Abstract
Background
Exposure to various types of stress can elevate craving for cocaine and hasten relapse among substance dependent individuals. This investigation evaluated the effects of social exclusion on brain activity in cocaine dependent individuals.
Method
Forty three individuals (18 crack-cocaine users, 25 controls) were recruited from the community to participate in functional neuroimaging study in which they performed a simulated 3 person ball-tossing game (Cyberball). Each participant was told that the other 2 players were in nearby MRI scanners. Task blocks included: Inclusion (likelihood of our participant receiving the ball = 50%), Exclusion (likelihood gradually decreases to 0%), and Rest. Self-worth variables (e.g self-esteem, locus of control) were measured before and after the ball-tossing game. General linear model-based statistics were used to measure the brain response to inclusion and exclusion within and between the groups with respect to rest.
Results
Relative to controls, cocaine users had significantly more activity during Exclusion versus Inclusion in 3 areas: the right medial frontal gyrus (Brodmann Area 9,10), left ventral lateral frontal gyrus (Brodmann Area 10,47) and right caudate. This was driven by a higher response to social exclusion in the cocaine users. There was no difference between groups in the brain reactivity to social inclusion.
Conclusion
Cocaine dependent individuals have an amplified brain response to social exclusion stress in cortical regions associated with emotional regulation, arousal, craving and perception of physical pain. These data suggest that there may be a neurological basis for the well-established relationship between social stress and addiction.
Keywords: Cocaine, Stress, Neuroimaging, Social exclusion, Cingulate, Pain, addiction, Prefrontal cortex, Ostracism
1. Introduction
In both clinical and preclinical literature, it is well established that exposure to various types of stress can elevate craving for cocaine and hasten relapse (Kreek and Koob, 1998; Sinha et al., 2006). One particularly potent form of stress is interpersonal/social relationship stress. Cocaine dependent individuals have an elevated physiological response to stressful interpersonal relationships relative to healthy controls (Chaplin et al., 2010) which is, in turn, associated with elevated craving for cocaine (Sinha et al. 1999, 2000, 2003; Back et al. 2005, 2010; Fox et al., 2008; Chaplin et al., 2010; Waldrop et al., 2010). Sinha and colleagues have demonstrated that exposure to psychosocial stress elicits craving at least as much as direct exposure to drug-related cues (Sinha et al., 1999).
A particularly potent form of interpersonal relationship stress is ostracism from a social group (Cacioppo and Hawkley, 2009). Exposure to social stressors is associated with elevated blood pressure and cortisol levels, along with an overall decrease in measures of self-worth (Wang et al., 2017; Cacioppo and Hawkley, 2009; Dickerson and Kemeny, 2004). One of the most well validated laboratory models of social ostracism uses a task known as “Cyberball” (Hartgerink et al., 2015). Eisenberger and colleagues (Eisenberger et al., 2003) were the first group to demonstrated that being socially excluded from this dynamic, computerized ball-tossing game led to elevation in the cingulate cortex, the anterior insula, and the right ventral prefrontal cortex – a network of brain regions which are now often grouped and referred to as the salience network (Seeley et al., 2007). These regions are implicated in physical pain as well as this form of social pain, and their activation by a social ostracism task is also supported by other models of social rejection (Kross et al., 2011).
In a recent metaanalysis, Wang et al. (2017) demonstrated that, of the 42 published studies that have used neuroimaging to investigate the effects of social ostracism, the results were highly overlapping. Social exclusion induced significantly more activity in many Salience Network regions than social inclusion, including the insula, anterior cingulate cortex (CC), and medial prefrontal cortex (MPFC). Additionally, exclusion-evoked activity in the insula, CC, and MPFC were all correlated with self-esteem assessments. This pattern existed across many studies despite subtle differences in the imaging modalities used, the experimental contrasts, and the clinical diagnosis of the participants.
Substance dependent individuals may be particularly vulnerable to the effects of social exclusion as the neural circuitry engaged during the Cyberball social ostracism task (e.g. MPFC, CC, Insula) overlaps with the limbic systems that are engaged by drug cues and precipitate relapse (Courtney et al., 2016; Hanlon et al., 2016; Garrison and Potenza, 2014; Bolla et al., 2004; Bonson et al 2002). Although limited data exists on the neural response to negative interpersonal relationships in cocaine users (Sinha et al., 2005; Potenza et al., 2012), it is possible that exposure to acute interpersonal stressors, such as ostracism from a peer-group, may engage reward circuitry of the brain, making an individual more sensitive to the reinforcing effects of a drug (Koob and Le Moal, 1997).
The primary aim of this investigation was to determine whether the brain response to social ostracism, particularly in Salience Network regions, was amplified in cocaine users. We tested the hypothesis that cocaine users would be disproportionately affected by social exclusion stress and that this would be manifest by significantly higher activation of these Salience Network structures involved in both social pain and craving during the task. A greater understanding of the neural basis for increased sensitivity to interpersonal stressors such as ostracism from a group among substance dependent individuals may have important implications for identifying vulnerability as well as potential treatment strategies.
