Abstract
Background
Leishmaniasis occurs with an incidence of 0.5–1.5 million new cases annually, and is also endemic in 88 countries across the world.
Aim
Presently, we aimed to investigate the status of Cutaneous Leishmaniasis (CL) in Golestan Province in North of Iran.
Methods
A retrospective survey was conducted on existed data of 6873 patients with CL who attended to Health Centers in Golestan Province during 2010 to 2017 years. Data were collected using patient sheets and online registry form of CL. Descriptive statistical methods such as mean and standard deviation, and Chi-square test were employed to report and analyze results. p-Value <0.05 was considered significant.
Results
CL cases were more common in men 3885 (56.7%) than women 2965 (43.3%) (p = 0.001). The most and least cases was reported in 2017 and 2104 as 18 and 3 CL cases, accordingly. CL was mainly happen in the November (3816 cases), December (2832 cases) months. Presently, CL gradually increases in the last three years from 2014 to 2017 years.
Conclusion
Decrease of exposurement, improve the nutrition and child's immunity can be more beneficial.
Keywords: Cutaneous, Incidence, Leishmaniasis, Registries
1. Background
Leishmaniasis presents a major public health problem that includes some of diseases caused by the protozoan parasites of the genus Leishmania (Karami et al., 2013) which is distinguish by long-term nodulo-ulcerative lesions healing spontaneously with continual scarring (Gürel et al., 2012). Leishmaniasis as a zoonotic and anthroponotic disease transmitted through the bite of the female Phlebotomus and Lutzomyia in the Old and New World, accordingly (Hailu et al., 2016). Rodents are animal reservoir hosts of the disease including Rhombomys opimus, Meriones libycus and M. nesokiain Iran (Karami et al., 2013). Leishmaniasis comprises three clinical forms including cutaneous, visceral, and mucocutaneous with the highest prevalence of cutaneous leishmaniasis in the Middle East (Blum and Hatz, 2009).
In general, over 350 million persons are at risk globally and 0.7–1.3 million new cases occur annually (Showler and Boggild, 2015). According to World Health Organization (WHO) reports, an incidence of 2 million new cases is annually occurred (comprising 0.5 million of visceral leishmaniasis (VL) and l.5 million of cutaneous leishmaniasis (CL). Given the numerous studies, the prevalence of leishmaniasis in Iran ranges from1.8 to 37.9% (Razmjou et al., 2009). Also 70% of CL cases occur in Iran, Algeria, Afghanistan, Colombia, Brazil, Sudan, Syria, Ethiopia, Nicaragua and Peru (Organization WH, 2010).
This disease is considered among the nine infectious diseases that impose remarkable health complications regarding medical burden, however it is ignored by the tropical countries (Hotez et al., 2004). Recently, CL has appeared as a considerable reason of morbidity in war-torn countries such as Iran and Afghanistan (Alavinia et al., 2009).
According to animal reservoir host, there are four region of disease in Iran country: The first one has been located in northeast and central of Iran (Golestan Province is located), a region with Rhombomys opimus and Phlebotomus papatasi as reservoir and vector of the disease. Other regions are west and southwest, Baluchistan Province in the southeast, and central and southern Iran. Golestan Province is well-known foci of ZCL in Iran country (Sofizadeh et al., 2016).
Incidence of leishmaniasis vary depending upon the different factors such as hosts, vectors, parasites environment, and more importantly the interaction among the aforementioned factors; therefore, national efforts have failed to eradicate leishmaniasis in Iran(Karami et al., 2013). Following the unsuccessful provincial and national efforts to control leishmaniasis in Iran, the cutaneous leishmaniasis control program has sought to explain the epidemiological features of the disease across the world (Khamesipour et al., 2006; Kishore et al., 2006; Minodier and Parola, 2007).
2. Objective
Thus, the present study was aimed at investigating the status of cutaneous leishmaniasis in Golestan Province, Iran.
