Abstract
Attention is a cognitive process crucial for human performance. It has four components: tonic alertness, phasic alertness, selective attention, and sustained attention. All the components of attention show homeostatic (time awake, sleep deprivation) and circadian (time of day) variations. The time course of the circadian rhythms in attention is important to program work and school-related activities. The components of attention reach their lowest levels during nighttime and early hours in the morning, better levels occur around noon, and even higher levels can be observed during afternoon and evening hours. However, this time course can be modulated by chronotype, sleep deprivation, age, or drugs. Homeostatic and circadian variations have also been found in other basic cognitive processes (working memory and executive functions), with a time course similar to that observed for attention. Data reviewed in this paper suggests the need to consider circadian rhythms, age, and chronotype of the person, when programming schedules for work, study, school start time, school testing, psychological testing, and neuropsychological assessment.
Keywords: Human circadian rhythms, cognitive processes, attention, alertness, selective attention, sustained attention
Introduction
Life evolved on our planet under the influence of environmental cycles. Earth rotation produces a daily cycle, in which light and temperature increase during the day and decrease during the night. Additionally, the Earth revolves around the sun producing another cycle manifested as the seasons of the year. Organisms on Earth adapted to the cycles in the environment through the development of internal oscillations, known as biological rhythms. The most studied biological rhythm is the circadian rhythm, with a period close to 24 h [1]. When an organism remains in a constant environment (constant darkness, constant temperature) for a prolonged interval, almost all physiological activities oscillate with a period close to, but still different from, 24 h [2]. In mammals, the lesion of the suprachiasmatic nuclei of the hypothalamus eliminates the circadian rhythms, indicating that this cerebral structure operates as a biological clock and as a pacemaker that keeps all physiological functions internally synchronized [3]. Most organisms adjust their circadian rhythm to the natural 24 h environmental cycle through synchronizers, such as the light-dark cycle, the temperature cycle, the feeding cycle, and the social stimulation cycle [4]. The light-dark cycle is the most potent synchronizer of circadian rhythms for the majority of species, including human beings [5]. The endogenous nature of the circadian rhythm is supported by the discovery of clock genes in many species, that are related to different parameters of the circadian rhythm [6]. Circadian rhythms are a biological property of all living organisms, these are oscillations in many physiological variables, such as body temperature, melatonin or cortisol secretion, or even in motor activity, and can be recorded in each member of a given species. Physiological variables can also show acute variations due to changes in the environment; for example, a mammal may run suddenly to escape from a predator, the intense exercise while running raises body temperature for several minutes, but then the effect dissipates and thus the body temperature resumes its circadian oscillation. These transitory changes affecting physiology produce a masking effect on circadian rhythms [7]. Many environmental factors may modify physiological variables producing a masking effect, sometimes even interfering with the recording of circadian rhythms. Therefore, studying circadian rhythms requires the recording of physiological variables and cognitive processes in constant conditions.
Circadian rhythms are also present in most of the physiology of human beings. In humans, there are circadian rhythms in body temperature, cardiac, pulmonary and metabolic activity, nervous system activity of many areas of the brain, the secretion of all hormones, such as melatonin or cortisol, and the sleep-wake cycle [8]. Circadian rhythms can be observed in the performance of most tasks and activities recorded thus far in humans, such as sensory, motor, reaction time, time estimation, memory tasks, verbal tasks, arithmetic calculations, and simulated driving tasks [9]. Performance increases during the day and decreases during the night. Variations in human performance may be the result of circadian rhythms in cognitive processes that are crucial for the execution of all that tasks [10]. One of these basic cognitive processes is attention. Patients with brain injury suffering a reduction of attention show an impairment in the performance of most tasks and neuropsychological tests [11]. This paper reviews the recent results on circadian rhythms in attention, as well as the implications of these rhythms for real-life conditions.
Homeostatic and Circadian Factors
Human physiology and cognitive performance in humans depend on two factors: homeostatic and circadian [12]. The homeostatic mechanisms maintain a set point level for any physiological function, while the circadian clock produces regular oscillations of the same function. As an example, even though environmental temperature shows variations, core body temperature tends to remain close to 36.5 degrees Celsius. Concurrently, a circadian rhythm in core body temperature of around one degree occurs, with higher values at daytime and lower values during nighttime. This rhythm appears to be irregular or noisy on a natural setting, this is due to changes in ambient temperature, exercise or food consumption, that affect body temperature moment by moment, thus producing masking effects; but the rhythm becomes very regular when the organism remains in constant environmental conditions. Sleep, sleepiness, and performance also change during the day due to the interaction between these two factors [13].
Homeostatic regulation of sleep and cognitive performance refers to the fact that after sleeping well, people wake up alert and active. However, as the day advances people report feeling less alert, at the end of the day sleepiness increases and they fall asleep. When people do not sleep well, they feel less alert and suffer an increase in daytime sleepiness. Alertness increases after the person sleeps efficiently again.
The circadian regulation of sleep and cognitive performance refers to the near 24 h period cycle present in alertness and sleepiness. During daytime hours, alertness is high and sleepiness is low, whereas the opposite occurs during nighttime. Sleepiness is commonly regarded to have detrimental effects on performance at school or work. But sleepiness is a self-report of the subjective feelings of a person during a specific physiological condition. In other terms, during nighttime the core body temperature and the brain activity diminish, this induces a reduction in cognitive processing that the person interprets as a sensation of subjective sleepiness.
Kleitman proposed a theory to explain the origin of cognitive performance rhythms. His theory asserts that the circadian rhythm in metabolic activity modulates brain activity, producing oscillations in cognitive performance [14]. Some cognitive processes show oscillations with a phase similar to the body temperature rhythm [15], but other cognitive processes show a 1 to 4 h phase delay with respect to the body temperature rhythm [16]. It is possible that several oscillators in the brain [17] drive the cycles of different cognitive processes, although all cycles remain coupled to the suprachiasmatic nuclei, which operates as the pacemaker of a multi-oscillatory circadian system.
