Highlights
-
•
Vestibular evoked myogenic potentials (VEMPs) are used to test the otolith organs in patients with vertigo and imbalance.
-
•
This review discusses the optimal procedures for recording VEMPs and the pitfalls commonly encountered by clinicians.
-
•
Better understanding of VEMP methodology should lead to improved quality of recordings.
Abbreviations: AC, air-conducted; AR, asymmetry ratio; AVS, acute vestibular syndrome; BAER, brainstem auditory evoked potential; BC, bone-conducted; BPV, benign positioning vertigo; BVP, bilateral vestibulopathy; CANVAS, cerebellar ataxia, neuropathy and vestibular areflexia syndrome; cVEMP, cervical vestibular evoked myogenic potential; dB, decibels, the logarithm of the relative power versus a reference; dBA, decibels, measured using an “A” weighting; Deg, degrees; ECG, electrocardiographic; EEG, electroencephalographic; EMG, electromyographic activity/electromyogram; FL, force level; HL, hearing level; IO, inferior oblique; MD, Meniere’s disease; nHL, normal hearing level; NIOSH, National Institutes of Occupational Safety and Health; oVEMP, ocular vestibular evoked myogenic potential; PCS, posterior circulation stroke; PICA, posterior inferior cerebellar artery; pkFL, peak force level; pkSPL, peak sound pressure level (3 dB higher than RMS for a sinusoid); PP, peak-to-peak; RMS, root mean square; SCD, superior canal dehiscence; SCM, sternocleidomastoid; SL, sensation level; SPL, sound pressure level, being the RMS value for a sinusoid; SCC, semicircular canal; SVH, subjective visual horizontal; UW, unilateral weakness; VEMP, vestibular evoked myogenic potential; vHIT, video head impulse test; VM, vestibular migraine; VN, vestibular neuritis; VS, vestibular schwannoma
Keywords: Otolith, Sound, Vibration, Vestibular, VEMP, Method
Abstract
Vestibular evoked myogenic potentials (VEMPs) are a useful and increasingly popular component of the neuro-otology test battery. These otolith-dependent reflexes are produced by stimulating the ears with air-conducted sound or skull vibration and recorded from surface electrodes placed over the neck (cervical VEMPs) and eye muscles (ocular VEMPs). VEMP abnormalities have been reported in various diseases of the ear and vestibular system, and VEMPs have a clear role in the diagnosis of superior semicircular canal dehiscence. However there is significant variability in the methods used to stimulate the otoliths and record the reflexes. This review discusses VEMP methodology and provides a detailed theoretical background for the techniques that are typically used. The review also outlines the common pitfalls in VEMP recording and the clinical applications of VEMPs.
Vestibular evoked myogenic potentials (VEMPs) are short-latency, vestibular-dependent reflexes that are recorded from the sternocleidomastoid (SCM) muscles in the anterior neck (cervical VEMPs or cVEMPs) and the inferior oblique (IO) extraocular muscles (ocular VEMPs or oVEMPs). They are evoked by short bursts of sound delivered through headphones or vibration applied to the skull. As these stimuli have been shown to preferentially activate the otolith organs rather than the semicircular canals, VEMPs are used clinically as measures of otolith function.
VEMPs can be recorded using standard evoked potential equipment with little modification and are therefore easily implemented in a range of clinical settings. As a result, VEMPs have become a standard component of the neuro-otology test battery over the past 20 years. However, there is a lack of consistency in recording procedures between clinics and differences in the quality of VEMP results reported in research papers. There is therefore a need for practical information about the basic requirements for VEMP recording as well as the theoretical basis underlying these requirements. In this review, we build upon previous published guidelines (Papathanasiou et al., 2014) and describe the optimal methods for stimulating the otoliths and recording cVEMPs and oVEMPs, as well as the reasoning behind, and evidence in support of, these methods. We also describe the common errors in VEMP recording that we encounter and how to avoid them, using illustrations that deliberately show small reflexes that can be difficult to record without optimal stimulation and recording conditions. Finally, we provide a brief description of the current clinical application of VEMPs in diagnosis of vertigo and imbalance.
1. Introduction
The cVEMP is a biphasic surface potential, with peaks at approx. 13 and 23 ms, recorded from electrodes arranged in a belly-tendon montage over the SCM muscle (Fig. 1). Cervical VEMPs were first described by Colebatch et al. (1994), who reported a click-evoked muscle reflex in the ipsilateral SCM, which was dependent upon vestibular, but not auditory, function. It scaled with stimulus intensity and the underlying SCM muscle activity and could be easily produced using standard evoked potential equipment and calibrated headphones. Intramuscular recordings later confirmed that the surface response is produced by a short inhibition of the SCM muscle (Colebatch and Rothwell, 2004). As air-conducted (AC) sound preferentially activates the saccule (Young et al., 1977, Zhu et al., 2014, Curthoys et al., 2016), cVEMPs evoked by this stimulus can be used as a test of saccular function.
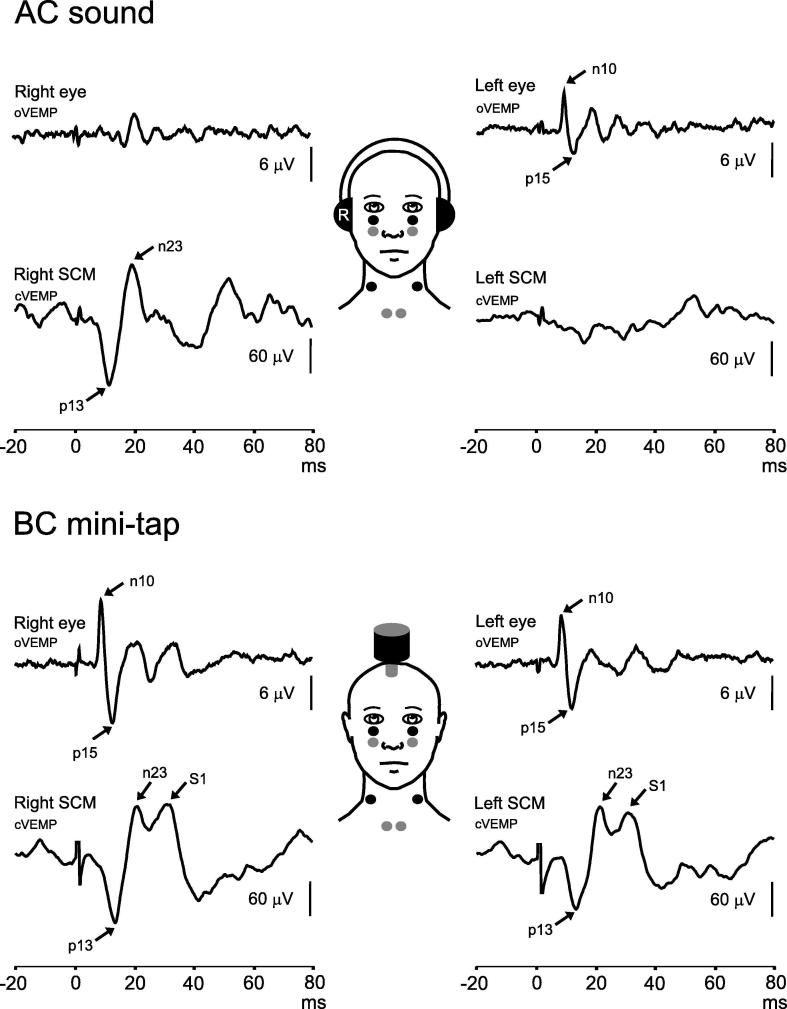
Fig. 1.
cVEMPs and oVEMPs evoked by air-conducted (AC) sound and bone-conducted (BC) vibration in a normal volunteer. Typical electrode positions used to record oVEMPs and cVEMPs are shown bilaterally and consist of bipolar montages made up of active (black circles) and reference (grey circles) pairs. In the top panel, cVEMPs (lower traces) evoked by AC sound can be seen on the right side, i.e. ipsilateral to stimulation, while oVEMPs (upper traces) can be seen on the left side, contralateral to stimulation. In the bottom panel, VEMPs evoked by BC stimulation applied to the forehead are shown. Responses can be seen bilaterally, as BC stimulation activates vestibular afferents in both ears. cVEMPs evoked by BC stimulation can have additional peaks following the p13-n23 response (labelled S1 here), which are thought to be stretch reflexes.
Ocular VEMPs were first described a decade after the cVEMP (Rosengren et al., 2005, Iwasaki et al., 2007, Todd et al., 2007). They are evoked by the same stimuli but are reflexes of the extraocular muscles and thus represent activation of the vestibulo-ocular reflex instead of the vestibulo-collic reflex. The oVEMP originates in the inferior oblique muscle and is produced by a brief excitation of the muscle (Weber et al., 2012). The response consists of a biphasic surface potential with peaks at approx. 10 and 15 ms, beginning with a negativity (Fig. 1). Like the cVEMP, oVEMPs scale with stimulus intensity and muscle contraction and are recorded with surface electrodes placed on the cheeks underneath the eyes while the patient looks upwards. In contrast to the cVEMP, the oVEMP is a contralateral reflex, recorded from the eye opposite the stimulated ear. oVEMPs are used clinically to assess the function of the utricle. Evidence suggests that the oVEMP is produced by otolith afferents in the superior vestibular nerve (which contains all utricular afferents and a small number of afferents from the anterior saccule). Given this, and the fact that sacculo-ocular pathways are thought to be weak, the oVEMP is considered a test of utricular function (Govender et al., 2015).
2. Optimal stimulus parameters: Air-conducted (AC) sound
2.1. Type and frequency
There is not one best VEMP stimulus, as there is significant variability in individual responses to stimuli of different shape and frequency (Taylor et al., 2012). AC sound is the most common VEMP stimulus modality. Clicks (0.1 ms square waves) were used in the initial report on cVEMPs and remain good stimuli, as they have a very fast onset and stimulate across a range of frequencies (approx. 1–4 kHz) (Burkard, 1984, Hood, 2015). Transmission to the saccule shows frequency tuning, with preferred frequency at approximately 500–1000 Hz (Young et al., 1977), and therefore AC tone bursts around these frequencies are also good stimuli. There are, however, mean differences in tuning with age and between cVEMPs and oVEMPs, whereby higher frequencies produce larger reflexes in older subjects and for oVEMPs (Taylor et al., 2012a, Piker et al., 2013). Frequency tuning can also change in some inner ear diseases, such as Meniere’s Disease (MD), whereby patients have larger responses to 1 kHz than 500 Hz stimulation (Rauch et al., 2004, Sandhu et al., 2012, Taylor et al., 2012c, Winters et al., 2012), and superior semicircular canal dehiscence (SCD), in which patients have broader tuning (Taylor et al., 2012c, Manzari et al., 2013).
2.2. Duration
Clicks are typically delivered with 0.1 ms duration, though can be longer. Likewise, tone bursts can be several milliseconds in duration. Increasing stimulus duration typically increases VEMP amplitude, as the total sound energy delivered to the inner ear is increased. However, the enhancement with increasing duration reverses after approx. 6–8 ms for cVEMPs and earlier for oVEMPs (Cheng and Murofushi, 2001a, Lim et al., 2013). In both cases, increases in duration must be tempered by decreases in intensity to keep the sound exposure of the cochlea within acceptable limits. We recommend stimulus durations of up to a maximum of 6 ms for tone bursts for clinical purposes, in part because some commercial systems used to perform VEMPs set minimum durations at this value. In addition to the trade-off between duration and intensity, there is a compromise related to frequency specificity, with longer stimuli providing greater specificity. For this reason stimuli up to 10 ms are sometimes used to test the tuning characteristics of the otolith organs, but are not recommended for routine use as they increase sound exposure. The same stimulus can be used for both cVEMPs and oVEMPs, however stimulus duration is a greater consideration for oVEMPs. As the reflex begins at approx. 6–7 ms, the latter part of long stimuli is likely to have little effect and stimulus artefact can obscure the initial wave due to the close proximity of the stimulation and recording sites, as shown in Fig. 2.
Fig. 2.
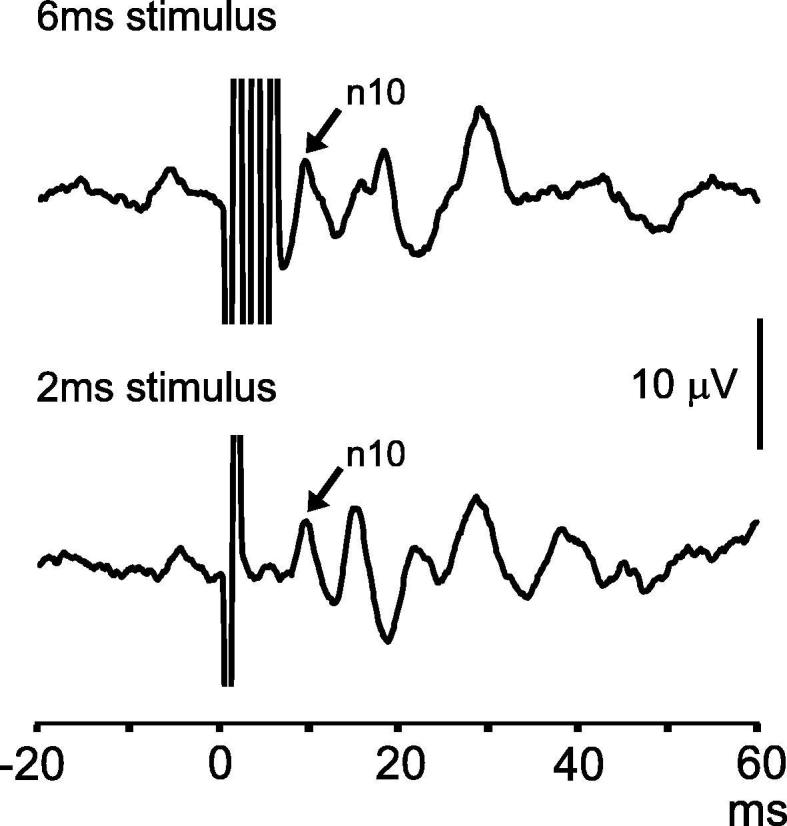
Effect of stimulus artefact on oVEMPs. In the upper trace, the oVEMP has been truncated by the bone-conducted stimulus artefact, while in the lower trace the whole response is visible.
2.3. Rise time
Rise time has a significant effect on the size and latency of the VEMP. The otolith organs may be sensitive to changes in acceleration over time (Curthoys et al., 2006, Jones et al., 2011) and thus VEMPs are larger and peak earlier if the onset of a stimulus (i.e. rise time) is short (Cheng and Murofushi, 2001b, Burgess et al., 2013). In fact, stimulus rise time is one of the major factors determining reflex latency and for this reason it is important to collect normal data for each stimulus.
2.4. Intensity and sound exposure
The optimal AC VEMP stimulus intensity is close to the upper limit of safe sound exposure for the cochlea. This is necessary as in normal humans the average threshold for a VEMP is around 114 dB pkSPL (Dennis et al., 2014) and this increases with age (Welgampola and Colebatch, 2001). Although the vestibular and auditory sensory organs are housed together within the bony labyrinth, the vestibular system is normally shielded from environmental sound, which is very efficiently directed to the cochlea. Only very loud sounds are sufficiently intense to activate the otolith hair cells. As such, there is only a relatively small intensity window for stimulating the vestibular system with AC sound: between the vestibular acoustic threshold and the upper limit of safe stimulation. Careful calibration of stimuli is therefore critical.
As loud sounds can potentially damage the cochlea, at present it seems reasonable to follow the well-argued recommendations of National Institutes of Occupational Safety and Health (1998) for daily exposure, except perhaps where local regulations require more stringent controls. Total sound energy appears to be an important variable as well as peak sound intensity. What is acceptable for diagnostic investigations may not necessarily be acceptable for research studies on normal volunteers.
NIOSH (1998) recommends a maximum daily exposure in the work environment of 85 dBA continuously over 8 h. This essentially sets a maximum for cumulative sound energy delivered to the ear. Higher intensities (up to 140 dB pkSPL) are acceptable for correspondingly shorter intervals. Exposure based on these guidelines does, however, assume that no other intense sounds have been encountered within the surrounding 24 h. These guidelines are equivalent to exposure to a 130 dBA SPL (RMS) stimulus for 1 s. Knowing the stimulus intensity, duration and the total stimuli delivered allows calculation of the 1 s equivalent sound energy (Colebatch and Rosengren, 2016). It is important to note that safe sound exposure is defined for SPL, not nHL (normal hearing level), and it is necessary to know how the manufacturer has defined nHL if this is used. If the ANSI standard applies, then, for a 500 Hz stimulus and TDH49 earphones, 13.5 dB needs to be added to the HL value to get the SPL equivalent, thereby allowing sound exposure to be calculated. For additional information see the following references (Colebatch and Rosengren, 2014, Portnuff et al., 2017).