3. Methods
3.1. Study design and procedure
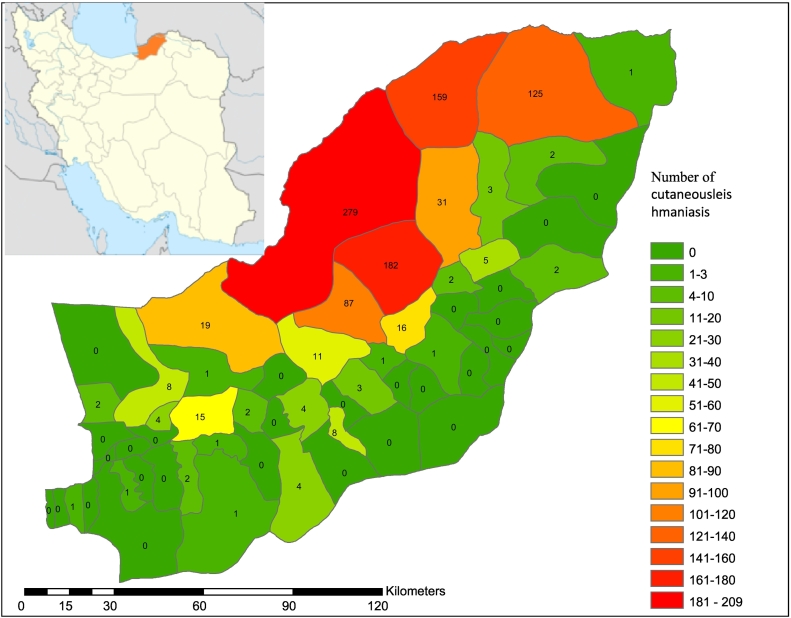
A retrospective survey of routinely collected data from the Health System of Golestan Province (North of Iran) was run during 2010 to 2017 years. All new cases of CL report by different cities of Golestan Provinces and register in the Iranian Health System in an online form. In detail, these forms are completed to assess the extent of the problem and at risk populations, promoting and concentrating control activities, and providing disease information to authorities. Fig. 1 indicates the hierarchy of the CL reporting process.
Fig. 1.
The sketch map of CL in Golestan Province in 2017 year (the last statistic).
3.2. Data instrument
To collect data, two forms were used. CL form was run to extracts data existed online in the Provincial Health System. Also, a patient sheet was used to gather demographic information such as age, gender, job, and residence (city and village).
3.3. Data collection
Data of the study was collected as follows: 1) active diagnosis by a physician, or 2) attending of patients to Rural and Urban Health Centers that supervised by the District Health Center in Golestan Province. Laboratory diagnosis was employed for all patients based on direct observation of Leishmania parasites by sampling skin lesions, preparing and coloring with Giemsa, and observing the form of Amastigote parasite. In total, 6873 samples were provided.
3.4. Data analysis
To analyze, descriptive statistics were employed to report the mean and standard deviation of variables. Chi-square test was run to investigate the qualitative variables. A flow chart was also used to present the Health System Structure in Iran.
4. Results
CL cases were more common in men 3885 (56.7%) than women 2965 (43.3%), more specifically in 2014 year as 60.8% compared to 39.2% that showed a significant statistical relationship (p = 0.001). Moreover, most of subjects were lived in the rural area 5605 (81.5%) compared to urban area 1228 (17.9%). In final, job analysis revealed the highest and lowest frequency of CL cases in the groups of children <7 years 2181 (32.8%) and rancher and farmer 26 (0.4%), respectively (Table 1).
Table 1.
Status of demographic characteristics of patients with CL during 2010 to 2017 years.
