Abstract
Neonatal hypoxic‐ischemic encephalopathy (NHIE) is a dramatic perinatal complication, associated with poor neurological prognosis despite neuroprotection by therapeutic hypothermia, in the absence of an available curative therapy. We evaluated and compared ready‐to‐use human umbilical cord blood cells (HUCBC) and bankable but allogeneic endothelial progenitors (ECFC) as cell therapy candidate for NHIE. We compared benefits of HUCBC and ECFC transplantation 48 hours after injury in male rat NHIE model, based on the Rice‐Vannucci approach. Based on behavioral tests, immune‐histological assessment and metabolic imaging of brain perfusion using single photon emission computed tomography (SPECT), HUCBC, or ECFC administration provided equally early and sustained functional benefits, up to 8 weeks after injury. These results were associated with total normalization of injured hemisphere cerebral blood flow assessed by SPECT/CT imaging. In conclusion, even if ECFC represent an efficient candidate, HUCBC autologous criteria and easier availability make them the ideal candidate for hypoxic‐ischemic cell therapy. Stem Cells Translational Medicine 2017;6:1987–1996
Keywords: Human umbilical cord blood cells, Endothelial colony‐forming cells, Neonatal hypoxic ischemic encephalopathy, Single photon emission computed tomography, Rat model
Significance Statement.
Neonatal hypoxic ischemic encephalopathy is a dramatic perinatal complication. Neurological and neurosensory sequelae are frequent in survivors, including motor or learning disabilities, cerebral palsy, or epilepsy. Facing the absence of effective curative therapy, many hopes have been credited in cell therapy strategies. Based on behavioral tests, immune‐histological assessment and metabolic imaging of brain perfusion using single photon emission computed tomography, we report in this work that cell transplantation of both human umbilical cord blood cells and endothelial progenitors provided equally early and sustained functional benefits, up to adulthood.
Introduction
Neonatal hypoxic‐ischemic encephalopathy (NHIE) is a dramatic consequence of perinatal asphyxia, causing neonatal mortality and severe morbidity including cerebral palsy, sensory sequelae, epilepsy, cognitive impairment, and learning disabilities. Cerebral hypoxia‐ischemia (HI) induces a cascade of events including inflammation, excitotoxic amino acids release, and oxidative stress, leading to cell and neuronal apoptosis and tissue necrosis. Therapeutic hypothermia proposed in severe NHIE has shown beneficial, but limited neuroprotective effects 1, 2, 3. Additional therapeutic strategies are under investigation.
Experimental studies using autologous or heterologous human umbilical cord blood cells (HUCBC) or mesenchymal stem cells demonstrated neuroprotective effects in NHIE models 4, 5, 6, 7. The mechanisms of their effects have been shown to involve paracrine and growth factors release, rather than engraftment and direct repair mechanisms 4, 5, 6. Long‐term consequences of such treatment on brain metabolism and functional neurologic abilities have been little investigated 8.
The neuroprotective effects of HUCBC may be linked to endothelial progenitor cells. These cells are a small fraction of the blood mononuclear cell population 9, 10 and play a critical role in vascular repair and tissue recovery by promoting the formation of new blood vessels in conditions of tissue ischemia. Endothelial colony‐forming cells (ECFC) are a homogeneous, well‐characterized population of endothelial progenitor cells with high proliferating capacity. They are considered to be relevant endothelial progenitors due to their specific vasculogenic activity 11, 12, 13. We and others have previously reported protective and regenerative effects of ECFC administration in an adult model of cerebral ischemia 14, 15. To date, the effects of ECFC administration have not been investigated in models of NHIE. Data from preclinical studies are still needed and are essential to design and support future clinical trials aiming to investigate the neuroprotective effects of autologous administration of cord blood cells in infants with severe NHIE. Indeed, the high concentration of active cells (progenitor, immune, and stem cells), the ease of access and well‐known cell processing techniques make cord blood a potential neuroprotective therapy for infants suffering NHIE 4, 5, 7, 16.
The aim of this study was to evaluate and compare the effects of HUCBC and ECFC administration after neonatal cerebral HI in the rat. Histologic, single photon emission computed tomography (SPECT) imaging used for brain metabolism and perfusion activity, and neurologic functions were assessed during the neonatal period and in adulthood.
Materials and Methods
The experimental protocol is presented in Figure 1.
Figure 1.
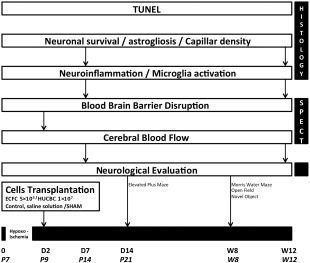
Experimental protocol. Abbreviations: ECFC, endothelial colony‐forming cells; HUCBC, human umbilical cord blood cells; P7, postnatal day 7; SPECT, single photon emission computed tomography.
Animal Care and Surgical Procedure of Cerebral HI
This study was approved by the local institutional Animal Care and Use Committee (CE14, Aix‐Marseille Université, agreement 3‐17012013) and was conducted according to the official edict presented by the French Ministry of Agriculture (Paris, France) and the recommendations of the Helsinki Declaration. The experiments were conducted in an authorized laboratory (C13‐055‐20). Pregnant Sprague‐Dawley rats (Charles Rivers, l'Arbresle, France, http://www.criver.com n = 23) were housed in a room with a 12‐hour light/dark cycle at a controlled temperature of 22°C. They had access to food and water ad libitum. After each delivery, litter sizes were adjusted to 10 pups per litter. Neonatal rats were carried by their mothers until they were weaned (day 21). They were then housed four per cage on standard rat chow (Safe‐UAR).
Only male rats (n = 122) were used in our study to avoid bias due to gender differences 17. Each neurological, histological, and isotopic imaging assessment was performed by a single investigator blinded to the experimental groups.
A variation of the Rice‐Vannucci model of NHIE was used 18, 19, 20. On postnatal day 7 (P7), rat pups (n = 99) were anesthetized with inhaled 3% sevoflurane. The right common carotid artery was permanently double‐ligated using a 5.0 surgical silk and severed. Pups were allowed to recover with their dams for 2 hours, and then placed into a hypoxia chamber in a water bath maintained at 37°C, under a constant flow of 1.5 l/minute humidified 8% oxygen/balance nitrogen for 90 minutes. SHAM rat pups (n = 23) underwent anesthesia, incision, and exposure of the right common carotid artery without ligature or hypoxia. At the end of the procedure, pups were returned to their cage and allowed to recover for 48 hours.
