of Tissue Engineering
Specific urinary tract cellular stress factors (urine, reduced blood perfusion, pathogens) makes the urinary bladder environment unfavorable for induced regeneration. Urine was proven to be one of the most influential factors that determined survival of implanted cells. In vitro studies carried out on urothelial cells and mesenchymal stem cells (MSC) with urine exhibited extreme cytotoxity 43, 44.
Cells seeded on a scaffold facing toward to the bladder lumen are directly exposed to stored urine, especially those located within inner biomaterial surface 21. Urine is rich in cationic substances, protamine sulfate and low molecular weight products that are mainly responsible for its high and nonselective cytotoxicity. When the protective uroplakin barrier is dysfunctional, as it is during early stages of urothelium regeneration, cytotoxic agents bind spontaneously to the anionic milieu of inner layers of the bladder mucosa. Thus, deep penetrating urine is believed to exert a significant impact on the survival of implanted stem cells 45. Since urine has a harmful effect on the cellular components of a tissue engineered bladder, it is important that the scaffold has adequate permeability since it might partially act as an isolation barrier. On the other hand, low porosity scaffolds have excellent resistance against fluid penetration and may prevent urine leakage from the graft, however, low porosity scaffolds inhibit seeded cell migration 46.
Interestingly, voided urine turned out to be a source of so‐called urine‐derived stem cells 47, 48. After isolation and expansion of this cell type, it was used for urinary‐tract regeneration 49. The influence of urine on urine‐derived stem cells after in vitro expansion has not been investigated yet; however, considering their origin, they must be more resistant to urine than other cell types. Investigation of the mechanism behind this possible resistance might be very useful to increase cell surveillance after implantation.
Many studies have confirmed the contribution of cells in bladder regeneration 50, 51. Diverse acellular scaffolds were found to be suitable only for regeneration of small defects using experimental models. From a clinical point of view, this is of no importance. In these cases, regeneration of the damaged area or an area of the resected bladder could be obtained by cells migrating from the surrounding native tissue under normal responses to tissue injury. Regeneration was observed mainly at the anastomosis edges of implanted grafts that were applied for augmentation in larger defects of the urinary bladder wall. Whereas the middle portion of the grafts were covered by overgrown fibrotic tissue 52. Cell seeded bioengineered grafts are believed to be more efficient at regenerative induction of the urinary bladder wall in comparison to biomaterial alone. Stem cells are an indispensable tool to modulate the host healing process resulting in gradual graft remodeling that finally may lead to regrowth of a layered structure of the neo‐bladder wall. Factors determining the restoration of the neo‐bladder's mechanical endurance and protection against urine are the regeneration process rate and efficiency 53. Highly advanced stem cell strategies addressing bladder regeneration are still under development. MSC, adipose stem cells, hair follicle stem cells, and stem cells derived from amniotic membrane were evaluated for this purpose; however, none were reported to have a specific superiority over another particular cell type 54. Therefore, MSC has become the most commonly applied stem cell of choice due to an abundance of niches, and well‐established isolation and in vitro propagation protocols 55. A number of different processes that determine regenerative outcomes were documented to be regulated by various MSC paracrine factors. Production and release of angiogenic factors such as VEGF and Ang1/Ang3 stimulates neovascularization and induces maturation of the newly regenerated area 56. Our previous work indicated that MSC upregulated anti‐inflammatory cytokines, that is, IL‐10 and Transforming Growth Factor beta (TGF‐β), while simultaneously attenuating pro‐inflammatory signals (IL‐1a, IL‐6, Transforming Growth Factor alpha [TGF‐α], Interferon gamma [INF‐γ]) 50. Apart from MSC, differentiated smooth muscle and urothelial cells were often evaluated for urinary bladder regeneration. These cells derived from bladder biopsies were successfully used for the regeneration of high‐pressure or poorly compliant bladders 1. A priori diseased cell source is a major limitation of autologous differentiated cell lines isolated from urinary tracts. Abnormal growth of isolated MSC was observed with less contractile ability and inferior adherence in neuropathic bladder dysfunction 57. Interstitial cystis, posterior urethral valves, epispadias, non‐neurogenic bladder dysfunctions, and other bladder conditions also reduce the proliferative capacity of isolated cells 58. The major disadvantage of bladder biopsy cells is the contraindications to use them for cell‐based management in patients with urothelial cancer. Isolated urothelial cells harboring activated oncogenes increases the risk of tumor formation within tissue engineered neo‐bladders due to the panurothelial nature of bladder cancer 59. Concentrating research efforts on MSC bladder regeneration will overcome this problem. These cells can be isolated from amniotic fluid, Wharton's jelly or the hair follicle; however, due to the availability and the ability of sufficient cell generation, bone‐marrow and adipose tissue are the most commonly used sources in bladder regeneration 60. MSC have the ability to differentiate into multiple cell types, including urothelial and smooth muscle cells 61, 62. Use of MSC for bladder regeneration includes the expansion of the cells and implantation with or without predifferentiation. Differentiation can be performed before or after scaffold seeding using a conditioned or chemically defined culture medium 63. Smaller defects do not require stem cell predifferentiation in vitro because after implantation into the bladder microenvironment, the cells can undergo differentiation into the desired cell type in vivo under the influence of trophic factors secreted by the urothelial and smooth muscle 64.
Whole bladder regeneration is more complex; stem cell predifferentiation could help in the restoration of bladder structure, but the differentiation efficiency is very low and the mechanism of this process is still unclear. In addition, little is known about the phenotypic stability of stem cell‐derived urothelial and smooth muscle cells following implantation 65.