| Variables | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Total | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | 398(45.7) | 716(40.5) | 279(40.6) | 289(42.9) | 141(39.2) | 317(46.8) | 339(40.8) | 486(49.2) | 2965(43.3) | 0.001* |
| Male | 472(54.3) | 1050(59.5) | 408(59.4) | 384(57.1) | 219(60.8) | 360(53.2) | 491(59.2) | 501(50.8) | 3885(56.7) | ||
| Residence | Urban | 128(14.7) | 294(16.4) | 134(19.5) | 137(20.4) | 67(18.6) | 127(18.8) | 167(20.1) | 174 (17.6) | 1228(17.9) | 0.001* |
| Rural | 742(85.3) | 1498(83.6) | 553(80.5) | 536(79.6) | 293(81.4) | 544(80.4) | 649 (78.2) | 790 (80) | 5605 (81.5) | ||
| Other place | – | – | – | – | – | 6(0.9) | 14(1.7) | 23(2.3) | 43(0.6) | ||
| Job | Rancher | 0(0) | 13(19.4) | 11(16.4) | 4(6) | 1(1.5) | 12(17.9) | 15(22.4) | 11(16.4) | 67 (100) | 0.001* |
| Farmer | 2(0.9) | 86(39.8) | 34(15.7) | 16(7.4) | 3(1.4) | 23(10.6) | 28(13) | 24(11.1) | 216 (100) | ||
| Rancher and farmer | 0(0.0) | 6(23.1) | 1(3.8) | 5(19.2) | 0(0.0) | 4(15.4) | 5(19.2) | 5(19.2) | 26 (100) | ||
| Fixed income | 45(8.2) | 152(27.7) | 82(15) | 50(9.1) | 18(3.3) | 55(10) | 72(13.1) | 74(13.5) | 548 (100) | ||
| Driver | 8 (5.5) | 33(22.8) | 11(7.6) | 26(17.9) | 10(6.9) | 20(13.8) | 16(11) | 21(14.5) | 145 (100) | ||
| Military | 27(5.7) | 191(40.2) | 51(10.7) | 29(6.1) | 60(12.6) | 24(5.1) | 54(11.4) | 39(8.2) | 475 (100) | ||
| Employee | 1(1.4) | 11(15.9) | 9(13) | 9(13) | 7(10.1) | 8(11.6) | 13(18.8) | 11(15.9) | 69 (100) | ||
| Housekeeper | 141(11.3) | 304(24.4) | 132(10.6) | 135(10.8) | 51(4.1) | 147(11.8) | 163(13.1) | 173(13.9) | 1246 (100) | ||
| Unemployed | 4(5.8) | 9(13) | 11(15.9) | 9(13) | 1(1.4) | 15(21.7) | 8(11.6) | 12(17.4) | 69 (100) | ||
| Student | 167(13.6) | 346(28.3) | 121(9.9) | 95(7.8) | 63(5.1) | 113(9.2) | 155(12.7) | 164(13.4) | 1224 (100) | ||
| Children ≤ 7 years | 272(12.5) | 546(25) | 161(7.4) | 211(9.7) | 125(5.7) | 222(10.2) | 247(11.3) | 397(18.2) | 2181 (100) | ||
| Other | 91(24.3) | 34(9.1) | 50(13.3) | 47(12.5) | 9(2.4) | 34(9.1) | 54(14.4) | 56(14.9) | 375 (100) | ||
In total, the most cases of CL were seen in the age group of 0–9 year in 2011 (649 cases) and 2017 (463 cases). Also, in the age group of 70<, the most and least cases were reported in 2017 and 2104 as 18 and 3 CL cases, accordingly. Also, a remarkable association was found between CL in different years and age groups (p = 0.001) (Table 2).
Table 2.
Frequency of CL cases in Golestan Province based on the age groups during 2010 to 2017 years.
