Abstract
1. We previously reported that flavone and flavanone interact spectrally with cytochrome P450 (P450 or CYP) 2A6 and 2A13 and other human P450s and inhibit catalytic activities of these P450 enzymes. In this study, we studied abilities of CYP1A1, 1A2, 1B1, 2A6, 2A13, 2C9, and 3A4 to oxidize flavone and flavanone.
2. Human P450s oxidized flavone to 6-and 5-hydroxylated flavones, seven uncharacterized mono-hydroxylated flavones, and five di-hydroxylated flavones. CYP2A6 was most active in forming 6-hydroxy-and 5-hydroxyflavones and several mono-and di-hydroxylated products.
3. CYP2A6 was also very active in catalyzing flavanone to form 2´-and 6-hydroxyflavanones, the major products, at turnover rates of 4.8 min−1 and 1.3 min−1, respectively. Other flavanone metabolites were 4´-, 3´-and 7-hydroxyflavanone, three uncharacterized mono-hydroxylated flavanones, and five mono-hydroxylated flavones, including 6-hydroxyflavone. CYP2A6 catalyzed flavanone to produce flavone at a turnover rate of 0.72 min−1 that was ~3-fold higher than that catalyzed by CYP2A13 (0.29 min−1).
4. These results indicate that CYP2A6 and other human P450s have important roles in metabolizing flavone and flavanone, two unsubstituted flavonoids, present in dietary foods. Chemical mechanisms of P450-catalyzed desaturation of flavanone to form flavone are discussed.
Keywords: P450 2A6, P450 2A13, Human, P450s, oxidation, flavone, flavanone
Introduction
Many plant flavonoids are found in nature (Tanaka et al., 2010; Tanaka and Brugliera, 2013) and these natural products show various biological properties, e.g. anti-allergic-, anti-inflammatory-, anti-oxidative, anti-microbial-, anti-tumorgenic-, and anti-mutagenic activities, thus preventing cancer, heart disease, bone loss, and a number of diseases (Arct and Pytowska, 2008; Kale et al., 2008; Walle et al., 2007; Zhang et al., 2005; Martens and Mithöfer, 2005). Different pathways of flavonoid metabolism in various plants may determine how these plants produce biologically active compounds and colored materials (Akashi et al., 1998; 1999; Tanaka and Brugliera, 2013; Tanaka et al., 2010; Du et al., 2010; Berim and Gang, 2013). The biological activities have been shown to vary with the number and substitution positions of hydroxyl and/or methoxy groups in flavonoid molecules (Hodek et al., 2002; Kim et al., 2005; Zhang et al., 2005; Wale, 2007; Walle and Walle, 2007; Walle et al., 2007; Shimada, 2017).
Inhibition of cytochrome P450 (P450 or CYP) enzymes by structurally diverse flavonoids has been extensively studied in our and other laboratories (Shimada et al., 2010; 2013; Kim et al., 2005; Moon et al., 2006; Breinholt et al., 2002; Doostdar et al., 2000). Human P450s 1A1, 1A2, 1B1, 2A6, 2A13, 2C9, and 3A4 are inhibited by various flavonoids (Dooster et al., 2000; Shimada et al., 2010; 2013; Tsujimoto et al., 2009), and these phenomena have been suggested to influence human health because these P450 enzymes play important roles in the activation and detoxication of endogenous and xenobiotic chemicals (Hodek et al., 2002; Guengerich, 2015; Walle et al., 2007; Guengerich et al., 2003; Shimada 2017). In fact, we and other investigators have reported that several oxidative metabolites of flavone, such as 3-, 5-, and 7-hydroxyflavone, 5,7-dihydroxyflavone, and 3,5,7-, 4,5,7-, and 5,6,7-trihydroxyflavone are more potent to interact with and inhibit human P450s than flavone itself (Shimada et al. 2010; 2013; Hodek et al., 2002; Tsujimoto et al., 2009). Little is, however, known how flavone is metabolized by human P450 enzymes to oxidative products (Uno et al., 2015), although several reports have indicated that flavanone is metabolized by rat liver microsomes and recombinant human CYP1A1, 1A2, and 2B6 to several oxidative metabolites (Kagawa et al., 2004; Nikolic et al., 2004; Uno et al., 2013).
In this study, we determined if flavone and flavanone are catalyzed by human P450s to oxidative metabolites using recombinant (purified) CYP1A1, 1A2, 1B1, 2A6, 2A13, 2C9, and 3A4 expressed in Escherichia coli. These P450 enzymes were reconstituted with NADPH-P450 reductase and b5 (in cases of CYP2A6, 2A13, 2C9, and 3A4) with the substrates flavone and flavanone, and the resulting products were analyzed with LC-MS/MS. Molecular docking analysis of interaction of flavone and flavanone with CYP2A6 and 2A13 is also reported.
Materials and methods
Chemicals
Flavone and flavanone (Figure 1) were obtained from Wako Pure Chemicals (Osaka, Japan); the oxidation products 5-, 6-, and 7-hydroxyflavone (OHF), 5,7-and 7,8-dihydroxyflavone (diOHF), and 6-and 7-hydroxyflavanone (OHFva) were obtained from Sigma-Aldrich (St. Louis, MO, USA) or Wako Pure Chemicals. 2´-, 3´-, and 4´-Hydroxyflavanones were kindly donated by Dr. Yukinori Yubuta (Tottori University). Other chemicals and reagents used in this study were obtained from the sources described previously or were of the highest quality commercially available (Shimada et al., 2016a: 2016b).
Figure 1.
Structures of flavone and flavanone.
Enzymes
In this study, we used only wild-type of human P450 enzymes (.1 designation). The expression in E. coli and purification of human P450 enzymes have been described previously (Parikh et al., 1997; Sandhu et al., 1993; 1994; Han et al., 2012). CYP1A1, 1A2, 1B1, 2A6, 2A13, 2C9, and 3A4, NADPH-P450 reductase, and b5 were purified from membranes of recombinant E. coli as described elsewhere (Parikh et al., 1997; Sandhu et al., 1993; 1994; Guengerich, 2015; Han et al., 2012; Shimada, 2017).
Oxidation of flavone and flavanone by P450 enzymes
Oxidative metabolism of flavone and flavanone by P450 enzymes was determined in a standard incubation mixture (0.25 ml) containing a reconstituted monooxygenase system consisted of each purified P450 (50 pmol), NADPH-P450 reductase (100 pmol), b5 (100 pmol, in the cases of CYP2A6, 2A13, 2C9, and 3A4), L-α-dilauroyl-sn-glycero-3-phosphocholine (DLPC) (50 µg), and an NADPH-generating system (0.5 mM NADP+, 5 mM glucose 6-phosphate, and 0.5 unit of yeast glucose 6-phosphate dehydrogenase/ml) (Yamazaki et al., 2002, Shimada et al., 2015). Flavone and flavanone were dissolved in (CH3)2SO as 10 mM stock solutions and were added at the final solvent concentration ≤0.5%, v/v.
Incubations were carried out at 37 °C for 20 min, following a pre-incubation time of 1 min before adding an NADPH-generating system. Each reaction was terminated by the addition of 0.5 ml of ice-cold CH3CN. The mixture was mixed vigorously (with a vortex device) and centrifuged at 10,000 × g for 5 min. The upper CH3CN layer was collected and stored at −20 °C; an aliquot of this layer was analyzed with LC-MS/MS.
LC-MS/MS analyses were performed using an HPLC system (ACQUITY UPLC I-Class system; Waters, Milford, MA) coupled to a tandem quadruple mass spectrometer (XevoTQ-S; Waters) by the methods described earlier, with modifications (Kakimoto et al., 2015; Shimada et al., 2016c). Chromatographic separation was performed on an octadecylsilane (C18) column (CORTECS C18, 100 × 2.1 mm i.d., 1.6 µm; Waters) at 45 ºC. The gradient elution was done using a mixture of solvents A (10 mM ammonium acetate containing 0.1% formic acid) and B (5% methanol in acetonitrile containing 0.1% formic acid) with B increasing from 20% (v/v) to 80% (v/v) over 16 minutes, at a flow rate of 0.2 ml/min.
