Abstract
Bioprinting is a quickly progressing technology, which holds the potential to generate replacement tissues and organs. Stem cells offer several advantages over differentiated cells for use as starting materials, including the potential for autologous tissue and differentiation into multiple cell lines. The three most commonly used stem cells are embryonic, induced pluripotent, and adult stem cells. Cells are combined with various natural and synthetic materials to form bioinks, which are used to fabricate scaffold‐based or scaffold‐free constructs. Computer aided design technology is combined with various bioprinting modalities including droplet‐, extrusion‐, or laser‐based bioprinting to create tissue constructs. Each bioink and modality has its own advantages and disadvantages. Various materials and techniques are combined to maximize the benefits. Researchers have been successful in bioprinting cartilage, bone, cardiac, nervous, liver, and vascular tissues. However, a major limitation to clinical translation is building large‐scale vascularized constructs. Many challenges must be overcome before this technology is used routinely in a clinical setting. Stem Cells Translational Medicine 2017;6:1940–1948
Keywords: Bioprinting, Stem cells, Tissue engineering, Artificial organs, Transplantation
Significance Statement.
This concise review presents the evolving technology bioprinting and its major components with a particular focus on bioink materials consisting of biomaterials and living cells. Various stem cells, used as a bioink material for bioprinting processes, are discussed thoroughly and their use in fabrication of different tissue types are expounded.
Essentials of Bioprinting
Bioprinting is the spatial patterning of living cells and other nonliving biologic materials using an additive manufacturing technique 1. The materials are assembled using a computer‐aided layer‐by‐layer deposition approach for fabrication of living tissue and organ analogs used in tissue engineering, regenerative medicine, pharmacokinetic, and other biological studies 2, 3. This technique allows for precise control of the micro architecture and macroarchitecture of tissues and organs, which is critical to the function of many biological tissues and organs. An assortment of bioinks are available including hydrogels, microcarriers, tissue spheroids, cell pellet, tissue strands, and decellularized matrix components. They are used with a variety of bioprinting processes, including droplet‐, extrusion‐, and laser‐based bioprinting 4. These diverse inks and techniques each have their own advantages and disadvantages 5, and allow for customization of a range of complex tissues including cartilage, bone, cardiac muscle, neural tissue, liver, and vasculature. Various bioinks are used to create each of these tissue types. Bioink can be defined as the bioprintable material consisting of living cells, proteins and other biologics loaded into a matrix. They mimic the extracellular matrix (ECM) to support cells. An ideal bioink material should be biocompatible, bioprintable, affordable, cell‐friendly, mechanically strong and structurally stable, and possesses the solidification ability by means of cross‐linking (i.e., physical, enzymatic and ionic) or aggregation of cells.
Cell Source
In order to bioprint tissues for transplantation, the cell component of the bioink should be autologous and patient specific. In addition, most tissues consist of multiple cell types, which all have various functions. Stem cells are thus a promising choice as they have the ability to differentiate into multiple cell types for fabrication of autologous tissues. Therefore, the use of stem cells is highly critical to process an appropriate bioink material. Bioprinting applications use multiple stem cell types including embryonic, induced pluripotent, and adult stem cells (Fig. 1).
Figure 1.
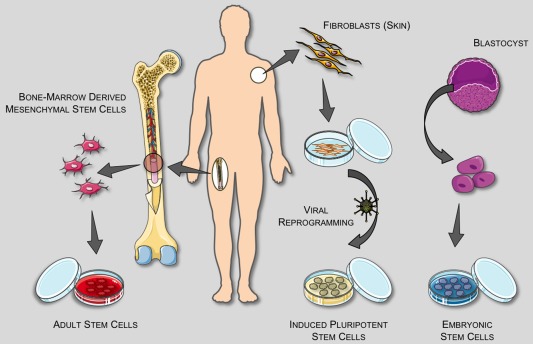
Stem cell sources. [Reproduced with permission from Christopherson, G. T. and Nesti, L. J. “Stem Cell Applications in Military Medicine” [Stem Cell Research & Therapy 2011;2:40]].
Embryonic Stem Cells
Embryonic stem cells (ESCs) are pluripotent stem cells isolated from the blastocyst stage of in vitro fertilized embryos 6. The embryos are cultured on a feeder layer of irradiated mouse fibroblasts with growth factors. Newer methods have been developed to culture cells without the mouse feeder layer to decrease the risk of viral transfer 7. Many ethical debates were sparked by the use of fertilized embryos and therefore other researchers starting using dead embryos 8 and single cell biopsy 9. ESCs proliferating in culture for at least 6 months without differentiating, that appear genetically normal, are considered an ESC line and can be frozen and sent to other laboratories for use. They can then undergo directed differentiation into various cell types. However, ESC use in research in the U.S. is currently limited.
Induced Pluripotent Stem Cells
Induced pluripotent stem cells (iPSCs) are adult fibroblasts genetically reprogrammed to have an embryonic like state 7. Mouse iPSCs were first described in 2006 and human iPSCs in 2007 10. Four transcription factors found to be important in ESCs were introduced into fibroblasts using viruses to generate iPSCs: Oct3/4, Sox2, c‐Myc, and Klf4. Oct3/4 expression levels determine the fate of the inner cell mass 11. Sox2 interacts with Oct3/4 to control gene expression and is important in maintaining pluripotency 12. C‐Myc plays an important role in growth control and differentiation of cells 13 whereas klf4 is important for stem cell renewal and maintenance of pluripotency 14. iPSCs are considered pluripotent, but have a lower differentiation capacity than ESCs and also carry an increased risk of teratoma formation 15, 16. Therefore, others have developed chemically‐iPSCs in mice 17.