| Age groups (year) | Year |
Total (%) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 (%) | 2011 (%) | 2012 (%) | 2013 (%) | 2014 (%) | 2015 (%) | 2016 (%) | 2017 (%) | |||
| 0–9 | 323 (37.1) | 649 (36.6) | 210 (30.6) | 256 (38) | 156 (43.3) | 258 (38.1) | 291 (35.1) | 463 (46.9) | 2606 (38) | 0.001a |
| 10–19 | 199 (22.9) | 384 (21.7) | 120 (17.5) | 84 (12.5) | 62 (17.2) | 110 (16.2) | 135 (16.3) | 126 (12.8) | 1220 (17.8) | |
| 20–29 | 176 (20.2) | 388 (21.9) | 161 (23.4) | 122 (18.1) | 72 (20) | 129 (19.1) | 164 (19.8) | 144 (14.6) | 1356 (19.8) | |
| 30–39 | 79 (9.1) | 153 (8.6) | 77 (11.2) | 82 (12.2) | 31 (8.6) | 61 (9) | 110 (13.3) | 100 (10.1) | 693 (10.1) | |
| 40–49 | 49 (5.6) | 97 (5.5) | 58 (8.4) | 62 (9.2) | 19 (5.3) | 54 (8) | 57 (6.9) | 70 (7.1) | 466 (6.8) | |
| 50–59 | 21 (2.4) | 61 (3.4) | 41 (6) | 43 (6.4) | 15 (4.2) | 30 (4.4) | 45 (5.4) | 44 (4.5) | 300 (4.4) | |
| 60–69 | 19 (2.2) | 29 (1.6) | 16 (2.3) | 14 (2.1) | 2 (0.6) | 26 (3.8) | 21 (2.5) | 22 (2.2) | 149 (2.2) | |
| 70< | 4 (0.5) | 12 (0.7) | 4 (0.6) | 10 (1.5) | 3 (0.8) | 9 (1.3) | 7 (0.8) | 18 (1.8) | 67 (1) | |
| Total | 870 (100) | 1773 (100) | 687 (100) | 673 (100) | 360 (100) | 677 (100) | 830 (100) | 987 (100) | 6857 (100) | |
Chi-square test.
Regarding month of the incidence, November (3816 cases), December (2832 cases) and October (2459 cases) were ranked as first, second and third month with the highest incidence (Table 3).
Table 3.
Frequency of CL cases in Golestan Province according to months of the year during 2010 to 2017 years.
| Months | Years |
p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||
| January | 116(13.3) | 226(12.6) | 57(8.3) | 53(7.9) | 1(0.3) | 116(17.1) | 69(8.3) | 113(11.4) | 0.001* |
| February | 28(3.2) | 69(3.9) | 18(2.6) | 23(3.4) | 0(0.0) | 39(5.8) | 29(3.5) | 33(3.3) | |
| March | 13(1.5) | 10(0.6) | 5(0.7) | 8(1.2) | 0(0.0) | 12(1.8) | 11(1.3) | 7(0.7) | |
| April | 8(0.9) | 11(0.6) | 11(1.6) | 16(2.4) | 7(1.9) | 3(0.4) | 4(0.5) | 6(0.6) | |
| May | 14(1.6) | 8(0.4) | 10(1.5) | 12(1.8) | 4(1.1) | 3(0.4) | 7(0.8) | 1(0.1) | |
| June | 3(0.3) | 5(0.3) | 13(1.9) | 9(1.3) | 1(0.3) | 5(0.7) | 10(1.20 | 3(0.3) | |
| July | 7(0.8) | 4(0.2) | 6(0.9) | 12(1.8) | 5(1.4) | 12(1.8) | 6(0.7) | 2(0.2) | |
| August | 12(1.4) | 4(0.2) | 44(6.4) | 24(3.6) | 9(2.5) | 29(4.3) | 38(4.6) | 8(0.8) | |
| September | 40(4.6) | 57(3.2) | 104(15.1) | 68(10.1) | 24(6.7) | 56(8.3) | 99(11.9) | 31(3.1) | |
| October | 174(20.0) | 390(21.8) | 167(24.3) | 111(16.5) | 72(20.0) | 81(12.0) | 154(18.6) | 161(16.3) | |
| November | 252(29.0) | 530(29.6) | 137(19.9) | 190(28.2) | 215(59.7) | 162(23.9) | 237(28.6) | 370(37.5) | |
| December | 203(23.3) | 478(26.7) | 115(16.7) | 147(21.8) | 22(6.1) | 159(23.5) | 166(20.0) | 252(25.5) | |
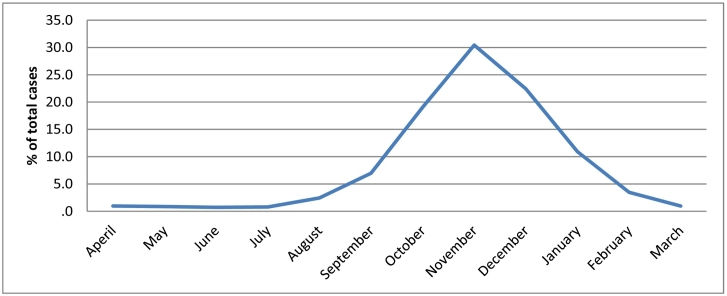
Graph 1 reported the months of CL status, such that increased from July, peaked in November and then decreased in March.