Methods Used to Assess Circadian Rhythms in Cognitive Processes in Humans
Methods in this field are important to separate the influence of homeostatic and circadian factors on physiology and performance. It is also important to identify homeostatic and circadian variations in physiology and cognitive performance without the influence of masking effects produced by changes in the environment, such as lighting, temperature, or by the activity of the person. Three main methods have been used to study circadian rhythms in physiology and cognitive performance of humans: time of day recordings, a constant routine protocol, and a forced desynchronization protocol [18].
Time of day recordings implies recording performance two or more times during the day, in individuals living in their normal environment. Typically, the recordings are made only during daytime hours, when the person is awake, to not disturb sleep. The few daytime recordings obtained through this type of protocol are insufficient to measure the circadian oscillations throughout the 24 h of the day. Results from these studies are usually highly variable and many studies obtain contradictory results. Due to the high variability of the results obtained with this protocol, larger samples are required to obtain significant differences at different times of day. Therefore, the results of this type of studies are very limited when attempting to draw firm conclusions regarding the circadian rhythms of cognitive processes [18].
A constant routine protocol implies measuring physiological and cognitive functions at regular intervals (for example, every hour), for at least 24 h. Masking conditions that can modify the circadian rhythms are kept constant, such as ambient temperature, light intensity, motor activity, and caloric consumption [19]. Participants remain awake and in a reclined position, ingest a small snack at regular intervals, and maintain a very low level of motor activity to minimize possible effects of food ingestion and motor activity on the circadian rhythm of core body temperature. This protocol is useful to assess homeostatic and circadian changes in cognitive performance.
A forced desynchronization protocol requires the participants to adjust their sleep-wake cycle to a period that is outside the entraining range of circadian rhythms (for example, 28 h). In this condition, the core body temperature continues oscillating with a period close to 24 h, and physiological and cognitive functions can be recorded while the individual is awake, at different phases of the circadian rhythm of the core body temperature [20]. This protocol permits the assessment of homeostatic and circadian changes in cognitive performance [21]. The last two protocols, constant routine and the forced desynchronization protocols, offer suitable controls for the variables that can mask the circadian rhythm, therefore the use of these protocols shows similar results about circadian rhythms in performance [18]. Both protocols allow the recording of circadian rhythms in physiological variables and cognitive performance throughout the 24 h of the day. Results obtained with these two protocols show circadian rhythms in physiology and cognitive performance in each individual recorded, so that significant results can be obtained even with small samples. For these reasons, this review includes mainly results using these two protocols.
The type of task is also very important to analyze circadian rhythms in specific cognitive processes. To assess human circadian rhythms in cognitive processes, performance is measured several times during the day, this may induce learning or fatigue effects. Tasks appropriate for this type of research should produce little or negligible effects on learning or fatigue, or the measurement conditions must be appropriate to minimize these effects. Very simple tasks may be inappropriate to assess specific cognitive processes. Some of these tasks require the participants to only respond to infrequent stimuli with long intervals between them. Continuous performance tasks, that require frequent and continuous responses, may allow the assessment of specific cognitive abilities [22]. On the other hand, exceedingly complex tasks may include multiple cognitive abilities, and separating the contribution of specific cognitive processes from the execution of the test may be very difficult [23]. Many neuropsychological tests suitable to the analysis of specific cognitive processes, cannot be used repeatedly at very short intervals, because the person may improve performance through learning. It is important to select tests appropriate for the study of human circadian rhythms, to design tasks that may accurately grasp specific components of cognitive processes, or devise other protocols suitable for the analysis of these rhythms. Cognitive performance may be influenced by other factors, such as motivation or emotion. Generally, motivational or emotional factors are kept constant and at a reduced level in these experiments. It is possible that these conditions may induce masking effects in natural environmental conditions, thus affecting the recording of circadian rhythms. More research is needed to study the possible effects of motivational or emotional factors on circadian rhythms: do these conditions induce only transitory masking effects? Are these conditions capable of modulating the amplitude, period or phase of the circadian rhythms in cognitive processes?
Circadian Rhythms in Attention
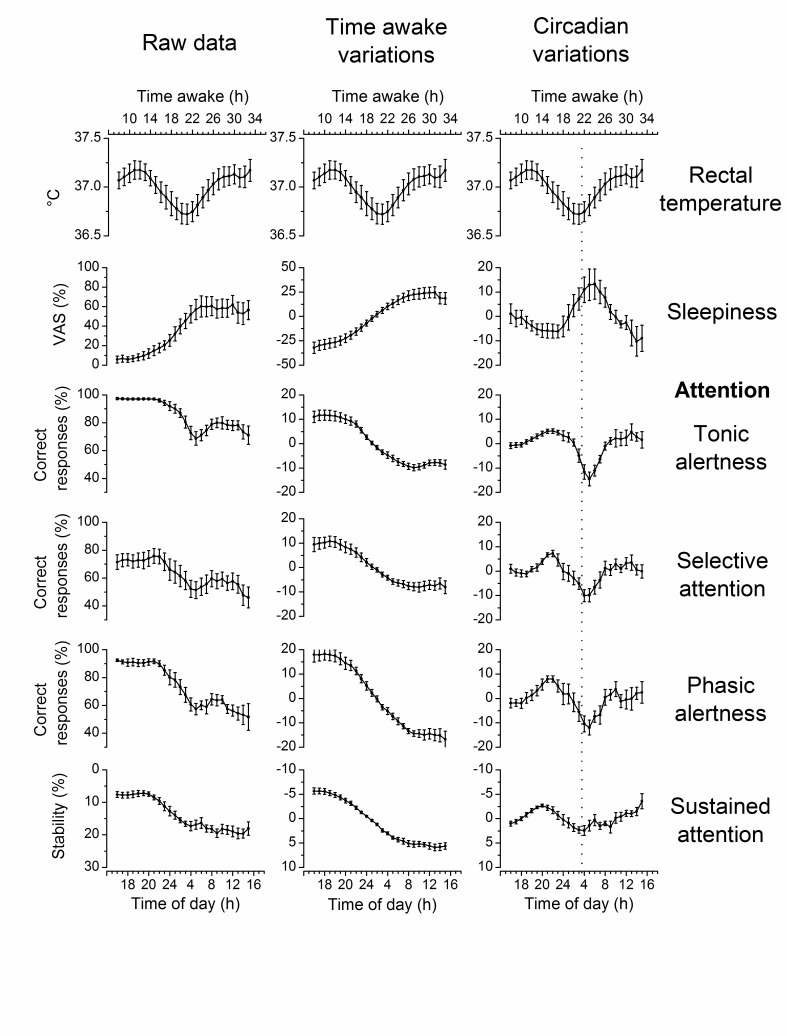
