Abstract
Endometrial regenerative cells (ERCs) are mesenchymal‐like stromal cells, and their therapeutic potential has been tested in the prevention of renal ischemic reperfusion injury, acute liver injury, ulcerative colitis, and immunosuppression. However, their potential in the induction of transplant tolerance has not been investigated. The present study was undertaken to investigate the efficacy of ERCs in inducing cardiac allograft tolerance and the function of stromal cell‐derived factor‐1 (SDF‐1) in the ERC‐mediated immunoregulation. The inhibitory efficacy of human ERCs in the presence or absence of rapamycin was examined in both mouse cardiac allograft models between BALB/c (H‐2d) donors and C57BL/6 (H‐2b) recipients and in vitro cocultured splenocytes. AMD3100 was used to inhibit the function of SDF‐1. Intragraft antibody (IgG and IgM) deposition and immune cell (CD4+ and CD8+) infiltration were measured by immunohistochemical staining, and splenocyte phenotypes were determined by fluorescence‐activated cell sorting analysis. The results showed that ERC‐based therapy induced donor‐specific allograft tolerance, and functionally inhibiting SDF‐1 resulted in severe allograft rejection. The negative effects of inhibiting SDF‐1 on allograft survival were correlated with increased levels of intragraft antibodies and infiltrating immune cells, and also with reduced levels of regulatory immune cells including MHC class IIlowCD86lowCD40lowdendritic cells, CD68+CD206+macrophages, CD4+CD25+Foxp3+T cells, and CD1dhighCD5highCD83lowIL‐10highB cells both in vivo and in vitro. These data showed that human ERC‐based therapy induces cardiac allograft tolerance in mice, which is associated with SDF‐1 activity, suggesting that SDF‐1 mediates the immunosuppression of ERC‐based therapy for the induction of transplant tolerance. Stem Cells Translational Medicine 2017;6:1997–2008
Keywords: Human endometrial regenerative cells, Stromal cell‐derived factor‐1, Cardiac transplantation, Allograft tolerance, Mice
Significance Statement.
Endometrial regenerative cells (ERCs) are newly identified stromal cells with advantages of noninvasively obtained method, abundant resources, highly proliferative rate, immunoregulatory function, and absence of tumorigenesis. Stromal cell‐derived factor‐1 (SDF‐1) is involved in many different physiological and pathological processes. This study demonstrates that human ERC‐based therapy can effectively suppress immune response and further induce allograft tolerance in a mouse cardiac transplantation model, and SDF‐1 secreted by ERCs plays an essential role in ERC‐mediated graft protection. Simultaneously, as xenograft, human ERCs are proven to be safe and effective in mice, which hold significant promise for therapeutic use in future clinical transplantation.
Introduction
Organ transplantation is an effective treatment for end‐stage organ failure, and the major focus of current transplant research is to achieve long‐term transplant survival with continuing function. At present, the worldwide median survival time of transplanted organs such as cardiac allografts has been greatly increased due to improvements in immunosuppressive treatments 1. However, the difficulty in achieving a balance between under‐ and over‐immunosuppression is accompanied by numerous issues, including rejections, infections, and malignancies 2. Therefore, an antigen‐specific transplant tolerance therapy is urgently needed. Mesenchymal stromal cells (MSCs) hold great potential as attractive candidates for cell therapy, as they have been shown to induce immune tolerance in organ transplantation 3, 4, 5, 6, 7. However, a large‐scale source of MSCs for clinical use has not been really established yet. The bone marrow (BM)‐derived MSCs have been used for both basic and clinical studies since 1976 when they were first isolated 8 but are limited by many factors, such as invasive procedures, low accessibility or low availability, and limited proliferation capacity 9. Much recently, both umbilical cord (UC) (especially Wharton's jelly)‐ and adipose‐derived MSCs have been demonstrated to be a viable clinical alternative to BM‐MSCs due to relatively easy harvest procedure 10, 11, but the availability of these MSCs is still an issue for large qualities needed in clinics as therapeutic cells. More seriously, these MSCs may be tumorigenic 12, 13 and promote tumor progression and metastasis 14, 15. Thus, there is a need for another type of stromal cells with a better risk‐to‐benefit profile.
Endometrial regenerative cells (ERCs) are mesenchymal‐like stromal cells obtained from menstrual blood and have many advantageous properties for clinical use: besides noninvasively obtained method, abundant resources, they also have highly proliferative rate, pluripotent differentiation activity, the ability to inhibit inflammatory responses, lack of immunogenicity, expandability to great quantities without karyotypic abnormalities or the loss of differentiation ability, and tumorigenesis 9, 16, 17, 18. We and others have reported the efficacy of ERCs in the treatment of myocardial infarction 19, heart failure 16, 20, critical limb ischemia 21, premature ovarian failure 22, multiple sclerosis 23, stroke 24, ulcerative colitis 25, ischemia‐reperfusion injury 26, and acute liver injury 27 in animal models. Recently, we have also demonstrated that ERCs attenuate antibody‐mediated allograft rejection by suppressing B cells and inhibiting the humoral response in cardiac transplantation 28. However, their roles and mechanisms in the inhibition of transplant rejection and induction of allograft tolerance have not been evaluated.
Stromal cell‐derived factor‐1 (SDF‐1), also known as CXC chemokine ligand 12 (CXCL12), and its highly conserved corresponding G protein‐coupled chemokine receptor CXC chemokine receptor 4 (CXCR4) play an important role in cell adhesion, chemotaxis, survival, and proliferation during physiological and pathological processes 29. Moreover, in the immune response, SDF‐1 can affect the generation of immune cells and is an important macrophage effector 30 that promotes macrophage polarization toward the anti‐inflammatory macrophage type 2 cell (M2) phenotype 31, and also repels effector T cells and recruits immunosuppressive regulatory T cells (Tregs) 32, 33. Furthermore, SDF‐1 can limit the inflammatory response by localizing mononuclear infiltrates at the site of injury 34. Just like MSCs, ERCs can secrete SDF‐1 in a substantial amount 35. Furthermore, the putative mature amino acid sequences and coding region nucleotide sequences of human and mouse SDF‐1 are more than 90% identical 36, suggesting that the activity of human SDF‐1 can be tested in a murine system.
The objective of this study was to investigate whether human ERC‐based therapy could induce cardiac allograft immune tolerance, and the contribution of SDF‐1 secreted by ERCs to their immunoregulation. Because ERCs are of human origin, the therapeutic approach used in our mouse transplant model holds significant promise for clinical transplantation.
Materials and Methods
Animals
Male adult C57BL/6 (B6) (H‐2b), BALB/c (H‐2d), and C3H (H‐2k) mice (10–12 weeks old) weighing 22–27 g were purchased from National Institutes for Food and Drug Control (Beijing, China, http://www.nicpbp.org.cn). The animals were housed under a conventional experimental environment at Tianjin General Surgery Institute (Tianjin, China). All the experiments were performed on the basis of protocols approved by the Animal Care and Use Committee of Tianjin Medical University (Tianjin, China), according to the Chinese Council on Animal Care guidelines.