Graph 1.
The frequency of CL in Golestan Province based on months during 2010–2017 years.
As presented by Fig. 1, Golestan Province is located in North of Iran. The last annual frequency of CL was revealed in Fig. 1 according to district of the Province in 2017 year. Districts with high prevalence that located in north and northeastern of the province are named Gonbad-e-Kaboos and MaravahTapeh.
5. Discussion
Prior to discuss, it has been well-documented that Golestan Province is an endemic area of rural CL in Iran, with Leishmania major as dominant parasite species in the infected areas (Hezari et al., 2016).
The mean age of subjects was 19.53 ± 1.72 year. At present study, most of cases (38%) were found in the age group of 0–9 years. In a study conducted by Hamzavi and Khademi, the most cases (24.7%) were seen in the age group of 20–29 years (Hamzavi and Khademi, 2015). In Delgado et al. survey, the mean age of cases was 35 years that is different with the current study (Delgado et al., 2008). The possible explanation for these differences might be likely associated with high incidence of CL in the Golestan Province. As an endemic disease in the Province, it is hypothesized that immaturity of the immune system, malnutrition of children, and abundant reservoirs and vectors are all factors that may impress the increased prevalence among young children (Karami et al., 2013; Zijlstra, 2016). Of course in a study conducted by Alavinia et al. (2009) in Northern Khorasan Province of Iran (Alavinia et al., 2009), the mean age of CL cases was 23.2 years that is partly similar to our subjects. This similarity can be due to the geographic proximity and being endemic of the disease in both Provinces.
In the current study, 43.3% of patients were female that is in relevance with Alavinia et al. (2009) study (42.9%) that they were investigated an endemic Province, as well. Other studies also reported remarkable statistical difference between gender and incidence of CL (Karami et al., 2013; Rafati et al., 2004; Aissi et al., 2015; Naeem et al., 2014; Lucero et al., 2015). Gender differences can be justified by the fact that male comprising the most of seasonal immigrants as worker, wearing less clothes than women, working in open environments and having probably more exposure to sandflies, especially in the rural society. Since, most of CL cases lived in the rural area worked usually as farmer that expose them to risk of sand flies bite, the aforementioned results are not surprising.
In terms of residence, most of CL cases were observed in the rural area. Alavinia et al. study conducted in a Province close to our Province yielded the same result (Alavinia et al., 2009). Soofizadeh et al. also argued that the highest incidence of CL cases existed in the rural district of Korand in Gonbad-e-Kavoos (Sofizadeh et al., 2016). CL remains yet a great public health problem in many rural areas of Iran due to increase the number of rodents as the most prominent reservoir hosts of this form of Leishmaniasis (Badirzadeh et al., 2013; Mohebali et al., 2007). Additionally, rural CL has increased in the last decade in the Golestan Province because of development of colony of gerbils, construction of residential houses in the vicinity of them, increasing population, increasing carriers, entering unprotected individuals into contaminated areas, and in brief, disposal of unhealthy waste (Sofizadeh et al., 2016).
The incidence of the disease has augmented in the last three years from 2014 to 2017 years. A slightly increasing in recent years was observed in Hamzavi and Khademi study that is associated with our findings (Hamzavi and Khademi, 2015). This might be resulted by the climate changes in the country due to gradual warming of the planet which is lucrative to the growth of sand flies. Although, more attention to CL as a public health problem by the Health System may be another reason of increase in the incidence rate and prevalence.