2. Materials and methods
2.1. 1 participants
Forty five individuals were recruited from the community (20 non-treatment seeking crack-cocaine users and 25 healthy non-drug using controls; right handed). Imaging data from two of the cocaine users were not viable due to artifacts; bringing the final sample to 43 participants (18 cocaine users and 25 controls). Control subjects did not have a history of substance abuse dependence other than nicotine and were chosen to match the Cocaine User population on the basis of gender and race. The participants were recruited via local media advertisements such as flyers and newspaper ads. During an initial visit, participants provided written informed consent to participate in procedures approved by the Wake Forest University School of Medicine Institutional Review Board. They then provided urine samples to test for current illicit drug use (i.e., cocaine, opiates, amphetamines, methamphetamine, barbiturates, benzodiazepines, and marijuana; Multipanel Urine Screen; Innovacon, Inc, San Diego, CA), and in women, for pregnancy (QuickVue urine HCG test; Quidel Inc, San Diego, CA). Additionally, participants were administered the Structured Clinical Interview for DSM-IV Axis-I disorders (SCID, First 1997) and the Alcohol Use Disorders Identification Test (Babor et al., 2001). Exclusionary criteria included a history of head trauma, a history of neurological disorders, systemic diseases that might affect the central nervous system including diabetes and cardiovascular disease, psychotropic prescription medication use in the 14 days prior to testing, Axis-I psychiatric disorders (other than cocaine dependence for the Cocaine users), left hand dominance (by observation and self-report), current dependence upon substances other than nicotine, claustrophobia, metal implants in their body, having experienced a gunshot wound or injury from metallic shrapnel, and being left-hand dominant. For inclusion, the results of the urine drug screen had to be compatible with the self-reported description of current and past drug use. Individuals classified in the cocaine user group were required to test negative for illicit drugs other than marijuana and cocaine and individuals classified as controls had to test negative for all illicit drugs. Participants who passed all inclusion criteria were scheduled for a second visit during which individuals underwent fMRI scanning while performing a social exclusion task (Williams et al., 2000). Cocaine users were asked to abstain from cocaine use at for at least 12 h prior to the scheduled fMRI scan visit.
2.2. Procedure
On the day of the functional MRI scan, participants provided urine samples to screen for illicit drug use and in women, for pregnancy. Additionally, participants completed the Spielberger State-Trait Anxiety Inventory (STAI) (Spielberger, 1984) and the Beck's Depression Inventory (BDI) (Beck et al., 1961). The participants also completed an inventory of self-worth (Need-Threat assessment) prior to the social ostracism task in the MRI scanner. All members of the cocaine group all had positive urine drug screens for cocaine (which is sensitive for approximately 72 h following last use) and acknowledged not having used cocaine the evening prior. Although the amount of craving was not quantified, the cocaine users reported that they were not actively craving cocaine upon arrival and did not exhibit signs of cocaine intoxication or withdrawal. To avoid potential confounds of nicotine withdrawal on functional brain activity (Wang et al., 2007; Xu et al., 2007), 1 h before the MRI scan participants were given a 5 min break with the opportunity to smoke. No Cocaine users nor Controls took advantage of this opportunity.
2.3. Cyberball task
This functional MRI experiment was modeled on a study which used a ball-tossing game, known as Cyberball, to induce a state of social exclusion in individuals (Williams et al., 2000). This task was adapted in order to optimize it for MRI data collection as well as to enhance the believability of the paradigm for our sample and context (Fig. 1). Briefly, the participants were informed that they would be playing a virtual ball-tossing game in the MRI scanner with two other players who were connected in nearby MRI scanners. This cover story was augmented by having the person pose for a picture of their face which would be shown to the other virtual players, and staged phone calls to the other experimenters making sure that their participants were ready to go. Additionally, while in the MRI scanner, just before the task began, the participant saw a visual display of phrases such as “Waiting to connect to MRI 1 …. “, “IP address secured”, “MRI 2 online.” All pictures were immediately destroyed and erased from the cache of the stimulus computer following the MRI paradigm in order to further protect the participant's identity.
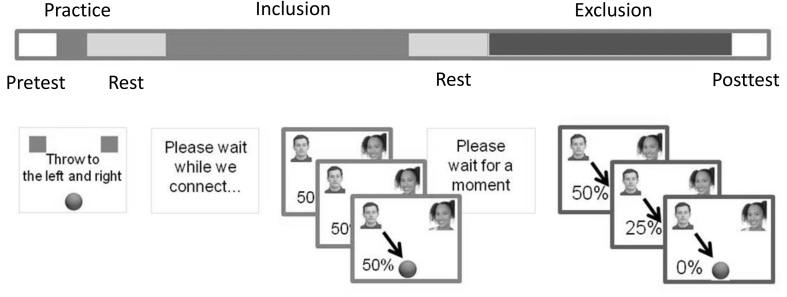
Fig. 1.
