Abstract
Skin injury to the face remains one of the greatest challenges in wound care due to the varied contours and complex movement of the face. Current treatment strategies for extensive facial burns are limited to the use of autografts, allografts, and skin substitutes, and these often result in scarring, infection, and graft failure. Development of an effective treatment modality will greatly improve the quality of life and social integration of the affected individuals. In this proof of concept study, we developed a novel strategy, called “BioMask”, which is a customized bioengineered skin substitute combined with a wound dressing layer that snugly fits onto the facial wounds. To achieve this goal, three-dimensional (3D) bioprinting principle was used to fabricate the BioMask that could be customized by patients’ clinical images such as computed tomography (CT) data. Based on a face CT image, a wound dressing material and cell-laden hydrogels were precisely dispensed and placed in a layer-by-layer fashion by the control of air pressure and 3-axis stage. The resulted miniature BioMask consisted of three layers; a porous polyurethane (PU) layer, a keratinocyte-laden hydrogel layer, and a fibroblast-laden hydrogel layer. To validate this novel concept, the bioprinted BioMask was applied to a skin wound on a pre-fabricated face-shaped structure in mice. Through this in vivo study using the 3D BioMask, skin contraction and histological examination showed the regeneration of skin tissue, consisting of epidermis and dermis layers, on the complex facial wounds. Consequently, effective and rapid restoration of aesthetic and functional facial skin would be a significant improvement to the current issues a facial wound patient experience.
Keywords: Bioprinting, CAD/CAM, skin wound, burn, skin regeneration, tissue engineering
1. Introduction
The integumentary system is the largest organ in the human body and has multiple functions such as preservation of body fluid, protection from infection, and control of body temperature. When damage to a large area of this system occurs, usually through burns, trauma, or skin necrosis, a skin graft is needed. Especially, extensive facial skin burn injuries have both physical and psychosocial implications [1, 2]. Current treatment methods for facial skin burn injuries mainly include the use of autologous skin grafts and alternative skin substitutes. Autologous skin grafts are the gold standard of care for skin burns and any full-thickness loss of skin integrity; however, it has been significantly limited by donor site availability and morbidity [3]. For example, flap reconstruction was used to match cosmetic features such as color, skin thickness, and texture but is rarely utilized in facial burns due to how common simultaneous injuries are to the neck and chest [4]. Small area skin wounds can be treated using commercial skin substitutes with cellular components and artificial skin grafts; however, facial skin tissue regeneration encounters a unique challenge in wound care [5]. The varied contour and continued movement of the face limit the effectiveness of skin substitutes.
In recent decades, the field of regenerative medicine and tissue engineering has emerged as an effective method for the skin regeneration [6–9]. Bioengineered skin substitutes can be created from both natural and synthetic materials and are most commonly placed directly on the skin wound site. These skin substitutes act as a barrier against microorganisms, and they act to promote wound healing at the site by releasing cytokines and growth factors. The skin substitutes should be able to adhere to the wound site rapidly and should also have similar mechanical properties to the native skin. When synthetic biomaterials are used, they should be non-toxic, non-immunogenic, and non-inflammatory and eventually degrade [10, 11]. Very recently, threedimensional (3D) bioprinting strategies have been introduced in the skin tissue engineering because of their capability to produce complex micro-architectures with cellular components [12–15]. Patient-specific bioengineered tissue constructs can also be prepared by 3D bioprinting a fabrication code obtained from the patient’s CT data [16]. To overcome the current limitation of patient’s different facial topography with a large area, 3D bioprinting technologies can be a powerful tool for patient-specific facial regeneration.
In this study, we developed a novel strategy, called “BioMask”, which is a customized bioengineered skin substitute combined with a wound dressing layer that snugly fits onto the facial wounds. The skin substitute can be designed to fit the exact contours of the patient’s face which can overcome limitations of traditional skin substitute products that are designed as simple flat sheets. In order to improve wound healing and reduce scarring the BioMask consists of three layers; a porous polyurethane (PU) layer, a keratinocyte-laden hydrogel layer, and a fibroblastladen hydrogel layer. It was hypothesized that the use of a patient-specific BioMask would improve regeneration of skin tissue, consisting of epidermis and dermis layers, on the complex facial wounds. The main objective was to achieve restoration of facial skin that is an improvement both aesthetically and functionally. We undertook a proof of concept study to demonstrate the printed BioMask for facial skin regeneration in a mouse model.