Cell Preparation and Transplantation
HUCBC samples (30–50 ml) from healthy human donors were collected, in compliance with the French law and after obtaining written informed consent forms from the parents. The cord blood cells were obtained from anonymous donations and 52% of the donors were male newborns. Preparation of the mononuclear cell fraction was performed by the Ficoll gradient technique (Amersham, Freiburg, Germany, http://www.amershambioscience.com). Blood samples were processed within 24 hours after collection. Before cell transplantation, HUCBC were washed three times with phosphate‐buffered saline (PBS) then suspended in PBS (1 × 107 cells per 0.5 milliliter).
ECFC were isolated as previously described from HUCBC 21. Before cell transplantation, ECFC were starved overnight in endothelial basal medium‐2/with 2% fetal calf serum, washed three times with PBS then suspended in PBS (5 × 105 cells per 0.5 milliliter).
Experimental Groups
Forty‐eight hours after the surgical procedure, HI pups were randomly allocated to three groups. Control pups (n = 35) were intraperitoneally injected with saline solution (500 µl) and the other interventional animal groups either with HUCBC (1 × 107/500 µl, n = 21) or ECFC (5 × 105/500 µl, n = 43). SHAM pups (n = 23) were intraperitoneally injected with saline solution (500 µl). Before cell or saline injections, pups were briefly anesthetized with 3% sevoflurane inhalation.
Neurological Assessment
Neurological assessment was performed at 14 days and 8 weeks after HI.
Elevated Plus Maze Test
The elevated plus maze (EPM) test was performed on P21 (n = 10/group) to evaluate animal anxiety as previously described 22. The EPM apparatus consists of two open arms (50 × 10 × 1 cm) and two closed arms (50 × 10 × 40 cm) crossing on a common central platform (10 × 10 cm) forming a plus shape, elevated to 50 cm above the floor. The anxiety‐related behaviors of each animal were video‐recorded for a period of 5 minutes and analyzed with the EthoVision XT7 (Noldus Information Technology, Leesburg, Virginia, http://www.noldus.com). Briefly, the rat was placed on the central platform with its head facing an open arm. The arm entry was defined as all four paws into an open or a closed arm. The total time each animal spent in any different section of the maze (open arms, central platform, and closed arms) was recorded. Results were expressed as mean ± SD percent of “open arms entries” (OAE ratio, number of OAE divided by the sum of entries in both open and closed arms), percent of “closed arms entries” (CAE ratio, number of CAE divided by the sum of entries in both open and closed arms), percent of “open arms time” (OAT%, time spent in open arms divided by the sum of time spent in both closed and open arms) and percent of “closed arms time” (CAT%, time spent in closed arms divided by the sum of time spent in both closed and open arms). The animal's natural tendency is to stay in closed spaces because of its unconditioned fear for open spaces and height.
Open Field Test
At 8 weeks, each rat (n = 10 in each group) was placed in an empty open field (OF; 100 × 100 cm) and allowed to freely explore the arena for 5 minutes. General locomotor behavior (total distance moved and velocity of movement) was analyzed with EthoVision XT 7.
Novel Object Recognition Test
At 8 weeks, the animal's exploratory behavior and its nonspatial learning and memory capacities were assessed with novel object recognition test 23, 24. Briefly, the rat (n = 10/group) was placed in an open apparatus (100 × 100 cm). Each animal's journey was video‐recorded and analyzed with the EthoVision XT7 software. The day before the test, animals received a habituation session of 30 minutes in the OF. On the testing day, each rat completed four 3‐minute sessions (intertrial interval of 30 minutes). During the first three sessions, the rat was allowed to explore the arena, which contained two identical objects. Total exploration time of the two objects was noted. For the fourth session, one of the original objects was replaced by a novel, different‐shaped object, then each rat was returned to the arena and was allowed to explore both objects (novel + familiar) for 3 minutes. The “object exploration” was considered when the rat sniffed at the novel object within a 1 cm‐distance or touched the novel object with its nose. The time spent on each object exploration was video‐recorded and a discrimination ratio was calculated (time spent to explore the novel object compared with the total exploration time). The latency period to the first exploration of the novel object was also measured.
Morris Water Maze Test
Spatial learning and memory performance were evaluated 8 weeks after HI lesion with the Morris water maze (MWM) test (n = 10/group) as previously described by Li et al. 25. The MWM consists of a black circular pool virtually divided in four equal quadrants located in a well‐lit white room with several spatial cues. Two centimeters beneath the water surface and hidden from the rat's view was a black circular platform.
( a) Spatial Acquisition Test. Training on spatial version of the MWM was carried out over five consecutive days. On each day, rats received four training trials in which the hidden platform was left at the same place. The mean latency time to find the platform was measured for individual animals on each day as a learning score. A different starting location was used in each trial, which consisted of a swim followed by a 30‐seconds platform rest. Rats that did not find the platform within 60 seconds were guided to it by the experimenter.
(b) Reference Memory Test (Probe Trial). To assess long‐term memory, 24 hours after the final trial, the platform was removed from its fixed location and the time spent in the target quadrant over 60 seconds was measured.
Histologic and Immunohistochemistry Assessment
Animals were deeply anesthetized 7 days and 12 weeks after HI with a lethal dose of pentobarbital (Clin Midy, Gentilly, France) and rat brains were fixed by trans‐cardiac perfusion with 4% phosphate‐buffered paraformaldehyde (PFA) (Sigma‐Aldrich, Saint‐Quentin Fallavier, France, http://www.sigmaaldrich.com). After decapitation, the brain was removed from the skull and post‐fixed for 24 hours in 4% PFA at 4°C and was successively cryopreserved, snap‐frozen, and stocked at −80°C. Frozen sections were cut with a sliding microtome (CM1900, Leica, France SA, http://www.leicabiosystems.com) and stored at −80°C. All sections were examined using a light and fluorescent microscope (Eclipse TE 2000‐U, Nikon France SA, https://www.nikoninstruments.com) equipped with a digital camera (DXM1200, Nikon France SA) and an image analyzer (Lucia 5.0 software, Nikon France SA). Histologic analysis was performed in 5–10 fields at the cortical level of ipsilateral hemisphere and in the corresponding location within contralateral hemisphere.
TUNEL Staining
Apoptotic cells were detected on 8 μm‐thick sections 7 days after injury by terminal deoxynucleotidyl transferase‐mediated 2′‐deoxyuridine 5′‐triphosphate nick‐end labeling (TUNEL) as previously described 26. Only cells containing apoptotic bodies are referred to as apoptotic cells. TUNEL positive cells were counted under high‐power microscope and expressed as number of positive cells per square mm section.
