Abstract
Purpose
A pooled analysis was conducted to evaluate tanezumab efficacy and safety in patients with osteoarthritis (OA), including subgroup analyses of at-risk patients with diabetes, severe OA symptoms, and those aged ≥65 years.
Patients and methods
Data from phase III placebo-controlled clinical trials of patients with moderate-to-severe OA of the knee or hip were pooled to evaluate tanezumab efficacy (four trials) and safety (nine trials). Patients received intravenous tanezumab, tanezumab plus an oral NSAID (naproxen, celecoxib, or diclofenac), active comparator (naproxen, celecoxib, diclofenac, or oxycodone), or placebo. Efficacy assessments included change from baseline to week 16 in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and physical function scores, Patient’s Global Assessment (PGA) of OA, and percentage of patients with ≥30%, ≥50%, ≥70%, and ≥90% improvement in WOMAC pain. Safety assessments included adverse event (AE) documentation and physical and neurologic examinations.
Results
Tanezumab significantly improved all efficacy end points in the overall population. Efficacy in at-risk patient subgroups was similar to the overall population. Incidence of AEs was highest in the tanezumab plus NSAID group and lowest in the placebo group. Incidence of AEs in the tanezumab monotherapy and active comparator groups was similar. Overall incidence of AEs was similar across subgroups. AEs of abnormal peripheral sensation were more frequently reported in tanezumab-treated patients compared with placebo or active comparator. Patients receiving active comparator had a slightly higher incidence of AEs suggestive of postganglionic sympathetic dysfunction.
Conclusion
Tanezumab consistently provided significant improvement of pain, physical function, and PGA in individuals with OA, including patients with diabetes, severe OA symptoms, or aged ≥65 years. No increased safety risk was observed in at-risk patient subgroups.
Trial registration
NCT00733902, NCT00744471, NCT00830063, NCT00863304, NCT00809354, NCT00864097, NCT00863772, NCT01089725, NCT00985621.
Keywords: tanezumab, efficacy, safety, osteoarthritis, nerve growth factor
Introduction
Osteoarthritis (OA) is a major cause of pain and locomotor disability.1 Despite a number of treatment options and guidelines for the management of pain associated with OA, many patients report dissatisfaction with or the need to change medications because adequate pain control is not achieved.1–3 NSAIDs and opioids are standard pharmacologic treatments for OA pain, but these are often associated with increased risk of adverse events (AEs), including gastrointestinal and cardiovascular AEs, multiorgan failure, and potential for dependence or addiction.1–3 The elderly and/or patients with diabetes, in particular, are more susceptible to these AEs than the rest of the population.4–6
Development of novel pharmacologic therapies targeting the function of key pain modulators may provide new treatment options with improved efficacy and/or safety.7–9 Nerve growth factor (NGF) is a neurotrophin and key mediator of pain, with a demonstrated role in pain signal transduction and pathophysiology.7–9 Tanezumab is a humanized anti-NGF monoclonal antibody that has high specificity and affinity for NGF, thereby blocking the binding of NGF to its receptors, TrkA and p75.7–9 In randomized clinical trials in patients with chronic pain conditions (OA and chronic low back pain), tanezumab provided clinically meaningful improvements by significantly reducing pain and improving physical function and Patient’s Global Assessment (PGA) of OA.10–24 During conduct of late-phase development studies, unexpected AEs requiring total joint replacement led the US Food and Drug Administration (USFDA) to impose a partial clinical hold on all NGF-inhibitor therapies in development (for all indications except for cancer pain). A blinded Adjudication Committee reviewed and adjudicated the joint-related AEs and determined tanezumab treatment in higher doses and in combination with NSAIDs was associated with an increase in rapidly progressive OA.25,26 The partial clinical hold was subsequently lifted and risk-mitigation strategies have been incorporated into anti-NGF antibody trial design.
In the current article, we performed a pooled analysis of data from previously completed phase III clinical trials to determine if tanezumab efficacy and safety (in terms of common AEs and AEs related to neurologic function) differ among specific at-risk subgroups of individuals with OA. The subgroups included patients with diabetes, severe OA symptoms at baseline, and patients aged ≥65 years.
Patients and methods
Study design
Overall, there have been nine placebo-controlled, phase III OA studies performed with tanezumab to date.10–14,21,23,26 Four of these studies had treatment periods that were completed prior to implementation of the partial clinical hold and, thus, their efficacy evaluations were not impacted.12–14 Individual patient data from these four studies were pooled to evaluate efficacy (Table S1).12–14 As a result, this efficacy analysis includes all phase II, placebo-controlled trials of tanezumab in patients with moderate-to-severe OA of the knee or hip that were completed prior to implementation of the partial clinical hold by the USFDA. Efficacy was assessed as the change from baseline to week 16 in three coprimary end points: Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain (an 11-point numeric rating scale [NRS]; greater scores represent greater pain intensity), WOMAC Physical Function (an 11-point NRS; greater scores represent worsening physical function), and PGA of OA (a 5-point scale ranging from 1 = very good to 5 = very poor). Other analyses included the percentage of patients with ≥30%, ≥50%, ≥70%, and ≥90% improvement relative to baseline on the WOMAC Pain subscale. Analyses were conducted in 1) the overall pooled population, and subgroups of patients with 2) diabetes (defined as patients who had a medical history of diabetes mellitus, hyperglycemia, insulin-requiring type 2 diabetes mellitus, type 1 diabetes mellitus, type 2 diabetes mellitus, or who had a baseline hemoglobin A1c ≥6.5) vs patients without diabetes, 3) severe OA symptoms at baseline (defined as baseline WOMAC pain score ≥7 on an 11-point NRS and WOMAC physical function score ≥7 and a score of “poor” or “very poor” in PGA of OA) vs less severe OA symptoms at baseline, and 4) aged ≥65 vs <65 years.
To evaluate safety, individual patient data from all nine phase III controlled OA studies were pooled (Table S1).10–14,21,23,26 These included the four studies pooled for efficacy evaluations plus five additional studies in which the treatment period had been impacted by the clinical hold. Safety assessments included AE documentation, physical and neurologic examinations, clinical laboratory test results, and vital signs. In each study, investigators performed standardized neurologic examinations at each visit.27,28 The patient was referred to a consulting neurologist if an AE of abnormal peripheral sensation (including pain in an extremity) or an AE suggestive of a new or worsened peripheral neuropathy was reported (Supplemental Text S1). To evaluate the safety of tanezumab related to the sympathetic nervous system, AEs consistent with decreased sympathetic nervous system function across treatment groups were analyzed (Supplemental Text S2). The AEs included in this list were intended to be sensitive, but not specific, for sympathetic dysfunction.
Subgroup analyses were also conducted on safety assessments using the same subgroups as those in the efficacy evaluations (patients with diabetes, patients without diabetes, patients with severe OA symptoms at baseline, patients with less severe OA symptoms at baseline, patients aged ≥65 years, and patients aged <65 years).
Detailed methodologies for the individual studies have been reported.10–14,21,23,26 All clinical trials were registered at ClinicalTrials.gov prior to respective trial initiation. Registration numbers are NCT00733902, NCT00744471, NCT00830063, NCT00863304, NCT00809354, NCT00864097, NCT00863772, NCT01089725, and NCT00985621 (Table S1). Studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The study protocols and informed consent documentation were reviewed and approved by an institutional review board at each site. Written informed consent was obtained from patients before initiation of any protocol-specified procedures.
Study populations
Study populations, treatments, and study designs have been reported for the individual studies (Table S1).10–14,21,23,26 Treatments in the four phase III studies included in efficacy analyses were intravenous (IV) tanezumab every 8 weeks, oral naproxen, or oral and IV placebo according to the study design (Table S1). For the nine phase III studies included in the safety analysis, treatments were IV or subcutaneous (SC) tanezumab monotherapy every 8 weeks (IV and SC groups at a same dose were pooled), oral NSAID monotherapy (naproxen, oral celecoxib, or oral diclofenac sustained release), controlled-release oral oxycodone, combined treatment of an oral NSAID (naproxen, celecoxib, or sustained-release diclofenac) with IV tanezumab every 8 weeks, or placebo (IV, SC, and/or oral; Table S1).
Inclusion and exclusion criteria for individual studies were generally similar and have been published within the individual studies (Table S1).10–14,21 Common inclusion criteria for all studies were patients aged ≥18 years, with body mass index ≤39 kg/m2, and diagnosis of hip or knee OA based on the American College of Rheumatology criteria (and, in most studies, radiographic confirmation with Kellgren–Lawrence grade ≥2 on a scale of 0–4).29,30 At screening, eligible patients reported WOMAC pain score ≥4 (on an 11-point NRS on which greater scores represent greater pain intensity) in the index joint, with or without analgesic medication. Exclusion criteria common to the individual studies were pregnancy or intention to become pregnant during the study; pain syndromes that could confound assessment of pain from OA (eg, fibromyalgia, systemic lupus erythematosus, or others); and significant cardiac, neurologic, or psychological conditions (Table S1).10–14,21
Statistical analyses
The efficacy population consisted of all patients evaluated for efficacy in the four phase III studies that included the intent-to-treat (ITT) population in one study and a modified ITT population in the other three studies.12–14 The ITT was defined as all randomized patients treated with at least one dose of study medication, whereas the modified ITT was all randomized patients treated with at least one dose of study medication, but excluded all patients from any study site found to have significant deviation from good clinical practices or patients for whom there were possible breaches in study blinding (identified through site audits by site monitors and by the sponsor Quality Assurance group).12–14 Because tanezumab 2.5 mg and naproxen were not used in any of the same studies in the efficacy analyses, these treatments could not be compared directly. The safety population consisted of all patients treated with one or more doses of tanezumab, placebo, or active comparator during one of the nine phase III studies.
Changes from baseline of the continuous end points were analyzed using an analysis of covariance model with factors of baseline, study, treatment, and study-by-treatment interaction. Subgroup analyses were performed using the same model as the overall analyses. Safety data were summarized with summary statistics.
Results
Patients
The pooled safety analysis consisted of 7,491 patients across the nine phase III studies. Of these patients, 1,171 (15.6%) had diabetes, 1,674 (22.3%) had severe OA symptoms, and 2,695 (36.0%) were aged ≥65 years at baseline. Baseline and demographic characteristics were generally similar across treatments in the safety analysis (Table 1). The total number of patients in the overall efficacy analysis in each treatment group was as follows: placebo =744, tanezumab 2.5 mg =327, tanezumab 5 mg =743, tanezumab 10 mg =748, and naproxen =417. The number (%) of diabetic patients in each treatment group was as follows: placebo =88 (11.8%), tanezumab 2.5 mg =50 (15.3%), tanezumab 5 mg =90 (12.1%), tanezumab 10 mg =75 (10.0%), and naproxen =60 (14.4%). The number of severe OA symptom patients in each treatment group was as follows: placebo =192 (25.8%), tanezumab 2.5 mg =88 (26.9%), tanezumab 5 mg =179 (24.1%), tanezumab 10 mg =170 (22.7%), and naproxen =113 (27.1%). The number of patients aged ≥65 years in each treatment group was as follows: placebo =275 (37.0%), tanezumab 2.5 mg =124 (37.9%), tanezumab 5 mg =269 (36.2%), tanezumab 10 mg =268 (35.8%), and naproxen =151 (36.2%). Baseline and demographic characteristics were generally similar across treatments in the efficacy analysis (Table S2).
Table 1.