Adult Stem Cells
Bone Marrow Stem Cells
Bone marrow stem cells (BMSCs) are a type of adult stem cell found in bone marrow. Adult stem cells are multipotent and reside in an area called the “stem cell niche.” They remain quiescent until they are activated to maintain normal tissues or repair diseased and injured tissues. They typically exist in small quantities and have a limited capacity to divide in vitro. It is thought that they would not induce rejection after transplantation of differentiated cells, thereby eliminating the need for immunosuppressive drugs that have many harsh side effects. Bone marrow contains both hematopoietic stem cells and stromal stem cells. The stromal stem cells make up a small portion of the bone marrow and can generate many tissue types 7. They require less in vitro manipulation than ESCs and iPSCs, and have a much lower rate of malignant transformation than iPSCs 18. However, their differentiation potential decreases with increasing age 19, 20 and harvest of BMSCs requires a painful procedure.
Adipose Derived Stem Cells
Adipose derived stem cells (ADSCs) are another type of adult stem cell abundant in white adipose (fat) tissue. They were first isolated from lipoaspirates in 2001 21, but can also be obtained via lipectomy. With over 235,237 liposuction procedures and 127,633 lipectomies performed in the U.S. in 2016 22, they offer easy accessibility. The infrapatellar fat pad is an alternative source of ADSCs, which show optimal results for cartilage and bone engineering 23. ADSCs are found in large numbers and have a longer lifespan than BMSCs 24. They offer up to a fivefold increase in stem cell yield compared to BMSCs 25. They hold great promise for autologous tissue fabrication.
INK
Bioinks are often adapted from hydrogels and derived from natural polymers. They are sensitive to harsh processing environments and often have high water content. Therefore, they are printed at lower temperatures than traditional three‐dimensional (3D) printing and mild crosslinking agents or conditions are used. These mild conditions also ensure cell viability. The components are also chosen for their structural, sacrificial, functional, or supportive characteristics. According to their base materials, bioinks can be classified into two major categories including scaffold‐based and scaffold‐free bioinks 26.
Scaffold‐Based Bioinks
Scaffold‐based bioinks consist of cells dispersed within hydrogels or decellularized matrix components (dECM), or seeded on microcarriers that help to create a conducive environment for cell proliferation as well as providing structural support. Both differentiated cells and stem cells can be used in bioinks. Ideal bioinks are accurate when printed, crosslinkable, maintain their properties after polymerization, biocompatible, and undergo controlled degradation and ECM production. The most common type of bioinks are hydrogels, which can be natural or synthetic. Natural hydrogels include collagen, fibrin, chitosan, and alginate. Synthetic polymers are artificial and therefore their properties are more controllable than natural polymers, but their long‐term effects on cells are unknown. Common synthetic hydrogels include methacrylated gelatin, Pluronic, and polyethylene glycol (Table 1). Researchers often combine various hydrogels and other components to improve bioink characteristics. Decellularized matrix components are a newer bioink source obtained by chopping tissue into small fragments, lysing the cells, and extracting the remaining ECM. However, ECM loses its mechanical and structural integrity during processing; therefore, a supportive frame is required during bioprinting 2. Microcarriers have recently been used in bioprinting to increase the cell density in bioinks. They are porous particles designed to promote cell attachment, survival, and expansion. They can easily be suspended in culture media due to their low density 41 and microcarrier/cell complexes can be embedded in hydrogels for use in bioprinting. However, limitations include nozzle clogging during bioprinting and possible toxic byproducts of degradation.
Table 1.
Various natural and synthetic hydrogels with their type, advantages, and disadvantages
| Hydrogel | Type | Advantages | Disadvantages | Cell type used | References |
|---|---|---|---|---|---|
| Agarose | Natural | High mechanical strength, low cost | Low cell adhesion | Human neural stem cells, porcine BMSCs | 27, 28, 29 |
| Alginate | Natural | Fast gelation, low cost, good stability | Poor cell attachment, easily clogs at high concentrations | Human neural stem cells, porcine BMSCs | 27, 28, 30 |
| Chitosan | Natural | Antibacterial & antifungal | Slow gelation, poor mechanical properties | Human neural stem cells, human ADSCs | 27, 29, 31 |
| Collagen I | Natural | Promotes cell attachment, good printing abilities, have RGD sequencea | Poor mechanical stability, slow gelation, easily clogs | Human amniotic fluid stem cells, human BMSCs | 29, 32 |
| Fibrin | Natural | Promotes angiogenesis, fast gelation | Poor mechanical stability, easily clogs | Human amniotic fluid stem cells, human BMSCs | 32, 33 |
| Gelatin | Natural | Reversible, promotes cell adhesion | Unstable/fragile, poor abilities without modification | Human cardiac progenitor cells | 34 |
| Hyaluronic acid (HA) | Natural | Promotes proliferation and angiogenesis, fast gelation | Rapid degradation, poor mechanical stability | Human cardiac progenitor cells, human BMSCs | 34, 35 |
| Matrigel | Natural | Promotes differentiation | Clogs easily, made from tumor cells | Human epithelial cells | 36 |
| Methacrylated gelatin/gelatin methacryloyl (GelMA) | Synthetic | Easily degradable, high mechanical strength | Slow gelation, requires ultraviolet (UV) light which causes cell damage | Porcine BMSCs, human BMSCs | 28, 37 |
| Pluronic | Synthetic | Reversible (good sacrificial ink) | Poor mechanical stability, rapid degradation, requires thermal control | Human endothelial cells, bovine chondrocytes | 39, 40 |
| Polyethylene glycol (PEG) | Synthetic | Good when combined with other components | Low cell proliferation & adhesion, poor mechanical strength, UV causes cell damage | Porcine BMSCs, human BMSCs | 28, 40 |
RGD = tripeptide Arg‐Gly‐Asp sequence which mediates cell attachment.
Abbreviations: ADSCs, adipose derived stem cells; BMSCs, bone marrow stem cells.