Attention is a cognitive process that refers to the capacity to interact effectively with our environment. Attention implies processing and selecting incoming stimulation, responding to each stimulus, and sustaining specific and efficient responses for minutes or hours. Many models attempt to explain attention; some, emphasize processing of stimuli; others, selection of stimuli; and others, responses to the environment [24]. To analyze the circadian rhythms of attention, this paper uses the neuropsychological model of Posner and Rafal [25], because this model includes all known conditions involving attention. According to this model, attention includes four components: tonic alertness, phasic alertness, selective attention, and sustained attention [25]. Tonic alertness refers to our capacity to respond to any event in the environment, it reflects the arousal or general activation level of the organism at any time. Phasic alertness is the capacity to respond to an event after a warning signal, it is crucial to be ready and respond to a change in the environment. Selective attention is the capacity to produce a specific response to a stimulus and a different response to another stimulus; it has to do with filtering out irrelevant information from the environment. Sustained attention refers to the capacity to efficiently continue responding during some time (minutes to hours) [23]. Several brain systems participate in these components of attention, mainly the reticular, the prefrontal, and the parietal systems [26]. The circadian variations in the components of attention may modulate changes in the performance of all human activities. When any of the components of attention are affected by brain damage or any other disorder, the performance of almost all tests and tasks results are compromised [11]. Figure 1 shows a typical recording of the circadian rhythm in rectal temperature, as well as homeostatic and circadian variations in subjective sleepiness, and each component of attention.
Figure 1.
Homeostatic (time awake) and circadian variations in rectal temperature, subjective sleepiness and the components of attention. Left-side graphs are raw data, middle graphs are smoothed data showing time awake variations, and right-side graphs are detrended data showing circadian variations. Rectal temperature raw data appear on the three columns. The dotted vertical line in the right-side graph represents the batyphase (time of day of the minimum value) of the rectal temperature circadian rhythm. Values are mean ± standard error of the mean. VAS = Visual Analog Scale. Data were taken from Valdez et al. (2005) [22] and Valdez et al. (2010) [46].
Alertness (Tonic and Phasic)
Many papers have documented homeostatic and circadian variations in alertness, using undemanding tasks such as a simple reaction task or vigilance tasks [27]. An example of a simple reaction task is the Psychomotor Vigilance Test, which is used in many studies on circadian rhythms to document changes in cognitive performance. Homeostatic and circadian changes have been demonstrated with this task using time of day, constant routine, and forced desynchronization protocols. Main changes observed are an increase in reaction time throughout the day and circadian variations in reaction time, with lower response latency during the day and higher response latency during the night. The number of lapses (omissions or responses with longer reaction times) also increases with time awake and show circadian variations, with fewer lapses during daytime and a higher frequency of lapses during nighttime [15,28,29]. Homeostatic and circadian variations in tonic and phasic alertness have been observed using a continuous performance task, on a constant routine protocol [22].
Attention requires processing of a single stimulus or several stimuli occurring successively. Processing new incoming information implies that attention should be shifted from one stimulus to another [30]. This process takes time and reduces the efficiency to process the new event [31]. When two stimuli occur with an interval of one second or longer between them, people have no problem to efficiently process both stimuli. But when they occur with a short interval between them (less than 500 ms), people respond accurately and rapidly to the first stimulus, but require more time or fail to process the second stimulus. So, a longer reaction time and less correct responses occur to the second stimulus occurring at an interval shorter than 500 ms, indicating a reduction in the capacity to process the new stimulus. These changes in the processing of a new stimulus have been measured through the Psychological Refractory Period (PRP) [32] and the Attentional Blink (AB) [33]. The PRP is an increase in the reaction time required to process a second stimulus occurring within a 500 ms interval after the first stimulus. Circadian variations have been observed in the PRP [34], this study recorded the PRP every 2 h, during 28 h, on a constant routine protocol. Two stimuli were used: Stimulus 1 (S1) was a 300 or 900 Hz tone, and stimulus 2 (S2) was an X or O letter. Three intervals were used between S1 and S2: 50, 200, or 1000 ms. Participants had to make a specific response to each stimulus. Results showed that accuracy was constant throughout sessions, whereas reaction times to both stimuli showed an increase during the night. Changes in time of day were observed in the PRP gradient, obtained by subtracting the reaction time to S2 at the 1000 ms interval from the reaction time to T2 at the 50 ms interval. This finding suggests that there are circadian oscillations in central processing time, which may explain the circadian variations in reaction time observed in many other studies. The changes in central processing time and reaction time are compatible with circadian variations in tonic alertness. The AB refers to a decrease in the accuracy to detect or identify a second stimulus (target 2, T2) occurring 200 to 500 ms after the first stimulus (target 1, T1). The AB is measured through a Rapid Serial Visual Presentation task, in which an array of stimuli is presented, including two targets (T1, T2) and a group of distractors. The first (T1) and second targets (T2) are presented within different intervals to measure the accuracy to T2 at each interval, between 100 and 800 ms. Typically, there is a decrease in the accuracy at 200 to 500 ms intervals, while longer intervals (600 to 800 ms) show a high level of accuracy, similar to the accuracy to T1. Task performance was recorded each hour, during 28 h, in a constant routine protocol. Homeostatic and circadian variations have been found in the accuracy of all the parameters of the RSVP task, used to assess the AB (T1 accuracy and T2 accuracy at all lags) [35]. T1 accuracy is related to the capacity to respond to independent stimuli, at separate times. A reduction in T1 accuracy was observed throughout the recording session, showing time awake (homeostatic) and time of day (circadian) effects. Circadian variations in T1 showed a phase delay of around 2 h with respect to the circadian rhythm in rectal temperature. Changes in T1 may be related to tonic alertness, which is the general capacity to respond to any stimulus.
