Abstract
This retrospective cohort study sought to assess the effectiveness of comprehensive geriatric assessment (CGA) for older patients at an HIV clinic in a large US city. We systematically reviewed medical records of all patients who underwent CGA from June 2013 to July 2017. In addition, physicians and social workers completed an anonymous survey about the impact of CGA on their patients. For the 76 patients (median age 67.2; Q1, Q3 = 60.9, 72.6) seen by geriatricians at the clinic, there were 184 recommendations, 54 instances of counseling, and 11 direct actions. Overall adherence to recommendations was 32.8%, 34.9% for patient-directed, and 31.7% for provider-directed recommendations. No demographic or CGA variables were associated with adherence. Despite this lack of adherence, surveyed providers reported that they usually or always followed recommendations; the most frequently cited barrier to implementation was lack of feasibility. Further research will be needed to determine how CGA can improve outcomes for this population.
Keywords: HIV positive, comprehensive geriatric assessment, adherence
Introduction
The advent of effective antiretroviral therapy has dramatically increased the life span of people living with HIV infection.1 As a consequence, the population of individuals with HIV is aging. According to the Centers for Disease Control and Prevention, in 2015 approximately 47% of those living with HIV infection in the United States were at least 50 years of age,2 and a study predicts that by 2020 this percentage will rise to 70%.3 Older people with HIV (OPH) have a higher risk of age-related comorbidities such as cardiovascular disease, type 2 diabetes mellitus, fractures, and impaired kidney function compared to HIV-uninfected individuals of the same age.4 In addition, they have higher rates of multimorbidity,5,6 low bone density,7 frailty, functional impairment,8 and geriatric (age-related) syndromes9 than their HIV-uninfected counterparts. The OPH have high rates of depression,10 loneliness,11 and social isolation,12 as well as increased levels of neurocognitive disorders13 and low rates of advance care planning.14 Research on the clinical care of OPH is limited, however, and although geriatric care has been proposed,15-17 there is no consensus regarding the role of geriatric specialists and the comprehensive geriatric assessment (CGA) in the care of older patients with HIV.18
Comprehensive geriatric assessment, the complete evaluation of a patient over multiple health, medical, and psychosocial domains, has not been formally studied in people living with HIV; CGA has been shown to have utility in other geriatric populations seen by subspecialists such as oncologists, however. One recent large meta-analysis found that CGAs may influence 21% to 49% of treatment decisions for older patients with cancer.19 Some individual studies show an even greater impact; CGA results influenced disease management decisions for 82% of patients in a study of 161 older patients with cancer.20 Comprehensive geriatric assessment has also enabled the detection of new clinically significant findings. For example, in a study, vision loss was newly detected in 18% and hearing impairment and depression newly detected in over 70% of older patients undergoing CGA.21 Furthermore, long-term outcomes have been shown to be affected by the CGA. One recent meta-analysis of 22 CGA-related studies showed that, among adults admitted to the hospital, those who undergo CGA were more likely to be alive at 12-month follow-up compared to those who did not undergo CGA.22
What Do We Already Know about This Topic?
The population with HIV is aging, and clinical programs are applying geriatric concepts to their care.
How Does Your Research Contribute to the Field?
This study documents that recommendations based on comprehensive geriatric assessment (CGA) for older people living with HIV (PLWH) are often not implemented.
What Are Your Research's Implications toward Theory, Practice, or Policy?
Classic CGA may require adaptation to meet the needs of older PLWH.
Geriatric programs and syndromes are gaining attention as adults with HIV age.18 Our geriatric HIV clinical program offers the opportunity to study the impact of CGA in a unique, long-standing, integrated, multidisciplinary HIV clinic. This study had 2 components: The first was to describe the recommendation patterns that arose from the CGA in a population of HIV-infected older adults, and to assess the adherence to recommendations during a 6-month follow-up. The second component of the study was a survey of clinic provider impressions of the value of geriatric consultation.
Methods
Setting
The HIV clinic at the New York-Presbyterian Hospital–Weill Cornell Medical Center (Center for Special Studies) follows approximately 2800 HIV-infected adults at 2 locations in New York City. All patients are followed by a designated HIV specialist (infectious diseases or internal medicine physician) and a social worker. Full-time psychiatrists and dieticians are also available at both locations.