Safe levels in children are likely to be lower than in adults, due to differences in the ear (Thomas et al., 2017). VEMP stimulus intensity is approx. 3 dB higher in children’s ears, and is linearly related to ear canal volume. However, the higher stimulus intensity is accompanied by larger cVEMP amplitudes, meaning that lower stimulus intensities in children are likely to be as effective as standard intensities in adults (Rodriguez et al., 2018, Rodriguez et al., 2019).
2.5. Stimulation protocol
It is common practice to test the ears consecutively using a standard, high intensity stimulus. For cVEMPs it is also possible to begin with a lower intensity stimulus (e.g. by 5 dB) in patients who are expected to have robust reflexes (younger subjects) or those in whom sound exposure is a particular issue, and progress to the maximum intensity in case of abnormality. In contrast, maximal stimulus intensity should be used initially for oVEMPs, as oVEMP thresholds are higher than those for cVEMP, and there are more absent responses.
Monaural stimulation is preferable to binaural stimulation for both reflexes to ensure that only responses from one ear or muscle are recorded during each trial. The cVEMP is usually only present in the ipsilateral SCM, however the reflex is not strictly unilateral. Stimulation of one ear can sometimes produce an inverted ‘crossed response’ in the contralateral SCM (Welgampola and Colebatch, 2001). Crossed responses are thought to be produced by weak activation of the utricle by AC sound, as the saccule has no known projection to the contralateral SCM (Uchino and Kushiro, 2011). Crossed responses can therefore interfere with ipsilateral cVEMPs if both ears are stimulated at once. Monaural stimulation is also the appropriate method when the SCM is activated using a head turn, as this increases activity in only one SCM at a time. Similarly, while the oVEMP is larger in the contralateral IO muscle, a small signal is often recorded on the ipsilateral side (Chihara et al., 2007, Murnane et al., 2011) and probably originates in other extraocular muscles. Bilateral stimulation would result in the addition of these ipsilateral and contralateral signals, potentially obscuring the asymmetry seen in unilateral vestibular loss.
If VEMP threshold is measured, the stimulus intensity is systematically lowered until the response disappears. Repeat trials are often needed near threshold to increase accuracy. In SCD patients, who have very low VEMP thresholds to AC sound, intensities can be skipped to reduce sound exposure and strain on the neck muscles. A good practice is to include a low intensity trial in routine cVEMP testing, near or below the lower limit of normal threshold, to detect potential cases of SCD or other third window disorders (Brantberg and Verrecchia, 2009).
3. Optimal stimulus parameters: Bone-conducted (BC) stimulation
Much less is known about the optimal stimulation characteristics for BC stimulation. The most important difference between AC and BC stimuli is that the latter is a bilateral stimulus. Healthy adults typically have BC cVEMPs and oVEMPs bilaterally, especially when stimulated in the midline of the skull. In patients with unilateral vestibular loss, the BC cVEMP is either absent on the affected side, or an inverted ‘crossed’ response is seen, with latency typically slightly earlier than the p13. The absence of a normal p13-n23 response in the ipsilateral SCM indicates loss of function. Likewise, for the BC oVEMP, patients with unilateral vestibular loss have oVEMPs opposite the normal ear but no response opposite the affected ear (as the reflex is contralateral). Thus the laterality of both reflexes allows clinical interpretation even though the stimulus activates both ears at once.
The initial report of BC cVEMPs described reflexes evoked by tendon hammer taps to the forehead (Halmagyi et al., 1995). Subsequent studies showed that tone-bursts delivered with a B-71 bone-conductor (Sheykholeslami et al., 2000, Welgampola et al., 2003) or electromechanical vibrator (Iwasaki et al., 2007) were also effective stimuli. BC stimulation has been more difficult to implement as existing evoked potential systems sometimes require modification to produce the stimulus. Taps are usually delivered with a triggered tendon hammer (or a standard tendon hammer modified with an accelerometer trigger) and require an option of an external trigger, while BC tone bursts usually require stronger amplification via an additional external amplifier. The same principles of a fast rise time and short duration apply to BC stimuli, however sound exposure is no longer relevant. The perceived sound loudness is much lower for effective BC sounds compared to AC, indicating their greater effectiveness for stimulating vestibular compared to cochlear receptors (Welgampola et al., 2003).
3.1. Stimulus frequency
Frequency tuning for BC stimuli is lower than for AC stimulation, and reflexes recorded at or above 1000 Hz are usually small, particularly given that stimuli are usually matched across frequency using constant force (Sheykholeslami et al., 2001, Welgampola et al., 2003, Zhang et al., 2012, Curthoys et al., 2016). It is also more difficult to deliver efficient BC vibration to the skull above these frequencies. A 500 Hz tone burst is a popular stimulus, in part because it has been comprehensively studied in animals (Curthoys et al., 2012). This frequency also lies within the optimal output range of many bone-conductors and stimulators, as it is commonly used to test bone-conducted hearing. Lower frequencies, such as 125 or 100 Hz, especially when delivered over 4 or 5 ms respectively (exact half sine waves), produce similar head acceleration as that produced by a tendon hammer, and can be delivered in a more controlled manner and with different intensities.
3.2. Stimulus intensity
The otolith organs are very sensitive to skull vibration and thus the optimum stimulus intensity for clinical purposes is typically a compromise between the output capability of the stimulator and patient comfort. It is important to ensure that the stimulus produces good responses in most normal people before testing patients. It is possible to produce a bruise with overly vigorous BC stimulation, thus care should be taken in the force of stimulus delivery and number of repetitions. Bone-conducted stimuli should be calibrated in force level (FL). An effective stimulus for a B-71 bone-conductor at the mastoid using 500 Hz is approx. 135 dB pkFL (Rosengren et al., 2009). Skull accelerations of approx. 0.1–0.4 g (measured at the first acceleration peak) when measured at the mastoid are effective and typically used (Rosengren et al., 2009, Zhang et al., 2012, Taylor et al., 2014a). Tendon hammer taps are typically more variable and are operator-dependent, however they produce robust oVEMPs and are often the easiest form of BC stimulation to implement (Rosengren et al., 2011).
3.3. Stimulus location
Stimuli can be delivered to the mastoid or in the midline of the skull, usually over the forehead. Tendon hammer taps usually produce good cVEMPs and oVEMPs when applied to either site, though the results can be variable due to operator inconsistency. In contrast B-71 bone-conductors placed on the mastoid typically produce good cVEMPs but poorer oVEMPs (Rosengren et al., 2011), and this bone-conductor is difficult to attach firmly to the forehead. A stronger electromechanical vibrator, such as a ‘minishaker’ (model 4810, Brüel and Kjaer, Denmark) or V201 shaker (Ling Dynamic Systems, Royston, England), typically evokes good reflexes from either site. The 4810 has the advantage that its output is much less affected by loading conditions than the V201 (Colebatch JG, unpublished observations).
Both cVEMPs and oVEMPs evoked by BC stimulation are sensitive to the direction of skull acceleration produced by the stimulus (Brantberg and Tribukait, 2002, Todd et al., 2008a, Rosengren et al., 2009, Jombik et al., 2011). Responses can change polarity and/or latency with different sites of stimulation. The effects are much greater for taps and low frequency tone bursts, but are still present with 500 Hz stimuli (Cai et al., 2011). When delivered to the anterior forehead around the hairline, stimuli that begin with acceleration toward the skull (as with a standard tendon hammer tap) produce earlier responses than those that begin with acceleration away from the skull, but the effects for other midline sites differ (Govender and Colebatch, 2017, Govender and Colebatch, 2018). When delivered to the mastoid in the interaural direction and with the stimulus accelerating towards the skull, the response on the side of the major projection (ipsilateral for cVEMPs, contralateral for oVEMPs) has the earlier response.
Apart from the above systematic effects of stimulus location, BC cVEMPs and oVEMPs are sometimes sensitive to small variations in vibrator placement. We suggest shifting the vibrator and trying nearby locations if BC VEMPs are very small or absent.
4. Which stimulus to use? AC versus BC
The best stimulus is that which produces the best responses and allows the clearest interpretation. The interpretation of VEMPs as saccular or utricular in origin involves a combination of stimulus specificity and pathway specificity. In terms of stimulus specificity, AC sound activates the otoliths in a differential manner, while BC stimulation does not. The saccule has the lowest threshold to AC sound, approx. 15–20 dB lower than the utricle, and much lower than the semicircular canals (Young et al., 1977, Zhu et al., 2011, Zhu et al., 2014, Curthoys et al., 2016). In contrast, BC stimulation appears to activate the saccule and utricle relatively equally (Curthoys et al., 2006, Curthoys et al., 2016). However, impulsive BC stimuli delivered to the mastoid may be more specific for the utricle (Govender et al., 2015). In terms of pathway specificity, the saccule and utricle have similar projections to the ipsilateral SCM muscles, but differential strength of projections to the extraocular muscles: utriculo-ocular projections are stronger than sacculo-ocular ones (Uchino and Kushiro, 2011).
Given the above, the best stimulus for cVEMPs is in most cases AC sound, because of its relative specificity for the saccule. The saccule will be preferentially activated by a loud AC stimulus and an absent cVEMP can be attributed to loss of saccular function (or impairment of pathways in the brainstem and cervical spinal cord involved in the generation of the response). AC sound is also the preferred stimulus for detecting SCD. The pattern of reflexes seen in patients is also consistent with animal research on saccular projections to the SCM muscle which show an ipsilateral inhibitory projection (Kushiro et al., 1999, Welgampola and Colebatch, 2001). The utricle has the same ipsilateral projection and an additional excitatory projection to the contralateral side (Uchino and Kushiro, 2011). There are several disadvantages of AC stimulation, including declining amplitudes and response rates with age, effects of conductive hearing loss, and potential effects of excessive sound exposure. BC stimulation is a good alternate stimulus in such cases, but is a test of general otolith function and not specific to the saccule. BC stimulation produces bilateral responses in patients with unilateral vestibular loss that are consistent with activation of both otolith organs (Brantberg, 2003), rendering their interpretation more difficult. BC cVEMPs can also be difficult to measure as the n23 wave sometimes merges with the later, non-vestibular waves.
For oVEMPs, the best stimulus is more complicated. BC stimulation produces more robust responses than AC sound and is therefore better for detection of vestibular loss, while AC sound is better for detection of third mobile windows such as SCD. BC stimulation produces large oVEMPs, probably because the stimulus activates both of the otolith organs and utriculo-ocular pathways are strong. In contrast, AC sound produces small oVEMPs, probably due to a combination of weak utricular activation by sound and weak sacculo-ocular projections (Isu et al., 2000, Uchino and Kushiro, 2011, Curthoys et al., 2016). Patient studies have shown that oVEMPs evoked by either stimulus are mediated by afferents in the superior vestibular nerve, which includes all utricular afferents and some saccular afferents from the anterior portion of the saccule (Iwasaki et al., 2009, Curthoys et al., 2011, Govender et al., 2011a, Govender et al., 2015). oVEMPs therefore reflect predominantly utricular function, particularly when evoked by BC stimuli.
oVEMPs are more difficult to elicit with AC sound than cVEMPs and studies have shown that oVEMP thresholds are 5–10 dB higher than cVEMP thresholds (Fig. 3) (Park et al., 2010, Rosengren et al., 2011, Rodriguez et al., 2019). The consequence of this is that AC oVEMPs are more often absent than cVEMPs and are particularly sensitive to the effects of age (Piker et al., 2011, Piker et al., 2013, Piker et al., 2015, Rosengren et al., 2011, Singh and Firdose, 2018). This has a significant impact on the clinical utility of the AC oVEMP to detect vestibular loss, as responses can be absent in substantial numbers of healthy older adults. In contrast, BC oVEMPs are present in most healthy adults (Iwasaki et al., 2008, Rosengren et al., 2011). For this reason BC stimulation is the better stimulus for detection of vestibular loss. For detection of vestibular hyperfunction, however, AC oVEMP amplitudes generally outperform those for BC oVEMPs, with higher sensitivity and specificity (Janky et al., 2013).
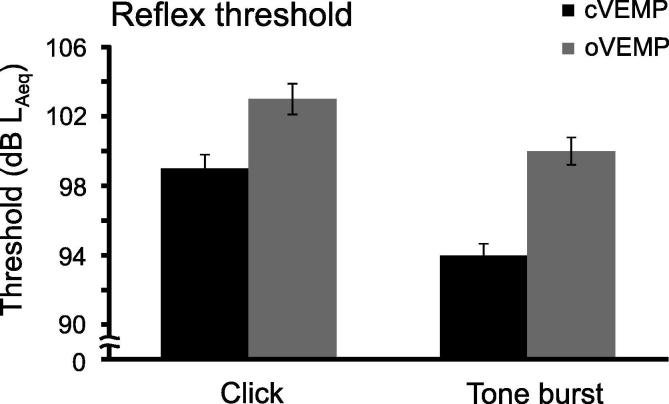
Fig. 3.
Threshold differences between cVEMPs and oVEMPs evoked by AC sound. oVEMP thresholds are approx. 5 dB higher than cVEMP thresholds, independent of the shape of stimulus used. In this example, data are from Rosengren et al. (2011), in which 61 normal volunteers were stimulated with 0.1 ms clicks and 500 Hz, 2 ms tone bursts, both at 105 dB LAeq. Error bars represent standard error.
5. How to record cVEMPs
The cVEMP is recorded from surface electrodes placed over the SCM during a tonic contraction of the muscle. During each trial, which typically lasts approx. 30 sec, stimuli are delivered to the ear (AC stimulation) or skull (BC stimulation) and the patient is asked to activate the SCM muscle/s of interest. The following section outlines in detail the optimal methods to record the cVEMP. Some of the recording principles also apply to the oVEMP and are mentioned below.
5.1. Electrode placement
Most laboratories follow the neurophysiological convention of placing the inverting electrode over the active recording site, resulting in upward deflections of negative polarities. Following this convention is helpful for comparison of traces across centres and publications, but not critical. The active electrode should be placed on or slightly above the middle of the anterior arm of SCM, as this is the approximate location of the motor point (Colebatch, 2012). Amplitudes will be largest and latencies shortest at this point (Colebatch, 2012, Rosengren et al., 2016). Placing electrodes significantly above or below this point can lead to prolonged latencies and, at the extremes of the muscle, polarity inversions (Rosengren et al., 2016). Care should also be taken when using a head turn technique that electrodes positioned with the head in neutral position are symmetrical and remain in place over the muscle belly after the head is turned. Isometric contraction is less likely to change relative electrode locations. For patients with SCM muscles that are difficult to discern, we recommend palpating or having the patient contract the muscle to reveal its location. If this does not work then an electrode can be placed at the midpoint of the distance between the mastoid and medial clavicle, but care is required to ensure that the muscle has not itself been subject to some pathology. The reference electrodes are usually placed bilaterally on the medial clavicles, though some recording systems have only one reference electrode available, which is usually placed in the midline on the upper sternum. The ground electrode is typically placed near the other electrodes or on the head.
Good neurophysiological practice should be followed regarding electrode impedance, in particular to prevent mains (50/60 Hz) electrical interference. Fortunately, impedance is generally not as critical as for evoked potentials of cortical origin (Taylor et al., 2014), however, electrical interference can occasionally be mistaken for EMG potentials and will artificially inflate estimates of contraction strength and so should be avoided.
5.2. EMG settings
The gain should be set to around 2000 for cVEMPs, with a sampling rate of approx. 2–5 kHz. Filter settings are typically from approx. 1–5 Hz (high pass, low cut, i.e. just above DC) to approx. 200 Hz to 1000 Hz (low pass, high cut). The main frequency content of the cVEMP is around 40–60 Hz, which is well within these limits.
The duration of each frame is restricted chiefly by the repetition rate. The cVEMP itself ends around 30–40 ms after stimulus onset, but is often followed by several additional waves. It is common to end the recording from 50 to 100 ms after stimulus onset. Pre-stimulus EMG should always be recorded if possible (at least 10–20 ms), even if it will not be used to measure the background tonic muscle contraction. The pre-stimulus EMG is used to gauge the level of background noise in the recording, from which the response peaks are detected. Reliable VEMPs are those that consistently exceed the residual background EMG seen in the pre-stimulus trace.
The usual repetition rate is typically around 5 Hz. Rates of up to and including 10 Hz are associated with good VEMPs, but above this there is a decrease in cVEMP amplitude (Sheykholeslami et al., 2001). Stimulus rate also affects the perceived loudness of a stimulus.
5.3. Number of repetitions and trials
While the optimal number of repetitions will depend upon the signal-to-noise ratio for each recording, the standard number of repetitions should be about 100–200. Fewer repetitions may be needed in patients with large reflexes, and the recording can be stopped early to reduce sound exposure if needed. More repetitions may be needed for patients with small or absent responses. In this case, we recommend performing two longer trials (e.g. at least 150–200 repetitions) rather than multiple shorter trials (e.g. 50–100 repetitions), as longer trials improve the signal-to-noise ratio by averaging out more of the background contraction (Fig. 4). When using tendon hammer taps, fewer repetitions are delivered, in part because the stimulus usually produces robust responses and can be uncomfortable if repeated too often.
Fig. 4.