MS/MS analysis was performed in a positive electrospray ionization mode with capillary voltage of 3000 V and cone voltage of 30 V. Collision energies of 20 eV, 30 eV, and 35 eV were used for analyzing flavanone and mono-hydroxylated flavanones, for flavone and mono-hydroxylated flavones, and for di-hydroxylated flavones, respectively. The selected reaction-monitoring mode was used to identify and quantify flavone and flavanone and their products. The product ions (m/z 223 for flavone, 239 for mono-hydroxylated flavones, 241 for mono-hydroxylated flavanones, and 255 for di-hydroxylated flavones) were scanned to obtain mass spectral chromatograms.
Other assays
P450 and protein contents were determined by the methods described previously (Omura and Sato, 1964; Brown et al., 1989).
Docking simulations of flavone and flavanone into CYP2A6 and 2A13
Crystal structures of CYP2A6 bound to 4,4´-dipyridyl disulfide (PBD 2FDY), an inhibitor of CYP2A6 (Fujita and Kamataki, 2001), and CYP2A13 bound to pilocarpine (PDB 3T3S) have been reported and were used in this study (Yano et al., 2006; DeVore et al., 2012; Shimada et al., 2016b). Simulations were carried out after removing each ligand from these P450 structures using the MMFF94x force field described in the MOE software (ver. 2015.10, Computing Group, Montreal, Canada). Ligand-interaction energies (U values) were obtained by use of the program ASEdock in MOE. Lower U values are indicative of stronger interaction between a chemical and the enzyme.
Kinetic analysis
Kinetic parameters were estimated by nonlinear regression analysis of hyperbolic plots using the program Kaleida-Graph (Synergy Software, Reading, PA, USA) or GraphPad Prism (GraphPad, La Jolla, CA, USA). Correlation coefficients were determined using the Curve Fit program of Cricket Graph (version III).
Results
Oxidation of Flavone by Human P450 Enzymes
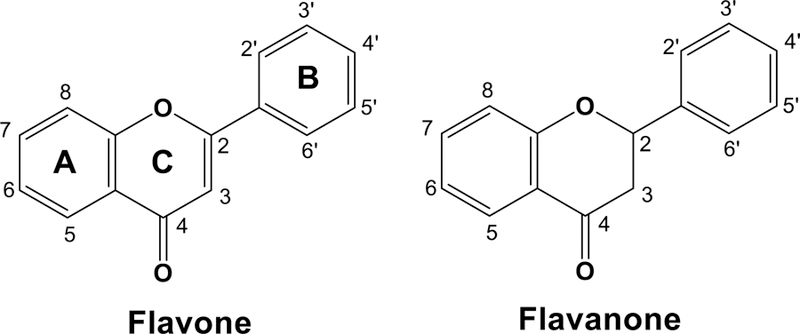
Flavone was incubated with reconstituted monooxygenase systems containing CYP1A2 and 2A6, and the products obtained were analyzed by LC-MS/MS for detecting mono-hydroxylated products using the transitions m/z 239>165 (solid line) and 239>129 (dotted line) (Figure 2). Because CYP2A6 produced a very little amount of mono-hydroxyflavone M2, only the chromatogram obtained with CYP1A2 is shown in Figure 2A. At least nine mono-hydroxylated products (OHF M1 and 5OHF were not included because their elution time was 3.7 min and 11.2 min, respectively) were formed on incubation of flavone with CYP1A2 and CYP2A6 and one of them (retention time, 6.6 min) was identified as 6-hydroxyflavone (6OHF) (Figures 2A and 2E). A number of fragment ions were detected in 6OHF (Figure 2E) and the major m/z 102.9 (phenylacetylene ion) and m/z 136.9 (hydroxylated quinoid-type ion) (Sasaki et al., 1966; Das et al., 1973; Kagawa et al., 2004 Nikolic and Breemen, 2004) were also found in the products oxidized at A-ring, such as standards 5OHF and 7OHF (results not shown), as well as 6OHF (Figure 2E). Similar patterns of these fragments were detected in uncharacterized OHF M4 and OHF M7 (Figure 2C and 2D, respectively) and also in OHF M1 (results not shown), suggesting that these metabolites are the products oxidized at A-ring. On the other hand, OHF M3 was found to contain major fragments of m/z 120.9 (quinoid-type ion) and m/z 118.8 (hydroxylated phenylacetylene ion) (Figure 2B), suggesting that the product is oxidized at B-ring and such fragment ions were also found in uncharacterized OHF M1, M5, and M6 (data not shown).
Figure 2.
Metabolism of flavone by human CYP1A2 and CYP2A6 to form mono-hydroxylated flavones. LC-MS chromatograms of formation of mono-hydroxylated metabolites (6OHF and M2, M3, M4, M5, M6, and M7) with CYP1A2 (A) are shown in the upper panels of the figure. (Formation of M-1 was not shown in these figures because the retention time of M-1 was 3. 7 min.) Detection of metabolites was made by using the transitions m/z 239>165 (solid line) and 239>129 (dotted line). The reconstructed mass chromatograms of mono-hydroxylated metabolites of OHF M3 (B), OHF M4 (C), and OHF M7 (D) detected with CYP2A6 and of standard 6OHF (E) are indicated in the lower part of the figure. The suggested structures of fragments of these metabolites are included in the figures.
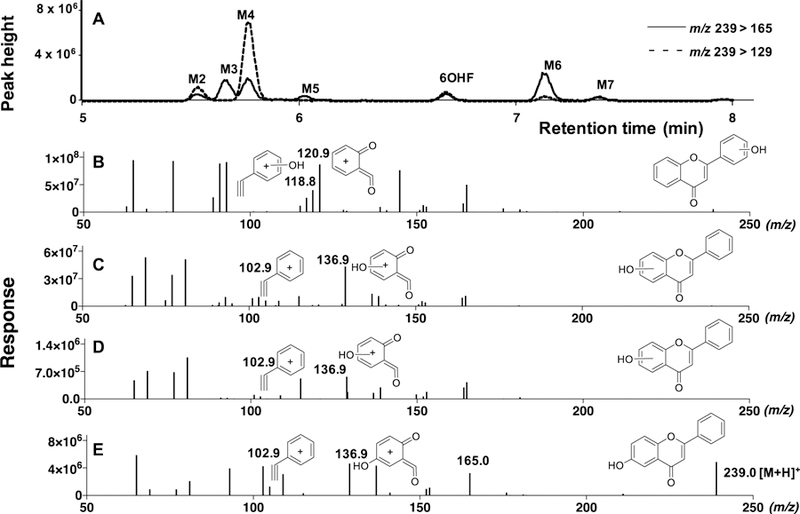
We also examined mass chromatograms with trace at m/z 255>153, showing formation of di-hydroxylated metabolites of flavone with CYP2A6 (Figure 3A). LC chromatographic separation showed the formation of three di-hydroxyflavones and comparison of fragmentation patters of these products was carried out with that of a standard 5,7-dihydroxyflavone (Figure 3E). The suggested chemical structures of fragment of m/z 102.9 (phenylacetylene ion) and m/z 152.9 (dihydroxylated quinoid-type ion) with 5,7diOHF (and also with 7,8diOHF, results not shown) was shown in Figure 3E and we found similar fragment patterns in diOHF M3 (Figure 3B) and diOHF M5 (Figure 3D), indicating that these products are di-oxidized at A-ring. Fragments with m/z 134.9 (dihydroxylated phenylacetylene ion) and m/z 121.0 (quinoid-type ion) in diOHF M4 were found to be different and were suggested to be di-oxidized at B-ring (Figure 3C).
Figure 3.
Metabolism of flavone by CYP2A6 to form di-hydroxylated flavones. LC-MS chromatograms of formation of di-hydroxylated metabolites (diOHF M3, diOHF M4, and diOHF M5) detected using the transitions m/z 255>153 are shown in Figure 3A. The reconstructed mass chromatograms of di-hydroxylated metabolites of diOHF M3 (B), diOHF M4 (C), and diOHF M5 (D) and of standard 5,7diOHF (E) are indicated in the lower part of the figure. The suggested structures of fragments of these metabolites are included in the figures.