2. Materials and Methods
2.1. Skin cell culture
Human epidermal keratinocytes and human dermal fibroblasts were obtained from Lonza (Walkersville, MD). Human epidermal keratinocytes were cultured and expanded in serum-free keratinocyte growth medium (Lonza), and human dermal fibroblasts were cultured and expanded in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, NY) with 10% (v/v) fetal bovine serum (FBS, Gibco BRL) and 1% (v/v) penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO). Cells were cultured at 37°C in a humidified 5% CO2 incubator. The medium was changed every 2–3 days until the use.
2.2. Bioink preparation for skin cell printing
For 3D bioprinting process, a composite hydrogel-based bioink composed of hyaluronic acid (HA, 3 mg/mL), glycerol (10% v/v), gelatin (30 mg/mL), and fibrinogen (20 mg/mL) in calciumfree high glucose DMEM was prepared. Briefly, HA and glycerol were mixed in DMEM, and the mixture was put in the orbital shaker for 48 h to make a homogeneous mixture. Gelatin and fibrinogen were then added to the mixture and dissolved at 37°C in the solution for 1 h. This composite hydrogel bioink was filtered using 0.45 μm sized syringe filter and stored at −20°C until the use. The reagents were obtained from Sigma-Aldrich and used as received unless stated otherwise.
2.3. 3D bioprinting workflow for BioMask fabrication
2.3.1. Image processing and BioMask design
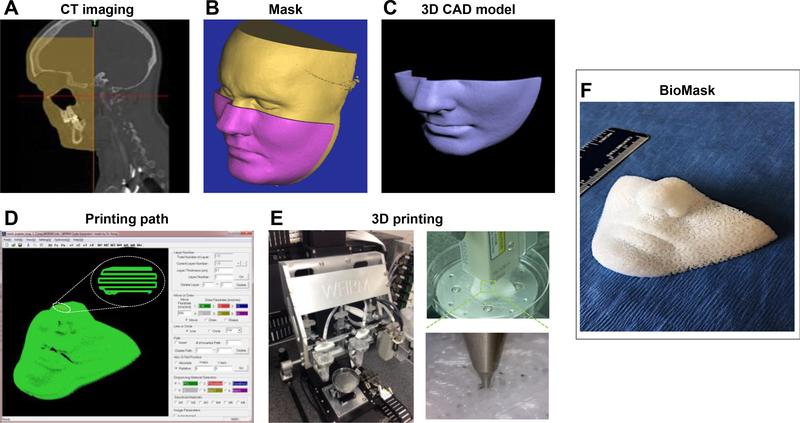
A 3D volumetric information for a human face was obtained from a computed tomography (CT) scan. The obtained digital imaging and communications in medicine (DICOM) format was transformed to a 3D computer-aided design (CAD) model using the Mimics® Software (Materialise NV, Leuven, Belgium). 3D computer-aided manufacturing (CAM) process, including slicing, tool path generation, and motion program generation, was performed to transfer into a 3D fabrication code compatible with the 3D bioprinting system. Using a custom software, a motion program was created by combining the 2D cross-sectional information. Figure 1 shows a workflow for 3D bioprinting process from medical imaging data to BioMask fabrication.
Fig 1.
Bioprinting workflow from medical imaging data to BioMask fabrication: (A) CT image, (B) image process, (C) 3D CAD model, (D) printing path generation, (E) printing process by ITOP system, and (F) 3D printed BioMask.
2.3.2. 3D bioprinter set-up
The in-house 3D integrated tissue-organ printing (ITOP) system [16] was used to fabricate BioMask constructs. The ITOP system consisted of an X, Y, Z-axis stage/controller and six dispensing modules that could process multiple types of cells and materials (Figure 1E). The three-axis stage system having 200 × 200 × 100 mm3 travel and the controller (Aerotech, Inc., Pittsburgh, PA) were used to provide motions for the printing process. The multiple dispensing modules had a precision pneumatic pressure controller (Musashi Engineering, Inc., Tokyo, Japan), a customized metal syringe, and micro-scale nozzles. A syringe heater (Musashi Engineering, Inc., Tokyo, Japan) was used to melt a thermoplastic polyurethane (PU).