Selective Attention
Homeostatic and circadian variations have been observed in selective attention associated with the melatonin circadian rhythm, on a constant routine protocol [36]. Homeostatic (time awake) and circadian (time of day) variations were also observed in selective attention using a continuous performance task, on a constant routine protocol [22].
Homeostatic and circadian variations were also observed in the processing of a second stimulus occurring at a short interval after the first stimulus (T2 accuracy at lag 2, 200 ms) and to successive independent stimuli (T2 accuracy at lag 8, 800 ms) [35]. The changes in information processing observed in this study, may be due to a reduction in the activation to process any stimulus or a reduction in the capacity to suppress the processing of distractors. Circadian variations in T2 showed a phase delay of around 2 h with respect to the circadian rhythm in rectal temperature. The reduction in the efficacy to process a second stimulus (T2) is compatible with changes in selective attention. Dual-task and task-switching performance (examples of multitasking) are affected by sleep deprivation [37-39] and time of day variations in these types of tasks have been observed [40-42].
Sustained Attention
To analyze sustained attention, it is necessary to measure three indices of this cognitive process: general stability of performance efficiency, time on task performance, and short-term stability [43]. General stability of performance efficiency can be measured as the variability (standard deviation) of correct responses throughout a task. Low variability means a high level of stability of efficiency and an increase in sustained attention. Circadian variations in the stability of performance efficiency have been observed, with high stability during daytime and low stability during nighttime and early in the morning [44]. Time on task performance can be measured by a linear regression coefficient of correct responses throughout the task. A zero linear coefficient value indicates maintenance of the same level of performance from start to end, meaning an increase in attention. This index of sustained attention decreases with time awake and sleep deprivation [45]. Short-term stability of efficiency can be measured by hit runs (sequences of correct responses) and error runs (sequences of errors). Longer and more frequent hit runs, or less frequent error runs indicate an increase in sustained attention. A progressive homeostatic decrease during the day has been observed in general stability and short-term stability (hit runs and error runs), using a continuous performance task, on a constant routine protocol, with a decrease between 04:00 to 07:00 h [46].
Time Course of Circadian Rhythms in Attention
The components of attention are at a low level in the morning (07:00 to 10:00 h), mainly because circadian rhythms reach their lowest point at this time of day; sleep inertia also contributes to this low level of execution. Attention improves towards noon (10:00 to 14:00 h), but then there is a decrease after lunch (14:00 to16:00 h) (post-lunch dip), execution gets better in the afternoon and early evening hours (16:00 to 22:00 h). Finally, attention decreases again at night (22:00 to 04:00 h) and reaches its lowest levels at dawn and early in the morning (04:00 to 07:00 h) (Figure 1). These changes in performance apply approximately to a person who usually sleeps from 23:00 to 07:00 h and with an intermediate chronotype. The time course of cognitive performance is important to program daily activities, such as school schedule, time allocated for studying, sports or work, as well as programming academic, medical, psychological, or neuropsychological tests [47].
Several conditions modulate the temporal course of cognitive performance: chronotype, sleep deprivation, age, and drugs. The chronotype is the pattern of preferences of a person: some individuals prefer and feel better when performing their activities in the morning (morning type), others prefer to do their activities in the evening (evening type) and other show no preference, they may carry out their activities at any time of day (intermediate type). Chronotype of an individual is a very stable trait [48]. Morning type persons show a phase advance and evening type persons show a phase delay of circadian rhythms [49]. These changes in circadian rhythms phase, may produce difficulties in learning or solving school tests in the afternoon for morning type students, and in the morning for evening type students. Attention in evening type students may improve after noon (around 12:00 to 16:00 h). Attention and other cognitive functions show a synchrony effect with the chronotype, morning type students show better performance during the morning, with worse execution in the evening, while evening type students show an inverse pattern [50]. Sleep deprivation affects the amplitude of the rhythms in cognitive performance [51]. The greater the sleep deprivation the greater the deterioration in execution throughout the day, with a greater decrease in the morning, the post-lunch dip, and in the first hours of the night. Evening type students have lower levels of attention during daytime. Their attention stays low during the morning because of the phase of their circadian rhythms. They go to bed very late and have to wake up early to comply with the start school time during weekdays, this reduction of sleep duration produces even lower levels of attention during daytime. It has been observed that evening type students obtain lower grades, compared to morning type students [52].
Age also modulates the time course of cognitive rhythms, adolescents tend to delay their sleep-wake cycle, while aged people tend to advance their sleep-wake cycle [53]. Some drugs can modulate the temporal course of circadian rhythms in cognitive performance, the effect depends on the dose and the timing of the ingestion. For example, taking caffeine in the morning increases alertness at that time of day and promotes a phase advance of circadian rhythms, while taking caffeine in the evening may induce alertness that interferes with bedtime, and promotes a delay of circadian rhythms [54].
Circadian Rhythms in Other Basic Cognitive Processes
Homeostatic and circadian variations have also been found in two other basic cognitive processes: working memory and executive functions. Working memory comprises the storage, retrieval, and use of information [55]. Working memory has four components: phonological storage, visuospatial storage, an episodic buffer and a central executive system [56]. Homeostatic and circadian variations are observed, both in the phonological storage and in the visuospatial storage working memory components, in a constant routine protocol [57,58]. The capacity to store phonological and visuospatial information decreased from 04:00 to 07:00 h. The oscillations in these components of working memory correlated with the circadian rhythm of core body temperature, with a phase delay of 1 to 3 h. The oscillations in the phonological store can explain the variations observed during the day in tasks such as reading comprehension and verbal learning. While the oscillations in the visuospatial store can explain the variations observed during the day in tasks that include space perception such as drawing, construction and arithmetic tasks.