Preparation of ERCs, Rapamycin, and AMD3100 for In Vivo Treatment
Human ERCs were isolated from the menstrual blood that was collected by using a sterilized menstrual cup on the first day of menstruation from healthy women (20–30 years old) under ethical approval from Tianjin Medical University (Tianjin, China). The ERC culture and expansion were the same as described previously 28. In brief, mononuclear cells from menstrual blood (approximately 10 ml) were suspended in Dulbecco's modified Eagle's medium high glucose supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, and were split into two 10 cm dishes that were cultured in a 37°C 5% CO2 incubator. The cultured cells were adherent to the surface of the dishes after overnight incubation. After 2 weeks of incubation, the cells displayed a spindle‐shape morphology, and the estimated adherent cell number at the start of culture was approximately 1 × 107. Typical cell surface markers of ERCs were analyzed by using a flow cytometry as previously described 9. For the treatment studies, the ERCs were intravenously injected into B6 recipients through the tail vein with a single dose of 1 × 106 cells per mouse 24 hours after cardiac transplantation. Rapamycin (RAPA) (Wyeth Pharmaceuticals, Soochow, China) was dissolved in 100% olive oil and was subcutaneously injected to the recipient B6 mice (2 mg/kg/day) for 13 days directly after cardiac transplantation. AMD3100 (Selleckchem, Shanghai, China, http://www.selleck.cn), the SDF‐1 receptor antagonist, was dissolved in PBS, and subcutaneously injected into the recipient B6 mice (5 mg/kg/day) for total 7 days right after cardiac transplantation.
Enzyme‐Linked Immunosorbent Assay
The level of SDF‐1 in the supernatant of ERCs used in this study was measured by the Elisa kit (R&D, Minneapolis, MN, https://www.rndsystems.com) according to the manufacturer's instructions. Approximately 3 ml of supernatant cultured with three million cells was collected as described previously 35. The optical density (OD) value was measured through the Microplate Reader (Tecan, Männedorf, Switzerland, http://www.tecan.com).
Heterotopic Cardiac Transplantation and Experimental Groups
Intra‐abdominal heterotopic cardiac transplantation was performed as previously described 37. The donor hearts from BALB/c (H‐2d) mice were transplanted to the B6 (H‐2b) recipients. Then the recipient B6 mice were randomly assigned to six groups: Group A, untreated; Group B, RAPA‐treated alone; Group C, ERCs‐treated alone; Group D, treatment of ERCs and AMD3100; Group E, ERCs‐RAPA combination group; Group F, combination treatment of ERCs, AMD3100, and RAPA. The heartbeat of the graft was monitored daily by direct abdominal palpation by a member of our team without prior knowledge of the treatment status of the animals. The degree of pulsation was scored as: A, beating strongly; B, noticeable decline in the intensity of pulsation; or C, complete cessation of pulsation. For the study of survival, histology and immunohistochemistry, heart grafts were harvested at the time of rejection or at postoperative day (POD) 100 (for the combination therapy group) (n = 6, each group). For the study of the population of different immune cells, splenocytes were harvested at POD 8 for assessment (n = 6, each group).
Graft Histology
Transplant cardiac graft samples were collected and fixated by 10% formalin. These were then embedded in paraffin and sectioned at 5 µm for hematoxylin and eosin (H&E) staining. The sections were examined for severity of rejection under light microscopy. Criteria for graft rejection included the presence of vasculitis, thrombosis, hemorrhage, and lymphocyte infiltration and were scored as previously described 38: 0, no change; 1, minimum change; 2, mild change; 3, moderate change; or 4, marked change compared with normal tissues.
Immunohistochemistry
The immunohistochemistry of intragraft antibody deposition and immune cell infiltration were performed using biotin‐conjugated rat anti‐mouse CD4 and CD8 monoclonal antibodies, and biotin‐conjugated goat anti‐mouse IgG and IgM antibodies (Abcom, Shanghai, China, http://www.abcam.cn). Nonspecific staining was assessed by negative control sections, which omitted the primary antibodies. Intragraft IgG and IgM deposition were quantified by the density of positive staining of an antibody titration series within a given section (mm2). Intragraft CD4+ and CD8+ cells were determined by quantifying all the positive staining cells within a given section (cells per mm2). Quantification was done by the ImageJ software (National Institutes of Health, Bethesda, MD, http://www.nih.gov).
Fluorescence‐Activated Cell Sorting Analysis
The spleens from mice of each group were collected, grinded, and passed through sterilized meshes (100 meshes) to obtain a homogeneous cell suspension. The red blood cells were lysed by red blood cell lysis solution. Then, the splenocytes were washed and dispensed for further using. Fluorescence‐activated cell sorting (FACS) analysis was used, as previously described 4, to determine phenotype of various immune cells in splenocytes stained with antibodies against CD1d, CD4, CD5, CD11c, CD19, CD25, CD40, CD68, CD83, CD86, CD206, Foxp3, IL‐10, and MHC class II. All fluorescent‐labeled antibodies were purchased from either eBioscience (eBioscience, San Diego, CA, http://www.eBioscience.com) or BioLegend (BioLegend, San Diego, CA, http://www.biolegend.com).
Mixed Lymphocyte Reaction
The function of tolerogenic dendritic cells (Tol‐DCs) in B6 recipients of each group was assessed by a one‐way mixed lymphocyte reaction (MLR) performed through the T cell proliferative response to alloantigen in a 96‐well plate, as described previously 3. In brief, at POD 8, splenic CD11c+DCs (5 × 104 cells per well) from B6 recipients, selected by CD11c microBeads (Miltenyi, Teterow, Germany, http://www.miltenyibiotec.com) through positive selection and pretreated with mitomycin C (50 μg/ml) (Solarbio, Beijing, China, http://www.solarbio.net.cn), were used as stimulators. Splenic T cells (5 × 105 cells per well) from BALB/c mice selected by CD3 microBeads (Miltenyi, Teterow, Germany, http://www.miltenyibiotec.com) through positive selection were used as responders. The proliferative response of T cells to the stimulator of DCs was examined after 96 hours of incubation and compared with those without DCs, then was assessed by Cell Counting Kit‐8 (CCK‐8) (Dojindo, Shanghai, China, http://www.dojindo.cn) through the Microplate Reader. The OD value at 450 nm was recorded (n = 6).
Cocultures of ERCs with Allogeneic Splenocytes
In order to examine the function of ERC‐derived SDF‐1, in the differentiation of Tol‐DCs, M2, Tregs, and regulatory B cells (Bregs), the coculture experiments were performed using ERCs and B6 splenocytes in a 96‐well plate in vitro. Splenocytes (2 × 105 cells per well) pretreated with or without AMD3100 (1 µg/ml) were cocultured with or without ERCs (1 × 104 cells per well), and were stimulated with various stimulators for 96 hours. For Tol‐DC, the stimulator was lipopolysaccharide ([LPS] 10 μg/ml; Saint Louis, Missouri, http://www.sigma-aldrich.com). For Tregs, stimulators were anti‐mouse CD3 (100 ng/ml) and CD28 (200 ng/ml) antibodies (eBioscience, San Diego, CA, http://www.eBioscience.com). For M2 and Bregs, stimulators were LPS (10 μg/ml) and interleukin (IL)‐4 (100 U/ml) (R&D, Minneapolis, MN, https://www.rndsystems.com). The percentage of each cell type was examined by FACS analysis (n = 6).