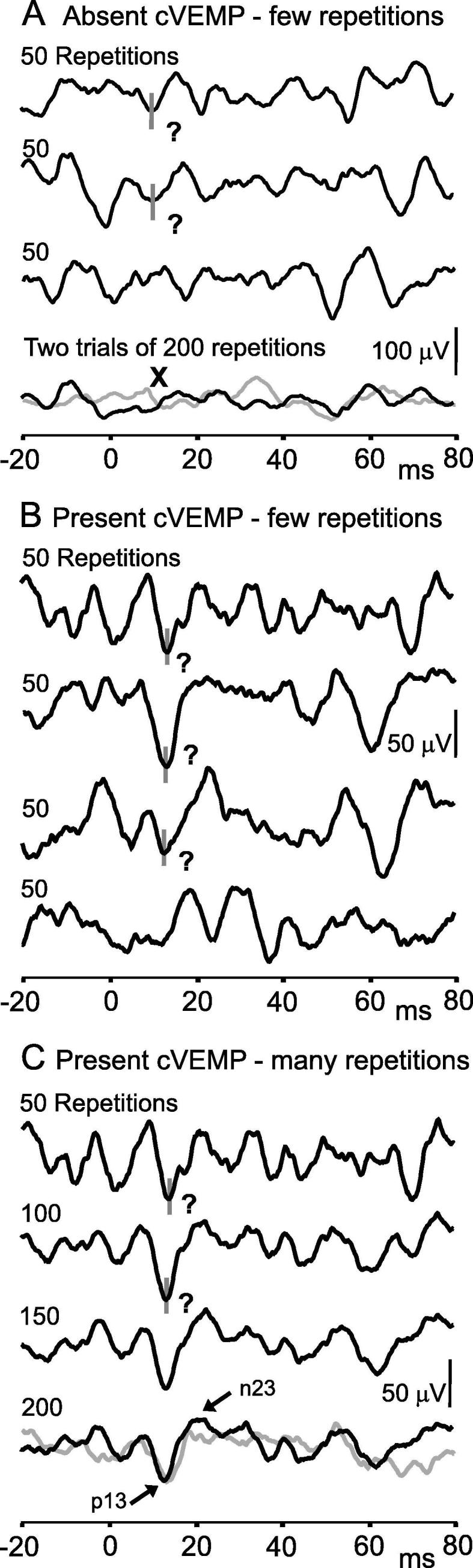
Effect of number of stimulus repetitions on cVEMPs. In part A, data are from a single subject stimulated with 500 Hz, 2 ms tone bursts at 105 dB LAeq. Data from a trial with 200 stimulus repetitions are shown, separated into the first three sets of 50 repetitions (1–49, 50–99 and 100–150 stimuli). Below these are the average of all 200 repetitions (black trace) and a repeat trial with 200 stimuli (grey trace). The first two 50 repetition trials gave the impression of a present response at the appropriate time (approx. 13 and 23 ms), but averaging more stimuli showed that the response was absent and the observed peaks were part of the background EMG. In parts B and C, data are from a different single subject tested with the same stimulus. Part B shows data from a trial with 200 stimulus repetitions, separated into four consecutive sets of 50 repetitions. These short trials again gave the impression of a possible cVEMP, but are too noisy to provide convincing evidence. Part C shows the same data presented with increasing numbers of stimulus repetitions. The cVEMP becomes clearer and the peaks reliably extend beyond the baseline noise level with 150–200 stimuli. A second trace of 200 stimuli is shown in grey, showing that the subject has quite small, but reproducible, cVEMPs.
It is worth noting that the sources of noise in cVEMP and oVEMP recordings differ. cVEMP recordings capture mostly EMG from SCM muscle, whereas other sources of electrical activity are usually small (and include EMG from other muscles, electrocardiographic [ECG] and electroencephalographic [EEG] activity and environmental noise). This is due to the large size and relative isolation of the SCM muscle. Thus the signal and most of the noise have the same origin, whereby the EMG ‘noise’ from the background contraction is paradoxically something that we need a moderate amount of (in order to detect the muscle inhibition of the cVEMP). In contrast, oVEMP recordings capture more EMG from the periocular and facial muscles than the extraocular muscles, which are the source of the signal. Thus the main sources of noise for oVEMPs are other nearby muscles, as well as EEG and environmental noise, all of which should be minimised.
We recommend recording two trials for each stimulus, even if the patient has clear responses. This helps to even out variability and produce more robust estimations of asymmetry. One additional reason for this is that patients with large reflexes are more sensitive to the effect of background contraction (Lim et al., 1995, Rosengren, 2015) and can therefore show larger variations between trials.
6. The effect of muscle contraction on cVEMPs
The strength of the SCM muscle contraction is an important factor to consider in cVEMP recording and measurement. This is because the cVEMP is produced by an inhibition of the muscle. Each cVEMP stimulus causes motor units in the SCM to reduce or pause their activity for several milliseconds (Colebatch and Rothwell, 2004). This has two important consequences. First, a pause, or gap, in activity can only be detected if the muscle is active and not at rest. Therefore the patient must make a sufficient contraction of the muscle during the test. The advantages and disadvantages of different methods of muscle contraction are discussed below. Second, a pause in activity represents a greater change if the muscle is very active than if it is only weakly active. This means that cVEMP amplitude will be greater during strong contractions than weak contractions. Indeed there is a largely linear relationship between the strength of background contraction and the peak-to-peak (PP) cVEMP amplitude (Colebatch et al., 1994, Rosengren, 2015). As the contraction may be different in the SCM muscles on the left and right sides, it can be a confounding factor in measuring symmetry of the reflex. It is therefore important to consider the impact of muscle contraction in cVEMP recordings, and methods of reducing the impact of muscle contraction are discussed below.
6.1. Methods of muscle contraction
There are several ways to produce a sufficient level of muscle contraction. A good method is to have the patient recline to approx. 30 deg. from supine and then lift the head against gravity. This typically produces a weak to moderate contraction of both SCM muscles, depending on the angle of the head (Rosengren, 2015). Lifting the head from a completely supine position will result in greater activity, but is not well-tolerated by patients, who will fatigue and may develop tremor, and is therefore not recommended. Lifting the head and facing forward allows examination of reflexes in both SCM muscles simultaneously. This can be helpful during AC stimulation, as there is sometimes an inverted ‘crossed response’ in the contralateral SCM, particularly in patients with SCD (Colebatch et al., 1994, Welgampola and Colebatch, 2001). This contraction method is also commonly used during BC stimulation, especially stimulation delivered to the forehead, as it allows reflexes to be measured from both muscles simultaneously. The contraction can be enhanced if necessary by applying pressure to the patient’s forehead.
An easy way to achieve stronger contractions is to have the patient lift and turn their head away from the stimulated ear. This typically produces moderate to strong contractions in the ipsilateral SCM (Rosengren, 2015), which often improves the recording, but decreases activity in the contralateral SCM, meaning responses can be recorded from only the ipsilateral (contracted) side. Using this method, responses for each SCM need to be recorded consecutively. This works well for AC stimulation, which requires the ears to be stimulated one at a time. For BC stimulation, which can be delivered to the forehead to stimulate the ears simultaneously, a trial for each side is necessary.
An alternate way of activating the SCM is to have the patient sit upright and turn the head away from the stimulated ear. A simple axial head rotation to the right or left approaching 90 deg can produce sufficient EMG for cVEMP recording, but sometimes falls short. To increase the contraction, patients can perform the head turn against resistance, by pressing their cheek against their own hand or the tester’s hand on the side opposite the tested ear/muscle. Care must be taken that the resulting force remains in axial rotation and does not shift to translation of the head, which may activate additional nearby muscles (Rosengren, 2015). Similar to above, the head turn method requires each side to be tested separately.
The choice of activation method will depend upon the preference for stronger contractions versus bilateral recordings, but also the available equipment and setting. Lack of space for a bed is a common reason for using the sit-and-turn method.
6.2. Methods of reducing the impact of muscle contraction
There are several ways of minimising the impact of muscle contraction on cVEMP amplitude and symmetry. Recording and measuring the background contraction is the best method of detecting asymmetric muscle contractions, and allows the trial to be repeated if necessary with more or less contraction. It also allows for arithmetic correction of the cVEMP amplitude to take the contraction strength into account.
Some VEMP systems provide an additional monitor which displays the muscle contraction strength and helps the patient maintain their contraction within predetermined upper and lower limits. Care should be taken that the limits are not set too narrow, as this increases the number of repetitions needed, producing longer contractions (increasing fatigue and potentially increasing sound exposure). Use of other external aids, such as a sphygmomanometer, to produce a sufficient contraction is an indirect method of controlling EMG and does not necessarily ensure that contractions are symmetric (Lee et al., 2008).
As many clinics or laboratories do not have access to equipment that measures the muscle contraction, it is important to note that, while not ideal, this does not necessarily prevent useful cVEMP testing. Without a measure of muscle contraction, it is more important to use standard recording procedures, including standard methods of muscle contraction. The tester should aim for equal activation on both sides by having the patient perform the same manoeuvre when testing each ear. Viewing the EMG during the contraction is sometimes possible, even if it cannot be measured, and can be used as a visual guide to control the patient’s effort. Finally, it is important to set appropriate upper normal limits for amplitude and asymmetry, to take into account the presence of this potential confounding variable. This ideally means collecting an age-appropriate normative sample, or if need be, using the limits provided by a published study using similar stimulation and recording techniques, which did not correct for muscle contraction. For example, Welgampola and Colebatch (2001) found the upper limit of normal for click-evoked cVEMPs to be 46% for raw PP amplitudes and 35% for amplitudes expressed as a ratio of the background contraction. McCaslin et al. (2014) reported an upper limit of 43% for raw amplitudes and under about 30% for corrected amplitudes (from Fig. 7 in (McCaslin et al., 2014)).
6.3. Measurement of muscle contraction
This can be done in real-time or offline after the recording, as long as the raw traces are saved. EMG should be measured over the pre-stimulus period so that the reflex is not included in the measurement. The pre-stimulus period should be at least 20 ms in duration, and there is no upper limit except that determined by the repetition rate. There are two methods for manipulating the pre-stimulus EMG so that it can be measured: full-wave rectification or root mean square (RMS). Full-wave rectification essentially takes the absolute value of all points along an EMG trace, while RMS is the square root of the arithmetic mean of the squares of EMG values. Both of these render the EMG wholly positive in polarity. As raw EMG traces contain roughly equal parts positive and negative polarity, averaging EMG over time typically produces a value near zero. This is, of course, the purpose of averaging, as it removes unwanted EMG ‘noise’ and reveals the reflex ‘signal’. It is not possible to measure the muscle contraction from the final average reliably. Averaging the rectified or RMS EMG over the pre-stimulus window produces a positive value for each frame, and these can then be averaged over all frames in a recording to provide an estimate of the background contraction strength (Fig. 5). The measures produce similar, but slightly different, values (RMS approx. 1.25 times rectified, (Colebatch, 2009)), and thus the method of EMG measurement should be specified in any research publication.
Fig. 5.
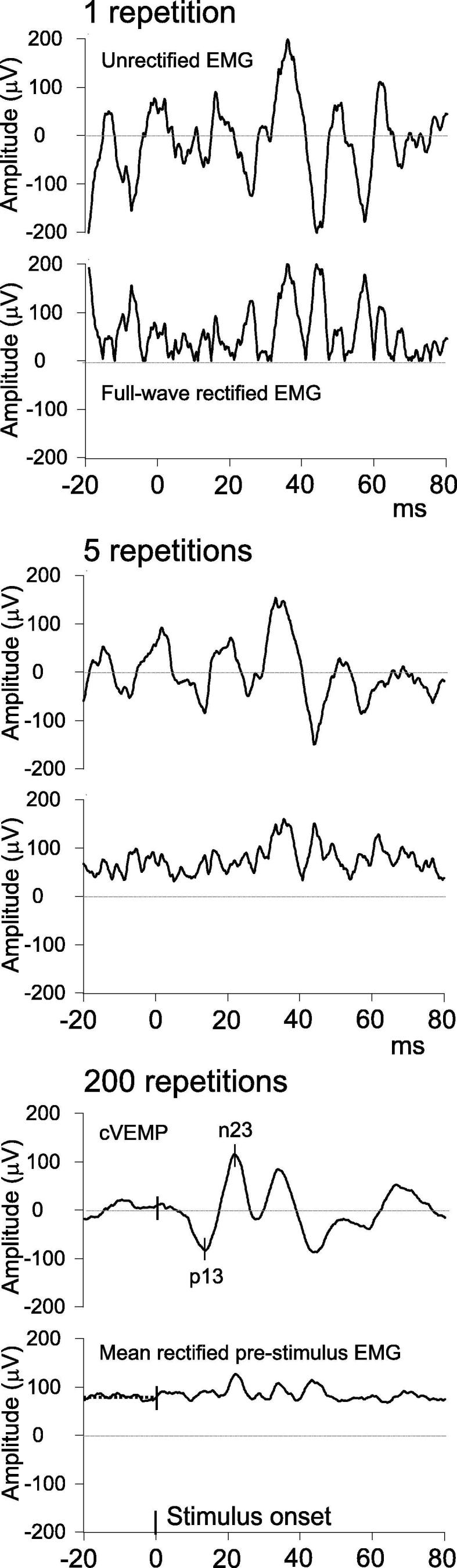
Measuring the SCM contraction using full-wave rectified EMG. The upper two traces show unrectified and full-wave rectified EMG following 1 stimulus repetition. It can be seen that the rectified trace is the absolute value of the unrectified trace. The middle two traces show the cVEMP recording after 5 stimulus repetitions, showing an intermediate step in the formation of the average. The lower two traces show the cVEMP and rectified EMG average after 200 stimulus repetitions. The cVEMP has a typical appearance, with peaks of 83 µV at 13.6 ms and 118 µV at 21.5 ms. The full-wave rectified EMG (after being averaged over 200 repetitions) is now averaged over the 20 ms baseline period (80 µV). The amplitude ratio for this subject is 2.5, calculated by dividing the peak-to-peak amplitude (201 µV) by the mean rectified EMG.
The obtained background EMG estimate is used to calculate an amplitude ratio by dividing the PP amplitude by the estimate of muscle contraction (Fig. 5, Fig. 6). Sometimes the EMG value is used to manipulate the raw cVEMP trace to create a ‘corrected’ trace (i.e. by dividing all points by the background contraction measurement). This produces traces that can be visually compared and from which the amplitude ratio can be directly measured. This is not necessary, but is provided by some manufacturers. However, this can obscure the presence of asymmetric contractions and thus we prefer to view the raw traces and calculate the ratio using the contraction strength separately. We suggest reporting both the raw amplitudes and background contraction measures, in addition to the ratio, in research publications, as these give more information about the conditions under which the cVEMP was recorded.
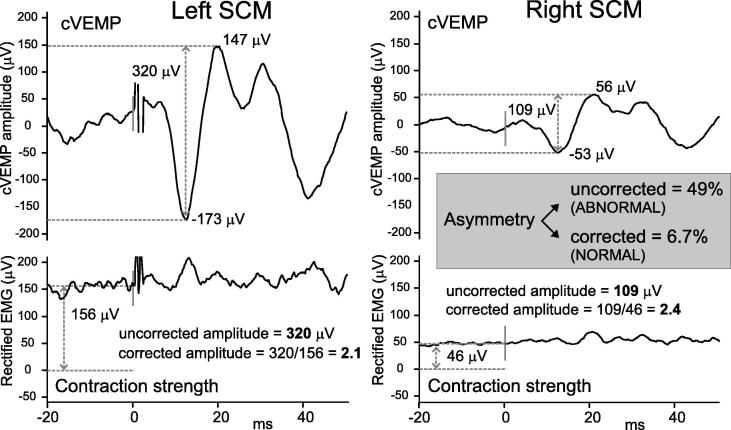
Fig. 6.
Effect of correction for muscle contraction on cVEMP amplitude. cVEMPs (upper traces) evoked by AC sound were measured from the left and right SCM muscles of a normal volunteer. Contraction strength (lower traces, mean pre-stimulus full-wave rectified EMG) was much lower in the right SCM (46 µV) than the left (156 µV), and as a result the uncorrected amplitudes were asymmetric across the two ears (109 vs 320 µV, asymmetry 49%). Using ratios as amplitude measures (i.e. corrected amplitudes) to correct for this contraction difference produced a normal asymmetry ratio of 6.7%.
The background contraction is usually measured using EMG from the same electrode pair used to record the cVEMP. Alternately, some measure EMG using an additional electrode, located close to the recording electrode over the muscle. This close bipolar montage typically leads to smaller estimates of muscle contraction, and much larger amplitude ratios. These are not directly comparable with results from other centres based upon the same electrodes.
7. Common pitfalls in cVEMP testing
This section contains detailed information about the most common problems we encounter in cVEMP testing, the effects they cause and the solutions we suggest to overcome them. We suggest that new users start with recording the cVEMP before trying to record the oVEMP as cVEMPs are often clearer.
Pitfall: The acoustic stimulus is not loud enough.