Baseline demographics for nine phase III OA studies pooled for safety analysis
| Population | Placebo | Tanezumab (all doses combined) | Tanezumab 2.5 mg | Tanezumab 5 mg | Tanezumab 10 mg | Tanezumab (all doses combined) + NSAIDa | Tanezumab 2.5 mg + NSAID | Tanezumab 5 mg + NSAID | Tanezumab 10 mg + NSAID | Active comparatorb |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | n=1,029 | n=3,666 | n=401 | n=1,581 | n=1,684 | n=1,530 | n=157 | n=686 | n=687 | n=1,266 |
| Female, n (%) | 656 (63.8) | 2,345 (64.0) | 243 (60.6) | 1,017 (64.3) | 1,085 (64.4) | 1,083 (70.8) | 121 (77.1) | 473 (69.0) | 489 (71.2) | 870 (68.7) |
| Age, years, mean ± SD | 60.3±10.4 | 60.8±10.2 | 61.5±10.0 | 60.8±10.1 | 60.7±10.3 | 61.8±9.9 | 62.1±9.7 | 61.8±10.0 | 61.7±9.8 | 60.8±9.7 |
| BMI, kg/m2, mean ± SD | 30.4±4.8 | 30.4±4.9 | 30.3±5.1 | 30.5±4.8 | 30.4±4.9 | 30.3±4.7 | 30.3±4.0 | 30.2±4.7 | 30.5±4.9 | 30.7±4.7 |
| Race, n (%) | ||||||||||
| White | 872 (84.7) | 2,920 (79.7) | 342 (85.3) | 1,243 (78.6) | 1,335 (79.3) | 1,176 (76.9) | 157 (100.0) | 519 (75.7) | 500 (72.8) | 1,006 (79.5) |
| Black | 127 (12.3) | 446 (12.2) | 46 (11.5) | 190 (12.0) | 210 (12.5) | 124 (8.1) | 0 (0.0) | 59 (8.6) | 65 (9.5) | 146 (11.5) |
| Asian | 11 (1.1) | 157 (4.3) | 7 (1.7) | 78 (4.9) | 72 (4.3) | 138 (9.0) | 0 (0.0) | 67 (9.8) | 71 (10.3) | 64 (5.1) |
| Other | 19 (1.8) | 143 (3.9) | 6 (1.5) | 70 (4.4) | 67 (4.0) | 92 (6.0) | 0 (0.0) | 41 (6.0) | 51 (7.4) | 50 (3.9) |
| Diabetesc | n=151 | n=571 | n=74 | n=255 | n=242 | n=241 | n=9 | n=112 | n=120 | n=208 |
| Female, n (%) | 92 (60.9) | 363 (63.6) | 48 (64.9) | 157 (61.6) | 158 (65.3) | 162 (67.2) | 8 (88.9) | 73 (65.2) | 81 (67.5) | 132 (63.5) |
| Age, years, mean ± SD | 62.9±10.0 | 63.3±9.0 | 63.1±7.9 | 63.7±9.0 | 62.8±9.3 | 63.4±8.6 | 59.7±7.0 | 63.6±9.0 | 63.4±8.4 | 62.1±7.9 |
| BMI, kg/m2, mean ± SD | 32.6±3.9 | 32.4±4.7 | 32.5±4.5 | 32.5±4.6 | 32.2±4.8 | 32.3±4.4 | 31.3±3.8 | 32.3±4.3 | 32.3±4.5 | 32.7±4.2 |
| Race, n (%) | ||||||||||
| White | 120 (79.5) | 411 (72.0) | 58 (78.4) | 180 (70.6) | 173 (71.5) | 142 (58.9) | 9 (100.0) | 65 (58.0) | 68 (56.7) | 152 (73.1) |
| Black | 23 (15.2) | 96 (16.8) | 11 (14.9) | 37 (14.5) | 48 (19.8) | 33 (13.7) | 0 (0.0) | 17 (15.2) | 16 (13.3) | 29 (13.9) |
| Asian | 4 (2.6) | 38 (6.7) | 4 (5.4) | 19 (7.5) | 15 (6.2) | 47 (19.5) | 0 (0.0) | 21 (18.8) | 26 (21.7) | 18 (8.7) |
| Other | 4 (2.6) | 26 (4.6) | 1 (1.4) | 19 (7.5) | 6 (2.5) | 19 (7.9) | 0 (0.0) | 9 (8.0) | 10 (8.3) | 9 (4.3) |
| No diabetes | n=878 | n=3,095 | n=327 | n=1,326 | n=1,442 | n=1,289 | n=148 | n=574 | n=567 | n=1,058 |
| Female, n (%) | 564 (64.2) | 1982 (64.0) | 195 (59.6) | 860 (64.9) | 927 (64.3) | 921 (71.5) | 113 (76.4) | 400 (69.7) | 408 (72.0) | 738 (69.8) |
| Age, years, mean ± SD | 59.9±10.4 | 60.4±10.3 | 61.1±10.5 | 60.3±10.2 | 60.3±10.4 | 61.5±10.1 | 62.2±9.9 | 61.4±10.1 | 61.3±10.1 | 60.6±10.0 |
| BMI, kg/m2, mean ± SD | 30.1±4.8 | 30.1±4.9 | 29.8±5.1 | 30.1±4.8 | 30.1±4.9 | 30.0±4.7 | 30.2±4.0 | 29.8±4.7 | 30.1±4.9 | 30.3±4.7 |
| Race, n (%) | ||||||||||
| White | 752 (85.6) | 2,509 (81.1) | 284 (86.9) | 1,063 (80.2) | 1,162 (80.6) | 1,034 (80.2) | 148 (100.0) | 454 (79.1) | 432 (76.2) | 854 (80.7) |
| Black | 104 (11.8) | 350 (11.3) | 35 (10.7) | 153 (11.5) | 162 (11.2) | 91 (7.1) | 0 (0.0) | 42 (7.3) | 49 (8.6) | 117 (11.1) |
| Asian | 7 (0.8) | 119 (3.8) | 3 (0.9) | 59 (4.4) | 57 (4.0) | 91 (7.1) | 0 (0.0) | 46 (8.0) | 45 (7.9) | 46 (4.3) |
| Other | 15 (1.7) | 117 (3.8) | 5 (1.5) | 51 (3.8) | 61 (4.2) | 73 (5.7) | 0 (0.0) | 32 (5.6) | 41 (7.2) | 41 (3.9) |
| Severe OA symptoms at baselined | n=284 | n=871 | n=108 | n=373 | n=390 | n=233 | n=15 | n=117 | n=101 | n=286 |
| Female, n (%) | 191 (67.3) | 603 (69.2) | 72 (66.7) | 266 (71.3) | 265 (67.9) | 182 (78.1) | 13 (86.7) | 87 (74.4) | 82 (81.2) | 210 (73.4) |
| Age, years, mean ± SD | 59.4±10.0 | 59.5±10.3 | 60.9±10.3 | 59.2±10.5 | 59.4±10.2 | 61.3±10.3 | 63.9±10.8 | 61.5±10.7 | 60.6±9.9 | 60.0±9.9 |
| BMI, kg/m2, mean ± SD | 31.5±4.5 | 31.4±4.9 | 32.0±4.6 | 31.4±4.9 | 31.3±4.9 | 31.3±4.6 | 31.9±3.9 | 30.8±4.6 | 31.9±4.6 | 31.0±4.9 |
| Race, n (%) | ||||||||||
| White | 224 (78.9) | 648 (74.4) | 93 (86.1) | 271 (72.7) | 284 (72.8) | 162 (69.5) | 15 (100.0) | 82 (70.1) | 65 (64.4) | 213 (74.5) |
| Black | 47 (16.5) | 139 (16.0) | 14 (13.0) | 56 (15.0) | 69 (17.7) | 17 (7.3) | 0 (0.0) | 9 (7.7) | 8 (7.9) | 49 (17.1) |
| Asian | 2 (0.7) | 34 (3.9) | 1 (0.9) | 19 (5.1) | 14 (3.6) | 29 (12.4) | 0 (0.0) | 12 (10.3) | 17 (16.8) | 13 (4.5) |
| Other | 11 (3.9) | 50 (5.7) | 0 (0.0) | 27 (7.2) | 23 (5.9) | 25 (10.7) | 0 (0.0) | 14 (12.0) | 11 (10.9) | 11 (3.8) |
| Less severe OA symptoms at baseline | n=745 | n=2,795 | n=293 | n=1,208 | n=1,294 | n=1,297 | n=142 | n=569 | n=586 | n=980 |
| Female, n (%) | 465 (62.4) | 1,742 (62.3) | 171 (58.4) | 751 (62.2) | 820 (63.4) | 901 (69.5) | 108 (76.1) | 386 (67.8) | 407 (69.5) | 660 (67.3) |
| Age, years, mean ± SD | 60.6±10.5 | 61.2±10.1 | 61.7±10.0 | 61.3±9.9 | 61.0±10.3 | 61.9±9.8 | 61.9±9.7 | 61.9±9.8 | 61.9±9.8 | 61.1±9.6 |
| BMI, kg/m2, mean ± SD | 30.0±4.8 | 30.1±4.9 | 29.7±5.2 | 30.2±4.8 | 30.2±4.9 | 30.1±4.7 | 30.1±4.0 | 30.1±4.8 | 30.2±4.9 | 30.6±4.7 |
| Race, n (%) | ||||||||||
| White | 648 (87.0) | 2,272 (81.3) | 249 (85.0) | 972 (80.5) | 1,051 (81.2) | 1,014 (78.2) | 142 (100.0) | 437 (76.8) | 435 (74.2) | 793 (80.9) |
| Black | 80 (10.7) | 307 (11.0) | 32 (10.9) | 134 (11.1) | 141 (10.9) | 107 (8.2) | 0 (0.0) | 50 (8.8) | 57 (9.7) | 97 (9.9) |
| Asian | 9 (1.2) | 123 (4.4) | 6 (2.0) | 59 (4.9) | 58 (4.5) | 109 (8.4) | 0 (0.0) | 55 (9.7) | 54 (9.2) | 51 (5.2) |
| Other | 8 (1.1) | 93 (3.3) | 6 (2.0) | 43 (3.6) | 44 (3.4) | 67 (5.2) | 0 (0.0) | 27 (4.7) | 40 (6.8) | 39 (4.0) |
| Aged ≥65 years | n=353 | n=1,293 | n=147 | n=551 | n=595 | n=613 | n=65 | n=269 | n=279 | n=436 |
| Female, n (%) | 227 (64.3) | 828 (64.0) | 88 (59.9) | 354 (64.2) | 386 (64.9) | 443 (72.3) | 49 (75.4) | 194 (72.1) | 200 (71.7) | 327 (75.0) |
| Age, years, mean ± SD | 71.4±5.3 | 71.5±5.3 | 71.7±5.2 | 71.4±5.1 | 71.5±5.5 | 71.4±5.0 | 71.4±4.8 | 71.6±5.0 | 71.3±5.0 | 71.2±5.2 |
| BMI, kg/m2, mean ± SD | 29.7±4.7 | 29.8±4.8 | 30.3±4.8 | 29.9±5.0 | 29.5±4.7 | 29.7±4.7 | 29.9±3.8 | 29.7±4.6 | 29.6±5.0 | 30.1±4.6 |
| Race, n (%) | ||||||||||
| White | 316 (89.5) | 1,069 (82.7) | 133 (90.5) | 445 (80.8) | 491 (82.5) | 472 (77.0) | 65 (100.0) | 205 (76.2) | 202 (72.4) | 362 (83.0) |
| Black | 30 (8.5) | 107 (8.3) | 11 (7.5) | 46 (8.3) | 50 (8.4) | 31 (5.1) | 0 (0.0) | 16 (5.9) | 15 (5.4) | 36 (8.3) |
| Asian | 4 (1.1) | 65 (5.0) | 1 (0.7) | 30 (5.4) | 34 (5.7) | 63 (10.3) | 0 (0.0) | 29 (10.8) | 34 (12.2) | 22 (5.0) |
| Other | 3 (0.8) | 52 (4.0) | 2 (1.4) | 30 (5.4) | 20 (3.4) | 47 (7.7) | 0 (0.0) | 19 (7.1) | 28 (10.0) | 16 (3.7) |
| Aged <65 years | n=676 | n=2,373 | n=254 | n=1,030 | n=1,089 | n=917 | n=92 | n=417 | n=408 | n=830 |
| Female, n (%) | 429 (63.5) | 1,517 (63.9) | 155 (61.0) | 663 (64.4) | 699 (64.2) | 640 (69.8) | 72 (78.3) | 279 (66.9) | 289 (70.8) | 543 (65.4) |
| Age, years, mean ± SD | 54.5±7.2 | 55.0±7.0 | 55.6±7.0 | 55.2±7.1 | 54.8±6.9 | 55.3±6.5 | 55.5±6.4 | 55.5±6.8 | 55.2±6.3 | 55.4±6.5 |
| BMI, kg/m2, mean ± SD | 30.8±4.8 | 30.8±4.9 | 30.2±5.3 | 30.8±4.7 | 30.9±5.0 | 30.7±4.7 | 30.5±4.1 | 30.5±4.8 | 31.0±4.8 | 31.0±4.7 |
| Race, n (%) | ||||||||||
| White | 556 (82.2) | 1,851 (78.0) | 209 (82.3) | 798 (77.5) | 844 (77.5) | 704 (76.8) | 92 (100.0) | 314 (75.3) | 298 (73.0) | 644 (77.6) |
| Black | 97 (14.3) | 339 (14.3) | 35 (13.8) | 144 (14.0) | 160 (14.7) | 93 (10.1) | 0 (0.0) | 43 (10.3) | 50 (12.3) | 110 (13.3) |
| Asian | 7 (1.0) | 92 (3.9) | 6 (2.4) | 48 (4.7) | 38 (3.5) | 75 (8.2) | 0 (0.0) | 38 (9.1) | 37 (9.1) | 42 (5.1) |
| Other | 16 (2.4) | 91 (3.8) | 4 (1.6) | 40 (3.9) | 47 (4.3) | 45 (4.9) | 0 (0.0) | 22 (5.3) | 23 (5.6) | 34 (4.1) |
Notes:
Naproxen, celecoxib, or diclofenac sustained release.
Naproxen, celecoxib, diclofenac sustained release, or oxycodone controlled release.
Patients who had a medical history of diabetes mellitus, hyperglycemia, insulin-requiring type 2 diabetes mellitus, type 1 diabetes mellitus, type 2 diabetes mellitus, or with baseline hemoglobin A1c ≥6.5.
Patients with severe OA symptoms had a baseline WOMAC pain score ≥7 on 11-point numeric rating scale, WOMAC physical function score ≥7, and score of “poor” or “very poor” in the PGA of OA.