As part of a program for people aging with HIV, a geriatrician has been consulting on patients onsite at the clinic since 2013; foundation support for 2½ years starting in 2015 enabled the addition of a second geriatrician along with other nonclinical offerings such as an arts program. HIV providers preferred an opt-in program with no fixed criteria such as age for referral. Instead, members of the patients’ primary care teams make geriatric referrals based on perceived need (such as cognitive or functional complaints), and consultations take place once a week during clinic sessions. Each consultation includes a CGA,22 comprised of a thorough review of each patient’s physical and mental health, as well as their daily functioning and strength of social support systems. Several validated scales are used in each patient’s CGA. The geriatrician writes a full consultation note (including the CGA results) in the electronic medical record and presents the case during the team’s afternoon rounds. Patients can opt to follow up with the geriatrician, and most are seen longitudinally every 6 to 12 months. This study examines only the results of the initial CGA.
Study Design
This study was a retrospective cohort study, conducted via chart review. All HIV-infected patients treated at the clinic who were seen at least once by a staff geriatrician between June 2013 and July 2017 were included in the study.
We abstracted demographic and clinical data from the first geriatric consultation visit that included a CGA. This included age, sex, race/ethnicity, HIV risk factors, marital status, insurance status (Medicaid versus non-Medicaid insurance), date of HIV diagnosis, and most recent HIV viral load. Measures from the CGA included standard, validated assessment tools: the Patient Health Questionnaire 4 (PHQ-4) depression/anxiety scales,23 Montreal Cognitive Assessment (MoCA),24 Gérontopôle Frailty Screening Tool,25 Veterans Aging Cohort Study (VACS) mortality score,26 as well as generic quality-of-life questions about health, pain, and function. The list of the items used in the clinic’s CGA is provided in Appendix.
Interventions during this initial visit were divided into 3 categories: (1) recommendations, (2) provision of information/counseling, or (3) direct action taken during the visit. Because direct actions (such as making a phone call for a patient) and counseling (eg, about aging or emotional responses to retirement) did not require a response and could not be assessed via chart review, only recommendations were evaluated to assess effectiveness of the CGA.
Recommendations were further divided into patient- or physician-directed recommendations, based on prior study models by Reuben et al.27 All interventions were then subdivided into the following categories: (1) medications, (2) screening/diagnostic test, (3) procedure, (4) referral/follow-up, (5) psychosocial intervention, (6) exercise and nutrition, (7) behavioral health and substance use, and (8) recommendation for home services. Examples of common recommendation types are provided in Table 1.
Table 1.
Examples of Recommendations from Comprehensive Geriatric Assessment.
| Subtype | Patient Directed | Physician Directed |
|---|---|---|
| Medication | Have spouse help with administration | Change dose/frequency |
| Screening test | NA | Order screening test (eg, bone density scan) |
| Procedural | Agree to dental work | NA |
| Referral/follow-up | Attend referral appointment | Recommend referral to specialist |
| Psychosocial | Increase socialization | Recommend family meeting |
| Physical | Stretching exercises | NA |
| Behavioral | Smoking cessation | NA |
| Home services | NA | Initiate home attendant services |
Abbreviation: NA, not applicable.
Adherence to recommendations was defined as chart evidence of fulfillment of the recommendation within 6 months following the date of the CGA. Two independent investigators, other than the geriatrician who performed the clinical assessment, reviewed each patient chart, including physician, social worker, and nutritionist visit notes; medication, laboratory, and radiology/procedure notes; and documentation of telephone encounters.
Any differences in coding of recommendations were discussed and reconciled to ensure consistency in categorizing interventions and determining adherence to recommendations. Provider-directed recommendations were considered “fulfilled” if the recommended event actually happened—for example, if the geriatrician recommended that the primary care provider order an echocardiogram for a patient, the recommendation would only be considered fulfilled if there was evidence in the chart that an echocardiogram was actually completed. The act of simply ordering the test would not constitute fulfillment of a provider-directed recommendation.
For the purposes of analysis, we examined both patient-directed adherence and provider-directed adherence as a whole and at the patient level. At the patient level, adherence was defined as following at least 1 patient- or provider-directed recommendation. Continuous data are summarized as median (first quartile, third quartile) with the inclusion of the overall range if appropriate. Pearson χ2 and Wilcoxon rank-sum tests assessed for comparison in the following variables based on whether or not a patient was “adherent”: sex, age, HIV viral load (dichotomized as <200 copies/mL versus ≥200 copies/mL), recipient of Medicaid, HIV risk factor (dichotomized as men who have sex with men versus other), MoCA score (dichotomized as <26 versus ≥26), Gérontopôle frailty assessment, PHQ-4 depression and anxiety scores, and VACS 5-year mortality risk. Additionally, the relationship between the total number of recommendations per patient and adherence rates was assessed by scatter plot and Spearman correlation. Data were analyzed using Stata (StataCorp 2011, Stata Statistical Software: Release 12; StataCorp LP). A P value of less than .05 was considered statistically significant; no adjustments were made for multiple comparisons.