Social exclusion task design. This was a modified version of Cyberball, a well-established task of social ostracism (Eisenberger et al., 2003) in which participants engage in a simulated ball tossing game with two other, fictitious, players. The participant is told that the other players are in nearby MRI scanners. During one phase of the task (Inclusion), the fictitious players throw the ball to the participant 50% of the time. During a second phase of the task (Exclusion), the participant is gradually ‘excluded’ as the frequency drops to 0%. Both before (Pretest) and after (Posttest) the functional MRI task, participants completed a Need-Threat inventory surveying sense of belonging, sense that their actions are meaningful, self-esteem, and locus of control.
Participants viewed the Cyberball game through MRI compatible goggles which were connected to a computer. Displayed on the computer screen were pictures of the two virtual players. One was displayed in the upper left hand and upper right hand corners of the computer screen who were said to be in nearby MRI scanners. To address potential concerns regarding gender and race effects on social exclusion, a picture of an African-American male and a Caucasian female were used as the virtual players for all participants. Our participant was able to “toss” a ball to the virtual player in the upper left hand corner by pushing the first (left), button or to the player in the upper right hand corner by pushing the second (right) key on the MRI compatible response box. Participants were required to toss the ball within 1 s or else the ball was taken from them and given to another player. In a brief training session the participants were introduced to the task and practiced throwing the ball to both the upper left and right hand corners of the screen using the response box.
Following the training session the pictures of the other players were revealed and a ball appeared on the screen to commence the game. The task was divided into blocks of inclusion, exclusion, and rest. During the inclusion period (3.5 min), the likelihood that the other players would toss the ball to our participant was 50%. There was a 30 s rest period before and after the Inclusion block in which the participants were told to remain alert with their eyes open. Following rest, the exclusion condition (3.5 min) began. During the exclusion block, the first 30 s were identical to the Inclusion block in which the likelihood that our participant would receive the ball was 50%. The probability slowly decreased in 10% increments every 30 s until the probability of our participant receiving the ball was 0%. This led to the perception of slowly being excluded from the game. For the last 60 s the participants were completely excluded by the two virtual players from the ball tossing game.
2.4. Inventory of self-worth variables
Both before and after engaging in the Cyberball task, participants were asked to complete the self-worth questionnaire (Need-Threat Inventory) adapted from previous Cyberball studies (Williams et al., 2000). This scale was used to determine the effects of social isolation on four fundamental areas of self-worth: belonging, locus of control, self-esteem, and meaningful actions. The questions were altered slightly and the form was named differently between the pre- and post-testing in order to minimize the likelihood the participants would remember what they reported in the pre-test. The questionnaire contained a number of questions that asked participants to assess their levels of the four domains of self-worth while engaging in the Cyberball game. Example questions assessing the four domains of self-worth included: belonging (ex: ‘‘I felt poorly accepted by the other participants,’’ ‘‘I felt as though I had made a ‘‘connection’’ or bonded with one or more of the participants during the Cyberball game,’’ ‘‘I felt like an outsider during the Cyberball game’‘), control (ex: ‘‘I felt that I was able to throw the ball as often as I wanted during the game,’’ ‘‘I felt somewhat frustrated during the Cyberball game,’’ ‘‘I felt in control during the Cyberball game’‘), self-esteem (ex: ‘‘During the Cyberball game, I felt good about myself,’’ ‘‘I felt that the other participants failed to perceive me as a worthy and likeable person,’’ ‘‘I felt somewhat inadequate during the Cyberball game’‘), and meaningful actions (ex: ‘‘I felt that my performance [e.g., catching the ball, deciding whom to throw the ball to] had some effect on the direction of the game,’’ ‘‘I felt non-existent during the Cyberball game,’’ ‘‘I felt as though my actions were meaningless during the Cyberball game’‘). Unless otherwise stated, all questions were rated on 9-point scales (1 = not at all, and 9 = very much so).
After participants finished the need-threat questionnaire of self-worth, the experimenter asked participants about their thoughts/feelings during the study. They were then thoroughly debriefed about the aims of the study, thanked, and given our contact information should they have any further questions. Educational materials and information about treatment services were also made available for our participants if they should be interested.
2.5. Functional MRI data acquisition
Images were acquired on a 1.5T General Electric scanner with a birdcage-type standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. Foam padding was used to limit head motion. High-resolution T1-weighted anatomical images (3D SPGR, TR = 10 ms, TE = 3 ms, voxel dimensions 1.0 × 1.0 × 1.5 mm, 256 × 256 voxels, 124 slices) were acquired for co-registration and normalization of functional images. During the Cyberball task a total of 230 co-planar functional images were acquired using a gradient echoplanar sequence (TR = 2100 ms, TE = 40 ms, voxel dimensions 3.75 × 3.75 × 5.0 mm, 64 × 64 voxels, 28 slices). The rest blocks were 31.5 s in length (15 vol). Inclusion and Exclusion blocks lasted 3.5 min (100 vol). The scanning planes were oriented parallel to the anterior–posterior commissure line and extended from the superior extent of motor cortex to the base of the cerebellum. Six volumes of data were acquired during the 20 s countdown period and immediately discarded to allow for equilibrium before selections began.