Abbreviations: BMI, body mass index; OA, osteoarthritis; PGA, Patient’s Global Assessment; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Efficacy
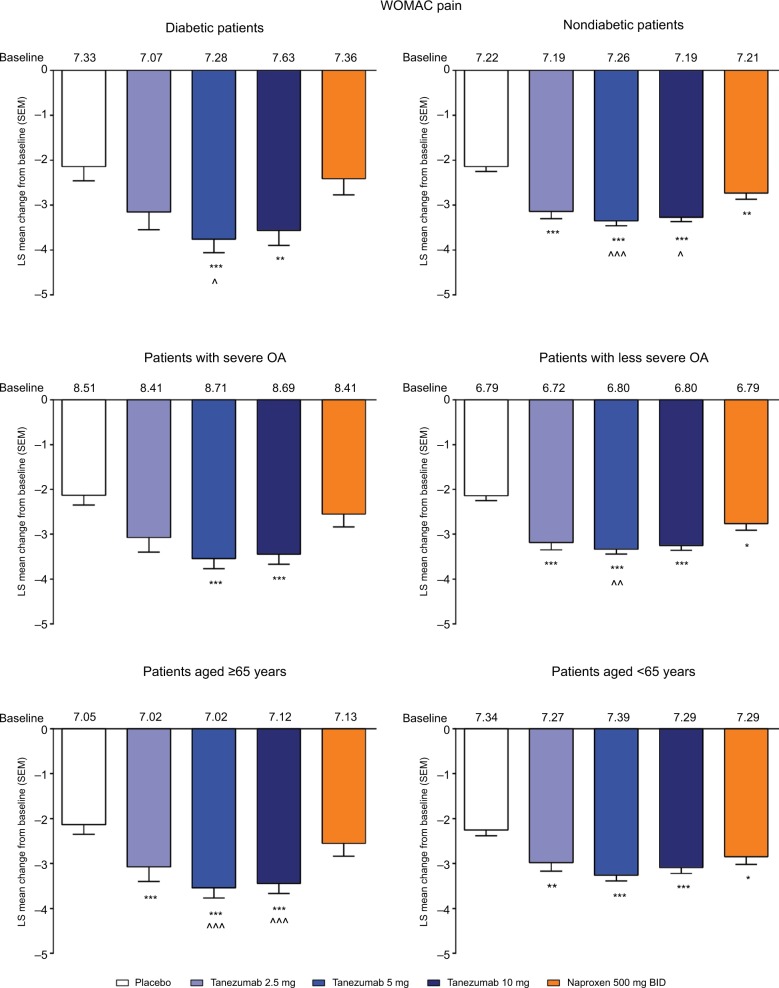
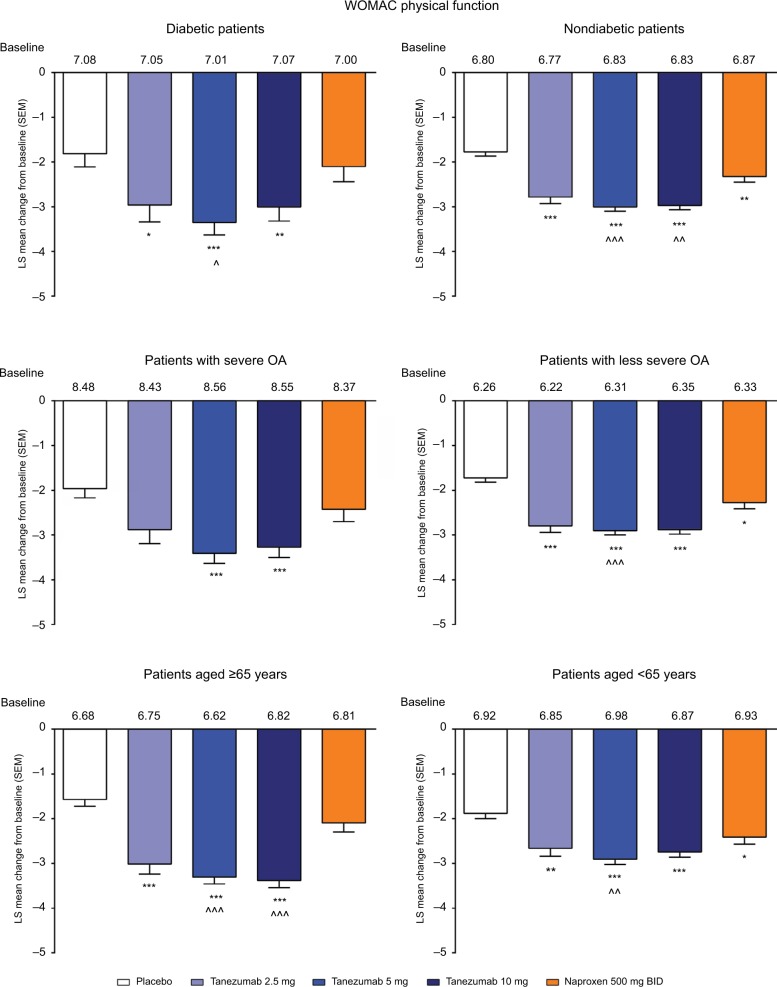
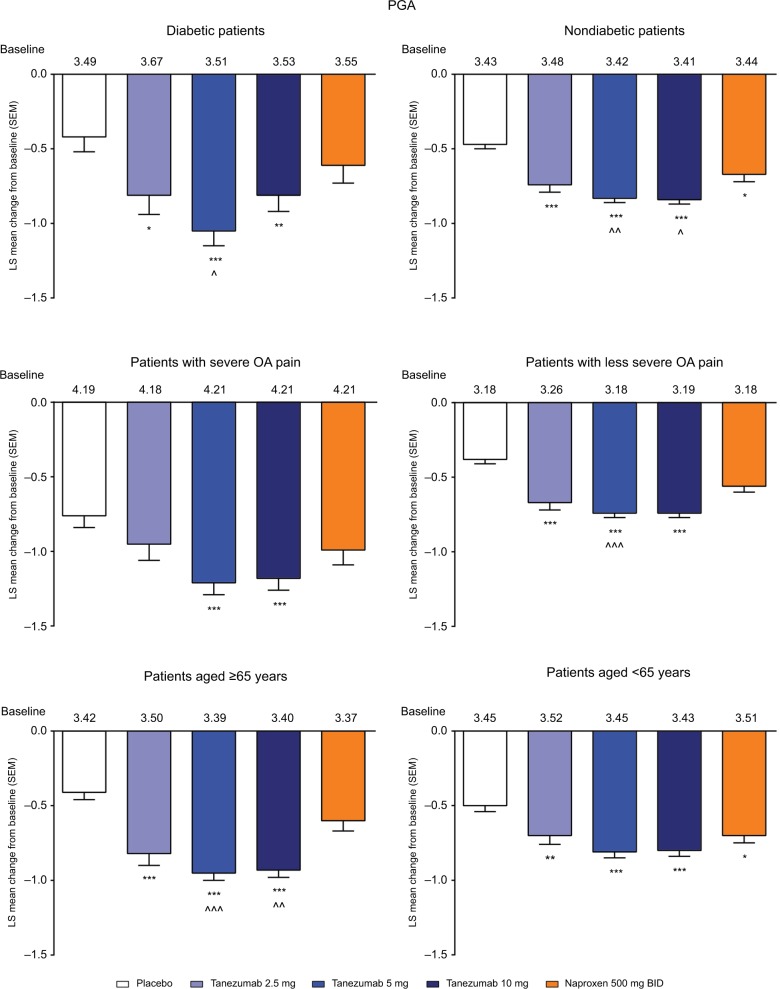
In the overall population across the four phase III studies pooled for efficacy, tanezumab 2.5–10 mg and naproxen 500 mg twice daily provided significantly greater improvement in WOMAC pain, WOMAC physical function, and PGA of OA compared with placebo at week 16 (Figure 1A–C). Treatment with tanezumab 5 and 10 mg also resulted in significantly greater improvement in these three end points compared with naproxen. Within the subgroups, tanezumab treatment, particularly the 5 and 10 mg dose groups, resulted in similar efficacy in patients with diabetes compared with nondiabetic patients, in patients with severe OA symptoms vs patients with less severe OA symptoms, and in patients aged ≥65 years compared with patients aged <65 years. The exception to this was the tanezumab 2.5 mg group, which was not significantly different from placebo for WOMAC pain in diabetic patients and patients with severe OA symptoms; for WOMAC physical function in patients with severe OA symptoms; and for PGA of OA in patients with severe OA symptoms. In addition, tanezumab 5 and 10 mg provided greater improvement vs naproxen in the subgroup analyses, although these differences were not always statistically significant at P<0.05, particularly when the numbers of patients were small (Figures 2–4).
Figure 1.
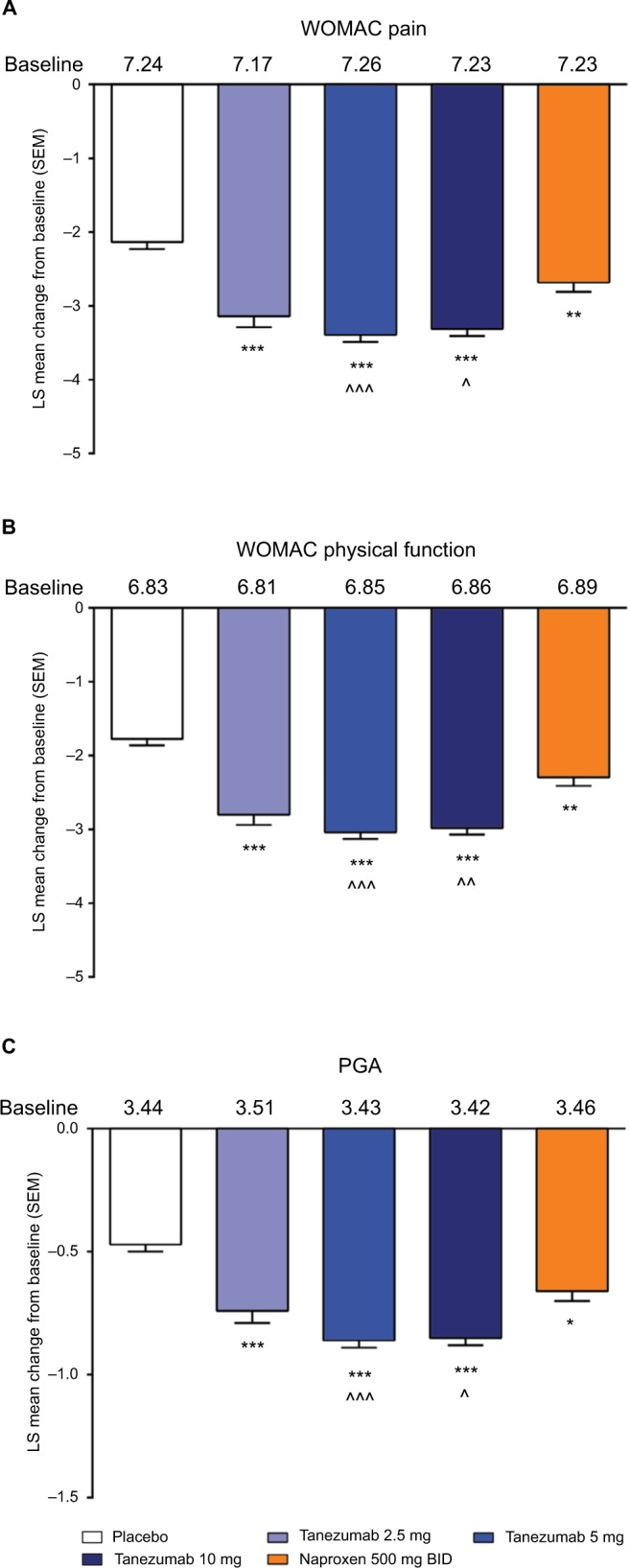
Least squares mean change in WOMAC pain (A), WOMAC physical function (B), and Patient’s Global Assessment (C) from baseline to week 16 for the overall population.
Notes: *P≤0.05; **P≤0.01; ***P≤0.001 vs placebo. ^P≤0.05; ^^P≤0.01; ^^^P≤0.001 vs naproxen.
Abbreviations: BID, twice daily; LS, least squares; PGA, Patient’s Global Assessment; SEM, standard error of the mean; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 2.
Least squares mean change in WOMAC pain from baseline to week 16 for the subgroup analyses.
Notes: *P≤0.05; **P≤0.01; ***P≤0.001 vs placebo. ^P≤0.05; ^^P≤0.01; ^^^P≤0.001 vs naproxen.
Abbreviations: BID, twice daily; LS, least squares; OA, osteoarthritis; SEM, standard error of the mean; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 3.
Least squares mean change in WOMAC physical function from baseline to week 16 for the subgroup analyses.
Notes: *P≤0.05; **P≤0.01; ***P≤0.001 vs placebo. ^P≤0.05; ^^P≤0.01; ^^^P≤0.001 vs naproxen.
Abbreviations: BID, twice daily; LS, least squares; OA, osteoarthritis; SEM, standard error of the mean; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 4.
Least squares mean change in PGA from baseline to week 16 for the subgroup analyses.
Notes: *P≤0.05; **P≤0.01; ***P≤0.001 vs placebo. ^P≤0.05; ^^P≤0.01; ^^^P≤0.001 vs naproxen.
Abbreviations: BID, twice daily; LS, least squares; OA, osteoarthritis; PGA, Patient’s Global Assessment; SEM, standard error of the mean; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
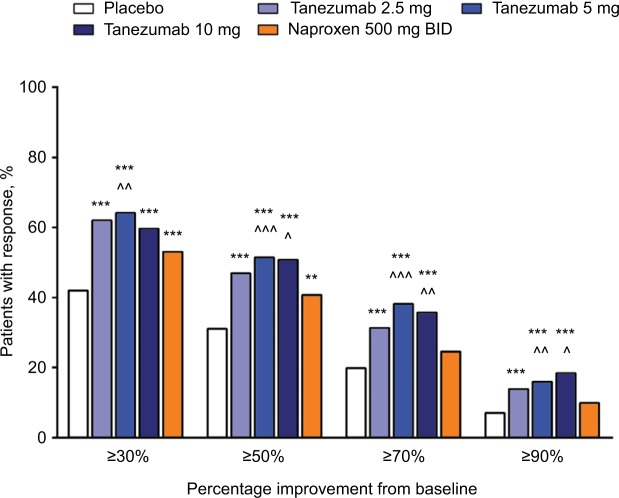
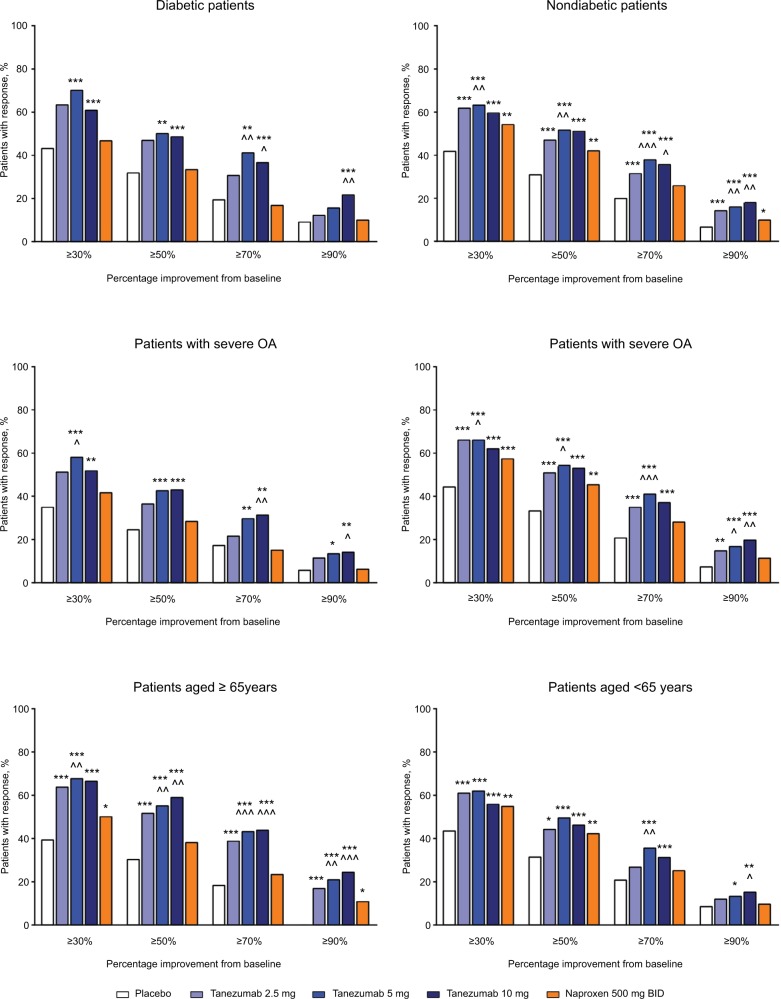
In the overall analysis, tanezumab 2.5–10 mg provided significantly greater improvement in the percentage of patients with WOMAC pain reduction ≥30%, ≥50%, ≥70%, and ≥90% compared with placebo (Figure 5). Treatment with tanezumab 5 or 10 mg also resulted in higher percentage of improvement in patients across all categories compared with naproxen, except tanezumab 10 mg at ≥30% response. For patients treated with naproxen 500 mg twice daily, significantly greater pain reduction vs placebo only occurred in the ≥30% and ≥50% response categories. In subgroup analyses, results were generally consistent with the overall analysis wherein tanezumab treatment, particularly the 5 and 10 mg dose groups, resulted in improvement in responder rates in all subgroups (Figure 6). Tanezumab treatment resulted in response rates that reached statistical significance vs both placebo and naproxen more often in nondiabetic patients (compared with diabetic patients), those patients with less severe (compared with severe) OA symptoms, and patients aged ≥65 years compared with patients aged <65 years (Figure 6).
