Abstract
Background
Recruitment to pediatric randomised controlled trials (RCTs) can be a challenge, with ethical issues surrounding assent and consent. Pediatric RCTs frequently recruit from a smaller pool of patients making adequate recruitment difficult. One factor which influences recruitment and retention in pediatric trials is patient and parent preferences for treatment.
Purpose
To systematically review pediatric RCTs reporting treatment preference.
Methods
Database searches included: MEDLINE, CINAHL, EMBASE, and COCHRANE.
Qualitative or quantitative papers were eligible if they reported: pediatric population, (0–17 years) recruited to an RCT and reported treatment preference for all or some of the participants/parents in any clinical area. Data extraction included: Number of eligible participants consenting to randomisation arms, number of eligible patients not randomised because of treatment preference, and any further information reported on preferences (e.g., if parent preference was different from child).
Results
Fifty-two studies were included. The number of eligible families declining participation in an RCT because of preference for treatment varied widely (between 2 and 70%) in feasibility, conventional and preference trial designs. Some families consented to trial involvement despite having preferences for a specific treatment. Data relating to ‘participant flow and recruitment’ was not always reported consistently, therefore numbers who were lost to follow-up or withdrew due to preference could not be extracted.
Conclusions
Families often have treatment preferences which may affect trial recruitment. Whilst children appear to hold treatment preferences, this is rarely reported. Further investigation is needed to understand the reasons for preference and the impact preference has on RCT recruitment, retention and outcome.
Keywords: Randomised controlled trial, Recruitment, Pediatric, Parent, Treatment preference
1. Introduction
Successful recruitment and retention is crucial in randomised controlled trials (RCT) research [[1], [2], [3]]. Recruitment problems can delay or prevent trial completion [[4], [5], [6], [7], [8], [9], [10], [11]], and post-randomisation drop-out can lead to the loss of statistical power to measure differences between treatment arms [4,7,9,12,13]. Exploration of recruitment and retention issues in trials is extensive. Factors highlighted as important during the design and implementation phases of RCTs include: trial design, incentives, patient characteristics, support for recruiters, and patient and recruiter preferences for treatment [1,2,4,[14], [15], [16], [17], [18]].
If patients have a preference for treatment offered in an RCT they may decline randomisation to access treatment outside the trial. The external validity of an RCT may be compromised if patients with treatment preferences decline to participate, and bias is possible if uneven numbers of participants drop-out or cross-over between treatment arms [19,20]. Preferences can also affect adherence to treatment arms in RCTs where blinding to trial interventions is not possible [21,22]. Trials recruiting adult patients have reported treatment preference as a barrier to recruitment [[23], [24], [25]], but there it is a lack of evidence in relation to the ways in which preferences for trial interventions might affect recruitment and retention in pediatric trial settings [18].
Systematic reviews investigating the effects of treatment preference in RCTs have largely focused on trials recruiting adult patients [26,27]. A systematic review published in 2005 investigated the effects of participants' and professionals' preferences on recruitment, retention, and treatment outcomes. This review extracted data from 34 RCTs, but only four of the included trials had recruited pediatric-patients. Preferences were found not to significantly affect trial validity, but preferences did influence patients’ willingness to participate [26]. The second systematic review published in 2008 focused on musculoskeletal trials, extracting data from 18 RCTs none of which recruited pediatric patients [27]. This review investigated the effect of preference on attrition and outcomes but did not investigate the effect of treatment preference on recruitment. It found that patient preferences for treatment were associated with treatment effects.
We cannot assume treatment preferences will have the same impact on recruitment to pediatric trials as has been shown in adult trials. Pediatric trials involve the combined preferences of parent(s), patient and recruiting clinicians, in addition to a more complex consent process [28,29]. There will also be variation in the extent to which young people participate in decision-making and the recruitment process, depending on the nature and severity of their illness [[30], [31], [32], [33], [34], [35]]. The purpose of this systematic review was to identify pediatric RCTs where treatment preferences are reported, and describe the impact of preference on recruitment and retention.
2. Methods
A review protocol was developed and registered with PROSPERO: https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015015942. The review protocol also included methodology relating to the syntheses of qualitative data extracted from papers identified via this systematic literature search, which will be submitted for publication separately [36].
2.1. Study eligibility and inclusion criteria
Scoping exercises were used to define and refine relevant search terms using the PICOC model: Population, Intervention, Comparison, Outcomes and Context [37]. Qualitative sub-studies embedded in RCTs or quantitative primary and secondary outcome papers were eligible for inclusion if they reported RCTs recruiting new-borns, children and young people aged 0–17 years to an RCT, in any clinical area. Eligible papers were also required to report treatment preferences for all or some of the participants/parents. Database searches were limited to 1950–2014 inclusive.
2.2. Search strategy
A search strategy was developed with guidance from University of Bristol data specialists (NIHR/CLAHRC West and Cochrane Collaboration group), the search strategy can be found in Supplemental Information, Appendix A. Database searches of MEDLINE, CINAHL, EMBASE, and COCHRANE were carried out. Searches of relevant reference lists, databases containing registered clinical trials (https://www.ukctg.nihr.ac.uk/https://clinicaltrials.gov/ct2/home http://www.anzctr.org.au/TrialSearch.aspx) and work not published in peer-reviewed journals (http://proquest.umi.com/login) were carried out.
Authors were contacted to establish whether full RCT results had been published, two provided copies of their papers [38,39] and three confirmed that they had not [[40], [41], [42], [43]].
2.3. Screening and data extraction
Each title and abstract was screened independently for inclusion by two researchers using the systematic review platform Covidence [44]. Discrepancies were documented, discussed and resolved in regular meetings by reviewers and a senior member of the study management team (EC) to ensure eligibility criteria were understood and screening queries resolved consistently. At the full text review stage papers were read in chronological order by two researchers (LB and AB, HK, RL or RP). Author(s) extracted relevant numeric data and/or descriptive reports of treatment preference into an Excel template (see Supplemental Information, Appendix B).
3. Results
3.1. Summary of included studies
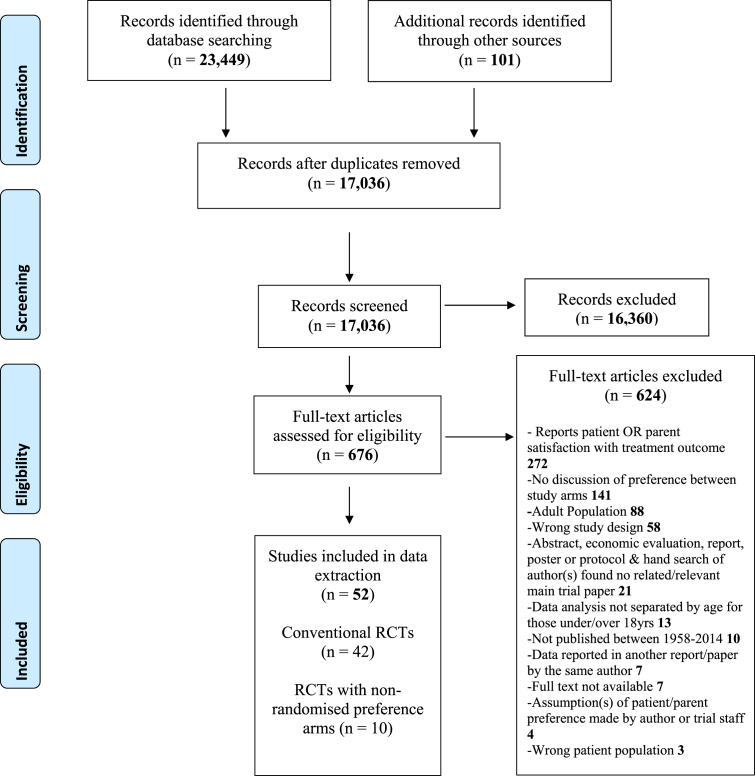
Database searches retrieved 23,449 papers, and additional searches yielded 101 papers. After deduplication, title and abstract screening was carried out on 17,036 papers, and 676 were read in full, with 52 papers eventually included in analyses (see Fig. 1). Table 1 describes the papers included in the systematic review. Twenty-seven papers reported data from RCTs conducted in the UK [28,29,[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]] and Europe [38,40,41,[60], [61], [62], [63], [64], [65], [66]], 16 from RCTs conducted in the USA and Canada [39,42,43,[67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79]], seven in countries outside of North America and Europe [30,[80], [81], [82], [83], [84], [85]], and two papers reported RCTs collecting data internationally [86,87]. Most papers were published from the year 2000 onwards (n = 42). Of the 52 papers included, 24 described ‘primary’ trial outcomes and 28 were ‘secondary’ papers which explored patient/parent experience of trial involvement, or reasons for declining, consenting, and recruitment. Searches were carried out to locate primary trial papers for secondary papers included in the review and 18 were located [[88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105]]. It was not possible to find all the primary trial papers because some secondary papers didn't explicitly use identifiable trial names or registration numbers. Of the 52 papers, seven reported findings from multiple trials [[28], [29], [30],39,48,52,70], and two were abstracts from poster presentations [40,41]. Forty-two of the papers reported ‘conventional’ RCTs [[28], [29], [30],[38], [39], [40], [41], [42], [43],[45], [46], [47],49,50,52,[54], [55], [56], [57], [58], [59], [60],62,64,65,[67], [68], [69], [70], [71],74,75,[77], [78], [79], [80], [81], [82], [83],[85], [86], [87]], two of which were in the feasibility or pilot stages [46,80]. Eight papers described RCTs with parallel ‘preference’ arms at trial outset [51,53,61,66,72,73,84,106], and two introduced preference arms due to slow recruitment [48,76].