2.6. Statistical analyses
Independent samples t-tests were used to compare controls and cocaine users on demographic variables (i.e., age, BDI, and STAI scores). Chi-square analyses were used to compare gender and ethnicity variables. For each of the four domains of self-worth (belonging, control, self-esteem, and meaningful actions), a 2 × 2 mixed model analysis of variance was used to assess the interaction between group (Controls, Users) and social exclusion (repeated: pre- and post-test values). The assessment of main effects and interactions were followed by post-hoc student t-tests. All behavioral data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 11.5. Significance was defined as p < 0.05.
2.7. Functional MRI preprocessing and data analysis
All imaging analyses were performed with SPM 8 (Wellcome Department of Imaging Neuroscience, London, UK) in the MATLAB 7.0 (Mathworks, Natick, MA) shell. The functional data from each participant were corrected for acquisition time (slice timing), realigned to the first volume (motion correction), normalized into a standardized neuroanatomical space (Montreal Neurological Institute brain template), smoothed using a Gaussian kernel of 8 mm, and high-pass filtered (128s) to remove low frequency noise. Inspection of motion correction revealed that all corrections were less than the 2 mm. A multiple linear regression analysis was performed for each participant corresponding to the periods of rest, Inclusion and Exclusion and convolved with a hemodynamic response function. Given the constraints of our design, in which the Inclusion block always precedes the Exclusion block, the data were temporally scaled to limit the contributions of negative drift. These regressors were convolving relevant event times with a canonical hemodynamic response function. For each individual, statistical contrast maps were created for the Inclusion relative to the Rest block, Exclusion relative to Rest, and Inclusion Relative to Exclusion.
The data were modeled in a 2 × 2 factorial design with group (controls, cocaine users) and condition (exclusion, inclusion) as the factors of interest and age and BDI scores included as covariates (as there were group differences in these metrics). In addition to F-tests, T-test were performed to identify brain activity associated with each of the Cyberball conditions. All reported results met significance at p < 0.05 corrected for multiple comparisons (determined by Monte Carlo simulation; voxel-level threshold of p < .05 for at least 90 contiguous voxels; Alphasim in REST toolbox).
3. Results
3.1. Demographics
Demographics of study participants are shown in Table 1. The control group consisted of 12 males and 13 females, 11 of whom were Caucasian, 13 African American, and one Asian. Controls were (mean ± SD) 33.5 ± 6.0 years old, had ‘minimal’ depressive symptoms, and anxiety scores below the level of clinical significance. The cocaine group consisted of 11 male and 7 females representing 14 African American and 4 Caucasian cocaine users They were 38.1 ± 6.1 years old, had ‘mild’ depressive symptoms on average, and anxiety scores below the level of clinical significance. There was no significant difference in gender, ethnic distribution, or state anxiety level. The cocaine users were however significantly older (t(41) = 6.0, p = 0.02) and had higher levels of depressive symptoms than controls (t(41) = 26.7, p < 0.001). All cocaine users endorsed using crack cocaine as their primary method, with 28% (5 of18) stating that they had used powdered cocaine at least once in the past 3 months. No one stated that they had ever injected cocaine.
Table 1.
Participant demographics.
| Demographics | Controls (n = 25) |
Cocaine users (n = 18) |
t | p |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Age (years) | 33.5 ± 6.0 | 38.1 ± 6.1 | 6.0 | 0.02 |
|
Gender (%) |
Χ2 |
p |
||
| Male | 44.0 | 61.1 | 1.22 | 0.27 |
| Female | 56.0 | 38.9 | ||
|
Ethnicity (%) |
Χ2 |
p |
||
| African American | 56.0 | 77.8 | 2.50 | 0.29 |
| Caucasian | 40.0 | 22.2 | ||
| Other | 4.0 | |||
|
Affect |
||||
| BDI | 2.4 ± 3.2 | 14.6 ± 11.3 | 26.7 | <0.001 |
| STAI | 24.8 ± 5.0 | 32.4 ± 12.1 | 8.1 | 0.01 |
BDI, Beck's Depression Inventory; STAI, Spielberger State-Trait Anxiety Index.