In most studies, the fate of cells seeded onto scaffolds and implanted into bladders is unknown. Our unpublished data has revealed that only a small number of implanted cells stained positively with PKH‐26 remained in the regenerated area after 3‐months follow‐up. Another important issue is the cell seeding density onto the scaffold. In the same study, we determined that significantly better results can be obtained when higher cell seeding densities (4 × 106/cm2 vs. 10 × 106/cm2) onto the scaffold were used. Thus, the cell number is a crucial factor for determining the regenerative outcome.
Determinants for successful urinary bladder regeneration using tissue engineering techniques are the selection of a suitable cell source, use of optimal cell density, and elaboration of efficient stem cell differentiation techniques.
Challenges for Future Translation into Clinic ‐ The Need for System Thinking
The ultimate aim of tissue engineering is to grow an in vitro living urinary bladder that can replace a native one 59. The realization of this goal must be postponed until biotechnology has reached an appropriate level of development. At present, tissue engineering tries to establish an optional solution for patients needing bladder replacement. At this initial stage of translational research, it is crucial to organize collaborative teams of research scientists and clinical urologists to support multidisciplinary insights at each step of future therapy development 66. This approach should allow us to reduce research costs, better address patients’ needs, and prevent us from pursuing any potential dead ends. Urology is one of the fastest growing segments in the tissue engineering market, which is rapidly expanding to be worth $11.5 billion by 2022 67.
Translation of tissue engineered based therapies into urology should be conducted using cooperative infrastructure consisting of tissue engineering facilities and urological departments. Synchronization of cell culturing with biomaterial scaffold fabrication requires an establishment of well‐coordinated procedures. Formation of tissue engineering departments belonging to urology clinics may be leveraged in similar way as the cell‐transplantation units of hematology‐oncology. This organizational set‐up combining stem cell harvesting, culturing, and transplantation has been proven to be safe and therapeutically efficient.
Quality control of the biomaterials used in various surgical applications is a key challenge for the tissue engineering industry. Tissue engineering based approaches are classified as advanced medical therapy, which needs to be authorized through central agencies 68. The European Union institutions agreed on a regulation on advanced therapies coordinated by European Medicines Agency 69. Analogously, in U.S., the Center for Biologics Evaluation and Research (CBER) regulates cellular and biomaterial based therapies. CBER uses both the Public Health Service Act, and the Federal Food Drug and Cosmetic Act as enabling statutes for oversight 70.
Tissue engineered based solutions for urinary bladder replacement consist of biomaterial and cellular components that are subject to detailed certification in order to meet good manufacturing standards. Therapy evaluation comprised, inter alia, the manufacture of biomaterials in in vitro cell culture, scaffold seeding, demonstration of conserved mechanical properties, and safety/efficacy in adequate animal models. Efforts in the future will most likely focus on increasing the safety of tissue engineered products. Strict regulations on translating new bladder replacement modalities into the clinic are necessary in the lights of best clinical practice. Nevertheless, with too many bureaucratic and time‐consuming burdens/procedures, we risk successful lab research and new findings rarely being translated into clinical practice.
Mechanical Properties
In the field of the urinary bladder regeneration, the graft's mechanical behavior corresponding to the native one is particularly important 71. The mechanical characteristic of an intact bladder wall should be taken as a benchmark in efforts to construct a tissue engineered bladder. Nevertheless, we need to take into account that the urinary bladder's standard mechanical properties are resultant of passive and active behavior, and dynamic changes during the emptying and filling phase. Designing and fabricating biomaterial which could mimic these sophisticated biomechanical properties is limited by available technology 21. Alternatively, the mechanical properties of the bowel wall which are applied during reconstructive urology as a gold standard can be considered as a starting point for ongoing research. Following this idea, we should aim to produce a graft with mechanical features that would in fact reflect improved parameters of the bowel wall, adopted for the specific environment of urinary tracts. Such a biomaterial that would be superior to the bowel wall would become a new gold standard in reconstructive urology.
Artificially designed scaffolds for urinary bladder replacement should be endurable on low but continuous volume changes, lacking the tendency to repeated kinking, especially with downward abdominal pressure and subsequent obstruction 72. Major difficulty during preoperative scaffold evaluation is predicting how the regenerating tissue affects the biomaterial matrix remodeling after implantation. The graft should obtain the intended mechanical characteristics after completed neo‐tissue formation. Hence, tensions generated by connective tissue and regenerated muscle cells need to be taken into consideration in planning the final graft structure 73.
Considering the urinary tract's hydrodynamic features, matters of concern are potential determinants of long‐term biomaterial fatigue, that is, continuous flow and related volume extensibility during the storage phase. Before implementation, a fatigue test could easily reveal the durability of the fabricated neo‐tissue; however, no published studies characterized fatigue in the preclinical tissue engineered graft 74. Of utmost importance in reconstructive therapies dedicated to young patients with a long‐life expectancy is fatigue analysis.
A well‐functioning bladder substitute should contain less than 100 ml urine after voiding. A residual urine volume of less than 100 ml was proven not to increase the risk of ascending infection 75. The physiological background of the neobladder in terms of elasticity and compliance suggests that the biomechanical specification would be challenging. Resultant parameters of biochemical specification during the storage phase should provide conditions to reversibly increase the bladder capacity up to 500 ml several times a day. The neobladder needs to sustain a constant low renal pelvic pressure as a main generator of ureteral back pressure. Urinary tract obstruction was the leading cause of long‐term renal function impairment, regardless of whether the patient had ileal conduit diversion or orthotopic ileal neobladder 76.