Scaffold‐Free Bioinks
Cells in scaffold‐free constructs are bioprinted without a supporting hydrogel and therefore cells are loaded in higher concentrations. Tissue strands, pellets, or spheroids can be created. They deposit their own ECM components, which provides support as well as facilitates cell to cell communication and maturation 2. This approach is only compatible with extrusion‐based bioprinting (EBB). Various methods have been used to facilitate the assembly of spheroids 42. They are bioprinted in close proximity and allowed to fuse during maturation. Several challenges exist with spheroids including the requirement of a delivery medium (sacrificial ink) for extrusion, premature fusion causing nozzle clogging, and gaps between printed spheroids leading to leaky tissues. Cells can instead be bioprinted into an inert hydrogel mold as a pellet and triggered to aggregate 43. Final tissue size is limited by mold dimensions, which prevents easy human clinical translation. To overcome this, tissue strands were developed. Using a custom nozzle, long strands can be printed without the use of a mold 44. Scale‐up tissues fabricated from cell aggregates will require vascularization as hypoxia occurs at diameters greater than 400 µm 45. Due to these limitations, most researchers still use scaffold‐based approaches 2.
Bioprinting Processes
A medley of different bioprinting processes can be used to achieve the desired additive manufacturing goal and tissue fabrication. The three main modalities include droplet‐, extrusion‐, and laser‐based bioprinting (LBB). These all have advantages and disadvantages, may be bioink specific, and are summarized in Table 2.
Table 2.
Types of bioprinting modalities and their respective characteristics. Droplet‐based bioprinting (DBB), extrusion‐based bioprinting (EBB), and laser‐based bioprinting (LBB). [data has been derived from 46]
| DBB | EBB | LBB | |
|---|---|---|---|
| Cost | Lowa | Medium | High |
| Viscosity | <15 mPa/s | <6 x107 mPa/sa | <300 mPa/s |
| Cell density | <106 cells/ml | High, spheroidsa | <108 cells/ml |
| Print speed | Medium | Slow | Fasta |
| Resolution | 50–100 µm | 100 µm | 20 µma |
| Common bioinks | Agarose, alginate, collagen, fibrin, methacrylated gelatin, polyethylene glycol | Alginate, hyaluronic acid, polyethylene glycol, agarose, collagen, gelatin, pluronic, matrigel, fibrina | Alginate, collagen, gelatin, matrigel |
| Cell viability | >85% | 80% | 95%a |
Denotes best for that characteristic.
Droplet‐Based Bioprinting
Droplet‐based bioprinting (DBB) includes inkjet, acoustic‐droplet‐ejection, and microvalve bioprinting (Fig. 2A) 47. Inkjet bioprinting was the first bioprinting technology developed and is the most commonly used type of droplet‐based bioprinter. It is based on standard two‐dimensional inkjet printing and a traditional printer can be modified 48. The bioink is stored in a cartridge and manipulated to form droplets using gravity, atmospheric pressure, and fluid mechanics. Inkjet bioprinting can be further broken down into three types: continuous, drop‐on‐demand, and electrohydrodynamic 47. Drop‐on‐demand requires high pressures to eject droplets through a nozzle, which can be harmful to cells whereas electrohydrodynamic jet bioprinters use an electric field to pull the bioink through limiting shear stress‐induced cell damage.
Figure 2.
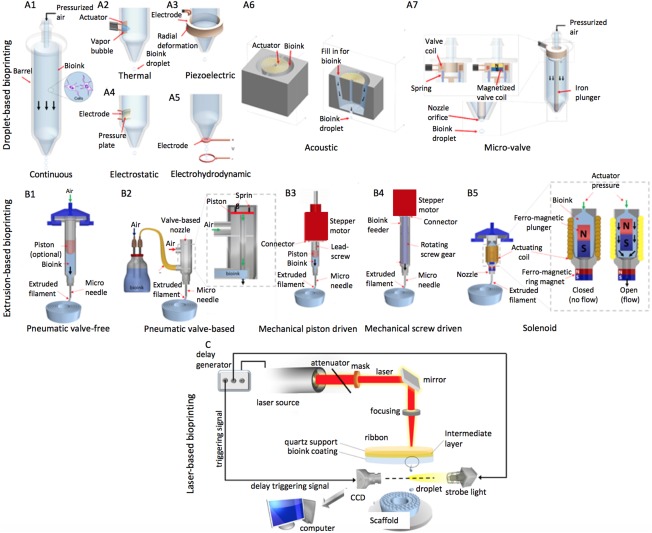
Bioprinting modalities. (A): Mechanisms of droplet‐based bioprinting. Inkjet bioprinting techniques: (A1): continuous‐ink‐jetting relies on Rayleigh‐Plateau instability, which breaks bioink jets into droplets; (A2): thermal drop‐on‐demand bioprinting uses a thermal actuator to locally heat bioink solutions to generate droplets; (A3): piezoelectric drop‐on‐demand bioprinting depends on radial deformation of a piezoelectric actuator to generate droplets; (A4): electrostatic bioprinting relies on deflection of pressure plate to generate droplets; (A5): electrohydrodynamic jetting uses an electric field, resulting from the electric potential difference between the printhead and the substrate, to pull a stream of bioink droplets through the printhead orifice. (A6): Acoustic‐droplet ejection relies on a gentle acoustic field generated by an acoustic actuator to eject droplets from an open pool of bioink solution. (A7): Microvalve (solenoid) bioprinting operates with an electromechanical valve to dispense droplets. (B): Mechanisms of extrusion‐based bioprinting. (B1): pneumatic microextrusion including (B2) valve‐free and (B3) valve‐based, (B4) mechanical microextrusion including (B5) piston‐ or (B6) screw‐driven and (B7) solenoid microextrusion. (C): Schematics of laser printing setup based on laser‐induced forward transfer: the upper donor slide is coated underneath with a thin laser energy absorbing layer and a layer of biological material to be transferred. The donor slide is placed above a second collector slide. Laser pulses are focused on the donor slide, evaporate the absorbing layer, and generate vapor pressure propelling the cell containing hydrogel toward the collector slide. Abbreviation: CCD, charge‐coupled device.