3.2. Drug use
Five members of the control group reported previous marijuana use limited to fewer than 50 lifetime uses, occurring more than 2 years prior to the study. No controls reported lifetime use of any other illegal substance. Three controls and 15 cocaine users were cigarette smokers (X2 = 16.9, p < 0.001). The Alcohol Use Disorders Inventory Test (AUDIT) score for the controls was 3.4 ± 3.1 and for cocaine users was 8.9 ± 7.2 (t(41) = 11.8, p < 0.001), none of whom had had a history of current or past alcohol dependence. The cocaine users have been using cocaine for a total (mean ± SD) of 15.1 ± 8.6 years, and 9.8 ± 7.1 years at the current level. The average age of first use was 21.1 ± 5.2 years of age. The cocaine users were currently using cocaine 3.9 ± 1.6 days per week and spending $220 ± 78 per week. All participants met criteria for cocaine dependence and crack-cocaine was the preferred method of use. Six of the cocaine users reported marijuana use in the past month (average ± SD = 5.4 ± 1.8 days/month). One participant reported past use of MDMA. Two participants reported past use of heroin. No participants reported past use of LSD, methamphetamine, or benzodiazepines. On the scanning day, all cocaine users had positive urine drug screens for cocaine, and four had a positive screen for marijuana. The other measured substances (e.g. opiates, amphetamines, methamphetamine, barbiturates, benzodiazepines) were all negative. All members of the control group had negative urine drug screens for all substances.
3.3. Behavioral response to social exclusion
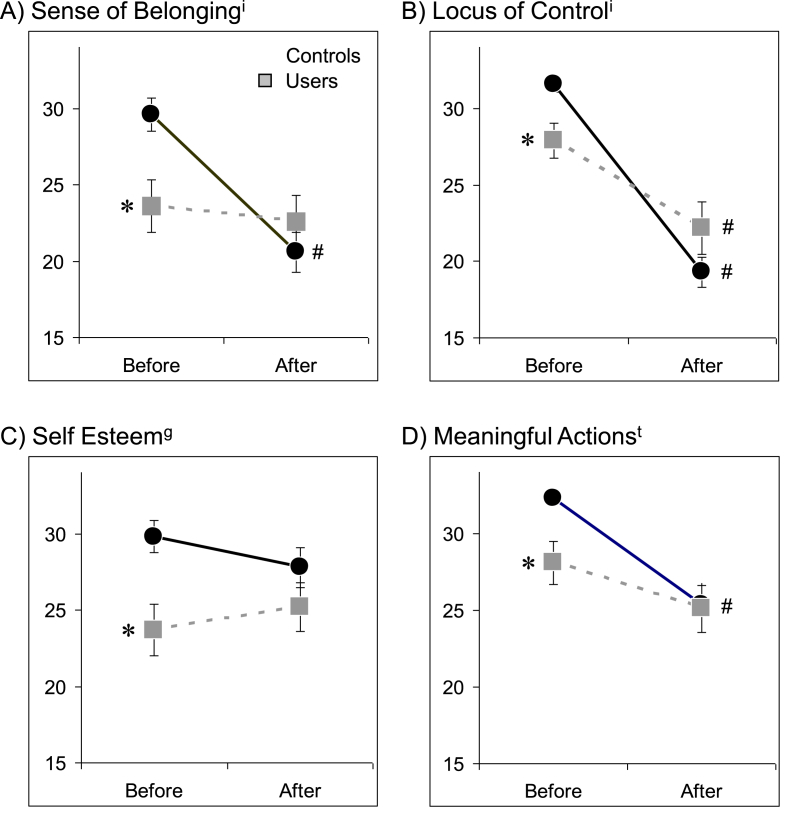
The mean (±SEM) responses on the four domains of the self-worth questionnaire in both controls and cocaine users are presented in Fig. 2. Social exclusion was associated with a main effect on 3 of the 4 measures of self-worth (sense of belonging: F = 12.30, p = 0.001; locus of control: F = 63.00, p < 0.001; sense of meaningful actions: F = 15.70, p < 0.001), with sense of belonging and meaningful actions being lower after exclusion and locus of control becoming more external. There was also a significant interaction between group and response to social exclusion for 2 of the 4 measures of self-worth (sense of belonging: F = 8.07, p = 0.006; locus of control: F = 10.19, p = 0.002). At baseline, Cocaine users had significantly lower scores on all four measures of self-worth relative to Controls. Following the social exclusion task however, there was no significant difference in sense of belonging, locus of control, or sense of meaningful actions.
Fig. 2.
The effect of the social exclusion task on self-worth assessments. The mean scores (±standard error) of the four components of the Need Threat Inventory (A) Sense of Belonging, B) Locus of Control, C) Self Esteem, D) Meaningful Actions) are plotted for both Controls (black circles) and Cocaine users (gray squares) both before and after the social exclusion task. A significant main effect of group, time point, or interaction between group and time point was present for all parameters. Post hoc significance testing is indicated for parameters that differ significantly: # within each group (p < 0.05, corrected) and * between each group (p < 0.05, corrected).
3.4. Brain response to social exclusion
3.4.1. Healthy controls
Relative to inclusion, social exclusion led to elevated BOLD signal in the dorsal anterior cingulate cortex (198 voxels; x,y,z = 2,12,23), and attenuated BOLD signal in the left lateral postcentral gyrus cortex (272 voxels; x,y,z = −38, 36,-2).