2.3.3. 3D bioprinting process
The printed BioMask consisted of three layers; porous PU layer as a wound dressing material, keratinocyte-laden hydrogel layer as an epidermis, and fibroblast-laden hydrogel layer as a dermis. With the 3D fabrication code based on patient’s facial medical data, a face-shape wound dressing layer was printed using PU (Tecoflex LM-95A, Lubrizol, Wickliffe, OH) material. Briefly, the PU beads were loaded into the metal syringe with affixed 200 μm metal nozzle (TECDIA, Tokyo, Japan) and placed in the ITOP system. The PU material was heated to 160°C for printing and dispensed by 1500 kPa of pneumatic pressure.
Human keratinocytes and fibroblasts were harvested with trypsin-EDTA and were spun down to make a cell pellet. The cell pellet was gently mixed with the composite hydrogel prepared in Section 2.2. The cell-laden hydrogels were transferred to a sterilized printing syringe. The syringe then was loaded in the ITOP system, and the cell-laden hydrogels were printed on the face-shaped PU construct sequentially through a 300 um Teflon nozzle at 60 kPa of pneumatic pressure. The cell densities of the epidermis layer containing human keratinocytes and dermis layer containing human fibroblasts were 1×107 cells/mL and 5×106 cells/mL, respectively. After the 3D printing process, thrombin solution (20U/ml, Sigma-Aldrich) was added to the printed BioMask constructs for cross-linking of the hydrogel. The fabricated 3D BioMask constructs were incubated at 37°C in a humidified 5% CO2 incubator, and the medium was changed every 2–3 days.
On days 3 and 7, the cell proliferation in the printed constructs was assessed via AlamarBlue® assay (Thermo Fisher Scientific, Waltham, MA). Briefly, the reagent was added in the culture medium using dilution factor of 10, and the constructs were incubated at 37°C in a humidified 5% CO2 incubator for 2 h. The absorbance at 570 nm was measured using a microplate reader (ELX 800, Bio-Tex Instruments, Winooski, VT).
2.4. Animal model development for facial skin regeneration
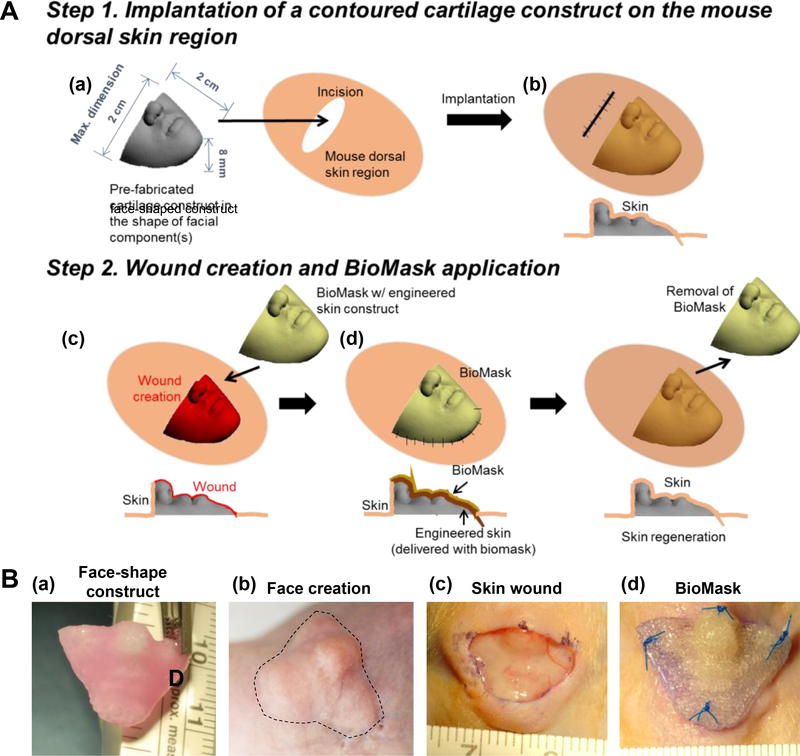
In order to simulate the skin regeneration on the face, a wound bed with a facial contour was needed. A 3D face-shaped structure (20 × 10 × 8 mm3) was implanted subcutaneously under the dorsal skin of athymic mice (NU/NU Nude, Charles River Laboratories, Wilmington, MA) prior to the application of the BioMask (Figure 3). For the 3D face-shaped structure, poly(ε-caprolactone) (PCL, MW = 43,000–50,000, Polysciences Inc., Warrington, PA) was printed through a 200-μm