Statistical Analysis
The enumeration data were presented as mean ± standard deviation (SD). Cardiac allograft survival time was accomplished through Kaplan‐Meier cumulative survival method, and survival differences between groups were determined by Log‐rank (Mantel‐Cox) test. The differences among multiple groups were analyzed using one‐way analysis of variance (ANOVA) after the normality test and followed by post hoc analysis with the least significant difference (LSD) test. p value (p) ≤.05 was considered statistically significant.
Results
SDF‐1 Is Required for ERC‐Based Therapy in the Induction of Donor‐Specific Cardiac Allograft Tolerance
To confirm ERCs used in this study could secret SDF‐1, given the same amount of cells and supernatant as the previous study 35, the level of SDF‐1 in the supernatant was measured by Elisa and its concentration was 9824 ± 1700 pg/ml, which is higher than that secreted by ERCs (6627 ± 5858 pg/ml) in the previous study.
It has been demonstrated that treatment with ERC monotherapy can significantly prolong cardiac allograft survival in mice 28. In this study, as shown in Figure 1, the mean survival time (MST) in the ERC monotherapy group was prolonged (MST in untreated group, 8.33 ± 0.82 days; MST in ERC monotherapy group, 19.67 ± 2.58 days; p < .001, Fig. 1A), which were the same as shown in our previous study 28, and the cardiac allografts achieved a long‐term survival of greater than 100 days in the ERCs‐RAPA combination group. However, the effect of ERCs was attenuated significantly when the function of SDF‐1 was inhibited through the use of AMD3100, as compared with the ERCs monotherapy group (10.17 ± 1.17 days vs. 19.67 ± 2.58 days, p < .001, Fig. 1A) and the RAPA combination treatment group (28.17 ± 1.72 days vs. 100 days, p < .001, Fig. 1A). Tolerance was donor‐specific since BALB/c (H‐2d) mouse skin grafts onto long‐term surviving tolerant B6 (H‐2b) recipients on POD100 were permanently accepted, whereas C3H (H‐2k) third party grafts were rejected in 11 days (data not shown). Collectively, these data indicate that the combination of ERCs with RAPA induces donor‐specific tolerance in this mouse model of cardiac allografts.
Figure 1.
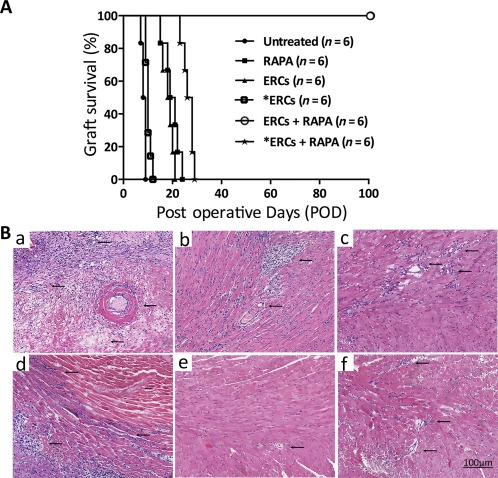
Stromal cell‐derived factor‐1 (SDF‐1) is required for ERC‐based therapy in prolongation of cardiac allograft survival. (A): Survival time of cardiac allografts in each B6 recipients receiving different immunosuppressive treatments. Data were shown as percentage of cardiac allograft survival. Statistical analysis was done by Log‐rank (Mantel‐Cox) test. p < .001 (ERCs vs. *ERCs: 19.67 ± 2.58 days vs. 10.17 ± 1.17 days), p < .001, (ERCs + RAPA vs. *ERCs + RAPA: 100 days vs. 28.17 ± 1.72 days). (B): Histology of cardiac allografts in each group of B6 recipients. Grafts were collected at the time of rejection or POD 100, fixed by formalin, and embedded by paraffin. Sections (5 μm) were stained with H&E staining. Cardiac grafts from untreated (Ba), RAPA monotherapy (Bb), ERC monotherapy (Bc), *ERCs (Bd), ERCs‐RAPA combination group (Be), or combination of *ERCs and RAPA (Bf) groups are presented and compared, n = 6. *ERCs indicated inhibition the function of SDF‐1 by AMD3100. Arrows indicate intravascular and/or interstitial changes in cardiac grafts (×400 magnification). Scale bars = 100 μm. Abbreviations: ERC, endometrial regenerative cell; POD, postoperative day; RAPA, rapamycin.
In addition, the pathological changes showed that cardiac grafts in the untreated group developed severe rejection, characterized by myocyte necrosis, as well as interstitial hemorrhage, lymphocytic infiltration, vasculitis, and intravascular thrombosis [Fig. 1Ba)]. Either RAPA [Fig. 1(Bb)]) or ERCs [Fig. 1(Bc)]) monotherapy alleviated the severity of pathological changes in the grafts. However, in the ERCs‐RAPA combination group, all of six grafts were functioning well with normal histology [Fig. 1(Be)]). And when the function of SDF‐1 was inhibited by AMD3100, the severity of graft pathological changes was worse than that of each corresponding group [Fig. 1(Bd), 1(Bf)]). These results demonstrate that ERC‐based therapy (ERCs‐RAPA combination therapy) can induce cardiac allograft tolerance with almost normal pathology. Moreover, inactivation of ERC‐derived SDF‐1 disrupts this effect of the combination therapy‐mediated graft tolerance.
SDF‐1 Mediates ERC‐Based Therapy in Attenuating Antibody‐Mediated Rejection and Acute Cellular Rejection
Intragraft alloreactive antibody (IgG or IgM) deposition and immune cell (CD4+ or CD8+) infiltration reflect the severity of antibody‐mediated rejection (AMR) and acute cellular rejection (ACR), which are two major types of rejection in cardiac allografts. As shown in Figures 2 and 3, when compared with the untreated group, the severity of immunohistological staining of both intragraft antibody deposition and cell infiltration in either the ERCs (IgG, p < .001; IgM, p = .002; CD4+, p < .001; CD8+, p < .001) or RAPA (IgG, p < .001; IgM, p < .001; CD4+, p < .001; CD8+, p < .001) monotherapy group was alleviated, and was further downregulated in the ERCs‐RAPA combination group (IgG, p < .001; IgM, p < .001; CD4+, p < .001; CD8+, p < .001). However, the inhibition of SDF‐1 significantly suppressed ERC‐mediated reduction of intragraft antibody deposition and immune cell infiltration, as compared with the corresponding groups (ERCs vs. ERCs + AMD3100: IgG, p < .001; CD4+, p < .001; CD8+, p = .002; ERCs + RAPA vs. ERCs + RAPA + AMD3100: IgG, p = .001; IgM, p < .001; CD4+, p = .022; CD8+, p < .001, Fig. 2B, 3B). One exception was noted for the intragraft IgM deposition between the ERC monotherapy group and the ERCs + SDF‐1 inhibitor group (5.88% ± 0.89% vs. 6.42% ± 0.80%, p = .24, Fig. 2B). Interestingly, the circulating IgG and IgM levels were not significantly different among these groups (data not shown). These results indicate that ERC‐based therapy can reduce AMR and ACR in cardiac allografts, and ERC‐induced graft protection is, at least in part, mediated by SDF‐1.
Figure 2.