3.4.1.1. Cocaine users
Relative to inclusion, social exclusion led to elevated BOLD signal in a large cluster which included the dorsal anterior cingulate cortex and the middle cingulate cortex (657 voxels; x,y,z = 5,-24, 44), as well as attenuated BOLD signal in the left lateral postcentral gyrus (103 voxels; x,y,z = −36,34,-16). These within group analyses between inclusion and exclusion blocks were modeled with respect to rest.
3.4.2. Between group analyses
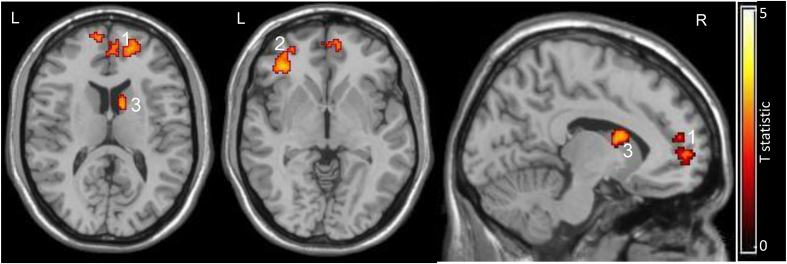
A direct comparison of cocaine users relative to controls during social exclusion versus inclusion revealed that cocaine users had significantly more activity in the medial frontal gyrus (Brodmann Area 9,10; 400 voxels; x,y,z = 6,58,-6), the left ventral lateral frontal gyrus (Brodmann Area 10,47; 193 voxels; x,y,z = −38, 36, −2), and the right caudate (297 voxels; x,y,z = 10,8,10) (Fig. 3). There were no areas in which the controls had a significantly larger response to exclusion relative to inclusion than the users. These between group analyses between inclusion and exclusion blocks were modeled with respect to rest.
Fig. 3.
Effects of social exclusion relative to inclusion in cocaine users relative to controls. In this between group analysis, the brain response to social exclusion was compared to inclusion. Rest was incorporated into the model. A direct comparison of cocaine users relative to controls during social exclusion versus inclusion revealed that cocaine users had more activity in 3 clusters than controls: 1) right medial frontal gyrus/pregenual cingulate (Brodmann Area 9,10; 400 voxels; x,y,z = 6,58,-6), 2) left ventral lateral frontal gyrus (Brodmann Area 10,47; 193 voxels; x,y,z = −38, 36, −2), and 3) right caudate (297 voxels; x,y,z = 10,8,10) (p < 0.05, corrected). There were no areas in which controls had more activity than the cocaine users. The left (L) side of the brain appears on the left side of the 2 transverse images. A right sagital section is shown as well (R). Clusters are numbered.
During Inclusion (relative to rest), there was no significant difference in the pattern of brain activity in the cocaine users versus the controls. During Exclusion (relative to rest), cocaine users had significantly more activity than controls in the cingulate gyrus (Brodmann Area 6,24,32; 536 voxels; x,y,z = −2, −14, 64) and the postcentral gyrus (Brodmann Area 2,3; 249 voxels; x,y,z = −54, −24, 48). There were no areas in which controls had more activity than the cocaine users.
3.4 Post hoc secondary analyses accounting for gender (Supplementary Fig. 1). As an exploratory analysis we evaluated the relationship between gender and the brain response to social exclusion versus inclusion in the controls and cocaine users. The global mean signal change was extracted for each individual via whole brain voxel-wise calculation from within subject contrast maps of Exclusion and for Inclusion. The average and standard deviations of these global signal values were then compiled for all individuals in each of the four groups (cocaine using men, cocaine using women, control men, control women). Group effect sizes were then calculated and compared between groups (G*Power 3.0.10). An assessment of the mean whole brain global signal change to exclusion versus inclusion demonstrated that there was a predictable order of the overall effect sizes between groups. A visual depiction of the rank order of the brain response to social ostracism is displayed in Supplementary Fig. 1 wherein the effect sizes were largest for the cocaine using (D = 0.94) and control women (0.89). While the cocaine using men (D = 0.69) and control men (D = 0.53) also had strong, but more modest changes in BOLD signal during Exclusion relative to Inclusion. Close inspection of these data also demonstrate that during Inclusion, the Cocaine using women and men had lower BOLD signal than controls – consistent with previous data demonstrating a general hypofrontal response to non-drug cue related fMRI tasks. As anticipated given the relatively low sample size, incorporating gender into the general linear model used in the primary contrasts did not yield a significant interaction between gender and drug using group.
3.5. Post hoc secondary analyses accounting for affect
To further explore the relationship between the elevated depression scores in the cocaine users and their response to social exclusion a posthoc analyses were conducted. There was a main effect of depressive symptoms on baseline scores on the self-worth inventory (F = 5.32, p = 0.02). There was not, however, a significant correlation between BDI scores and brain activity related to social exclusion. This analysis was done by extracting the average beta value (parameter estimate) from the three functional ROIs that were significantly greater in cocaine users relative to controls during the Cyberball social exclusion task. These values were then correlated with scores on the Beck's Depression Inventory (BDI) and Speilberger Test of Anxiety Inventory (STAI) and corrected for multiple comparisons to determine if there was a significant relationship between baseline affect in cocaine users and their neural response to social exclusion. There were also no significant correlations between STAI scores and brain activity during social exclusion. None of the measures of baseline self-worth, or demographic variables were independent predictors of the brain response to social exclusion in these ROIs.