Executive functions refer to the ability to program and regulate human behavior, they are thus crucial for decision making, self-control, and problem-solving. Executive functions include the following components: initiative, inhibition, flexibility, planning, prevision, self-monitoring, verification and correction [59]. Cognitive inhibition shows changes with time of day in a Go/NoGo task, on a time of day protocol [60] and on a constant routine protocol [29]. Circadian variations in inhibition and cognitive flexibility have been observed by means of two types of Stroop-type tasks, in one the test items are read aloud [61], and in the other a computerized Stroop-type task is used [62]. Homeostatic and circadian variations occurred in cognitive inhibition and flexibility, with the lowest values occurring around 04:00 to 07:00 h. The performance in a Go/NoGo task diminishes with time awake, with no time of day variations, although execution decreased when the person woke up near the time of the lowest core body temperature level (bathyphase) [63]. On the other hand, changes with time awake and time of day were observed in self-monitoring, using a constant routine protocol [64]. Self-monitoring decreased from around 04:00 to 07:00 h. The reduction of the components of attention during nighttime make people prone to commit errors which may result in accidents, due to the concurrent reduction of self-monitoring, interfering with the correction of errors.
Many components of these basic cognitive processes (attention, working memory, and executive functions) change with sleep deprivation and time of day. A sequential participation of these functions throughout time awake and time of day may explain the homeostatic and circadian variations in all components of cognitive processes [23]. Accordingly, during daytime the body temperature is high, sleepiness is low, and cognitive performance is high. During the first hours of the night body temperature begins to decrease and sleepiness increases due to time awake (sleep deprivation), at this stage the more primitive processes (tonic alertness, arousal) may be affected first, interfering with other basic cognitive processes and producing some errors in performance. At this time executive functions are still operating, thus maintaining behavior directed to the goals and compensating for the decrease in tonic alertness. As the night advances, body temperature gets lower, sleepiness increases, and other components of attention, such as working memory and some basic components of executive functions are affected, producing more frequent and more serious errors. The last stage involves a steep decrease in executive functions, such as self-monitoring, impeding a compensation for the reduction in cognitive processing, and producing an impairment in decision making and problem-solving, with an increased risk of severe errors and accidents. Finally, the person falls asleep, ceasing to respond to the environment [10].
The time course of working memory and executive functions is similar to that observed in the components of attention, suggesting that cognitive performance tends to be higher during daytime hours and to be lower during nighttime and early in the morning. Also suggests possible circadian rhythms in the brain areas crucial for these cognitive processes. These rhythms in cognitive processes keep a phase relation to the rhythm in body temperature, indicating that they are coordinated with the rest of the physiological rhythms of the body, through the suprachiasmatic nucleus, which operates as a pacemaker of the circadian rhythms. Table 1 summarizes studies on circadian rhythms in attention, working memory, and executive functions.
Table 1. Circadian rhythms in attention and other basic cognitive processes (working memory and executive functions).
| Basic cognitive process / component | Protocol | Task | Highest cognitive performance | Lowest cognitive performance | Study |
| Attention | |||||
| Tonic alertness | Forced desynchronization protocol | Psychomotor vigilance task (simple reaction time task) | Day (rhythm of body temperature) 6 lapses 300 ms reaction time |
Night (rhythm of body temperature) 12 lapses 450 ms reaction time |
Wright et al, 2002 [15] |
| Tonic alertness | Constant routine protocol | Continuous performance task | 20:00-23:00 h 90% correct responses 400 ms reaction time |
04:00-07:00 h 70% correct responses 450 ms reaction time |
Valdez et al, 2005 [22] |
| Tonic alertness | Constant routine protocol | Psychological Refractory Period | 23:00 h 250 ms measure of speed of central processing |
07:00 h 370 ms measure of speed of central processing |
Bratzke et al, 2007 [34] |
| Tonic alertness | Constant routine protocol | Attentional blink (Rapid Serial Visual Presentation task) | 20:00-23:00 h 80% correct responses |
05:00-08:00 h 65% correct responses |
Gallegos et al, 2018 [35] |
| Tonic alertness | Forced desynchronization protocol | Psychomotor vigilance task (simple reaction time task) | Day (rhythm of melatonin) 2 lapses 250 ms reaction time |
Night (rhythm of melatonin) 8 lapses 400 ms reaction time |
McHill et al, 2018 [28] |
| Tonic alertness | Constant routine protocol | Psychomotor vigilance task (simple reaction time task) | 17:00-21:00 h 0 lapses 265 ms reaction time |
05:00-09:00 h 7 lapses 295 ms reaction time |
Zeeuw et al, 2018 [29] |
| Phasic alertness | Constant routine protocol | Continuous performance task | 20:00-23:00 h 80% correct responses 440 ms reaction time |
04:00-07:00 h 60% correct responses 500 ms reaction time |
Valdez et al, 2005 [22] |
| Selective attention | Constant routine protocol | Spatial-configuration search task (Figures) | Day (rhythm of melatonin) 700 ms reaction time |
Night (rhythm of melatonin) 850 ms reaction time |
Horowitz et al, 2003 [36] |
| Selective attention | Constant routine protocol | Spatial-configuration search task (Numbers) | Day (rhythm of melatonin) 1400 ms reaction time |
Night (rhythm of melatonin) 1650 ms reaction time |
Horowitz et al, 2003 [36] |
| Selective attention | Constant routine protocol | Continuous performance task | 20:00-23:00 h 75% correct responses 500 ms reaction time |
04:00-07:00 h 55% correct responses 550 ms reaction time |
Valdez et al, 2005 [22] |
| Selective attention | Constant routine protocol | Attentional blink (Rapid Serial Visual Presentation task) | 20:00-23:00 h 75% correct responses |
04:30-08:00 h 60% correct responses |
Gallegos et al, 2018 [35] |
| Sustained attention | Constant routine protocol | Continuous performance task | 20:00-23:00 h 15 hit runs 3 error runs |
04:00-09:00 h 10 hit runs 10 error runs |
Valdez et al, 2010 [46] |
| Working memory | |||||