Effect: This causes high rates of small or absent responses in patients. The problem is often detected in the early stages of implementation, when clinicians quickly notice they are not getting responses in many patients (Fig. 7).
Fig. 7.
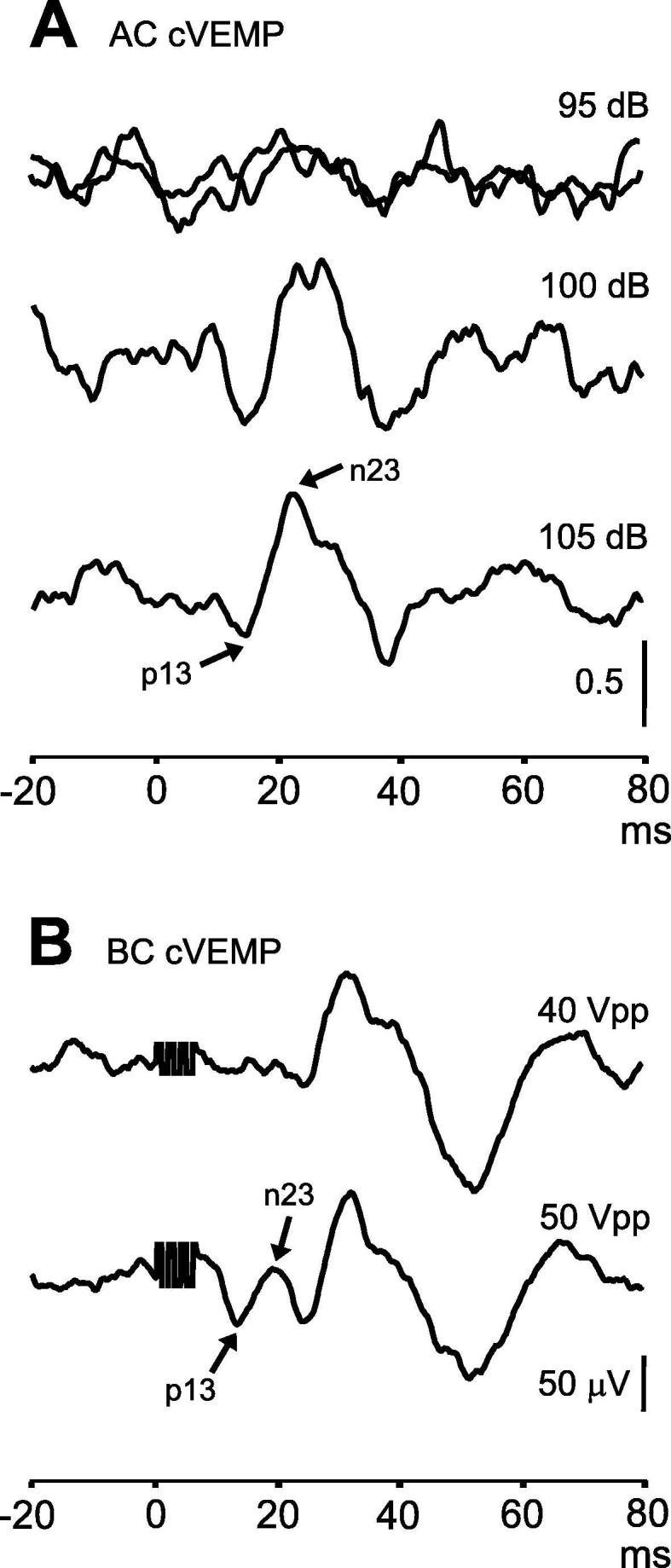
Effect of stimulus intensity on cVEMPs. In part A, the subject had no response to two trials of AC sound delivered at 95 dB (tone bursts of 500 Hz, 2 ms). Increasing the stimulus intensity to 100 and 105 dB revealed a normal reflex. In part B, a different subject had no response to BC stimulation delivered to the forehead in the midline with stimulus intensity 40 Vpp, but had a normal response to a stronger stimulus.
Solution: The solution to this pitfall is twofold. First, we recommend using professionally calibrated stimuli, whether the equipment is part of a commercial machine or custom setup. Sound pressure level (SPL) is the best measure for AC stimuli, as it allows calculation of sound exposure and emphasises that hearing is not required. Sensation level (SL) is not relevant to the VEMP, unlike the brainstem auditory evoked response (BAER). If only normal hearing level (nHL) is available, a stimulus of 95 dB nHL is a good starting point, but higher intensities may be required. However sound exposure still needs to be measured (which requires SPL calibration). Total sound exposure for each ear must remain within safe guidelines (see above). Repetition rate is less important than total stimuli in this calculation.
Second, it is good practice to collect normative data from a reasonable sample of volunteers who match the intended target patient population before administering the test to patients. The reflex evoked by AC sound should be present in nearly all normal adults under 60 years of age. The rate of absent AC cVEMP is typically about 5–15% in subjects older than 60 years (Welgampola and Colebatch, 2001, Ochi and Ohashi, 2003, Basta et al., 2007, Rosengren et al., 2011, Piker et al., 2013), however, depending upon stimulation and recording methods, as well as age, it can be higher.
Pitfall: The muscle activity in SCM is insufficient.
Effect: Very weak contractions can cause small (less than 20 µV peak to peak) or absent cVEMPs or delayed peaks. This is sometimes seen when patients sit and turn their head to contract the muscle, or when using non-standard methods to contract the muscles (or no contraction at all). It may not be recognised if contraction strength is not measured.
Solution: If the reflex is small or absent and insufficient contraction is a possibility, we suggest repeating the trial with increased activity (Fig. 8). Rosengren (2015) suggested a minimum of 80 µV mean rectified EMG, but this will differ depending on the method used to activate the muscle and the measurement technique used. If the head turn method is used, the tester can provide appropriate resistance by placing a hand on the patient’s cheek and pressing against the desired turning direction. Use of one of the standard methods of contracting the muscle described above will increase the chance of producing sufficient EMG. This is especially important if the muscle contraction cannot be measured.
Fig. 8.
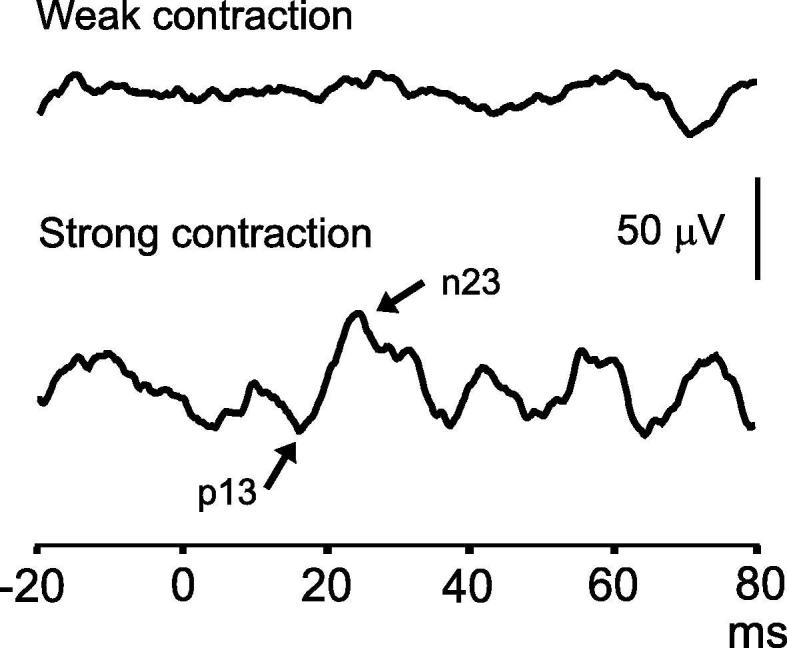
Effect of muscle contraction on cVEMPs. This subject had an absent cVEMP evoked by AC sound with a weak SCM contraction of 61 µV mean rectified EMG, but a small normal reflex with a stronger contraction of 127 µV.
Pitfall: The muscle activity in SCM is excessive.
Effect: It is not always a case of ‘the more the better’. Very strong background contractions may leave residual EMG peaks that can be mistaken for small cVEMP peaks. The aim of averaging is to remove the peaks and troughs of EMG that are not time-locked to the stimulus. With unlimited stimulus repetitions, the background EMG would average out to a flat line. However, cVEMP recordings typically have around 100–200 repetitions, and the background contraction does not always disappear by the time the recording is finished. This is not a problem when the cVEMP is moderate or large in size, as the reflex grows in proportion to the background contraction and its peaks are detectible even if there is residual background EMG. However, it can be a problem when the cVEMP is very small or absent, and much time can be spent trying to distinguish small peaks from residual EMG noise (Fig. 4).
Solution: We advise against using maximal contractions of the SCM muscle and instead prefer to ask patients to perform a moderate contraction. We recommend a bed angle of around 20–40 deg if the patient is supine, as lifting the head from a fully recumbent position is very difficult and produces contractions that are often too strong, producing fatigue and tremor. The bed can be adjusted if required for individual patients. When the lift and turn method produces excessive EMG, a good first option is to ask the patient to lift their head straight without turning, then finally if the muscle activity still too strong, we suggest raising the backrest closer to a near-sitting-upright position with the head lifted straight. In addition, it is better to record fewer trials with greater numbers of repetitions than many short trials. Longer trials allow time for minimisation of the background contraction. A cVEMP should be reproducible over two trials of 200 stimuli with a moderate contraction (Fig. 4).
Pitfall: Insufficient number of stimulus repetitions.
Effect: The effect is the same as for excessive muscle activity. cVEMPs of moderate or large size tend to become clear with only few stimulus repetitions, while very small responses require more averaging to be clearly distinguishable from the background contraction.
Solution: We recommend two trials of at least 150–200 stimuli when responses are very small or absent (Fig. 4).
Pitfall: Measuring noise (excessive amplification).
Effect: Responses appear to be very variable and not reproducible.
Solution: Often users expect cVEMP amplitudes of a few µV, similar to BAERs, and use excessive amplification. Due to either too weak a stimulus or contraction (see above) there is no response, but the high levels of amplification lead to spurious responses (noise). In a young adult, using standard AC or BC stimuli as described above, with adequate muscle contraction, responses of the order of 100 µV peak-peak are expected for the cVEMP. A rule of thumb is that the putative response should always be clearly larger than any pre-stimulus waveforms.
Pitfall: Failure to account for neck muscle activation.
Effect: This can cause false asymmetries in cVEMP amplitude if one SCM has a significantly stronger contraction than the other.
Solution: There are several potential solutions to this problem. The best way to avoid the problem is to monitor the background contraction in real time to enable production of roughly equal EMG levels on both sides. Viewing the contraction level immediately after a trial is also helpful as it allows a repeat trial with greater or less contraction if necessary. Measuring the level of contraction allows calculation of a ‘corrected’ amplitude (a ratio: PP amplitude/background contraction) which can be used to compare the two sides and removes the effect of muscle contraction (Fig. 6). Recording systems which do not measure the background contraction often have a window displaying continuous EMG, which can be used as a rough visual guide to the contraction level, especially in the hands of an experienced tester. If there is no information about the strength of muscle contraction, testers should make sure their asymmetry cut-off values reflect this (i.e. normal limits should be based on their own data collected under the same stimulation and recording conditions, or, as a last resort, on published data collected without correction for muscle contraction).
Pitfall: Measuring the SCM muscle contraction level from the final average.
Effect: This will produce very small values, which will approach zero with increasing numbers of repetitions. Corrected cVEMP amplitudes calculated using such measures of EMG are subject to large errors. Measurement of background contraction typically involves calculating the full-wave rectified EMG or RMS EMG from the pre-stimulus interval for the individual trials. As the process of averaging gradually removes the variations in EMG from the recording, revealing only the stimulus-locked reflex, the final average no longer contains the raw EMG required for reliable measurements.
Solution: Calculations of pre-stimulus EMG should be made using the individual frames in a recording, before they are averaged together. The value for each frame can then be averaged over all repetitions. This is performed automatically by some VEMP systems, and can be done offline if the individual traces can be saved.
8. How to record oVEMPs
In contrast to the cVEMP, the oVEMP is a crossed excitatory reflex of the inferior oblique extraocular muscle. It is recorded from a pair of surface electrodes placed underneath the eyes during up-gaze. Apart from the obvious major differences (organ and muscle of origin, polarity and laterality), oVEMPs are similar muscle reflexes to cVEMPs and therefore have similar stimulation and recording requirements. The following section discusses the optimal methods used to record the oVEMP, focusing on the parameters that differ from the cVEMP.
8.1. Electrode montage
The traditional oVEMP electrode montage consists of an active recording electrode just beneath the midpoint of the eye and a reference electrode a cm or two just below it on the cheek. This montage was designed to maximise the pick-up of electrical activity from the IO muscle while minimising the contribution from other extraocular muscles (Todd et al., 2007). This is important because the oVEMP in the IO muscle is a unilateral (crossed) reflex, but this does not seem to be the case for other extraocular muscles, such as the lateral or medial rectus muscles (Govender et al., 2011). Contamination from other muscles may weaken the laterality of the reflex and reduce its clinical utility. The standard closely-spaced differential montage is associated with smaller amplitudes than montages with reference located much further away; for example, on the chin (Piker et al., 2011, Zuniga et al., 2014), sternum (Vanspauwen et al., 2017) or earlobes (Rosengren et al., 2005). This is because the standard montage prioritizes specificity over amplitude (some of the IO signal will be subtracted out, but so will activity from nearby extraocular muscles). In contrast, the latter montages are likely to include activity from a variety of extraocular muscles. Indeed, mapping studies of vestibular evoked activity over the scalp have shown that extraocular muscle activity can be recorded widely (Todd et al., 2008).
There have been recent reports of greater oVEMP amplitudes using more lateral active recording electrodes in normal subjects, with reference electrode located either beneath the eye (Govender et al., 2016) or on the medial canthus (Sandhu et al., 2013, Makowiec et al., 2017, Vanspauwen et al., 2017). However, Piker et al. (2018) showed that the medial canthus is not an electrically indifferent site. Using a remote reference, a waveform with opposite polarity to the oVEMP can be recorded from the medial canthus, possibly originating in nearby medial rectus muscles. The electrical activity recorded using the lateral (active) – medial canthus (reference) montage therefore may reflect the combination of these two waveforms. The size of the reflex is an important consideration, especially given that many patients have small or absent responses (in particular with AC stimulation). However, the best montage will be the one with the greatest ability to detect vestibular loss. Studies of patients with known vestibular loss, which compare the asymmetry of the n10-p15 response in patients and controls for both montages will ultimately establish the relative clinical utility of the montages. A recent study by Leyssens et al. (2017), which included a small sample of neuro-otology patients with mixed diagnoses, provided promising preliminary evidence in this regard. Four of their patients had unilateral vestibular loss (2 vestibular neuritis, 1 schwannoma and 1 labyrinthectomy) and both the standard and lateral-medial canthus montages showed absent responses from the affected ear, while the latter montage produced larger amplitudes from the normal ear.
8.2. Recording parameters
Similar to the cVEMP, we suggest always recording some pre-stimulus EMG if possible (at least 10–20 ms). A good sample of pre-stimulus EMG will help differentiate oVEMP peaks from residual background noise, especially if oVEMPs are small. The clinically-relevant oVEMP peaks occur at approx. 10 and 15 ms, however the oVEMP consists of a series of negative and positive peaks, which can sometimes continue for 30–40 ms after stimulus onset. It is therefore common the end the oVEMP recording at or after 50 ms following stimulus onset. The oVEMP has higher frequency content than the cVEMP (around 100 Hz vs 40 Hz), therefore the filter setting should be set the same or broader than for the cVEMP. Amplifier gain should also be set higher for oVEMPs (20,000× = 50 μV/V), as the oVEMP is an order of magnitude smaller than the cVEMP. As mentioned above, most of the noise in an oVEMP recording comes from the periocular and facial muscles, such as orbicularis oculi and masseter. As such, it is important to ask patients to relax their face and not talk or clench their jaws during the recording. The optimum number of repetitions may be different to the cVEMP. AC cVEMPs are often very small and require many repetitions (up to 200 or more), while BC oVEMPs may be clear after approx. 50 repetitions. The optimal number will become clear after testing some normal subjects with your own stimuli.
8.3. Effect of gaze
oVEMPs are generally recorded from beneath the eyes while the patient looks upwards. Although many extraocular muscles probably generate oVEMPs, as they all contribute to vestibular evoked eye movements, the largest and clearest signal that can be recorded with surface electrodes comes from the IO muscle (Weber et al., 2012), which is located below the eye. The oVEMP becomes larger as the patient looks upwards (Fig. 9), and between neutral and maximal up-gaze there is a near linear relationship between reflex amplitude and angle of gaze (Govender et al., 2009, Murnane et al., 2011). This is due to two factors: increased tonic muscle activity and proximity to the recording electrodes (Rosengren et al., 2013). As the main role of the IO is to rotate and elevate the eye, increasing upwards gaze increases the tonic contraction of the muscle. Up-gaze also brings the muscle belly closer to the surface electrodes, increasing the strength of signal recorded.
Fig. 9.