Figure 5.
Percentage of patients with ≥30%, ≥50%, ≥70%, and ≥90% improvement on the WOMAC pain subscale at week 16 (overall group).
Notes: **P≤0.01; ***P≤0.001 vs placebo. ^P≤0.05; ^^P≤0.01; ^^^P≤0.001 vs naproxen.
Abbreviations: BID, twice daily; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 6.
Percentage of patients with ≥30%, ≥50%, ≥70%, and ≥90% improvement on the WOMAC pain subscale at week 16 in the subgroups.
Notes: *P≤0.05; **P≤0.01; ***P≤0.001 vs placebo. ^P≤0.05; ^^P≤0.01; ^^^P≤0.001 vs naproxen.
Abbreviations: BID, twice daily; OA, osteoarthritis; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Safety
In the nine phase III studies pooled for safety analyses, incidence rates of AEs, withdrawals due to AEs, and serious AEs in patients treated with tanezumab were similar to patients receiving active comparator but higher compared with placebo-treated patients (Table 2). Combination of tanezumab with NSAIDs was associated with slightly higher rates of these AEs than with tanezumab monotherapy, placebo, or active comparator. In general, safety in the subgroups was consistent with the overall analysis. In the overall analysis, the most common AEs (ie, those reported by ≥5% of patients in any group) were arthralgia, headache, pain in extremity, paresthesia, peripheral edema, OA, nasopharyngitis, and hypoesthesia (Table 3). Percentages of patients reporting these AEs were generally similar across all treatments and subgroups. The small numbers of patients in some subgroups contributed to some AEs reaching the threshold of ≥5% in any treatment in some subgroups.
Table 2.
Summary of adverse events, serious adverse events, and adverse events leading to discontinuation
| Incidence, n (%) | Placebo | Tanezumab (all doses combined) | Tanezumab 2.5 mg | Tanezumab 5 mg | Tanezumab 10 mg | Tanezumab (all doses combined) + NSAIDa | Tanezumab 2.5 mg+ NSAID | Tanezumab 5 mg + NSAID | Tanezumab 10 mg + NSAID | Active comparatorb |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | n=1,029 | n=3,666 | n=401 | n=1,581 | n=1,684 | n=1,530 | n=157 | n=686 | n=687 | n=1,266 |
| AEs | 461 (44.8) | 2,165 (59.1) | 225 (56.1) | 936 (59.2) | 1,004 (59.6) | 1,006 (65.8) | 71 (45.2) | 463 (67.5) | 472 (68.7) | 731 (57.7) |
| SAEs | 25 (2.4) | 151 (4.1) | 10 (2.5) | 69 (4.4) | 72 (4.3) | 148 (9.7) | 12 (7.6) | 62 (9.0) | 74 (10.8) | 69 (5.5) |
| AEs leading to discontinuation | 29 (2.8) | 255 (7.0) | 12 (3.0) | 97 (6.1) | 146 (8.7) | 204 (13.3) | 8 (5.1) | 88 (12.8) | 108 (15.7) | 100 (7.9) |
| Diabetesc | n=151 | n=571 | n=74 | n=255 | n=242 | n=241 | n=9 | n=112 | n=120 | n=208 |
| AEs | 67 (44.4) | 329 (57.6) | 42 (56.8) | 150 (58.8) | 137 (56.6) | 165 (68.5) | 4 (44.4) | 75 (67.0) | 86 (71.7) | 132 (63.5) |
| SAEs | 3 (2.0) | 30 (5.3) | 4 (5.4) | 16 (6.3) | 10 (4.1) | 22 (9.1) | 0 (0.0) | 7 (6.3) | 15 (12.5) | 15 (7.2) |
| AEs leading to discontinuation | 5 (3.3) | 38 (6.7) | 2 (2.7) | 10 (3.9) | 26 (10.7) | 31 (12.9) | 1 (11.1) | 11 (9.8) | 19 (15.8) | 21 (10.1) |
| No diabetes | n=878 | n=3,095 | n=327 | n=1,326 | n=1,442 | n=1,289 | n=148 | n=574 | n=567 | n=1,058 |
| AEs | 394 (44.9) | 1,836 (59.3) | 183 (56.0) | 786 (59.3) | 867 (60.1) | 841 (65.2) | 67 (45.3) | 388 (67.6) | 386 (68.1) | 599 (56.6) |
| SAEs | 22 (2.5) | 121 (3.9) | 6 (1.8) | 53 (4.0) | 62 (4.3) | 126 (9.8) | 12 (8.1) | 55 (9.6) | 59 (10.4) | 54 (5.1) |
| AEs leading to discontinuation | 24 (2.7) | 217 (7.0) | 10 (3.1) | 87 (6.6) | 120 (8.3) | 173 (13.4) | 7 (4.7) | 77 (13.4) | 89 (15.7) | 79 (7.5) |
| Severe OA symptoms at baselined | n=284 | n=871 | n=108 | n=373 | n=390 | n=233 | n=15 | n=117 | n=101 | n=286 |
| AEs | 124 (43.7) | 477 (54.8) | 66 (61.1) | 199 (53.4) | 212 (54.4) | 162 (69.5) | 6 (40.0) | 88 (75.2) | 68 (67.3) | 174 (60.8) |
| SAEs | 6 (2.1) | 43 (4.9) | 5 (4.6) | 19 (5.1) | 19 (4.9) | 22 (9.4) | 0 (0.0) | 10 (8.5) | 12 (11.9) | 21 (7.3) |
| AEs leading to discontinuation | 8 (2.8) | 56 (6.4) | 7 (6.5) | 20 (5.4) | 29 (7.4) | 36 (15.5) | 1 (6.7) | 19 (16.2) | 16 (15.8) | 25 (8.7) |
| Less severe OA symptoms at baseline | n=745 | n=2,795 | n=293 | n=1,208 | n=1,294 | n=1,297 | n=142 | n=569 | n=586 | n=980 |
| AEs | 337 (45.2) | 1,688 (60.4) | 159 (54.3) | 737 (61.0) | 792 (61.2) | 844 (65.1) | 65 (45.8) | 375 (65.9) | 404 (68.9) | 557 (56.8) |
| SAEs | 19 (2.6) | 108 (3.9) | 5 (1.7) | 50 (4.1) | 53 (4.1) | 126 (9.7) | 12 (8.5) | 52 (9.1) | 62 (10.6) | 48 (4.9) |
| AEs leading to discontinuation | 21 (2.8) | 199 (7.1) | 5 (1.7) | 77 (6.4) | 117 (9.0) | 168 (13.0) | 7 (4.9) | 69 (12.1) | 92 (15.7) | 75 (7.7) |
| Aged ≥65 years | n=353 | n=1,293 | n=147 | n=551 | n=595 | n=613 | n=65 | n=269 | n=279 | n=436 |
| AEs | 153 (43.3) | 802 (62.0) | 76 (51.7) | 353 (64.1) | 373 (62.7) | 407 (66.4) | 29 (44.6) | 187 (69.5) | 191 (68.5) | 267 (61.2) |
| SAEs | 13 (3.7) | 76 (5.9) | 7 (4.8) | 31 (5.6) | 38 (6.4) | 61 (10.0) | 3 (4.6) | 28 (10.4) | 30 (10.8) | 29 (6.7) |
| AEs leading to discontinuation | 12 (3.4) | 109 (8.4) | 6 (4.1) | 39 (7.1) | 64 (10.8) | 85 (13.9) | 3 (4.6) | 37 (13.8) | 45 (16.1) | 47 (10.8) |
| Aged <65 years | n=676 | n=2,373 | n=254 | n=1,030 | n=1,089 | n=917 | n=92 | n=417 | n=408 | n=830 |
| AEs | 308 (45.6) | 1,363 (57.4) | 149 (58.7) | 583 (56.6) | 631 (57.9) | 599 (65.3) | 42 (45.7) | 276 (66.2) | 281 (68.9) | 464 (55.9) |
| SAEs | 12 (1.8) | 75 (3.2) | 3 (1.2) | 38 (3.7) | 34 (3.1) | 87 (9.5) | 9 (9.8) | 34 (8.2) | 44 (10.8) | 40 (4.8) |
| AEs leading to discontinuation | 17 (2.5) | 146 (6.2) | 6 (2.4) | 58 (5.6) | 82 (7.5) | 119 (13.0) | 5 (5.4) | 51 (12.2) | 63 (15.4) | 53 (6.4) |
Notes:
Naproxen, celecoxib, or diclofenac sustained release.
Naproxen, celecoxib, diclofenac sustained release, or oxycodone controlled release.
Patients who had a medical history of diabetes mellitus, hyperglycemia, insulin-requiring type 2 diabetes mellitus, type 1 diabetes mellitus, type 2 diabetes mellitus, or with baseline hemoglobin A1c ≥6.5.
Patients with severe OA symptoms had a baseline WOMAC pain score ≥7 on 11-point numeric rating scale, WOMAC physical function score ≥7, and score of “poor” or “very poor” in the PGA of OA.
Abbreviations: AE, adverse event; OA, osteoarthritis; PGA, Patient’s Global Assessment; SAE, serious adverse event; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Table 3.