Fig. 1.
PRISMA [107] Systematic search of literature reporting treatment preference in pediatric RCTs.
Table 1.
Included studies (n = 52).
| Conventional RCTs (n = 42) | |||
|---|---|---|---|
| Author | Paper type (primary or secondary papera) | Participant age | Aim |
| Allen 2013 [80] | Primary (Feasibility) | 13–17yrs | Assessed feasibility of recruiting young women into an RCT of caseload midwifery. |
| Allmark 2006 [45] | Secondary Primary paper Azzopardi 2009 [90] | ≥36wks | Compared intensive care plus total-body cooling for 72 h with intensive care without cooling among term infants with asphyxial encephalopathy. |
| Banks 2012 [46] | Primary (Pilot) | 5–16yrs | Assessed feasibility of carrying out a fully powered RCT comparing; care of childhood obesity intervention (COCO) and a primary care clinic intervention (PCC). |
| Barratt 2013 [81] | Secondary Primary paper Wake 2009 [104] | 5–10yrs | In-depth understanding of why families chose not to participate in a community-based study on childhood obesity. |
| Bauchner 1996 [67] | Primary | 3mth-6yrs | Do parents prefer antibiotic administration for treatment of acute otitis media by a single intramuscular (IM) injection or standard oral therapy for 10 days. |
| Blickman 2013 [68] | Primary | 1–12yrs | Assessed the impact of a Certified Child Life Specialist (CCLS) on parent satisfaction, staff satisfaction, child satisfaction, and parent and staff perceptions of child pain and distress in a pediatric imaging department. |
| Byrne-Davis 2010 [47] | Secondary Primary paper Vora 2013 [103] | 2–11yrs | Examined how recruitment looked to an observer and how it felt to parents, (of children with low-risk acute lymphoblastic leukemia) to identify how doctors' communication could promote or inhibit optimal recruitment. |
| Caldwell 2003 [30] | Secondary (Multiple RCTs) | Not stated | Explored parents' attitudes to children's participation in trials, identifying factors that influenced decision making and perceived risks and benefits. RCTs included oncology and renal: interventions not defined. |
| Carvalho 2013 [82] | Secondary Primary paper Moreira 2013 [99] | <3yrs | The understanding and perceptions of mothers regarding the informed consent and randomisation processes linked to an RCT that compared behavior management techniques for pediatric dental sedation. |
| Chappuy 2014 [38] | Secondary | Children - age not stated | Parental and child understanding of RCT participation (Acute lymphoblastic leukemia FRALLE 2000A protocol) and evaluations of the readability of written documents provided. |
| Duncan 2004 [69] | Primary | 11mths-12yrs | Effectiveness of osteopathic manipulation, acupuncture or wait list control as a 6-month therapeutic adjunct for children with spastic cerebral palsy. |
| Eiser 2005 [49] | Secondary Primary paper Mitchell 2005 [98] | 4–16yrs | Mothers' (of children newly diagnosed with Acute Lymphoblastic Leukemia: ALL) views regarding consent to randomised controlled trials. |
| Forsander 1995 [60] | Primary | 12–15yrs | Evaluation of family attitudes in relation to the two 3wk care systems for diabetes management: early discharge from ward to training apartment and treatment on a ward in pediatric clinic. |
| Glogowska 2001 [50] | Secondary Primary paper Glogowska 2000 [94] | 3–4yrs | Reported attitudes of parents whose child took part in a speech and language therapy RCT comparing immediate treatment and watchful waiting. |
| Harth 1990 [83] | Secondary Primary paper Van Asperen 1992 [101] | 6mths-3yrs | Double-blind, placebo-controlled trial of ketotifen, a new and unlicensed (for Australia) oral asthma drug. |
| Hissink Muller 2011 [40] | Secondary (poster presentation) Primary paper Hissink Muller 2017 [96] | Children - age not stated | Comparison of three treatment strategies, and feedback relating to treatment preferences among parents of patients with recent onset juvenile idiopathic arthritis. |
| Hissink Muller 2012 [41] | Secondary (poster presentation) Primary paper Hissink Muller 2017 [96] | 12–18yrs | Comparison of three treatment strategies, and feedback relating to equipoise among parents and patients with recent onset juvenile idiopathic arthritis. |
| Johnson 2007 [42] | Secondary | 10–18yrs (and adults) | Assessed participant and parent experiences in the parenteral insulin arm of the Diabetes Prevention Trial (DPT-Type 1). |
| Johnson 2009 [43] | Secondary | 10–18yrs (and adults) | Assessed the experiences of participants and parents of children in the oral insulin study of the Diabetes Prevention Trial (DPT-Type 1). |
| Jollye 2009 [52] | Secondary (Multiple RCTs) | Neonates | Explored the thoughts and feelings of parents in their decision-making process, in either choosing or declining to participate in neonatal RCTs. |
| Levi 2000 [70] | Secondary (Multiple RCTs) | 2–18yrs | Retrospective parent perceptions of communication of their child's cancer diagnosis and the informed consent process. |
| Miner 2007 [71] | Primary | 6mth-17yrs | To determine if nebulized fentanyl is a feasible alternative to IV fentanyl for the treatment of acute pain in children presenting to the emergency department (ED) with painful conditions. |
| Payne 2004 [54] | Secondary | 3–12yrs | Views and preferences for anesthetic related issues important to parents (and adults) who took part in a prospective RCT. |
| (PENTA) Paediatric European Network for Treatment of AIDS 1999 [86] | Secondary (double-blind) | Children - age not stated | Described parents' experience of their child being enrolled in a HIV infection RCT, including the degree to which it interfered with life, and their feelings about use of deferred (placebo) and immediate antiretroviral treatment. |
| Rovers 2000 [62] | Primary | 16-24mths | The effectiveness of ventilation tubes on the language development in infants with persistent otitis media with effusion (OME) compared to watchful waiting (WW). |
| Sammons 2007 [55] | Secondary Primary paper Atkinson 2007 [89] | 6mth-16yrs | Parental views on the informed consent process, information provided, reasons for taking part and willingness to participate in future research. Compared motives of British and European parents. |
| Sandler 2014 [56]z | Primary | 12–18yrs | Effectiveness of 3 methods of orthodontic anchorage supplementation, reporting orthodontists' and patients' values. |
| Sartain 2002 [57] | Primary | 6wks-12yrs | Assessed the clinical effectiveness of a pediatric hospital at home service compared to conventional hospital care. |
| Schuttelaar 2010 [64] | Primary | ≤16yrs | Compared the level of care from nurse practitioners with care delivered by dermatologists. |
| Sederberg-olsen 1998 [65] | Secondary (double blind) Primary paper Balle 1998 [91] | 1–10yrs | Evaluated the efficacy of amoxicillin-clavulanate and penicillin-V in the treatment of secretory otitis media (SOM). |