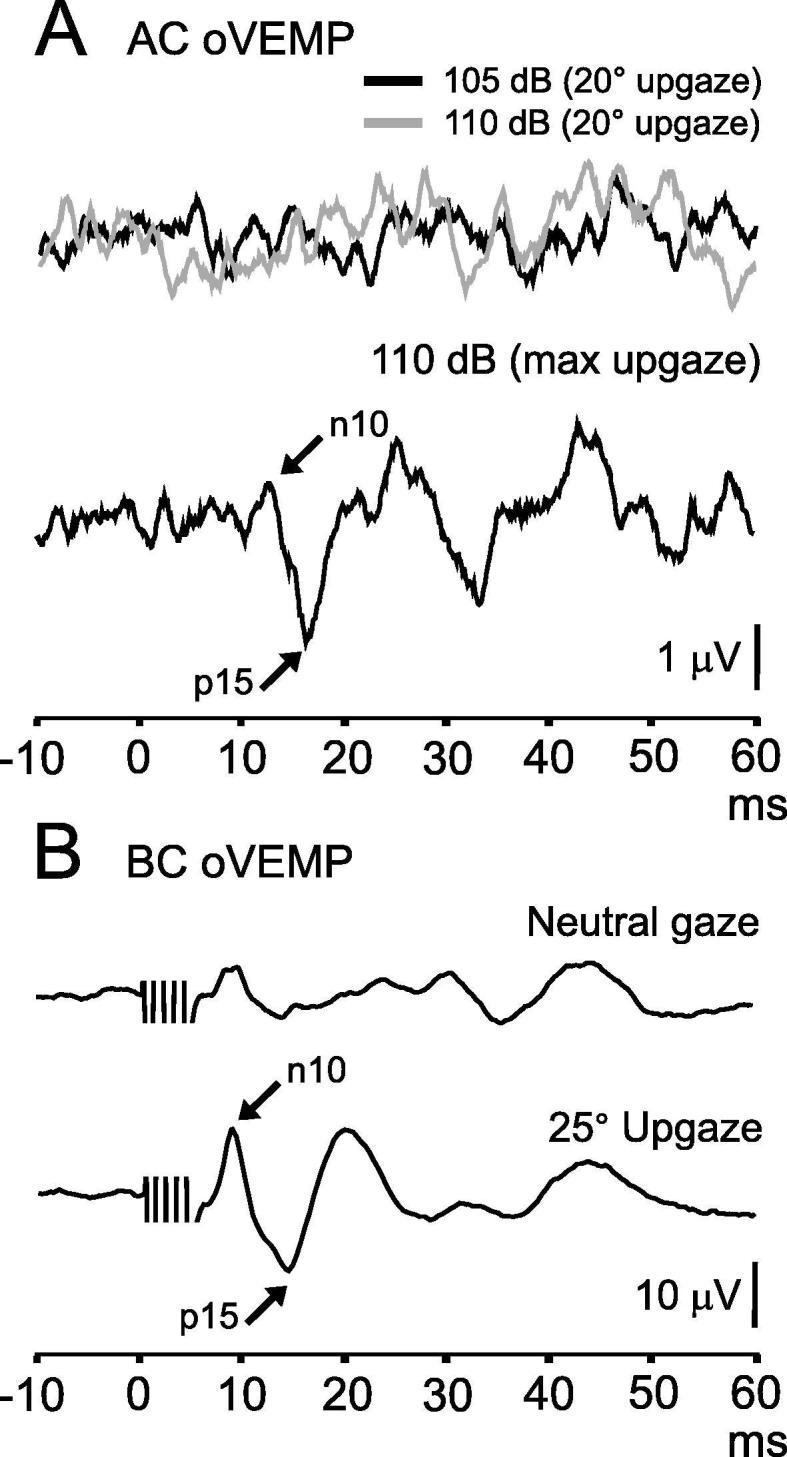
Effect of vertical gaze on oVEMPs. Part A shows oVEMPs evoked by AC tone bursts (500 Hz, 2 ms). The top two overlaid traces show absent responses to stimuli delivered at two intensities (105 and 110 dB LAeq) recorded with gaze elevated to 20 degrees. Raising the angle of gaze to maximal up-gaze revealed a small oVEMP. Part B shows oVEMPs evoked by BC stimulation with gaze straight ahead and elevated to 25 degrees. oVEMPs are larger with up-gaze.
We recommend using maximal comfortable up-gaze for most clinical purposes, as this maximises the amplitude of the oVEMP. However, in some experimental contexts it is useful to have a constant angle of gaze between successive trials to minimise variability. Lack of sufficient vertical gaze can contribute to poor results in elderly patients and those with gaze palsies. There are also potential effects of nystagmus on oVEMP amplitude. Optokinetic nystagmus and alcohol-mediated gaze-evoked nystagmus have both been shown to reduce oVEMP amplitude in normal experimental subjects (Rosengren et al., 2014). Horizontal nystagmus, such as that produced by acute unilateral vestibular loss, may also impact oVEMP recording (Shin et al., 2012, Yang et al., 2017).
9. Common pitfalls in oVEMP testing
Pitfall: Insufficient up-gaze.
Effect: This can cause small or absent responses (see Fig. 9).
Solution: Apart from carefully explaining to patients where you would like them to direct their gaze, and watching them during the test to ensure they comply, it can be useful to provide a visual target that is suitable for most people. A vertical line of tape with points marked in degrees is also a good idea and enables patients to choose their own target. Alternately, the examiner can hold their hand or finger out as a target, which also allows the angle to be customized for each patient.
Pitfall: The acoustic stimulus is not loud enough.
Effect: Insufficient intensity of AC sound produces high rates of small or absent responses in patients. As the threshold to sound is higher for the oVEMP than the cVEMP, this is a particular problem for the oVEMP.
Solution: In normal subjects, oVEMP threshold is approx. 5–10 dB higher than cVEMP threshold. This means that a stimulus that is only marginally weak for the cVEMP can be insufficient for the oVEMP. The solution is the same as that discussed above for the cVEMP and involves careful calibration and collection of normal data. As mentioned above, BC stimulation is a stronger stimulus and good alternative, although if diagnosing SCD is the main object, this should rarely be necessary.
Pitfall: Over-interpretation of absent AC oVEMPs.
Effect: This will result in over-diagnosis of otolith (utricular) abnormalities.
Solution: We suggest caution in interpreting absent AC oVEMPs, especially in elderly patients, or those with small AC cVEMPs. AC oVEMPs are more sensitive than BC oVEMPs in detecting disease, but are associated with more false positive (abnormal) results. Interpretation in light of other balance and auditory tests is advised.
10. cVEMP and oVEMP reflex measurement
Amplitudes and latencies are measured at the response peaks, which occur at approx. 13 and 23 ms for the cVEMP and 10 and 15 ms for the oVEMP, depending on the stimulus. The difference between the peak amplitudes is taken to give the PP amplitude. For cVEMPs, if a measure of SCM muscle activity is available, a ‘corrected amplitude’ can be calculated to take the muscle contraction strength into account. This is done by dividing the PP amplitude by the measure of contraction strength to form a (unit-less) ratio. For oVEMPs, some laboratories prefer to use only n10 amplitude. To compare the two ears, the interaural asymmetry ratio (IAR) is calculated using the Jongkees formula ((right − left)/(right + left)) (or its absolute value, which will have a different normal range) either on the raw PP amplitude or on the amplitude ratio. If threshold is measured, it is reported in dB.
It is very important to have at least some normative data collected locally, in order to estimate the upper and lower limits for amplitude, latency and symmetry. The latency parameter is particularly affected by stimulus shape and rise time.
Asymmetries in VEMP amplitude are usually readily interpretable. Except in third mobile window disorders and early Meniere’s disease (Young et al., 2002), the side with the smaller amplitude is usually the abnormal side. Bilateral vestibular loss is more difficult to detect, as cVEMPs and oVEMPs can be very small or absent in some normal people. It is reasonable to set a lower limit of normal amplitude at the 5th percentile of normative values, as a means of detecting abnormal VEMPs in patients with bilateral vestibular loss (Agrawal et al., 2013, Tarnutzer et al., 2018).
11. Equipment required for recording VEMPs
Most evoked potential systems capable of recording brainstem auditory evoked potentials are capable of recording VEMPs, but are not ideal. The common limitations are that only clicks are available, no calibration in SPL is given, the maximum stimulus intensity is limited, and rectified EMG levels cannot be measured. BC stimulation through an electromechanical vibrator, such as a minishaker, requires an additional dedicated power amplifier. Recent research suggests that portable, smart phone-based devices may become available in time, providing AC and tendon hammer (impulsive) stimuli (MacDougall et al., 2018).
For research applications, a suitable amplification system, interface and collection programs are required for recording. Stimulation requires calibrated headphones for AC sound (and output limits to prevent inadvertent overstimulation and damage to hearing), a modified tendon hammer with a sensitive trigger or electromechanical vibrator (optional but desirable) for BC stimulation, and suitable power amplifiers.
12. Clinical application of VEMPs
cVEMPs and oVEMPs are now widely used to test otolith function in patients with vertigo and imbalance. They are used to reveal loss of otolith function, i.e. in conditions where damage to the inner ear, vestibular nerve, or central vestibular pathways occurs, such as in Meniere’s disease (MD), vestibular neuritis (VN), vestibular schwannoma (VS) or stroke. They are also commonly used to detect enhancement of otolith activation by sound and vibration, such as in third mobile window disorders like SCD. Like other evoked potentials, VEMPs are also sensitive to slowing of conduction along the neural pathways, and thus latency prolongation can be another useful test parameter. Caution is warranted in interpreting latency delay, as it can also be caused by technical factors, such as electrode placement. VEMP abnormalities should be interpreted in light of measures of semicircular canal function and hearing, taking into account the potential false positive rate of each VEMP. The typical patterns of abnormality encountered in common neuro-otological conditions are outlined below.
12.1. VEMPs in acute vestibular syndrome (AVS)
A sudden disabling episode of spontaneous vertigo lasting one or more days could represent vestibular neuritis, an innocuous self-limiting illness, or posterior circulation stroke (PCS), a life threatening cause. VN is associated with distinctive physical signs and vestibular test abnormalities, while PCS is accompanied by diverse physical signs and test profiles. Thus in AVS, it is common to first seek the cardinal features of VN and, if they are absent, then investigate for stroke.
Acute VN can affect the superior, inferior or both divisions of the vestibular nerve or even the ampullary nerves individually (Walther and Blödow, 2013, Magliulo et al., 2014, Taylor et al., 2016). The superior vestibular nerve demonstrates the highest prevalence of abnormalities, and the whole nerve is affected in 50–55% of patients with VN (Magliulo et al., 2014, Taylor et al., 2016). An absent or reduced AC and BC oVEMP in the presence of a preserved AC cVEMP is a common finding in VN (Fig. 10). The prevalence of oVEMP abnormalities is close to ∼70% compared to ∼40% for the cVEMP (Taylor et al., 2016). Labyrinthitis and labyrinthine infarction can present with sudden sensorineural hearing loss and vertigo. Some patients will demonstrate vestibular test abnormalities that map to the common cochlear artery, which supplies the cochlea, saccule and posterior canal. In a case series of 27 subjects, while the prevalence of posterior canal dysfunction was high (74%), prevalence of AC cVEMP asymmetry (30%) was lower than expected, probably since the participants were not tested acutely (Pogson et al., 2016).
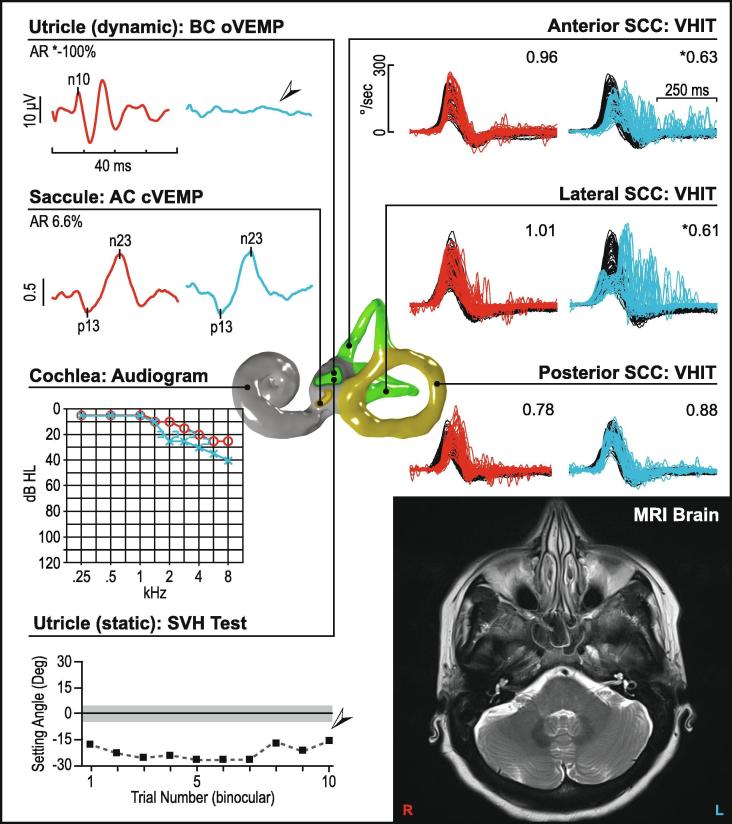
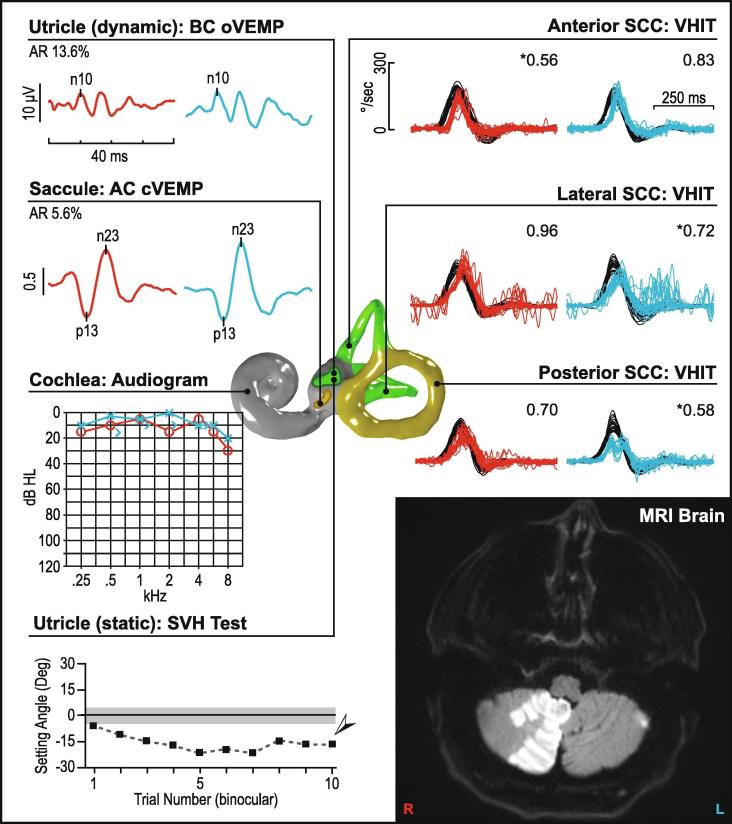
Fig. 10.
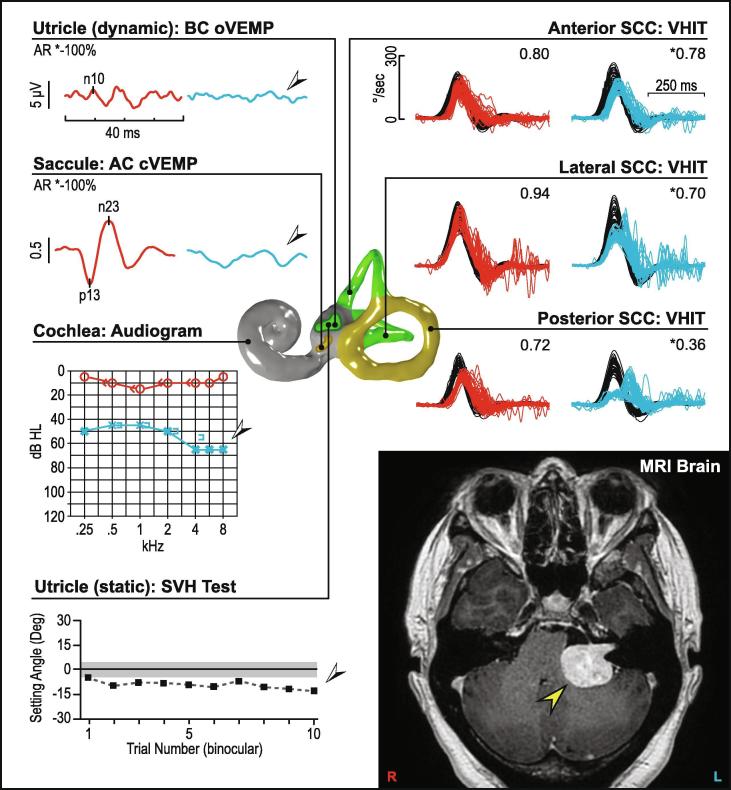
Vestibular neuritis (left ear). This figure illustrates a pattern of abnormality consistent with the superior portion of the vestibular nerve being affected in a patient with vestibular neuritis. In the affected ear oVEMPs are absent, while cVEMPs are preserved bilaterally. Video head impulses (vHITs) are reduced in the anterior and lateral semicircular canals, while posterior canals are unaffected. The audiogram shows essentially normal and symmetric hearing with a mild sloping sensorineural hearing loss, which is age-consistent. Subjective visual horizontal testing is significantly abnormal showing a leftward bias. MRI of the brain is normal. In Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, right ear results are in red, left ear results are in blue. vHIT gains are listed in the upper-right corner of each recording. cVEMP amplitude is shown as a corrected amplitude (ratio), after dividing the peak-to-peak value by the mean rectified EMG. Asymmetry ratios are given for VEMPs and calorics. Arrows and asterisks indicate results which fall outside the normal limits. [Abbreviations: AR; asymmetry ratio, dB HL; Decibels hearing level, Deg; Degree, kHz; Kilohertz, SCC; Semicircular canal, SVH; subjective visual horizontal, UW; unilateral weakness.]