Incidence of adverse events in ≥5% of patients in any group
| Incidence, n (%) | Placebo | Tanezumab (all doses combined) | Tanezumab 2.5 mg | Tanezumab 5 mg | Tanezumab 10 mg | Tanezumab (all doses combined) + NSAIDa | Tanezumab 2.5 mg + NSAID | Tanezumab 5 mg + NSAID | Tanezumab 10 mg + NSAID | Active comparatorb |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | n=1,029 | n=3,666 | n=401 | n=1,581 | n=1,684 | n=1,530 | n=157 | n=686 | n=687 | n=1,266 |
| Arthralgia | 36 (3.5) | 319 (8.7) | 22 (5.5) | 127 (8.0) | 170 (10.1) | 160 (10.5) | 7 (4.5) | 80 (11.7) | 73 (10.6) | 73 (5.8) |
| Headache | 48 (4.7) | 154 (4.2) | 19 (4.7) | 66 (4.2) | 69 (4.1) | 60 (3.9) | 9 (5.7) | 29 (4.2) | 22 (3.2) | 55 (4.3) |
| Hypoesthesia | 9 (0.9) | 136 (3.7) | 15 (3.7) | 54 (3.4) | 67 (4.0) | 73 (4.8) | 2 (1.3) | 36 (5.2) | 35 (5.1) | 27 (2.1) |
| Pain in extremity | 25 (2.4) | 159 (4.3) | 10 (2.5) | 47 (3.0) | 102 (6.1) | 57 (3.7) | 2 (1.3) | 22 (3.2) | 33 (4.8) | 27 (2.1) |
| Paresthesia | 17 (1.7) | 212 (5.8) | 14 (3.5) | 88 (5.6) | 110 (6.5) | 114 (7.5) | 4 (2.5) | 48 (7.0) | 62 (9.0) | 29 (2.3) |
| Peripheral edema | 8 (0.8) | 171 (4.7) | 10 (2.5) | 69 (4.4) | 92 (5.5) | 101 (6.6) | 2 (1.3) | 42 (6.1) | 57 (8.3) | 25 (2.0) |
| Osteoarthritis | 18 (1.7) | 101 (2.8) | 10 (2.5) | 40 (2.5) | 51 (3.0) | 93 (6.1) | 3 (1.9) | 50 (7.3) | 40 (5.8) | 39 (3.1) |
| Nasopharyngitis | 15 (1.5) | 93 (2.5) | 8 (2.0) | 44 (2.8) | 41 (2.4) | 83 (5.4) | 10 (6.4) | 42 (6.1) | 31 (4.5) | 46 (3.6) |
| Diabetesc | n=151 | n=571 | n=74 | n=255 | n=242 | n=241 | n=9 | n=112 | n=120 | n=208 |
| Abdominal pain upper | 0 (0.0) | 2 (0.4) | 0 (0.0) | 1 (0.4) | 1 (0.4) | 8 (3.3) | 1 (11.1) | 5 (4.5) | 2 (1.7) | 2 (1.0) |
| Arthralgia | 3 (2.0) | 47 (8.2) | 5 (6.8) | 23 (9.0) | 19 (7.9) | 28 (11.6) | 1 (11.1) | 13 (11.6) | 14 (11.7) | 5 (2.4) |
| Blood creatine phosphokinase increased | 2 (1.3) | 14 (2.5) | 2 (2.7) | 5 (2.0) | 7 (2.9) | 9 (3.7) | 0 (0.0) | 6 (5.4) | 3 (2.5) | 5 (2.4) |
| Cough | 1 (0.7) | 8 (1.4) | 0 (0.0) | 3 (1.2) | 5 (2.1) | 5 (2.1) | 1 (11.1) | 3 (2.7) | 1 (0.8) | 1 (0.5) |
| Decreased appetite | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.4) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Diarrhea | 6 (4.0) | 17 (3.0) | 4 (5.4) | 11 (4.3) | 2 (0.8) | 11 (4.6) | 0 (0.0) | 5 (4.5) | 6 (5.0) | 2 (1.0) |
| Fatigue | 0 (0.0) | 12 (2.1) | 2 (2.7) | 4 (1.6) | 6 (2.5) | 3 (1.2) | 1 (11.1) | 1 (0.9) | 1 (0.8) | 0 (0.0) |
| Headache | 7 (4.6) | 23 (4.0) | 2 (2.7) | 13 (5.1) | 8 (3.3) | 7 (2.9) | 1 (11.1) | 2 (1.8) | 4 (3.3) | 8 (3.8) |
| Hypoesthesia | 2 (1.3) | 25 (4.4) | 1 (1.4) | 9 (3.5) | 15 (6.2) | 11 (4.6) | 0 (0.0) | 5 (4.5) | 6 (5.0) | 4 (1.9) |
| Nasopharyngitis | 2 (1.3) | 13 (2.3) | 1 (1.4) | 7 (2.7) | 5 (2.1) | 12 (5.0) | 0 (0.0) | 7 (6.3) | 5 (4.2) | 8 (3.8) |
| Nausea | 4 (2.6) | 13 (2.3) | 0 (0.0) | 9 (3.5) | 4 (1.7) | 8 (3.3) | 1 (11.1) | 4 (3.6) | 3 (2.5) | 7 (3.4) |
| Osteoarthritis | 1 (0.7) | 16 (2.8) | 2 (2.7) | 8 (3.1) | 6 (2.5) | 6 (2.5) | 0 (0.0) | 4 (3.6) | 2 (1.7) | 11 (5.3) |
| Pain in extremity | 2 (1.3) | 25 (4.4) | 1 (1.4) | 6 (2.4) | 18 (7.4) | 11 (4.6) | 0 (0.0) | 5 (4.5) | 6 (5.0) | 7 (3.4) |
| Paresthesia | 5 (3.3) | 30 (5.3) | 2 (2.7) | 15 (5.9) | 13 (5.4) | 20 (8.3) | 0 (0.0) | 7 (6.3) | 13 (10.8) | 4 (1.9) |
| Peripheral edema | 1 (0.7) | 27 (4.7) | 3 (4.1) | 13 (5.1) | 11 (4.5) | 12 (5.0) | 0 (0.0) | 5 (4.5) | 7 (5.8) | 4 (1.9) |
| Red blood cell sedimentation rate increased | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal colic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Upper respiratory tract infection | 1 (0.7) | 23 (4.0) | 2 (2.7) | 11 (4.3) | 10 (4.1) | 11 (4.6) | 0 (0.0) | 5 (4.5) | 6 (5.0) | 5 (2.4) |
| Urinary tract infection | 4 (2.6) | 24 (4.2) | 3 (4.1) | 7 (2.7) | 14 (5.8) | 12 (5.0) | 0 (0.0) | 6 (5.4) | 6 (5.0) | 9 (4.3) |
| No diabetes | n=878 | n=3,095 | n=327 | n=1,326 | n=1,442 | n=1,289 | n=148 | n=574 | n=567 | n=1,058 |
| Arthralgia | 33 (3.8) | 272 (8.8) | 17 (5.2) | 104 (7.8) | 151 (10.5) | 132 (10.2) | 6 (4.1) | 67 (11.7) | 59 (10.4) | 68 (6.4) |
| Headache | 41 (4.7) | 131 (4.2) | 17 (5.2) | 53 (4.0) | 61 (4.2) | 53 (4.1) | 8 (5.4) | 27 (4.7) | 18 (3.2) | 47 (4.4) |
| Hypoesthesia | 7 (0.8) | 111 (3.6) | 14 (4.3) | 45 (3.4) | 52 (3.6) | 62 (4.8) | 2 (1.4) | 31 (5.4) | 29 (5.1) | 23 (2.2) |
| Nasopharyngitis | 13 (1.5) | 80 (2.6) | 7 (2.1) | 37 (2.8) | 36 (2.5) | 71 (5.5) | 10 (6.8) | 35 (6.1) | 26 (4.6) | 38 (3.6) |
| Osteoarthritis | 17 (1.9) | 85 (2.7) | 8 (2.4) | 32 (2.4) | 45 (3.1) | 87 (6.7) | 3 (2.0) | 46 (8.0) | 38 (6.7) | 28 (2.6) |
| Pain in extremity | 23 (2.6) | 134 (4.3) | 9 (2.8) | 41 (3.1) | 84 (5.8) | 46 (3.6) | 2 (1.4) | 17 (3.0) | 27 (4.8) | 20 (1.9) |
| Paresthesia | 12 (1.4) | 182 (5.9) | 12 (3.7) | 73 (5.5) | 97 (6.7) | 94 (7.3) | 4 (2.7) | 41 (7.1) | 49 (8.6) | 25 (2.4) |
| Peripheral edema | 7 (0.8) | 144 (4.7) | 7 (2.1) | 56 (4.2) | 81 (5.6) | 89 (6.9) | 2 (1.4) | 37 (6.4) | 50 (8.8) | 21 (2.0) |
| Upper respiratory tract infection | 22 (2.5) | 117 (3.8) | 17 (5.2) | 58 (4.4) | 42 (2.9) | 38 (2.9) | 0 (0.0) | 18 (3.1) | 20 (3.5) | 36 (3.4) |
| Severe OA symptoms at baselined | n=284 | n=871 | n=108 | n=373 | n=390 | n=233 | n=15 | n=117 | n=101 | n=286 |
| Arthralgia | 9 (3.2) | 70 (8.0) | 7 (6.5) | 28 (7.5) | 35 (9.0) | 23 (9.9) | 2 (13.3) | 13 (11.1) | 8 (7.9) | 21 (7.3) |
| Back pain | 4 (1.4) | 23 (2.6) | 5 (4.6) | 11 (2.9) | 7 (1.8) | 11 (4.7) | 0 (0.0) | 6 (5.1) | 5 (5.0) | 6 (2.1) |
| Headache | 14 (4.9) | 30 (3.4) | 4 (3.7) | 14 (3.8) | 12 (3.1) | 10 (4.3) | 0 (0.0) | 4 (3.4) | 6 (5.9) | 12 (4.2) |
| Hypertriglyceridemia | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 2 (0.9) | 1 (6.7) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| Hypoesthesia | 1 (0.4) | 25 (2.9) | 2 (1.9) | 10 (2.7) | 13 (3.3) | 9 (3.9) | 0 (0.0) | 4 (3.4) | 5 (5.0) | 4 (1.4) |
| Joint swelling | 1 (0.4) | 15 (1.7) | 3 (2.8) | 5 (1.3) | 7 (1.8) | 9 (3.9) | 0 (0.0) | 6 (5.1) | 3 (3.0) | 1 (0.3) |
| Nausea | 3 (1.1) | 11 (1.3) | 1 (0.9) | 6 (1.6) | 4 (1.0) | 4 (1.7) | 0 (0.0) | 2 (1.7) | 2 (2.0) | 19 (6.6) |
| Nasopharyngitis | 3 (1.1) | 16 (1.8) | 0 (0.0) | 8 (2.1) | 8 (2.1) | 14 (6.0) | 0 (0.0) | 9 (7.7) | 5 (5.0) | 12 (4.2) |
| Oral infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Osteoarthritis | 6 (2.1) | 25 (2.9) | 5 (4.6) | 8 (2.1) | 12 (3.1) | 21 (9.0) | 0 (0.0) | 10 (8.5) | 11 (10.9) | 8 (2.8) |
| Osteoporotic fracture | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pain in extremity | 3 (1.1) | 34 (3.9) | 6 (5.6) | 8 (2.1) | 20 (5.1) | 6 (2.6) | 0 (0.0) | 2 (1.7) | 4 (4.0) | 3 (1.0) |
| Paresthesia | 6 (2.1) | 38 (4.4) | 3 (2.8) | 19 (5.1) | 16 (4.1) | 19 (8.2) | 0 (0.0) | 10 (8.5) | 9 (8.9) | 4 (1.4) |
| Peripheral edema | 0 (0.0) | 41 (4.7) | 3 (2.8) | 15 (4.0) | 23 (5.9) | 16 (6.9) | 0 (0.0) | 10 (8.5) | 6 (5.9) | 4 (1.4) |
| Rhinorrhea | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 2 (0.9) | 1 (6.7) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| Urinary tract infection | 7 (2.5) | 39 (4.5) | 5 (4.6) | 17 (4.6) | 17 (4.4) | 8 (3.4) | 1 (6.7) | 4 (3.4) | 3 (3.0) | 10 (3.5) |
| Less severe OA symptoms at baseline | n=745 | n=2,795 | n=293 | n=1,208 | n=1,294 | n=1,297 | n=142 | n=569 | n=586 | n=980 |
| Arthralgia | 27 (3.6) | 249 (8.9) | 15 (5.1) | 99 (8.2) | 135 (10.4) | 137 (10.6) | 5 (3.5) | 67 (11.8) | 65 (11.1) | 52 (5.3) |
| Headache | 34 (4.6) | 124 (4.4) | 15 (5.1) | 52 (4.3) | 57 (4.4) | 50 (3.9) | 9 (6.3) | 25 (4.4) | 16 (2.7) | 43 (4.4) |
| Hypoesthesia | 8 (1.1) | 111 (4.0) | 13 (4.4) | 44 (3.6) | 54 (4.2) | 64 (4.9) | 2 (1.4) | 32 (5.6) | 30 (5.1) | 23 (2.3) |
| Nasopharyngitis | 12 (1.6) | 77 (2.8) | 8 (2.7) | 36 (3.0) | 33 (2.6) | 69 (5.3) | 10 (7.0) | 33 (5.8) | 26 (4.4) | 34 (3.5) |
| Osteoarthritis | 12 (1.6) | 76 (2.7) | 5 (1.7) | 32 (2.6) | 39 (3.0) | 72 (5.6) | 3 (2.1) | 40 (7.0) | 29 (4.9) | 31 (3.2) |
| Pain in extremity | 22 (3.0) | 125 (4.5) | 4 (1.4) | 39 (3.2) | 82 (6.3) | 51 (3.9) | 2 (1.4) | 20 (3.5) | 29 (4.9) | 24 (2.4) |
| Paresthesia | 11 (1.5) | 174 (6.2) | 11 (3.8) | 69 (5.7) | 94 (7.3) | 95 (7.3) | 4 (2.8) | 38 (6.7) | 53 (9.0) | 25 (2.6) |
| Peripheral edema | 8 (1.1) | 130 (4.7) | 7 (2.4) | 54 (4.5) | 69 (5.3) | 85 (6.6) | 2 (1.4) | 32 (5.6) | 51 (8.7) | 21 (2.1) |
| Aged ≥65 years | n=353 | n=1,293 | n=147 | n=551 | n=595 | n=613 | n=65 | n=269 | n=279 | n=436 |
| Arthralgia | 13 (3.7) | 107 (8.3) | 6 (4.1) | 42 (7.6) | 59 (9.9) | 61 (10.0) | 2 (3.1) | 34 (12.6) | 25 (9.0) | 27 (6.2) |
| Back pain | 5 (1.4) | 44 (3.4) | 3 (2.0) | 21 (3.8) | 20 (3.4) | 25 (4.1) | 0 (0.0) | 15 (5.6) | 10 (3.6) | 9 (2.1) |
| Nasopharyngitis | 8 (2.3) | 30 (2.3) | 1 (0.7) | 13 (2.4) | 16 (2.7) | 29 (4.7) | 5 (7.7) | 14 (5.2) | 10 (3.6) | 10 (2.3) |
| Osteoarthritis | 7 (2.0) | 44 (3.4) | 4 (2.7) | 15 (2.7) | 25 (4.2) | 28 (4.6) | 1 (1.5) | 20 (7.4) | 7 (2.5) | 15 (3.4) |
| Pain in extremity | 6 (1.7) | 63 (4.9) | 5 (3.4) | 16 (2.9) | 42 (7.1) | 19 (3.1) | 1 (1.5) | 6 (2.2) | 12 (4.3) | 7 (1.6) |
| Paresthesia | 4 (1.1) | 62 (4.8) | 5 (3.4) | 25 (4.5) | 32 (5.4) | 33 (5.4) | 1 (1.5) | 11 (4.1) | 21 (7.5) | 8 (1.8) |
| Peripheral edema | 6 (1.7) | 70 (5.4) | 3 (2.0) | 28 (5.1) | 39 (6.6) | 42 (6.9) | 1 (1.5) | 18 (6.7) | 23 (8.2) | 12 (2.8) |
| Urinary tract infection | 8 (2.3) | 51 (3.9) | 6 (4.1) | 26 (4.7) | 19 (3.2) | 29 (4.7) | 0 (0.0) | 14 (5.2) | 15 (5.4) | 29 (6.7) |
| Aged <65 years | n=676 | n=2,373 | n=254 | n=1,030 | n=1,089 | n=917 | n=92 | n=417 | n=408 | n=830 |
| Arthralgia | 23 (3.4) | 212 (8.9) | 16 (6.3) | 85 (8.3) | 111 (10.2) | 99 (10.8) | 5 (5.4) | 46 (11.0) | 48 (11.8) | 46 (5.5) |
| Headache | 31 (4.6) | 110 (4.6) | 14 (5.5) | 45 (4.4) | 51 (4.7) | 40 (4.4) | 7 (7.6) | 22 (5.3) | 11 (2.7) | 34 (4.1) |
| Hypoesthesia | 5 (0.7) | 86 (3.6) | 11 (4.3) | 33 (3.2) | 42 (3.9) | 53 (5.8) | 2 (2.2) | 28 (6.7) | 23 (5.6) | 17 (2.0) |
| Nasopharyngitis | 7 (1.0) | 63 (2.7) | 7 (2.8) | 31 (3.0) | 25 (2.3) | 54 (5.9) | 5 (5.4) | 28 (6.7) | 21 (5.1) | 36 (4.3) |
| Osteoarthritis | 11 (1.6) | 57 (2.4) | 6 (2.4) | 25 (2.4) | 26 (2.4) | 65 (7.1) | 2 (2.2) | 30 (7.2) | 33 (8.1) | 24 (2.9) |
| Pain in extremity | 19 (2.8) | 96 (4.0) | 5 (2.0) | 31 (3.0) | 60 (5.5) | 38 (4.1) | 1 (1.1) | 16 (3.8) | 21 (5.1) | 20 (2.4) |
| Paresthesia | 13 (1.9) | 150 (6.3) | 9 (3.5) | 63 (6.1) | 78 (7.2) | 81 (8.8) | 3 (3.3) | 37 (8.9) | 41 (10.0) | 21 (2.5) |
| Peripheral edema | 2 (0.3) | 101 (4.3) | 7 (2.8) | 41 (4.0) | 53 (4.9) | 59 (6.4) | 1 (1.1) | 24 (5.8) | 34 (8.3) | 13 (1.6) |
| Upper respiratory tract infection | 18 (2.7) | 104 (4.4) | 17 (6.7) | 47 (4.6) | 40 (3.7) | 30 (3.3) | 0 (0.0) | 15 (3.6) | 15 (3.7) | 29 (3.5) |
Notes: OA as an adverse event represents worsening of OA in a joint previously diagnosed with OA or OA in a new/different joint.