metal nozzle at 800 kPa of air pressure. All animal procedures were performed in accordance with a protocol approved by the Wake Forest University Institutional Animal Care and Use Committee (IACUC). Under general anesthesia, the dorsal surface was cleaned and sterilized with betadine and wiped with 70% alcohol. A dorsal longitudinal incision was made, and a subcutaneous pocket was created. The 3D face-shaped structure was placed into the subcutaneous space, and the incision was closed in a routine fashion. All animals were observed once daily after implantation until sacrifice.
Fig 3.
(A) Schematic illustration of facial skin wound animal model creation and implantation: (a) fabrication and (b) implantation of pre-fabricated face-shaped construct, (c) wound creation on the face-shaped construct after 4-week implantation, and (d) BioMask application. (B) Surgical procedure of BioMask application: (a) face-shape construct, (b) face creation after 4-week implantation, (c) 70% skin wound on the face-shaped construct, and (d) BioMask application.
2.5. Creation of the wound bed and BioMask application
The full-thickness wound was created in the face-shaped dorsal skin of mice. Under general anesthesia, the dorsal surface was cleaned and sterilized with betadine and wiped with 70% alcohol. The skin (dermis and epidermis layers) was then trimmed and a 10 × 20 mm2 wound on the face-shaped structure was created. The 3D BioMask was placed on the wound site and fixed using the suture. The bandage was applied to the wound site using antibiotic ointment and Tegaderm (3D, Maplewood, MN). After 7 days of BioMask application, the PU layer was removed. The skin regeneration was observed at 7 and 14 days after implantation. A total of 12 animals were used in this study (2 groups × 2 time points × 3 animals per group).
2.6. Wound contraction
To evaluate the wound contraction, we measured the wound area on the face-shaped structure just after the wound creation and at the designed time points using Image J software (NIH, Bethesda, DC).
2.7. Histological evaluation
For histological analysis, the retrieved skin tissues from each group were fixed in 10% formaldehyde solution at room temperature for 24 h. Subsequently, the samples were embedded in paraffin and sectioned into 4-μm sections. Deparaffinized sections were stained with hematoxylin and eosin (H&E) for morphological analysis.
2.8. Statistical analysis
Data from the cell proliferation and wound contraction were analyzed using ANOVA. Differences were considered significant if P < 0.05.
