Summary
The mission of the California Institute of Regenerative Medicine (CIRM) is to accelerate treatments to patients with unmet medical needs. In September 2016, CIRM sponsored a workshop held at the University of California, Los Angeles, to discuss regenerative medicine approaches for treatment of lung diseases and to identify the challenges remaining for advancing such treatments to the clinic and market approval. Workshop participants discussed current preclinical and clinical approaches to regenerative medicine in the lung, as well as the biology of lung stem cells and the role of stem cells in the etiology of various lung diseases. The outcome of this effort was the recognition that whereas transient cell delivery approaches are leading the way in the clinic, recent advances in the understanding of lung stem cell biology, in vitro and in vivo disease modeling, gene editing and replacement methods, and cell engraftment approaches raise the prospect of developing cures for some lung diseases in the foreseeable future. In addition, advances in in vitro modeling using lung organoids and “lung on a chip” technology are setting the stage for high quality small molecule drug screening to develop treatments for lung diseases with complex biology. Stem Cells Translational Medicine 2017;6:1823–1828
Keywords: Lung, Regenerative medicine, Stem cells, Clinical trials, Gene therapy, Organoids, Mesenchymal stromal cells, Engraftment, Lung diseases, Cellular therapeutics, Induced pluripotent stem cells, Bioengineering, In vivo imaging, Disease modeling
Introduction
Although lung diseases remain a major source of morbidity and mortality throughout the world, progress has been slow, both in basic research for understanding the biological basis for lung disease, as well as clinical research for diagnostics, prevention, and treatment. The economic cost of asthma, chronic obstructive pulmonary disease (COPD), and pneumonia was $106 billion in the U.S. in 2009, including $81 billion in direct health expenditures and $25 billion in indirect costs of mortality 1. Although there have been incremental improvements in the development of therapies for symptoms of lung diseases such as COPD and asthma, disease‐modifying treatments remain scarce. Stem cells and regenerative medicine approaches hold great promise for the development of new therapies and patient‐specific disease models to increase understanding of disease pathophysiology. Here we review the state of the field and highlight next steps and challenges for the field.
Lung Stem Cells and Associated Diseases
The lung is a structurally complex organ comprised of several spatial regions, each with its own stem and progenitor cells and types of niches. Diseases of the lung are complex in etiology, affecting distinct regions and cell types, thus it is critical to understand the repair processes in each area of the lung (Fig. 1).
Figure 1.
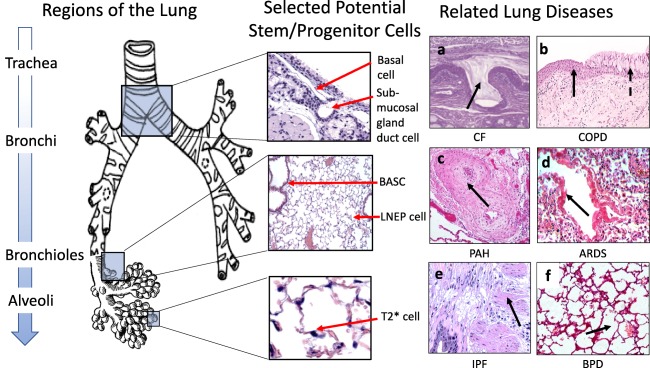
Regions of the lung, their potential stem cell niches and related lung diseases. Left: Regions of the bronchial tree are shown from proximal (top) to distal (bottom). Middle: The normal histology of regions highlighted in the blue boxes are shown, indicating putative, region‐specific stem/progenitor cells and their niches. Right: Lung diseases shown are (a): cystic fibrosis with mucus plugging and abnormal submucosal gland duct (arrow), (b): chronic obstructive pulmonary disease with squamous metaplasia (solid arrow) and mucus metaplasia (dotted arrow), (c): pulmonary arterial hypertension with intimal thickening (arrow), (d): acute respiratory distress syndrome with hyaline membranes (arrow), inflammation, and edema, (e): idiopathic pulmonary fibrosis with fibrotic foci (arrow) and extensive lung remodeling and (f): bronchopulmonary dysplasia with alveolar simplification. Abbreviations: ARDS, acute respiratory distress syndrome; BASC, bronchioalveolar stem cell; BPD, bronchopulmonary dysplasia; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; LNEP, lineage‐negative epithelial stem/progenitor cell; PAH, pulmonary arterial hypertension; T2*, type 2 alveolar cell*.