Provider Survey
Provider survey study data were collected and managed using the Research Electronic Data Capture electronic data capture system hosted at Weill Cornell Medical College.28 Full-time clinic social workers and physicians were eligible for the survey portion of the study. The medical director of the clinic e-mailed a description of the survey to potential participants (10 HIV specialists, 4 psychiatrists, and 10 social workers) using institutional review board (IRB)–approved language, along with a link to the survey.
Consenting participants first completed a form that contained questions about their clinical role, sex, ethnicity, and age. Next, the participant was taken to a survey about geriatric consultation that was analyzed separately from the demographic information in order to preserve participant anonymity. The survey included questions about the number of referrals, length of time employed at the clinic, likelihood of referring, usefulness of the consultations, and barriers to implementation of recommendations. Most questions used a multiple-choice format, with options to write in explanations (eg, reasons why recommendations were not followed other than those listed).
Ethical Approval and Informed Consent
The retrospective study protocol was approved by the IRB of Weill Cornell Medical College (IRB 1505016187), and the requirement for informed consent was waived because the study was felt to have minimal risk to participants and the research team performing the chart reviews did not have contact with the study participants. The provider questionnaire aspect of the study was approved separately by the same board (IRB 1412015778), which permitted participants to implicitly grant consent by agreeing to complete the survey after reading the key elements of informed consent that were included in a recruitment email.
Results
Comprehensive Geriatric Assessment
Geriatricians evaluated 76 patients whose median age was 67.2 years (Q1, Q3 = 60.9, 72.6; range 50.0-84.2) at the time of evaluation (see Table 2). Fifty-two (68.4%) patients were male, 31 (40.8%) were African American, 26 (34.2%) were white, and 17 (22.4%) were Latino/a. Median time since HIV diagnosis was 21.5 years (Q1, Q3 = 17.7, 25.1). In terms of risk factors for HIV acquisition, 41 patients (53.9%) were men who have sex with men, 12 (15.8%) reported injection drug use, and 19 (25%) reported heterosexual sex as their only risk factor. Forty-three (56.6%) patients reported that they lived alone, and 49 (64.5%) used Medicaid as their primary insurance. Of 73 patients with available HIV viral load data, 66 (90.4%) had values <200 copies/mL, of whom 51 were below the level of detection (<20 copies/mL).
Table 2.
Characteristics of Patients Who Underwent Comprehensive Geriatric Assessment (n = 76).
| Characteristics | N (%) or Median (Q1, Q3) |
|---|---|
| Age (years) | 67.2 (60.9, 72.6) |
| Male sex | 52 (68.4%) |
| Race/ethnicity | |
| African American | 31 (40.8%) |
| White | 26 (34.2%) |
| Latino | 17 (22.4%) |
| Years since HIV diagnosis | 21.5 (17.7, 25.1) |
| HIV risk factor | |
| Men who have sex with men | 41 (53.9%) |
| Injection drug use | 12 (15.8%) |
| Heterosexual | 19 (25.0%) |
| Lives alone | 43 (56.6%) |
| Medicaid as primary insurance | 49 (64.5%) |
| HIV-1 viral load <20 copies/mL | 51 (67.1%) |
| Anxiety (PHQ-4) subscore | 0.5 (0, 4) |
| Depression (PHQ-4) subscore | 1.0 (0, 3) |
| MoCA | 23 (18, 26) |
| VACS 5-year mortality risk (%) | 22 (11.9, 38) |
Abbreviations: MoCA, Montreal Cognitive Assessment; PHQ, Patient Health Questionnaire; VACS, Veterans Aging Cohort Study.
The median PHQ-4 depression subscore was 1 (Q1, Q3 = 0, 3; range, 0-6). The median PHQ-4 anxiety subscore was 0.5 (Q1, Q3 = 0, 4; range, 0-6). The median MoCA score was 23 (Q1, Q3 = 18, 26; range, 6-30). The median VACS 5-year mortality risk was 22% (Q1, Q3 = 11.9, 38; range, 3.7-75.8).
Initial consultation for the 76 patients resulted in several different types of consultant behaviors. These were classified as direct actions (n = 11), counseling/provision of information (n = 54), and distinct recommendations (n = 63 patient-directed recommendations and n = 120 provider-directed recommendations, or 183 in total). Only the recommendations outcomes were analyzed. Table 1 gives examples of the different classes of recommendations.