The mechanical properties of an in vitro constructed neobladder are determined by the biomaterial and cellular components. Preclinical studies comprised of research involving animal models and bioreactors are required in order to obtain optimal mechanical characteristics for the tissue engineered bladder wall. Using this concept, a urinary tract‐specific physiological environment can be provided during neotissue maturation, ultimately improving data collection to better plan the clinical setting.
Vascularization
For adequate clinical use, a tissue engineered urinary neobladder should have a minimal capacity of approximately 100 ml. Consequently, if the topography of the neobladder is considered to be the irregular sphere, it would be an area of about 130 cm2 and a thickness of several millimeters.
Available biomaterial and cell culturing technologies provide solutions to engineer cell seeded scaffolds that fulfil the objectives of structural design. However, a major hurdle in growing neo‐organs lies in the formation of vascular networks capable of perfusing regenerating tissue after implantation 77.
The wall of the neobladder needs to be prevascularized to avoid graft necrosis since diffusion alone is not sufficient to maintain tissue oxygenation. In order to obtain sufficient functional results, an abundance of vascular networks is required to guarantee blood supply to metabolically active bladder walls rich in muscle 78. A recognized cause of continual bladder loss of function is the deteriorated microvasculature 79.
Although spontaneous vasculogenesis and angiogenesis take place after biomaterial implantation, these processes are not effective and fast enough to become a reliable option in terms of therapeutic application 80. Attempts to stimulate vascularization by enriching implants with pro‐angiogenic factors, that is, vascular endothelial growth factor (VEGF), matrix metalloprotease (MMP), basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF), angiotensin (Ang), and hepatocyte growth factor (HGF) have demonstrated limited use due to the time‐consuming nature, and the possible lack of ability to shape the growing vascular network architecture 81. One promising approach is prevascularization of a tissue construct with networks of well‐formed capillaries prior to transplantation. In order to achieve this aim, the best choice at present is de novo design and generation of the vascular network with the use of a customizable three‐dimensional (3D) bioprinting technology 82. Bertassoni et al. developed technology that prints a template using agarose fibers to fabricate microchannel networks that can be populated with endothelial cells 83. Capturing the normal architecture of a bladder wall and applying it as a template for the fabrication of a collagen‐based vascularized scaffold is possible in the long term, as recently demonstrated by Huling et al. 84. Attention should be focused on the characteristics of lower urinary tracts during vascular network design for any bladder substitution. With regards to these requirements, the arrangement of an artificially created vascular network must allow blood vessels to maintain patency and guarantee network sealing during wall stretching of the neobladder.
During partial bladder reconstruction, vascularization of scaffold could be achieved by alternatively implanting scaffolds into areas of the body of favorable local conditions for neo‐vascularization. Following this idea, the peritoneal cavity might serve as a natural bioreactor 85.
Following the critical stage after implantation of the prevascularized neobladder is to perform anastomosis with arterial and venous blood vessels in the host. Vascular network inosculation should take place within several minutes in order to increase cell survival and prevent necrosis 86. This pivotal problem is still waiting to be addressed by any methodology, nevertheless, urological surgeons would certainly prefer grafts that provide suturable vascular pedicles.
In regenerative medicine, we might use the opportunity to use pharmacodynamics for purposes other than those originally intended. Accordingly, well known PDE5 inhibitors for the treatment of erectile dysfunction might be useful in tissue engineering based reconstructive urology because of their pro‐angiogenic activity. In a small animal model, orally administrated PDE5 inhibitors facilitated the recruitment of endothelial progenitor cells from the blood and bone marrow, and contributed to the promotion of new capillary formation within the neobladder 87.
Fibrotic Reaction
Implantation of a biomaterial into the body always triggers foreign body response 88. This process is step‐wise, maintained by overlapping prolonged inflammation and wound healing, which drives gradual fibrotic encapsulation of the engrafted biomaterial. Considering a neobladder, the foreign body's surface will range from a few to tens of hundred square centimeters and as such, presents a risk of triggering extensive local scarring.
Fibrous tissue overgrowth first limits in vivo functionality and longevity of implantable constructs 89. Second, extensive local fibrosis would be likely initially spread in the pelvis and gradually ascend into the peritoneal cavity, resulting in severe adhesion formation.
Abdominal adhesions are related to chronic abdominal pain and might cause potentially life‐threatening situations, including mechanical ileus and bowel necrosis 90.
Altering the fibrotic response toward an engineered neobladder will not be a straightforward task. Different concepts to alleviate excessive tissue response have been proposed. Given the exposed mosaic signaling architecture regulating progression of fibrosis, a number of potential targets have been described 91. Multifactorial regulation is a major challenge. Selective targeting of TGF‐β as a factor pathway failed to hamper the fibrotic reaction in the clinical setting 91, 92, 93. Similarly, unsuccessful attempts to use IFN‐gamma endothelin agonist or antagonists of IL‐17 as an anti‐fibrotic treatment demonstrated the necessity for coordinated suppression of more than one pro‐fibrotic cascade 94, 95, 96.
From a urologist's perspective, the most efficient first line therapy overcoming biomaterial related fibrosis would be a pharmacological one. Despite rich experience in antifibrotic treatment, mainly gained from research on pulmonary fibrosis, there are a few management options which could affect the formation of a fibrous capsule around a neobladder. Pirfenidone, a medication with multidirectional antifibrotic action, might be appropriate as a perioperative treatment after performing urinary diversion with the use of biomaterials 97. Steroid and nonsteroid drugs slowed down pathologic‐fibrosis in many clinical settings. Unfortunately, since these agents may influence the behavior of cancer cells, they must be avoided until proper clinical evaluation is conducted 98.