3. Results and Discussion
3.1. BioMask fabrication
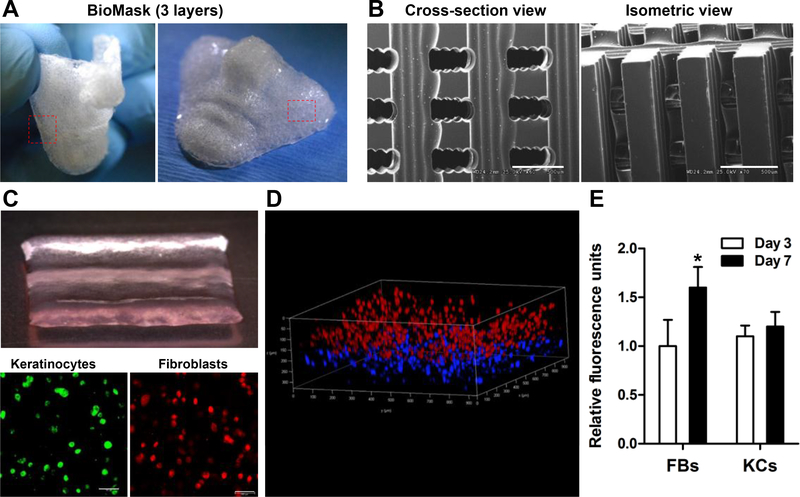
With currently available skin grafts, it is difficult to fully cover the whole facial area due to the complex topography [5]. Here, we investigated the facial skin tissue regeneration using the BioMask fabricated by 3D bioprinting strategies. The BioMask consisted of three layers; porous PU layer, keratinocyte-laden hydrogel layer, and fibroblast-laden hydrogel layer (Figure 2). Many researchers reported that the cellular components and their interactions are required for the skin regeneration [14]. Thus, there have been multiple attempts and fabrication trials for skin graft using both keratinocytes and dermal fibroblasts. For the BioMask fabrication, the composite hydrogel-based bioink previously optimized [16] was used for cell bioprinting. Gelatin was used to increase the viscosity, HA enhanced the dispensing uniformity, and glycerol prevented the nozzle clogging. Fibrinogen provided the structural stability after bioprinting. In addition, PU was selected as a supporting wound dressing material because it has high elastic property compared to other biocompatible thermoplastic polymers [17]. Furthermore, PU has been reported to have a wound dressing effect during skin regeneration. Thus, the printed BioMask was flexible and easy to handle (Figure 2A).
Fig 2.
3D printed BioMask: (A) Gross appearance, (B) SEM images, (C) three layers; porous PU layer, keratinocyte-laden hydrogel layer, and fibroblast-laden hydrogel layer, (D) confocal microscopic image, and (E) cell proliferation assay by AlamarBlue assay kit.
The BioMask featured cross-sectional and isometric pores which average 500 μm throughout (Figure 2B). The cell-laden hydrogels were printed simultaneously while still maintaining their structural integrity (Figure 2C). Confocal microscopic imaging was used to ensure that the keratinocytes and fibroblasts were printed in two distinct layers (Figure 2D). The overall pressure exerted on the cells in order to dispense the hydrogel did not affect the cell viability (data not shown). The cell proliferation of the fibroblasts and keratinocytes in the printed constructs was measured at 3 and 7 days (Figure 2E). The fluorescent intensity in the fibroblastladen layer was increased at 7 days while no cell proliferation was observed in the keratinocyteladen layer.
3.2. Animal model creation and BioMask application
One of the most important steps of the study was to design a model in which a facial defect could be replicated on. Unfortunately, there are no animal models established for testing the facial skin regeneration. A basic animal model could not be used because the animal lacks the unique shape and structure of the human face. This unique shape of the human face will ultimately affect the wound healing process. In this proof of concept study, we have successfully developed the novel facial wound animal model to validate the BioMask application. We created the face-shaped structure subcutaneously on the mouse dorsal skin. After 4 weeks of implantation, the faceshaped structure was fully integrated with adjacent tissues in mice. This unique model has the advantage of providing the exact contours of the patient, which traditional animal models were unable to accomplish.
Figure 3A shows the process of animal model creation with 3D printed facial structure. Right after implantation, the facial structure did not fully settle down. Meanwhile, the implanted facial structure could be easily moved inside the mouse subcutaneously. However, the facial structure finally settled down with the exact facial shape after 10 days of implantation. Furthermore, the implanted facial structure in the animal model did not dislocate or move subcutaneously throughout the four weeks after implantation.
In this novel animal model, we were able to apply the BioMask in the skin wound on the facial structure (70% of facial skin wound was created) (Figure 3B). When skin wound was created on the facial structure, the tissue ingrowth into the implanted structure and vascular network were observed under the dermis layer. The BioMask, consisting of PU support layer, bioengineered epidermis, and dermis layers, was placed on the facial skin wound bed. The BioMask proved to be an excellent combination with the wound bed due to the high flexibility of PU.