Dr. Mark Krasnow (Stanford University) described lineage tracing experiments that identified a rare subset of mature surfactant‐secreting alveolar type 2 (AT2 cells) called AT2* cells. These cells have the ability to either self‐renew or to give rise to alveolar type 1 (AT1) cells, thus fulfilling a stem cell function in the distal lung. Although normally quiescent, AT2* cells become activated to proliferate and differentiate in response to lung injury, providing a repair mechanism. Experimental depletion of AT2* cells in mice resulted in formation of fibrosis reminiscent of idiopathic pulmonary fibrosis (IPF), suggesting that at least some cases of IPF may be due to stem cell deficiency or exhaustion. If so, replacement of AT2* stem cells could provide an avenue for IPF treatment. In support of this hypothesis, telomerase mutations have been found to be associated with some hereditary forms of IPF, and telomerase deficiency is known to lead to stem cell exhaustion.
Dr. Hal Chapman (UCSF) discussed a mouse stem cell population found in distal airways. ΔNp63+Krt5+ cells are distal airway stem cells that expand from lineage negative epithelial progenitors (LNEPs) after influenza H1N1 infection in mice, and subsequently differentiate to mature epithelium upon downregulation of Notch signaling 2. Failure to downregulate Notch signaling results in the formation of alveolar cysts. A cell type similar to Krt5+ exists in human lungs, and also proliferates in response to H1N1 infection, thus may represent another cell type ripe for exploitation.
Approaches to Monogenic Lung Diseases
Cystic Fibrosis
Among the workshop presentations, cystic fibrosis (CF) was viewed as a “shining example” of a monogenic respiratory disorder that may clearly benefit from stem cell therapeutics, as highlighted by Dr. William Skach (Cystic Fibrosis Foundation). CF is a fatal respiratory disease caused by an autosomal recessive mutation in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR), a chloride transporter that regulates water transport in epithelial cells of multiple organs, including the lung. Due to the development of effective clinical methods to enhance mucociliary clearance, the median survival of CF patients is now over 40 years, and is expected to increase due to recent FDA approvals of several CFTR modulators 3. However, drug treatments are not necessarily curative and they are only effective in a subset of patients bearing specific CFTR mutations. Lung transplantation, while potentially curative, is only available for some patients, and comes with the risks of lifelong immune suppression and the potential for transplant rejection and infections.
The recent development of simple and efficient gene editing or replacement methods has raised the prospect of gene correction of CFTR mutations in autologous lung airway epithelial progenitor cells as a cure for CF 4. Dr. Amy Firth (USC) described the use of CRISPR/Cas9 gene editing to correct CF patient induced pluripotent stem cells (iPSC), followed by ex vivo differentiation to functionally corrected lung epithelial cells. A longer‐term goal is to differentiate such gene‐corrected iPSCs to lung progenitor cells that could be engrafted back into the lungs of CF patients. To achieve this goal, some of the challenges to be addressed include the identification and characterization of the human lung progenitors that repopulate and restore normal airway epithelium, development of methods to differentiate iPSC to that particular lung progenitor, and identification of the appropriate stem cell niche and ability to manipulate it to encourage engraftment. Alternative approaches under development include methods for efficiently gene editing lung progenitor cells in vivo (Table 1).
Table 1.