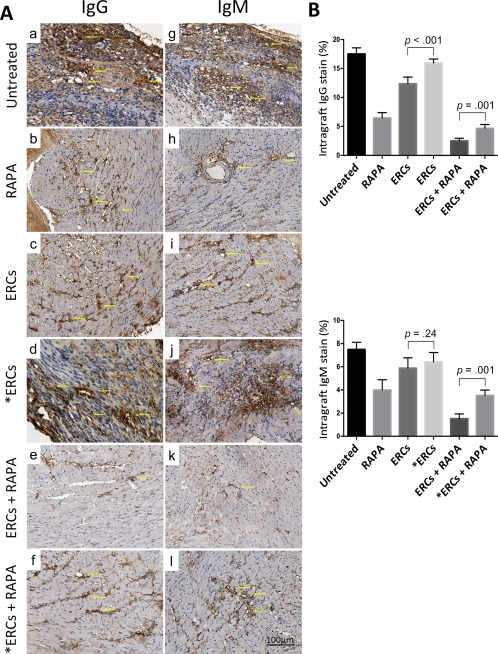
Stromal cell‐derived factor‐1 (SDF‐1) mediates the role of ERC‐based therapy in reducing antibody‐mediated rejection in cardiac allografts. (A): Immunohistological staining of intragraft IgG (Aa–Af) and IgM (Ag–Al) antibody deposition of each group. Grafts were collected at the time of rejection or postoperative day (POD) 100. Arrows show positive staining (×400 magnification). (B): Intragraft IgG and IgM antibody deposition of each group were presented by the percentage of positive staining within a given section (mm2). Grafts were collected at the time of rejection or POD 100. *ERCs indicated inhibition the function of SDF‐1 by AMD3100. Statistical analysis was done by one‐way analysis of variance followed by the least significant difference test, n = 6. Scale bars = 100 μm. Abbreviations: ERC, endometrial regenerative cell; RAPA, rapamycin.
Figure 3.
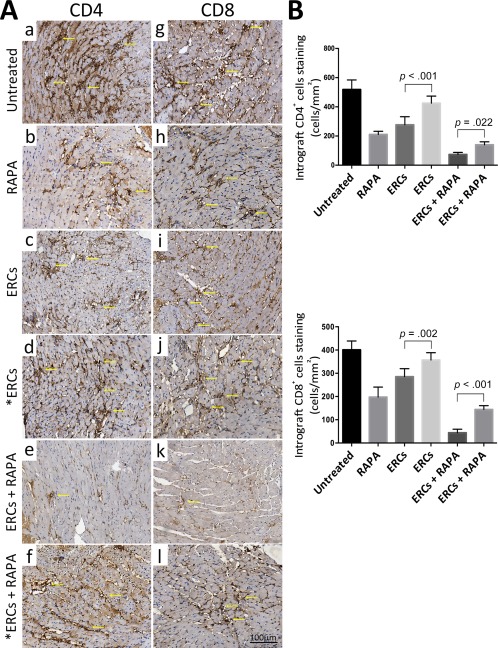
Stromal cell‐derived factor‐1 (SDF‐1) mediates the role of ERC‐based therapy in reducing acute cellular rejection in cardiac allografts. (A): Immunohistological staining of CD4+ (Aa–Af) and CD8+ (Ag–Al) cells infiltration of each group. Grafts were collected at the time of rejection or POD 100. Arrows show positive staining (×400 magnification). (B): Intragraft CD4+ and CD8+ cell infiltration of each group was presented by quantitating all the positive staining cells within a given section (cells per mm2). Grafts were collected at the time of rejection or POD 100. *ERCs indicated inhibition the function of SDF‐1 by AMD3100. Statistical analysis was done by one‐way analysis of variance followed by the least significant difference test, n = 6. Scale bars = 100 μm. Abbreviations: ERC, endometrial regenerative cell; RAPA, rapamycin.
SDF‐1 Mediates ERC‐Based Therapy in Increasing the Percentage of Tol‐DCs
To explore the effect of each treatment therapy on DCs, the Tol‐DC population in splenocytes gated by CD11c was investigated by expressing low levels of antigen presenting‐related markers (MHC class II, CD86, CD40) through FACS analysis. As expected, the expression of all these markers in the ERC or RAPA monotherapy group were lower than those of the untreated group (ERCs vs. untreated: MHC class II, p < .001; CD86, p < .001; CD40, p < .001; RAPA vs. untreated: MHC class II, p < .001; CD86, p < .001; CD40, p < .001), and were further lowered in the ERCs‐RAPA combination group (MHC class II, p < .001; CD86, p < .001; CD40, p < .001). Moreover, the effect of inhibiting the function of SDF‐1 on Tol‐DC development was analyzed in both the ERCs monotherapy group and the ERCs‐RAPA combination group. We found that the Tol‐DC population was significantly decreased compared with corresponding groups (ERCs vs. ERCs + AMD3100: MHC class II, p < .001; CD86, p < .001; CD40, p < .001; ERCs + RAPA vs. ERCs + RAPA + AMD3100, MHC class II, p < .001; CD86, p < .001; CD40, p < .001, Fig. 4A–4C).
Figure 4.
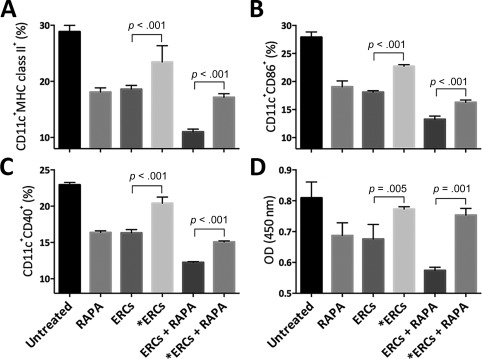
Stromal cell‐derived factor‐1 (SDF‐1) mediates the effect of ERC‐based therapy in increasing the percentage of tolerogenic dendritic cell (Tol‐DCs) in transplant recipients. Splenocytes were harvested from B6 recipients at postoperative day 8, followed by double‐staining gated by anti‐mouse CD11c antibody, and then the percentage of surface MHC class II (A), CD86 (B), and CD40 (C) were measured by fluorescence‐activated cell sorting (FACS) analysis. Statistical analysis was done by one‐way analysis of variance (ANOVA) followed by the least significant difference (LSD) test, n = 6. (D): CD11c+ DCs were isolated by CD11c microBeads from splenocytes collected from the B6 recipients and were treated with mitomycin. The function of these DCs (stimulators) was measured by the stimulation of CD3+ T‐cell (responders) proliferation index (OD value) in one‐way mixed lymphocyte reaction. Statistical analysis was done by one‐way ANOVA followed by the LSD test, n = 6. *ERCs indicated inhibition the function of SDF‐1 by AMD3100. Abbreviations: ERC, endometrial regenerative cell; OD, optical density; RAPA, rapamycin.
To further confirm the function of CD11c+ DC populations from B6 recipients in each group, one‐way MLR was performed through measuring the index of antigen‐stimulated CD3+ T‐cell proliferation from splenocytes of the BALB/c mouse. As shown in Figure 4D, T‐cell proliferation index measured by the OD value in the ERC (p = .001) or RAPA (p = .001) monotherapy group was lower than that of the untreated group, and further lowered in the ERCs‐RAPA combination group (p < .001). Also, when the function of SDF‐1 was inhibited, the T‐cell proliferation was significantly higher than the corresponding groups (ERCs vs. ERCs + AMD3100, p = .005, ERCs + RAPA vs. ERCs + RAPA + AMD3100, p < .001, Fig. 4D). Taken together, SDF‐1 mediates ERC mono‐ and combination therapy to enhance the generation and function of Tol‐DCs.