Most research data is generated by analyzing disease related pathological fibrosis, but elective implantation of a neobladdder has advantages that should be exploited further. First, planned procedures give opportunity to intervene from the very beginning and counteract the activation of fibrotic mechanisms. Second, anti‐fibrotic agents could be incorporated within an implanted scaffold, which may exert a strong local impact on the host healing process. Alternatively, developing a minimal invasive laparoscopic or robotic extraperitoneal technic of graft implantation might also limit perioperative site scarring and reduce the risk of severe abdominal adhesion.
Innervation
Lower urinary tracts are innervated with cholinergic, peptidergic, and nitrergic nerve fibers of peripheral autonomic and somatic nerve systems 99. Balanced interplay between the activity of these neuronal networks regulate a proper bladder function. According to the principles of artificial organ engineering, the restoration of bladder innervation might be achieved by regeneration of neuronal components and shaping of the neuronal network, or by replacing it with an artificially designed material 100. The solution of this important problem was, however, hardly ever highlighted in experimental settings 101. We are currently focused on inducible regeneration of the urothelial and smooth muscle layers that alone do not guarantee proper bladder function. During further research, the need for neuronal network substitute will emerge to re‐establish ascending and descending neuronal transmission as well as autonomic neobladder activity.
Tissue engineering has developed technology to culture neurons that might be applied in attempts to create an artificially designed neuronal network within the neobladder wall.
Ma et al. provided one of the first reports demonstrating that neural progenitor cells cultured in 3D matrix spontaneously recapitulated functional synapses 102. Ban et al. proved that cultivated neurons preserved the capacity of generating signal transduction networks 103. The major obstacle, however, is to constitute a cell source in the human body from where neuronal cells could be extracted without harm. An interesting strategy with translational potential is to harvest neuronal precursors by stereotactic biopsy from niches in adult central nervous system (CNS) with preserved neurogenesis 104. Alternatively, a variety of stem cells might be considered for differentiation into neurons as it has been already proposed 105, 106.
To be consistent with the above‐proposed scenario, forming a neuronal network might be guided by biochemical, biophysical, and topographical signals provided by a scaffold that could be individually rendered by neuronal modeling experts. Moe et al. used a customizable multi‐architecture chip based on Polydimethylsiloxane that could control differentiation of neuronal progenitor cells 107.
Although de novo regeneration of a convolutional neural network within an artificial urinary bladder is far beyond tissue engineering technology, this difficulty can be approached differently. Biocompatible current conductive material might be applied as a replacement for a neuronal network in order to propagate action potentials generated in computer‐controlled implantable stimulator. This computer unit would additionally couple signaling between the neobladder and the host's neuronal system. It should also be noticed that further advances in a tissue engineered graft innervation are inherently dependent from research on urinary bladder neurohistology which still needs to be better defined.
The Microenvironment of Tissue Engineered Graft
Tissue engineered urinary diversions are inspired by the biology of urinary tracts. The biomimetic approach aims to create a graft milieu that is similar to the physiological environment of the developing tissue 108. The basis of this strategy is to speed up graft intake in a positive manner and to induce subsequent remodeling. The microenvironment of the tissue engineered graft is built from two inherent components: cellular and biomaterial that both interfere with the host systems 109. Before implantation, cell seeded grafts grown in incubators with strictly controllable surroundings provide cultivated cells an optimal microenvironment. After implantation, these cells are suddenly exposed to a much more complex microenvironment laden with pro‐inflammatory mediators and activated immunoreactive cells 110. In this scenario, the microenvironmental niche should play a supportive role for the self‐renewal, survival, and differentiation of the applied cell populations, and act as a shield to damaging factors. Thus, tissue engineering methods should provide a stable cell‐friendly microenvironment to engrafted cells after implantation 111. The response on this requirement could be a cocoon like architecture of the biomaterial matrix that protects the graft's cellular component from damaging agents. Coupling of immunomodulatory agents to biomaterial scaffolds might change them into “immunomodulating” biomaterials that could actively shape host response 112. Hypothetically, along with the better understanding of paracrine signaling pathways it could be possible to guide behavior of host immune cells and direct them to support graft intake.
Summary
Tissue engineered grafts designed to replace urinary bladder will become in future new gold standard of reconstructive urology. Apart from the above‐mentioned challenges that are the focus and attention of major researchers, there are many other clinically relevant issues that need to be raised: sterilization of tissue engineered constructs, biomaterial‐associated thrombosis, risk factors for abscess formation within a large‐sized artificial graft, graft adaptation for robotic or laparoscopic implantation, imaging modalities for evaluating a graft's remodeling, impact of aging on the regenerative capacity of human urinary tracts since all preclinical trials are planned on young, and large animal models. These secondary issues also require to be widely discussed before we can safely translate tissue engineering based solutions into reconstructive urology.
Author Contributions
J.A. and M.P.: conception and design, manuscript writing; S.V.V.B. and T.K.: manuscript writing; T.D.: conception and design, manuscript writing. administrative support.
Disclosure of Potential Conflicts of Interest
T.D. received honoraria as a Speaker for Astellas, Speaker for Bayer. The other authors indicated no potential conflicts of interest.