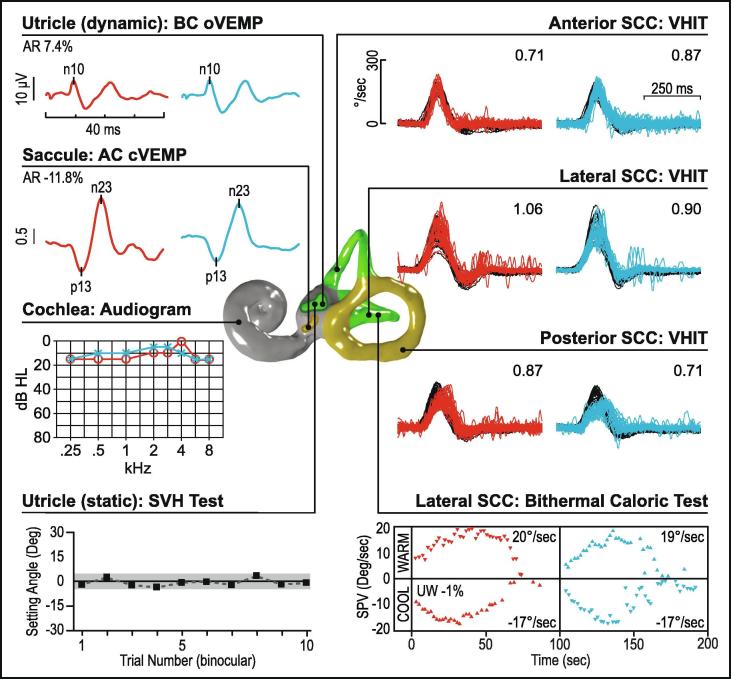
cVEMP or oVEMP abnormalities in PCS are influenced by stroke location. Brainstem ischaemic lesions causing unilaterally abnormal cVEMPs are mostly located in the areas of the vestibular nuclei and spinal accessory nerve in the lateral medulla, in the anterolateral parts of the pyramidal tract fibers, and in the tegmental area of the pons and the vestibular nuclei (Oh et al., 2016). Surprisingly, some rostral lesions up to the midbrain were also associated with abnormal cVEMPs, implying the existence of descending pathways that influence cVEMPs. Since oVEMPs represent the crossed utriculo-ocular pathways that ascend in the pons and midbrain, it is not surprising that midbrain lesions commonly show abnormal oVEMPs in response to contralateral ear stimulation (80%) and are associated with a contraversive ocular tilt response. oVEMPs are also abnormal in 57% of pontine and 47% of medullary lesions. Most of these patients had lesions in the region of the MLF, the crossed ventral tegmental tract, oculomotor nuclei and the interstitial nucleus of Cajal. Unilateral cerebellar infarctions can produce normal VEMP reflexes (as shown in Fig. 11), but may show abnormal oVEMPs, especially when clinical ocular tilt reaction is evident, indicating involvement of otolith ocular pathways (Oh et al., 2013).
Fig. 11.
Right posterior inferior cerebellar artery (PICA) stroke. VEMP reflexes and audiogram are normal bilaterally. Right anterior and left lateral semicircular canal vHIT gains are reduced in the right anterior and left lateral and posterior semicircular canals. Subjective visual horizontal testing is significantly abnormal showing a leftward bias away from the affected side. MRI of the brain shows an area of diffusion restriction within the right medial cerebellar hemisphere in the right PICA territory.
12.2. VEMPs in episodic vertigo
Diagnosis of recurrent positional vertigo from benign positional vertigo (BPV) relies solely on demonstration of canal plane nystagmus on provocative testing and does not require VEMPs to assist the diagnosis. Elevated rates of VEMP abnormality in BPV patients compared to control groups have been reported in the literature (Kim et al., 2015), possibly in part pointing to secondary BPV from an underlying vestibular disorder.
MD and vestibular migraine (VM) are two common causes of recurrent spontaneous vertigo. While typical MD is associated with aural symptoms and typical VM with headaches, photophobia or motion sensitivity, some patients can present with isolated vertigo, necessitating assessment of vestibular function. MD is associated with a distinctive profile of audio-vestibular test abnormalities that include low-frequency hearing loss, asymmetric AC cVEMPs and oVEMPs, asymmetric caloric tests and relative sparing of the video head impulse test (vHIT) (Fig. 12), while vestibular migraine patients have either normal tests or no specific pattern of test abnormalities (Fig. 13).
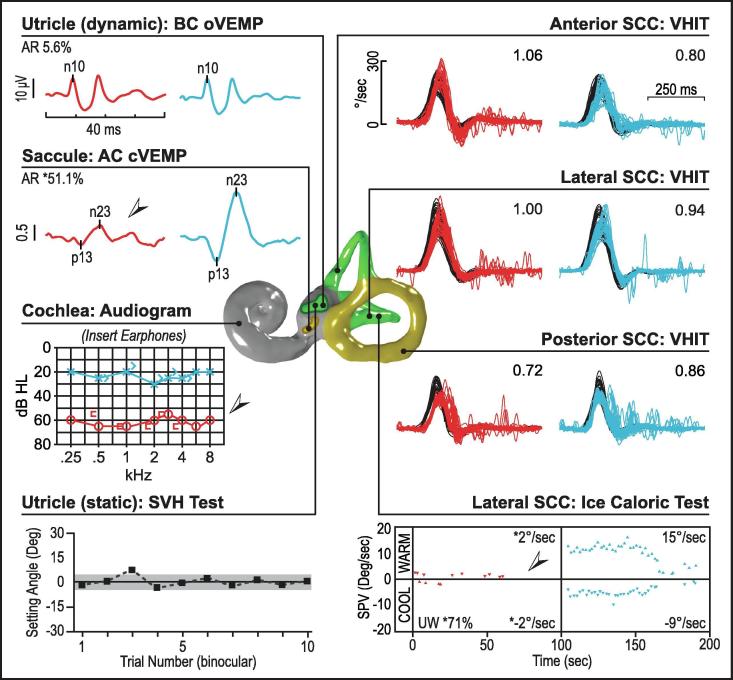
Fig. 12.
Ménière's disease (right ear). In this patient with Ménière’s, cVEMP reflexes are reduced in the affected ear while the oVEMP reflexes are preserved bilaterally. vHITs and subjective visual horizontal tests are normal. The audiogram shows a significant asymmetry with the affected ear showing a flat moderately-severe sensorineural hearing loss. Caloric testing also shows a significant asymmetry, where the right ear shows negligible vestibular response to ice-water irrigation.
Fig. 13.
Vestibular migraine. In this patient with vestibular migraine all audiovestibular test results are normal and symmetric.
In MD, 30–50% of subjects demonstrate asymmetric AC cVEMPs and oVEMPs, yet BC cVEMPs and oVEMPs are spared, implying greater involvement of the saccule (Taylor et al., 2011). Effects of endolymphatic hydrops on stapes footplate motion and changes in inner ear resonance have been proposed to underlie these selective abnormalities affecting AC VEMPs (Huang et al., 2011, Taylor et al., 2011). When healthy controls and subjects with VM underwent VEMP testing using stimulus frequencies of 250–2000 Hz, both groups demonstrated the highest cVEMP amplitudes in response to a 500 Hz stimulus and the highest oVEMP amplitudes to 500 Hz or 1000 Hz stimuli. Patients with MD had best responses at 1000 Hz, for both cVEMP and oVEMP (Taylor et al., 2012). This tuning shift could be useful when seeking to diagnose MD. Thus it is common practice to use a 500 Hz stimulus when responses to a click stimulus yield symmetric responses. Tuning shifts can also occur with advancing age (Piker et al., 2013), therefore it is best to compare against age-matched normative tuning data.
12.3. VEMPs in chronic dizziness or imbalance
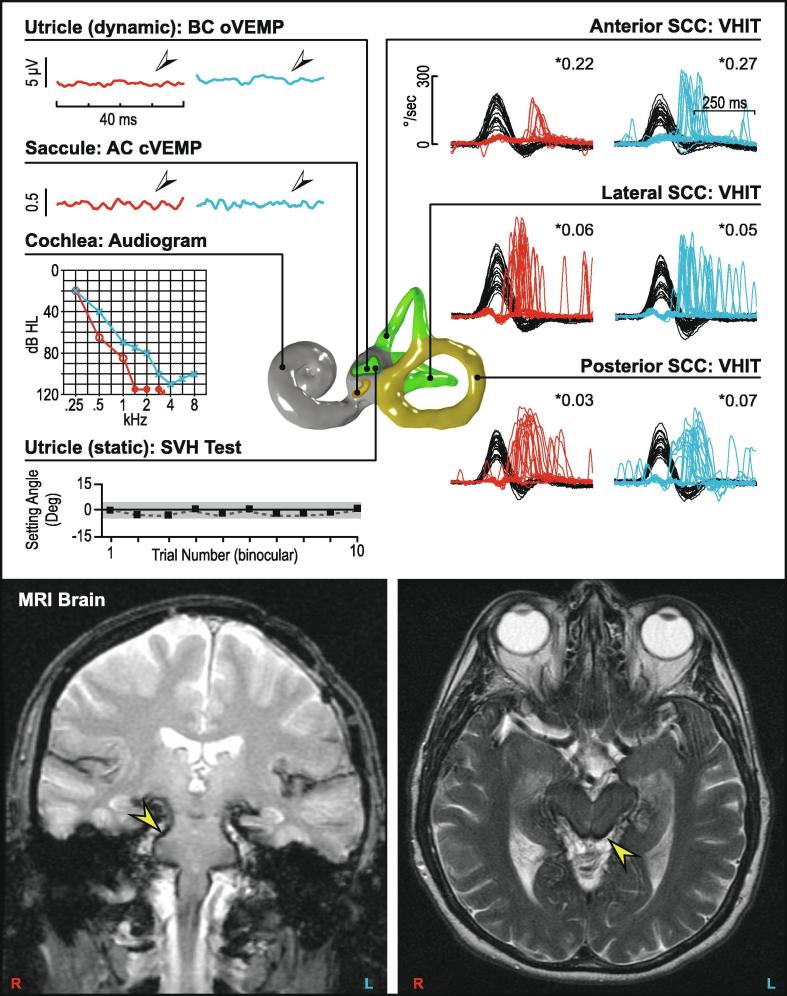
Bilateral vestibulopathy (BVP) presents with ataxia, oscillopsia and imbalance. Since the diagnosis of definite BVP requires impairment of horizontal canal function (Strupp et al., 2017), the prevalence of abnormalities on caloric tests and vHIT is likely to be inflated in subjects diagnosed with BVP comparison to VEMP abnormalities. VEMPs are considered to be only complementary to vHIT, caloric and sinusoidal rotation tests when diagnosing bilateral vestibular failure, however VEMP characteristics may provide clues to the aetiology of BVP. VEMPs may be preserved in cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS) (Rust et al., 2017) but are likely to be impaired in bilateral MD and gentamicin toxicity (Agrawal et al., 2013) as well as superficial siderosis (Ushio et al., 2006) (Fig. 14).
Fig. 14.
Bilateral vestibular loss (superficial siderosis). Bilateral audiovestibular loss in a patient with superficial siderosis. The oVEMP and cVEMP reflexes are absent bilaterally, with vHIT results showing significantly reduced gains and catch-up saccades in all six semicircular canals. The audiogram shows a sloping mild to profound sensorineural hearing loss bilaterally. MRI of the brain shows haemosiderin deposition over the brainstem and spinal cord (arrows). Subjective visual horizontal test is normal.
Uncompensated unilateral vestibular loss from VN, MD, VS or other structural causes could also present with chronic dizziness. Here, the collective information obtained from VEMPs, canal function tests and audiometry will guide the clinician to seek specific causes of unilateral loss. In VS, similar rates of asymmetrical cVEMPs and oVEMPs have been reported (Taylor et al., 2015). Small schwannomas show normal responses, whereas large schwannomas are more likely to affect both cVEMPs and oVEMPs together (Fig. 15). Some patients with abnormal VEMPs in the presence of VS have symmetrical hearing and would thus be missed by audiometric screening protocols. Using VEMPs, vHIT and audiometry in the subject presenting with imbalance will optimise detection of an undiagnosed schwannoma.
Fig. 15.
Unilateral vestibular loss (left vestibular schwannoma). The audio vestibular test results are reduced on the left side, while the right-sided test results are normal. MRI of the brain shows a left-sided vestibular schwannoma compressing the brainstem.
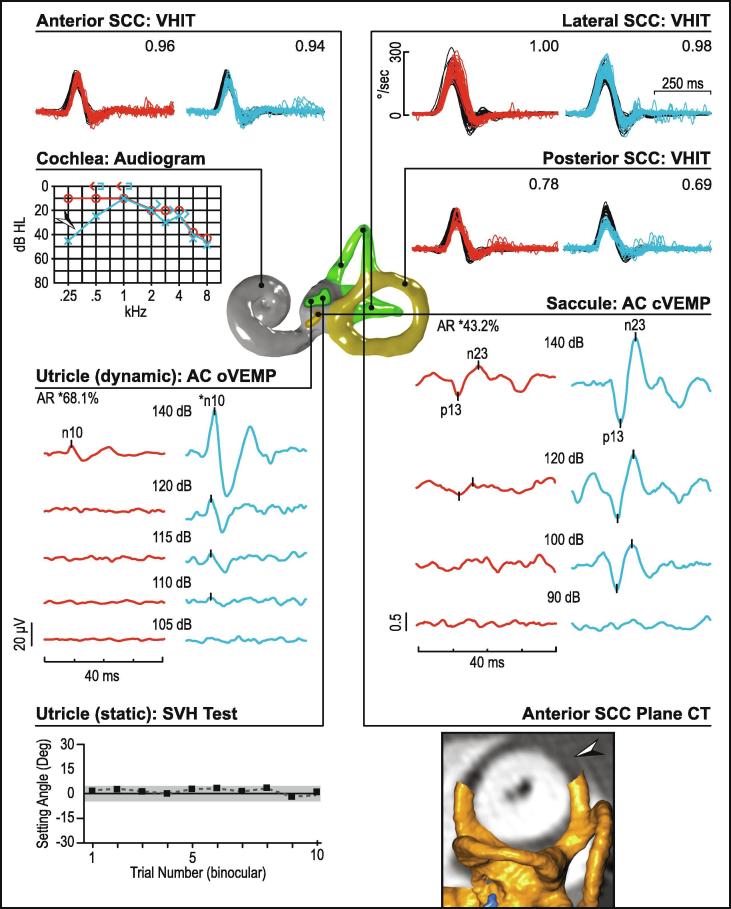
12.4. VEMPs in superior canal dehiscence and third window syndromes
In a structurally normal ear, sound energy conveyed through the ossicular chain reaches the inner ear through the oval window, passes through the incompressible perilymph within the scala vestibule and scala tympani to produce outward motion of the round window. The presence of a third mobile window such as SCD results in shunting of sound pressure away from the cochlea and through the vestibule thus resulting in vestibular hyper-sensitivity to sound and reduced hearing. Both cVEMPs and oVEMPs are augmented in SCD, with high amplitude and low threshold (Fig. 16) (Rosengren et al., 2008, Welgampola et al., 2008). BC VEMPs are still abnormal, yet the threshold reductions are less marked for BC oVEMP and cVEMP (Welgampola et al., 2008). The best one step screening test for SCD may be the AC oVEMP which demonstrates enlarged amplitudes in dehiscent ears although only cVEMP thresholds have strong supporting evidence in the literature as to their value in SCD (Fife et al., 2017). cVEMPs, which are inhibitory potentials, have amplitudes that are likely to saturate when using standard stimulus intensities and require lower stimulus levels to improve their pick up rates for SCD using amplitude criteria (Fife et al., 2017). A high frequency tone-burst of 2,000 and 4,000 Hz can be particularly effective (Manzari et al., 2013). Comparison against age-matched normative data is important since VEMP thresholds, amplitudes and tuning characteristics are influenced by age. SCD alters the waveforms, amplitudes, and latencies of oVEMPs to midline BC stimulation; typically the BC oVEMP in response to midline (Fz) taps is either delayed or double peaked (Taylor et al., 2014b, Holmeslet et al., 2015, Verrecchia et al., 2016). Successful plugging of SCD normalises oVEMP thresholds and amplitudes (Welgampola et al., 2008). Posterior canal dehiscence (Aw et al., 2010, Gopen et al., 2010) and large vestibular aqueduct syndrome (Taylor et al., 2012) are also associated with large VEMP amplitudes and low reflex thresholds. In large vestibular aqueduct syndrome however, VEMP thresholds may rise and amplitudes fall as end-organ damage sets in.