SDF‐1 Mediates ERC‐Based Therapy in Decreasing the Percentage of Total Macrophages and Increasing the Percentage of M2
To determine whether each treatment therapy influences the percentage of total macrophages and M2, anti‐mouse CD68 antibody was used to measure total macrophages, and combination with anti‐mouse CD206 antibody was used to measure M2 phenotype in splenocytes. As shown in Figure 5, either ERCs or RAPA monotherapy suppressed the percentage of total macrophages, but increased the percentage of M2 compared with the untreated group (ERCs vs. untreated: total macrophages, p < .001; M2, p < .001; RAPA vs. untreated: total macrophages, p < .001; M2, p < .001), and more markedly in the ERCs‐RAPA combination group (total macrophages, p < .001; M2, p < .001). Similar to the other results, when the function of SDF‐1 was inhibited, the effect of ERCs monotherapy and ERCs‐RAPA combination group on decreasing total macrophages (ERCs vs. ERCs + AMD3100, p < .001; ERCs + RAPA vs. ERCs + RAPA + AMD3100, p < .001, Fig. 5B) and increasing M2 phenotype (ERCs vs. ERCs + AMD3100, p < .001; ERCs + RAPA vs. ERCs + RAPA + AMD3100, p < .001, Fig. 5C) was reduced significantly. Taken together, SDF‐1 mediates ERC‐based therapy in decreasing the percentage of total macrophages and increasing the percentage of M2 phenotype.
Figure 5.
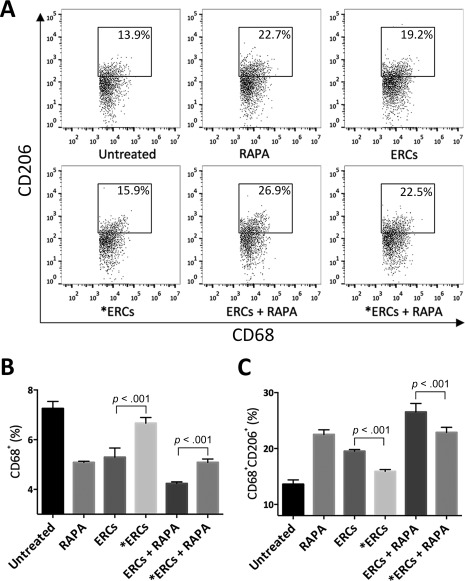
Stromal cell‐derived factor‐1 (SDF‐1) mediates ERC‐based therapy in decreasing the percentage of total macrophages and but increasing the percentage of macrophage type 2 (M2) in transplant recipients. Splenocytes were collected from the B6 recipients in each group at postoperative day 8, followed by single‐staining with anti‐mouse CD68 antibody to measure the percentage of total macrophages, and together with anti‐mouse CD206 antibody gating by anti‐mouse CD68 antibody to measure the percentage of M2, and then were determined by FACS analysis. (A): Dot plots of CD68+CD206+ M2. (B): Percentage of CD68+ total macrophages. (C): Percentage of CD68+CD206+ M2. *ERCs indicated inhibition the function of SDF‐1 by AMD3100. Statistical analysis was done by one‐way analysis of variance followed by the least significant difference test, n = 6. Abbreviations: ERC, endometrial regenerative cell; RAPA, rapamycin.
SDF‐1 Mediates ERC‐Based Therapy by Increasing the Percentage of Both Tregs and Bregs
We have previously demonstrated that ERCs enhance the generation of Tregs 26, 27 in vivo. To explore the role of SDF‐1 on ERC‐mediated immunoregulation, the percentage of Tregs gated by anti‐mouse CD4 antibody in the splenocytes of each group was investigated, followed by double positive staining of anti‐mouse CD25 and Foxp3 antibodies. As shown in Figure 6A and 6B, the percentage of Tregs in either the ERCs (p < .001) or RAPA (p < .001) monotherapy group was higher than that of the untreated group, also was even higher in the ERCs‐RAPA combination group (p < .001). The percentage of Tregs in the two groups with SDF‐1 inhibitor was significantly decreased compared with corresponding groups (ERCs vs. ERCs + AMD3100: p < .001; ERCs + RAPA vs. ERCs + RAPA + AMD3100: p < .001, Fig. 6A, 6B).
Figure 6.
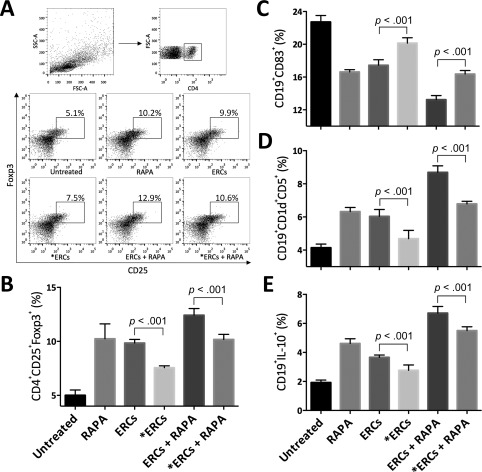
Stromal cell‐derived factor‐1 (SDF‐1) mediates ERC‐based therapy in increasing the percentage of both regulatory T cell (Tregs) and regulatory B cell (Bregs) in allograft recipients. Splenocytes were collected from B6 recipients in each group at postoperative day 8. For the Tregs, the percentage of CD25+Foxp3+ cells in CD4+ gating population was determined by fluorescence‐activated cell sorting analysis. (A): Dot plots of CD4+CD25+Foxp3+ T cells. (B): Percentage of CD4+CD25+Foxp3+ T cells. For the Bregs, the percentage of CD83+ (C), CD1d+CD5+ (D), or IL‐10+ (E) cells in CD19+ gating population was measured. *ERCs indicated inhibition the function of SDF‐1 by AMD3100. Statistical analysis was done by one‐way analysis of variance followed by the least significant difference test, n = 6. Abbreviations: ERC, endometrial regenerative cell; RAPA, rapamycin.
In addition, CD19+ B cells were isolated from splenocytes of B6 recipients from each group to identify the percentage of Bregs. As expected (Fig. 6C–6E), the percentage of Bregs in the splenocytes from the ERCs‐RAPA combination group showed significantly lower expression of B cell maturation marker CD83, higher expression of both CD1d and CD5 positive markers, and higher expression of IL‐10 compared with the ERCs or RAPA monotherapy groups, and especially compared with the untreated group (ERCs vs. untreated: CD83, p < .001; CD1dCD5, p < .001; IL‐10, p < .001; RAPA vs. untreated: CD83, p < .001; CD1dCD5, p < .001; IL‐10, p < .001). In the groups with AMD3100, the percentage of Bregs was markedly decreased compared with the corresponding groups (ERCs vs. ERCs + AMD3100: CD83, p < .001; CD1dCD5, p < .001; IL‐10, p < .001; ERCs + RAPA vs. ERCs + RAPA + AMD3100: CD83, p < .001; CD1dCD5, p < .001; IL‐10, p < .001, Fig. 6C–6E). These data demonstrate that SDF‐1 mediates the ERC‐induced increase the percentage of Tregs and Bregs.