Immunofluorescent Staining
After nonspecific protein binding blocking, slices were incubated (1/100, 2 hours, 4°C) with the primary antibody (eNOS: 610297, BD Pharmingen, http://www.bdbiosciences.com; NeuN: MAB377, Millipore, http://www.merckmillipore.com; glial fibrillary acidic protein, GFAP: M0761, Dako, http://www.agilent.com) and were incubated secondary antibodies. Cerebral vessel density (eNOS), neuronal survival (NeuN), and astrogliosis (GFAP) are expressed as ipsilateral‐to‐contralateral (i/c) ratio.
Immunohistochemistry
After endogen peroxydase activity blocking and nonspecific protein binding blocking, sections were incubated with mouse monoclonal anti‐iNOS (1/100, 4 hours, BD Pharmingen, 610297) and then incubated with the biotinylated secondary antibody and visualized with 3,3′‐diaminobenzidine (Thermofischer, http://www.thermofisher.com). Neuroinflammation is expressed as i/c ratio.
Single Photon Emission Computed Tomography
Radiotracers
Hexamethylpropyleneamine oxime kit (HMPAO, Cerestab, General Electrics, Velizy‐Villacoublay, France, http://www.gehealthcare.com) was radiolabeled with fresh [99mTc] pertechnetate solution (500 MBq/1 ml) following the manufacturer's instructions.
SPECT Data Acquisition
Seven days (P14) and 12 weeks after HI insult, 20 MBq of [99mTc]Tc‐HMPAO were injected through the tail vein to assess cerebral blood flow (CBF). Thirty minutes after [99mTc]Tc‐HMPAO injection, the animals were anesthetized with 1.5% sevoflurane and a cerebral SPECT/CT imaging was acquired for 20 minutes (NanoSPECT/CT+ camera, Bioscan Europe Ltd., Paris, France).
SPECT Image Analysis
Images analysis was performed using the 3D‐ROI module part of InVivoScope software v2.0p4 (InviCRO, Boston, https://www.invicro.com). Two volumes of interest (VOI) were drawn over right (ipsilateral) and left (contralateral) cerebral hemisphere for each animal in the axial section. Radioactivity inside each VOI was quantified and corrected by the tissue volume (MBq/mm3). We then calculated the i/c ratios (i/c, %). Image color scales were normalized in order to illustrate CBF.
Statistical Analysis
Values were reported as mean ± SD unless otherwise indicated. Physiological parameters were analyzed by unpaired t test. TUNEL and immunoassaying data were tested for normality and were compared with unpaired t test with Bonferroni correction for post hoc intergroup comparisons. Behavioral and morphological outcomes were compared between the groups using one‐way analysis of variance (ANOVA) followed by post hoc Bonferroni test. Statistical analyses were performed with Prism software v5.03 (GraphPad Software, La Jolla, CA). A p value <.05 was considered statistically significant.
Results
Survival rate: Survival rate after hypoxic‐ischemic injury was 96%.
Locomotor behavior: To analyze locomotor behavior, OF activity was performed at W8 in all experiment groups. Physical abilities were similar in all groups (data not shown).
Neurological Assessment
HUCBC or ECFC Administration Equally Rescues Hypoxic‐Ischemic‐Induced Short‐Term Anxiety‐Like Behavior Abnormalities
The EPM test was performed on day P21 (n = 10/group). The animal's natural tendency is to stay in enclosed spaces, as was the case with the SHAM group (91% of time spent in closed arms). After HI injury, we observed a higher OAE ratio in the Control group compared with the SHAM group (OAE ratio, Control: 0.568 ± 0.08, SHAM: 0.290 ± 0.06; p < .05, n = 10, Fig. 2A) and a higher percent of time in the open arms (OAT%, Control: 0.357 ± 0.1, SHAM: 0.091 ± 0.02, p < .05, n = 10, Fig. 2B). After cellular therapy, we observed a significant decrease in OAT ratio in the HUCBC (0.091 ± 0.04, p < .05, n = 10) and ECFC groups (0.094 ± 0.02, p < .05, n = 10) compared with the Control group (Fig. 2B). There was no significant difference between HUCBC, ECFC, and SHAM animals.
Figure 2.
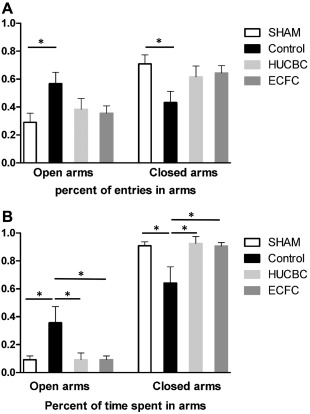
Evaluation of HUCBC or ECFC administration on anxiety‐like behavior measured by the elevated plus maze test at post‐natal day 21. (A): Percent of entries in open arms and in closed arms. (B): Percent of time spent in open arms and in closed arms. (mean ± SD; *, p < .05 compared with Control; n = 10 in each group). Abbreviations: ECFC, endothelial colony‐forming cells; HUCBC, human umbilical cord blood cells.
HUCBC or ECFC Administration Equally Rescues Hypoxic‐Ischemic‐Induced Long‐Term Cognitive Deficits
Morris Water Maze Test. Control rats showed significant spatial and learning memory deficits 8 weeks after HI as compared with SHAM rats. (a) Spatial acquisition test (Fig. 3A): During the five training days, the mean escape latencies to find the platform in the HUCBC and ECFC groups were significantly shorter than in Control rats (HUCBC: 29.1 ± 6.6 seconds; ECFC: 25.8 ± 7.2 seconds; Control: 44.6 ± 3.1 seconds, n = 10 in each group, HUCBC vs. Control p < .01; ECFC vs. Control p < .05). There were no significant differences between the HUCBC, ECFC, and SHAM groups. (b) Reference memory test (probe trial, Fig. 3B): Twenty‐four hours after the last training session, the time spent in the target quadrant (without the platform) was measured. The SHAM, HUCBC, and ECFC groups showed longer time spent in the target quadrant than Control rats (SHAM vs. Control, p < .01; ECFC and HUCBC vs. Control, p < .05) There was no difference between the ECFC, HUCBC, and SHAM groups.
Figure 3.