Fig. 16.
Superior semicircular canal dehiscence syndrome (left ear). In the affected ear there are abnormally large oVEMP and cVEMP reflexes with low reflex thresholds while the audiogram shows a low-frequency air-bone gap with increased air-conducted thresholds in dB SPL). Temporal bone CT scan shows a dehiscence over the left anterior (superior) canal. All other audiovestibular tests results fall within normal limits.
12.5. Conclusion
VEMP testing offers valuable diagnostic information in patients presenting with acute vestibular syndrome, recurrent spontaneous vertigo, chronic imbalance and third window syndromes. Their contribution to diagnosis is influenced by the use of adequate stimuli, correct testing technique and comparison of results against control data gathered using the same techniques in an age matched population.
Acknowledgments
Acknowledgements
The authors would like to thank Prof. G. Michael Halmagyi for helpful comments on the manuscript. Dr Sally Rosengren was supported by the National Health and Medical Research Council of Australia (GNT1104772).
Conflict of interest statement
The authors have no conflicts to declare.
References
- Agrawal Y., Bremova T., Kremmyda O., Strupp M. Semicircular canal, saccular and utricular function in patients with bilateral vestibulopathy: analysis based on etiology. J. Neurol. 2013;260(3):876–883. doi: 10.1007/s00415-012-6724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S.T., Welgampola M.S., Bradshaw A.P., Todd M.J., Magnussen J.S., Halmagyi G.M. Click-evoked vestibulo-ocular reflex distinguishes posterior from superior canal dehiscence. Neurology. 2010;75(10):933–935. doi: 10.1212/WNL.0b013e3181f11dfd. [DOI] [PubMed] [Google Scholar]
- Basta D., Todt I., Ernst A. Characterization of age-related changes in vestibular evoked myogenic potentials. J. Vestib. Res. 2007;17(2–3):93–98. [PubMed] [Google Scholar]
- Brantberg K. Familial early-onset progressive vestibulopathy without hearing impairment. Acta Otolaryngol. 2003;123(6):713–717. doi: 10.1080/00016480310002500. [DOI] [PubMed] [Google Scholar]
- Brantberg K., Tribukait A. Vestibular evoked myogenic potentials in response to laterally directed skull taps. J. Vestib. Res. 2002;12(1):35–45. [PubMed] [Google Scholar]
- Brantberg K., Verrecchia L. Testing vestibular-evoked myogenic potentials with 90-dB clicks is effective in the diagnosis of superior canal dehiscence syndrome. Audiol. Neurootol. 2009;14(1):54–58. doi: 10.1159/000153435. [DOI] [PubMed] [Google Scholar]
- Burgess A.M., Mezey L.E., Manzari L., MacDougall H.G., McGarvie L.A., Curthoys I.S. Effect of stimulus rise-time on the ocular vestibular-evoked myogenic potential to bone-conducted vibration. Ear Hear. 2013;34(6):799–805. doi: 10.1097/AUD.0b013e318294e3d2. [DOI] [PubMed] [Google Scholar]
- Burkard R. Sound pressure level measurement and spectral analysis of brief acoustic transients. Electroencephalogr. Clin. Neurophysiol. 1984;57(1):83–91. doi: 10.1016/0013-4694(84)90010-5. [DOI] [PubMed] [Google Scholar]
- Cai K.Y., Rosengren S.M., Colebatch J.G. Cervical and ocular vestibular evoked myogenic potentials are sensitive to stimulus phase. Audiol. Neurootol. 2011;16(5):277–288. doi: 10.1159/000321988. [DOI] [PubMed] [Google Scholar]
- Cheng P.W., Murofushi T. The effect of rise/fall time on vestibular-evoked myogenic potential triggered by short tone bursts. Acta Otolaryngol. 2001;121(6):696–699. doi: 10.1080/00016480152583638. [DOI] [PubMed] [Google Scholar]
- Cheng P.W., Murofushi T. The effects of plateau time on vestibular-evoked myogenic potentials triggered by tone bursts. Acta Otolaryngol. 2001;121(8):935–938. [PubMed] [Google Scholar]
- Chihara Y., Iwasaki S., Ushio M., Murofushi T. Vestibular-evoked extraocular potentials by sound: Another clinical test for vestibular air-conducted function. Clin. Neurophysiol. 2007;118(12):2745–2751. doi: 10.1016/j.clinph.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G. Properties of rectified averaging of an evoked-type signal: theory and application to the vestibular-evoked myogenic potential. Exp. Brain Res. 2009;199(2):167–176. doi: 10.1007/s00221-009-1993-0. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G. Mapping the vestibular evoked myogenic potential (VEMP) J. Vestib. Res. 2012;22(1):27–32. doi: 10.3233/VES-2011-0438. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G., Halmagyi G.M., Skuse N.F. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J. Neurol. Neurosurg. Psychiatry. 1994;57(2):190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch J.G., Rosengren S.M. Safe levels of acoustic stimulation: comment on “effects of acoustic stimuli used for vestibular evoked myogenic potential studies on the cochlear function”. Otol. Neurotol. 2014;35(5):932–933. doi: 10.1097/MAO.0000000000000289. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G., Rosengren S.M. Safe levels of acoustic stimulation for vemps: comment on “sudden bilateral hearing loss after cervical and ocular vestibular evoked myogenic potentials”. Otol. Neurotol. 2016;37(1):117–118. doi: 10.1097/MAO.0000000000000912. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G., Rothwell J.C. Motor unit excitability changes mediating vestibulocollic reflexes in the sternocleidomastoid muscle. Clin. Neurophysiol. 2004;115(11):2567–2573. doi: 10.1016/j.clinph.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Curthoys I.S., Iwasaki S., Chihara Y., Ushio M., McGarvie L.A., Burgess A.M. The ocular vestibular-evoked myogenic potential to air-conducted sound; probable superior vestibular nerve origin. Clin. Neurophysiol. 2011;122(3):611–616. doi: 10.1016/j.clinph.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Curthoys I.S., Kim J., McPhedran S.K., Camp A.J. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp. Brain Res. 2006;175(2):256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- Curthoys I.S., Vulovic V., Burgess A.M., Sokolic L., Goonetilleke S.C. The response of guinea pig primary utricular and saccular irregular neurons to bone-conducted vibration (BCV) and air-conducted sound (ACS) Hear. Res. 2016;331:131–143. doi: 10.1016/j.heares.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Curthoys I.S., Vulovic V., Sokolic L., Pogson J., Burgess A.M. Irregular primary otolith afferents from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound. Brain Res. Bull. 2012;89(1–2):16–21. doi: 10.1016/j.brainresbull.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Dennis D.L., Govender S., Chen P., Todd N.P., Colebatch J.G. Differing response properties of cervical and ocular vestibular evoked myogenic potentials evoked by air-conducted stimulation. Clin. Neurophysiol. 2014;125(6):1238–1247. doi: 10.1016/j.clinph.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife T.D., Colebatch J.G., Kerber K.A., Brantberg K., Strupp M., Lee H., Walker M.F., Ashman E., Fletcher J., Callaghan B., Gloss D.S., 2nd Practice guideline: Cervical and ocular vestibular evoked myogenic potential testing: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017;89(22):2288–2296. doi: 10.1212/WNL.0000000000004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopen Q., Zhou G., Poe D., Kenna M., Jones D. Posterior semicircular canal dehiscence: first reported case series. Otol. Neurotol. 2010;31(2):339–344. doi: 10.1097/MAO.0b013e3181be65a4. [DOI] [PubMed] [Google Scholar]
- Govender S., Cheng P.Y., Dennis D.L., Colebatch J.G. Electrode montage and gaze effects on ocular vestibular evoked myogenic potentials (oVEMPs) Clin. Neurophysiol. 2016;127(8):2846–2854. doi: 10.1016/j.clinph.2016.05.365. [DOI] [PubMed] [Google Scholar]
- Govender S., Colebatch J.G. Effects of midline sagittal location on bone-conducted cervical and ocular vestibular evoked myogenic potentials. J. Appl. Physiol. (1985) 2017;122(6):1470–1484. doi: 10.1152/japplphysiol.01069.2016. [DOI] [PubMed] [Google Scholar]
- Govender S., Colebatch J.G. Location and phase effects for ocular and cervical vestibular-evoked myogenic potentials evoked by bone-conducted stimuli at midline skull sites. J. Neurophysiol. 2018;119(3):1045–1056. doi: 10.1152/jn.00695.2017. [DOI] [PubMed] [Google Scholar]
- Govender S., Dennis D.L., Colebatch J.G. Vestibular evoked myogenic potentials (VEMPs) evoked by air- and bone-conducted stimuli in vestibular neuritis. Clin. Neurophysiol. 2015;126(10):2004–2013. doi: 10.1016/j.clinph.2014.12.029. [DOI] [PubMed] [Google Scholar]
- Govender S., Rosengren S.M., Colebatch J.G. The effect of gaze direction on the ocular vestibular evoked myogenic potential produced by air-conducted sound. Clin. Neurophysiol. 2009;120(7):1386–1391. doi: 10.1016/j.clinph.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Govender S., Rosengren S.M., Colebatch J.G. Vestibular neuritis has selective effects on air- and bone-conducted cervical and ocular vestibular evoked myogenic potentials. Clin. Neurophysiol. 2011;122(6):1246–1255. doi: 10.1016/j.clinph.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Govender S., Rosengren S.M., Todd N.P., Colebatch J.G. Ocular vestibular evoked myogenic potentials produced by impulsive lateral acceleration in unilateral vestibular dysfunction. Clin. Neurophysiol. 2011;122(12):2498–2504. doi: 10.1016/j.clinph.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Halmagyi G.M., Yavor R.A., Colebatch J.G. Tapping the head activates the vestibular system: a new use for the clinical reflex hammer. Neurology. 1995;45(10):1927–1929. doi: 10.1212/wnl.45.10.1927. [DOI] [PubMed] [Google Scholar]
- Holmeslet B., Foss O.A., Bugten V., Brantberg K. Ocular vestibular-evoked myogenic potentials (oVEMPs) in response to bone-conducted vertex vibration. Clin. Neurophysiol. 2015;126(3):608–613. doi: 10.1016/j.clinph.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Hood L.J. Auditory brainstem response: estimation of hearing sensitivity. In: Katz J., Chasin M., English K.M., Hood L.J., Tillery K.L., editors. Handbook of Clinical Audiology. Wolters Kluwer; Philadelphia: 2015. pp. 249–266. [Google Scholar]
- Huang C.H., Wang S.J., Young Y.H. Localization and prevalence of hydrops formation in Meniere's disease using a test battery. Audiol. Neurootol. 2011;16(1):41–48. doi: 10.1159/000312199. [DOI] [PubMed] [Google Scholar]
- Isu N., Graf W., Sato H., Kushiro K., Zakir M., Imagawa M., Uchino Y. Sacculo-ocular reflex connectivity in cats. Exp. Brain Res. 2000;131(3):262–268. doi: 10.1007/s002219900292. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Chihara Y., Smulders Y.E., Burgess A.M., Halmagyi G.M., Curthoys I.S., Murofushi T. The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clin. Neurophysiol. 2009;120(3):588–593. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., McGarvie L.A., Halmagyi G.M., Burgess A.M., Kim J., Colebatch J.G., Curthoys I.S. Head taps evoke a crossed vestibulo-ocular reflex. Neurology. 2007;68(15):1227–1229. doi: 10.1212/01.wnl.0000259064.80564.21. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Smulders Y.E., Burgess A.M., McGarvie L.A., Macdougall H.G., Halmagyi G.M., Curthoys I.S. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin. Neurophysiol. 2008;119(9):2135–2147. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Janky K.L., Nguyen K.D., Welgampola M., Zuniga M.G., Carey J.P. Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Otol. Neurotol. 2013;34(1):127–134. doi: 10.1097/MAO.0b013e318271c32a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jombik P., Spodniak P., Bahyl V. Direction-dependent excitatory and inhibitory ocular vestibular-evoked myogenic potentials (oVEMP) produced by oppositely directed accelerations along the midsagittal axis of the head [corrected] Exp. Brain Res. 2011;211(2):251–263. doi: 10.1007/s00221-011-2681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.A., Jones S.M., Vijayakumar S., Brugeaud A., Bothwell M., Chabbert C. The adequate stimulus for mammalian linear vestibular evoked potentials (VsEPs) Hear. Res. 2011;280(1–2):133–140. doi: 10.1016/j.heares.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Oh S.Y., Kim J.S., Yang T.H., Yang S.Y. Persistent otolith dysfunction even after successful repositioning in benign paroxysmal positional vertigo. J. Neurol. Sci. 2015;358(1–2):287–293. doi: 10.1016/j.jns.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Kushiro K., Zakir M., Ogawa Y., Sato H., Uchino Y. Saccular and utricular inputs to sternocleidomastoid motoneurons of decerebrate cats. Exp. Brain Res. 1999;126(3):410–416. doi: 10.1007/s002210050747. [DOI] [PubMed] [Google Scholar]
- Lee K.J., Kim M.S., Son E.J., Lim H.J., Bang J.H., Kang J.G. The Usefulness of Rectified VEMP. Clin. Exp. Otorhinolaryngol. 2008;1(3):143–147. doi: 10.3342/ceo.2008.1.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssens L., Heinze B., Vinck B., Van Ombergen A., Vanspauwen R., Wuyts F.L., Maes L.K. 'Standard' versus 'nose reference' electrode placement for measuring oVEMPs with air-conducted sound: Test-retest reliability and preliminary patient results. Clin. Neurophysiol. 2017;128(2):312–322. doi: 10.1016/j.clinph.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Lim C.L., Clouston P., Sheean G., Yiannikas C. The influence of voluntary EMG activity and click intensity on the vestibular click evoked myogenic potential. Muscle Nerve. 1995;18(10):1210–1213. doi: 10.1002/mus.880181021. [DOI] [PubMed] [Google Scholar]
- Lim L.J., Dennis D.L., Govender S., Colebatch J.G. Differential effects of duration for ocular and cervical vestibular evoked myogenic potentials evoked by air- and bone-conducted stimuli. Exp. Brain Res. 2013;224(3):437–445. doi: 10.1007/s00221-012-3323-1. [DOI] [PubMed] [Google Scholar]
- MacDougall H.G., Holden J., Rosengren S.M., Chiarovano E. muVEMP: A Portable Interface to Record Vestibular Evoked Myogenic Potentials (VEMPs) With a Smart Phone or Tablet. Front. Neurol. 2018;9:543. doi: 10.3389/fneur.2018.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliulo G., Gagliardi S., Ciniglio Appiani M., Iannella G., Re M. Vestibular neurolabyrinthitis: a follow-up study with cervical and ocular vestibular evoked myogenic potentials and the video head impulse test. Ann. Otol. Rhinol. Laryngol. 2014;123(3):162–173. doi: 10.1177/0003489414522974. [DOI] [PubMed] [Google Scholar]
- Makowiec K., McCaslin D.L., Jacobson G.P., Hatton K., Lee J. Effect of electrode montage and head position on air-conducted ocular vestibular evoked myogenic potential. Am. J. Audiol. 2017;26(2):180–188. doi: 10.1044/2017_AJA-16-0108. [DOI] [PubMed] [Google Scholar]
- Manzari L., Burgess A.M., McGarvie L.A., Curthoys I.S. An indicator of probable semicircular canal dehiscence: ocular vestibular evoked myogenic potentials to high frequencies. Otolaryngol. Head Neck Surg. 2013;149(1):142–145. doi: 10.1177/0194599813489494. [DOI] [PubMed] [Google Scholar]
- McCaslin D.L., Fowler A., Jacobson G.P. Amplitude normalization reduces cervical vestibular evoked myogenic potential (cVEMP) amplitude asymmetries in normal subjects: proof of concept. J. Am. Acad. Audiol. 2014;25(3):268–277. doi: 10.3766/jaaa.25.3.6. [DOI] [PubMed] [Google Scholar]
- Murnane O.D., Akin F.W., Kelly K.J., Byrd S. Effects of stimulus and recording parameters on the air conduction ocular vestibular evoked myogenic potential. J. Am. Acad. Audiol. 2011;22(7):469–480. doi: 10.3766/jaaa.22.7.7. [DOI] [PubMed] [Google Scholar]
- National Institutes of Occupational Health and Safety. Occupational Noise Exposure: Revised criteria 1998, Cincinatti OH: US Department of Health and Human Services, 1998.