| Phonological storage | Constant routine protocol | Phonological working memory task | 18:00-23:00 h 90% correct responses |
05:00-08:00 h 75% correct responses |
Ramirez et al, 2006 [57] |
| Phonological storage | Constant routine protocol | Verbal N-back task | Night (rhythm of melatonin) 75% correct responses |
Day (rhythm of melatonin) 40% correct responses |
Groeger et al, 2008 [58] |
| Phonological storage | Constant routine protocol | Verbal N-back task | 15:00-21:00 h 95% correct responses |
05:00-09:00 h 78% correct responses |
Zeeuw et al, 2018 [29] |
| Visuospatial storage | Constant routine protocol | Visuospatial working memory task | 18:00-23:00 h 85% correct responses |
05:00-08:00 h 75% correct responses |
Ramirez et al, 2006 [57] |
| Visuospatial storage | Constant routine protocol | Spatial N-back task | Day (rhythm of melatonin) 70% correct responses |
Night (rhythm of melatonin) 35% correct responses |
Groeger et al, 2008 [58] |
| Executive functions | |||||
| Cognitive inhibition | Forced desynchronization protocol | Go/no-go task | Day (rhythm of body temperature) 20% commission errors |
Night (rhythm of body temperature) 30% commission errors |
Harrison et al, 2007 [63] |
| Cognitive inhibition | Constant routine protocol | Computarized Stroop-type task with shifting criteria | 18:00-23:00 h 85% correct responses 500 ms reaction time |
03:00-06:00 h 70% correct responses 570 ms reaction time |
Garcia et al, 2012 [62] |
| Cognitive inhibition | Constant routine protocol | Stroop task with shifting criteria (48 words) |
18:00-23:00 h 43 s time to complete the task |
03:00-06:00 h 50 s time to complete the task |
Ramirez et al, 2012 [61] |
| Cognitive inhibition | Constant routine protocol | Go/no-go task | 15:00-21:00 h 75% commission errors |
05:00-09:00 h 93% commission errors |
Zeeuw et al, 2018 [29] |
| Cognitive flexibility | Constant routine protocol | Computarized Stroop-type task with shifting criteria | 18:00-23:00 h 65% correct responses |
03:00-06:00 h 45% correct responses |
Garcia et al, 2012 [62] |
| Cognitive flexibility | Constant routine protocol | Stroop task with shifting criteria (48 words) |
18:00-23:00 h 50 s time to complete the task |
03:00-06:00 h 57 s time to complete the task |
Ramirez et al, 2012 [61] |
| Self-monitoring | Constant routine protocol | Tracking task | 18:00-23:00 h 4 circles to adjust to changes in path |
05:00-09:00 h 14 circles to adjust to changes in path |
Garcia et al, 2016 [64] |
Notes: This table includes only studies with constant routine or forced desynchronization protocols. In the forced desynchronization protocol, time of day is expressed as subjective day or night, according to the phase of the circadian rhythm of body temperature or melatonin.
Implications
Circadian rhythms of attention may affect the performance in at least two common settings: work and school. Circadian rhythms in this cognitive process may interfere with activities performed during the night and early morning. Working on a night shift produces a misalignment between the circadian clock and the behavioral cycles, resulting in an impairment of cognitive performance [65]. Night shift workers may be especially at risk to commit errors and suffer accidents. But morning shift workers may also be at risk, especially during morning hours. These risks may increase with extended working periods, sleep deprivation and fatigue. Workers of these shifts tend to make some adjustments to counteract the reduction of attention during nighttime and early in the morning, by eating snacks, taking short naps when feasible, and consuming caffeinated beverages. Although these measures tend to offer a transitory increase in alertness, they may not be effective to improve phasic alertness, selective attention or sustained attention.
Circadian rhythms in attention can also affect school learning. According to the results described, tonic alertness, phasic alertness, selective attention, and sustained attention improve towards the afternoon and evening [47], thus at this time of day we are in a better position to process information in activities such as reading, understanding texts, analyzing information, carrying out numerical calculations. Therefore, the best time to study occurs at night, approximately two hours before bedtime. On the other hand, all the components of attention are still at low levels at early hours in the morning. Students may be at a disadvantage for learning efficiently at the beginning of their school schedule, commonly programmed at 07:00 or 08:00 h in the morning. They may have difficulties to learn and process information during the first hours of classes. Many teachers have observed that some students can not actively participate in school activities during the first hours of classes, with several even falling asleep in that interval. Paradoxically, the more difficult subject matters are scheduled in the early morning hours, because it is often assumed that the students would be refreshed and active after sleeping. The reduction of attention during morning may be even greater because of a sleep reduction during weekdays. This sleep reduction occurs because during weekdays adolescents go to bed late but wake up early to comply with the school start time [66].
According to data reviewed in this paper, healthy individuals show acceptable levels of cognitive performance from 10:00-14:00 h and from 16:00-22:00 h, so school testing should be programmed during these intervals. However, these intervals may be modified by factors such as chronotype, age, sleep deprivation, or drugs [47]. Chronotype refers to the preference of an individual to sleep and perform its activities early in the morning (morning type), or sleep and perform its activities late in the evening (evening type), while other individuals show no extreme preferences to do their activities (intermediate type) [67]. Morning type persons show a phase advance, while evening type persons show a phase delay of their circadian rhythms [68]. According to the chronotype, morning type persons show better performance in the morning than in the evening, while evening type persons show better performance in the evening than in the morning [69]. These results suggest that for evening type persons, the circadian increase in attention may occur until noon or even later. Aged people tend to be morning type individuals [70], and tend to perform better in the morning, with lower performance in the afternoon and evening [71]. Sleep deprivation deteriorates performance throughout the day [72]. It is necessary to consider which timing is more appropriate for studying and school testing for the individual, according to his/her own circadian rhythm parameters, chronotype, and age; as well as to promote better sleep in students and in the general population.