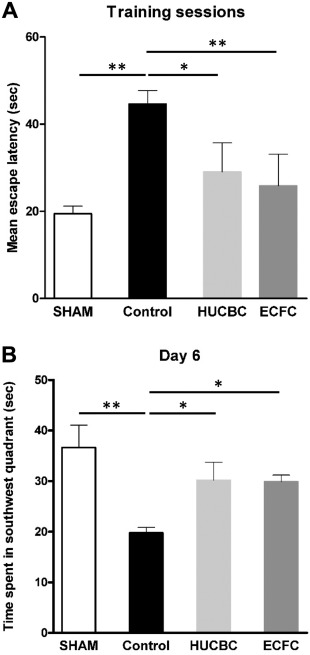
Evaluation of cognitive function with the Morris water maze test at post‐natal 8 weeks. (A): Spatial acquisition test (Training sessions). (B): Reference memory test (day 6). (mean ± SD; *, p < .05 compared with Control; **, p < .01 compared with Control; n = 10 in each group; one‐way analysis of variance followed by post hoc Bonferroni test). Abbreviations: ECFC, endothelial colony‐forming cells; HUCBC, human umbilical cord blood cells.
Novel Object Recognition Test. HI resulted in cognitive deficit involving nonspatial working memory and exploratory capacities during the novel object recognition test, with a discrimination ratio significantly reduced in the Control group compared with the SHAM group, (respectively, 0.55 ± 0.1 and 0.92 ± 0.03; p < .05; n = 10/group, Fig. 4A). HUCBC and ECFC administration significantly increased exploratory and memory performance compared with the Control group, with a higher discrimination ratio (HUCBC: 0.88 ± 0.02, p < .05; n = 10; ECFC: 0.89 ± 0.03, p < .05; n = 10, Fig. 4A). The mean escape latency to first exploration of the novel object was significantly increased in the Control group compared with the SHAM group (respectively 24.0 ± 3.2 seconds and 8.31 ± 2.2 seconds, p < .01, n = 10 in each group, Fig. 4B) and was reduced in the HUCBC (11.28 ± 1.8 seconds, p < .01, n = 10, Fig. 4B) and ECFC (10.15 ± 2.6 seconds, p < .05, n = 10, Fig. 4B) groups. There was no difference between the ECFC, HUCBC, and SHAM groups.
Figure 4.
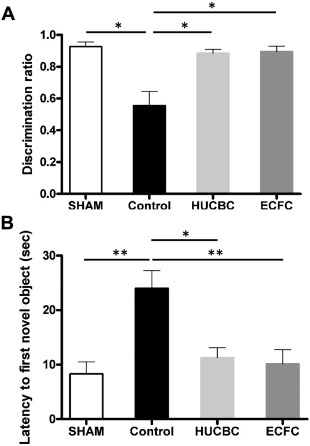
Evaluation of HUCBC and ECFC administration on Novel Object Recognition test at post‐natal 8 weeks. (A): Discrimination ratio. (B): The mean escape latency to first exploration of the novel object. (mean ± SD; *, p < .05 compared with Control, **, p < .01 compared with Control; n = 10 in each group; one‐way analysis of variance followed by post hoc Bonferroni test). Abbreviations: ECFC, endothelial colony‐forming cells; HUCBC, human umbilical cord blood cells.
Histological and Immunochemical Assessment
HUCBC or ECFC Administration Decreased Apoptosis and Neuroinflammation Activation 7 Days After Neonatal HI
Seven days post‐HI, the HUCBC group revealed a significantly lower cortical neuroinflammation than the Control group (HUCBC vs. Control: 0.9 ± 0.15 vs. 1.6 ± 0.34; p = .038; n = 5 per group, Fig. 5A). The ECFC group revealed a trend to a lower neuroinflammation in the ipsilateral cortex than the Control group but this difference was not statistically significant (ECFC vs. Control; 1.1 ± 0.11 vs. 1.6 ± 0.34; p = .08, n = 5 per group, Fig. 5A).
Figure 5.
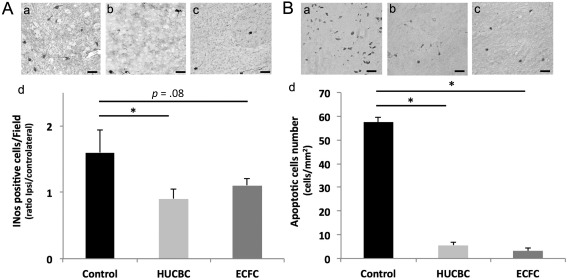
Effects of HUCBC and ECFC administration on neuroinflammation (A) and apoptosis (B) 7 days after neonatal HI. (A): Representative photographs of iNOS immunohistochemistry in ipsilateral cortical brain section of Control (Aa), HUCBC (Ab), and ECFC (Ac) groups. (Ad): Quantification of iNOS immunopositive cells (ipsilateral/contralateral ratio), Scale bar: 40 µm. (B): Representative photographs of TUNEL immunohistochemistry in ipsilateral cortical brain section of Control (Ba), HUCBC (Bb), and ECFC (Bc) groups. (Bd): quantification of apoptotic cells in the ipsilateral hemisphere. (mean ± SD; *, p < .05 compared with Control, **, p < .005 compared with Control; n = 5–6 in each group; unpaired t test followed by post‐hoc Bonferroni test). Abbreviations: ECFC, endothelial colony‐forming cells; HUCBC, human umbilical cord blood cells.
Similarly, 7 days after HI, apoptotic cell number (Fig. 5B) was significantly lower in the HUCBC (5.5 ± 1.2 cells/mm2; p = .005, n = 6) and ECFC groups (3.2 ± 1.3 cells/mm2, p = .006, n = 6) than in the Control group (57.5 ± 2.1 cells/mm2, n = 6)
HUCBC and ECFC Administration Equally Improved Early and Late Neuronal Survival and Prevented the Formation of Astrocytic Scar
Neuronal survival: HI induced a significant and extended decrease of NeuN‐immunopositive cells in the ipsilateral hemisphere 7 days (0.99 ± 0.08 vs. 0.80 ± 0.07 in the SHAM and Control groups respectively, p = .026, n = 4 in each group, Fig. 6Aa, 6Ac) and 12 weeks after HI insult (0.99 ± 0.15 vs. 0.74 ± 0.1 in the SHAM and Control groups respectively, p = .025, n = 4/group, Fig. 6Ab, 6Ad).
Figure 6.