| Shilling 2011 [28] | Secondary (Multiple RCTs) MASCOT: funding extension application rejected & trial closed prematurely [97] MENDS [88] POPs [still recruiting] TIPIT [108] |
MASCOT: 6–15yrs MENDS: 3–15yrs POP: 4–18yrs TIPIT: < 28wks |
Identify strategies to improve recruitment and trial conduct, by comparing practitioners' and parents' accounts of the invitation to enter a child into clinical trials. |
| Snowdon 1997 [58] | Secondary Primary paper UK Collaborative ECMO Trial Group [95] | Neonates | Exploration of parental reactions to random allocation of treatment in a neonatal RCT comparing two methods of life support; conventional management (CM) and extracorporeal membrane oxygenation (ECMO). Recruitment was stopped early, because data showed a clear advantage with ECMO. |
| Spandorfer 2005 [74] | Primary Loss of clinical equipoise and declining accrual rates led to trial termination. | 8wk-3yrs | Compare oral rehydration therapy (ORT) and intravenous fluid therapy (IVF) in the treatment of viral gastroenteritis. |
| Sureshkumar 2012 [85] | Secondary Primary paper Craig 2009 [92] | <18yrs | To identify modifiable and unmodifiable factors associated with parental consent to a trial investigating long-term, low-dose antibiotics in preventing recurrent urinary tract infection. |
| Tercyak 1998 [75] | Secondary Primary paper Diabetes Control Complications Trial Research Group [93] | 11–18yrs | Identify reasons/characteristics of adolescents who refuse or consent to participate in an RCT of intensive therapy (IT) for insulin-dependent diabetes mellitus. |
| Willey 2005 [59] | Primary | 4–16yrs | Efficacy of oral or rectal route administered analgesia for post-operative pain. |
| Williams 2013 [77] | Primary | 2–17yrs | Compared cast versus splint for distal radial buckle fractures in children in terms of parental and patient satisfaction, convenience and preference. |
| WoodgateZ 2010 [39] | Secondary (Multiple RCTs) | 6mth-15yrs | In-depth understanding of Canadian parents' participation in decisions about childhood cancer clinical trials. |
| Woolfall 2013 [29] | Secondary (Multiple RCTs) MASCOT [97] funding extension application rejected & trial closed prematurely. MENDS [88] POPs [still recruiting] TIPIT [108] |
MASCOT: 6–15yrs MENDS: 3–15yrs POP: 4–18yrs TIPIT: < 28wks |
Explored how a parent's understanding of a trial might be associated with the way that the trial was explained during the discussion with a practitioner. |
| Wright 2005 [87] | Primary Recruitment was expected to take 3yrs but took 6yrs. |
4–10yrs | Investigated early application hip spica compared with external fixation in pediatric femoral fractures. Recruitment was expected to take 3yrs but took 6yrs. |
| Wynn 2010 [78] | Secondary Primary paper Wang 2011 [105] |
<18mths | In response to slow recruitment study coordinators evaluated factors that affected enrollment and accrual. |
| Young 2006 [79] | Secondary | 7–17yrs | Reported results of two studies of social phobia, assessing the extent to which parental reluctance toward medication resulted in pre-treatment attrition in; behavioural, fluoxetine and placebo groups. |
| RCTs with non-randomised preference arms (n = 10) | |||
| Cunningham 2011 [48] | Secondary Trial 1: preference arm added and trial terminated early due to inadequate sample size. | Adolescents (age not stated) | Reported two RCTs, both terminated early due to inadequate sample size. Trial 1: Multi-center Orthodontic RCT which compared two different methods of treating a specific type of malocclusion in adolescents. (Trial 2: RCT, no preference data). |
| Gowers 2010 [51] | Primary | 12–18yrs | Compared the clinical effectiveness of inpatient against outpatient treatment and of generalist against specialist management. |
| Lock 2010 [53] | Primary Trial extended from 5 to 7yrs to increase patient recruitment. | 4–15yrs | An embedded qualitative study informed the development of the RCT, it explored patient/parent(s) preferences for different treatment options in patients with recurrent sore throats who had recently been referred to ENT clinic. Extended from 5 to 7yrs to increase patient recruitment. |
| Mattila 2007 [61] | Primary | ≤2yrs | Assessed adenoidectomy in connection with tympanostomy compared with tympanostomy only in preventing otitis media in children. |
| Paradise 1984 [72] | Primary | 3–15yrs | Assessed the efficacy of tonsillectomy and adenoidectomy. |
| Paradise 1990 [73] | Primary | 1–15yrs | Assessed the efficacy of adenoidectomy, comparing surgical and non-surgical management, with equivalent non-randomised preference arms. |
| Reddihough 1998 [84] | Primary | 12-36mths | Compared conductive education (CE) program with equivalent intensity traditional neurodevelopmental programs of rehabilitation for young children with Cerebral Palsy. |
| Rovers 2001 [106] | Primary | 9–12mths | Compared ventilation tubes (VT) and watchful waiting (WW) in the management of patients with otitis media with effusion. The generaliszability of randomised patients with eligible non-randomised patients was studied via preference arms. |
| Weinstein 2013 [76] | Primary Preference arms added after 3yrs of recruitment. | 10–15yrs | The effectiveness of bracing, compared with observation in preventing progression of the curve to 50° or more in idiopathic scoliosis patients, with equivalent non-randomised preference arms. |
| Van Wijk 2014 [66] | Secondary Primary paper Van Wijk 2014 [102] | 4.5–6.5mths | Primary: Effectiveness of helmet therapy for positional skull deformation compared with the natural course of the condition Secondary: Assess parents' decision for helmet therapy in infants with skull deformation. |
Primary papers were defined as those reporting primary RCT outcome(s). Secondary papers were those reporting embedded/related studies (e.g. qualitative) describing patient/parent experience of trial involvement, reasons for decline, consenting and recruitment.
3.2. Impact of treatment preference on recruitment – conventional RCTs
Table 2 describes data on preference from all included papers. Seventeen papers reported the number of eligible families declining participation because of a preference for treatment, this ranged from 2 to 50% in conventional trials [49,[54], [55], [56], [57],64,65,68,69,74,75,78,79,83,[85], [86], [87]], and 4–70% in the two pilot/feasibility phase trials [46,80]. Eleven RCTs reported the preferences of families who opted for trial participation [38,[40], [41], [42], [43],49,[58], [59], [60],67,77], these treatment preferences were either expressed at enrolment or after randomisation. Five trials reported withdrawal after randomisation [57,62,71,74,82]. Families either withdrew consent or refused their allocated intervention, but only one of these trials specifically attributed this to a preference for the alternate treatment arm [71].
Table 2.
Number of eligible participants recruited to trial, and those not randomised due to treatment preference.