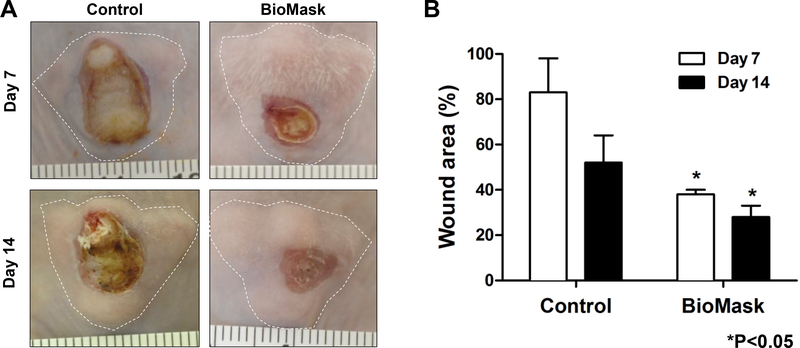
3.3. Wound contraction measurement
To evaluate the wound contraction, we measured the wound area on the face-shaped structure just after the wound creation and then at 7 and 14 days after implantation. Grossly, the wound region in the BioMask group was fully covered with newly regenerated skin while the faceshaped construct was exposed without the skin coverage in the non-treated control group (Figure 4A). There was a significant decrease in wound area percent at both 7 and 14-day time points when compared to the non-treated control group (Figure 4B). This indicates that the bioengineered skin grafts composed of keratinocytes and fibroblasts were well applied to the wound region on the face-shaped structure, resulting in accelerating wound healing.
Fig 4.
Wound contraction measurement: (A) Gross images of skin wound and (B) % wound area at 7 and 14 days (*P<0.05 compared with control). Control: non-treated group.
3.4. Histological evaluation
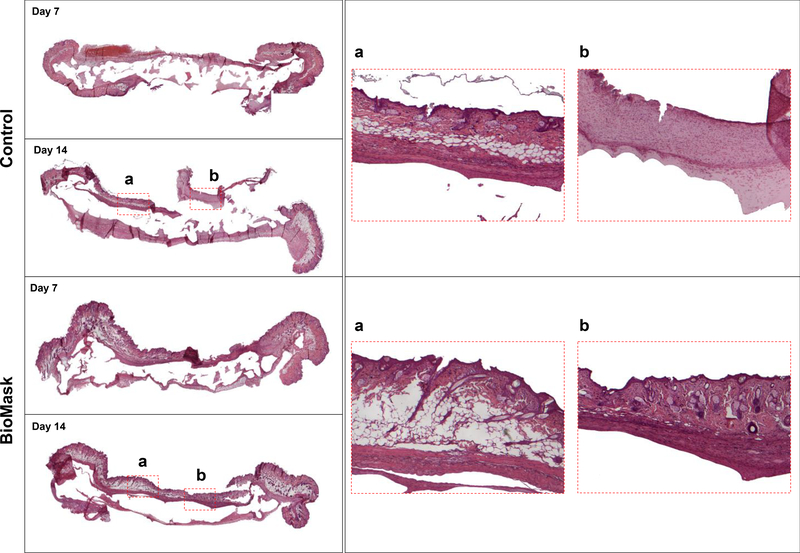
In the histological analysis using H&E staining, the result shows that the regenerated skin tissue differed depending on each group and the time associated with regeneration (Figure 5). Almost no epidermal tissue was regenerated on the non-treated control group at 7 days after implantation; however, the BioMask group at 7 days after implantation had partial epidermal regeneration. Furthermore, non-treated control group shows a limited skin regeneration at 14 days after implantation. In the BioMask group, skin tissue was regenerated and integrated well with surrounding tissue at 14 days after implantation. The result indicates that the bioengineered skin grafts could be delivered by 3D printed BioMask concept for the facial skin regeneration.
Fig 5.
Histological examination of skin regeneration on the face-shaped constructs at 7 and 14 days after BioMask application. Control: non-treated group.