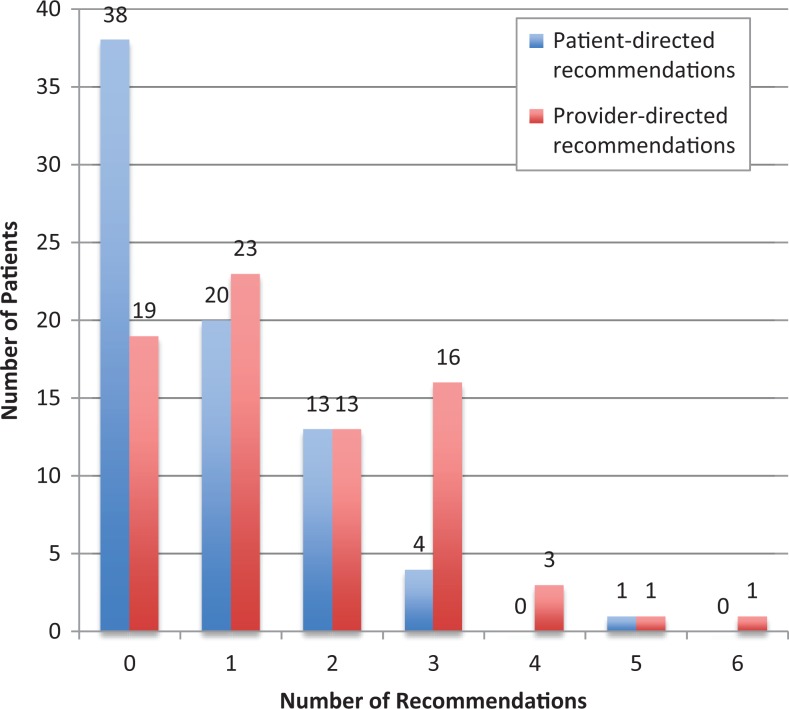
Geriatricians made at least 1 recommendation for 67 (88.5%) patients, of which 38 (50.0%) patients received at least 1 patient-directed recommendation, and 57 (75.0%) patients received at least 1 provider-directed recommendation (Figure 1). The median number of patient-directed recommendations per patient was 0.5 (Q1, Q3 = 0, 1; range, 0-5) and the median number of provider-directed recommendations was 1 (Q1, Q3 = 0.75, 3; range, 0-6). The median number of total recommendations per patient was 2 (Q1, Q3 = 1, 3; range, 0-7). Overall, 22 (34.9%) of 63 patient-directed recommendations and 38 (31.7%) of 120 provider-directed recommendations were fulfilled (Table 3).
Figure 1.
Number of patient-directed and provider-directed recommendations per patient.
Table 3.
Recommendation Adherence by Type.
| Recommendation Type | Patient- Directed Adherence (%) | Provider- Directed Adherence (%) | Total Adherence (%) |
|---|---|---|---|
| Medication | 2/9 (22.2) | 20/59 (33.9) | 22/68 (32.4) |
| Screening/diagnostic test | NA | 6/28 (21.4) | 6/28 (21.4) |
| Procedure | 1/1 (100) | NA | 1/1 (100) |
| Referral/follow-up | 7/15 (46.7) | 10/21 (47.6) | 17/36 (45.7) |
| Psychosocial | 4/14 (28.6) | 0/2 (0.0) | 4/16 (25.0) |
| Physical | 3/10 (30.0) | NA | 3/10 (30.0) |
| Behavioral | 5/14 (35.7) | NA | 5/14 (35.7) |
| Home services | NA | 2/10 (20.0) | 2/10 (20.0) |
| Total | 22/63 (34.9) | 38/120 (31.7) | 60/183 (32.8) |
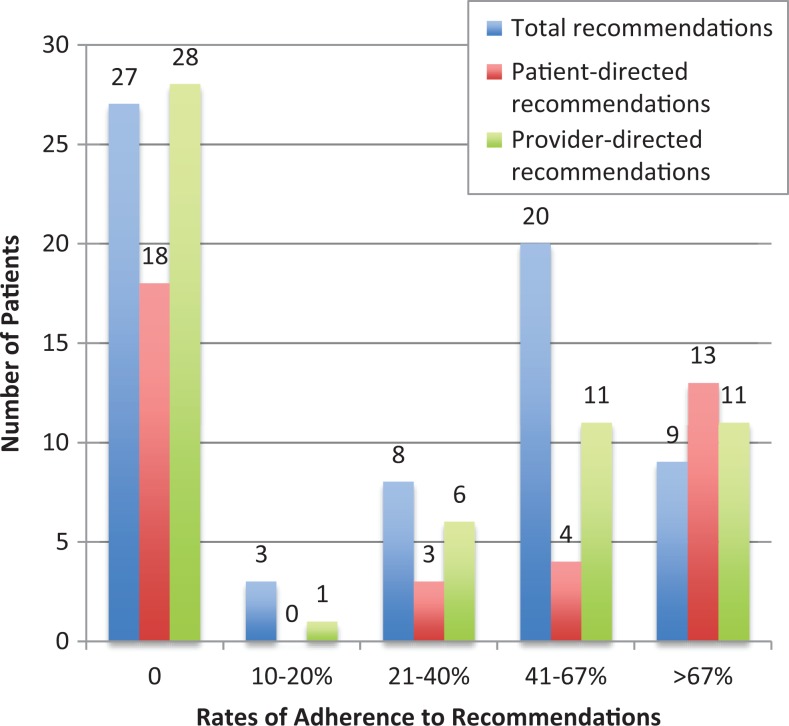
Of the 67 patients who received at least 1 patient- or provider-specific recommendation, there was adherence to at least 1 recommendation in 40 (59.7%). Median total adherence was 33.3% (Q1, Q3 = 0%, 66.7%; range, 0%-100%; Figure 2). Of the 38 patients who received at least 1 patient-directed recommendation, 20 (52.6%) were adherent to at least 1 recommendation (Figure 2). Median adherence was 33.3% (Q1, Q3 = 0%, 100%; range 0%-100%). Of 57 patients for whom at least 1 provider-directed