| Conventional RCTs (n = 42) | |||
|---|---|---|---|
| Author | Number of eligible participants consenting to randomisation arms | Number of eligible patients not randomised because of treatment preference n (%) | Is preference expressed by patients (in addition to parents) |
| Allen 2013 | 1 (10%) (Feasibility) | 7 (70%) | Yes (only patient preference reported) |
| Allmark 2010 | 325 (81%) | Unclear, preference reported qualitatively [45] ‘30 declined’ ‘45 other reasons’ [90] |
n/a neonates |
| Banks 2012 | 76 (50%) (Pilot) | 6 (4%) | No |
| Barratt 2013 | 258 (33%) | Not reported. 9 (26%) of non-responders reported concern with being in either the intervention or control group, but only 37/305 non-responders replied to question. |
No |
| Bauchner 1996 | 648 (total eligible not reported) | Not reported. Parents were asked their preference at enrollment and 551 (85%) of those randomised preferred single-dose therapy over standard therapy. |
n/a children under 6yrs |
| Blickman 2013 | 142 (88%) | 4 (2%) | Unclear (patients aged 4yrs + were asked to complete a standardised study instrument) |
| Byrne-Davis 2010 | 521 (71%) [103] | Not reported, preference reported qualitatively [47] 215 (29%) not randomly assigned; 97 refused, 7 had Down's syndrome, 4 because of toxic effects, 28 other reason, 79 unknown [103] |
No |
| Caldwell 2003 | Not reported (multiple trials) | Not reported, preference reported qualitatively. | Participant age not stated. |
| Carvalho 2013 | Unclear 48 'recruited' [82] 44 (100%) 'randomised' [99] | Not reported, preference reported qualitatively [82] 3 (7%) parents refused allocated interventions post-randomisation in x 2 trial arms [99] |
No |
| Chappuy 2014 | Not reported | Not reported. Some Parents felt that standard treatment was the best arm for their child because it was less risky |
Participant age not stated. |
| Duncan 2004 | 50 different participants randomised. Total eligible not reported. | 8 (between 12 and 16%) | No |
| Eiser 2005 |
1621 (90%) [98] | 181 (10%) declined randomisation (opted for PRED; 165 DEXA; 16) [98] Preference reported qualitatively, 16 (32%) ‘agreed reluctantly to randomisation’ [49]. |
No |
| Forsander 1995 | 38 (93%) | Not reported Immediately after randomisation 3 families in the control arm reported that they would have preferred the family therapeutic care arm. |
No |
| Glogowska 2001 | 159 (69%) [94] | Not reported, preference reported qualitatively [50] Declined trial in total 70 (31%) [94] |
n/a children under 4yrs |
| Harth 1990 | 72 (55%) | 40 (30%) families declined because of ‘concern about side effects of the new drug’ (ketotifen) 60 declined in total. | n/a children under 3yrs |
| Hissink Muller 2011 | Not reported | Not reported. 41% participating parents reported a preference for therapy with methotrexate and etanercept and 6% had hoped against assignment to this group. Primary aversion was highest (25%) in the prednisone group [40]. Declined trial n = 38 (29%) [96]. |
No |
| Hissink Muller 2012 | Not reported | Not reported. 65% participating parents reported a preference for therapy with etanercept. 5 parents and 2 patients participated in the study to access treatment with etanercept, as initial treatment was not possible nor reimbursed in daily practice. |
Yes |
| Johnson 2007 | Not reported | Not reported. Participating families stated: Close monitoring arm - 27% parents and 70% participants were glad to be in that arm. 74% parents and 35% participants sometimes wished they had been assigned the intervention arm. Intervention arm - 53% parents and 21% participants were glad to be in that arm. 25% parents and 47% participants sometimes wished they had been assigned the closely monitored arm. |
Yes |
| Johnson 2009 | Not reported | Not reported. Participating families were blinded to treatment but were asked which treatment arm they would have preferred. 60% parents and 53% participants chose the capsule condition. 8% parents and 21% participants chose the no intervention condition. Very few participants and parents (3%) chose the insulin injection condition. |
Yes |
| Jollye 2009 | Not reported, multiple trials. | Not reported, preference reported qualitatively. | n/a neonates |
| Levi 2000 | Not reported, multiple trials. | Unclear. 3 (13.6%) stated they declined participation because they felt more comfortable with a “tried and true” method. |
No |
| Miner 2007 | 41 (82%) | Unclear. Declined randomised 9 (18%) reasons not reported. After allocation 4 (10%) parents requested that their child receive nebulized fentanyl rather than the assigned IV fentanyl. |
No |
| Payne 2004 | Unclear Calculated as; 322 (69%) of eligible patients. Paper reports recruitment rate of 75% |
59 (50%) ‘Around half of the eligible participants who refused to participate did so because there was a 50% chance of the child being randomised to the inhalational induction arm’. | No |
| (PENTA) Paediatric European Network for Treatment of AIDS 1999 | 197 | 4 (3%) parents stated explicitly that they were concerned with the use of placebo. | No |
| Rovers 2000 | 187 | Not reported. 19 (10%) parents withdrew consent straight after randomisation (15 in ventilation tubes arm and 4 in watchful waiting arm). 10 (5%) children in the watchful waiting arm were treated with ventilation tubes. |
n/a children under 2yrs |
| Sammons 2007 | Unclear 245 'randomised’ [55] 252 (85%) ‘randomised’ [89] |
25 (9%) declining families stated they wanted a specific treatment (IV; 20 or oral; 5) [55] 43 (15%) declined to take part; n = 6 (2%) excluded post randomisation reasons: 4 withdrawn by parents/2 by clinician (no further detail provided) [89]. |
No |
| Sandler 2014 | 78 (87%) | 7 (8%) Three did not want to wear headgear for anchorage, three did not want the Nance button palatal arches, but only one patient did not want to take part because he or she was unhappy at “the thought of temporary anchorage devices”. | No |
| Sartain 2002 | 399 (86%) | 10 (2%) 7 families withdrew from ‘hospital care’ arm because they wanted the ‘hospital at home’ arm |
Yes |
| Schuttelaar 2010 | 160 | 4 (2%) Preferred only dermatologist (n = 2), preferred only nurse practitioner (n = 2). | No |
| Sederberg-olsen 1998 | 429 | 120 (10%) parents insisted that the child had grommet insertion performed at the time of randomisation. | No |
| Shilling 2011 | MASCOT: 63 [97] MENDS: 146 (84%) [88] POP: [still recruiting] TIPIT: 153 (57%) [108] |
Unclear, preference reported qualitatively. MASCOT Assessed for eligibility (n = 898), Not registered (n = 732), Excluded (n = 103) [97]. MMENDS 27 (16%) assessed for eligibility but not randomised: ‘declined 11’ ‘other 16’ [88]. TIPIT 57 (21%) assessed for eligibility but not randomised: ‘refused’ [105]. |
Yes |
| Snowdon 1997 | 185 (79%) [95] | Unclear. ‘majority of parents had a keen preference for ECMO treatment arm’. Preference reported qualitatively [58]. 48 (21%) were registered but not randomised; 14 died, 19 improved and 15 parents refused trial participation [95]. |
n/a neonates |
| Spandorfer 2005 | 73 | 24 (7%) A further 3 parents refused participation after randomisation to oral rehydration therapy before starting treatment. |
n/a children under 3yrs |
| Sureshkumar 2012 | 412 (37%) [85] | 214 (19%) Prefer antibiotics 71/Prefer no antibiotics 143 [85]. Primary paper reports patients excluded because ‘participation refused by parent’ 1935 [92] |
No |
| Tercyak 1998 | 56 | 2 (5%) | Yes (only patient preference reported) |
| Willey 2005 | 31 | Not reported. 19/31 patients completed a preference questionnaire/10 (43%) preference for oral, 2 (9%) for suppositories, 7 (30%) no preference/preference for oral more pronounced among girls 5 (83%). |
Yes |
| Williams 2013 | 94 | Not reported. A significantly larger percentage of parents and patients in the cast group reported that they would not choose the same method of immobilization again at all time points (baseline, days; 1, 3, 7, 21 after injury). |
No |
| Woodgate 2010 | Not reported (multiple trials) | Not reported, preference reported qualitatively. | n/a neonates |
| Woolfall 2013 | MASCOT: 63 [97] MENDS: 146 (84%) [88] POP: [still recruiting] TIPIT: 153 (57%) [108] |
Unclear, preference reported qualitatively. MASCOT Assessed for eligibility (n = 898), Not registered (n = 732), Excluded (n = 103) [97]. MMENDS 27 (16%) assessed for eligibility but not randomised: ‘declined 11’ ‘other 16’ [88]. TIPIT 57 (21%) assessed for eligibility but not randomised; ‘refused’ [105]. |
No |
| Wright 2005 | 108 (46%) | 41 (33%) | No |
| Wynn 2010 | 234 (29%) | 2% unwilling to take placebo. | n/a children under 2yrs |
| Young 2006 | Not reported. | 125 ‘Reluctance toward medication treatment accounted for 44.7% of study refusals and was disproportionately common among ethnic minority families’. |
No |
| RCTs with non-randomised preference arms (n = 10) | |||
| Cunningham 2011 | Not reported. (multiple trials) | Not reported. A small number of patients who were eligible declined the trial as they had a treatment preference. These were patients allocated to both intervention groups, so one treatment option was not preferred to the other. Preference arms added. |
Unclear |
| Gowers 2010 | 170 (68%) | 28 (11%) Not randomised, patient preference. |
Yes |
| Lock 2010 | 268 (26%) | 286 (28%) declined any follow up, authors assumed that all had a patient preference. 461 (45%) opted for preference arms in cohort. |
Only in qualitative sample. Authors did not attempt to differentiate between parent/child preferences in RCT/preference samples. |
| Mattila 2007 | 137 (45%) | 169 (55%) opted for preference arms. | n/a children under 2yrs |
| Paradise 1984 | 91 (49%) | 96 (51%) opted for preference arms. | No |
| Paradise 1990 | 99 (46%) | 114 (54%) opted for preference arms. | No |
| Reddihough 1998 | 34 (49%) | 32 (46%) declined randomisation. | n/a children under 3yrs |
| Rovers 2001 |
187 (48%) | 133 (34%) opted for non-randomised cohort arms. 66 (17%) refused randomisation/follow up via cohort. |
n/a children under 1yrs |
| Van Wijk 2014 | 84 (21%) | 186 (46%) opted for preference arms. | n/a children under 1yrs |
| Weinstein 2013 | 155 (14%) | 228 (21%) opted for preference arms. 297 (27%) declined all follow-up due to preference. 216 (20%) no to randomisation. |
No |
3.3. Impact of treatment preference – RCTs with non-randomised preference arms
Eight papers reported RCTs which used non-randomised ‘preference arms’ in addition to randomised treatment arms from the outset [51,53,61,63,72,73,84,102]. All of these trials reported the number of eligible families declining randomisation arms because of a preference for treatment, this ranged from 11 to 55%. One of these trials was extended by two years to increase recruitment to randomised trial arms [53]. Two additional trials introduced preference arms because families declined participation because of preferences for treatment [48,76].