The current state of the field for lung stem cell therapies
| Lung disease | Approach for stem cell‐based therapy | Current stage of development | Challenges/Next steps |
|---|---|---|---|
| CF | • Gene corrected autologous iPSC, differentiated to lung airway progenitor cells for transplant to lung | • Research |
• Differentiation to appropriate cell type • “Making space” in the niche for engraftment • Engraftment efficiency and durability |
| hPAP | • Gene corrected autologous HSC, differentiated to macrophages for transplant to lung | • Preclinical development |
• Translation of gene correction from mouse to human HSC • Using the best gene correction technology • IND for the gene corrected cell product |
| BPD |
• Cord‐blood derived MSC delivered to lung airway • MSC (source of MSC not specified) |
• NCT02381366 Phase I–II U.S. • NCT01828957 Phase II Korea • NCT02443961 Phase I Spain |
• Safety of MSC treatment in pre‐term infants • Efficacy in placebo controlled trials • Understanding mechanism of action |
| ARDS |
• Allogeneic bone marrow derived MSC administered intravenously • Allogeneic MSC administered intravenously |
• NCT02097641 Phase II U.S. • NCT02804945 Phase II U.S. |
• Optimal source of MSCs • Evidence for efficacy • Understanding mechanism of action |
| IPF |
• Allogeneic bone marrow‐derived MSC administered intravenously • Placental MSC administered intravenously • Autologous bone‐marrow derived MSC administered endobronchially |
• NCT02013700 Phase I U.S. • NCT01385644 Phase I Australia • NCT01919827 Phase I Spain |
• Safety of MSC treatment in IPF patients • Better understanding of disease etiology • Understanding mechanism of action • Efficacy in placebo controlled trials |
| PAH |
• eNOS‐transfected autologous endothelial progenitor cells delivered to the pulmonary artery • Allogeneic cardiosphere derived cells (CDC) administered via central intravenous delivery |
• NCT00469027 Phase I Canada • NCT03001414 Phase II Canada • NCT03145298 Phase Ia/b U.S. |
• Patient recruitment • Manufacturing costs (eNOS‐EPC) • Understanding mechanism of action • Safety in PAH patients (CDC) • Efficacy in placebo controlled trials |
Abbreviations: ARDS, acute respiratory distress syndrome; BPD, bronchopulmonary dysplasia; CF, cystic fibrosis; eNOS, endothelial nitric oxide synthase; hPAP, hereditary pulmonary alveolar proteinosis; HSC, hematopoietic stem cells; IPF, idiopathic pulmonary fibrosis; iPSC, induced pluripotent stem cells; MSC, mesenchymal stem cells; PAH, pulmonary arterial hypertension.
Pulmonary Alveolar Proteinosis
Dr. Bruce Trapnell (Cincinnati Children's Hospital) presented results of preclinical studies of the rare monogenic respiratory disorder, hereditary pulmonary alveolar proteinosis (hPAP). hPAP is characterized by abnormal accumulation of surfactant in alveolar macrophages and pulmonary alveoli. Alveolar macrophages normally require granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) signaling to stimulate surfactant clearance; however, in hPAP this function is prevented by mutations in the genes encoding the GM‐CSF receptor (CSF2R) α or β chains. As a result, alveoli and lung macrophages become filled with surfactant lipids, causing restrictive lung impairment and respiratory failure.
Prompted by the dysfunctional status of hPAP macrophages, Trapnell and his team developed a strategy for transplanting pulmonary macrophages in mice 5. A normal copy of the CSF2R gene was introduced into bone marrow derived CD34+ hematopoietic stem and progenitor cells by a viral vector. The corrected stem cells were then differentiated to form macrophages that were then transplanted into the lung of Csf2Rb–/– knockout mice, which faithfully recapitulate human hPAP. In the transplanted mice, the gene‐marked macrophages remained localized in the alveoli and did not migrate to other organs. Moreover, their lungs showed improvements in surfactant homeostasis, alveolar stability, and lung function. The overall survival of the mice improved, and no side effects were observed 5. Trapnell is now working toward translating this proof of concept study to clinical trials (Table 1).
Mesenchymal Stromal Cell Treatment for Inflammatory Lung Disease
Mesenchymal stromal cells (MSCs) have anti‐inflammatory properties that have prompted their testing in clinical trials for a variety of chronic inflammatory diseases. Since MSC‐based approaches generally do not require modification of specific genes or molecules, they may provide a generalizable therapeutic intervention for the many lung diseases caused by or secondary to inflammatory responses. MSCs do not themselves engraft, and therefore do not provide sustained benefits; nevertheless, high intensity acute inflammatory conditions in the lung, such as acute respiratory distress syndrome (ARDS) or sepsis/septic shock, may benefit from administration of MSCs, as we discuss below.