Significantly, majority CL cases were happen in the autumn and in the months of November and December. Soofizadeh et al. survey implemented in Golestan Province indicated also similar results such that the greatest number of CL cases was seen in the months of October and November (Sofizadeh et al., 2016). Studies reported that some condition are required to reproduce sandfly including degree of 23–28 °C and moisture of 70–100% that are commonly happen in the last months of the summer and early autumn. Since, the incubation period varies from 1 to 2 months (Shirzadi et al., 2015); then, it is expected to be occurred in the autumn season in the Golestan Province.
CL cases mainly occurred in the north and northeastern of the province including Gonbad-e-Kavous and Maraveh Tapeh district where vary mainly with other districts of the province in terms of climate and topology. Similarly, Soofizadeh et al. found the same results in a study that conducted in the Golestan Province (Sofizadeh et al., 2016). It seems that high number of CL cases in the two aforementioned districts might be due to the plenty of wild rodents as the reservoir of disease and vicinity of their colonies to people residence.
6. Conclusion
Presently, findings revealed that CL cases were usually happen in children aged <9 years and autumn. Therefore, decrease of exposurement, improve the nutrition and their immunity appeared to be likely useful.
Limitation of the study
Two approaches of active and passive case-finding for diagnosing CL have been implementing in the country. Although, cases were mostly ZCL that lived in the rural area with active case-finding screening; however, data of some patients who lived in the urban area might be missed due to treatment in the private physician's clinics.
Conflict of interest
None.
Acknowledgments
Acknowledgement
The authors wish to thank the personnel who working at District Health Network for providing data of the study.
Funding
This work was supported by the Golestan University of Medical Sciences.
References
- Aissi W., Ben K.H., Habboul Z., Ben I.S., Harrat Z., Bouratbine A. Epidemiological, clinical and biological features of infantile visceral leishmaniasis at Kairouan hospital (Tunisia): about 240 cases. Bull. Soc. Pathol. Exot. 2015;108(4):265–271. doi: 10.1007/s13149-015-0438-1. [DOI] [PubMed] [Google Scholar]
- Alavinia S., Arzamani K., Reihani M., Jafari J. Some epidemiological aspects of cutaneous leishmaniasis in Northern Khorasan Province, Iran. Iran. J. Arthropod. Borne Dis. 2009;3(2):50. [PMC free article] [PubMed] [Google Scholar]
- Badirzadeh A., Mohebali M., Ghasemian M., Amini H., Zarei Z., Akhoundi B. Cutaneous and post kala-azar dermal leishmaniasis caused by Leishmania infantum in endemic areas of visceral leishmaniasis, northwestern Iran 2002–2011: a case series. Pathogens Glob. Health. 2013;107(4):194–197. doi: 10.1179/2047773213Y.0000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J.A., Hatz C.F. Treatment of cutaneous leishmaniasis in travelers 2009. J. Travel Med. 2009;16(2):123–131. doi: 10.1111/j.1708-8305.2008.00286.x. [DOI] [PubMed] [Google Scholar]
- Delgado O., Silva S., Coraspe V., Rivas M.A., Rodriguez-Morales A.J., Navarro P. Cutaneous leishmaniasis imported from Colombia to Northcentral Venezuela: implications for travel advice. Travel Med. Infect. Dis. 2008;6(6):376–379. doi: 10.1016/j.tmaid.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Gürel M.S., Yesilova Y., Ölgen M.K., Özbel Y. Cutaneous leishmaniasis in Turkey. Turkiye Parazitol. Derg. 2012;36(2):121. doi: 10.5152/tpd.2012.29. [DOI] [PubMed] [Google Scholar]
- Hailu T., Yimer M., Mulu W., Abera B. Challenges in visceral leishmaniasis control and elimination in the developing countries: a review. J. Vector Borne Dis. 2016;53(3):193. [PubMed] [Google Scholar]