- Ochi K., Ohashi T. Age-related changes in the vestibular-evoked myogenic potentials. Otolaryngol. Head Neck Surg. 2003;129(6):655–659. doi: 10.1016/s0194-5998(03)01578-x. [DOI] [PubMed] [Google Scholar]
- Oh S.Y., Kim H.J., Kim J.S. Vestibular-evoked myogenic potentials in central vestibular disorders. J. Neurol. 2016;263(2):210–220. doi: 10.1007/s00415-015-7860-y. [DOI] [PubMed] [Google Scholar]
- Oh S.Y., Kim J.S., Lee J.M., Shin B.S., Hwang S.B., Kwak K.C., Kim C., Jeong S.K., Kim T.W. Ocular vestibular evoked myogenic potentials induced by air-conducted sound in patients with acute brainstem lesions. Clin. Neurophysiol. 2013;124(4):770–778. doi: 10.1016/j.clinph.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Papathanasiou E.S., Murofushi T., Akin F.W., Colebatch J.G. International guidelines for the clinical application of cervical vestibular evoked myogenic potentials: an expert consensus report. Clin. Neurophysiol. 2014;125(4):658–666. doi: 10.1016/j.clinph.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Park H.J., Lee I.S., Shin J.E., Lee Y.J., Park M.S. Frequency-tuning characteristics of cervical and ocular vestibular evoked myogenic potentials induced by air-conducted tone bursts. Clin. Neurophysiol. 2010;121(1):85–89. doi: 10.1016/j.clinph.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Piker E.G., Baloh R.W., Witsell D.L., Garrison D.B., Lee W.T. Assessment of the clinical utility of cervical and ocular vestibular evoked myogenic potential testing in elderly patients. Otol. Neurotol. 2015;36(7):1238–1244. doi: 10.1097/MAO.0000000000000793. [DOI] [PubMed] [Google Scholar]
- Piker E.G., Jacobson G.P., Burkard R.F., McCaslin D.L., Hood L.J. Effects of age on the tuning of the cVEMP and oVEMP. Ear Hear. 2013;34(6):e65–73. doi: 10.1097/AUD.0b013e31828fc9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piker E.G., Jacobson G.P., Makowiec K.F., Atabek P.M., Krolewicz S. The medial canthus reference electrode is not electrically indifferent to the ocular vestibular evoked myogenic potential. Otol. Neurotol. 2018;39(10):e1069–e1077. doi: 10.1097/MAO.0000000000001978. [DOI] [PubMed] [Google Scholar]
- Piker E.G., Jacobson G.P., McCaslin D.L., Hood L.J. Normal characteristics of the ocular vestibular evoked myogenic potential. J. Am. Acad. Audiol. 2011;22(4):222–230. doi: 10.3766/jaaa.22.4.5. [DOI] [PubMed] [Google Scholar]
- Pogson J.M., Taylor R.L., Young A.S., McGarvie L.A., Flanagan S., Halmagyi G.M., Welgampola M.S. Vertigo with sudden hearing loss: audio-vestibular characteristics. J. Neurol. 2016;263(10):2086–2096. doi: 10.1007/s00415-016-8214-0. [DOI] [PubMed] [Google Scholar]
- Portnuff C.D.F., Kleindienst S., Bogle J.M. Safe use of acoustic vestibular-evoked myogenic potential stimuli: protocol and patient-specific considerations. J. Am. Acad. Audiol. 2017;28(8):708–717. doi: 10.3766/jaaa.16071. [DOI] [PubMed] [Google Scholar]
- Rauch S.D., Zhou G.W., Kujawa S.G., Guinan J.J., Herrmann B.S. Vestibular evoked myogenic potentials show altered tuning in patients with Meniere’s disease. Otol. Neurotol. 2004;25(3):333–338. doi: 10.1097/00129492-200405000-00022. [DOI] [PubMed] [Google Scholar]
- Rodriguez A.I., Thomas M.L.A., Fitzpatrick D., Janky K.L. Effects of high sound exposure during air-conducted vestibular evoked myogenic potential testing in children and young adults. Ear Hear. 2018;39(2):269–277. doi: 10.1097/AUD.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A.I., Thomas M.L.A., Janky K.L. Air-conducted vestibular evoked myogenic potential testing in children, adolescents, and young adults: thresholds, frequency tuning, and effects of sound exposure. Ear Hear. 2019;40(1):192–203. doi: 10.1097/AUD.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren S.M. Effects of muscle contraction on cervical vestibular evoked myogenic potentials in normal subjects. Clin. Neurophysiol. 2015;126(11):2198–2206. doi: 10.1016/j.clinph.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., Aw S.T., Halmagyi G.M., Todd N.P., Colebatch J.G. Ocular vestibular evoked myogenic potentials in superior canal dehiscence. J. Neurol. Neurosurg. Psychiatry. 2008;79(5):559–568. doi: 10.1136/jnnp.2007.126730. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., Colebatch J.G., Borire A., Straumann D., Weber K.P. cVEMP morphology changes with recording electrode position, but single motor unit activity remains constant. J. Appl. Physiol. (1985) 2016;120(8):833–842. doi: 10.1152/japplphysiol.00917.2015. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., Colebatch J.G., Straumann D., Weber K.P. Why do oVEMPs become larger when you look up? Explaining the effect of gaze elevation on the ocular vestibular evoked myogenic potential. Clin. Neurophysiol. 2013;124(4):785–791. doi: 10.1016/j.clinph.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., Govender S., Colebatch J.G. Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone-conducted stimuli: comparative properties and effects of age. Clin. Neurophysiol. 2011;122(11):2282–2289. doi: 10.1016/j.clinph.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., McAngus Todd N.P., Colebatch J.G. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin. Neurophysiol. 2005;116(8):1938–1948. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., Todd N.P., Colebatch J.G. Vestibular evoked myogenic potentials evoked by brief interaural head acceleration: properties and possible origin. J. Appl. Physiol. (1985) 2009;107(3):841–852. doi: 10.1152/japplphysiol.00296.2009. [DOI] [PubMed] [Google Scholar]
- Rosengren S.M., Weber K.P., Hegemann S.C., Roth T.N. The effect of alcohol on cervical and ocular vestibular evoked myogenic potentials in healthy volunteers. Clin. Neurophysiol. 2014;125(8):1700–1708. doi: 10.1016/j.clinph.2013.12.096. [DOI] [PubMed] [Google Scholar]
- Rust H., Peters N., Allum J.H.J., Wagner B., Honegger F., Baumann T. VEMPs in a patient with cerebellar ataxia, neuropathy and vestibular areflexia (CANVAS) J. Neurol. Sci. 2017;378:9–11. doi: 10.1016/j.jns.2017.04.029. [DOI] [PubMed] [Google Scholar]
- Sandhu J.S., George S.R., Rea P.A. The effect of electrode positioning on the ocular vestibular evoked myogenic potential to air-conducted sound. Clin. Neurophysiol. 2013;124(6):1232–1236. doi: 10.1016/j.clinph.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Sandhu J.S., Low R., Rea P.A., Saunders N.C. Altered frequency dynamics of cervical and ocular vestibular evoked myogenic potentials in patients with Meniere’s disease. Otol Neurotol. 2012;33(3):444–449. doi: 10.1097/MAO.0b013e3182488046. [DOI] [PubMed] [Google Scholar]
- Sheykholeslami K., Habiby Kermany M., Kaga K. Frequency sensitivity range of the saccule to bone-conducted stimuli measured by vestibular evoked myogenic potentials. Hear. Res. 2001;160(1–2):58–62. doi: 10.1016/s0378-5955(01)00333-1. [DOI] [PubMed] [Google Scholar]
- Sheykholeslami K., Murofushi T., Kermany M.H., Kaga K. Bone-conducted evoked myogenic potentials from the sternocleidomastoid muscle. Acta Otolaryngol. 2000;120(6):731–734. doi: 10.1080/000164800750000252. [DOI] [PubMed] [Google Scholar]
- Shin B.S., Oh S.Y., Kim J.S., Kim T.W., Seo M.W., Lee H., Park Y.A. Cervical and ocular vestibular-evoked myogenic potentials in acute vestibular neuritis. Clin. Neurophysiol. 2012;123(2):369–375. doi: 10.1016/j.clinph.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Singh N.K., Firdose H. Characterizing the age and stimulus frequency interaction for ocular vestibular-evoked myogenic potentials. Ear Hear. 2018;39(2):251–259. doi: 10.1097/AUD.0000000000000482. [DOI] [PubMed] [Google Scholar]
- Strupp M., Kim J.S., Murofushi T., Straumann D., Jen J.C., Rosengren S.M., Della Santina C.C., Kingma H. Bilateral vestibulopathy: diagnostic criteria consensus document of the Classification Committee of the Barany Society. J. Vestib. Res. 2017;27(4):177–189. doi: 10.3233/VES-170619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnutzer A.A., Bockisch C.J., Buffone E., Weber K.P. Hierarchical cluster analysis of semicircular canal and otolith deficits in bilateral vestibulopathy. Front. Neurol. 2018;9:244. doi: 10.3389/fneur.2018.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.L., Blaivie C., Bom A.P., Holmeslet B., Pansell T., Brantberg K., Welgampola M.S. Ocular vestibular-evoked myogenic potentials (oVEMP) to skull taps in normal and dehiscent ears: mechanisms and markers of superior canal dehiscence. Exp. Brain Res. 2014;232(4):1073–1084. doi: 10.1007/s00221-013-3782-z. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Bradshaw A.P., Halmagyi G.M., Welgampola M.S. Tuning characteristics of ocular and cervical vestibular evoked myogenic potentials in intact and dehiscent ears. Audiol. Neurootol. 2012;17(4):207–218. doi: 10.1159/000336959. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Bradshaw A.P., Magnussen J.S., Gibson W.P., Halmagyi G.M., Welgampola M.S. Augmented ocular vestibular evoked myogenic potentials to air-conducted sound in large vestibular aqueduct syndrome. Ear Hear. 2012;33(6):768–771. doi: 10.1097/AUD.0b013e31825ce613. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Kong J., Flanagan S., Pogson J., Croxson G., Pohl D., Welgampola M.S. Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. J. Neurol. 2015;262(5):1228–1237. doi: 10.1007/s00415-015-7697-4. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., McGarvie L.A., Reid N., Young A.S., Halmagyi G.M., Welgampola M.S. Vestibular neuritis affects both superior and inferior vestibular nerves. Neurology. 2016;87(16):1704–1712. doi: 10.1212/WNL.0000000000003223. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Schulin M., Goonetilleke S., Welgampola M.S. Does electrode impedance affect the recording of ocular vestibular-evoked myogenic potentials? J. Am. Acad. Audiol. 2014;25(10):969–974. doi: 10.3766/jaaa.25.10.5. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Wijewardene A.A., Gibson W.P., Black D.A., Halmagyi G.M., Welgampola M.S. The vestibular evoked-potential profile of Meniere’s disease. Clin. Neurophysiol. 2011;122(6):1256–1263. doi: 10.1016/j.clinph.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Zagami A.S., Gibson W.P., Black D.A., Watson S.R., Halmagyi M.G., Welgampola M.S. Vestibular evoked myogenic potentials to sound and vibration: characteristics in vestibular migraine that enable separation from Meniere’s disease. Cephalalgia. 2012;32(3):213–225. doi: 10.1177/0333102411434166. [DOI] [PubMed] [Google Scholar]
- Thomas M.L.A., Fitzpatrick D., McCreery R., Janky K.L. Big stimulus, little ears: safety in administering vestibular-evoked myogenic potentials in children. J. Am. Acad. Audiol. 2017;28(5):395–403. doi: 10.3766/jaaa.15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd N.P., Rosengren S.M., Aw S.T., Colebatch J.G. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin. Neurophysiol. 2007;118(2):381–390. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Todd N.P., Rosengren S.M., Colebatch J.G. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by impulsive transmastoid accelerations. Clin. Neurophysiol. 2008;119(7):1638–1651. doi: 10.1016/j.clinph.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Todd N.P., Rosengren S.M., Colebatch J.G. A source analysis of short-latency vestibular evoked potentials produced by air- and bone-conducted sound. Clin. Neurophysiol. 2008;119(8):1881–1894. doi: 10.1016/j.clinph.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Uchino Y., Kushiro K. Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci. Res. 2011;71(4):315–327. doi: 10.1016/j.neures.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Ushio M., Iwasaki S., Sugasawa K., Murofushi T. Superficial siderosis causing retrolabyrinthine involvement in both cochlear and vestibular branches of the eighth cranial nerve. Acta Otolaryngol. 2006;126(9):997–1000. doi: 10.1080/00016480500540535. [DOI] [PubMed] [Google Scholar]
- Vanspauwen R., Wuyts F.L., Krijger S., Maes L.K. Comparison of different electrode configurations for the oVEMP with bone-conducted vibration. Ear Hear. 2017;38(2):205–211. doi: 10.1097/AUD.0000000000000372. [DOI] [PubMed] [Google Scholar]
- Verrecchia L., Westin M., Duan M., Brantberg K. Ocular vestibular evoked myogenic potentials to vertex low frequency vibration as a diagnostic test for superior canal dehiscence. Clin. Neurophysiol. 2016;127(4):2134–2139. doi: 10.1016/j.clinph.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Walther L.E., Blödow A. Ocular vestibular evoked myogenic potential to air conducted sound stimulation and video head impulse test in acute vestibular neuritis. Otol Neurotol. 2013;34(6):1084–1089. doi: 10.1097/MAO.0b013e318280da47. [DOI] [PubMed] [Google Scholar]
- Weber K.P., Rosengren S.M., Michels R., Sturm V., Straumann D., Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J. Physiol. 2012;590(13):3091–3101. doi: 10.1113/jphysiol.2011.226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgampola M.S., Colebatch J.G. Vestibulocollic reflexes: normal values and the effect of age. Clin. Neurophysiol. 2001;112(11):1971–1979. doi: 10.1016/s1388-2457(01)00645-9. [DOI] [PubMed] [Google Scholar]
- Welgampola M.S., Myrie O.A., Minor L.B., Carey J.P. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70(6):464–472. doi: 10.1212/01.wnl.0000299084.76250.4a. [DOI] [PubMed] [Google Scholar]
- Welgampola M.S., Rosengren S.M., Halmagyi G.M., Colebatch J.G. Vestibular activation by bone conducted sound. J. Neurol. Neurosurg. Psychiatry. 2003;74(6):771–778. doi: 10.1136/jnnp.74.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters S.M., Berg I.T., Grolman W., Klis S.F. Ocular vestibular evoked myogenic potentials: frequency tuning to air-conducted acoustic stimuli in healthy subjects and Meniere’s disease. Audiol. Neurootol. 2012;17(1):12–19. doi: 10.1159/000324858. [DOI] [PubMed] [Google Scholar]
- Yang T.H., Chen H.L., Young Y.H. Pathological eye movements influence on the recordings of ocular vestibular-evoked myogenic potential. Acta Otolaryngol. 2017;137(8):807–813. doi: 10.1080/00016489.2017.1286037. [DOI] [PubMed] [Google Scholar]
- Young E.D., Fernandez C., Goldberg J.M. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol. 1977;84(5–6):352–360. doi: 10.3109/00016487709123977. [DOI] [PubMed] [Google Scholar]
- Young Y.H., Wu C.C., Wu C.H. Augmentation of vestibular evoked myogenic potentials: an indication for distended saccular hydrops. Laryngoscope. 2002;112(3):509–512. doi: 10.1097/00005537-200203000-00019. [DOI] [PubMed] [Google Scholar]
- Zhang A.S., Govender S., Colebatch J.G. Tuning of the ocular vestibular evoked myogenic potential to bone-conducted sound stimulation. J. Appl. Physiol. (1985) 2012;112(8):1279–1290. doi: 10.1152/japplphysiol.01024.2011. [DOI] [PubMed] [Google Scholar]
- Zhu H., Tang X., Wei W., Maklad A., Mustain W., Rabbitt R., Highstein S., Allison J., Zhou W. Input-output functions of vestibular afferent responses to air-conducted clicks in rats. J. Assoc. Res. Otolaryngol. 2014;15(1):73–86. doi: 10.1007/s10162-013-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Tang X., Wei W., Mustain W., Xu Y., Zhou W. Click-evoked responses in vestibular afferents in rats. J. Neurophysiol. 2011;106(2):754–763. doi: 10.1152/jn.00003.2011. [DOI] [PubMed] [Google Scholar]
- Zuniga M.G., Davalos-Bichara M., Schubert M.C., Carey J.P., Janky K.L. Optimizing ocular vestibular evoked myogenic potential testing for superior semicircular canal dehiscence syndrome: electrode placement. Audiol Neurootol. 2014;19(4):239–247. doi: 10.1159/000360124. [DOI] [PMC free article] [PubMed] [Google Scholar]