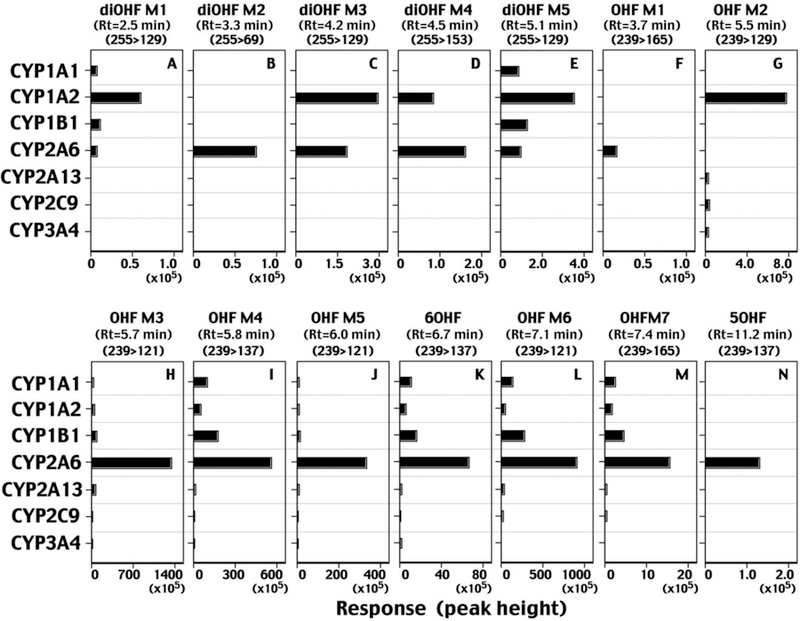
Kinetic analysis of formation of several oxidation products of flavone was determined using incubation time of 20 min and protein concentration of 0.1 µM CYP2A6 in the reconstituted monooxygenase system (Supplemental Figure 1); our preliminary studies showed that formation of flavone and flavanone metabolites by CYP 2A6 increased linearly for about 40 min and P450 concentrations up to 0.2 µM (results not shown). CYP2A6 oxidized flavone to OHF M3 at a high rate, followed by formation of OHF M6, OHF M4, and OHF M5, having Km values of 15, 39, 14, and 21 µM, respectively (Supplemental Figure 1A). The Km values of formation of other flavone oxidation products of 6OHF, OHF M2, OHF M7, and OHF M5 by CYP2A6 were 20, 24, 28, and 10 µM, respectively (Supplemental Figure 1B).
Formation of flavone metabolites (5OHF, 6OHF, three diOHFs, and seven uncharacterized mono-hydroxylated ones) by seven human P450 enzymes was determined using LC-MS/MS (Figure 4). Since we did not have standards of several mono-and di-hydroxylated products, the formation of these products was compared with peak integral response catalyzed by individual P450 enzymes (see horizontal axis). The most abundant peak formed on incubation of flavone with CYP2A6 was OHF M3 (maximum value, 1,400 × 105), followed by OHF M6 (1,000 × 105), OHF M4 (600 × 105), OHF M5 (500 × 105), and 6OHF (60 × 105), indicating that formation of the former four mono-hydroxylated products was greater than 6OHF, although quantities could not be determined except that formation of 6OHF was calculated to be 0.22 nmol/min/nmol CYP2A6 (Table 1). In many cases, CYP2A6 was most active in catalyzing flavone to oxidative products, except that formation of diOHF M1, M3, and M4 and OHF M2 with CYP1A2 was equal to or more than for CYP2A6 (Figure 4).
Figure 4.
Metabolism of flavone by human P450 enzymes. Formation of mono-hydroxylated and di-hydroxylated metabolites of flavone by individual forms of P450s is shown in the figure. Detection of individual metabolites on LC-MS/MS analysis was made using the indicated m/z and the response (intensity) was shown using peak height on the chromatograms.
Table 1.
Oxidation of flavone by human P450 enzymes
| P450 | Flavone oxidation | ||
|---|---|---|---|
| Hydroxyflavone formation (nmol/min/nmol P450) | |||
| 5OHF | 6OHF | 7OHF | |
| CYP1A1 | <0.001 | 0.031 ± 0.004 | <0.001 |
| CYP1A2 | <0.001 | 0.016 ± 0.001 | <0.001 |
| CYP1B1 | <0.001 | 0.053 ± 0.002 | <0.001 |
| CYP2A6 | 0.007±0.002 | 0.22 ± 0.01 | <0.001 |
| CYP2A13 | <0.001 | 0.003 ± 0.001 | <0.001 |
| CYP2C9 | <0.001 | 0.001± 0.001 | <0.001 |
| CYP3A4 | <0.001 | 0.002 ± 0.001 | <0.001 |
Abbreviations used in this table: 5OHF, 5-hydroxyflavobe; 6OHF, 6-hydroxyflavone; 7OHF, 7-hydroxyflavone.
Data are presented a means and SD (range) of duplicate determinations.
The turnover numbers for formation of 5OHF, 6OH, and 7OHF by seven forms of human P450 enzymes were determined. Two metabolites (5OHF, 6OHF) were formed by CYP2A6 at the highest rates, and 7OHF was not formed by any of these P450 enzymes (Table 1).
Oxidation of Flavanone by Human P450 Enzymes
Flavanone was incubated with CYP2A6 and products formed were determined with LC-MS/MS analysis using the transition m/z 223>129 (Figure 5). HPLC chromatograms showed a major peak of retention time at 8.7 min (Figure 5C), similar to that of standard flavone (Figure 5A). Comparison of mass fragments derived from chromatographic peaks from standard flavone (Figure 5B) and incubates with CYP2A6 (Figure 5D) indicated oxidation of flavanone at C2 or C3 position of the C-ring to form flavone by CYP2A6. The suggested chemical structures of fragments of m/z 102.9 (phenylacetylene ion), m/z 121.0 (quinoid-type ion), and m/z 92.0 ion (after abstraction of CO) were included in the figure (Figure 5D).
Figure 5.
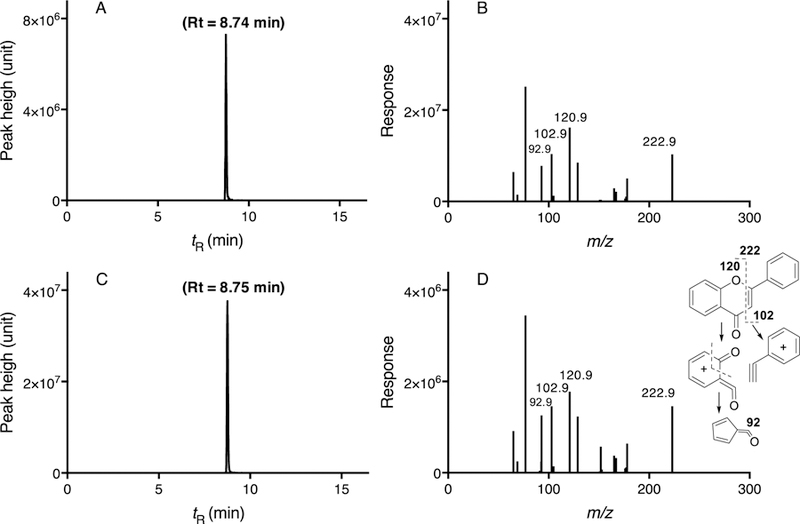
Metabolism of flavanone to flavone by CYP2A6. LC-MS/MS chromatograms of flavone standard (A) and incubates of flavanone with CYP2A6 (C) and the mass fragments of flavone standard (B) and flavanone metabolites with CYP2A6 (D) are shown. The suggested structures of fragments of flavanone metabolism are included in the figure.