ARDS
ARDS is characterized by severe inflammation in the lungs, and presents as severe hypoxemia and bilateral opacities on chest x‐ray that are not explained by heart failure. The usual causes are pneumonia, sepsis, aspiration, and major trauma. There are ∼200,000 cases in the U.S. per year, with a high mortality rate.
Dr. Michael Matthay (UCSF) described preclinical data in which administration of bone marrow‐derived MSCs reduced endotoxin‐induced lung injury in mouse and sheep models, improving survival and lung function and markedly reducing inflammation and microbial infections. No safety issues were raised in a phase I clinical trial for allogeneic MSC treatment of ARDS patients, which prompted initiation of a multi‐center, randomized blinded placebo‐controlled phase II trial enrolling 60 patients, to further assess safety and obtain preliminary efficacy data (Table 1) 6.
Idiopathic Pulmonary Fibrosis
IPF is an unpredictable and fatal chronic lung scarring disease. Dr. Marilyn Glassberg (University of Miami) presented results of a 2014 randomized exploratory phase I clinical trial to evaluate safety, tolerability, and potential efficacy of allogeneic human bone marrow‐derived MSC delivered via intravenous infusion for patients with confirmed IPF 7. Among the nine participants enrolled in the trial, no treatment‐emergent serious adverse events were reported. Two non‐treatment related deaths occurred during the study in two subjects receiving the highest dosage of cells (200 million) due to progression of IPF (i.e., disease worsening and/or acute exacerbation). At 60 weeks post‐infusion, improvements in lung function parameters were observed, although they were not statistically significant due to the small number of participants. Specifically, a 3.0% mean decline in percent predicted forced vital capacity and 5.4% mean decline in percent predicted carbon monoxide diffusing capacity (DLCO) was reported 7.
Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is a multifactorial disease of prematurity that causes impaired lung development. Dr. Bernard Thébaud (Ottawa Hospital Research Institute, OHRI) presented an experimental stem cell‐based approach to treat BPD with umbilical cord‐derived mesenchymal stem cells, which as a “young” population of cells, may have superior therapeutic benefit as compared to “old” cells available for autologous stem cell transplants, as shown in parabiosis experiments 8. Thébaud's pre‐clinical studies of US‐MSCs showed feasibility, safety, and structural and functional short‐term and long‐term benefit in neonatal rodents 9. The group is now conducting pre‐clinical testing in a baboon model in collaboration with Dr. Seidner. Ventilation delivered to the lungs of preterm baboons for two weeks induces radiological and histological features reminiscent of human BPD, establishing a unique large animal model for acquiring critical preclinical safety and efficacy data (Table 1).
Endothelial Progenitor Cells + eNOS for Pulmonary Arterial Hypertension
Dr. Duncan Stewart (OHRI) presented a transient gene overexpression strategy for treating pulmonary arterial hypertension (PAH), a disease characterized by massive microvascular loss that causes increased blood pressure in the lungs. Endothelial nitric oxide synthetase (eNOS) is an enzyme that produces nitric oxide, a vascular relaxation factor that also plays an important role in vascular repair and regeneration, thus could potentially alleviate the high pulmonary arterial pressure in PAH. In a phase I trial to test safety and preliminary efficacy, autologous endothelial progenitor cells were transiently transfected with a plasmid expressing eNOS, and introduced into the pulmonary artery of PAH patients. Stewart reported difficulty in recruiting patients due to rarity of PAH, restrictive eligibility criteria, and the need for ICU admission and special instrumentation. However, the treatment was well tolerated, and patients showed significant improvements in long‐term 6‐minute walk distance, though no significant effect on hemodynamic scores 10. A phase II trial using an improved “minicircle” DNA vector and multiple cell dosing is planned to start in early‐2017. The trial will entail up to eight monthly cell injections and use minicircle DNA vectors to avoid innate immune responses caused by bacterial sequences of plasmids (Table 1).