recommendation was made, 29 (50.9%) followed at least 1 recommendation (Figure 2). Median adherence was 16.7% (Q1, Q3 = 0%, 66.7%; range 0%-100%). The most common subcategories were medication-related (37.2% of total recommendations), referrals (19.1% of total recommendations), and screening/diagnostic tests (15.3% of total recommendations).
Figure 2.
Frequency of adherence to recommendations.
We compared specific variables in patients stratified by adherence to at least 1 recommendation (versus adherence to 0 recommendations). We found no relationship between the following variables and adherence to at least 1 recommendation: sex (P = .41), age (P = .34), viral load <200 copies/mL (P = .18), Medicaid usage (P = .41), HIV risk factor (P = .45), MoCA less than 26 (P = .21), frailty (P = .59), depression subscore (P = .73), anxiety subscore (P = .13), and VACS mortality risk (P = .45). Additionally, total number of recommendations was not correlated with percentage adherence (Spearman r = 0.044; P = .72).
Survey
Altogether, 21 people started the provider survey. Two participants filled out the demographic form but did not proceed to the survey about the geriatric program; their demographic information was not included. Data from 10 physicians (6/10 HIV specialists and 4/4 psychiatrists) and 9 (of 10) social workers were analyzed. Two physicians declined to provide their sex or ethnic group (1 listed their age as 80). Median age of physicians was 57.5 (range, 37-80) and that of the social workers was 38 (range, 27-66). Reanalyzing the data without the ages of those who did not give other demographic data did not change the median age.
Table 4 summarizes provider responses to geriatric consultation. Of note, both social workers and physicians identified recommendations (to themselves, others on the team and patients) as a major focus of the consultation; all but 1 in each group said that they implemented recommendations either usually or always.
Table 4.