Several oxidative products other than flavone were detected on incubation of flavanone with CYP1A2 using the transitions m/z 241>121 (Figure 6A), 241>131 (Figure 6B), and 241>137 (Figure 6C) on LC-MS/MS analyses. CYP1A2 produced four mono-hydroxylated flavanones, 4´OHFra, 3´OHFra, 2´OHFra, and OHFra M2 with m/z 241>121 (Figure 6A) and 7OH, 6OH, OHFraM1, and OHFraM3 with m/z 241>131 (Figure 6B) and 241>137 (Figure 6C). Major fragments obtained from products (e.g. 4´OHFva and 3´OHFva) that were the oxidation products with modification flavanone at the B-ring contained m/z 120.9, 118.9, and 92.9 (as indicated in the suggested chemical structures in Figure 6); these fragment patterns were well correlated with those of standard products and incubates of flavanone with CYP1A2, e.g. 4´OHFva, 3´OHFva, and OHFva M2 (Figures 6D, 6E, and 6F). Similarly, oxidation products at A-ring contained major fragments of m/z 136.9 and 102.9, and these fragments of standard 6OHFra, 7OHFra, and OHFva M3 were well correlated with those from the incubates of flavanone with CYP1A2 and CYP2A6 (Figures 6G, 6H, and 6I). Correlations of these fragment patterns in standard mono-hydroxylated flavanones and those in products from incubates with CYP1A2 and CYP2A6 were made (Supplemental Table 1). It was evident that the fragment patterns obtained from oxidation products at A-ring, such as 6OHFva and 7OHFva, were well correlated with those of OHFva M1 and OHFva M3, when the intensities (peak height) were compared with major fragments of m/z 102.9, 131.0, 136.9, 80.9, 108.9, 106.8, 77.2, 78.8, and other minor fragments (total of 37 fragments) (Supplemental Table 1). On the other hand, the patterns obtained from oxidation products at B-ring, such as 2´-, 3´-, and 4´-OHFva, were well correlated with that of OHFva M2, when fragments of m/z 120.9, 92.9, 64.9, 118.9, 64.9, 90.9, 146.8, 152.2, 164.8, and others (total 37 fragments) are considered (Supplemental Table 1). These results suggest that M1 and M3 are the products oxidized at A-ring and M2 is at B-ring.
Figure 6.
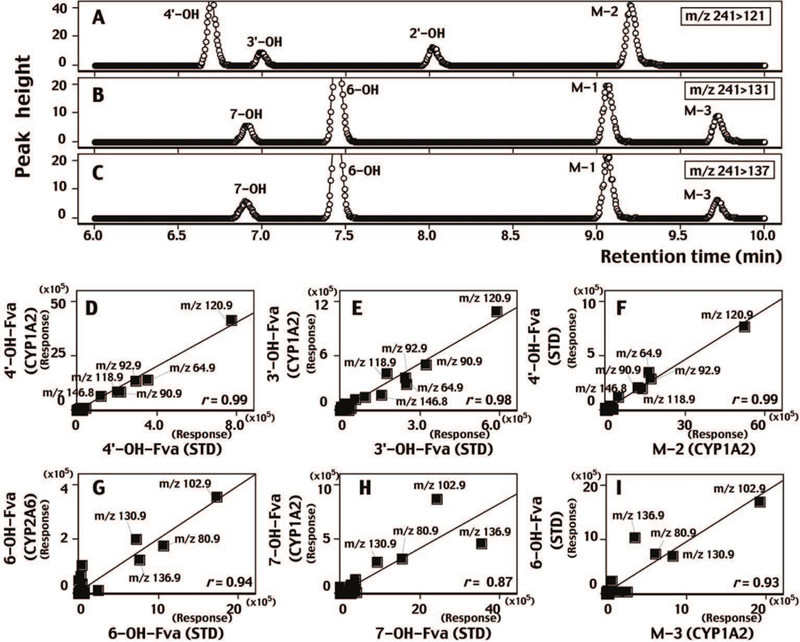
LC-MS/MS analysis of metabolism of flavanone by CYP1A2 by detecting m/z 241>121 (A), m/z 241>131 (B), and m/z 241>137 (C). Fragments of standard 4´-hydroxyflavanone (4’-OH-Fva, D and F), 3´-hydroxyflavanone (3’-OH-Fva, E), 6-hydroxyflavanone (6-OH-Fva, G and I), and 7-hydroxyflavanone (6-OH-Fva, H) were plotted for respective metabolites obtained with CYP1A2 (D, E, F, and H) and with CYP2A6 (G). Unidentified M-2 and M-3 metabolites were plotted with standards 4’-OH-Fva and 6-OH-Fva, respectively. The suggested structures of fragments of these metabolites are included in the figures.
Kinetic analysis showed that Km values of formation of 2´OHFva, flavone, 6OHFva, and 4´OHFva were 12, 18, 11, and 13 µM, respectively (Supplemental Figure 2A). The Km values of other flavanone products, OHFva M1, OHFva M2, 3’OHFva, OHFva M3, and 7OHFva, were 49, 41, 19, 39, and 15 µM, respectively (Supplemental Figure 2B).
Flavanone metabolism by seven human P450 enzymes was determined and compared (Figure 7). As in a case for flavone metabolism, we compared relative intensities of oxidation of flavanone by measuring peak height at indicated fragments (m/z) in the figure. Again, CYP2A6 was found to be very active in catalyzing flavanone and the results suggest that the major oxidation products—2´OHFva, 6OHFva, and flavone, which had higher peak response (vide infra)— were the major oxidation products with CYP2A6, and the turnover number were determined to be 4.8, 1.3, and 0.72 nmol/min/nmol CYP2A6, respectively (Table 2). Formation of flavone from flavanone was found in CYP2A13, with a turnover number of 0.29 min−1, but other P450s examined were not so active in this reaction (Figure 7 and Table 2). Interestingly, CYP2A6 also produced several oxidation products with minor quantities on incubation of flavanone, e.g. OHF M3, OHF M4, OHF M5, OHF M6, and 6OHF. CYP1A2 catalyzed oxidation of flavanone to form 3´OHFva-, 4´OHFva -, 7OHFva, OHFva M2, and OHFva M3 more efficiently than did CYP2A6 (Figure 7).
Figure 7.
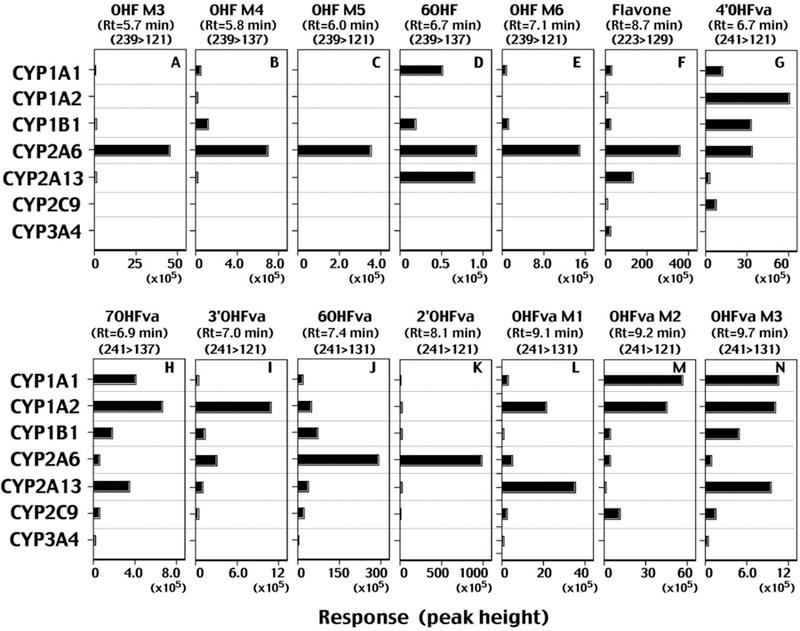
Metabolism of flavanone by human P450 enzymes. Formation of flavone and mono-hydroxylated flavanones by individual forms of P450s are shown in the figure. Detection of individual metabolites on LC-MS/MS analysis was made using the indicated m/z and the response (intensity) was shown using peak height on the chromatograms.
Table 2.