3.4. Patient or parent preference
Nine papers explicitly reported the treatment preferences of patients, as well as their parents [28,[41], [42], [43],53,57,59,75,80]. Child/parental views on a preferred treatment arm differed on three occasions [28,42,53]. Twelve papers reported findings from trials involving children under the age of six years, so did not include information on preference from children [45,50,58,[61], [62], [63],67,68,74,78,83,84].
3.5. Clinician preferences for trial treatments
Most studies did not comment on why families held a treatment preference, but six papers reported different forms of clinician preference for a particular treatment which may have influenced patient preference [28,41,63,77,84,85]. Two trials stated that staff experienced discomfort with children's medication/intervention being selected by a process of randomisation [28,84], one highlighted that ‘consent was more likely when the recruiting physician was a member of the research team’ [85] and in another, a parent whose child was randomised to a splint treatment arm was told the day after randomisation by a clinician outside the RCT that ‘all buckle fractures need to be casted’ [77]. Finally, one trial reported that parents who refused randomisation did so because of; ‘a desire to have decisional control, and they trusted their physician's choice of treatment more than a computer's choice’ [109]. These findings suggest that recruiters and treating clinicians may be an important influence on parent and patient treatment preferences when families consider RCT participation.
4. Discussion
To our knowledge, this is the first systematic review that has specifically investigated whether treatment preference influences recruitment into pediatric trials. The review has shown that families often have preferences for treatment at recruitment, and some families consent to trial involvement despite having preferences for a specific treatment. The number of eligible families declining participation in an RCT because of preference for treatment varied widely: From 2 to 70% in feasibility RCTs, from 2 to 50% in conventional main RCTs, and from 11 to 55% in trials with preference arms. Declining accrual rates and a loss of clinical equipoise led to the closure of two trials [48,74], and two required extensions because of slow recruitment [53,87].
Several trials included in this systematic review introduced preference arms to improve recruitment. Patient preference trials (PPTs) and comprehensive cohort designs [110,111], (in which participants with a preference are offered their treatment of choice, and those without a preference have their treatment allocated randomly) offer the opportunity to investigate the effects of preference on recruitment, validity and treatment outcome [26,27,112]. Although this is one way of dealing with patients’ preferences for treatment, this design has a number of disadvantages. PPTs often require larger numbers of patients. In extending trial duration to meet recruitment targets for the randomised arms, they may reduce external validity and generalisability of results. Also, such designs do not necessarily improve informed consent [53,110,111,[113], [114], [115]].
A key strength of this review is that a large number of papers were screened for inclusion by two reviewers at all stages in the review process. This review was enriched by the inclusion of a wide range of papers, including data from papers reporting primary trial outcomes, and papers reporting qualitative findings on patient or parent experiences of trial involvement, and reasons for decline, consenting and recruitment. Limitations include the fact that seven papers reported findings from multiple trials in one paper [[28], [29], [30],39,48,52,70], and many of the papers reporting qualitative findings did not include full CONSORT flow diagrams, therefore data on those who were lost to follow-up or withdrew due to preference could not be extracted. The effect that treatment preference has on retention in pediatric trials requires further investigation. If trial acronyms or references were provided in secondary papers, we carried out a search for each related primary RCT outcome paper, but only 18/28 additional papers were located. Data relating to ‘participant flow and recruitment’ was not always reported consistently in primary RCT outcome papers. One paper reported that 76 participants were allocated to treatment arms, but only 68 then entered the RCT, presumably eight withdrew post-randomisation but reasons for this were not provided [46]. A lack of standardised detail in the reporting of recruitment and retention methodology in RCTs has also been highlighted previously in a systematic review of behavioural interventions recruiting dyads (adult patients and their support person) [116].
Parental reasons for strongly held treatment preferences include concerns about side effects and attitudes towards new ‘experimental’ or ‘placebo’ interventions [55,117,118]. Although altruism is often cited as a reason for RCT participation, there is also poor parental understanding of the process of randomisation and perceived personal benefit for their child [14,119]. In pediatric trials, parents and children are often both involved in receiving information about the trial and making a decision about whether to take part, with support from a recruiting clinician [120,121]. Our findings showed that parents' preferences are reported more frequently than children's preferences. Only nine papers reported child preference, even though the majority of included trials were conducted with children and young people who were old enough to assent to RCT involvement and express their views on treatment.
Children's preferences for treatment differed from parental views on three occasions [28,42,53]. Older children and teenagers have reported different views from their parents on the acceptability of treatment and participation in asthma research protocols [122]. This is not consistent with guidance suggesting young people's voices need to be more widely heard [35,123], or approaches to communication which aim to support personal autonomy instead of isolated ‘independence’ of choice in decision-making [124,125].
Although this systematic review was not seeking to report clinician preference for treatment in pediatric RCTs, a small number of studies did report that members of the recruiting/treating teams held preferences. The impact of clinician preference has been described as affecting pediatric trials [26,126]. In one trial 63% of parents said the doctors recruiting them had influenced their decision to participate [55]. Clinician preference has also been shown to influence recruitment in adult trials [4,[127], [128], [129]]. More research should be carried out to investigate the influence of recruiting professionals’ preferences for treatment on the decision-making process of families.
5. Conclusions
This systematic review shows that treatment preference can be a barrier to recruitment to pediatric RCTs. In some cases this can result in the need to change the design of the trial (introduction of preference arms), extend recruitment or result in trial closure. Further investigation is needed to understand the impact treatment preference has on retention, and on the outcomes under investigation in pediatric trials. Exploration of the reasons for parent and child preferences would also be beneficial to ensure that families are fully informed when making decisions about RCT participation.
Conflicts of interest
The authors have no conflicts of interest relevant to this article to disclose.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Funding source
This work was undertaken with the support of the Medical Research Council (MRC) ConDuCT-II Hub (Collaboration and innovation for Difficult and Complex randomised controlled Trials In Invasive procedures - MR/K025643/1).