SDF‐1 Plays a Role in ERC‐Mediated the Generation of Tol‐DCs, M2, Tregs, and Bregs In Vitro
To further directly confirm the effects of ERCs and their secretion of SDF‐1 on in vitro generations of Tol‐DCs (CD11c+MHC class II+), M2 (CD68+CD206+), Tregs (CD4+CD25+Foxp3+), and Bregs (CD19+IL‐10+), we designed an in vitro coculture experiment with ERCs and splenocytes from B6 mouse pretreated with or without AMD3100. The percentage of each type of cell was measured by FACS analysis. As shown in Figure 7, ERCs significantly increased the generations of these cells as compared with the control group (without ERCs) (Tol‐DCs, p < .001; M2, p < .001; Tregs, p < .001; Bregs, p < .001, Fig. 7). When the function of SDF‐1 was inhibited by AMD3100, the beneficial effect of ERCs was attenuated (ERCs vs. ERCs + AMD3100: Tol‐DCs, p < .001; M2, p < .001; Tregs, p < .001; Bregs, p < .001, Fig. 7). These data suggest that ERCs mediate the generation of Tol‐DC, M2, Tregs, and Bregs, and that SDF‐1 is a critical determinant of ERC‐mediated immunosuppression.
Figure 7.
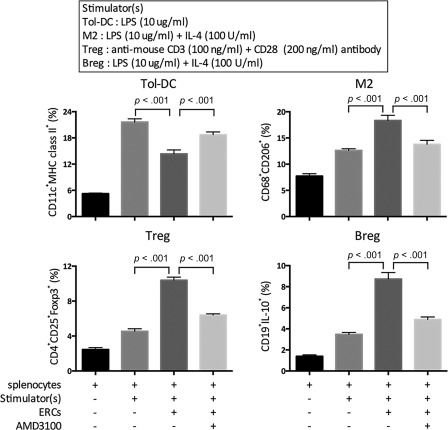
Stromal cell‐derived factor‐1 plays an important role in ERC‐mediated generation of Tol‐DC, M2, Treg, Breg cells in vitro. Splenocytes obtained from B6 mice pretreated with or without AMD3100 were cocultured with ERCs and different stimulators for 96 hours. The percentage of Tol‐DCs (CD11c+MHC class II+), M2 (CD68+CD206+), Tregs (CD4+CD25+Foxp3+), Bregs (CD19+IL‐10+) was measured by fluorescence‐activated cell sorting analysis. Statistical analysis was done by one‐way analysis of variance followed by the least significant difference test, n = 6. Abbreviations: Breg, regulatory B cell; ERC, endometrial regenerative cell; LPS, lipopolysaccharide; M2, macrophage type 2; Tol‐DCs, tolerogenic dendritic cells; Treg, regulatory T cell.
Discussion
ERCs are a type of stromal cell noninvasively obtained from adult female menstrual blood. These cells have a highly proliferative rate and immunoregulatory function. As we know, the immune rejection following organ transplantation presents a major therapeutic challenge in maintaining graft function and survival. Our previous study shows that ERCs can prolong survival time of cardiac allografts through suppressing the function of B cells 28. Moreover, in this study we have demonstrated that ERC‐based therapy with RAPA can achieve a greater long‐term survival and induce immune tolerance in a mouse model of cardiac allografts.
SDF‐1, with the characteristic of evolutionary conservatism 36, is secreted by many organs and tissues 39 and possesses functions in several human physiological and pathological processes. SDF‐1 can act as both chemotaxis and angiogenic factors and has been shown to influence stem cell homing, cardiac myocyte survival and regeneration, ventricular remodeling, and revascularization in both acute myocardial infarction and chronic heart failure in animal studies 40, 41, 42, 43, 44, 45, 46. In transplantation immunity, it has been reported that SDF‐1 incorporated by alginate encapsulant could support long‐term allo‐ and xeno‐islet transplantation without systemic immune suppression 33. The present study further demonstrates that SDF‐1, secreted by ERCs, plays an important role in ERC‐mediated immune regulation and allograft protection in cardiac transplantation.
Similar to the previous study 35, the ERCs used in this study can also secrete SDF‐1 in a great quantity, which also demonstrate that the stability of different batches of ERCs in SDF‐1 secretion. Simultaneously, we found that ERCs act on immune cells through SDF‐1 by inducing the generation of M2, Tol‐DCs, Tregs, and Bregs both in vivo and in vitro, suppressing IgG and IgM antibody deposition, and reducing CD4+ and CD8+ cell infiltration in vivo. As two important antigen presenting cells in the innate immune system, macrophages and DCs play a significant role in antigen phagocytosis and presentation, and their differentiation and maturation will further affect the formation and differentiation of adaptive immune cells 47, 48, 49. Tol‐DCs induced by ERCs present an immature DC phenotype, with low expression of antigen‐presenting costimulatory molecules such as MHC class II, CD86, and CD40. These Tol‐DCs could decrease the proliferation of reactive T cells and also act as guardians for the induction and maintenance of peripheral T‐cell tolerance 50, which are important for the induction and maintenance of transplantation tolerance. But when the function of SDF‐1 is inhibited, the effect of ERCs is weakened. Macrophages are likely the most plastic cells among hematopoietic origin cells, and can differentiate down different pathways depending on stimuli in the surrounding environment 51. For the induction of transplantation tolerance, both of the total macrophages decrease and M2 increase are important. We have reported that ERCs can decrease the percentage of total macrophages and promote the differentiation of macrophages to M2 in previous study 26, and in the present study, we further demonstrate that ERCs come into effect through SDF‐1 partly. In the adaptive immune system, T cells and B cells play important roles in cellular immunity and humoral immunity, respectively. Through blood circulation, IgG and IgM antibodies secreted by mature B cells can deposit on the allografts and CD4+ and CD8+ cells can infiltrate into the allografts, both of them leading to graft rejection. We demonstrated that ERCs can alleviate the occurrence of these rejections to different degrees, and when SDF‐1 is inhibited, this effect of ERCs is weakened. Furthermore, Tregs and Bregs are important immunoregulatory cells that secrete IL‐10 and TGF‐β, which play critical roles not only in the anti‐inflammatory response, but also in the tolerance induction function 52, 53, 54 in transplantation models. ERCs can also increase the percentage of Tregs and Bregs, and when the function of SDF‐1 is inhibited, this effect of ERCs is weakened.
Interestingly, human ERCs as a xenograft, the current study demonstrated that ERCs injected into mouse transplant recipients were not rejected, and played a critical role in the induction of tolerance in cardiac allografts, as they prolonged the survival time of allografts and induced the percentage of tolerogenic or regulatory cells. This phenomenon could be related, but not limited to the lack of antigen recognition factors such as MHC class II, as well as the low costimulatory molecule expression on ERCs 17. Some of the relevant studies may give some explanations. It has been found that ERCs could suppress peripheral blood mononuclear cell (PBMC) proliferation in the MLR 21. Simultaneously, in our previous cardiac allograft transplant model, it has been demonstrated that ERCs could suppress B cells maturation and activation, and inhibit humoral response 28. Both of them demonstrated the immunosuppressive ability of ERCs. In addition, in our previous mouse acute liver injury model with an ERC tracking study, fluorescently‐labeled ERCs could be observed not only in the injured hepatic lobules, but also in the spleen with strong fluorescent 27. Which means as cells with heterologous expression, after transfusing into mouse, human ERCs appear to possess the ability to escape detection by the mouse immune system and further migrate to the spleen, where they could come into effect. And in the present study, we found that ERCs can also induce the percentage of immune tolerogenic or regulatory cells both in vivo and in vitro. Based on all the above data, we speculate that human ERCs not only have ability to avoid destruction by mouse transplant recipients, but also regulate the mouse immune system.