Oxidation of flavanone by human P450 enzymes
| P450 | Flavanone oxidation | |||||||
|---|---|---|---|---|---|---|---|---|
| Formation of flavone and hydroxylated products (nmol/min/nmol P450) | ||||||||
| Flavone | 6OHF | 7OHF | 6OHFva | 7OHFva | 2´-OHFva | 3´-OHFva | 4’´-OHFva | |
| CYP1A1 | 0.053 ± 0.003 | 0.002 ± 0.001 | <0.001 | 0.058 ± 0.008 | 0.007 ± 0.003 | 0.008 ± 0.004 | 0.004 ± 0.002 | 0.074 ± 0.002 |
| CYP1A2 | 0.008 ± 0.001 | <0.001 | 0.002 ± 0.002 | 0.19 ± 0.02 | 0.009 ± 0.001 | 0.074 ± 0.012 | 0.117 ± 0.023 | 0.33 ± 0.05 |
| CYP1B1 | 0.046 ± 0.002 | 0.002 ± 0.001 | <0.001 | 0.33 ± 0.05 | 0.003 ± 0.001 | 0.046 ± 0.009 | 0.013 ± 0.002 | 0.19 ± 0.04 |
| CYP2A6 | 0.72 ± 0.06 | 0.003 ± 0.001 | <0.001 | 1.29 ± 0.08 | <0.001 | 4.76 ± 0.32 | 0.031 ± 0.003 | 0.20 ± 0.01 |
| CYP2A13 | 0.29 ± 0.02 | 0.004 ± 0.001 | <0.001 | 0.17 ± 0.01 | 0.005 ± 0.001 | 0.027 ± 0.002 | 0.011 ± 0.004 | 0.013 ± 0.001 |
| CYP2C9 | 0.009 ± 0.002 | <0.001 | <0.001 | 0.091± 0.001 | <0.001 | 0.012 ± 0.001 | 0.003± 0.001 | 0.040± 0.001 |
| CYP3A4 | 0.057 ± 0.011 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Abbreviations used: 6OHF, 6-hydroxyflavone; 7OHF, 7-hydroxyflavone; 6OHFva, 6-hydroxyflavanone; 7OHFva, 7-hydroxyflavanone 2´OHFva, 2´-hydroxyflavanone; 3´OHFva, 3´-hydroxyflavanone; 4´OHFva, 4´-hydroxyflavanone.
Data are presented as means and SD (range) of duplicate determinations.
Molecular Docking of Interaction of Flavone and Flavanone with CYP2A6 and 2A13
These results suggest that CYP2A6 is highly active in catalyzing oxidation of flavone and flavanone to the observed products. We determined molecular interactions of these flavonoids with CYP2A6 and compared those with CYP2A13 (Figures 8 and 9). Crystal structures of CYP2A6 bound to 4,4´-dipyridyl disulfide (PBD 2FDY) (Yano et al., 2006) and CYP2A13 bound to pilocarpine (PDB 3T3S) (DeVore et al., 2012) were used for analyses.
Figure 8.
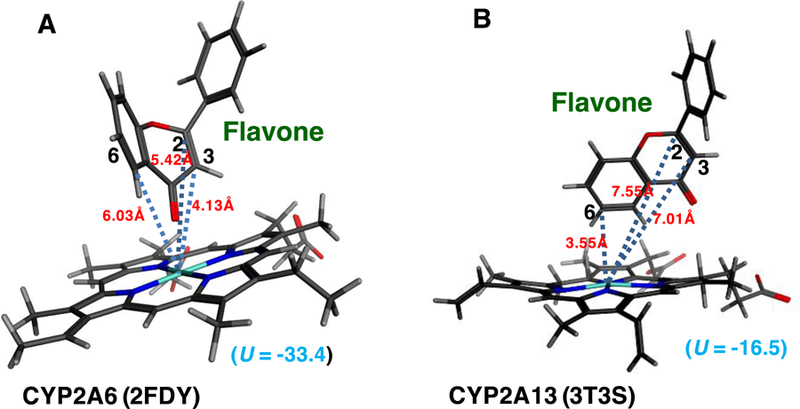
Molecular simulation of interaction of flavone with active sites of CYP2A6 (A) and CYP2A13 (B).
Figure 9.
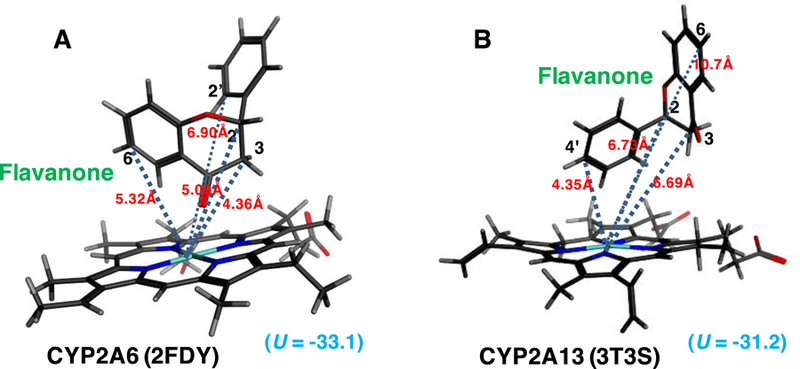
Molecular simulation of interaction of flavanone with active sites of CYP2A6 (A) and CYP2A13 (B).
The distances between C2, C3, and C6 of flavone and iron center of CYP2A6 2FDY were calculated to be 5.42, 4.13, and 6.03Å, respectively, and those of the flavone and the CYP2A13 3T3S iron were 7.55, 7.01, and 3.55Å, respectively (Figures 8A and 8B). The ligand-interaction energy (U value) of interaction of flavone with active site of CYP2A6 was found to be –33.4, which was low compared with that of CYP2A13 (U = −16.5).
On the other hand, the molecular interaction of flavanone with CYP2A6 and 2A13 was found to be different in that the A-ring of flavanone was far from the iron center of CYP2A13 as compared with that of CYP2A6 (Figure 9). Distances between C2, C3, C6, C2´, and C4´ of flavanone and the iron center of CYP2A6 was calculated to be 5.09, 4.36, 5.32, 6.90, and 9.28Å, respectively, and C2, C3, C6, C2´, and C4´ of flavanone the CYP2A13 was 6.73, 6.69, 10.7, 4.35, and 4.35Å, respectively (Figures 9A and 9B). The interaction of flavanone with active site of CYP2A6 (U value = −33.1) was not so different from that of CYP2A13 (U = −31.2).
Discussion
Formation of flavone from flavanone has been reported in several laboratories using rat liver microsomes and recombinant human CYP1A1, 1A2, and 2B6 enzymes (NiKolic and van Breemen, 2004; Kagawa et al., 2004). Similar reactions have been observed with substituted flavanones with plant P450s, where CYP93G2 (Oryza sativa (rice)), CYP93B1 (Glycyrrhiza echinata,(licorice)) and CYP93B10 (Medicago truncatula) function as a flavone 2-hydroxylase (F2H), while CYP93G1 (O. sativa), CYP93B2 (Gerbera cultivars), CYP93B6 (Perilla frutescens), and CYP93B3 (Antirrhinum majus, snapdragon) catalyze flavone synthase II (FNSII) activities to desaturate flavanones to form the respective flavones (Lam et al., 2014; Akashi et al., 1998; 1999; Kitada et al., 2001; Du et a., 2010; Fliegmann et al., 2010).
Mechanistically there are two major routes of formation of flavone from flavanone by P450s (Figure 10). In both cases the reaction begins with hydrogen abstraction from the C2 or C3 carbon of C-ring. Oxygen rebound gives 2-or 3-hydroxyflavanone. 3-Hydroxyflavanone was reported as a product of flavanone by Nikolic and van Breeman (2004). 2-Hydroxynaringenin was reported as a product of naringenin, as well as a (glycosylated) ring-opened product (Du et al., 2010) (expected because the 2-hydroxyflavanone is a hemiketal). (The assignments of these cited structures were based only on mass spectral fragmentation.) An alternate pathway involves a direct desaturation, as shown in Figure 10. We favor this latter possibility, in that we did not detect any 2-or 3-hydroxy flavanone products (although we can only assume that OHFva M1 and M3 are not 2-or 3-hydroxylated products; these products were suggested to be the oxidation products at A-ring of flavanone (Figure 6 and Supplemental Table 1). Kagawa et al. (2004) reported a kinetic deuterium isotope effect of 1.5 for the conversion of flavanone to flavone by rat liver microsomes, but this result is not revealing as to what the mechanism is or, if desaturation occurs without hydroxylation, which hydrogen atom is abstracted first. Clear evidence has been observed for P450-catalyzed desaturation reactions in the absence of dehydration (Guengerich and Kim, 1991; Wang et al., 1991; Kaminsky et al., 1980; Johnson et al., 2017; Guengerich, 2001; Rettie et al., 1987; Guan et al., 1998; Fasco et al., 1978; Nagata et al., 1986). Usually desaturation is a minor pathway relevant to hydroxylation in mammals, but in the case of human CYP27C1 the hydroxylations of vitamin A1 are very minor pathways relative to desaturation (Johnson et al., 2017). In the present case, also, no hydroxylated products were detected. (However, P450s catalyzing predominantly only desaturases are known in plants (Morikawa et al., 2006; Arnqvist et al., 2008) and yeast (Skaggs et al., 1996; Kelly et al., 1997). Non-heme desaturases are important in oxidation of fatty acids and some other alkanes, and a few residues have been shown to alter the balance of desaturation and hydroxylation (Broun et al, 1998; Broadwater et al., 2002).