Conclusions and Outlook
Attention is a cognitive process crucial for the performance of all human activities, being it learning, working, engaging in sports, arts, social interaction, or recreational activities. Any condition affecting the efficiency of these processes, such as sleep deprivation or circadian rhythms, interferes with human performance. Circadian rhythms have been found in all the components of attention, both in tonic alertness and phasic alertness, as well as in selective attention and sustained attention (concentration). Our attention improves during the day, while it reaches its lowest level during the night and early morning hours. The circadian rhythm in attention modulates the performance in all the activities that the human being performs, so during the night and early morning hours the performance at work and productivity decreases, with an increase in the probability of errors and accidents. In addition, the decrease in attention at these times of the day also affects work performance and school learning.
Scheduling of human activities is generally based on economic or social considerations. Work and school schedules do not consider human physiology, sleep needs, or circadian rhythms. Data examined in this paper suggests the need to program schedules compatible with human physiology and biological rhythms. Circadian rhythms in physiology and cognitive processes do not easily adjust to night shift work or to an early school start time. These work and study schedules result in sleep reduction, lower levels of attention, low work or study performance, and an increase in the risk of errors and accidents. Scheduling for other activities, such as school testing, psychological, or neuropsychological assessment, must also be programmed according to circadian rhythms, age, and chronotype of the person.
Declaration of interest statement
The author hereby states that there is no financial interest or benefit arising from the direct application of this research; therefore, there is no conflict of interest whatsoever.
References
- Moore-Ede MC, Sulzman FM, Fuller CA. The clocks that time us. Cambridge: Harvard University Press; 1982. [Google Scholar]
- Foster RG, Kreitzman L. Rhythms of life: the biological clocks that control the daily lives of every living thing. New Haven (CT): Yale University Press; 2005. [Google Scholar]
- Evans J, Silver R. The suprachiasmatic nucleus and the circadian timekeeping system of the body In: Pfaff D, Volkov N. Neuroscience in the 21st Century. New York (NY): Springer; 2016. pp. 2241–88. [Google Scholar]
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90(3):1063–102. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Buxton OM. Human circadian timing system and sleep-wake regulation In: Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 6th ed. Philadelphia (PA): Elsevier Saunders; 2017. pp. 362–76. [Google Scholar]
- Kumar V. Biological timekeeping: clocks, rhythms and behaviour. New York, NY. India: Springer; 2017. [Google Scholar]
- Rietveld WJ, Minors DS, Waterhouse JM. Circadian rhythms and masking: an overview. Chronobiol Int. 1993;10(4):306–12. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Circadian physiology. 3rd ed. Boca Raton (FL): CRC Press/Taylor & Francis Group; 2016. [Google Scholar]
- Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007;24(7):755–89. [DOI] [PubMed] [Google Scholar]
- Valdez P. Homeostatic and circadian regulation of cognitive performance. Biol Rhythm Res. 2019;50(1):85–93. [Google Scholar]
- Cohen RA. The neuropsychology of attention. 2nd ed. Boston (MA): Springer; 2014. [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25(2):131–43. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Sleep and wakefulness. 2nd ed. Chicago (IL): The University of Chicago Press; 1963. [Google Scholar]
- Wright KP, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283(6):R1370–7. [DOI] [PubMed] [Google Scholar]
- Gabehart RJ, Van Dongen HP. Circadian rhythms in sleepiness, alertness, and performance In: Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 6th ed. Philadelphia (PA): Elsevier Saunders; 2017. pp. 388–95. [Google Scholar]
- Muto V, Jaspar M, Meyer C, Kusse C, Chellappa SL, Degueldre C, et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 2016;353(6300):687–90. [DOI] [PubMed] [Google Scholar]
- Blatter K, Cajochen C. Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. Physiol Behav. 2007;90(2–3):196–208. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17(1):4–13. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–81. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol Regul Integr Comp Physiol. 1999;277(3):R640–9. [DOI] [PubMed] [Google Scholar]
- Valdez P, Ramírez C, García A, Talamantes J, Armijo P, Borrani J. Circadian rhythms in components of attention. Biol Rhythm Res. 2005;36(1–2):57–65. [Google Scholar]
- Valdez P, Reilly T, Waterhouse J. Rhythms of mental performance. Mind Brain Educ. 2008;2(1):7–16. [Google Scholar]
- Nobre K, Kastner S. The Oxford handbook of attention. 1st ed. New York (NY): Oxford University Press; 2014. [Google Scholar]
- Posner M, Rafal R. Cognitive theories of attention and the rehabilitation of attentional deficits In: Meier M, Benton A, Diller L. Neuropsychological rehabilitation. New York (NY): Guilford Press; 1987. pp. 182–201. [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35(1):73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHill AW, Hull JT, Wang W, Czeisler CA, Klerman EB. Chronic sleep curtailment, even without extended (>16-h) wakefulness, degrades human vigilance performance. Proc Natl Acad Sci USA. 2018;115(23):6070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw J, Wisniewski S, Papakonstantinou A, Bes F, Wahnschaffe A, Zaleska M, et al. The alerting effect of the wake maintenance zone during 40 hours of sleep deprivation. Sci Rep. 2018;8(11012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116(2):220–44. [DOI] [PubMed] [Google Scholar]
- Telford CW. The refractory phase of voluntary and associative responses. J Exp Psychol. 1931;14(1):1–36. [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18(3):849–60. [DOI] [PubMed] [Google Scholar]
- Bratzke D, Rolke B, Ulrich R, Peters M. Central slowing during the night. Psychol Sci. 2007;18(5):456–61. [DOI] [PubMed] [Google Scholar]