Survey Participants’ Assessment of Value of Geriatric Consultation.a
| Survey Question | Physicians, n = 10 | Social Workers, n = 9 |
|---|---|---|
| Years at HIV clinic (median; range) | 13.5 (5-27) | 7 (2-15) |
| Patients referred in prior 12 months (median; range) | 5 (2-12) | 3 (0-6) |
| Likelihood to refer (n) | ||
| Extremely or very likely | 7 | 6 |
| Somewhat likely | 2 | 2 |
| Not at all likely | 1 | 1 |
| Usefulness of consult (n) | ||
| Not useful | 0 | 0 |
| A little or somewhat useful | 3 | 0 |
| Very or extremely useful | 7 | 9 |
| Purpose of consult (n) | ||
| Making or confirming diagnosis | 3 | 4 |
| Providing direct assistance | 1 | 5 |
| Providing recommendations to me | 8 | 8 |
| Providing recommendations to other team member | 6 | 7 |
| Recommendations to patient | 8 | 9 |
| Counseling the patient or caregiver | 8 | 6 |
| Other | 1 | 4 |
| How often they implemented recommendations (n) | ||
| Never or rarely | 1 | 0 |
| Sometimes | 0 | 1 |
| Usually or always | 9 | 8 |
| Commonest reason recommendation not followed (n) | ||
| Not applicable | 3 | 4 |
| I did not agree with recommendations | 0 | 0 |
| Recommendations weren’t feasible | 5 | 4 |
| Too many recommendations | 0 | 0 |
| Recommendations weren’t clear | 0 | 0 |
| Other | 2 | 1 |
| Significance of barriersb (mean, range) | ||
| Patient’s cognitive ability/executive functioning | 3.22 (2-5) | 4.67 (4-5) |
| Patient’s mental health | 3.44 (2-4) | 3.88 (3-5) |
| Patient’s physical health | 3.22 (2-4) | 3.63 (2-5) |
| Strength of patient’s social support system | 3.25 (1-4) | 4.67 (4-5) |
| Too many recommendations at one time | 2.22 (1-4) | 2.75 (1-5) |
| Patient’s health beliefs or behaviors | 3.33 (2-4) | 3.25 (1-5) |
| Usefulness of geriatrician’s attendance on roundsc (mean, range) | 3.78 (2-4) | 4.00 (3-5) |
a “Other” comments are listed in Box 1.
b Scale of 1-5: 1 = not significant, 5 = very significant.
c Scale of 1-5: 1 = not at all useful, 5 = extremely useful.
The most common reason for not following recommendations was that they were not feasible. Neither group felt that the number of recommendations was a major barrier to implementation, an impression confirmed by the chart review. Social workers appeared to be more concerned than physicians about cognition and social supports as barriers to implementation, but given the small number of participants, no test of statistical significance was performed.
Discussion
This study examines the recommendation patterns based on CGA in OPH. Overall, approximately one-third of all recommendations based on the CGA results were fulfilled, which is somewhat lower than adherence reported in the literature; for example, a study in the general population reported rates of adherence approaching 80% in older patients.27 In our study, for approximately half of the 67 patients who received recommendations, there was adherence to at least 1 recommendation overall; this proportion remained consistent when analyzing patient- and provider-directed recommendations separately. Of note, there were no apparent demographic or CGA-related differences in patients with adherence to 0 versus 1 or more recommendations. Sex, age, HIV viral load, Medicaid usage, HIV risk factors, MoCA score, frailty status, depression score, anxiety score, and VACS mortality risk were not significantly different between these groups, possibly due to small sample size. Additionally, there was no relationship between adherence rates and the number of recommendations per patient; this is in contrast to prior studies which found that there was a negative correlation between number of provider-directed recommendations and adherence.27,29
Box 1.
Answers Listed under “Other” in This Table
Purpose of consult:
Social workers: Increase understanding of patients’ limitation; the consultation validates and supports SW goals of care and provides guidance for appropriate interventions; reiterating recommendations to patient; of particular importance around cognitive.
Physicians: Help with resources for patients.
Commonest reason recommendations not followed:
Social workers: Social barriers
Physicians: Patient declined to follow the recommendation; would be more helpful if geriatrics consult would directly implement some recommendations, that is, physical therapy or age related, that is, ophthalmology/hearing tests.
Comparison of chart review and survey data elicited some contradictions. Clinicians responded that they valued the geriatric assessments for their recommendations and felt that they followed through with recommendations. Objectively, however, this was not the case. Social workers uniformly felt the geriatric assessments were valuable; although most physicians scored the assessments positively, they were not unanimous in their support.
There are a number of reasons that might explain why CGA was less successful for OPH in our study than for published data on the general population. As consultants, geriatricians could not control the outcomes. This was no doubt a contributor, but as much of the CGA literature describes consultation, it cannot completely explain this study’s lower success rates. There may have been differences in patient characteristics, in the quality of the CGA, or in the methodology such as documentation or operational definitions of adherence. It is possible that the patient population needed time to develop trust in providers, and a new consultant may not have had much influence, or that the problems identified by the geriatricians required a longer time to solve.
This was a young population by geriatric standards and it is likely that CGA requires translation to a new population that is aging differently. A recent editorial announced, “Geriatric-HIV Medicine is born,”15 but it is having some growing pains. Our understanding of the HIV-aging phenomenon has evolved from a recognition of the changing demographics, to the documentation of multimorbidity and aging-related syndromes, to the recognition that geriatric principles can inform the care of the growing population of older patients living with HIV at all levels—from ambulatory to long-term care.30 This study offers evidence to suggest that traditional geriatric care is unlikely to achieve maximum success if duplicated directly into the care of OPH. Rather, geriatric care is likely to require some adaptation if it is to be successful. We are using these data to explore such adaptations with clinic staff.