4. Discussion
The emotional pain associated with social exclusion is a very powerful aspect of the human experience. Previous neuroimaging studies have demonstrated that the “emotional pain” associated with social exclusion is associated with activity in the same network of brain regions involved in processing physical pain (Eisenberger et al., 2003). In the present study we have replicated many of those findings in healthy individuals and also extended those observations to a population of substance abusers that may be particularly vulnerable to the effects of social exclusion. Consistent with our hypothesis, cocaine users exhibit a greater functional brain response to social exclusion than controls. These differences were largest in the anterior middle cingulate cortex, an area involved in both negative affect and pain (Shackman et al., 2011) and the medial prefrontal cortex including Brodmann areas 9/10. These data suggest that the well-known sensitivity to social exclusion observed in substance abusers may be related to an underlying affective dysregulation.
4.1. Social pain and the cingulate
In response to social exclusion the cocaine users had a significantly larger neural response in the cingulate cortex than the healthy controls. This extends data from a previous social ostracism task which demonstrated that, in healthy controls, the cingulate cortex was one of the brain regions most affected by exclusion from a simulated ball-tossing game (Eisenberger et al., 2003). The consistency of these results suggests that, beyond its traditional role in processing physical pain and expectation monitoring, the zone between the anterior and middle cingulate gyrus may also be involved in processing social pain. This is compatible with a recent a review of literature on the cingulate cortex which suggests that the cingulate cortex represents a hub where information about pain, punishment and negative experiences are linked to motor centers responsible for expressing emotion on the face (Shackman et al., 2011). In this interpretation it is possible that the anterior middle cingulate cortex represents a sensitized hub in cocaine users where the elevated response to social pain in cocaine users is linked to elevated activity in downstream limbic related circuitry.
4.2. Lateral prefrontal cortex and substance abuse
In addition to the anterior middle cingulate cortex, the cocaine users in this study also had a significantly stronger response than controls in the lateral prefrontal cortex (Brodmann Area 10) during social exclusion. Brodmann Area 10 is the largest consistent cytoarchitectural area of the brain. The lateral aspects of BA 10 are involved in multiple of cognitive processes including sustained attention, prospective planning, set-shifting, and complex decision making (Ramnani and Owen, 2004). Despite a relatively low number of cells, this area is highly arborized, and receives afferents predominantly from neighboring prefrontal regions (Petrides and Pandya, 2007). This has led to the interpretation that this area is involved primarily with information integration such as prospective or intentional thinking (Okuda et al., 2007), exploratory decisions in the presence of uncertainty (Daw et al., 2006), and evaluating action outcomes (Koechlin and Hyafil, 2007). Consistent with this interpretation, multiple imaging studies have documented that the poor performance of cocaine users on cognitive tasks is related to aberrant activity in BA10 (see: Goldstein and Volkow, 2002).
4.3. Although activity in the lateral prefrontal cortex during cognitive tasks is well-established
This study demonstrates that cocaine users have elevated activity in this ‘cognitive integration’ area when faced with a social exclusion task. One interpretation is that cocaine users have more difficulty integrating the change in social structure (inclusion to exclusion) than the controls. This observation supports recent findings that suggest cognitive dysfunction in cocaine users is related to their real-life social dysfunction (Goldstein and Volkow, 2002). Another point of interest is that BA10 and the orbitofrontal cortex are the only cortical areas with an elevated BOLD response after direct cocaine administration (Cunha et al., 2011) and resting state connectivity is impaired in this region relative to healthy controls (Kufahl et al., 2005; Gu et al., 2010). While this cannot account for the Exclusion versus Inclusion differences between the groups, it does suggest that this brain area may be more reactive in individuals that have years of exposure to cocaine.
4.4. Elevated activity in the caudate nucleus
In addition to an increase in these cortical areas, in the present study cocaine users also had an elevated BOLD response in the caudate nucleus. The caudate nucleus in humans is functionally heterotopic, wherein the ventral aspects of the caudate are involved in limbic arousal and have high connectivity to the medial prefrontal and orbital prefrontal cortex, whereas the dorsal lateral aspects of the caudate are involved in cognitive processing and habit formation. These dorsal lateral aspects of the caudate have higher connectivity with the dorsolateral prefrontal cortex (Haber and Knutson, 2010).