- Gallegos C, García A, Ramírez C, Borrani J, Azevedo CV, Valdez P. Circadian and homeostatic modulation of the attentional blink. Chronobiol Int. 2018:1–10. Epub 2018 Nov 29. 10.1080/07420528.2018.1543315 [DOI] [PubMed] [Google Scholar]
- Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Searching night and day: a dissociation of effects of circadian phase and time awake on visual selective attention and vigilance. Psychol Sci. 2003;14(6):549–57. [DOI] [PubMed] [Google Scholar]
- Bohnen HG, Gaillard AW. The effects of sleep loss in a combined tracking and time estimation task. Ergonomics. 1994;37(6):1021–30. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. 2001;10(2):85–92. [DOI] [PubMed] [Google Scholar]
- Couyoumdjian A, Sdoia S, Tempesta D, Curcio G, Rastellini E, De Gennaro L, et al. The effects of sleep and sleep deprivation on task-switching performance. J Sleep Res. 2010;19(1-Part-I):64–70. [DOI] [PubMed] [Google Scholar]
- Rodgers MD, Holding D. Dual-task efficiency throughout the day. Hum Perform. 1991;4(3):187–98. [Google Scholar]
- Van Eekelen AP, Kerkhof GA. No interference of task complexity with circadian rhythmicity in a constant routine protocol. Ergonomics. 2003;46(15):1578–93. [DOI] [PubMed] [Google Scholar]
- Bratzke D, Rolke B, Steinborn MB, Ulrich R. The effect of 40 h constant wakefulness on task-switching efficiency. J Sleep Res. 2009;18(2):167–72. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Valentino DA, Arruda JE. Measures of variations in performance during a sustained attention task. J Clin Exp Neuropsychol. 2002;24(6):828–39. [DOI] [PubMed] [Google Scholar]
- Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- Lisper HO, Kjellberg A. Effects of 24-hour sleep deprivation on rate of decrement in a 10-minute auditory reaction time task. J Exp Psychol. 1972;96(2):287–90. [DOI] [PubMed] [Google Scholar]
- Valdez P, Ramírez C, García A, Talamantes J, Cortez J. Circadian and homeostatic variation in sustained attention. Chronobiol Int. 2010;27(2):393–416. [DOI] [PubMed] [Google Scholar]
- Valdez P. Ramírez, García A. Circadian rhythms in cognitive performance: implications for neuropsychological assessment. ChronoPhysiology Ther. 2012;2:81–92. [Google Scholar]
- Kantermann T, Eastman CI. Circadian phase, circadian period and chronotype are reproducible over months. Chronobiol Int. 2018;35(2):280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Hahn CS, Hasher L, Wiprzycka UJ, Zelazo PD. Time of day, intellectual performance, and behavioral problems in morning versus evening type adolescents: is there a synchrony effect? Pers Individ Dif. 2007;42(3):431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Weber M. Sleep deprivation and cognitive performance In: Bianchi MT. Sleep deprivation and disease. New York (NY): Springer; 2014. pp. 209–29. [Google Scholar]
- van der Vinne V, Zerbini G, Siersema A, Pieper A, Merrow M, Hut RA, et al. Timing of examinations affects school performance differently in early and late chronotypes. J Biol Rhythms. 2015;30(1):53–60. [DOI] [PubMed] [Google Scholar]
- Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51(8):1085–91. [DOI] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, McHill AW, Chinoy ED, Snider JA, Bessman SC, et al. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med. 2015;7(305):305ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working memory In: Bower GH. Psychology of learning and motivation. New York (NY): Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baddeley A. Working memory: theories, models, and controversies. Annu Rev Psychol. 2012;63(1):1–29. [DOI] [PubMed] [Google Scholar]
- Ramírez C, Talamantes J, García A, Morales M, Valdez P, Menna-Barreto L. Circadian rhythms in phonological and visuospatial storage components of working memory. Biol Rhythm Res. 2006;37(5):433–41. [Google Scholar]
- Groeger J, Viola A, Lo J, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31(8):1159–67. [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 5th ed. New York (NY): Oxford University Press; 2012. [Google Scholar]
- Manly T, Lewis GH, Robertson IH, Watson PC, Datta AK. Coffee in the cornflakes: time-of-day as a modulator of executive response control. Neuropsychologia. 2002;40(1):1–6. [DOI] [PubMed] [Google Scholar]
- Ramírez C, García A, Valdez P. Identification of circadian rhythms in cognitive inhibition and flexibility using a Stroop task. Sleep Biol Rhythms. 2012;10(2):136–44. [Google Scholar]
- García A, Ramírez C, Martínez B, Valdez P. Circadian rhythms in two components of executive functions: cognitive inhibition and flexibility. Biol Rhythm Res. 2012;43(1):49–63. [Google Scholar]
- Harrison Y, Jones K, Waterhouse J. The influence of time awake and circadian rhythm upon performance on a frontal lobe task. Neuropsychologia. 2007;45(8):1966–72. [DOI] [PubMed] [Google Scholar]
- García A, Ramírez C, Valdez P. Circadian variations in self-monitoring, a component of executive functions. Biol Rhythm Res. 2016;47(1):7–23. [Google Scholar]
- Chellappa SL, Morris CJ, Scheer FA. Daily circadian misalignment impairs human cognitive performance task-dependently. Sci Rep. 2018;8(1):3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez P, Ramírez C, García A. Delaying and extending sleep during weekends: sleep recovery or circadian effect? Chronobiol Int. 1996;13(3):191–8. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- Kerkhof GA, Van Dongen HP. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci Lett. 1996;218(3):153–6. [DOI] [PubMed] [Google Scholar]
- May CP. Synchrony effects in cognition: the costs and a benefit. Psychon Bull Rev. 1999;6(1):142–7. [DOI] [PubMed] [Google Scholar]
- Mecacci L, Zani A, Rocchetti G, Lucioli R. The relationships between morningness-eveningness, ageing and personality. Pers Individ Dif. 1986;7(6):911–3. [Google Scholar]
- May CP, Hasher L, Stoltzfus ER. Optimal time of day and the magnitude of age differences in memory. Psychol Sci. 1993;4(5):326–30. [Google Scholar]
- Kim DJ, Lee HP, Kim MS, Park YJ, Go HJ, Kim KS, et al. The effect of total sleep deprivation on cognitive functions in normal adult male subjects. Int J Neurosci. 2001;109(1–2):127–37. [DOI] [PubMed] [Google Scholar]