Limitations
One major limitation of this study is that it was conducted via retrospective chart review. Determining adherence to recommendations was therefore limited by provider documentation; if explicit evidence of fulfillment was not present in the chart, the recommended intervention was considered to have never occurred, leading to possible underestimation of adherence. Furthermore, we conservatively required documentation that diagnostic tests, for example, were actually completed rather than just ordered by a clinician. In the future, prospective studies of this type may be useful to more accurately determine adherence to both patient- and provider-directed recommendations.
Additionally, although this study serves as a comprehensive analysis of the nature of recommendations and adherence to these recommendations, clinicians rarely documented in the electronic health record the reasons for not adhering to recommendations. Furthermore, there was no control group to compare patient outcomes such as mortality or quality of life. We are unable to conclude how CGA ultimately affects patient outcomes.
Conclusion
In this retrospective cohort study and survey, we found that CGA of patients cared for in a large US city HIV clinic resulted in diverse recommendations for which documentation of adherence was present in the medical record less than one-third of the time, even though most clinicians perceived that the recommendations were being followed and found value in the consultations. Further studies are needed to determine (1) the true rates of adherence to recommendations, using prospective data and comprehensive documentation methods; (2) the reasons for patient and provider nonadherence; (3) effective adaptations of CGA to meet the needs of OPH; and (4) the impact of CGA—whether those cases reviewed within the CGA paradigm when compared to non-CGA cases have better clinical outcomes.
Acknowledgments
The authors acknowledge the clinical staff of the Center for Special Studies for their support of this study.
Appendix
Components of Comprehensive Geriatric Assessment
General history
Goals, what’s important to the patient
Basic and Instrumental Activities of Daily Living (including continence, falls)
VACS (calculated ahead of time)26
PHQ-423 (depression, anxiety)
Frailty screen (Gérontopôle)25
Strength (handgrip)
MoCA24
Questions about quality of life and pain
Impact of hearing, visual impairment
FRAX (calculated ahead of time) (https://www.sheffield.ac.uk/FRAX)
Physical exam, including observing gait
Scales are part of the medical record
Authors’ Note: Dr. Bitas contributed to acquisition of data, analysis, interpretation of data, and preparation of manuscript. Dr. Jones contributed to study methods, acquisition of data, and analysis. Dr. Singh contributed to study concept and design and methods. Dr. Ramirez did study design, methods, and preparation of manuscript. Dr. Siegler and Dr. Glesby contributed to study concept and design, methods, data acquisition, analysis and interpretation, and preparation of manuscript.
Eugenia L. Siegler, MD, and Marshall Glesby, MD, PhD, contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Siegler receives book royalties from Springer Publishing Company. Drs. Siegler and Glesby receive research support to their institution from Gilead Sciences for an investigator-initiated study. Dr. Jones received fees from Gilead Sciences for consulting work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Siegler was supported by the Fan Fox and Leslie R. Samuels Foundation. Research reported in this publication was supported by the National Center for Advancing Translational Science of the National Institute of Health under Award Number UL1TR000457.