Acoustic‐droplet ejection bioprinting applies an acoustic field to eject droplets from a pool instead of a nozzle 49. A piezoelectric substrate is used to generate acoustic waves and droplets are ejected when the force from the waves overcomes the surface tension. As a result, cells are not exposed to the stress of inkjet printing; however, the acoustic field can be easily disrupted leading to poor depositional control. In microvalve bioprinting, an electromechanical valve is used to generate droplets 50. The bioink is housed within a pressurized fluid chamber and gated by a microvalve, then dispersed in a continuous manner or drop on demand depending on the pressure and gating time. Cell damage is limited, but larger droplets (50–300 µm) lead to a lower resolution. Highly complex constructs can be created with all types of droplet based bioprinters 51. These bioprinters tend to be affordable and user friendly 47.
Extrusion‐Based Bioprinting
EBB uses the shear thinning behavior of bioink materials and has progressed significantly in the past decade (2). Bioink is deposited from a fluid dispensing system under control of a computer and can be dispensed in cylindrical lines rather than droplets (Fig. 2B). Fluid deposition is driven by a pneumatic, mechanical, or solenoid system. Post‐printing cell viability is usually around 80% but can be as high as 97% with optimization of process parameters 2. Cell survival is decreased with increasing pressure, nozzle gauge, and shear stress. Computer aided design (CAD) software is easily incorporated and the continuous deposition improves structural integrity 52. EBB also offers greater printing speed, facilitating scalability and clinical translation, and a larger variety of inks are able to be used. However, resolution is typically limited to 100 µm 53.
Laser‐Based Bioprinting
LBB uses a donor layer comprised of a ribbon structure (Fig. 2C). A laser pulse creates a bubble at the interface and propels the bioink to form a droplet 54. Mechanical stress is reduced because the technology is nozzle free and cells do not have direct contact with the printer (unlike the other bioprinting modalities), leading to high cell viability of >95% 51. Additionally, highly viscous materials can be printed and resolution is the best of all methods. It is also highly precise and enables cells to be placed within 5 µm of the template 55. Despite these benefits, the cellular effects of laser exposure are not known, lasers are expensive compared to the other systems, and the systems are large and complex.
Bioprinted Tissues
Cartilage
Osteoarthritis affects 54.4 million people in the U.S. 56. Bioprinting technologies are unique in that they enable the precise patterning of multiple cell types and materials to re‐create the native structure of cartilage. Yu et al. fabricated scalable tissue strands using chondrocytes which were then bioprinted using a coaxial extrusion system to form larger tissues upon cell fusion, such as a cartilage patch 44. Zhang et al. seeded MSCs in a 3D printed poly ɛ‐caprolactone scaffold and placed them in meniscectomy defects in rabbits 57. The scaffolds increased fibrocartilage tissue regeneration and mechanical strength, suggesting their potential as an alternative meniscal substitute. Nguyen et al. bioprinted iPSCs combined with irradiated chondrocytes and hyaline cartilage tissue which formed hyaline like cartilage with type II collagen expression (Fig. 3A) 58. Recently, another group compared three different bioinks loaded with BMSCs (a) GelMA, (b) GelMA + chondroitin sulfate aminoethyl methacrylate (CS‐AEMA), and (c) GelMA + CS‐AEMA + hyaluronic acid methacrylate 38. The cells were then differentiated into chondrocytes post‐printing. Enhanced viability and chondrogenic differentiation was seen as well as accuracy of the method, suggesting this as a model for engineering cartilage tissue.
Figure 3.
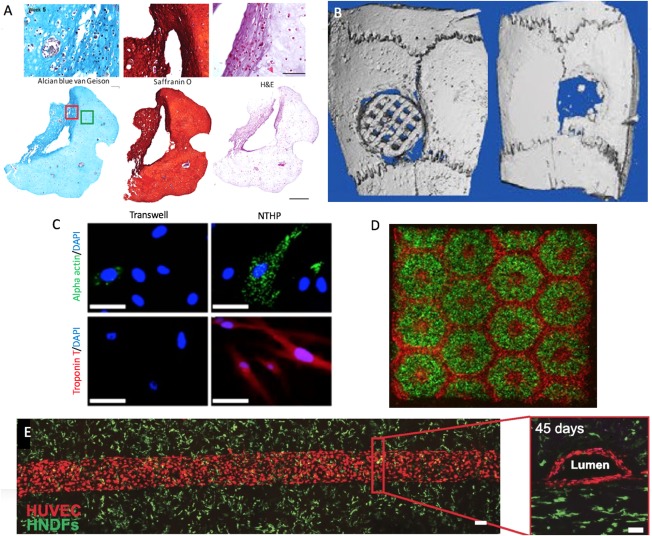
Bioprinted tissues. (A): 3D‐bioprinted chondrocyte‐derived iPSCs at week 5 of differentiation, sections stained for GAGs, Safranin O for cartilage (with nuclear counterstain), and H&E for extracellular matrix (with nuclear counterstain) (the scale bar represents 100 μm or 500 μm) (Reproduced with permission from 58). (B): Micro‐CT images of polylactic acid/hydroxyapatite scaffold (left) versus bone defect without scaffold (right) after 4 weeks in vivo. (Adapted and reproduced with permission from 58). (C): Immunocytochemistry of MSCs for cardiac proteins in the transwell versus nanothin and highly porous membrane methods (Adapted and reproduced with permission from 59). (D): 3D bioprinting of hydrogel based hepatic construct. Images (×5) showing patterns of fluorescently labeled hiPSC‐HPCs (green) in 5% (wt/vol) GelMA and supporting cells (red) in 2.5% (wt/vol) GelMA with 1% GMHA on day 0 (Scale bars, 500 µm; Reproduced with permission from 60). (E): Top‐down (left) and cross‐sectional (right) confocal microscopy images of bioprinted vascular networks supporting human neonatal dermal fibroblast‐laden (green) matrix and HUVEC (red) lined channels obtained after 45 days of perfusion culture. (Scale bar: 100 µm; Reproduced with permission from 61). Abbreviations: DAPI, 4′6‐diamidino‐2‐phenylindole; H&E, hematoxylin and eosin; HNDFS, human neonatal dermal fibroblasts; HUVECs, human umbilical vein endothelial cells; NTHP, nanothin and highly porous.