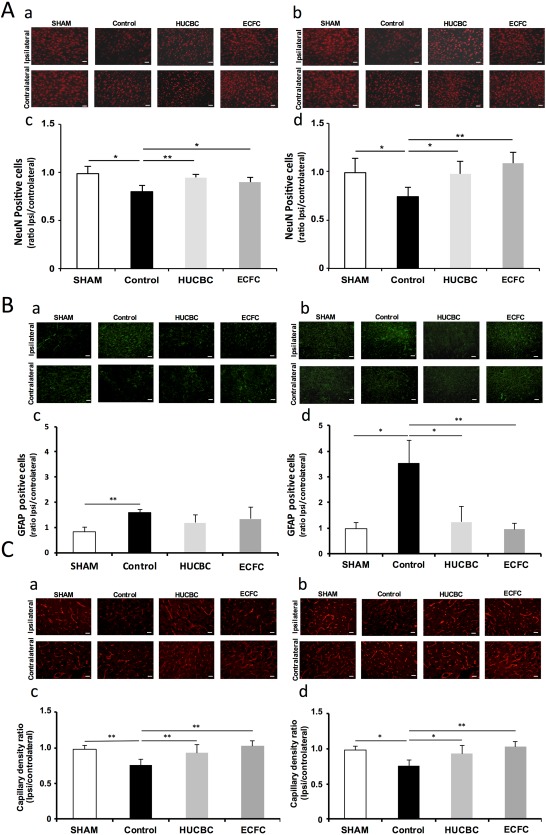
Effects of HUCBC and ECFC administration on neuronal survival (A), astrogliosis (B), and capillary density (C) 7 days (a, c) and 12 weeks (b, d) after neonatal HI. Representative NeuN immunofluorescence (red) at P14 (Aa) and W12 (Ac), Representative glial fibrillary acidic protein (GFAP) immunofluorescence (green) at P14 (Ba) and W12 (Bc) and Representative eNOS immunofluorescence (red) at P14 (Ca) and W12 (Cc). Ipsilateral/contralateral ratio of NeuN‐immunopositive cells at P14 (Ab) and W12 (Ad), ipsilateral/contralateral ratio of GFAP‐immunopositive cells at P14 (Bb) and W12 (Bd) and ipsilateral/contralateral ratio of eNOS‐immunopositive cells at P14 (Cb) and W12 (Cd), (mean ± SD; *, p < .05 compared with Control; **, p < .01 compared with Control, n =5 in each group; unpaired t test followed by post hoc Bonferroni test; scale bars = 20 µm). Abbreviations: ECFC, endothelial colony‐forming cells; HUCBC, human umbilical cord blood cells.
Seven days after HI (Fig. 6Aa, 6Ac), NeuN‐positive cells i/c ratios were significantly increased in the HUCBC (0.95 ± 0.03, p = .0094, n = 4) and ECFC (0.90 ± 0.05, p = .043, n = 4) groups in comparison with the Control group.
Twelve weeks after HI (Fig. 6Ab, 6Ad), NeuN‐positive cells i/c ratios were still significantly increased in the HUCBC group (0.98 ± 0.13, p = .022, n = 4) and in the ECFC group (1.09 ± 0.11, p < .001; n = 4) in comparison with the Control group.
No Difference was Observed Between the SHAM, ECFC, and HUCBC Groups 7 Days and 12 Weeks After HI
Astrogliosis: HI induced a significant and extended increase in astrogliosis, expressed as i/c GFAP ratio, 7 days (0.82 ± 20 vs. 1.59 ± 0.12 in the SHAM and Control groups respectively, p < .001, n = 5/group, Fig. 6Ba, 6Bc) and 12 weeks after HI (0.98 ± 0.24 vs. 3.53 ± 0.9 in the SHAM and Control groups respectively, p = .004, n = 5/group, Fig. 6Bb, 6Bd).
Seven days after insult (Fig. 6Ba, 6Bc), no significant decrease in astrogliosis was observed in the ECFC and HUCBC groups compared with the Control group; whereas at W12 (Fig. 6Bb, 6Bd) we observed a huge delayed decrease in astrogliosis in the ECFC (0.96 ± 0.23, p < .001, n = 5) and HUCBC (1.24 ± 0.63, p < .05, n = 5) groups compared with the Control group. No difference was observed between the SHAM, ECFC, and HUCBC at W12.
HUCBC and ECFC Administration Both Resulted in an Early and Sustained Increase in Cerebral Capillary Density, from Day 7 to 12 Weeks After Neonatal HI
In comparison with SHAM animals, HI was associated with a significant reduction in cerebral capillary density (i/c ratios) 7 days (0.97 ± 0.03 vs. 0.73 ± 0.04 in SHAM and Control animals respectively, p < .001, n = 4/group, Fig. 6Ca, 6Cc) and 12 weeks after HI insult (0.98 ± 0.05 vs. 0.76 ± 0.08 in SHAM and Control animals respectively, p = .0014, n = 4/group, Fig. 6Cb, 6Cd).
Seven Days After HI. cerebral capillary density was significantly higher in the HUCBC (1.00 ± 0.06; p < .001; n = 5) group and in the ECFC (1.09 ± 0.08; p < .001; n = 6) group in comparison with the Control group. No difference was observed between the SHAM, ECFC, and HUCBC groups. (Fig. 6Ca, 6Cc).
Twelve Weeks After HI. cerebral capillary density was significantly higher in the HUCBC (0.93 ± 0.11; p = .034, n = 6) group and in the ECFC (1.02 ± 0.08; p < .001, n = 6) group in comparison with the Control group. No difference was observed between the SHAM, ECFC, and HUCBC groups (Fig. 6Cb, 6Cd).
Single Photon Emission Computed Tomography
Cerebral Blood Flow
Although no significant difference was found between conditions at P14, at W12 we observed in the Control group a significant decrease in CBF i/c ratios (77% ± 2%, n = 5) compared with the SHAM (99% ± 3%, n = 5, p = .019) group and a significant improvement in the HUCBC (95% ± 2%, p = .017, n = 5) and ECFC (96% ± 2%, p = .014, n = 7) groups compared with the Control group (Fig. 7).
Figure 7.
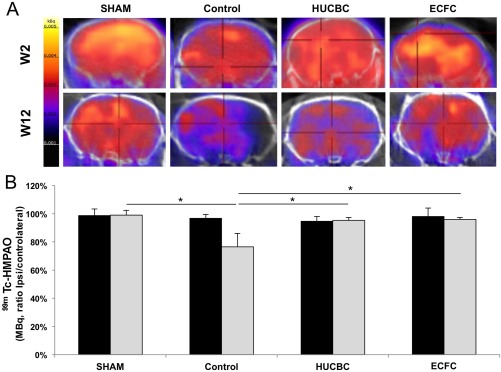
Effects of HUCBC and ECFC administration on cerebral blood flow (CBF) SPECT/CT 2 weeks and 12 weeks after hypoxia‐ischemia. (A): Brain representative tomographic images of 99mTc‐HMPAO SPECT/CT assessing CBF 2 weeks (top row) and 12 weeks (bottom row) after hypoxia‐ischemia. (B): Quantification of 99mTc‐HMPAO activity in ischemic hemisphere (% of contralateral activity) 2 weeks (black bars) and 12 weeks (gray bars) after hypoxia‐ischemia (mean ± SD; *, p < .05 compared with Control; n = 4–5 in each group; one‐way analysis of variance followed by post‐hoc Bonferroni test). Abbreviations: ECFC, endothelial colony‐forming cells; HMPAO, hexamethylpropyleneamine oxime; HUCBC, human umbilical cord blood cells.