In Vitro Modeling
IPSCs have become an established tool for performing drug screening and studies of disease mechanisms. The use of iPSC lines made from hundreds or even thousands of individuals is a powerful tool for studying disease heterogeneity, whereas single iPSC lines can be used to study the potential effectiveness of therapeutics on individual patient in a cell‐based assay. Moreover, “disease in a dish” models can identify critical cellular and molecular contributors to disease and provide a means to vary them independently. They can be useful in situations where animal models fail to adequately recapitulate human pathologies, and immortalized cell lines fail to mimic tissue dysfunction central to the disease.
iPSC‐Derived Cells for Studying Disease Mechanisms
Of the monogenic lung diseases, CF, ciliopathies, and Alpha 1 anti‐trypsin deficiency have all been successfully modeled with iPSC. However, complex lung disease models using iPSC have not yet been reported. Recapitulating a complex disease in culture can be challenging, and may require certain environmental conditions or screening for disruptions of pathways by gene expression analysis to detect the disease phenotype. For diseases with a high degree of heterogeneity, establishing patient specific cell lines may be needed to cost‐effectively represent disease variation.
Differentiating lung tissue from iPSCs is a complex multistep process, requiring passage through definitive endoderm, anterior foregut endoderm, and lung progenitors, to finally obtain mature polarized airway epithelium. Efficiency of differentiation to lung tissue can be variable due to differences in iPSC quality, differentiation protocols, reagents, and criteria for defining mature cells 11. Workshop participants recommended that lung researchers develop a standard lung differentiation protocol to facilitate data comparisons from lab to lab.
iPSC‐Derived Cells for Drug Development
iPSCs provide an opportunity for phenotypic drug screening that requires no knowledge of the molecular pathways involved in the disease phenotype. Compounds are screened using high throughput automated robotic platforms with the objective of identifying hits that correct the cellular disease phenotype. Discovering pharmacological targets, pharmacologically profiling a cellular or biochemical pathway of interest, and assessing the effects of compounds on phenotypes are all possible with iPSC screening. For example, Dr. Brigitte Gomperts (UCLA) presented a phenotypic model of scarring using iPSC derived from IPF patients that her laboratory used to screen for compounds to prevent the phenotype.
New Technical Advances to Aid the Field
Tissue Engineering
Another promising area for stem cells in treating lung disease is tissue engineering, where stem cells provide a source of proliferating, multi‐potent cells used to seed scaffolds. Major challenges include generating adequate surface area for gas exchange and maintaining barrier function between blood and air. Engineered tissue must possess proper mechanical characteristics, and not be emphysematous or fibrotic, for example.
Dr. Laura Niklason (Yale University) reviewed first generation efforts in which decellularized lung matrices were repopulated with human lung cells differentiated from adult lung tissue or iPSCs. When implanted into rodents, such tissue engineered lungs were capable of exchanging gas for 4–5 hours before alveolar barriers failed, leading to bleeding into the airways 12. Similar results have been reported by others 13. While these initial results are exciting, lung tissue engineering efforts are still at the research stage, and will require further work before they can be considered for preclinical development.
Organoid models of various lung tissues have the potential to advance technologies such as patient‐specific precision screens, 3D confocal drug screening, and microfluidics 14. Dr. Jennifer Sucre (Vanderbilt University) emphasized that structure is itself a biological signal; therefore, lung models must retain structural features unique to the human lung 15. Dr. Brigitte Gomperts (UCLA), reviewed the diverse lung tissues that have been differentiated from cell lines, including tracheospheres, bronchiolar organoids, bronchioalveolar spheres, alveolospheres, proximal and distal airway, and distal airway scaffolds onto which many cell types can be patterned. To date, organoid‐based disease models have been developed for CF, lung fibrosis 16, and BPD 17. Targeted correction of the CFTR gene in organoids derived from CF patients has also been reported 18, 19, 20.
Combining organoids with microfluidics devices has created the “lung on a chip,” consisting of epithelial and endothelial chambers in which breathing motions simulate cyclic stretch 16, 21. Multiple pathologic states have been modeled this way, including asthma, COPD, airway response to injury, sepsis, and pulmonary edema 22. The chips can be used as a platform for drug/toxin screen, and linked in series with other organs.
Vector Development
Because lung diseases can involve so many different lung regions and cell types, including multiple types of stem/progenitor cells, the appropriate choice of gene therapy vectors to modify cells is critical to the success of stem cell therapies for lung diseases. Dr. John Engelhardt (University of Iowa) reviewed several diverse gene therapy vectors suitable for cell‐based strategies for treating respiratory disorders, focusing on CF.