References
- 1. Atala A, Bauer SB, Soker S et al.Tissue‐engineered autologous bladders for patients needing cystoplasty. Lancet 2006;15:1241–1246. [DOI] [PubMed] [Google Scholar]
- 2. Dobruch J, Daneshmand S, Fisch M et al. Gender and bladder cancer: A collaborative review of etiology, biology, and outcomes. Eur Urol 2016;69:300–310. [DOI] [PubMed] [Google Scholar]
- 3. Troussard G, Shariat SF, Dragomir A et al. Conditional survival after radical cystectomy for bladder cancer: Evidence for a patient changing risk profile over time. Eur Urol 2014;66:361–370. [DOI] [PubMed] [Google Scholar]
- 4. Takenaka A. Current status of robot‐assisted radical cystectomy: What is the real benefit? Yonago Acta Med 2015;58:95–99. [PMC free article] [PubMed] [Google Scholar]
- 5. Alfred Witjes J, Lebret T, Compérat EM et al. Updated 2016 EAU guidelines on muscle‐invasive and metastatic bladder cancer. Eur Urol 2017;71:462–475. [DOI] [PubMed] [Google Scholar]
- 6. Witjes JA, Compérat E, Cowan NC et al. European Association of Urology. EAU guidelines on muscle‐invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur Urol 2014;65:778–792. [DOI] [PubMed] [Google Scholar]
- 7. Jäger W, Thomas C, Haag S et al. Early vs delayed radical cystectomy for ‘high‐risk' carcinoma not invading bladder muscle: Delay of cystectomy reduces cancer‐specific survival. BJU Int 2011;108:E284–288. [DOI] [PubMed] [Google Scholar]
- 8. van Hemelrijck M, Thorstenson A, Smith P et al. Risk of in‐hospital complications after radical cystectomy for urinary bladder carcinoma: Population‐based follow‐up study of 7608 patients. BJU Int 2013;112:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clifford TG, Shah SH, Bazargani ST et al. Prospective evaluation of continence following radical cystectomy and orthotopic urinary diversion using a validated questionnaire. J Urol 2016;196:1685–1691. [DOI] [PubMed] [Google Scholar]
- 10. Ahmed K, Khan SA, Hayn MH et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot‐assisted radical cystectomy: Results from the International Robotic Cystectomy Consortium. Eur Urol 2014;65:340–347. [DOI] [PubMed] [Google Scholar]
- 11. Buscarini M, Pasin E, Stein JP. Complications of radical cystectomy. Minerva Urol Nefrol 2007;59:67–87. [PubMed] [Google Scholar]
- 12. Vasdev N, Moon A, Thorpe AC. Metabolic complications of urinary intestinal diversion. Indian J Urol 2013;29:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krajewski W, Piszczek R, Krajewska M et al. Urinary diversion metabolic complications—underestimated problem. Adv Clin Exp Med 2014;23:633–638 [DOI] [PubMed] [Google Scholar]
- 14. Park J, Ahn H. Radical cystectomy and orthotopic bladder substitution using ileum. Korean J Urol 2011;52:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh V, Yadav R, Sinha RJ et al. Prospective comparison of quality‐of‐life outcomes between ileal conduit urinary diversion and orthotopic neobladder reconstruction after radical cystectomy: A statistical model. BJU Int 2014;113:726–732. [DOI] [PubMed] [Google Scholar]
- 16. Zehnder P, Dhar N, Thurairaja R et al. Effect of urinary tract infection on reservoir function in patients with ileal bladder substitute. J Urol 2009;181:2545–2549. [DOI] [PubMed] [Google Scholar]
- 17. Kassouf W, Hautmann RE, Bochner BH et al. A critical analysis of orthotopic bladder substitutes in adult patients with bladder cancer: Is there a perfect solution? Eur Urol 2010;58:374–383. [DOI] [PubMed] [Google Scholar]
- 18. Studer UE. Life is good with orthotopic bladder substitutes! BJU Int 2014;113:686–687. [DOI] [PubMed] [Google Scholar]
- 19. Shariat SF, Milowsky M, Droller MJ. Bladder cancer in the elderly. Urol Oncol 2009;27:653–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shariat SF, Sfakianos JP, Droller MJ et al. The effect of age and gender on bladder cancer: A critical review of the literature. BJU Int 2010;105:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kloskowski T, Pokrywczyńska M, Drewa T. Artificial urinary conduit construction using tissue engineering methods. Cent European J Urol 2015;68:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drewa T. The artificial conduit for urinary diversion in rats: A preliminary study. Transplant Proc 2007;39:1647–1651 [DOI] [PubMed] [Google Scholar]
- 23. Geutjes P, Roelofs L, Hoogenkamp H et al. Tissue engineered tubular construct for urinary diversion in a preclinical porcine model. J Urol 2012;188:653–660. [DOI] [PubMed] [Google Scholar]
- 24. Basu J, Jayo MJ, Ilagan RM et al. Regeneration of native–like neo–urinary tissue from nonbladder cell sources. Tissue Eng Part A 2012;18:1025–1034. [DOI] [PubMed] [Google Scholar]
- 25. Liao W, Yang S, Song C et al. Tissue–engineered tubular graft for urinary diversion after radical cystectomy in rabbits. J Surg Res 2013;182:185–191. [DOI] [PubMed] [Google Scholar]
- 26. Sloff M, Simaioforidis V, Tiemessen DMJ et al. Tubular constructs as artificial urinary conduits. J Urol 2016;196:1279–1286. [DOI] [PubMed] [Google Scholar]
- 27. Kates M, Singh A, Matsui H et al. Tissue‐engineered urinary conduits. Curr Urol Rep 2015;16:1–8. [DOI] [PubMed] [Google Scholar]