Discussion
Using a rat neonatal model of brain HI, we demonstrated that HUCBC or ECFC administration similarly (a) limited cellular apoptosis, neuroinflammation, and astrocytic reaction, (b) restored cerebral capillary density, and (c) improved neuronal cell survival. Long‐term CBF and neurologic functions were definitively improved as well.
Administration of HUCBC after neonatal cerebral HI in rats limits the severity of brain injury and improves long‐term neurologic functions. Meier et al. were the first to describe improved neurologic functions in rats with neonatal cerebral HI after intraperitoneal infusion of HUCBC (1 × 107 HUCB cells), 24 hours after cerebral injury 7, and a preservation of somatosensory functions in the ipsilateral hemisphere at P48 27. These effects have been demonstrated in studies using different doses, administration route, or administration timing of HUCBC after neonatal cerebral insult 4, 5, 6, 28, 29. Yasuhara et al. have shown improved motor coordination as early as the 7th day after intravenous administration of low doses of HUCBC (1.5 × 104) 4. Pimentel‐Coelho demonstrated that intraperitoneal injection of 2 × 106 HUCBC 3 hours after the ischemic episode improved sensorimotor reflexes up to 10 days after injection. Most of these effects were associated with decreased neuroinflammation and less apoptosis reaction 6, 30. The mechanism by which HUCBC do limit brain injury is unclear. Cord blood contains different cell types with various functions including mesenchymal cells, stem cells, progenitor cells, immune cells (T‐regulatory lymphocytes), and endothelial progenitor cells which contribute to the neuroprotective effects. It has been proposed that such effects result from in situ trophic/growth factors release rather than from engraftment process 4, 6, 31, and studies found a limited number, or an absence of HUCBC in the injured brain 4, 5, 7, 28. Our findings provide further evidence on the neuroprotective effects of HUCBC which sustain in adulthood.
The relevant findings of this study concern the significant neuroprotective effects of ECFC administration after neonatal cerebral HI, which have never been evaluated as cell therapy for neonatal cerebral HI. ECFC are considered to be the relevant endothelial progenitors due to their specific vasculogenic activity 11, 12. We previously showed that intravenous administration of ECFC contributed to vascular recovery, limited tissue injury after hind limb ischemia, and transient cerebral ischemia in adult rodents 14, 15, 32, 33, 34, 35. In all these models, ECFC administration was associated with a decrease in apoptosis, a growth factor release enhancement and a neovascularization stimulation.
Beneficial effects of the administration of ECFC or HUCBC 3 days after cerebral HI in pups sustained in adulthood. Compared with the Control group, ECFC and HUCBC administration limited neuronal death, neuroinflammation and preserved capillary density. We observed that cell therapy did not prevent early glial cell proliferation, supposed to be protective, but prevent delayed glial scar, reinforcing the hypothesis of a temporary astroglial border that could limit ischemia to surrounding tissues 36. The effects of astrogliosis and microglia on inflammation and oxidative stress reactions are debated 37, 38, but a sustained microglial activation has been shown to affect tissue integrity after ischemic injury through secreting cytotoxic cytokines and free radicals 39, 40, 41, 42, 43.
We originally used µSPECT/CT imaging to follow‐up brain metabolism after cerebral HI from neonatal period to adulthood. Brain perfusion is a critical parameter of cerebral tissue physiology; maintenance of brain metabolism is finely tuned and needs a constant provision of oxygen and glucose. Cerebral SPECT/CT imaging showed a huge decrease in brain perfusion in Control rats compared with the SHAM group at the adult stage (W12). These alterations were associated with significant impaired neurologic functions and brain damages. Although no difference was observed on day 7 after cerebral HI, adult HUCBC‐ or ECFC‐treated animals recovered brain metabolism and perfusion with less extended brain damages and better neurological functions. Patho‐physiological processes including cell activity and cell proliferation, tissue inflammation or regenerative processes may maintain tissue metabolism and explain unchanged brain perfusion soon after cerebral HI as observed in adult cerebral HI models 44. All of these findings make CBF as evaluated by SPECT/CT imaging a reliable tool to assess long‐term consequences of neonatal HI and the effects of experimental therapies.
The current study provides further evidence on the neuroprotective effects of HUCBC and ECFC after neonatal cerebral HI in the rat. The experimental design included heterologous cell therapy which questions the reliability of the model. However, some evidence argues for the effectiveness of these cells. Neuroprotective effects of cord blood cells have been demonstrated in immunodeficient rodents 4, 45 and in a model of NHIE in lamb after autologous administration of cord blood cells 46. We did not investigate the effects of hypothermia as used in clinical practice because our aim was to compare the effects of both HUCBC and ECFC. The strength of our study was to investigate long‐term consequences of HUCBC and ECFC administration in a model of neonatal cerebral HI using histologic, neurologic and SPECT imaging tools. We found similar long‐term neuroprotective effects between both cell therapies which underlines the robustness of cell therapies as therapeutic candidate for neonatal HI.
Clinical Implications
Caution is required when extrapolating our findings to infants since current findings were observed in a specific experimental model; but evidence argues for their potential relevance for NHIE. The Rice‐Vannucci model is considered a reference for studying neonatal HI brain, since cortical maturity at 7 days post‐delivery in rats is similar to that of human newborns 19, 47. We observed, as previously reported 4, 5, 7, 16, 28, 29, that neonatal cerebral HI induced long‐term neurofunctional and cognitive deficits with significant impaired spatial and nonspatial memory skills and behavior disorders in stress conditions, as can be seen in children born with NHIE. The rat model is a valuable model for studying pathophysiological mechanisms of NHIE and for evaluating the efficacy and tolerance of cellular therapies. Our findings and previous studies provide sufficient data on the effectiveness and the safety of using these cells in NHIE conditions 48, 49, 50.