Bone
Bony defects most commonly occur after trauma or tumor resection. Currently, hardware is placed or bone grafts are used to reconstruct defects, but many limitations exist. Pati et al. added ECM to their scaffolds to mimic the bony microenvironment and showed upregulation of four osteoblastic genes as well as increased calcium deposition compared to bare scaffolds 62. In vivo testing showed greater bone formation. Gao et al. used thermal inkjet printing to study osteogenesis of printed BMSCs in polyethylene glycol demethyacrylate, bioactive glass, and hydroxyappetite (HA) 36. The HA group showed the highest cell viability and compressive modulus at 21 days as well as the most collagen production and highest alkaline phosphatase activity. Patel et al. also suggested that the addition of HA enhances BMSC differentiation by promoting endogenous osteogenic signals 63. In vivo study of 3D‐printed polylactic acid/HA scaffolds seeded with BMSCs showed they have good osteogenic capability with no difference in inflammation (Fig. 3B) 64.
Cardiac Muscle
Coronary artery disease is a leading cause of morbidity and mortality in the U.S. 65. Bioprinted cardiac patches can be used to help heal damaged myocardium after a heart attack. Human cardiac derived cardiomyocyte progenitor cells (hCPCs) were printed with alginate and had 92% viability after 1 day and 89% after 7 days 35. They also retained their commitment to a cardiac lineage with enhanced gene expression of early transcription factors, suggesting they could be used in cardiac tissue engineering. Gaebel fabricated a cardiac patch using laser‐induced‐forward‐transfer (LIFT) consisting of polyester urethane urea with human umbilical vein endothelial cells (HUVECs) and MSCs 66. The patches were transferred into infarcted rat hearts and increased vessel formation as well as functional improvement was seen in the LIFT group. Another group used dECM‐based bioink with hCPCs or turbinate MSCs to fabricate cardiac patches 67. Patterned patches (containing both cell types) reduced cardiac hypertrophy and fibrosis, increased migration to the infarcted area, and improved neo‐muscle and capillary function in in vivo. Nanothin cell sheets can also be printed with various cells in different layers. Human BMSCs and rat cardiomyocytes were cocultured on a printed cellulose acetate membrane and remained highly viable and transferable suggesting that this platform would be effective for therapeutic use (Fig. 3C) 59.
Neural Tissue
Peripheral nerve injuries are common after trauma with 1.4 million injuries occurring per year in the U.S. 68. The gold standard for a nerve conduit is use of an autograft; however, this has limitations including donor site morbidity. Due to this, a number of approaches have been studied including bioprinting. Owens et al. used multicellular cylindrical units composed of BMSCs and Schwann cells to fabricate fully cellular bioprinted nerve grafts 69. They demonstrated the recovery of both motor and sensory function with long‐term functionality at 40 weeks in a rat sciatic nerve injury model. Other groups have worked on creating functional neural tissues. Gu et al. used a novel alginate, carboxymethyl‐chitosan, and agarose bioink with neural stem cells and showed the differentiated neurons form synaptic contacts, establish networks, are spontaneously active, and show increased calcium response to bicuculline 28. Another group printed neural stem cells in thermoresponsive biodegradable polyurethane and studied the constructs in a zebrafish traumatic brain injury model 70. Function was improved after implantation of one form of the constructs, suggesting this bioink may offer new possibilities in neural tissue engineering.
Liver
Many patients with end‐stage‐liver‐disease die waiting for an organ because donors are scarce, leading to alternative strategies for liver replacement. One group bioprinted iPSCs and differentiated them into hepatocytes showing that stem cells can maintain their pluripotency after bioprinting 71. Another group printed ADSCs and were able to successfully convert them into a hepatogenic lineage with expression of liver genes 31. A new cross‐linking system was used in their study, which improved gene expression profiles. Ma et al. successfully printed a hexagonal structure of liver cells and supporting cells (Fig. 3D) 60. Other groups have developed liver models with organized hepatic structures which may be used for drug testing 72. These organs‐on‐a‐chip will likely be the first successes in organ bioprinting 73.
Vasculature
Vasculature network formation is a major limiting factor in the creation of scaled‐up tissues and organs. Constructs larger than 200–400 µm require vasculature, as this is the maximum diffusion distance 74. All three printing modalities have difficulty creating complex hollow structures. One approach to create vasculature is to use a sacrificial material. Initially agarose was used and it was removed with a vacuum, but this compromised channel structures 75. Therefore, other groups have used Pluronic, which is a solution below 4ºC. Kolesky et al. printed Pluronic channels and endothelialized them with HUVECs 39. They showed that the bioprinted vasculature remains stable during long‐term perfusion (45 days) 61. On the other hand, direct bioprinting can be used. Dolati et al. used a coaxial nozzle to print scaffold free perfusable vascular conduits which were mechanically strong and cell viability was high 76. Since capillaries are too small to be printed, angiogenesis is relied on to create fine interconnections between bioprinted microvascular channels (100 µm; Fig. 3E) 77.
Transplantation of Bioprinted Tissues
Challenges and Requirements
Surgical challenges similar to allogenic organ transplants including cellular ischemia, vascular anastomoses, and size match will persist. However, ischemia time should be limited since the organ can be perfused in laboratory settings. Any construct greater than 100–200 μm requires vascularization 73. Fabrication of an organ with vascularity is a major area of research currently. Additionally, size match can be planned pre‐operatively with imaging such as CT scans and CAD. The implanted tissue or organ must consist of biocompatible materials that integrate with native cells, allow for vascular ingrowth, and avoid immune response. Many ethical dilemmas and regulatory issues will likely develop as this technology progresses 78.