Figure 10.
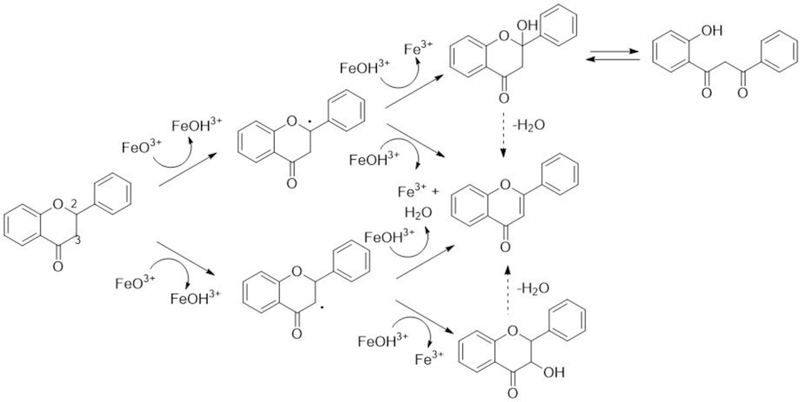
Proposed mechanisms for oxidation of flavanone to form flavone by P450 enzymes.
Our results showed that CYP2A6 oxidized flavanone to form 2´-and 6-hydroxyflavanone at turnover rates of 4.8 and 1.3 nmol/min/nmol CYP2A6, respectively. Uno et al. (2013; 2015) have reported the turnover number of formation of 2´-and 6-hydroxyflavanone at 0.32 min−1 and 0.25−1, respectively, by wild-type CYP2A6 and did not report formation of flavone from flavanone. It should also be noted that CYP2A6, 1A1, 1A2, and 2A13 produced 6-hydroxyflavone as well as 6-hydroxyflavanone on incubation of flavanone, although formation of the former metabolite was very low (Table 2). It is not known whether 6-hydroxyflavone is formed via the oxidation of flavone or 6-hydroxyflavanone when incubated flavanone with P450 enzymes. The former reaction, flavone 6-hydroxylation, was catalyzed by CYP2A6, 1A1, 1A2, and 2A13 at rates of 0.22, 0.031, 0.016, and 0.003 nmol/min/nmol P450 when flavone was used as a substrate (Table 1).
LC-MS/MS analysis using positive electrospray ionization mode gave us information about chemical structures of a variety of metabolites that formed through metabolism of flavone and flavanones. Major fragment ions of m/z 102.9 (phenylacetylene ion) and m/z 136.9 (hydroxylated quinoid-type ion) (Sasaki et al., 1966; Das et al., 1973; Kagawa et al., 2004 Nikolic and Breemen, 2004) were detected in mono-hydroxyflavone metabolites that are oxidized at A-ring, such as 5-, 6-, and 7-hydroxyflavones and also uncharacterized OHF M1, M4, and M7 (Figure 2). The OHF M1, M3, M5, and M6 that contained major fragments of m/z 120.9 (quinoid-type ion) and m/z 118.8 (hydroxylated phenylacetylene ion) were suggested to be the products oxidized at B-ring (Figure 2). The formation of di-hydroxyflavones was measured with fragments of m/z 102.9 (phenylacetylene ion) and m/z 152.9 (dihydroxylated quinoid-type ion) for the di-oxidized at A-ring and m/z 134.9 (dihydroxylated phenylacetylene ion) and m/z 121.0 (quinoid-type ion) for the products di-oxidized at B-ring (Figure 3). The formation of flavone from flavanone by P450s was determined using fragments of m/z 102.9 (phenylacetylene ion), m/z 121.0 (quinoid-type ion), and also m/z 92.0 ion (minus CO from quinoid-type) (Sasaki et al., 1966; Das et al., 1973; Kagawa et al., 2004; Nikolic and Breemen, 2004). Flavanone oxidation was also determined using fragment ions of m/z 120.9, 118.9, and 92.9 for the products oxidized at B-ring and fragments of m/z 136.9 and 102.9 for the oxidation products at A-ring.
Molecular docking analysis suggested that there are differences in interaction of flavanone with the iron center of CYP2A6 and 2A13. The A-ring of flavanone was located close to the iron in CYP2A6 while B-ring of flavanone was close to the iron center of CYP2A13, when CYP2A6 2FDY (Yano et al., 2006) and CYP2A13 3T3S (DeVore et al., 2012) were used for analysis (Figure 9). We did not find such differences in molecular docking analysis of interaction of flavone with these P450 enzymes, although the ligand-interaction energy (U value) of interaction of flavone with active site of CYP2A6 was found to be lower (U = –33.4) than that of CYP2A13 (U = −16.5) (Figure 8). Flavanone does not have an apparent conjugated double bond at the C-ring which might control any free rotation of the B-ring, in comparison with the case of flavone. This could facilitate different docking manners into a small substrate pocket of CYP2A6 (Figure 9) and a relatively larger pocket of CYP2A13 due to the poor planar configuration of flavanone (DeVore and Scott, 2012). It is not known at present whether or not these differences in molecular interaction of flavone and flavanone with CYP2A6 and 2A13 relate to the catalytic differences in the oxidation of flavone and flavanone by these P450s
Our present studies show the importance of CYP2A6 in the oxidation of flavone and flavanone, and we have previously shown that both CYP2A6 and CYP2A13 are the major human P450 enzymes in the oxidation of acenaphthene, acnaphthene, pyrene, 1-nitropyrene, 1-acetylpyrene, and naphthalene, phenanthrene, biphenyl, and their derivatives, 2,5,2´,5´-tetrachlorobiphenyl, and 1-chloropyrene (Shimada et al., 2015; 2016a; 2016b; 2016c). These results, in conjunction with our present observations, suggest that both CYP2A6 and 2A13 enzymes play important roles of metabolism of wide range of environmental and natural and endogenous chemicals that show various pharmacological and toxicological effects in humans.
In conclusion, present results showed that human P450 enzymes oxidize flavone and flavanone, two dietary unsubstituted flavonoids, to various hydroxylated products that were detected LC-MS/MS analysis. Among the seven forms of P450 enzymes examined, CYP2A6 had the highest catalytic activities towards both flavonoids. Flavone was oxidized at higher rates by CYP2A6 and CYP1A2 to form uncharacterized seven mono-and five di-hydroxylated metabolites as well as 5-and 6-hydroxyflavones. Four mono-hydroxylated products—OHF M3, OHF M6, OHF M4, and OHF M5—were suggested to be formed by CYP2A6 more rapidly than 6OHF (turnover rate of 0.22−1). Interestingly, CYP2A6 was more active in catalyzing the oxidation of flavanone than flavone and we found that the turnover rate (nmol product/min/nmol CYP2A6) was the highest in forming 2´OHFva (4.76 min−1), followed by 6OHFva (1.29 min−1) and flavone (0.72 min−1). The formation of flavone from flavanone by CYP2A6 was the highest among the seven human P450 enzymes examined. The chemical mechanisms of desaturation of flavanone to form flavone are proposed in this study.