Contributors’ Statement Page
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100335.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bower P. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials. 2014;15:399. doi: 10.1186/1745-6215-15-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treweek S. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2) doi: 10.1136/bmjopen-2012-002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healy P. Identifying trial recruitment uncertainties using a james lind alliance priority setting partnership - the PRioRiTy (prioritising recruitment in randomised trials) study. Trials. 2018;19(1):147. doi: 10.1186/s13063-018-2544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross S. Barriers to participation in randomised controlled trials: a systematic review. J. Clin. Epidemiol. 1999;52(12):1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 5.Paramasivan S. Key issues in recruitment to randomised controlled trials with very different interventions: a qualitative investigation of recruitment to the SPARE trial (CRUK/07/011) Trials. 2011;12:78. doi: 10.1186/1745-6215-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard L. Why is recruitment to trials difficult? An investigation into recruitment difficulties in an RCT of supported employment in patients with severe mental illness. Contemp. Clin. Trials. 2009;30(1):40–46. doi: 10.1016/j.cct.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toerien M. A review of reporting of participant recruitment and retention in RCTs in six major journals. Trials. 2009;10:52. doi: 10.1186/1745-6215-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treweek S. Meeting the challenges of recruitment to multicentre, community-based, lifestyle-change trials: a case study of the BeWEL trial. Trials. 2013;14:436. doi: 10.1186/1745-6215-14-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald A.M. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schandelmaier S. Premature discontinuation of randomized trials in critical and emergency care: a retrospective cohort study. Crit. Care Med. 2016;44(1):130–137. doi: 10.1097/CCM.0000000000001369. [DOI] [PubMed] [Google Scholar]
- 11.Sully B.G., Julious S.A., Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials. 2013;14:166. doi: 10.1186/1745-6215-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everitt B.S. Analysis of drop-out data in treatment trials. Br. J. Psychiatry. 1998;173:271. doi: 10.1192/bjp.173.3.271a. [DOI] [PubMed] [Google Scholar]
- 13.Mills E.J. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 14.Wulf F., Krasuska M., Bullinger M. Determinants of decision-making and patient participation in paediatric clinical trials: a literature review. Open J. Pediatr. 2012;2:1–17. [Google Scholar]
- 15.Rick J. Systematic techniques for assisting recruitment to trials (START): study protocol for embedded, randomized controlled trials. Trials. 2014;15(1):407. doi: 10.1186/1745-6215-15-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houghton C. Factors that impact on recruitment to randomised trials in health care: a qualitative evidence synthesis (Protocol) Cochrane Database Syst. Rev. 2017;(5) doi: 10.1002/14651858.MR000045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan J.L. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials. 2014;15:5. doi: 10.1186/1745-6215-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treweek S. Strategies to improve recruitment to randomised trials. Cochrane Database Syst. Rev. 2018;2 doi: 10.1002/14651858.MR000013.pub6. MR000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McPherson K., Britton A. The impact of patient treatment preferences on the interpretation of randomised controlled trials. Eur. J. Cancer. 1999;35(11):1598–1602. doi: 10.1016/s0959-8049(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 20.King M. Conceptual framework and systematic review of the effects of participants' and professionals' preferences in randomised controlled trials. Health Technol. Assess. 2005;9(35):1–186. doi: 10.3310/hta9350. iii-iv) [DOI] [PubMed] [Google Scholar]
- 21.Torgerson D.J., Klaber-Moffett J., Russell I.T. Patient preferences in randomised trials: threat or opportunity? J. Health Serv. Res. Pol. 1996;1(4):194–197. doi: 10.1177/135581969600100403. [DOI] [PubMed] [Google Scholar]
- 22.Steidtmann D. Patient treatment preference as a predictor of response and attrition in treatment for chronic depression. Depress. Anxiety. 2012;29(10):896–905. doi: 10.1002/da.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donovan J. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ. 2002;325(7367):766–770. doi: 10.1136/bmj.325.7367.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donovan J. Prostate testing for cancer and treatment (ProtecT) feasibility study. Health Technol. Assess. 2003;7(14):1–88. doi: 10.3310/hta7140. [DOI] [PubMed] [Google Scholar]
- 25.Donovan J.L. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J. Clin. Epidemiol. 2009;62(1):29–36. doi: 10.1016/j.jclinepi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 26.King M. Impact of participant and physician intervention preferences on randomized trials: a systematic review. J. Am. Med. Assoc. 2005;293(9):1089–1099. doi: 10.1001/jama.293.9.1089. [DOI] [PubMed] [Google Scholar]
- 27.Preference Collaborative Review Group . vol. 337. 2008. (Patients' Preferences within Randomised Trials: Systematic Review and Patient Level Meta-Analysis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shilling V. Processes in recruitment to randomised controlled trials of medicines for children (RECRUIT): a qualitative study. Health Technol. Assess. 2011;15(15):1–116. doi: 10.3310/hta15150. [DOI] [PubMed] [Google Scholar]
- 29.Woolfall K. Parents' agendas in paediatric clinical trial recruitment are different from researchers' and often remain unvoiced: a qualitative study. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0067352. e67352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caldwell P.H., Butow P.N., Craig J.C. Parents' attitudes to children's participation in randomized controlled trials. J. Pediatr. 2003;142(5):554–559. doi: 10.1067/mpd.2003.192. [DOI] [PubMed] [Google Scholar]
- 31.Caldwell P.H. Clinical trials in children. Lancet. 2004;364(9436):803–811. doi: 10.1016/S0140-6736(04)16942-0. [DOI] [PubMed] [Google Scholar]
- 32.Coyne I. Children's participation in consultations and decision-making at health service level: a review of the literature. Int. J. Nurs. Stud. 2008;45(11):1682–1689. doi: 10.1016/j.ijnurstu.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Pritchard-Jones K., Europe S. Clinical trials for children with cancer in Europe - still a long way from harmonisation: a report from SIOP Europe. Eur. J. Cancer. 2008;44(15):2106–2111. doi: 10.1016/j.ejca.2008.07.026. Oxford, England : 1990. [DOI] [PubMed] [Google Scholar]
- 34.Simon C.M. Comparison of the informed consent process for randomized clinical trials in pediatric and adult oncology. J. Clin. Oncol. 2004;22(13):2708–2717. doi: 10.1200/JCO.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Nuffield Council on Bioethics . Nuffield Council on Bioethics: London ESP Colour Ltd; 2015. Children and Clinical Research: Ethical Issues. [Google Scholar]
- 36.Beasant L. 2015. A Systematic Review and Qualitative Synthesis of Literature Reporting Treatment Preference in Paediatric Randomised Controlled Trials. [Google Scholar]
- 37.Petticrew M., Roberts H. Blackwell Publishing; USA: 2006. (Systematic Reviews in the Social Sciences A PRACTICAL GUIDE). 350 Main Street, Malden, MA 02148-5020. [Google Scholar]
- 38.Chappuy H.F., A. C, De Haut De Sigy A. Parental comprehension and decision in informed consent in pediatric clinical trials. [French] Revue d'Oncologie Hematologie Pediatrique. 2014;2(2014376989):65–69. [Google Scholar]
- 39.Woodgate R.L., Yanofsky R.A. Parents' experiences in decision making with childhood cancer clinical trials. Cancer Nurs. 2010;33(1):11–18. doi: 10.1097/NCC.0b013e3181b43389. [DOI] [PubMed] [Google Scholar]
- 40.Hissink Muller P.C.E. Parental preferences for treatment: preliminary report from a randomised comparison of treatment strategies in (early) juvenile idiopathic arthritis (BeSt for Kids trial) Pediatr. Rheumatol. 2011;9:8. [Google Scholar]
- 41.Hissink Muller P.C.E. Randomized clinical trial in pediatric rheumatology: are parents and patients in equipoise? Arthritis Rheum. 2012;64:S849. [Google Scholar]
- 42.Johnson S.B. Participant and parent experiences in the parenteral insulin arm of the diabetes prevention trial for type 1 diabetes. Diabetes Care. 2007;30(9):2193–2198. doi: 10.2337/dc06-2422. [DOI] [PubMed] [Google Scholar]
- 43.Johnson S.B. Participant and parent experiences in the oral insulin study of the diabetes prevention trial for type 1 diabetes. Pediatr. Diabetes. 2009;10(3):177–183. doi: 10.1111/j.1399-5448.2008.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Covidence . 2013. A Product by Alfred Health. [Google Scholar]