In addition, our present study also showed that the immunoregulatory of ERCs is critically mediated by SDF‐1. ERCs secrete important cytokines such as protective TGF‐β 35 and SDF‐1. It has been known that TGF‐β is influential in the induction of immune tolerance by MSCs 55, 56, and SDF‐1 is essential for stem cells homing to the injured tissues and organs 57. Based on the results of this study and our previous ERCs migration study mentioned above 27, we speculate that, under the guidance of SDF‐1/CXCR4 axis, human ERCs injected into mouse recipients, on the one hand, migrate to the allograft directly to come into the protective effect, and on the other hand, migrate to the lymphoid organs such as spleen, at which point the ERCs could promote immune cells to become an immune tolerogenic phenotype. Besides, according to the previous study 58, SDF‐1 secreted by ERCs may also affect the generation of immune cells, such as M2, directly. Both of them together help the ERCs achieve immunoregulation.
In this study, we demonstrated that human ERC‐based therapy can effectively suppress the immune response and further induce allograft tolerance in a mouse cardiac transplantation model, and SDF‐1 secreted by ERCs plays an important role in ERC‐mediated graft protection. Although the results are encouraging and promising, more in‐depth studies, such as the impact of RAPA on biological activities of ERCs including the secretion of SDF‐1, and the role of ERCs in combination with other immunosuppressive drugs in the prevention of graft rejection are warranted.
Recently, human ERCs have been used in clinical trials in patients with multiple sclerosis, Duchenne muscular dystrophy, and early heart failure. The results showed no immediate immunological reactions, and the patients remained in good health 23, 59, 60. Considering that human ERCs were proven to be safe and effective in mice, human ERCs hold significant promise for therapeutic use in future clinical transplantation.
Conclusion
To our knowledge, this study is the first to investigate the efficacy of ERC‐based therapy in mediating allograft tolerance following transplantation. Our studies have demonstrated that SDF‐1 plays an essential role in ERC‐mediated immunoregulation both in vivo and in vitro. More importantly, as a xenograft, human ERCs could be accepted by mice, which proves its hypoimmunogenicity, along with its features of noninvasively obtained method, abundant resources, highly proliferative rate, immunoregulatory function and absence of tumorigenesis. It suggests that human ERCs could be an excellent candidate to be used in prevention of transplant rejection in the clinical settings.
Author Contributions
X. Lan and X.X.: conception and design, performance of the research, data analysis and interpretation, manuscript writing; G.W.: conception and design, data analysis and interpretation, manuscript writing; S.L., X. Li, B.Z., G.S. and Y.Z.: performance of the research, data analysis and interpretation; C.D.: Data analysis and interpretation, manuscript writing; H.W.: conception and design, financial support, administrative support, manuscript writing, final approval of manuscript; X. Lan, X.X., G.W., S.L., X. Li, B.Z., G.S., Y.Z., C.D., and H.W.: read and approved the final manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
We are grateful to Dr. Yongliang Sun for his support of some consumptive material used in this study. This work was supported by grants to H.W. from National Natural Science Foundation of China (81471584 and 81273257), Tianjin Application Basis and Cutting‐Edge Technology Research Grant (14JCZDJC35700), Li Jieshou Intestinal Barrier Research Special Fund (LJS_201412), and Tianjin Medical University Talent Fund.
References
- 1. Lund LH, Edwards LB, Kucheryavaya AY et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty‐Second Official Adult Heart Transplantation Report–2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1244–1254. [DOI] [PubMed] [Google Scholar]
- 2. Söderlund C, Rådegran G. Immunosuppressive therapies after heart transplantation–The balance between under‐ and over‐immunosuppression. Transplant Rev (Orlando) 2015;29:181–189. [DOI] [PubMed] [Google Scholar]
- 3. Ge W, Jiang J, Baroja ML et al. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant 2009;9:1760–1772. [DOI] [PubMed] [Google Scholar]
- 4. Wang H, Qi F, Dai XC et al. Requirement of B7‐H1 in mesenchymal stem cells for immune tolerance to cardiac allografts in combination therapy with rapamycin. Transpl Immunol 2014;31:65–74. [DOI] [PubMed] [Google Scholar]
- 5. Reinders ME, de Fijter JW, Roelofs H et al. Autologous bone marrow‐derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: Results of a phase I study. Stem Cells Translational Medicine 2013;2:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. English K, Wood KJ. Mesenchymal stromal cells in transplantation rejection and tolerance. Cold Spring Harb Perspect Med 2013;3:a015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo K, Ikehara S, Meng X. Mesenchymal stem cells for inducing tolerance in organ transplantation. Front Cell Dev Biol 2014;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 1976;4:267–274. [PubMed] [Google Scholar]
- 9. Meng X, Ichim TE, Zhong J et al. Endometrial regenerative cells: A novel stem cell population. J Transl Med 2007;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minteer D, Marra KG, Rubin JP. Adipose‐derived mesenchymal stem cells: Biology and potential applications. Adv Biochem Eng Biotechnol 2013;129:59–71. [DOI] [PubMed] [Google Scholar]
- 11. Dalous J, Larghero J, Baud O. Transplantation of umbilical cord‐derived mesenchymal stem cells as a novel strategy to protect the central nervous system: Technical aspects, preclinical studies, and clinical perspectives. Pediatr Res 2012;71:482–490. [DOI] [PubMed] [Google Scholar]
- 12. Tolar J, Nauta AJ, Osborn MJ et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 2007;25:371–379. [DOI] [PubMed] [Google Scholar]
- 13. Jeong JO, Han JW, Kim JM et al. Malignant tumor formation after transplantation of short‐term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res 2011;108:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamat P, Schweizer R, Kaenel P et al. Human adipose‐derived mesenchymal stromal cells may promote breast cancer progression and metastatic spread. Plast Reconstr Surg 2015;136:76–84. [DOI] [PubMed] [Google Scholar]
- 15. Karnoub AE, Dash AB, Vo AP et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449:557–563. [DOI] [PubMed] [Google Scholar]
- 16. Hida N, Nishiyama N, Miyoshi S et al. Novel cardiac precursor‐like cells from human menstrual blood‐derived mesenchymal cells. Stem Cells 2008;26:1695–1704. [DOI] [PubMed] [Google Scholar]
- 17. Patel AN, Park E, Kuzman M et al. Multipotent menstrual blood stromal stem cells: Isolation, characterization, and differentiation. Cell Transplant 2008;17:303–311. [DOI] [PubMed] [Google Scholar]
- 18. Patel AN, Silva F. Menstrual blood stromal cells: The potential for regenerative medicine. Regen Med 2008;3:443–444. [DOI] [PubMed] [Google Scholar]