Supplementary Material
Acknowledgements
This study was supported in part by JSPS KAKENHI [16K09119] (to K. K.), [15K07770] (to S. T.), [16K21710] (to H. N), [17K08426] (to N. M.), and [17K08425] (to H. Y.), National Research Foundation of Korea [NRF-2016R1D1A1B03932002] (to D. K.), and United States Public Health Service grant [R01 GM118122] (to F. P. G.).
Abbreviations used:
- P450 or CYP
cytochrome P450
- b5
cytochrome b5
- OHF
mono-hydroxyflavone
- diOHF
di-hydroxyflavone
- OHFva
mono-hydroxyflavanone
Footnotes
Declaration of interest Statement
The authors declare no conflict of interest associated with this manuscript.
References
- Akashi T Aoki T and Ayabe S (1998) Identification of a cytochrome P450 cDNA encoding (2S)-flavanone 2-hydroxylase of licorice (Glycyrrhiza echinata L.; Fabaceae) which represents licodione synthase and flavone synthase II. FEBS Lett 431:287–90. [DOI] [PubMed] [Google Scholar]
- Akashi T Fukuchi-Mizutani M Aoki T Ueyama Y Yonekura-Sakakibara K Tanaka Y Kusumi T and Ayabe S (1999) Molecular cloning and biochemical characterization of a novel cytochrome P450, flavone synthase II, that catalyzes direct conversion of flavanones to flavones. Plant Cell Physiol 40:1182–1186. [DOI] [PubMed] [Google Scholar]
- Arnqvist L Persson M Jonsson L Dutta PC and Sitbon F (2008) Overexpression of CYP710A1 and CYP710A4 in transgenic Arabidopsis plants increases the level of stigmasterol at the expense of sitosterol. Planta 227, 309–317. [DOI] [PubMed] [Google Scholar]
- Arct J and Pytkowska K (2008) Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol 26, 347–357. [DOI] [PubMed] [Google Scholar]
- Berim A and Gang DR. (2013) The roles of a flavone-6-hydroxylase and 7-O-demethylation in the flavone biosynthetic network of sweet basil. J Biol Chem 288:1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breinholt VM Offord EA Brouwer C Nielsen SE Brøsen K and Friedberg T (2002) In vitro investigation of cytochrome P450-mediated metabolism of dietary flavonoids. Food Chem Toxicol 40:609–616. [DOI] [PubMed] [Google Scholar]
- Broun P Shanklin J Whittle E and Somerville C (1998) Catalytic plasticity of fatty acid modification enzymes underlying chemical diversity of plant lipids. Science 282, 1315–1317. [DOI] [PubMed] [Google Scholar]
- Broadwater JA., Whittle E and Shanklin J (2002) Desaturation and hydroxylation. Residues 148 and 324 of Arabidopsis FAD2, in addition to substrate chain length, exert a major influence in partitioning of catalytic specificity. J. Biol. Chem 277, 15613–15620. [DOI] [PubMed] [Google Scholar]
- Brown RE Jarvis KL and Hyland KJ (1989) Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem 180: 136–139. [DOI] [PubMed] [Google Scholar]
- Devore NM and Scott EE (2012) Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone binding and access channel in human cytochrome P450 2A6 and 2A13 enzymes. J Biol Chem 287: 26576–26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVore NM Meneely KM Bart AG Stephens ES Battaile KP and Scott EE (2012) Structural comparison of cytochromes P450 2A6, 2A13, and 2E1 with pilocarpine. FEBS J 279: 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doostdar H Burke MD and Mayer RT (2000) Bioflavonoids: selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology 144, 31–38. [DOI] [PubMed] [Google Scholar]
- Du Y Chu H Chu IK and Lo C (2010) CYP93G2 is a flavanone 2-hydroxylase required for C-glycosylflavone biosynthesis in rice. Plant Physiol 154:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasco MJ Dymerski PP Wos JD, and Kaminsky LS (1978) A new warfarin metabolite: structure and function. J Med Chem 21, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Fliegmann J Furtwängler K Malterer G Cantarello C Schüler G Ebel J and Mithöfer A (2010) Flavone synthase II (CYP93B16) from soybean (Glycine max L.). Phytochemistry 71:508–514. [DOI] [PubMed] [Google Scholar]
- Fujita K and Kamataki T (2001) Screening of organosulfur compounds as inhibitors of human CYP2A6. Drug Metab Dispos 29: 983–9. [PubMed] [Google Scholar]
- Guan X Fisher MB Lang DH Zheng YM Koop DR and Rettie AE (1998) Cytochrome P450-dependent desaturation of lauric acid: isoform selectivity and mechanism of formation of 11-dodecenoic acid. Chem-Biol Interact 110, 103–121. [DOI] [PubMed] [Google Scholar]
- Guengerich FP (2001) Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol 14, 611–650. [DOI] [PubMed] [Google Scholar]
- Guengerich FP and Kim DH (1991) Enzymatic oxidation of ethyl carbamate to vinyl carbamate and its role as an intermediate in the formation of 1,N6-ethenoadenosine. Chem Res Toxicol 4, 413–421. [DOI] [PubMed] [Google Scholar]
- Guengerich FP Chun Y-J Kim D Gillam EMJ and Shimada T (2003) Cytochrome P450 1B1: A target for inhibition in anticarcinogenesis strategies. Mut. Res 523–524, 173–182. [DOI] [PubMed] [Google Scholar]
- Guengerich FP (2015) Human cytochrome P450 enzymes, in Cytochrome P450: Structure, Mechanism, and Biochemistry (Ortiz de Montellano PR, Ed.) 4th ed., pp 523–785, Springer, New York. [Google Scholar]
- Han S Choi S Chun YJ Yun CH Lee CH Shin HJ Na HS Chung MW and Kim D (2012) Functional characterization of allelic variants of polymorphic human cytochrome P450 2A6 (CYP2A6*5, *7, *8, *18, *19, and *35). Biol Pharm Bull 35: 394–9. [DOI] [PubMed] [Google Scholar]
- Hodek P Trefil P and Stiborová M (2002) Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem.-Biol. Interact 139, 1–21. [DOI] [PubMed] [Google Scholar]
- Johnson KM Phan TT Albertolle ME and Guengerich FP (2017) Human mitochondrial cytochrome P450 27C1 is localized in skin and preferentially desaturates trans-retinol to 3,4-dehydroretinol. J Biol Chem, 292, 13672–13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H Takahashi T Ohta S and Harigaya Y (2004) Oxidation and rearrangements of flavanones by mammalian cytochrome P450. Xenobiotica 34:797–810. [DOI] [PubMed] [Google Scholar]
- Kakimoto K Nagayoshi H Inazumi N Tani A Konishi Y Kajimura K Ohura T Nakano T Tang N Hayakawa K and Toriba A (2015) Identification and characterization of oxidative metabolites of 1-chloropyrene. Chem Res Toxicol 28:1728–36. [DOI] [PubMed] [Google Scholar]
- Kale A Gawande S and Kotwal S. (2008) Cancer phytotherapeutics: role for flavonoids at the cellular level. Phytother Res 22, 567–577. [DOI] [PubMed] [Google Scholar]
- Kaminsky LS Fasco MJ and Guengerich FP (1980) Comparison of different forms of purified cytochrome P-450 from rat liver by immunological inhibition of regio-and stereoselective metabolism of warfarin. J Biol Chem 255, 85–91. [PubMed] [Google Scholar]
- Kelly SL Lamb DC Baldwin BC Corran AJ and Kelly DE (1997) Characterization of Saccharomyces cerevisiae CYP61, sterol delta22-desaturase, and inhibition by azole antifungal agents. J Biol Chem y 272, 9986–9988. [DOI] [PubMed] [Google Scholar]
- Kim H-J Lee SB Park S-K Kim HM Park YI and Dong M-S (2005) Effects of hydroxy group numbers on the B-ring of 5,7-dihydroxyflavones on the differential inhibition of human CYP 1A and CYP1B1 enzymes. Arch. Pharm. Res 28, 1114–1121. [DOI] [PubMed] [Google Scholar]
- Kitada C Gong Z Tanaka Y Yamazaki M and Saito K (2001) Differential expression of two cytochrome P450s involved in the biosynthesis of flavones and anthocyanins in chemo-varietal forms of Perilla frutescens. Plant Cell Physiol 42:1338–1344. [DOI] [PubMed] [Google Scholar]