ORCID iD: Eugenia L. Siegler, MD  https://orcid.org/0000-0001-9449-5873
https://orcid.org/0000-0001-9449-5873
Marshall Glesby, MD, PhD  https://orcid.org/0000-0002-2145-148X
https://orcid.org/0000-0002-2145-148X
References
- 1. Costagliola D. Demographics of HIV and aging. Curr Opin HIV AIDS. 2014;9(4):294–301. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. HIV Surveillance Report 2016. Atlanta, GA: Centers for Disease Control and Prevention; 2017;28:125. [Google Scholar]
- 3. Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–818 doi:10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDs. 2013;27(1):5–16. doi:10.1089/apc.2012.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayer KH, Loo S, Crawford PM, et al. Excess clinical comorbidity among HIV-infected patients accessing primary care in us community health centers. Public Health Rep. 2018;133(1):109–118. doi:10.1177/0033354917748670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126. doi:10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 7. Erlandson KM, Lake JE, Sim M, et al. Bone mineral density declines twice as quickly among HIV-infected women compared with men. J Acquir Immune Defic Syndr. 2018;77(3):288–294. doi:10.1097/QAI.0000000000001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khoury AL, Morey MC, Wong TC, et al. Diminished physical function in older HIV-infected adults in the Southeastern U.S. despite successful antiretroviral therapy. PLoS One. 2017;12(6):e0179874 doi:10.1371/journal.pone.0179874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greene M, Covinsky KE, Valcour V, et al. Geriatric syndromes in older HIV-infected adults. J Acquir Immune Defic Syndr. 2015;69(2):161–167. doi:10.1097/QAI.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Applebaum A, Brennan M. Mental health and depression In: Brennan M, Karpiak SE, Shippy RA, Cantor MH, eds. Older Adults with HIV: An In-Depth Examination of an Emerging Population. New York, NY: Nova Science Publishers; 2009:27–34. [Google Scholar]
- 11. Greene M, Hessol NA, Perissinotto C, et al. Loneliness in older adults living with HIV. AIDS Behav. 2018;22(5):1475–1484. doi:10.1007/s10461-017-1985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shippy R, Karpiak S. The aging HIV/AIDS population: fragile social networks. Aging Mental Health. 2005;9(3):246–254. doi:10.1080/13607860412331336850. [DOI] [PubMed] [Google Scholar]
- 13. Watkins CC, Treisman GJ. Cognitive impairment in patients with AIDS – prevalence and severity. HIV AIDS (Auckl). 2015;7:35–47. doi:10.2147/HIV.S39665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erlandson K, Allshouse A, Duong S, MaWhinney S, Kohrt W, Campbell T. HIV, aging, and advance care planning: are we successfully planning for the future? J Palliat Med. 2012;15(10):1124–1129. doi:10.1089/jpm.2011.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guaraldi G, Rockwood K. Geriatric-HIV medicine is born. Clin Infect Dis. 2017;65(3):507–509. doi:10.1093/cid/cix316. [DOI] [PubMed] [Google Scholar]
- 16. Justice A. HIV and aging: time for a new paradigm. Current HIV/AIDS Rep. 2010;7(2):69–76. doi:10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 17. Work Group for HIV and Aging Consensus Project. Summary report from the Human Immunodeficiency Virus and Aging Consensus Project: treatment strategies for clinicians managing older individuals with the human immunodeficiency virus. J Am Geriatr Soc. 2012;60(5):974–979. doi:10.1111/j.1532-5415.2012.03948.x. [DOI] [PubMed] [Google Scholar]
- 18. Singh HK, Del Carmen T, Freeman R, Glesby MJ, Siegler EL. From one syndrome to many: incorporating geriatric consultation into HIV Care. Clin Infect Dis. 2017;65(3):501–506. doi:10.1093/cid/cix311. [DOI] [PubMed] [Google Scholar]
- 19. Caillet P, Laurent M, Bastuji-Garin S, et al. Optimal management of elderly cancer patients: usefulness of the comprehensive geriatric assessment. Clin Interv Aging. 2014;9:1645–1660. doi:10.2147/CIA.S57849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaïbi P, Magné N, Breton S, et al. Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients. Crit Rev Oncol Hematol. 2011;79(3):302–307. doi:10.1016/j.critrevonc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 21. Mann E, Koller M, Mann C, van der Cammen T, Steurer J. Comprehensive geriatric assessment (CGA) in general practice: results from a pilot study in Vorarlberg, Austria. BMC Geriatrics. 2004;4:4 doi:10.1186/1471-2318-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211 doi:10.1002/14651858.CD006211.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Löwe B, Wahl I, Rose M, et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. 2010;122(1-2):86–95. doi:10.1016/j.jad.2009.06.019 [DOI] [PubMed] [Google Scholar]
- 24. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25. Vellas B, Balardy L, Gillette-Guyonnet S, et al. Looking for frailty in community-dwelling older persons: the Gérontopôle Frailty Screening Tool (GFST). J Nutr Health Aging. 2013;17(7):629–631. doi:10.1007/s12603-013-0363-6 [DOI] [PubMed] [Google Scholar]
- 26. Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149–163. doi:10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reuben DB, Maly RC, Hirsch SH, et al. Physician implementation of and patient adherence to recommendations from comprehensive geriatric assessment. Am J Med. 1996;100(4):444–451. [DOI] [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sears C, Charlson M. The effectiveness of a consultation: compliance with initial recommendations. Am J Med. 1983;74(5):870–876. doi:10.1016/0002-9343(83)91079-3. [DOI] [PubMed] [Google Scholar]
- 30. Siegler EL, Brennan-Ing M. Adapting systems of care for people aging with HIV. J Assoc Nurses AIDS Care. 2017;2 8(5):698–707. doi:10.1016/j.jana.2017.05.006. [DOI] [PubMed] [Google Scholar]