- 28. Bohne AW, Urwiller KL. Experience with urinary bladder regeneration. J Urol 1957;77:725–732. [DOI] [PubMed] [Google Scholar]
- 29. Portilla Sanchez R, Blanco FL, Santamarina A et al. Vesical regeneration in the human after total cystectomy and implantation of a plastic mould. Br J Urol 1958;30:180–188. [DOI] [PubMed] [Google Scholar]
- 30. Tsulukidze A, Murvanidze D, Dvali R et al. Formation of a bladder by a plastic shell after total cystectomy. Br J Urol 1964;36:102–105. [DOI] [PubMed] [Google Scholar]
- 31. Tsuji I, Kuroda K, Fujieda J et al. Clinical experiences of bladder reconstruction using preserved bladder and gelatin sponge bladder in the case of bladder cancer. J Urol 1967;98:91–92. [DOI] [PubMed] [Google Scholar]
- 32. Tsuji I, Kuroda K, Fujieda J et al. A clinical and experimental study on cystoplasty not using the intestine. J Urol 1963;89:214–225. [DOI] [PubMed] [Google Scholar]
- 33. Orikasa S, Tsuji I. Enlargement of contracted bladder by use of gelatin sponge bladder. J Urol 1970;104:107–110. [DOI] [PubMed] [Google Scholar]
- 34. Fujita K. The use of resin‐sprayed thin paper for urinary bladder regeneration. Invest Urol 1978;15:355–357. [PubMed] [Google Scholar]
- 35. Taguchi H, Ishizuka E, Saito K. Cystoplasty by regeneration of the bladder. J Urol 1977;118:752–756. [DOI] [PubMed] [Google Scholar]
- 36. Kelami A. Duroplasty of the urinary bladder—results after two to six years. Eur Urol 1975;1:178–181. [PubMed] [Google Scholar]
- 37. Arikan N, Ozdiler E, Yaman O et al. Augmentation duracystoplasty in neurogenic bladder dysfunction. Int J Urol 1995;2:172–175. [DOI] [PubMed] [Google Scholar]
- 38. Caione P, Boldrini R, Salerno A et al. Bladder augmentation using acellular collagen biomatrix: A pilot experience in exstrophic patients. Pediatr Surg Int 2012;28:421–428. [DOI] [PubMed] [Google Scholar]
- 39. Moon SJ, Kim DH, Jo JK et al. Bladder reconstruction using bovine pericardium in a case of enterovesical fistula. Korean J Urol 2011;52:150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoo JJ, Meng J, Oberpenning F et al. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology 1998;51:221–225. [DOI] [PubMed] [Google Scholar]
- 41. Oberpenning F, Meng J, Yoo JJ et al. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 1999;17:149–155. [DOI] [PubMed] [Google Scholar]
- 42. Joseph DB, Borer JG, De Filippo RE et al. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: Phase II study in children and adolescents with spina bifida. J Urol 2014;191:1389–1395. [DOI] [PubMed] [Google Scholar]
- 43. Davis NF, Callanan A, McGuire BB et al. Evaluation of viability and proliferative activity of human urothelial cells cultured onto xenogenic tissue‐engineered extracellular matrices. Urology 2011;77:1007–1007. [DOI] [PubMed] [Google Scholar]
- 44. Adamowicz J, Kloskowski T, Tworkiewicz J et al. Urine is a highly cytotoxic agent. Does it influence on the stem cell therapies in urology. Transplant Proc 2012;44:1439–1441. [DOI] [PubMed] [Google Scholar]
- 45. Rajasekaran M, Stein P, Parsons CL. Toxic factors in human urine that injure urothelium. Int J Urol 2006;13:409–414. [DOI] [PubMed] [Google Scholar]
- 46. Kloskowski T, Kowalczyk T, Nowacki M et al. Tissue engineering and ureter regeneration: Is it possible? Int J Artif Organs 2013;36:392–405. [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, McNeill E, Tian H et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol 2008;180:2226–2233. [DOI] [PubMed] [Google Scholar]
- 48. Kloskowski T, Nowacki M, Pokrywczyńska M et al. Urine‐ a waste or the future of regenerative medicine? Med Hypotheses 2015;84:344–349. [DOI] [PubMed] [Google Scholar]
- 49. Wu S, Liu Y, Bharadwaj S et al. Human urine‐derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011;32:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pokrywczynska M, Jundzill A, Bodnar M et al. Do mesenchymal stem cells modulate the milieu of reconstructed bladder wall? Arch Immunol Ther Exp (Warsz) 2013;61:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lam Van Ba O, Aharony S, Loutochin O et al. Bladder tissue engineering: A literature review. Adv Drug Deliv Rev 2015;82–83:31–37. [DOI] [PubMed] [Google Scholar]
- 52. Dorin RP, Pohl HG, De Filippo RE et al. Tubularized urethral replacement with unseeded matrices: What is the maximum distance for normal tissue regeneration? World J Urol 2008;26:323–326. [DOI] [PubMed] [Google Scholar]
- 53. Kim JH, Lee SR, Song YS et al. Stem cell therapy in bladder dysfunction: Where are we? And where do we have to go? Biomed Res Int 2013;2013:930713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Orabi H, Bouhout S, Morissette A et al. Tissue engineering of urinary bladder and urethra: Advances from bench to patients ScientificWorldJournal 2013;2013:154564. [DOI] [PMC free article] [PubMed]
- 55. Jundziłł A, Pokrywczyńska M, Adamowicz J et al. Vascularization potential of electrospun poly(L‐Lactide‐co‐Caprolactone) scaffold: The impact for tissue engineering. Med Sci Monit 2017;23:1540–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kusuma GD, Carthew J, Lim R et al. Effect of the microenvironment on mesenchymal stem cells paracrine signalling: Opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26:617–631. [DOI] [PubMed] [Google Scholar]