Immune Acceptance
Patients who receive allogenic organ transplants are subjected to lifelong immunosuppression to prevent immune rejection. These drugs have many associated side effects including increased infection and cancer risk. Their levels must be monitored regularly and sometimes they do not prevent rejection, subjecting patients to prolonged steroid courses and hospitalizations. Steroids have additional side effects of hyperglycemia, weight gain, osteoporosis, and decreased muscle function 79. Adult stem cells offer the ability for autologous tissue production which may avoid the need for immunosuppression. In addition, supporting materials and bioink components will need to be biocompatible with low inflammatory response to prevent tissue inflammation and macrophage recruitment.
Monitoring
Traditional organ transplant and free flap patients are initially placed in intensive care units for close monitoring postoperatively. This will likely remain a necessity as the surgeries will be similar in technique and duration, therefore leading to similar physiology and patient monitoring requirements. Even though immunosuppression will likely not be needed, the grafts will still be monitored for rejection. Overall organ function can be monitored with laboratory testing or imaging. For example, blood urea nitrogen, creatinine, and electrolytes can be used to monitor kidney function or cardiac enzymes (creatinine kinase and troponin) and echocardiogram to monitor heart function. Biomedical devices or implants also have an increased infection risk with increased morbidity and mortality 80. Any sign of infection would require prompt initiation of antimicrobial therapy.
Future Perspectives
One of the major hurdles to fabrication of large scale organs for transplantation is the incorporation of vasculature at multiple scales 81. Macroscale vasculature will be needed for surgical anastomosis and creation of this will likely be accomplished by advances in bioprinting techniques. These larger vessels will then need to be integrated with microscale capillaries. Since direct bioprinting of capillaries is not feasible due to current resolution of bioprinting techniques, microvascular channels can be bioprinted with adjacent endothelial cells to allow angiogenesis to create capillary networks. In addition, growth factors can be incorporated in the media to enhance angiogenesis.
Before clinical application, stem cell processing must be standardized as well as the quality of stem cells used. There are currently several different processes, which are still evolving. As native tissues and organs are made of multiple cell types, robust and efficient stem cell differentiation protocols are required. The lack of standardized differentiation protocols leads to varying results between groups. Additionally, differentiation requires prolonged culture periods leading to risk of contamination and use of antimicrobials and antimycotics. Human forms of all serum reagents used in cell culture must be developed to prevent transfer of zoonoses. Core manufacturing facilities for stem cell processing and tissue biofabrication must be developed and isolation facilities will need to be created in clinics in order to facilitate transition into clinical use. Bioprinted tissues will face many regulatory hurdles as they will likely be monitored as a device, biologic, and/or drug by the Food and Drug Administration.
Conclusion
There have been many advances in bioprinting technologies over the past several decades, including scaled‐up tissues and integration of vascularization. Multiple tissue types have been fabricated from both primary and stem cells including cartilage, bone, cardiac muscle, neural tissue, liver, and vasculature. However, many hurdles must still be overcome before wide‐scale clinical applicability is achieved. Technical challenges of creating human‐scale tissues with physiologically‐relevant vasculature and cell distributions in additional to economic and ethical obstacles currently limit clinical implementation. Nonetheless, these novel technologies have great potential to fabricate replacement tissues and organs in the future. Organ‐on‐a‐chip models will likely serve as an intermediate step toward the creation of large scale vascularized organs.
Author Contributions
A.N.L.: literature review and analysis, manuscript writing; D.J.R.: manuscript writing, financial support; A.D.: manuscript writing; I.T.O.: conception and design, final approval of manuscript, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This work was supported by the U.S. National Science Foundation CMMI Awards 1349716 (ITO) and 1462232 (ITO), Diabetes in Action Research and Education Foundation Grant 426 (ITO), the Osteology Foundation Grant 15‐042 (ITO), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under BIRCWH award K12HD055882 “Career Development Program in Women's Health Research at Penn State” (DJR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the abovementioned funding agencies.
References
- 1. Ozbolat IT, Peng W, Ozbolat V. Application areas of 3D bioprinting. Drug Discov Today 2016;21:1257–1271. [DOI] [PubMed] [Google Scholar]
- 2. Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion‐based bioprinting. Biomaterials 2016;76:321–343. [DOI] [PubMed] [Google Scholar]
- 3. Guillemot F, Mironov V, Nakamura M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B'09). Biofabrication 2010;2:010201. [DOI] [PubMed] [Google Scholar]
- 4. Hospodiuk M, Dey M, Sosnoski D et al. The bioink: A comprehensive review on bioprintable materials. Biotechnol Adv 2017;35:217–239. [DOI] [PubMed] [Google Scholar]
- 5. Ozbolat IT, Moncal KK, Gudapati H. Evaluation of bioprinter technologies. Addit Manuf 2017;13:179–200. [Google Scholar]
- 6. Thomson JA, Itskovitz‐Eldor J, Shapiro SS et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 7.National Institutes of Health, U.S. Department of Health and Human Services. Available at https://stemcells.nih.gov/info/basics/3.htm. Accessed March 1, 2017.