- 19. Jiang Z, Hu XY, Yu H et al. Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. J Cell Mol Med 2013;17:1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bockeria L, Bogin V, Bockeria O et al. Endometrial regenerative cells for treatment of heart failure: A new stem cell enters the clinic. J Transl Med 2013;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy MP, Wang H, Patel AN et al. Allogeneic endometrial regenerative cells: An “Off the shelf solution” for critical limb ischemia? J Transl Med 2008;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai DM, Wang FY, Yao XF et al. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med 2015;13:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhong ZH, Patel AN, Ichim TE et al. Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med 2009;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borlongan CV, Kaneko Y, Maki M et al. Menstrual blood cells display stem cell‐like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev 2010;19:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lv YC, Xu XX, Zhang B et al. Endometrial regenerative cells as a novel cell therapy attenuate experimental colitis in mice. J Transl Med 2014;12:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun P, Liu J, Li WW et al. Human endometrial regenerative cells attenuate renal ischemia reperfusion injury in mice. J Transl Med 2016;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu S, Shi G, Xu X et al. Human endometrial regenerative cells alleviate carbon tetrachloride‐induced acute liver injury in mice. J Transl Med 2016;14:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu X, Li X, Gu X et al. Prolongation of Cardiac Allograft Survival by Endometrial Regenerative Cells: Focusing on B‐Cell Responses. Stem Cells Translational Medicine 2016;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Si XY, Li JJ, Yao T et al. Transforming growth factor‐beta1 in the microenvironment of ischemia reperfusion‐injured kidney enhances the chemotaxis of mesenchymal stem cells to stromal cell‐derived factor‐1 through upregulation of surface chemokine (C‐X‐C motif) receptor 4. Mol Med Rep 2014;9:1794–1798. [DOI] [PubMed] [Google Scholar]
- 30. Wan X, Xia WK, Gendoo Y et al. Upregulation of stromal cell‐derived factor 1 (SDF‐1) is associated with macrophage infiltration in renal ischemia‐reperfusion injury. PLoS One 2014;9:e114564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beider K, Bitner H, Leiba M et al. Multiple myeloma cells recruit tumor‐supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget 2014;5:11283–11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Papeta N, Chen T, Vianello F et al. Long‐term survival of transplanted allogeneic cells engineered to express a T cell chemorepellent. Transplantation 2007;83:174–183. [DOI] [PubMed] [Google Scholar]
- 33. Chen T, Yuan J, Duncanson S et al. Alginate encapsulant incorporating CXCL12 supports long‐term allo‐ and xenoislet transplantation without systemic immune suppression. Am J Transplant 2015;15:618–627. [DOI] [PubMed] [Google Scholar]
- 34. McCandless EE, Wang Q, Woerner BM et al. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol 2006;177:8053–8064. [DOI] [PubMed] [Google Scholar]
- 35. Wang H, Jin P, Sabatino M et al. Comparison of endometrial regenerative cells and bone marrow stromal cells. J Transl Med 2012;10:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shirozu M, Nakano T, Inazawa J et al. Structure and chromosomal localization of the human stromal cell‐derived factor 1 (SDF1) gene. Genomics 1995;28:495–500. [DOI] [PubMed] [Google Scholar]
- 37. Wang H, Hosiawa KA, Min W et al. Cytokines regulate the pattern of rejection and susceptibility to cyclosporine therapy in different mouse recipient strains after cardiac allografting. J Immunol 2003;171:3823–3836. [DOI] [PubMed] [Google Scholar]
- 38. Wang H, Hosiawa KA, Garcia B et al. Attenuation of acute xenograft rejection by short‐term treatment with LF15‐0195 and monoclonal antibody against CD45RB in a rat‐to‐mouse cardiac transplantation model. Transplantation 2003;75:1475–1481. [DOI] [PubMed] [Google Scholar]
- 39. Bleul CC, Fuhlbrigge RC, Casasnovas JM et al. A highly efficacious lymphocyte chemoattractant, stromal cell‐derived factor 1 (SDF‐1). J Exp Med 1996;184:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang JM, Wang JN, Yang JY et al. Mesenchymal stem cells over‐expressing SDF‐1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg 2009;36:644–650. [DOI] [PubMed] [Google Scholar]
- 41. Ascione R, Rowlinson J, Avolio E et al. Migration towards SDF‐1 selects angiogenin‐expressing bone marrow monocytes endowed with cardiac reparative activity in patients with previous myocardial infarction. Stem Cell Res Ther 2015;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schuh A, Kroh A, Konschalla S et al. Myocardial regeneration by transplantation of modified endothelial progenitor cells expressing SDF‐1 in a rat model. J Cell Mol Med 2012;16:2311–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Penn MS, Pastore J, Miller T et al. SDF‐1 in myocardial repair. Gene Ther 2012;19:583–587. [DOI] [PubMed] [Google Scholar]
- 44. Dong F, Harvey J, Finan A et al. Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation 2012;126:314–324. [DOI] [PubMed] [Google Scholar]
- 45. Mayorga M, Kiedrowski M, Shamhart P et al. Early upregulation of myocardial CXCR4 expression is critical for dimethyloxalylglycine‐induced cardiac improvement in acute myocardial infarction. Am J Physiol Heart Circ Physiol 2016;310:H20–28. [DOI] [PubMed] [Google Scholar]
- 46. Zhang M, Mal N, Kiedrowski M et al. SDF‐1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 2007;21:3197–3207. [DOI] [PubMed] [Google Scholar]
- 47. Mazzarella G, Petillo O, Margarucci S et al. Role of monocyte/macrophage population in immune response. Monaldi Arch Chest Dis 1998;53:92–96. [PubMed] [Google Scholar]
- 48. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–252. [DOI] [PubMed] [Google Scholar]
- 49. Lee HK, Iwasaki A. Innate control of adaptive immunity: Dendritic cells and beyond. Semin Immunol 2007;19:48–55. [DOI] [PubMed] [Google Scholar]
- 50. Jonuleit H, Schmitt E, Steinbrink K et al. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol 2001;22:394–400. [DOI] [PubMed] [Google Scholar]
- 51. Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol 2013;120:163–184. [DOI] [PubMed] [Google Scholar]
- 52. Kondelkova K, Vokurkova D, Krejsek J et al. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove) 2010;53:73–77. [DOI] [PubMed] [Google Scholar]
- 53. Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev 2008;224:201–214. [DOI] [PubMed] [Google Scholar]
- 54. Walker LS. Treg and CTLA‐4: Two intertwining pathways to immune tolerance. J Autoimmun 2013;45:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoo SW, Chang DY, Lee HS et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF‐beta. Neurobiol Dis 2013;58:249–257. [DOI] [PubMed] [Google Scholar]
- 56. Patel SA, Meyer JR, Greco SJ et al. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: Role of mesenchymal stem cell‐derived TGF‐beta. J Immunol 2010;184:5885–5894. [DOI] [PubMed] [Google Scholar]
- 57. Petit I, Jin D, Rafii S. The SDF‐1‐CXCR4 signaling pathway: A molecular hub modulating neo‐angiogenesis. Trends Immunol 2007;28:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sanchez‐Martin L, Estecha A, Samaniego R et al. The chemokine CXCL12 regulates monocyte‐macrophage differentiation and RUNX3 expression. Blood 2011;117:88–97. [DOI] [PubMed] [Google Scholar]
- 59. Ichim TE, Alexandrescu DT, Solano F et al. Mesenchymal stem cells as anti‐inflammatories: Implications for treatment of Duchenne muscular dystrophy. Cell Immunol 2010;260:75–82. [DOI] [PubMed] [Google Scholar]
- 60. Ichim TE, Solano F, Lara F et al. Combination stem cell therapy for heart failure. Int Arch Med 2010;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