NHIE is the second cause of neonatal death worldwide 51 and is associated with high risk of long‐term neurocognitive disabilities in surviving infants. Therapeutic hypothermia, which is the only protective therapy proposed in such a situation, has limited neuroprotective effects 1, 2, 3, 52, 53. Combined therapies are currently evaluated, out of which cell therapy using HUCBC has promising potential neuroprotective effects. HUCBC are easily and immediately available at the patient's bedside just after birth. Recently, in only one study, HUCBC administrations have been shown to be feasible and well tolerated in newborns with severe NHIE 54. Using ECFC from cord blood is more debated. Their use in clinical practice remains tricky since ECFC are allogeneic and take several days to be produced. We have characterized cord blood stem cells of neonates with neonatal asphyxia and compared them with those from healthy newborn infants (NEOCORD, NCT01284673). We observed that neonatal asphyxia seemed to enrich cord blood stem cell content, with a higher quantity of endothelial progenitor cells found in cord blood of asphyxiated neonates (unpublished data). All these arguments confirm our choice to establish and set up a clinical study (NEOSTEM, NCT02881970). Three other studies are ongoing (NCT01506258, NCT02256618, NCT02605018) and the neonatal physician community has placed much hope in these strategies.
Conclusion
In the current study, we reported that a single HUCBC or ECFC administration after neonatal cerebral HI in rat equally limited glial cell proliferation, prevented apoptosis and neuronal loss and improved capillary growth, CBF using µSPECT/CT imaging, and neurologic functions up to young adulthood. Our findings provide further evidence on the effectiveness and tolerability of HUCBC as the potential candidate for clinical HI cell therapy.
Author Contributions
I.G.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; P.G. and A.R.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; F.B. and U.S.: conception and design, final approval of manuscript; F.D‐G.: conception and design, administrative support, final approval of manuscript; F.S.: conception and design, administrative support, provision of study material or patients, final approval of manuscript; B.G.: conception and design, financial support, administrative support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
We thank Samantha Fernandez and Lionel Pellegrini for their excellent technical support.
References
- 1. Azzopardi DV, Strohm B, Edwards AD et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–1358. [DOI] [PubMed] [Google Scholar]
- 2. Shankaran S, Laptook AR, Ehrenkranz RA et al. Whole‐body hypothermia for neonates with hypoxic‐ischemic encephalopathy. N Engl J Med 2005;353:1574–1584. [DOI] [PubMed] [Google Scholar]
- 3. Gluckman PD, Wyatt JS, Azzopardi D et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet 2005;365:663–670. [DOI] [PubMed] [Google Scholar]
- 4. Yasuhara T, Hara K, Maki M et al. Mannitol facilitates neurotrophic factor up‐regulation and behavioural recovery in neonatal hypoxic‐ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med 2010;14:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pimentel‐Coelho PM, Magalhaes ES, Lopes LM et al. Human cord blood transplantation in a neonatal rat model of hypoxic‐ischemic brain damage: Functional outcome related to neuroprotection in the striatum. Stem Cells Dev 2010;19:351–358. [DOI] [PubMed] [Google Scholar]
- 6. Rosenkranz K, Kumbruch S, Tenbusch M et al. Transplantation of human umbilical cord blood cells mediated beneficial effects on apoptosis, angiogenesis and neuronal survival after hypoxic‐ischemic brain injury in rats. Cell Tissue Res 2012;348:429–438. [DOI] [PubMed] [Google Scholar]
- 7. Meier C, Middelanis J, Wasielewski B et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res 2006;59:244–249. [DOI] [PubMed] [Google Scholar]
- 8. Bae SH, Kong TH, Lee HS et al. Long‐lasting paracrine effects of human cord blood cells on damaged neocortex in an animal model of cerebral palsy. Cell Transplant 2012;21:2497–2515. [DOI] [PubMed] [Google Scholar]
- 9. Ingram DA, Mead LE, Tanaka H et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004;104:2752–2760. [DOI] [PubMed] [Google Scholar]
- 10. Chen JZ, Zhang FR, Tao QM et al. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–280. [DOI] [PubMed] [Google Scholar]
- 11. Kawamoto A, Asahara T. Role of progenitor endothelial cells in cardiovascular disease and upcoming therapies. Catheter Cardiovasc Interv 2007;70:477–484. [DOI] [PubMed] [Google Scholar]
- 12. Yoder MC, Mead LE, Prater D et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007;109:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bompais H, Chagraoui J, Canron X et al. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood 2004;103:2577–2584. [DOI] [PubMed] [Google Scholar]
- 14. Moubarik C, Guillet B, Youssef B et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev 2011;7:208–220. [DOI] [PubMed] [Google Scholar]
- 15. Pellegrini L, Bennis Y, Guillet B et al. Therapeutic benefit of a combined strategy using erythropoietin and endothelial progenitor cells after transient focal cerebral ischemia in rats. Neurol Res 2013;35:937–947. [DOI] [PubMed] [Google Scholar]
- 16. Fan LW, Lin S, Pang Y et al. Hypoxia‐ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res 2005;165:80–90. [DOI] [PubMed] [Google Scholar]
- 17. Arteni NS, Pereira LO, Rodrigues AL et al. Lateralized and sex‐dependent behavioral and morphological effects of unilateral neonatal cerebral hypoxia‐ischemia in the rat. Behav Brain Res 2010;210:92–98. [DOI] [PubMed] [Google Scholar]
- 18. Levine S. Anoxic‐ischemic encephalopathy in rats. Am J Pathol 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- 19. Vannucci RC, Connor JR, Mauger DT et al. Rat model of perinatal hypoxic‐ischemic brain damage. J Neurosci Res 1999;55:158–163. [DOI] [PubMed] [Google Scholar]
- 20. Vannucci SJ, Hagberg H. Hypoxia‐ischemia in the immature brain. J Exp Biol 2004;207:3149–3154. [DOI] [PubMed] [Google Scholar]
- 21. Cuccuini W, Poitevin S, Poitevin G et al. Tissue factor up‐regulation in proinflammatory conditions confers thrombin generation capacity to endothelial colony‐forming cells without influencing non‐coagulant properties in vitro. J Thromb Haemost 2010;8:2042–2052. [DOI] [PubMed] [Google Scholar]
- 22. Ming‐Yan H, Luo YL, Zhang XC et al. Hypoxic‐ischemic injury decreases anxiety‐like behavior in rats when associated with loss of tyrosine‐hydroxylase immunoreactive neurons of the substantia nigra. Braz J Med Biol Res 2012;45:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartolini L, Casamenti F, Pepeu G. Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol Biochem Behav 1996;53:277–283. [DOI] [PubMed] [Google Scholar]