- 8. Zhang X, Stojkovic P, Przyborski S et al. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells 2006;24:2669–2676. [DOI] [PubMed] [Google Scholar]
- 9. Chung Y, Klimanskaya I, Becker S et al. Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature 2006;439:216–219. [DOI] [PubMed] [Google Scholar]
- 10. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 11. Okumura‐Nakanishi S, Saito M et al. Oct‐3/4 and Sox2 regulate Oct‐3/4 gene in embryonic stem cells. J Biol Chem 2005;280:5307–5317. [DOI] [PubMed] [Google Scholar]
- 12. Zhang S, Cui W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells 2014;6:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffman B, Liebermann D. Apoptotic signaling by c‐MYC. Oncogene 2008;27:6462–6472. [DOI] [PubMed] [Google Scholar]
- 14. Zhang P, Andrianakos R, Yang Y et al. Kruppel‐like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating Nanog gene expression. J Biol Chem 2010;285:9180–9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic instability of iPSCs: Challenges towards their clinical applications. Stem Cell Rev Rep 2016;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewandowski J, Kurpisz M. Techniques of human embryonic stem cell and induced pluripotent stem cell derivation. Arch Immunol Ther Exp (Warsz) 2016;64:349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hou P, Li Y, Zhang X et al. Pluripotent stem cells induced from mouse somatic cells by small‐molecule compounds. Science 2013;341:651–654. [DOI] [PubMed] [Google Scholar]
- 18. Marquez‐Curtis LA, Janowska‐Wieczorek A, McGann LE et al. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015;71:181–197. [DOI] [PubMed] [Google Scholar]
- 19. Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev 2010;16:445–453. [DOI] [PubMed] [Google Scholar]
- 20. Im G‐I. Bone marrow‐derived stem/stromal cells and adipose tissue‐derived stem/stromal cells: Their comparative efficacies and synergistic effects. J Biomed Mater Res Part A 2017;105:2640–2648. [DOI] [PubMed] [Google Scholar]
- 21. Zuk PA, Zhu M, Mizuno H et al. Multilineage cells from human adipose tissue: Implications for cell‐based therapies. Tissue Eng 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 22.ASPS. Available at https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/2016‐plastic‐surgery‐statistics‐report.pdf. Accessed March 27, 2017.
- 23. Tangchitphisut P, Srikaew N, Numhom S et al. Infrapatellar fat pad: An Alternative source of adipose‐derived mesenchymal stem cells. Arthritis 2016;2016:4019873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Locke M, Windsor J, Dunbar PR. Human adipose‐derived stem cells: Isolation, characterization and applications in surgery. ANZ J Surg 2009;79:235–244. [DOI] [PubMed] [Google Scholar]
- 25. Bunnell BA, Flaat M, Gagliardi C et al. Adipose‐derived stem cells: Isolation, expansion and differentiation. Methods 2008;45:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ozbolat IT. Scaffold‐based or scaffold‐free bioprinting: Competing or complementing approaches? J Nanotechnol Eng Med 2015;6:024701. [Google Scholar]
- 27. Gu Q, Tomaskovic‐Crook E, Lozano R et al. Functional 3D neural mini‐tissues from printed gel‐based bioink and human neural stem cells. Adv Healthc Mater 2016;5:1429–1438. [DOI] [PubMed] [Google Scholar]
- 28. Andrew CD, Susan EC, Emily MR et al. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016;8:045002. [DOI] [PubMed] [Google Scholar]
- 29. Duarte Campos DF, Blaeser A, Korsten A et al. The stiffness and structure of three‐dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages. Tissue Eng Part A 2014;21:740–756. [DOI] [PubMed] [Google Scholar]
- 30. Ahn SH, Lee HJ, Lee J‐S et al. A novel cell‐printing method and its application to hepatogenic differentiation of human adipose stem cell‐embedded mesh structures. Sci Rep 2015;5:13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ye K, Felimban R, Traianedes K et al. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS One 2014;9:e99410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skardal A, Mack D, Kapetanovic E et al. Bioprinted amniotic fluid‐derived stem cells accelerate healing of large skin wounds. Stem Cells Translation Medicine 2012;1:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song SJ, Choi J, Park YD et al. A three‐dimensional bioprinting system for use with a hydrogel‐based biomaterial and printing parameter characterization. Artif Organs 2010;34:1044–1048. [DOI] [PubMed] [Google Scholar]
- 34. Gaetani R, Doevendans PA, Metz CHG et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012;33:1782–1790. [DOI] [PubMed] [Google Scholar]
- 35. Gao G, Schilling AF, Yonezawa T et al. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three‐dimensional scaffold and human mesenchymal stem cells. Biotechnol J 2014;9:1304–1311. [DOI] [PubMed] [Google Scholar]
- 36. Fan R, Piou M, Darling E et al. Bio‐printing cell‐laden Matrigel–agarose constructs. J Biomater Appl 2016;31:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marco C, Joanna I, Krisztina S et al. 3D bioprinting of BM‐MSCs‐loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016;8:035002. [DOI] [PubMed] [Google Scholar]
- 38. Kolesky DB, Truby RL, Gladman AS et al. 3D bioprinting of vascularized, heterogeneous cell‐laden tissue constructs. Adv Mat 2014;26:3124–3130. [DOI] [PubMed] [Google Scholar]
- 39. Müller M, Becher J, Schnabelrauch M et al. Nanostructured pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015;7:035006. [DOI] [PubMed] [Google Scholar]
- 40. Gao G, Yonezawa T, Hubbell K et al. Inkjet‐bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J 2015;10:1568–1577. [DOI] [PubMed] [Google Scholar]
- 41. Riccardo L, Jetze V, Josep AP et al. Biofabrication of tissue constructs by 3D bioprinting of cell‐laden microcarriers. Biofabrication 2014;6:035020. [DOI] [PubMed] [Google Scholar]
- 42. Mehesz AN, Brown J, Hajdu Z et al. Scalable robotic biofabrication of tissue spheroids. Biofabrication 2011;3:025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tasoglu S, Demirci U. Bioprinting for stem cell research. Trends Biotechnol 2013;31:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu Y, Moncal KK, Li J et al. Three‐dimensional bioprinting using self‐assembling scalable scaffold‐free “tissue strands” as a new bioink. Sci Rep 2016;6:28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Achilli T‐M, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi‐cellular spheroids. Exp Opin Biol Ther 2012;12:1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dababneh AB, Ozbolat IT. Bioprinting technology: A current state‐of‐the‐art review. J Manuf Sci Eng 2014;136:061016. [Google Scholar]
- 47. Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet‐based bioprinting: Past, present and future. Biomaterials 2016;102:20–42. [DOI] [PubMed] [Google Scholar]
- 48. Mattimore JP, Groff RE, Burg T et al. A General Purpose Driver Board for the HP26 Ink‐Jet Cartridge With Applications to Bioprinting: Proceedings of the IEEE SoutheastCon 2010 (SoutheastCon), March 18–21, 2010; Concord, NC; 2010:510–513. [Google Scholar]
- 49. Demirci U, Montesano G. Single cell epitaxy by acoustic picolitre droplets. Lab Chip 2007;7:1139–1145. [DOI] [PubMed] [Google Scholar]
- 50. Faulkner‐Jones A, Greenhough S, King JA et al. Development of a valve‐based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication 2013;5:015013. [DOI] [PubMed] [Google Scholar]
- 51. Mandrycky C, Wang Z, Kim K et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv 2016;34:422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ozbolat I, Gudapati H. A review on design for bioprinting. Bioprinting 2016;3–4:1–14. [Google Scholar]
- 53. Duan B, Hockaday LA, Kang KH et al. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res Part A 2013;101A:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guillemot F, Guillotin B, Fontaine A et al. Laser‐assisted bioprinting to deal with tissue complexity in regenerative medicine. MRS Bull 2011;36:1015–1019. [Google Scholar]
- 55. Schiele NR, Chrisey DB, Corr DT. Gelatin‐based laser direct‐write technique for the precise spatial patterning of cells. Tissue Eng Part C Methods 2011;17:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CDC. Available at https://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Accessed April 18, 2017.