Conclusion
We developed the BioMask concept for patient-specific facial skin regeneration using 3D bioprinting and tissue engineering strategies. The 3D printed BioMask composed of two different skin cell types for the epidermis and dermis layers. Additionally, elastic PU polymer was coprinted to support the bioengineered skin constructs and to provide the wound dressing effect. To validate the printed BioMask, we also developed the novel in vivo animal model for the simulation of skin tissue regeneration on a human face. Future studies could be used to expand upon this strategy with the inclusion melanocytes to avoid cosmetic complications with patchy skin loss and discoloration between the bioengineered skin and native skin pigmentation. The BioMask approach has great potential to offer the effective and rapid restoration of aesthetic and functional facial skin. This would have a significant impact on the affected individuals.
Acknowledgments
Funding sources
This study was supported, in part, by the US National Institutes of Health (1P41EB023833–01).
Footnotes
Conflicts of interest
The authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A, The hidden cost of skin scars: quality of life after skin scarring, J Plast Reconstr Aesthet Surg 61(9) (2008) 1049–58. [DOI] [PubMed] [Google Scholar]
- [2].Demling RH, DeSanti L, Management of partial thickness facial burns (comparison of topical antibiotics and bio-engineered skin substitutes), Burns 25(3) (1999) 256–61. [DOI] [PubMed] [Google Scholar]
- [3].Cichowski A, Kawecki M, Glik J, Klama-Baryla A, Labus W, Maj M, Nowak M, Dworak A, Sieron AL, Literature review concerning cell and skin substitute cultures obtained by means of tissue engineering used in the treatment of burns, Pol Przegl Chir 86(4) (2014) 202–10. [DOI] [PubMed] [Google Scholar]
- [4].Wong S, Melin A, Reilly D, Head and Neck Reconstruction, Clin Plast Surg 44(4) (2017) 845–856. [DOI] [PubMed] [Google Scholar]
- [5].Cheng X, Yoo JJ, Hale RG, Biomask for skin regeneration, Regen Med 9(3) (2014) 245–8. [DOI] [PubMed] [Google Scholar]
- [6].Ramanathan G, Singaravelu S, Muthukumar T, Thyagarajan S, Perumal PT, Sivagnanam UT, Design and characterization of 3D hybrid collagen matrixes as a dermal substitute in skin tissue engineering, Mater Sci Eng C Mater Biol Appl 72 (2017) 359–370. [DOI] [PubMed] [Google Scholar]
- [7].Naves LB, Dhand C, Almeida L, Rajamani L, Ramakrishna S, In vitro skin models and tissue engineering protocols for skin graft applications, Essays Biochem 60(4) (2016) 357–369. [DOI] [PubMed] [Google Scholar]
- [8].Chua AW, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ, Skin tissue engineering advances in severe burns: review and therapeutic applications, Burns Trauma 4 (2016) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K, Skin tissue engineering--in vivo and in vitro applications, Adv Drug Deliv Rev 63(4–5) (2011) 352–66. [DOI] [PubMed] [Google Scholar]
- [10].Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S, Dennis V, Singh SR, Pillai SR, Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review, Int J Mol Sci 17(12) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mohd Hilmi AB, Halim AS, Vital roles of stem cells and biomaterials in skin tissue engineering, World J Stem Cells 7(2) (2015) 428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Augustine R, Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks, Prog Biomater (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ng WL, Qi JTZ, Yeong WY, Naing MW, Proof-of-concept: 3D bioprinting of pigmented human skin constructs, Biofabrication 10(2) (2018) 025005. [DOI] [PubMed] [Google Scholar]
- [14].Pourchet LJ, Thepot A, Albouy M, Courtial EJ, Boher A, Blum LJ, Marquette CA, Human Skin 3D Bioprinting Using Scaffold-Free Approach, Adv Healthc Mater 6(4) (2017). [DOI] [PubMed] [Google Scholar]
- [15].Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P, Design and fabrication of human skin by three-dimensional bioprinting, Tissue Eng Part C Methods 20(6) (2014) 473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity, Nat Biotechnol 34(3) (2016) 312–9. [DOI] [PubMed] [Google Scholar]
- [17].Mathur AB, Collier TO, Kao WJ, Wiggins M, Schubert MA, Hiltner A, Anderson JM, In vivo biocompatibility and biostability of modified polyurethanes, J Biomed Mater Res 36(2) (1997) 246–57. [DOI] [PubMed] [Google Scholar]