- 57. Lin HK, Cowan R, Moore P et al. Characterization of neuropathic bladder smooth muscle cells in culture. J Urol 2004;171:1348–1352. [DOI] [PubMed] [Google Scholar]
- 58. Subramaniam R, Hinley J, Stahlschmidt J et al. Tissue engineering potential of urothelial cells from diseased bladders. J Urol 2011;86:2014–2020. [DOI] [PubMed] [Google Scholar]
- 59. Adamowicz J, Kowalczyk T, Drewa T. Tissue engineering of urinary bladder—current state of art and future perspectives. Cent European J Urol 2013;66:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Drewa T, Adamowicz J, Sharma A. Tissue engineering for the oncologic urinary bladder. Nat Rev Urol 2012;9:561–572. [DOI] [PubMed] [Google Scholar]
- 61. Yang B, Zheng JH, Zhang YY. Myogenic differentiation of mesenchymal stem cells for muscle regeneration in urinary tract. Chin Med J (Engl) 2013;126:2952–2959. [PubMed] [Google Scholar]
- 62. Liu J, Huang J, Lin T et al. Cell‐to‐cell contact induces human adipose tissue‐derived stromal cells to differentiate into urothelium‐like cells in vitro. Biochem Biophys Res Commun 2009;390:931–936. [DOI] [PubMed] [Google Scholar]
- 63. Tian H, Bharadwaj S, Liu Y et al. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: Potential for urological tissue engineering. Tissue Eng Part A 2010;16:1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bury MI, Fuller NJ, Meisner JW et al. The promotion of functional urinary bladder regeneration using anti‐inflammatory nanofibers. Biomaterials 2014;35:9311–9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pokrywczynska M, Adamowicz J, Sharma AK et al. Human urinary bladder regeneration through tissue engineering—an analysis of 131 clinical cases. Exp Biol Med (Maywood) 2014;239:264–271. [DOI] [PubMed] [Google Scholar]
- 66. Williams DF. 25 Tissue engineering: The multidisciplinary epitome of hope and despair. In: Paton R, McNamara LA, eds. Studies in Multidisciplinarity. Elsevier, 2005;3:483–524.
- 67.Arundhati Parmar Medical Device Business. Available at http://www.mddionline.com/article/tissue-engineering-market-be-worth-115-billion-2022. Accessed April 12, 2017.
- 68.Regenerative medicine and the regulation of advanced therapies medicinal products. Available at https://www.hta.gov.uk/policies/regenerative-medicine-and-regulation-advanced-therapies-medicinal-products-atmps. Accessed April 12, 2017.
- 69.Multidisciplinary: Cell therapy and tissue engineering. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000405.jsp&mid=WC0b01ac058002958a. Accessed April 12, 2017.
- 70.About the Center for Biologics Evaluation and Research. Available at http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER. Accessed April 12, 2017.
- 71. Miftahof RN, Nam HG. Biomechanics of the Human Urinary Bladder. Springer 2013.
- 72. Kollhoff DM, Cheng EY, Sharma AK. Urologic applications of engineered tissue. Regen Med 2011;6:757–765. [DOI] [PubMed] [Google Scholar]
- 73. Kheradmandi M, Vasheghani‐Farahani E, Ghiaseddin A et al. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J Biomed Mater Res A 2016;104:1720–1727. [DOI] [PubMed] [Google Scholar]
- 74. Martin C, Sun W. Fatigue damage of collagenous tissues: Experiment, modeling and simulation studies. J Long Term Eff Med Implants 2015;25:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McNeill SA, Hargreave TB, Geffriaud‐Ricouard C et al. Postvoid residual urine in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Pooled analysis of eleven controlled studies with alfuzosin. Urology 2001;57:459–465. [DOI] [PubMed] [Google Scholar]
- 76. Jin XD, Roethlisberger S, Burkhard FC et al. Long‐term renal function after urinary diversion by ileal conduit or orthotopic ileal bladder substitution. Eur Urol 2012;61:491–497. [DOI] [PubMed] [Google Scholar]
- 77. Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 2011;63:300–311. [DOI] [PubMed] [Google Scholar]
- 78. Scheepe JR, Amelink A, Wolffenbuttel KP et al. Influence of sildenafil on blood oxygen saturation of the obstructed bladder. BMC Urol 2014;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scheepe JR, Amelink A, de Jong BW et al. Changes in bladder wall blood oxygen saturation in the overactive obstructed bladder. J Urol 2011;186:1128–1133. [DOI] [PubMed] [Google Scholar]
- 80. Sun X, Altalhi W, Nunes SS. Vascularization strategies of engineered tissues and their application in cardiac regeneration. Adv Drug Deliv Rev 2016;96:183–194. [DOI] [PubMed] [Google Scholar]
- 81. Moon JJ, West JL. Vascularization of engineered tissues: Approaches to promoteangio‐genesis in biomaterials. Curr Top Med Chem 2008;8:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bae H, Puranik AS, Gauvin R et al. Building vascular networks. Sci Transl Med 2012;4:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bertassoni LE, Cecconi M, Manoharan V et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014;14:2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huling J, Ko IK, Atala A et al. Fabrication of biomimetic vascular scaffoldsfor 3D tissue constructs using vascular corrosion casts. Acta Biomater 2016;32:190–197. [DOI] [PubMed] [Google Scholar]