- 57. Zhang W, Lian Q, Li D et al. Cartilage repair and subchondral bone migration using 3D printing osteochondral composites: A one‐year‐period study in rabbit trochlea. BioMed Res Int 2014;2014:746138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nguyen D, Hägg DA, Forsman A et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep 2017;7:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ryu S, Yoo J, Jang Y et al. Nanothin coculture membranes with tunable pore architecture and thermoresponsive functionality for transfer‐printable stem cell‐derived cardiac sheets. ACS Nano 2015;9:10186–10202. [DOI] [PubMed] [Google Scholar]
- 60. Ma X, Qu X, Zhu W et al. Deterministically patterned biomimetic human iPSC‐derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci 2016;113:2206–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kolesky DB, Homan KA, Skylar‐Scott MA et al. Three‐dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci USA 2016;113:3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pati F, Song T‐H, Rijal G et al. Ornamenting 3D printed scaffolds with cell‐laid extracellular matrix for bone tissue regeneration. Biomaterials 2015;37:230–241. [DOI] [PubMed] [Google Scholar]
- 63. Patel M, Patel KJ, Caccamese JF et al. Characterization of cyclic acetal hydroxyapatite nanocomposites for craniofacial tissue engineering. J Biomed Mater Res Part A 2010;94A:408–418. [DOI] [PubMed] [Google Scholar]
- 64. Zhang H, Mao X, Du Z et al. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical‐size rat calvarial defect model. Sci Technol Adv Mater 2016;17:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.CDC. Available at https://www.cdc.gov/heartdisease/heart_attack.htm. Accessed April 18, 2017.
- 66. Gaebel R, Ma N, Liu J et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 2011;32:9218–9230. [DOI] [PubMed] [Google Scholar]
- 67. Jang J, Park H‐J, Kim S‐W et al. 3D printed complex tissue construct using stem cell‐laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017;112:264–274. [DOI] [PubMed] [Google Scholar]
- 68. Taylor CA, Braza D, Rice JB et al. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehab 2008;87:381–385. [DOI] [PubMed] [Google Scholar]
- 69. Owens CM, Marga F, Forgacs G et al. Biofabrication and testing of a fully cellular nerve graft. Biofabrication 2013;5:045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hsieh F‐Y, Hsu S‐h. 3D bioprinting: A new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis 2015;11:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Faulkner‐Jones A, Fyfe C, Cornelissen D‐J et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte‐like cells for the generation of mini‐livers in 3D. Biofabrication 2015;7:044102. [DOI] [PubMed] [Google Scholar]
- 72. Robert C, Kamal E, Honglu W et al. Biofabrication of a three‐dimensional liver micro‐organ as an in vitro drug metabolism model. Biofabrication 2010;2:045004. [DOI] [PubMed] [Google Scholar]
- 73. Lee SY, Kim HJ, Choi D. Cell sources, liver support systems and liver tissue engineering: Alternatives to liver transplantation. Int J Stem Cells 2015;8:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotech 2014;32:773–785. [DOI] [PubMed] [Google Scholar]
- 75. Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci Am 2009;300:64–71. [DOI] [PubMed] [Google Scholar]
- 76. Dolati F, Yu Y, Zhang Y et al. In vitro evaluation of carbon‐nanotube‐reinforced bioprintable vascular conduits. Nanotechnology 2014;25:145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ozbolat IT. Bioprinting scale‐up tissue and organ constructs for transplantation. Trends Biotechnol 2015;33:395–400. [DOI] [PubMed] [Google Scholar]
- 78. Ravnic DJ, Leberfinger AN, Koduru SV et al. Transplantation of bioprinted tissues and organs: Technical and clinical challenges and future perspectives. Ann Surg 2017;266:48–58. [DOI] [PubMed] [Google Scholar]
- 79. Sparham S, Charles P. Controversies in diagnosis and management of community‐acquired pneumonia. Med J Aust 2017;206:316. [DOI] [PubMed] [Google Scholar]
- 80. Yan L, Zhang L, Ma H et al. A single B‐repeat of staphylococcus epidermidis accumulation‐associated protein induces protective immune responses in an experimental biomaterial‐associated infection mouse model. Clin Vaccine Immunol 2014;21:1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Datta P, Ayan B, Ozbolat IT. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater 2017;51:1–20. [DOI] [PubMed] [Google Scholar]