- 45.Allmark P., Mason S. Improving the quality of consent to randomised controlled trials by using continuous consent and clinician training in the consent process. J. Med. Ethics. 2006;32(8):439–443. doi: 10.1136/jme.2005.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks J. Evaluating the transferability of a hospital-based childhood obesity clinic to primary care: a randomised controlled trial. Br. J. Gen. Pract. 2012;62(594):e6–12. doi: 10.3399/bjgp12X616319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrne-Davis L.M. Balancing high accrual and ethical recruitment in paediatric oncology: a qualitative study of the 'look and feel' of clinical trial discussions. BMC Med. Res. Methodol. 2010;10:101. doi: 10.1186/1471-2288-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham S. In search of the sample: recent experiences of a trial team in orthodontics. Contemp. Clin. Trials. 2011;32(4):530–534. doi: 10.1016/j.cct.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Eiser C. Mothers' attitudes to the randomized controlled trial (RCT): the case of acute lymphoblastic leukaemia (ALL) in children. Child Care Health Dev. 2005;31(5):517–523. doi: 10.1111/j.1365-2214.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 50.Glogowska S.R. Who's afraid of the randomised controlled trial? Parents' views of an SLT research study. Int. J. Lang. Commun. Disord. 2001;36(s1):499–504. doi: 10.3109/13682820109177936. [DOI] [PubMed] [Google Scholar]
- 51.Gowers S.G. A randomised controlled multicentre trial of treatments for adolescent anorexia nervosa including assessment of cost-effectiveness and patient acceptability - the TOuCAN trial. Health Technol. Assess. 2010;14(15):1–98. doi: 10.3310/hta14150. [DOI] [PubMed] [Google Scholar]
- 52.Jollye An exploratory study to determine how parents decide whether to enrol their infants into neonatal clinical trials. J. Neonatal Nurs. 2009;15:18–24. [Google Scholar]
- 53.Lock C. North of England and Scotland Study of Tonsillectomy and Adeno-tonsillectomy in Children(NESSTAC): a pragmatic randomised controlled trial with a parallel non-randomised preference study. Health Technol. Assess. 2010;14(13):1–164. doi: 10.3310/hta14130. (iii-iv) [DOI] [PubMed] [Google Scholar]
- 54.Payne K. Day-case anaesthesia: what would the patient prefer? Clin. Manag. 2004;12(3):133–139. [Google Scholar]
- 55.Sammons H.M. What motivates British parents to consent for research? A questionnaire study. BMC Pediatr. 2007;7:12. doi: 10.1186/1471-2431-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandler J. Effectiveness of 3 methods of anchorage reinforcement for maximum anchorage in adolescents: a 3-arm multicenter randomized clinical trial. Am. J. Orthod. Dentofacial Orthop. 2014;146(1):10–20. doi: 10.1016/j.ajodo.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Sartain S.A. Randomised controlled trial comparing an acute paediatric hospital at home scheme with conventional hospital care. Arch. Dis. Child. 2002;87(5):371–375. doi: 10.1136/adc.87.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snowdon C., Garcia J., Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Soc. Sci. Med. 1997;45(9):1337–1355. doi: 10.1016/s0277-9536(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 59.Willey S.E., Griffiths D.M., Nightingale J.J. Prospective randomised controlled trial comparing rectal versus oral paracetamol and diclofenac in children following appendicectomy. Acute Pain. 2005;7(1):33–35. [Google Scholar]
- 60.Forsander G. Family attitudes to different management regimens in diabetes mellitus. Practical Diabetes International. 1995;12(2):80–85. [Google Scholar]
- 61.Mattila V.M., Parkkari J., Rimpela A. Adolescent survey non-response and later risk of death. A prospective cohort study of 78609 persons with 11 year follow-up. BMC Public Health. 2007;7 doi: 10.1186/1471-2458-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rovers M.M. The effect of ventilation tubes on language development in infants with otitis media with effusion: a randomized trial. Pediatrics. 2000;106(3):E42. [PubMed] [Google Scholar]
- 63.Rovers M.M. Generalizability of trial results based on randomized versus nonrandomized allocation of OME infants to ventilation tubes or watchful waiting. J. Clin. Epidemiol. 2001;54(8):789–794. doi: 10.1016/s0895-4356(01)00340-7. [DOI] [PubMed] [Google Scholar]
- 64.Schuttelaar M.L. A randomized controlled trial in children with eczema: nurse practitioner vs. dermatologist. Br. J. Dermatol. 2010;162(1):162–170. doi: 10.1111/j.1365-2133.2009.09502.x. [DOI] [PubMed] [Google Scholar]
- 65.Sederberg-Olsen J. Problems in recruiting patients to controlled trials on children with secretory otitis media: a demographic comparison of excluded versus included patients. Int. J. Pediatr. Otorhinolaryngol. 1998;43(3):229–233. doi: 10.1016/s0165-5876(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 66.van Wijk R.M. Parents' decision for helmet therapy in infants with skull deformation. Childs Nerv Syst. 2014;30(7):1225–1232. doi: 10.1007/s00381-014-2399-2. [DOI] [PubMed] [Google Scholar]
- 67.Bauchner H. Therapy for acute otitis media. Preference of parents for oral or parenteral antibiotic. Arch. Pediatr. Adolesc. Med. 1996;150(4):396–399. doi: 10.1001/archpedi.1996.02170290062010. [DOI] [PubMed] [Google Scholar]
- 68.Blickman J.G. Child life services in pediatric radiology: a randomized controlled trial. Pediatr. Radiol. 2013;43:S573–S574. doi: 10.1007/s00247-014-3005-1. [DOI] [PubMed] [Google Scholar]
- 69.Duncan B. Parental perceptions of the therapeutic effect from osteopathic manipulation or acupuncture in children with spastic cerebral palsy. Clin. Pediatr. 2004;43(4):349–353. doi: 10.1177/000992280404300406. [DOI] [PubMed] [Google Scholar]
- 70.Levi R.B. Diagnosis, disclosure, and informed consent: learning from parents of children with cancer. J. Pediatr. Hematol. Oncol. 2000;22(1):3–12. doi: 10.1097/00043426-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 71.Miner J.R. Randomized clinical trial of nebulized fentanyl citrate versus i.v. fentanyl citrate in children presenting to the emergency department with acute pain. Acad. Emerg. Med. 2007;14(10):895–898. doi: 10.1197/j.aem.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 72.Paradise J.L. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and nonrandomized clinical trials. N. Engl. J. Med. 1984;310(11):674–683. doi: 10.1056/NEJM198403153101102. [DOI] [PubMed] [Google Scholar]
- 73.Paradise J.L. Efficacy of adenoidectomy for recurrent otitis media in children previously treated with tympanostomy-tube placement. Results of parallel randomized and nonrandomized trials. J. Am. Med. Assoc. 1990;263(15):2066–2073. [PubMed] [Google Scholar]
- 74.Spandorfer P.R. Oral versus intravenous rehydration of moderately dehydrated children: a randomized, controlled trial. Pediatrics. 2005;115(2):295–301. doi: 10.1542/peds.2004-0245. [DOI] [PubMed] [Google Scholar]
- 75.Tercyak K.P., Jr. Offering a randomized trial of intensive therapy for IDDM to adolescents. Reasons for refusal, patient characteristics, and recruiter effects. Diabetes Care. 1998;21(2):213–215. doi: 10.2337/diacare.21.2.213. [DOI] [PubMed] [Google Scholar]
- 76.Weinstein S.L. Effects of bracing in adolescents with idiopathic scoliosis. N. Engl. J. Med. 2013;369(16):1512–1521. doi: 10.1056/NEJMoa1307337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams K.G. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr. Emerg. Care. 2013;29(5):555–559. doi: 10.1097/PEC.0b013e31828e56fb. [DOI] [PubMed] [Google Scholar]
- 78.Wynn L. Recruitment of infants with sickle cell anemia to a Phase III trial: data from the BABY HUG study. Contemp. Clin. Trials. 2010;31(6):558–563. doi: 10.1016/j.cct.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young B.J. Pretreatment attrition and childhood social phobia: parental concerns about medication. J. Anxiety Disord. 2006;20(8):1133–1147. doi: 10.1016/j.janxdis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Allen J. Is a randomised controlled trial of a maternity care intervention for pregnant adolescents possible? An Australian feasibility study. BMC Med. Res. Methodol. 2013;13:138. doi: 10.1186/1471-2288-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barratt R. Why families choose not to participate in research: feedback from non-responders. J. Paediatr. Child Health. 2013;49(1):57–62. doi: 10.1111/jpc.12020. [DOI] [PubMed] [Google Scholar]
- 82.Carvalho A.A., Costa L.R. Mothers' perceptions of their child's enrollment in a randomized clinical trial: poor understanding, vulnerability and contradictory feelings. BMC Med. Ethics. 2013;14:52. doi: 10.1186/1472-6939-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harth S.C., Thong Y.H. Sociodemographic and motivational characteristics of parents who volunteer their children for clinical research: a controlled study. BMJ. 1990;300(6736):1372–1375. doi: 10.1136/bmj.300.6736.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reddihough D.S. Efficacy of programmes based on Conductive Education for young children with cerebral palsy. Dev. Med. Child Neurol. 1998;40(11):763–770. doi: 10.1111/j.1469-8749.1998.tb12345.x. [DOI] [PubMed] [Google Scholar]
- 85.Sureshkumar P. Parental consent to participation in a randomised trial in children: associated child, family, and physician factors. Clin. Trials. 2012;9(5):645–651. doi: 10.1177/1740774512453219. [DOI] [PubMed] [Google Scholar]