Among potential gene therapy vectors, parvovirus vectors, which include adeno‐associated virus and human bocavirus (HBoV1), may be closest to the clinic 23, 24. RNA viral vectors, which include integrating and non‐integrating lentiviral vectors with a packaging capacity up to 8 kb transgene cassettes, represent another promising class of gene therapy vectors 25. Different viral vectors offer different risks, such as viral‐elicited immune response, and benefits, such as the ability to deliver homologous DNA fragments. Moreover, each animal model species may require its own vector toolbox and serotype to efficiently test in vivo gene correction strategies.
Animal Models
Improved animal models for lung diseases are needed for pre‐clinical testing of stem cell and drug therapies. In particular, attempting to emulate human biology in small animals for testing cell transplantation presents particular challenges in terms of cell dosing, biodistribution, and durability. Hence many researchers are developing larger animal models for preclinical testing.
For CF, larger animal models such as the pig mimic the human disease well, although accessibility to this model is limited by its expense. Dr. Engelhardt has developed a ferret model of CF as a more tractable alternative, which also has the advantage of having mucous‐producing goblet cells and submucosal glands, like humans 26. Dr. Firth has established ferret iPSCs to evaluate the ability of autologous cells to repair the proximal and distal airway epithelium following controlled experimental injury. The ferret model also can be used to test the differentiation capacity of ferret iPSCs in vitro and in vivo, as well as the engraftment potential of their derivatives.
Creating a Niche
For effective engraftment of transplanted cells to take place, it may be necessary to deplete resident stem cells in order to generate physical space for the transplanted cells. In addition, a permissive milieu that includes appropriate matrices, cytokines, and growth factors must be established without exacerbating underlying disease or damaging lung tissue. Dr. Barry Stripp (Cedars Sinai Medical Center) discussed several examples from the recent literature showing that preconditioning with naphthalene or other cell damaging treatments stimulates regeneration of clonally derived patches of cells at the site of injection 2, 27, 28. Dr. Yair Reisner (Weizmann Institute of Science) commented that the lung stem cell niche is similar to the bone marrow stem cell niche in that it can be opened for stem cell engraftment by first damaging the tissue to stimulate stem cell proliferation, then irradiating to kill the endogenous stem cells 28. These and other studies indicate that stem cell ablation may be needed for robust repopulation by newly introduced cells. However, since the ablation method itself can be damaging to the tissue, the ideal method for opening the lung stem cell niche remains to be determined.
Tracking Cells In Vivo in Clinical Trials
For cell replacement to succeed in patients, it will be important to monitor the biodistribution of cell products after they are introduced into patients. Improved cell imaging technologies will accelerate clinical trials by improving cell delivery and dosing, illuminating mechanisms of actions, and providing improved surveillance of inflammation. To this end, Eric Ahrens (UCSD) has developed a non‐invasive, highly sensitive technology for detecting and quantifying cell therapy products in vivo using 19F MRI 29. MRI is suitable for use in humans because it does not require radionuclides or ionizing radiation. However unlike current 1H labeling technologies, 19F MRI requires only one scanning session, saving money and time. Proof of concept studies in rodents and pigs have demonstrated its capacity to monitor biodistribution of dendritic cell, T cells, and stem cells in various organs and tissues 30. This approach has been used in human clinical trials, is agnostic to the cell type or disease, and can be broadly applied to monitor cellular biodistribution 31.
Conclusion and Next Steps
Early clinical trials using cell therapies for lung disease have used nonengrafting adult stem and progenitor cells (MSC and EPC) to provide transient paracrine benefits. These cells have been shown to be safe when administered to critically ill patients. The next step will be to determine if they are unambiguously efficacious in treating or curing lung diseases and, if so, to demonstrate their mechanism of action for different diseases.
The next generation of stem cell‐based lung therapies will likely entail repair or replacement of damaged lung tissue by permanently engrafting lung stem cells, which if successful, could provide cures for some lung diseases. For the lung, the “low‐hanging fruit” for such next generation therapies are likely to be monogenic lung diseases such as CF and hPAP, in which approaches to correct or replace a defective gene in a stem cell are feasible, either ex vivo followed by transplantation and engraftment, or by gene modification of stem cells in vivo. The recent emergence of a new generation of gene editing and gene replacement methods promises to accelerate the development of therapies for monogenic disorders in particular.