Naproxen, celecoxib, or diclofenac sustained release.
Naproxen, celecoxib, diclofenac sustained release, or oxycodone controlled release.
Patients who had a medical history of diabetes mellitus, hyperglycemia, insulin-requiring type 2 diabetes mellitus, type 1 diabetes mellitus, type 2 diabetes mellitus, or with baseline hemoglobin A1c ≥6.5.
Patients with severe OA symptoms had a baseline WOMAC pain score ≥7 on 11-point numeric rating scale, WOMAC physical function score ≥7, and score of “poor” or “very poor” in the PGA of OA.
Abbreviations: OA, osteoarthritis; PGA, Patient’s Global Assessment; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
AEs of abnormal peripheral sensation were more frequently reported in tanezumab-treated patients than in patients receiving placebo or active comparator (Table 4). The majority of patients receiving tanezumab (monotherapy or in combination with NSAIDs) whose final neurologic consultations were categorized as having a new or worsening peripheral neuropathy based on clinically significant signs or diagnostic tests were diagnosed with some form of mononeuropathy, predominantly carpal tunnel syndrome or radiculopathy. Few patients were diagnosed with a polyneuropathy. Frequencies of these types of AEs in the subgroups were consistent with the overall population. In general, patients with diabetes, those with severe OA symptoms, and patients aged ≥65 years did not have more of these types of AEs than nondiabetic patients, patients with less severe OA symptoms, and patients aged <65 years, respectively.
Table 4.
Incidence of adverse events of abnormal peripheral sensation occurring in two or more patients in at least one groupa
| Incidence, n (%) | Placebo | Tanezumab (all doses combined) | Tanezumab 2.5 mg | Tanezumab 5 mg | Tanezumab 10 mg | Tanezumab (all doses combined) + NSAIDb | Tanezumab 2.5 mg + NSAID | Tanezumab 5 mg + NSAID | Tanezumab 10 mg + NSAID | Active comparatorc |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | n=1,029 | n=3,666 | n=401 | n=1,581 | n=1,684 | n=1,530 | n=157 | n=686 | n=687 | n=1,266 |
| Allodynia | 0 (0.0) | 15 (0.4) | 0 (0.0) | 2 (0.1) | 13 (0.8) | 4 (0.3) | 1 (0.6) | 1 (0.1) | 2 (0.3) | 1 (0.1) |
| Burning sensation | 1 (0.1) | 41 (1.1) | 2 (0.5) | 15 (0.9) | 24 (1.4) | 20 (1.3) | 0 (0.0) | 8 (1.2) | 12 (1.7) | 7 (0.6) |
| Decreased vibratory sense | 4 (0.4) | 22 (0.6) | 0 (0.0) | 13 (0.8) | 9 (0.5) | 13 (0.8) | 0 (0.0) | 6 (0.9) | 7 (1.0) | 2 (0.2) |
| Demyelinating polyneuropathy | 0 (0.0) | 3 (0.1) | 0 (0.0) | 2 (0.1) | 1 (0.1) | 3 (0.2) | 1 (0.6) | 0 (0.0) | 2 (0.3) | 0 (0.0) |
| Dysesthesia | 4 (0.4) | 19 (0.5) | 1 (0.2) | 4 (0.3) | 14 (0.8) | 8 (0.5) | 0 (0.0) | 3 (0.4) | 5 (0.7) | 4 (0.3) |
| Hyperesthesia | 1 (0.1) | 28 (0.8) | 1 (0.2) | 8 (0.5) | 19 (1.1) | 11 (0.7) | 0 (0.0) | 4 (0.6) | 7 (1.0) | 0 (0.0) |
| Hypoesthesia | 9 (0.9) | 136 (3.7) | 15 (3.7) | 54 (3.4) | 67 (4.0) | 73 (4.8) | 2 (1.3) | 36 (5.2) | 35 (5.1) | 27 (2.1) |
| Neuralgia | 0 (0.0) | 4 (0.1) | 0 (0.0) | 2 (0.1) | 2 (0.1) | 3 (0.2) | 0 (0.0) | 2 (0.3) | 1 (0.1) | 1 (0.1) |
| Neuritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.3) | 1 (0.1) |
| Neuropathy peripheral | 0 (0.0) | 28 (0.8) | 2 (0.5) | 12 (0.8) | 14 (0.8) | 16 (1.0) | 0 (0.0) | 6 (0.9) | 10 (1.5) | 4 (0.3) |
| Paresthesia | 17 (1.7) | 212 (5.8) | 14 (3.5) | 88 (5.6) | 110 (6.5) | 114 (7.5) | 4 (2.5) | 48 (7.0) | 62 (9.0) | 29 (2.3) |
| Polyneuropathy | 1 (0.1) | 3 (0.1) | 0 (0.0) | 2 (0.1) | 1 (0.1) | 8 (0.5) | 1 (0.6) | 4 (0.6) | 3 (0.4) | 2 (0.2) |
| Sensory disturbance | 0 (0.0) | 10 (0.3) | 0 (0.0) | 4 (0.3) | 6 (0.4) | 4 (0.3) | 0 (0.0) | 2 (0.3) | 2 (0.3) | 1 (0.1) |
| Sensory loss | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.2) | 0 (0.0) | 2 (0.3) | 1 (0.1) | 0 (0.0) |
| Diabetesd | n=151 | n=571 | n=74 | n=255 | n=242 | n=241 | n=9 | n=112 | n=120 | n=208 |
| Allodynia | 0 (0.0) | 3 (0.5) | 0 (0.0) | 1 (0.4) | 2 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Burning sensation | 0 (0.0) | 10 (1.8) | 0 (0.0) | 4 (1.6) | 6 (2.5) | 7 (2.9) | 0 (0.0) | 3 (2.7) | 4 (3.3) | 2 (1.0) |
| Decreased vibratory sense | 0 (0.0) | 3 (0.5) | 0 (0.0) | 2 (0.8) | 1 (0.4) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 1 (0.5) |
| Hyperesthesia | 0 (0.0) | 3 (0.5) | 0 (0.0) | 1 (0.4) | 2 (0.8) | 2 (0.8) | 0 (0.0) | 1 (0.9) | 1 (0.8) | 0 (0.0) |
| Hypoesthesia | 2 (1.3) | 25 (4.4) | 1 (1.4) | 9 (3.5) | 15 (6.2) | 11 (4.6) | 0 (0.0) | 5 (4.5) | 6 (5.0) | 4 (1.9) |
| Neuropathy peripheral | 0 (0.0) | 4 (0.7) | 1 (1.4) | 1 (0.4) | 2 (0.8) | 2 (0.8) | 0 (0.0) | 1 (0.9) | 1 (0.8) | 1 (0.5) |
| Paresthesia | 5 (3.3) | 30 (5.3) | 2 (2.7) | 15 (5.9) | 13 (5.4) | 20 (8.3) | 0 (0.0) | 7 (6.3) | 13 (10.8) | 4 (1.9) |
| Polyneuropathy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 2 (1.8) | 0 (0.0) | 1 (0.5) |
| No diabetes | n=878 | n=3,095 | n=327 | n=1,326 | n=1,442 | n=1,289 | n=148 | n=574 | n=567 | n=1,058 |
| Allodynia | 0 (0.0) | 12 (0.4) | 0 (0.0) | 1 (0.1) | 11 (0.8) | 4 (0.3) | 1 (0.7) | 1 (0.2) | 2 (0.4) | 0 (0.0) |
| Burning sensation | 1 (0.1) | 31 (1.0) | 2 (0.6) | 11 (0.8) | 18 (1.2) | 13 (1.0) | 0 (0.0) | 5 (0.9) | 8 (1.4) | 5 (0.5) |
| Decreased vibratory sense | 4 (0.5) | 19 (0.6) | 0 (0.0) | 11 (0.8) | 8 (0.6) | 12 (0.9) | 0 (0.0) | 6 (1.0) | 6 (1.1) | 1 (0.1) |
| Dysesthesia | 3 (0.3) | 18 (0.6) | 1 (0.3) | 3 (0.2) | 14 (1.0) | 8 (0.6) | 0 (0.0) | 3 (0.5) | 5 (0.9) | 3 (0.3) |
| Hyperesthesia | 1 (0.1) | 25 (0.8) | 1 (0.3) | 7 (0.5) | 17 (1.2) | 9 (0.7) | 0 (0.0) | 3 (0.5) | 6 (1.1) | 0 (0.0) |
| Hypoesthesia | 7 (0.8) | 111 (3.6) | 14 (4.3) | 45 (3.4) | 52 (3.6) | 62 (4.8) | 2 (1.4) | 31 (5.4) | 29 (5.1) | 23 (2.2) |
| Neuralgia | 0 (0.0) | 4 (0.1) | 0 (0.0) | 2 (0.2) | 2 (0.1) | 3 (0.2) | 0 (0.0) | 2 (0.3) | 1 (0.2) | 0 (0.0) |
| Neuropathy peripheral | 0 (0.0) | 24 (0.8) | 1 (0.3) | 11 (0.8) | 12 (0.8) | 14 (1.1) | 0 (0.0) | 5 (0.9) | 9 (1.6) | 3 (0.3) |
| Paresthesia | 12 (1.4) | 182 (5.9) | 12 (3.7) | 73 (5.5) | 97 (6.7) | 94 (7.3) | 4 (2.7) | 41 (7.1) | 49 (8.6) | 25 (2.4) |
| Polyneuropathy | 1 (0.1) | 3 (0.1) | 0 (0.0) | 2 (0.2) | 1 (0.1) | 6 (0.5) | 1 (0.7) | 2 (0.3) | 3 (0.5) | 1 (0.1) |
| Sensory disturbance | 0 (0.0) | 9 (0.3) | 0 (0.0) | 4 (0.3) | 5 (0.3) | 3 (0.2) | 0 (0.0) | 2 (0.3) | 1 (0.2) | 1 (0.1) |
| Sensory loss | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.2) | 0 (0.0) | 2 (0.3) | 1 (0.2) | 0 (0.0) |
| Severe OA symptoms at baselinee | n=284 | n=871 | n=108 | n=373 | n=390 | n=233 | n=15 | n=117 | n=101 | n=286 |
| Burning sensation | 0 (0.0) | 5 (0.6) | 0 (0.0) | 1 (0.3) | 4 (1.0) | 4 (1.7) | 0 (0.0) | 3 (2.6) | 1 (1.0) | 0 (0.0) |
| Decreased vibratory sense | 2 (0.7) | 4 (0.5) | 0 (0.0) | 1 (0.3) | 3 (0.8) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (0.3) |
| Dysesthesia | 2 (0.7) | 3 (0.3) | 0 (0.0) | 1 (0.3) | 2 (0.5) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| Hyperesthesia | 0 (0.0) | 4 (0.5) | 0 (0.0) | 1 (0.3) | 3 (0.8) | 1 (0.4) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Hypoesthesia | 1 (0.4) | 25 (2.9) | 2 (1.9) | 10 (2.7) | 13 (3.3) | 9 (3.9) | 0 (0.0) | 4 (3.4) | 5 (5.0) | 4 (1.4) |
| Neuralgia | 0 (0.0) | 2 (0.2) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neuritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.9) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 0 (0.0) |
| Neuropathy peripheral | 0 (0.0) | 6 (0.7) | 2 (1.9) | 0 (0.0) | 4 (1.0) | 3 (1.3) | 0 (0.0) | 1 (0.9) | 2 (2.0) | 0 (0.0) |
| Paresthesia | 6 (2.1) | 38 (4.4) | 3 (2.8) | 19 (5.1) | 16 (4.1) | 19 (8.2) | 0 (0.0) | 10 (8.5) | 9 (8.9) | 4 (1.4) |
| Polyneuropathy | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 3 (1.3) | 0 (0.0) | 2 (1.7) | 1 (1.0) | 1 (0.3) |
| Less severe OA symptoms at baseline | n=745 | n=2,795 | n=293 | n=1,208 | n=1,294 | n=1,297 | n=142 | n=569 | n=586 | n=980 |
| Allodynia | 0 (0.0) | 15 (0.5) | 0 (0.0) | 2 (0.2) | 13 (1.0) | 4 (0.3) | 1 (0.7) | 1 (0.2) | 2 (0.3) | 1 (0.1) |
| Burning sensation | 1 (0.1) | 36 (1.3) | 2 (0.7) | 14 (1.2) | 20 (1.5) | 16 (1.2) | 0 (0.0) | 5 (0.9) | 11 (1.9) | 7 (0.7) |
| Decreased vibratory sense | 2 (0.3) | 18 (0.6) | 0 (0.0) | 12 (1.0) | 6 (0.5) | 12 (0.9) | 0 (0.0) | 6 (1.1) | 6 (1.0) | 1 (0.1) |
| Demyelinating polyneuropathy | 0 (0.0) | 3 (0.1) | 0 (0.0) | 2 (0.2) | 1 (0.1) | 3 (0.2) | 1 (0.7) | 0 (0.0) | 2 (0.3) | 0 (0.0) |
| Dysesthesia | 2 (0.3) | 16 (0.6) | 1 (0.3) | 3 (0.2) | 12 (0.9) | 7 (0.5) | 0 (0.0) | 3 (0.5) | 4 (0.7) | 4 (0.4) |