- 85. Laschke MW, Menger MD. Prevascularization in tissue engineering: Current concepts and future directions. Biotechnol Adv 2016;34:112–121. [DOI] [PubMed] [Google Scholar]
- 86. Cheng G, Liao S, Kit Wong H et al. Engineered blood vessel networks connect to host vasculature via wrapping‐and‐tapping anastomosis. Blood 2011;118:4740–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sahara M, Sata M, Morita T et al. A phosphodiesterase‐5 inhibitor vardenafil enhances angiogenesis through a protein kinase G‐dependent hypoxia‐inducible factor‐1/vascular endothelial growth factor pathway. Arterioscler Thromb Vasc Biol 2010;30:1315–1324. [DOI] [PubMed] [Google Scholar]
- 88. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008;20:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Porras AM, Hutson HN, Berger AJ et al. Engineering approaches to study fibrosis in 3‐D in vitro systems. Curr Opin Biotechnol 2016;40:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Holmdahl L, Risberg B, Beck DE et al. Adhesions: Pathogenesis and prevention‐panel discussion and summary. Eur J Surg Suppl 1997;577:56–62. [PubMed] [Google Scholar]
- 91. Boccafoschi F, Mosca C, Cannas M. Cardiovascular biomaterials: When the inflammatory response helps to efficiently restore tissue functionality? J Tissue Eng Regen Med 2014;8:253–267. [DOI] [PubMed] [Google Scholar]
- 92. Meng XM, Nikolic‐Paterson DJ, Lan HY. TGF‐β: The master regulator of fibrosis. Nat Rev Nephrol 2016;12:325–338. [DOI] [PubMed] [Google Scholar]
- 93. Hawinkels LJ, Ten Dijke P. Exploring anti‐TGF‐β therapies in cancer and fibrosis. Growth Factors 2011;29:140–152. [DOI] [PubMed] [Google Scholar]
- 94. Opitz CF, Ewert R, Kirch W et al. Inhibition of endothelin receptors in the treatment of pulmonary arterial hypertension: Does selectivity matter? Eur Heart J 2008;29:1936–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Raghu G, Brown KK, Bradford WZ et al. Idiopathic Pulmonary Fibrosis Study Group. A placebo‐controlled trial of interferon gamma‐1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. [DOI] [PubMed] [Google Scholar]
- 96. Miossec P, Kolls JK. Targeting IL‐17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 2012;11:763–776. [DOI] [PubMed] [Google Scholar]
- 97. Badylak S. Host Response to Biomaterials: The Impact of Host Response on Biomaterial Selection. Elsevier 2015.
- 98. Rosenbloom J, Mendoza FA, Jimenez SA. Strategies for anti‐fibrotic therapies. Biochim Biophys Acta 2013;1832:1088–1103. [DOI] [PubMed] [Google Scholar]
- 99. Eastham JE, Gillespie JI. The concept of peripheral modulation of bladder sensation. Organogenesis 2013;9:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Adamowicz J, Drewa T, Tworkiewicz J et al. Schwann cells—a new hope in tissue engineered urinary bladder innervation. A method of cell isolation. Cent European J Urol 2011;64:87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Drewa T, Adamowicz J, Lysik J et al. Chitosan scaffold enhances nerve regeneration within the in vitro reconstructed bladder wall: An animal study. Urol Int 2008;81:330–334. [DOI] [PubMed] [Google Scholar]
- 102. Ma W, Fitzgerald W, Liu QY et al. CNS stem and progenitor cell differentiation into functional neuronal circuits in three‐dimensional collagen gels. Exp Neurol 2004;190:276–288. [DOI] [PubMed] [Google Scholar]
- 103. Ban J, Bonifazi P, Pinato G et al. Embryonic stem cell‐derived neurons form functional networks in vitro. Stem Cells 2007;25:738–749. [DOI] [PubMed] [Google Scholar]
- 104. López‐Juárez A, Howard J, Ullom K et al. Gsx2 controls region‐specific activation of neural stem cells and injury‐induced neurogenesis in the adult subventricular zone. Genes Dev 2013;27:1272–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Guan M, Xu Y, Wang W et al. Differentiation into neurons of rat bone marrow‐derived mesenchymal stem cells. Eur Cytokine Netw 2014;25:58–63. [DOI] [PubMed] [Google Scholar]
- 106. Zheng YH, Xiong W, Su K et al. Multilineage differentiation of human bone marrow mesenchymal stem cells in vitro and in vivo. Exp Ther Med 2013;5:1558–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Moe AA, Suryana M, Marcy G et al. Microarray with micro‐ and nano‐topographies enables identification of the optimal topography for directing the differentiation of primary murine neural progenitor cells. Small 2012;8:3050–3061. [DOI] [PubMed] [Google Scholar]
- 108. Godier AF, Marolt D, Gerecht S et al. Engineered microenvironments for human stem cells. Birth Defects Res C Embryo Today 2008;84:335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wanjare M, Huang NF. Regulation of the microenvironment for cardiac tissue engineering. Regen Med 2017;12:187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Huang NF, Li S. Regulation of the matrix microenvironment for stem cell engineering and regenerative medicine. Ann Biomed Eng 2011;39:1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nakayama KH, Hou L, Huang NF. Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering. Adv Healthc Mater 2014;3:628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vishwakarma A, Bhise NS, Evangelista MB et al. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol 2016;34:470–82. [DOI] [PubMed] [Google Scholar]