- Hamzavi Y., Khademi N. Trend of cutaneous leishmaniasis in Kermanshah Province, West of Iran from 1990 to 2012. Iran. J. Parasitol. 2015;10(1):78. [PMC free article] [PubMed] [Google Scholar]
- Hezari F., Niyyati M., Tabaei S.J.S., Mohebali M., Vaziri V.M., Behniafar H. Frequency of cutaneous leishmaniasis and species identification in suspected individuals from Golestan Province, Northern Iran in 2014. Iran. J. Public Health. 2016;45(10):1348. [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Remme J.H., Buss P., George G., Morel C., Breman J.G. Combating tropical infectious diseases: report of the disease control priorities in developing countries project. Clin. Infect. Dis. 2004;38(6):871–878. doi: 10.1086/382077. [DOI] [PubMed] [Google Scholar]
- Karami M., Doudi M., Setorki M. Assessing epidemiology of cutaneous leishmaniasis in Isfahan, Iran. J. Vector Borne Dis. 2013;50(1):30. [PubMed] [Google Scholar]
- Khamesipour A., Rafati S., Davoudi N., Maboudi F., Modabber F. Leishmaniasis vaccine candidates for development: a global overview. Indian J. Med. Res. 2006;123(3):423. [PubMed] [Google Scholar]
- Kishore K., Kumar V., Kesari S., Dinesh D. Vector control in leishmaniasis. Indian J. Med. Res. 2006;123(3):467. [PubMed] [Google Scholar]
- Lucero E., Collin S.M., Gomes S., Akter F., Asad A., Das A.K. Effectiveness and safety of short course liposomal amphotericin B (AmBisome) as first line treatment for visceral leishmaniasis in Bangladesh. PLoS Negl. Trop. Dis. 2015;9(4):36–99. doi: 10.1371/journal.pntd.0003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minodier P., Parola P. Cutaneous leishmaniasis treatment. Travel Med. Infect. Dis. 2007;5(3):150–158. doi: 10.1016/j.tmaid.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mohebali M., Fotouhi A., Hooshmand B., Zarei Z., Akhoundi B., Rahnema A. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103(1):33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Naeem A.T., Mahmoudi S., Saboui F., Hajjaran H., Pourakbari B., Mohebali M. Clinical features and laboratory findings of visceral leishmaniasis in children referred to Children Medical Center Hospital, Tehran, Iran during 2004–2011. Iran. J. Parasitol. 2014;9(1):1. [PMC free article] [PubMed] [Google Scholar]
- Organization WH . March 2010. Control of the Leishmaniases: Report of a Meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva; pp. 22–26. [Google Scholar]
- Rafati N., Shapori-Moghadam A., Ghorbani R. Epidemiological survey of cutaneous leishmaniasis in Damghan (1999–2005) Sci. J. Semnan Univ. Med. Sci. 2004;2(1):247–253. [Google Scholar]
- Razmjou S., Hejazy H., Motazedian M.H., Baghaei M., Emamy M., Kalantary M. A new focus of zoonotic cutaneous leishmaniasis in Shiraz, Iran. Trans. R. Soc. Trop. Med. Hyg. 2009;103(7):727–730. doi: 10.1016/j.trstmh.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Shirzadi M.R., Mollalo A., Yaghoobi-Ershadi M.R. Dynamic relations between incidence of zoonotic cutaneous leishmaniasis and climatic factors in Golestan Province, Iran. J. Arthropod. Borne Dis. 2015;9(2):148. [PMC free article] [PubMed] [Google Scholar]
- Showler A.J., Boggild A.K. Cutaneous leishmaniasis in travellers: a focus on epidemiology and treatment in 2015. Curr. Infect. Dis. Rep. 2015;17(7):37. doi: 10.1007/s11908-015-0489-2. [DOI] [PubMed] [Google Scholar]
- Sofizadeh A., Vatandoost H., Rassi Y., Hanafi-Bojd A.A., Rafizadeh S. Spatial analyses of the relation between rodent's active burrows and incidence of zoonotic cutaneous leishmaniasis in Golestan province, northeastern of Iran. J. Arthropod. Borne Dis. 2016;10(4):569. [PMC free article] [PubMed] [Google Scholar]
- Zijlstra E.E. Visceral leishmaniasis: a forgotten epidemic. Arch. Dis. Child. 2016;101(6):561–567. doi: 10.1136/archdischild-2015-309302. [DOI] [PubMed] [Google Scholar]