| Hyperesthesia | 1 (0.1) | 24 (0.9) | 1 (0.3) | 7 (0.6) | 16 (1.2) | 10 (0.8) | 0 (0.0) | 3 (0.5) | 7 (1.2) | 0 (0.0) |
| Hypoesthesia | 8 (1.1) | 111 (4.0) | 13 (4.4) | 44 (3.6) | 54 (4.2) | 64 (4.9) | 2 (1.4) | 32 (5.6) | 30 (5.1) | 23 (2.3) |
| Neuralgia | 0 (0.0) | 2 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 3 (0.2) | 0 (0.0) | 2 (0.4) | 1 (0.2) | 1 (0.1) |
| Neuropathy peripheral | 0 (0.0) | 22 (0.8) | 0 (0.0) | 12 (1.0) | 10 (0.8) | 13 (1.0) | 0 (0.0) | 5 (0.9) | 8 (1.4) | 4 (0.4) |
| Paresthesia | 11 (1.5) | 174 (6.2) | 11 (3.8) | 69 (5.7) | 94 (7.3) | 95 (7.3) | 4 (2.8) | 38 (6.7) | 53 (9.0) | 25 (2.6) |
| Polyneuropathy | 1 (0.1) | 2 (0.1) | 0 (0.0) | 1 (0.1) | 1 (0.1) | 5 (0.4) | 1 (0.7) | 2 (0.4) | 2 (0.3) | 1 (0.1) |
| Sensory disturbance | 0 (0.0) | 8 (0.3) | 0 (0.0) | 3 (0.2) | 5 (0.4) | 3 (0.2) | 0 (0.0) | 1 (0.2) | 2 (0.3) | 0 (0.0) |
| Sensory loss | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.2) | 0 (0.0) | 2 (0.4) | 1 (0.2) | 0 (0.0) |
| Aged ≥65 years | n=353 | n=1,293 | n=147 | n=551 | n=595 | n=613 | n=65 | n=269 | n=279 | n=436 |
| Allodynia | 0 (0.0) | 4 (0.3) | 0 (0.0) | 0 (0.0) | 4 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Burning sensation | 1 (0.3) | 15 (1.2) | 1 (0.7) | 5 (0.9) | 9 (1.5) | 6 (1.0) | 0 (0.0) | 1 (0.4) | 5 (1.8) | 4 (0.9) |
| Decreased vibratory sense | 1 (0.3) | 11 (0.9) | 0 (0.0) | 6 (1.1) | 5 (0.8) | 6 (1.0) | 0 (0.0) | 1 (0.4) | 5 (1.8) | 2 (0.5) |
| Demyelinating polyneuropathy | 0 (0.0) | 2 (0.2) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 3 (0.5) | 1 (1.5) | 0 (0.0) | 2 (0.7) | 0 (0.0) |
| Dysesthesia | 1 (0.3) | 5 (0.4) | 0 (0.0) | 1 (0.2) | 4 (0.7) | 3 (0.5) | 0 (0.0) | 1 (0.4) | 2 (0.7) | 3 (0.7) |
| Hyperesthesia | 0 (0.0) | 8 (0.6) | 0 (0.0) | 3 (0.5) | 5 (0.8) | 4 (0.7) | 0 (0.0) | 2 (0.7) | 2 (0.7) | 0 (0.0) |
| Hypoesthesia | 4 (1.1) | 50 (3.9) | 4 (2.7) | 21 (3.8) | 25 (4.2) | 20 (3.3) | 0 (0.0) | 8 (3.0) | 12 (4.3) | 10 (2.3) |
| Neuropathy peripheral | 0 (0.0) | 12 (0.9) | 2 (1.4) | 4 (0.7) | 6 (1.0) | 7 (1.1) | 0 (0.0) | 1 (0.4) | 6 (2.2) | 1 (0.2) |
| Paresthesia | 4 (1.1) | 62 (4.8) | 5 (3.4) | 25 (4.5) | 32 (5.4) | 33 (5.4) | 1 (1.5) | 11 (4.1) | 21 (7.5) | 8 (1.8) |
| Polyneuropathy | 0 (0.0) | 2 (0.2) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 3 (0.5) | 0 (0.0) | 1 (0.4) | 2 (0.7) | 2 (0.5) |
| Sensory disturbance | 0 (0.0) | 4 (0.3) | 0 (0.0) | 0 (0.0) | 4 (0.7) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Aged <65 years | n=676 | n=2,373 | n=254 | n=1,030 | n=1,089 | n=917 | n=92 | n=417 | n=408 | n=830 |
| Allodynia | 0 (0.0) | 11 (0.5) | 0 (0.0) | 2 (0.2) | 9 (0.8) | 4 (0.4) | 1 (1.1) | 1 (0.2) | 2 (0.5) | 0 (0.0) |
| Burning sensation | 0 (0.0) | 26 (1.1) | 1 (0.4) | 10 (1.0) | 15 (1.4) | 14 (1.5) | 0 (0.0) | 7 (1.7) | 7 (1.7) | 3 (0.4) |
| Decreased vibratory sense | 3 (0.4) | 11 (0.5) | 0 (0.0) | 7 (0.7) | 4 (0.4) | 7 (0.8) | 0 (0.0) | 5 (1.2) | 2 (0.5) | 0 (0.0) |
| Dysesthesia | 3 (0.4) | 14 (0.6) | 1 (0.4) | 3 (0.3) | 10 (0.9) | 5 (0.5) | 0 (0.0) | 2 (0.5) | 3 (0.7) | 1 (0.1) |
| Hyperesthesia | 1 (0.1) | 20 (0.8) | 1 (0.4) | 5 (0.5) | 14 (1.3) | 7 (0.8) | 0 (0.0) | 2 (0.5) | 5 (1.2) | 0 (0.0) |
| Hypoesthesia | 5 (0.7) | 86 (3.6) | 11 (4.3) | 33 (3.2) | 42 (3.9) | 53 (5.8) | 2 (2.2) | 28 (6.7) | 23 (5.6) | 17 (2.0) |
| Neuralgia | 0 (0.0) | 4 (0.2) | 0 (0.0) | 2 (0.2) | 2 (0.2) | 2 (0.2) | 0 (0.0) | 1 (0.2) | 1 (0.2) | 1 (0.1) |
| Neuritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 1 (0.1) |
| Neuropathy peripheral | 0 (0.0) | 16 (0.7) | 0 (0.0) | 8 (0.8) | 8 (0.7) | 9 (1.0) | 0 (0.0) | 5 (1.2) | 4 (1.0) | 3 (0.4) |
| Paresthesia | 13 (1.9) | 150 (6.3) | 9 (3.5) | 63 (6.1) | 78 (7.2) | 81 (8.8) | 3 (3.3) | 37 (8.9) | 41 (10.0) | 21 (2.5) |
| Polyneuropathy | 1 (0.1) | 1 (0.04) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 5 (0.5) | 1 (1.1) | 3 (0.7) | 1 (0.2) | 0 (0.0) |
| Sensory disturbance | 0 (0.0) | 6 (0.3) | 0 (0.0) | 4 (0.4) | 2 (0.2) | 3 (0.3) | 0 (0.0) | 2 (0.5) | 1 (0.2) | 1 (0.1) |
| Sensory loss | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.3) | 0 (0.0) | 2 (0.5) | 1 (0.2) | 0 (0.0) |
Notes:
Adverse events of abnormal peripheral sensation include allodynia, axonal neuropathy, burning sensation, decreased vibratory sense, demyelinating polyneuropathy, dysesthesia, formication, hyperesthesia, hyperpathia, hypoesthesia, hypoesthesia facial, hypoesthesia oral, intercostal neuralgia, neuralgia, neuritis, neuropathy peripheral, paresthesia, paresthesia oral, peripheral sensorimotor neuropathy, peripheral sensory neuropathy, polyneuropathy, polyneuropathy chronic, sensory disturbance, sensory loss, and thermohypoesthesia.
Naproxen, celecoxib, or diclofenac sustained release.
Naproxen, celecoxib, diclofenac sustained release, or oxycodone controlled release.
Patients who had a medical history of diabetes mellitus, hyperglycemia, insulin-requiring type 2 diabetes mellitus, type 1 diabetes mellitus, type 2 diabetes mellitus, or with baseline hemoglobin A1c ≥6.5.
Patients with severe OA symptoms had a baseline Western Ontario and McMaster Universities Osteoarthritis (WOMAC) pain score ≥7 on 11-point numeric rating scale, WOMAC physical function score ≥7, and score of “poor” or “very poor” in the PGA of OA.
Abbreviations: OA, osteoarthritis; PGA, Patient’s Global Assessment; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
AEs suggestive of postganglionic sympathetic dysfunction (AEs of decreased sympathetic function such as bradycardia, orthostatic hypotension, nausea, diarrhea, or vomiting) occurred at similar rates in patients receiving placebo (4.3%), tanezumab (4.8%), or tanezumab + NSAID (5.2%) in the overall population; patients treated with an active comparator had a slightly higher incidence (7.5%) of these events (Table 5). Overall frequency of these AEs was generally similar in patients with diabetes vs patients without diabetes, in patients with severe OA symptoms at baseline vs patients with less severe OA symptoms, and in patients aged ≥65 years compared with patients aged <65 years. The frequency of these events by treatment within the subgroups generally was consistent with that of the overall population, but somewhat higher in patients treated with active comparator compared with the other treatments.
Table 5.
Summary of decreased sympathetic function adverse eventsa
| Incidence, n (%) | Placebo | Tanezumab (all doses combined) | Tanezumab (all doses combined) + NSAIDb | Active comparatorc |
|---|---|---|---|---|
| Overall | n=1,029 | n=3,666 | n=1,530 | n=1,266 |
| Decreased sympathetic function AEs | 44 (4.3) | 175 (4.8) | 80 (5.2) | 95 (7.5) |
| Diabetesd | n=151 | n=571 | n=241 | n=208 |
| Decreased sympathetic function AEs | 9 (6.0) | 36 (6.3) | 17 (17.1) | 13 (6.3) |
| No diabetes | n=878 | n=3,095 | n=1,289 | n=1,058 |
| Decreased sympathetic function AEs | 35 (4.0) | 139 (4.5) | 63 (4.9) | 82 (7.8) |
| Severe OA symptoms at baselinee | n=284 | n=871 | n=233 | n=286 |
| Decreased sympathetic function AEs | 7 (2.5) | 31 (3.6) | 15 (6.4) | 29 (10.1) |
| Less severe OA symptoms at baseline | n=745 | n=2,795 | n=1,297 | n=980 |
| Decreased sympathetic function AEs | 37 (5.0) | 144 (5.2) | 65 (5.0) | 66 (6.7) |
| Aged ≥65 years | n=353 | n=1,293 | n=613 | n=436 |
| Decreased sympathetic function AEs | 11 (3.1) | 74 (5.7) | 35 (5.7) | 42 (9.6) |
| Aged <65 years | n=676 | n=2,373 | n=917 | n=830 |
| Decreased sympathetic function AEs | 33 (4.9) | 101 (4.3) | 45 (4.9) | 53 (6.4) |
Notes:
Decreased sympathetic function AEs include blood pressure orthostatic decreased, bradycardia, dizziness postural, presyncope, sinus bradycardia, syncope, heart rate decreased, orthostatic hypotension, anhidrosis, hypohidrosis, abdominal discomfort, diarrhea, early satiety, fecal incontinence, nausea, vomiting, ejaculation delayed, ejaculation disorder, ejaculation failure, hypertonic bladder, micturition urgency, respiratory distress, respiratory failure, nocturia, urinary frequency (pollakiuria), urinary hesitation, and urinary incontinence.
Naproxen, celecoxib, or diclofenac sustained release.
Naproxen, celecoxib, diclofenac sustained release, or oxycodone controlled release.
Patients who had a medical history of diabetes mellitus, hyperglycemia, insulin-requiring type 2 diabetes mellitus, type 1 diabetes mellitus, type 2 diabetes mellitus, or with baseline HbA1c ≥6.5.
Patients with severe OA symptoms had a baseline WOMAC pain score ≥7 on 11-point numeric rating scale, WOMAC physical function score ≥7, and score of “poor” or “very poor” in the PGA of OA.