A pressing challenge for developing cell therapeutics aimed at lung diseases will be achieving a better understanding of those with complex etiologies, such as PF, BPD, COPD, and PAH (Table 1). Of these, if any are found to have defective lung stem cells, as was hypothesized to be the case for pulmonary fibrosis, then methods to replace or reactivate such stem cells may prove to be effective treatments. In addition, diseases in which tissue is absent or damaged would theoretically be amenable to regenerative repair. Another challenge will be to gain a better understanding of the lung stem cell niche(s), and to develop engraftment strategies that will create space for stem cells without severely damaging the lung. Finally, standardizing sources of cells and protocols will help accelerate and improve the rigor of clinical testing.
Author Contributions
L.K., N.D.W., and B.G.: conception and design, manuscript writing, final approval of manuscript
Disclosure of Potential Conflicts of Interest
Dr. Brigitte Gomperts is a founder and consultant to InSpira LLC. The other authors indicated no potential conflicts of interest.
Acknowledgments
We thank the workshop participants for their valuable contributions: Denise Al‐Alam (Children's Hospital Los Angeles), Eric Ahrens (UCSD), Hal Chapman (UCSF), Greg Cosgrove (Pulmonary Fibrosis Foundation), Larry Couture (Orbsen Therapeutics), Ramona Doyle (UCSF), John Engelhardt (University of Iowa), Amy Firth (USC), Paul Frech (CIRM), Marilyn Glassberg (University of Miami), Tracy Grikscheit (Children's Hospital Los Angeles), Valeria Hatcher (PFF advocate), Mark Krasnow (Stanford), Steven Kesten (SKC Life Sciences), Michael Matthay (UCSF), Parviz Minoo (USC), Laura Niklason (Yale University), Pat Olson (CIRM), Scott Randell (University of North Carolina), Yair Reisner (Weizmann Institute of Science), Kelly Shepard (CIRM), Bill Skach (Cystic Fibrosis Foundation), Isabel Stenzel Byrnes (CF advocate), Duncan Stewart (Ottawa Hospital Research Institute), Barry Stripp (Cedars Sinai Medical Center), Jennifer Sucre (Vanderbilt University), Bernard Thébaud (Ottawa Hospital Research Institute), Bruce Trapnell (Cincinnati Children's Hospital), Preethi Vijayaraj (UCLA), and David Warburton (Children's Hospital Los Angeles). This workshop was supported by CIRM educational grant EDUC1.3 to Brigitte Gomperts.
CIRM was established in November 2004 with the passage of Proposition 71, the California Stem Cell Research and Cures Act. The statewide ballot measure, which provided $3 billion in funding for stem cell research at California universities and research institutions, was overwhelmingly approved by voters, and called for the establishment of an entity to make grants and provide loans for stem cell research.
The Proceedings of the California Stem Cell Agency is a series of commentaries, articles, interviews, webinars, forums, and concise reviews on a wide range of topics in regenerative medicine.
This article was published online on August 9, 2017. An adjustment was made to the Conflicts of Interest section. This notice is included in the online version to indicate that it has been corrected [August 22, 2017].
References
- 1.National Heart, Lung, and Blood Institute. Available at https://www.nhlbi.nih.gov/about/documents/factbook/2012/chapter4. Archived at http://www.webcitation.org/6qpdGRox8. Last accessed July 22, 2017.