- 24. Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one‐trial object recognition in rodents. Neurosci Biobehav Rev 2007;31:673–704. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Liang G, Wang S et al. Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology 2007;53:942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Codaccioni JL, Velly LJ, Moubarik C et al. Sevoflurane preconditioning against focal cerebral ischemia: Inhibition of apoptosis in the face of transient improvement of neurological outcome. Anesthesiology 2009;110:1271–1278. [DOI] [PubMed] [Google Scholar]
- 27. Geissler M, Dinse HR, Neuhoff S et al. Human umbilical cord blood cells restore brain damage induced changes in rat somatosensory cortex. PLoS One 2011;6:e20194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Paula S, Vitola AS, Greggio S et al. Hemispheric brain injury and behavioral deficits induced by severe neonatal hypoxia‐ischemia in rats are not attenuated by intravenous administration of human umbilical cord blood cells. Pediatr Res 2009;65:631–635. [DOI] [PubMed] [Google Scholar]
- 29. de Paula S, Greggio S, Marinowic DR et al. The dose‐response effect of acute intravenous transplantation of human umbilical cord blood cells on brain damage and spatial memory deficits in neonatal hypoxia‐ischemia. Neuroscience 2012;210:431–441. [DOI] [PubMed] [Google Scholar]
- 30. Pimentel‐Coelho PM, Mendez‐Otero R. Cell therapy for neonatal hypoxic‐ischemic encephalopathy. Stem Cells Dev 2010;19:299–310. [DOI] [PubMed] [Google Scholar]
- 31. Rosenkranz K, Kumbruch S, Lebermann K et al. The chemokine SDF‐1/CXCL12 contributes to the ‘homing' of umbilical cord blood cells to a hypoxic‐ischemic lesion in the rat brain. J Neurosci Res 2010;88:1223–1233. [DOI] [PubMed] [Google Scholar]
- 32. Bennis Y, Sarlon‐Bartoli G, Guillet B et al. Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J Thromb Haemost 2012;10:1914–1928. [DOI] [PubMed] [Google Scholar]
- 33. Hache G, Garrigue P, Bennis Y et al. ARA290, A Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors' Angiogenic Potential and Homing Ability. Shock 2016;46:390–397. [DOI] [PubMed] [Google Scholar]
- 34. Garrigue P, Giacomino L, Bucci C et al. Single photon emission computed tomography imaging of cerebral blood flow, blood‐brain barrier disruption, and apoptosis time course after focal cerebral ischemia in rats. Int J Stroke 2016;11:117–126. [DOI] [PubMed] [Google Scholar]
- 35. Garrigue P, Hache G, Bennis Y et al. EPO pretreatment of ECFCs enhances functional recovery after transplantation in a rat model of cerebral ischemia through an increase of their homing abilities: A SPECT/CT study. J Nucl Med 2016;57:1798–1804. [DOI] [PubMed] [Google Scholar]
- 36. Renault‐Mihara F, Okada S, Shibata S et al. Spinal cord injury: Emerging beneficial role of reactive astrocytes' migration. Int J Biochem Cell Biol 2008;40:1649–1653. [DOI] [PubMed] [Google Scholar]
- 37. Robel S, Bardehle S, Lepier A et al. Genetic deletion of cdc42 reveals a crucial role for astrocyte recruitment to the injury site in vitro and in vivo. J Neurosci 2011;31:12471–12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 2009;32:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dinkel K, Dhabhar FS, Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci USA 2004;101:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Butovsky O, Talpalar AE, Ben‐Yaakov K et al. Activation of microglia by aggregated beta‐amyloid or lipopolysaccharide impairs MHC‐II expression and renders them cytotoxic whereas IFN‐gamma and IL‐4 render them protective. Mol Cell Neurosci 2005;29:381–393. [DOI] [PubMed] [Google Scholar]
- 41. Lehnardt S, Massillon L, Follett P et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll‐like receptor 4‐dependent pathway. Proc Natl Acad Sci USA 2003;100:8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaur C, Rathnasamy G, Ling EA. Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J Neuroimmune Pharmacol 2013;8:66–78. [DOI] [PubMed] [Google Scholar]
- 43. Bain JM, Ziegler A, Yang Z et al. TGFbeta1 stimulates the over‐production of white matter astrocytes from precursors of the “brain marrow” in a rodent model of neonatal encephalopathy. PLoS One 2010;5:e9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin A, Mace E, Boisgard R et al. Imaging of perfusion, angiogenesis, and tissue elasticity after stroke. J Cereb Blood Flow Metab 2012;32:1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kidani Y, Miki Y, Nomimura N et al. The therapeutic effect of CD133(+) cells derived from human umbilical cord blood on neonatal mouse hypoxic‐ischemic encephalopathy model. Life Sci 2016;157:108–115. [DOI] [PubMed] [Google Scholar]
- 46. Aridas JD, McDonald CA, Paton MC et al. Cord blood mononuclear cells prevent neuronal apoptosis in response to perinatal asphyxia in the newborn lamb. J Physiol 2016;594:1421–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rice JE, 3rd , Vannucci RC, Brierley JB. The influence of immaturity on hypoxic‐ischemic brain damage in the rat. Ann Neurol 1981;9:131–141. [DOI] [PubMed] [Google Scholar]
- 48. Taupin P. Transplantation of two populations of stem cells to improve engraftment: WO2008060932. Expert Opin Ther Pat 2010;20:1259–1263. [DOI] [PubMed] [Google Scholar]
- 49. Rocha V, Crotta A, Ruggeri A et al. Double cord blood transplantation: Extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best Pract Res Clin Haematol 2010;23:223–229. [DOI] [PubMed] [Google Scholar]
- 50. Brunstein CG. Umbilical cord blood transplantation for the treatment of hematologic malignancies. Cancer Control 2011;18:222–236. [DOI] [PubMed] [Google Scholar]
- 51. Lawn JE CS, Zupan J; Lancet Neonatal Survival Steering Team . 4 million neonatal deaths: When? Where? Why? Lancet 2005;365:891–900. [DOI] [PubMed] [Google Scholar]
- 52. Moon CJ, Youn YA, Yum SK et al. Cytokine changes in newborns with therapeutic hypothermia after hypoxic ischemic encephalopathy. J Perinatol 2016;36:1092–1096. [DOI] [PubMed] [Google Scholar]
- 53. McAdams RM, Juul SE. Neonatal encephalopathy: Update on therapeutic hypothermia and other novel therapeutics. Clin Perinatol 2016;43:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cotten CM, Murtha AP, Goldberg RN et al. Feasibility of autologous cord blood cells for infants with hypoxic‐ischemic encephalopathy. J Pediatr 2014;164:973–979 e971. [DOI] [PMC free article] [PubMed] [Google Scholar]