Abbreviations: AEs, adverse events; OA, osteoarthritis; PGA, Patient’s Global Assessment; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Discussion
Pain is a major public health challenge because it affects tens of millions of individuals in the USA each year.31 This leads to substantial impacts on morbidity, mortality, and disability, as well as placing significant demands on the health care system and creating a large economic burden.31 Substantial disparities exist in pain prevalence and rates of undertreatment across population groups with vulnerable populations, such as the elderly who more commonly suffer from inadequate pain treatment.31 In addition, pain is associated with societal issues that extend beyond individuals and their suffering, such as in the opioid epidemic.31 Pharmacologic therapies for OA pain, such as acetaminophen, oral or topical NSAIDs, and tramadol produce inadequate pain relief for reasons of efficacy and/or safety.1 In a recent prospective multinational, longitudinal real-world study of patients with knee OA, more than half (54%) of patients reported inadequate pain relief, significant functional loss, and lower quality of life.32 Similarly, in an analysis of a health plan claims database of patients with new prescriptions for OA treatment, switching, discontinuation, and augmenting therapy were common, which could indicate that these patients had inadequate pain relief or could not tolerate the treatment.33 Thus, management of the pain associated with OA of the hip or knee remains a significant unmet medical need.34
This pooled analysis allowed examination of tanezumab efficacy and safety in a number of subgroups of interest, namely patients with diabetes, those with severe OA symptoms at baseline, and elderly patients. These patients were of particular interest because pain treatment for them can be particularly difficult. For example, elderly patients have increased sensitivity to medications and higher potential for complications and AEs.35,36 Several of the body changes associated with aging, such as slowing of the gastrointestinal transit time, increased fat to lean body weight ratio, and alterations in liver metabolism and renal excretion, could alter the effects of some drugs (eg, continuous-release enteral drugs) or may affect drug absorption or distribution.35 In addition, the elderly are likely to have comorbidities that may require treatment, thereby increasing the possibility of drug–drug interactions.36 Similarly, patients with diabetes must be carefully managed since they are at increased risk for renal and cardiovascular complications and may be susceptible to increased blood pressure with the use of NSAIDs.5 In addition, chronic musculoskeletal (knee and back) pain is prevalent among patients with diabetes and contributes to poorer overall diabetes self-management and increased difficulty with self-care activities.37 Severe OA pain may result in patients switching or discontinuing therapies due to dissatisfaction.33 In some cases, patients may be switched to opioid analgesics, and this requires careful clinical management to avoid the risks associated with opioid abuse, addiction, and diversion.38 However, opioids may provide only modest OA pain relief, and no studies to date have reported long-term pain relief with opioids.23,39 With increased concerns about the opioid epidemic, it is particularly important to find alternative therapies that are safe and effective in this population. Tanezumab may offer an effective and well-tolerated alternative in these difficult to treat and vulnerable populations.
In the current pooled analysis of efficacy across four phase III studies of tanezumab in the treatment of OA, tanezumab provided significant improvement in WOMAC pain and physical function scores and in PGA of OA. These improvements were significantly greater than those with placebo and generally significantly greater compared with the active comparator naproxen. In addition, tanezumab treatment resulted in significantly higher percentage of patients having ≥30%, ≥50%, ≥70%, and ≥90% improvement on the WOMAC pain subscale than patients treated with placebo. Tanezumab 5 and 10 mg provided significantly greater improvement than naproxen across all categories (except ≥30% response for tanezumab 10 mg).
Results in the individual subgroups evaluated in this pooled analysis were similar to the overall population. Tanezumab treatment resulted in good clinical efficacy in all the subgroups, with consistently significantly greater improvement than placebo in WOMAC pain, WOMAC physical function, and PGA of OA. Specifically, efficacy was generally similar in patients with diabetes vs those without diabetes and in elderly patients relative to younger patients. Tanezumab 5 and 10 mg also provided significantly greater improvement vs naproxen in the elderly. Of particular interest were the efficacy results in patients with more severe OA symptoms at study entry, since these individuals are in most need of pain relief and may be the most difficult to treat. Tanezumab efficacy in this subgroup of patients with severe OA symptoms was comparable with that observed in patients with less severe OA symptoms. The current tanezumab OA studies are focused on patients with more severe or refractory pain; therefore, it is important that tanezumab has demonstrated consistent efficacy in patients with severe OA.
In addition to the subgroups discussed here, it should be noted that an analysis was done to determine whether there was a BMI by treatment interaction at study end point for both WOMAC pain and WOMAC physical function scores. This analysis utilized three separate subgroups including patients with baseline BMI <25 kg/m2, BMI 25 to <30 kg/m2, and BMI ≥30 kg/m2. However, no significant interaction was found (data not shown).
Overall, a greater number of AEs were observed when tanezumab was combined with NSAIDs than with tanezumab monotherapy, placebo, or NSAID alone. The most commonly reported AEs were generally consistent across subgroups. AEs of abnormal peripheral sensation were reported more frequently in patients receiving tanezumab than in patients treated with placebo or active comparator. In general, most patients whose final neurologic consultations were categorized as new or worsening peripheral neuropathy based on clinically significant signs or diagnostic tests were diagnosed with some form of mononeuropathy (predominantly carpal tunnel syndrome) or radiculopathy; few patients were diagnosed with a polyneuropathy. Since these results are not the expected pattern for a neurotoxic compound, which typically causes length-dependent polyneuropathy in affected patients, the association of tanezumab with symptoms of mononeuropathy suggests that these presentations may be a result of NGF inhibition acting to unmask these conditions.21 Tanezumab was not associated with any increase in AEs associated with decreased sympathetic nervous system function.
The overall incidence of AEs was also similar across subgroups. Safety profiles among patients with diabetes, patients with severe OA symptoms, and patients aged ≥65 years were similar to the overall population. As noted, patients with diabetes and the elderly can be more prone to adverse effects of some pain treatments. In general, the rates of AEs in the subgroups evaluated in this pooled analysis were similar to the overall population, indicating that tanezumab did not adversely affect these more vulnerable patients compared with other patients. For those in the diabetes subgroup, the results are also consistent with a study of tanezumab in diabetic nerve pain in which tanezumab had significant efficacy without an increase in AEs.40 The most commonly reported AEs and rates of these events in the subgroups were similar to those of the overall population. AEs of abnormal peripheral sensation or AEs of decreased sympathetic nervous system function were not reported more frequently by patients in any of the subgroups. It is important to point out that patients with diabetes did not have an increase in AEs of abnormal peripheral sensation and had comparable nerve safety despite the susceptibility of these patients to concomitant diabetic neuropathy.
Conclusion
In conclusion, this pooled analysis indicated that tanezumab provided significant improvement in WOMAC pain, WOMAC physical function, and PGA of OA. Tanezumab efficacy in the subgroups of patients with diabetes, those with severe OA symptoms, and those aged ≥65 years was similar to the overall population. The incidence of AEs was also similar across subgroups, indicating that there was no increase in safety concerns in these vulnerable patient populations. This profile indicates that tanezumab has significant potential to be a treatment option that will add to the management of chronic OA pain in diverse patient populations.
Data sharing statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices 1) for indications that have been approved in the USA and/or EU or 2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supplementary Materials
Acknowledgments
These studies were funded by Pfizer Inc. Medical writing and/or editorial support was provided by Christina McManus, PhD, Joseph Oleynek, and Matt Soulsby, PhD, CMPP, of Engage Scientific Solutions and was funded by Pfizer Inc and Eli Lilly and Company.
Footnotes
Author contributions
LT interpreted the data, revised the manuscript, and gave approval for publication; AEB, DR, and TJS acquired the data, interpreted the data, revised the manuscript, and provided approval for publication; HN analyzed and interpreted the data, revised the manuscript, and provided approval for publication; MTB acquired the data, interpreted the data, revised the manuscript, and provided approval for publication; CRW acquired the data, interpreted the data, revised the manuscript, and provided approval for publication. All authors agreed to be accountable for all aspects of the work.
Disclosure
LT, HN, MTB, and CRW are employees of and hold stock and/or stock options in Pfizer Inc; AEB is currently a speaker and serves on advisory boards for Pfizer Inc; TJS received funding for the ongoing clinical research studies from Pfizer Inc and Regeneron; the authors report no other conflicts of interest in this work.
References
- 1.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Lee J, Shin CM, Lee DH, Park BJ. Risk of gastrointestinal bleeding and cardiovascular events due to NSAIDs in the diabetic elderly population. BMJ Open Diabetes Res Care. 2015;3(1):e000133. doi: 10.1136/bmjdrc-2015-000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sowers JR, White WB, Pitt B, et al. Celecoxib Rofecoxib Efficacy and Safety in Comorbidities Evaluation Trial (CRESCENT) Investigators The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med. 2005;165(2):161–168. doi: 10.1001/archinte.165.2.161. [DOI] [PubMed] [Google Scholar]
- 6.Wehling M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol. 2014;70(10):1159–1172. doi: 10.1007/s00228-014-1734-6. [DOI] [PubMed] [Google Scholar]
- 7.Abdiche YN, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci. 2008;17(8):1326–1335. doi: 10.1110/ps.035402.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hefti FF, Rosenthal A, Walicke PA, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27(2):85–91. doi: 10.1016/j.tips.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115(1):189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balanescu AR, Feist E, Wolfram G, et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann Rheum Dis. 2014;73(9):1665–1672. doi: 10.1136/annrheumdis-2012-203164. [DOI] [PubMed] [Google Scholar]
- 11.Brown MT, Herrmann DN, Goldstein M, et al. Nerve safety of tanezumab, a nerve growth factor inhibitor for pain treatment. J Neurol Sci. 2014;345(1–2):139–147. doi: 10.1016/j.jns.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2012;13(8):790–798. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum. 2013;65(7):1795–1803. doi: 10.1002/art.37950. [DOI] [PubMed] [Google Scholar]
- 14.Ekman EF, Gimbel JS, Bello AE, et al. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol. 2014;41(11):2249–2259. doi: 10.3899/jrheum.131294. [DOI] [PubMed] [Google Scholar]
- 15.Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185(5):1716–1721. doi: 10.1016/j.juro.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 16.Gimbel JS, Kivitz AJ, Bramson C, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014;155(9):1793–1801. doi: 10.1016/j.pain.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Katz N, Borenstein DG, Birbara C, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152(10):2248–2258. doi: 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013;154(7):1009–1021. doi: 10.1016/j.pain.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagashima H, Suzuki M, Araki S, Yamabe T, Muto C, Tanezumab Investigators Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartilage. 2011;19(12):1405–1412. doi: 10.1016/j.joca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Schnitzer TJ, Ekman EF, Spierings EL, et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis. 2015;74(6):1202–1211. doi: 10.1136/annrheumdis-2013-204905. [DOI] [PubMed] [Google Scholar]
- 22.Schnitzer TJ, Lane NE, Birbara C, Smith MD, Simpson SL, Brown MT. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage. 2011;19(6):639–646. doi: 10.1016/j.joca.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Spierings EL, Fidelholtz J, Wolfram G, Smith MD, Brown MT, West CR. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain. 2013;154(9):1603–1612. doi: 10.1016/j.pain.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 24.Spierings EL, Fidelholtz J, Wolfram G, Smith MD, Brown MT, West CR. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee: response. Pain. 2014;155(11):2432–2433. doi: 10.1016/j.pain.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Hochberg MC, Tive LA, Abramson SB, et al. When is osteonecrosis not osteonecrosis?: adjudication of reported serious adverse joint events in the Tanezumab clinical development program. Arthritis Rheumatol. 2016;68(2):382–391. doi: 10.1002/art.39492. [DOI] [PubMed] [Google Scholar]
- 26.Pfizer Inc Tanezumab arthritis Advisory Committee Briefing document. 2012. [Accessed February 04, 2019]. Available from: https://wayback.archive-it.org/7993/20170114000618/http://www.fda.gov/downloads/Adviso-ryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvi-soryCommittee/UCM295205.pdf.
- 27.Apfel SC, Schwartz S, Adornato BT, et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic poly-neuropathy: a randomized controlled trial. rhNGF clinical investigator group. JAMA. 2000;284(17):2215–2221. doi: 10.1001/jama.284.17.2215. [DOI] [PubMed] [Google Scholar]
- 28.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O’Brien PC. Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology. 1995;45(6):1115–1121. doi: 10.1212/wnl.45.6.1115. [DOI] [PubMed] [Google Scholar]
- 29.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria Committee of the American rheumatism association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 30.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Institute of Medicine Relieving pain in America: a blueprint for transforming prevention, care, education, and research. 2011. [Accessed February 04, 2019]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK91497/
- 32.Conaghan PG, Peloso PM, Everett SV, et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology (Oxford) 2015;54(2):270–277. doi: 10.1093/rheumatology/keu332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gore M, Sadosky A, Leslie D, Tai KS, Seleznick M. Patterns of therapy switching, augmentation, and discontinuation after initiation of treatment with select medications in patients with osteoarthritis. Clin Ther. 2011;33(12):1914–1931. doi: 10.1016/j.clinthera.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Cannon GW. Osteoarthritis: disease burden and unmet patient needs. [Accessed February 04, 2019]. Available from: https://wayback.archive-it.org/7993/20170405050406/https://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4290s1-02-01-Merck-Cannon.pdf.
- 35.American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons Pharmacological management of persistent pain in older persons. Pain Med. 2009;10(6):1062–1083. doi: 10.1111/j.1526-4637.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 36.Fitzcharles MA, Lussier D, Shir Y. Management of chronic arthritis pain in the elderly. Drugs Aging. 2010;27(6):471–490. doi: 10.2165/11536530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Krein SL, Heisler M, Piette JD, Makki F, Kerr EA. The effect of chronic pain on diabetes patients’ self-management. Diabetes Care. 2005;28(1):65–70. doi: 10.2337/diacare.28.1.65. [DOI] [PubMed] [Google Scholar]
- 38.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30(8):489–505. doi: 10.2165/11533440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Bramson C, Herrmann DN, Carey W, et al. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015;16(6):1163–1176. doi: 10.1111/pme.12677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.