- Lam PY Zhu FY Chan WL Liu H and Lo C (2014) Cytochrome P450 93G1 is a flavone synthase II that channels lavanones to the biosynthesis of tricin O-linked conjugates in rice. Plant Physiol 165:1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S and Mithöfer A (2005) Flavones and flavone synthases. Phytochemistry 66:2399–407. [DOI] [PubMed] [Google Scholar]
- Moon YJ Wang X and Morris ME (2006) Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 20, 187–210. [DOI] [PubMed] [Google Scholar]
- Morikawa T Mizutani M Aoki N Watanabe B Saga H Saito S Oikawa A Suzuki H Sakurai N Shibata D Wadano A Sakata K and Ohta D (2006) Cytochrome P450 CYP710A encodes the sterol C-22 desaturase in Arabidopsis and tomato. The Plant cell 18, 1008–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K Liberato DJ Gillette JR and Sasame HA (1986) An unusual metabolite of testosterone. 17b-4,6-Androstadiene-3-one. Drug Metabolism and Disposition 14, 559–565. [PubMed] [Google Scholar]
- Nikolic D and van Breemen RB (2004) New metabolic pathways for flavanones catalyzed by rat liver microsomes. Drug Metab Dispos 32:387–397. [DOI] [PubMed] [Google Scholar]
- Omura T and Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239: 2370–2378. [PubMed] [Google Scholar]
- Parikh A Gillam EMJ and Guengerich FP (1997) Drug metabolism by Escherichia coli expressing human cytochromes P450. Nat Biotechnol 15: 784–788. [DOI] [PubMed] [Google Scholar]
- Rettie AE Rettenmeier AW Howald WN and Baillie TA (1987) Cytochrome P-450 catalyzed formation of d4-VPA, a toxic metabolite of valproic acid. Science 235, 890–893. [DOI] [PubMed] [Google Scholar]
- Sandhu P Baba T and Guengerich FP (1993) Expression of modified cytochrome P450 2C10 (2C9) in Escherichia coli, purification, and reconstitution of catalytic activity. Arch Biochem Biophys 306; 443–450. [DOI] [PubMed] [Google Scholar]
- Sandhu P Guo Z Baba T Martin MV Tukey RH and Guengerich FP (1994) Expression of modified human cytochrome P450 1A2 in Escherichia coli: Stabilization, purification, spectral characterization, and catalytic activities of the enzyme. Arch Biochem Biophys 309: 168–177. [DOI] [PubMed] [Google Scholar]
- Sasaki S Itagaki Y Kurokawa T Watanabe E and Aoyama T (1966) The mass spectra of flavonoids. J Mass Spectro Soc Japan 14: 82–92 (in Japanese). [Google Scholar]
- Shimada T (2017) Inhibition of carcinogen-activating cytochrome P450 enzymes by xenobiotic chemicals in relation to antimutagenicity and anticarcinogenicity. Toxicol Res 33: 79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T Tanaka K Takenaka S Murayama N Martin MV Foroozesh MK Yamazaki H Guengerich FP and Komori M (2010) Structure-function relationships of inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2C9, and 3A4 by 33 flavonoid derivatives. Chem Res Toxicol 23: 1921–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T Kim D Murayama N Tanaka K Takenaka S Nagy LD Folkman LM, Foroozesh MK Komori M Yamazaki H and Guengerich FP (2013) Binding of diverse environmental chemicals with human cytochromes P450 2A13, 2A6, and 1B1 and enzyme inhibition. Chem Res Toxicol 26: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T Takenaka S Murayama N Yamazaki H Kim JH Kim D Yoshimoto FK Guengerich FP and Komori M (2015) Oxidation of acenaphthene and acenaphthylene by human cytochrome P450 enzymes. Chem Res Toxicol 28: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T Takenaka S Murayama N Kramlinger VM Kim JH Kim D Liu J Foroozesh MK Yamazaki H Guengerich FP and Komori M (2016a) Oxidation of pyrene, 1-hydroxypyrene, 1-nitropyrene and 1-acetylpyrene by human cytochrome P450 2A13. Xenobiotica 46: 211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T Takenaka S Kakimoto K Murayama N Lim YR Kim D, Foroozesh MK Yamazaki H Guengerich FP and Komori M (2016b) Structure-function studies of naphthalene, phenanthrene, biphenyl, and their derivatives in interaction with and oxidation by cytochromes P450 2A13 and 2A6. Chem. Res. Toxicol 29: 1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T Kakimoto K Takenaka S Koga N Uehara S Murayama N Yamazaki H Kim D, Guengerich FP and Komori M (2016c) Roles of human CYP2A6 and monkey CYP2A24 and 2A26 cytochrome P450 enzymes in the oxidation of 2,5,2´,5´-tetrachlorobiphenyl. Drug Metab Disp 44: 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs BA Alexander JF Pierson CA Schweitzer KS Chun KT Koegel C Barbuch R and Bard M (1996) Cloning and characterization of the Saccharomyces cerevisiae C-22 sterol desaturase gene, encoding a second cytochrome P-450 involved in ergosterol biosynthesis. Gene 169, 105–109. [DOI] [PubMed] [Google Scholar]
- Tanaka Y Brugliera F Kalc G Senior M Dyson B Nakamura N Katsumoto Y and Chandler S (2010) Flower color modification by engineering of the flavonoid biosynthetic pathway: practical perspectives. Biosci Biotechnol Biochem 74:1760–9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y and Brugliera F (2013) Flower colour and cytochromes P450. Philos Trans R Soc Lond B Biol Sci 368: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M Horie M Honda H Takara K and Nishiguchi K (2009) The structure-activity correlation on the inhibitory effects of flavonoids on cytochrome P450 3A activity. Biol Pharm Bull 32, 671–676. [DOI] [PubMed] [Google Scholar]
- Uno T Obe Y Ogura C Goto T Yamamoto K Nakamura M Kanamaru K Yamagata H and Imaishi H (2013) Metabolism of 7-ethoxycoumarin, safrole, flavanone and hydroxyflavanone by cytochrome P450 2A6 variants. Biopharm Drug Dispos 34:87–97. [DOI] [PubMed] [Google Scholar]
- Uno T Ogura C Izumi C Nakamura M Yanase T Yamazaki H Ashida H Kanamaru K Yamagata H and Imaishi H (2015) Point mutation of cytochrome P450 2A6 (a polymorphic variant CYP2A6.25) confers new substrate specificity towards flavonoids. Biopharm Drug Dispos 36:552–563. [DOI] [PubMed] [Google Scholar]
- Walle T (2007) Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin Cancer Biol 17, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle UK and Walle T (2007) Bioavailable flavonoids: cytochrome P450-mediated metabolism of methoxyflavones. Drug Metab. Dispos 35, 1985–1989. [DOI] [PubMed] [Google Scholar]
- Walle T Ta N Kawamori T Wen X Tsuji PA and Walle UK (2007) Cancer chemopreventive properties of orally bioavailable flavonoids—methylated versus unmethylated flavones. Biochem Pharmacol 73, 1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RW Kari PH Lu AY Thomas PE Guengerich FP and Vyas KP (1991) Biotransformation of lovastatin. IV. Identification of cytochrome P450 3A proteins as the major enzymes responsible for the oxidative metabolism of lovastatin in rat and human liver microsomes. Arch Biochem Biophys 290, 355–361. [DOI] [PubMed] [Google Scholar]
- Yamazaki H Nakamura Komatsu T Ohyama K Hatanaka N Asahi S Shimada N Guengerich FP Shimada T Nakajima M and Yokoi T (2002) Roles of NADPH-P450 reductase and apo-and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr Purif 24: 329–337. [DOI] [PubMed] [Google Scholar]
- Yano JK Denton TT Cerny MA Zhang X Johnson EF and Cashman JR (2006) Synthetic inhibitors of cytochrome P-450 2A6: inhibitory activity, difference spectra, mechanism of inhibition, and protein cocrystallization. J Med Chem 49:6987–7001. [DOI] [PubMed] [Google Scholar]
- Zhang S Yang X Coburn RA Morris ME (2005) Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem Pharmacol 70, 627–639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