- 2. Vaughan AE, Brumwell AN, Xi Y et al. Lineage‐negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 2015;517:621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elborn JS, Horsley A, MacGregor G et al. Current strategies for the long‐term assessment, monitoring, and management of cystic fibrosis patients treated with CFTR modulator therapy. J Cyst Fibros 2017;16:163–164. [DOI] [PubMed] [Google Scholar]
- 4. Firth AL, Menon T, Parker GS et al. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 2015;12:1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki T, Arumugam P, Sakagami T et al. Pulmonary macrophage transplantation therapy. Nature 2014;514:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu KD, Wilson JG, Zhuo H et al. Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate‐severe acute respiratory distress syndrome. Ann Intensive Care 2014;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glassberg MK, Minkiewicz J, Toonkel RL et al. Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): A phase I, safety, clinical trial. Chest 2016;151:971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conboy IM, Conboy MJ, Wagers AJ et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–764. [DOI] [PubMed] [Google Scholar]
- 9. Pierro M, Ionescu L, Montemurro T et al. Short‐term, long‐term and paracrine effect of human umbilical cord‐derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 2013;68:475–484. [DOI] [PubMed] [Google Scholar]
- 10. Granton J, Langleben D, Kutryk MB et al. Endothelial NO‐synthase gene‐enhanced progenitor cell therapy for pulmonary arterial hypertension: The PHACeT trial. Circ Res 2015;117:645–654. [DOI] [PubMed] [Google Scholar]
- 11. Hawkins F, Kotton DN. Embryonic and induced pluripotent stem cells for lung regeneration. Ann Am Thorac Soc 2015;12(suppl 1):S50–53. [DOI] [PubMed] [Google Scholar]
- 12. Petersen TH, Calle EA, Zhao L et al. Tissue‐engineered lungs for in vivo implantation. Science 2010;329:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ott HC, Clippinger B, Conrad C et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 2010;16:927–933. [DOI] [PubMed] [Google Scholar]
- 14. Dye BR, Hill DR, Ferguson MAH et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife 2015;4:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metzger RJ, Klein OD, Martin GR et al. The branching programme of mouse lung development. Nature 2008;453:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson DC, Alva‐Ornelas JA, Sucre JMS et al. Development of a three‐dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med 2016;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sucre JMS, Wilkinson D, Vijayaraj P et al. A three‐dimensional human model of the fibroblast activation that accompanies bronchopulmonary dysplasia identifies Notch‐mediated pathophysiology. Am J Physiol Lung Cell Mol Physiol 2016;310:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dekkers JF, Wiegerinck CL, de Jonge HR et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 2013;19:939–945. [DOI] [PubMed] [Google Scholar]
- 19. Saini Y, Proper SP, Dornbos P et al. Loss of Hif‐2alpha rescues the Hif‐1alpha deletion phenotype of neonatal respiratory distress in mice. PLoS One 2015;10:e0139270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crane AM, Kramer P, Bui JH et al. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports 2015;4:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benam KH, Novak R, Nawroth J et al. Matched‐comparative modeling of normal and diseased human airway responses using a microengineered breathing lung chip. Cell Syst 2016;3:456–466 e454. [DOI] [PubMed] [Google Scholar]
- 22. Konar D, Devarasetty M, Yildiz DV et al. Lung‐on‐a‐chip technologies for disease modeling and drug development. Biomed Eng Comput Biol 2016;7(suppl 1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samulski RJ, Muzyczka N. AAV‐mediated gene therapy for research and therapeutic purposes. Annu Rev Virol 2014;1:427–451. [DOI] [PubMed] [Google Scholar]
- 24. Yan Z, Keiser NW, Song Y et al. A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol Ther 2013;21:2181–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schott JW, Morgan M, Galla M et al. Viral and synthetic RNA vector technologies and applications. Mol Ther 2016;24:1513–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yan Z, Stewart ZA, Sinn PL et al. Ferret and pig models of cystic fibrosis: Prospects and promise for gene therapy. Hum Gene Ther Clin Dev 2015;26:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghosh M, Dwyer‐Nield LD, Kwon JB et al. Tracheal dysplasia precedes bronchial dysplasia in mouse model of N‐nitroso trischloroethylurea induced squamous cell lung cancer. PLoS One 2015;10:e0122823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosen C, Shezen E, Aronovich A et al. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med 2015;21:869–879. [DOI] [PubMed] [Google Scholar]
- 29. Bonetto F, Srinivas M, Heerschap A et al. A novel (19)F agent for detection and quantification of human dendritic cells using magnetic resonance imaging. Int J Cancer 2011;129:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srinivas M, Boehm‐Sturm P, Aswendt M et al. In vivo 19F MRI for cell tracking. J Vis Exp 2013;81:e50802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahrens ET, Heifer BM, O'Hanlon CF et al. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine‐19 MRI. Magn Reson Med 2014;72:1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]