- 86.Paediatric European Network for Treatment of AIDS (PENTA) Parents' attitudes to their HIV-infected children being enrolled into a placebo-controlled trial: the PENTA 1 trial. Paediatric European Network for Treatment of AIDS. HIV Med. 1999;1:25–31. doi: 10.1046/j.1468-1293.1999.00005.x. [DOI] [PubMed] [Google Scholar]
- 87.Wright J.G. Treatments for paediatric femoral fractures: a randomised trial. Lancet. 2005;365(9465):1153–1158. doi: 10.1016/S0140-6736(05)71878-X. [DOI] [PubMed] [Google Scholar]
- 88.Appleton R.E. The use of MElatonin in children with neurodevelopmental disorders and impaired sleep: a randomised, double-blind, placebo-controlled, parallel study (MENDS) Health Technol. Assess. 2012;16(40):i–239. doi: 10.3310/hta16400. [DOI] [PubMed] [Google Scholar]
- 89.Atkinson M. Comparison of oral amoxicillin and intravenous benzyl penicillin for community acquired pneumonia in children (PIVOT trial): a multicentre pragmatic randomised controlled equivalence trial. Thorax. 2007;62(12):1102–1106. doi: 10.1136/thx.2006.074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Azzopardi D.V. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 2009;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 91.Balle V. Treatment of children with secretory otitis media (SOM) with amoxicillin and clavulanic acid (Spektramox) or penicillin-V (Primcillin). Bacteriological findings in the nasopharynx before and after treatment. Int. J. Pediatr. Otorhinolaryngol. 1998;45(1):77–82. doi: 10.1016/s0165-5876(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 92.Craig J.C. Antibiotic prophylaxis and recurrent urinary tract infection in children. N. Engl. J. Med. 2009;361(18):1748–1759. doi: 10.1056/NEJMoa0902295. [DOI] [PubMed] [Google Scholar]
- 93.Diabetes, C.C.T.R., Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 94.Glogowska M. Randomised controlled trial of community based speech and language therapy in preschool children. BMJ. 2000;321(7266):923–926. doi: 10.1136/bmj.321.7266.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.UK Collaborative ECMO Trial Group UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996;348(9020):75–82. [PubMed] [Google Scholar]
- 96.Hissink Muller P.C. A comparison of three treatment strategies in recent onset non-systemic Juvenile Idiopathic Arthritis: initial 3-months results of the BeSt for Kids-study. Pediatr Rheumatol Online J. 2017;15(1):11. doi: 10.1186/s12969-017-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lenney W. Management of Asthma in School age Children on Therapy (MASCOT): a randomised, double-blind, placebo-controlled, parallel study of efficacy and safety. Health Technol. Assess. 2013;17(4):1–218. doi: 10.3310/hta17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitchell C.D. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br. J. Haematol. 2005;129(6):734–745. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 99.Moreira T.A. Combined oral midazolam-ketamine better than midazolam alone for sedation of young children: a randomized controlled trial. Int. J. Paediatr. Dent. 2013;23(3):207–215. doi: 10.1111/j.1365-263X.2012.01246.x. [DOI] [PubMed] [Google Scholar]
- 100.Ng S.M. TIPIT: a randomised controlled trial of thyroxine in preterm infants under 28 weeks' gestation. Trials. 2008;9:17. doi: 10.1186/1745-6215-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Asperen P.P. A multicentre randomized placebo-controlled double-blind study on the efficacy of Ketotifen in infants with chronic cough or wheeze. J. Paediatr. Child Health. 1992;28(6):442–446. doi: 10.1111/j.1440-1754.1992.tb02714.x. [DOI] [PubMed] [Google Scholar]
- 102.van Wijk R.M. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741. doi: 10.1136/bmj.g2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vora A. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. doi: 10.1016/S1470-2045(12)70600-9. [DOI] [PubMed] [Google Scholar]
- 104.Wake M. Outcomes and costs of primary care surveillance and intervention for overweight or obese children: the LEAP 2 randomised controlled trial. BMJ. 2009;339:b3308. doi: 10.1136/bmj.b3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang W.C. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rovers M.M. Generalisability of clinical trials in otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 2001;60(1):29–40. doi: 10.1016/s0165-5876(01)00504-3. [DOI] [PubMed] [Google Scholar]
- 107.Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ng S.M. An explanatory randomised placebo controlled trial of levothyroxine supplementation for babies born <28 weeks' gestation: results of the TIPIT trial. Trials. 2013;14:211. doi: 10.1186/1745-6215-14-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wiley F.M. Parents' perceptions of randomization in pediatric clinical trials. Children Cancer Group. Cancer Pract. 1999;7(5):248–256. doi: 10.1046/j.1523-5394.1999.75010.x. [DOI] [PubMed] [Google Scholar]
- 110.Torgerson D.J., Sibbald B. Understanding controlled trials. What is a patient preference trial? BMJ. 1998;316(7128):360. doi: 10.1136/bmj.316.7128.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brewin C.R., Bradley C. Patient preferences and randomised clinical trials. BMJ. 1989;299(6694):313–315. doi: 10.1136/bmj.299.6694.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Janevic M.R. The role of choice in health education intervention trials: a review and case study. Soc. Sci. Med. 2003;56(7):1581–1594. doi: 10.1016/s0277-9536(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 113.Donovan J.L. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI) Trials. 2016;17(1):283. doi: 10.1186/s13063-016-1391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Torgerson D., Moffett J.K. Patient preference and validity of randomized controlled trials. J. Am. Med. Assoc. 2005;294(1):41–42. doi: 10.1001/jama.294.1.41-b. author reply 42. [DOI] [PubMed] [Google Scholar]
- 115.Walter S. Beyond the treatment effect: evaluating the effects of patient preferences in randomised trials. Stat. Methods Med. Res. 2014;26(1):489–507. doi: 10.1177/0962280214550516. [DOI] [PubMed] [Google Scholar]
- 116.Trivedi R.B. Recruitment and retention rates in behavioral trials involving patients and a support person: a systematic review. Contemp. Clin. Trials. 2013;36(1):307–318. doi: 10.1016/j.cct.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 117.Kaur G. A survey of facilitators and barriers to recruitment to the MAGNETIC trial. Trials. 2016;17(1):607. doi: 10.1186/s13063-016-1724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rothmier J.D., Lasley M.V., Shapiro G.G. Factors influencing parental consent in pediatric clinical research. Pediatrics. 2003;111(5):1037–1041. doi: 10.1542/peds.111.5.1037. [DOI] [PubMed] [Google Scholar]
- 119.Kodish E. Communication of randomization in childhood leukemia trials. J. Am. Med. Assoc. 2004;291(4):470–475. doi: 10.1001/jama.291.4.470. [DOI] [PubMed] [Google Scholar]
- 120.Snethen J.A. Family patterns of decision-making in pediatric clinical trials. Res. Nurs. Health. 2006;29(3):223–232. doi: 10.1002/nur.20130. [DOI] [PubMed] [Google Scholar]
- 121.Unguru Y., Sill A.M., Kamani N. The experiences of children enrolled in pediatric oncology research: implications for assent. Pediatrics. 2010;125(4):e876–e883. doi: 10.1542/peds.2008-3429. [DOI] [PubMed] [Google Scholar]
- 122.Brody J.L. Comparisons of adolescent and parent willingness to participate in minimal and above-minimal risk pediatric asthma research protocols. J. Adolesc. Health. 2005;37(3):229–235. doi: 10.1016/j.jadohealth.2004.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lees A. Positioning children's voice in clinical trials research: a new model for planning, collaboration, and reflection. Qual. Health Res. 2017;27(14):2162–2176. doi: 10.1177/1049732317726760. [DOI] [PubMed] [Google Scholar]
- 124.Gillies K., Entwistle V.A. Supporting positive experiences and sustained participation in clinical trials: looking beyond information provision. J. Med. Ethics. 2012;38(12):751–756. doi: 10.1136/medethics-2011-100059. [DOI] [PubMed] [Google Scholar]
- 125.Ashcroft R. Children's consent to research participation: social context and personal experience invalidate fixed cutoff rules. Am. J. Bioeth. 2003;3(4):16–18. doi: 10.1162/152651603322614436. [DOI] [PubMed] [Google Scholar]
- 126.Kaur G. Psychology. University of Liverpool; 2016. Recruitment to randomised controlled trials with children. ProQuest Dissertations & Theses Global: Health & Medicine. ProQuest document ID 1937388378. [Google Scholar]
- 127.Rooshenas L. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians' practices across six randomised controlled trials. PLoS Med. 2016;13(10) doi: 10.1371/journal.pmed.1002147. e1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siminoff L.A., Fetting J.H., Abeloff M.D. Doctor-patient communication about breast cancer adjuvant therapy. J. Clin. Oncol. 1989;7(9):1192–1200. doi: 10.1200/JCO.1989.7.9.1192. [DOI] [PubMed] [Google Scholar]
- 129.Bowen J., Hirsch S. Recruitment rates and factors affecting recruitment for a clinical trial of a putative anti-psychotic agent in the treatment of acute schizophrenia. Hum. Psychopharmacol. Clin. Exp. 1992;7:337–341. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.