From the perspective of addiction, the caudate is generally involved with goal directed and motivated behavior (Balleine et al., 2007) and is active during cue-elicited craving (Garavan et al., 2000; Kilts et al., 2004). The caudate has increased BOLD signal when users report feeling the rush and high from cocaine administration (Breiter et al., 1997; Risinger et al., 2005). Relevant to this study, in a study with patients in treatment for cocaine abuse, Sinha and colleagues found that when cocaine users were instructed to imagine stressful situations while in the scanner, they had increased BOLD signal in the caudate. Activation in the caudate during this stress task was also associated with an increase in cocaine craving (Sinha et al., 2005).
4.5. Gender
In the present investigation of social exclusion on brain activity and subjective measured of well-being, post-hoc exploratory analyses of the data revealed that the effect of social exclusion on the brain was higher in the women than the men, and within each gender class the cocaine users had a higher response than the controls. From one perspective this is surprising given that previous studies in healthy controls have failed to find effects of gender on the brain response to social exclusion (Eisenberger et al., 2003). From another perspective however, it is well known that women experience greater subjective distress and have a higher heart rate response to stress than men (Kirschbaum et al., 1992; Kajantie and Phillips, 2006; Farb et al., 2011). Additionally among cocaine users women have a larger subjective response (Back et al., 2005; Fox et al., 2008) and brain response (Potenza et al., 2012) to stressors as compared to male cocaine users. Our inability to detect an effect of gender in the full factorial design can likely be attributed to insufficient power – which is expected given that this study was not initially intended to evaluate gender specific effects. That said, the predictable difference in effect sized between gender and drug using groups suggests that further research should be done in this area. Specifically, more studies are necessary to fully evaluate potential interactions between the brain response to social exclusion, gender, and craving, especially in context of recent data that has been emerging on this topic (Potenza et al., 2012).
As with many human imaging experiments in substance abusers, it is difficult to attribute the differences in behavioral and brain responses to social exclusion solely to the effects of cocaine use. This is particularly true in the present sample as many of the participants also used tobacco regularly (15 of 18 were cigarette smokers), smoked marijuana occasionally (4 of 18 had positive USD for marijuana on the study visit), and consumed alcohol at high rates. Differences in the response to social exclusion observed in Cocaine users may also be influenced by other common comorbid factors such as low socioeconomic status, high rates of medical and psychiatric comorbidity. In addition to higher smoking and alcohol use rates, the cocaine users in the present study had significantly higher scores on measures of baseline affect than the controls. Although none of the Cocaine users met DSM-IV criteria for major depressive disorder or generalized anxiety disorder, these underlying baseline symptoms of depression and anxiety may have influenced the response to social exclusion. To address the role of baseline affect in our cocaine users secondary post-hoc analyses were conducted. While these analyses demonstrated a positive correlation between baseline depressive symptoms and the brain response to social exclusion in BA10 and the middle cingulate in the cocaine users, when corrected for multiple comparisons this did not remain significant. Again, however, the study was not initially designed to evaluate this hypothesis, and thus future studies may want to consider this as variable to consider in the future.
4.6. Limitations
Although the results of the current investigation support and extend previous investigations, there are a few limitations which must be considered when interpreting the results. As with many human drug abuse investigations, it is difficult to isolate a socially and demographically matched control group that does not have a history of drug abuse. Although the cocaine users were not dependent on any illegal substance other than cocaine, nor did they have a history of diagnosis with another Axis 1 disorder, they were more likely to smoke cigarettes, had higher levels of baseline depressive and anxiety symptoms, and a lower IQ than the controls. Among the smokers, it is possible that their performance on the task may have been affected by mild withdrawal from cigarettes, based on a prior report that visual information processing deficits can be detected as early at 30 min after the last cigarette (Hendricks et al., 2006). While differences in these variables may be viewed as a weakness of this investigation from a scientific perspective, these factors are known to coexist with substance abuse disorders in the population. This increases the likelihood that these findings will generalize to the population of cocaine dependent individuals as a whole. The cocaine users in this study also all had a positive urine drug screen for cocaine. It is not clear if these results would be different in a group of treatment seeking cocaine users that were trying to maintain abstinence from cocaine, or a group of cocaine users that had not used cocaine recently. It is possible that those cohorts may have differential sensitivity to the effects of social exclusion. Furthermore, the observation that affective symptoms are correlated with the brain response to social exclusion adds an element to the literature on social ostracism that had not been addressed previously. This opens an interesting avenue to investigate the role of subclinical depressive symptoms on brain activity during social stress in non-drug using populations as well.
There are several other important questions that could not be robustly addressed by this study, including the role of gender on the neural response to social exclusion, the impact of social ostracism on craving, and the effect of positive urine drug screens on the results. To avoid any deleterious effects of nicotine withdrawal on performance, all participants were given the opportunity to smoke upon request up to an hour prior to image acquisition (Wang et al., 2007; Xu et al., 2007). All cocaine users in the current study had positive urine screens for cocaine at the time of scanning. They were asked to abstain the night before and there was no evidence of acute intoxication present in any of the participants. This decision was made based on the knowledge that users with positive urines are less impaired on multiple cognitive performance measures than users with negative urine drug screens (Kudielka and Kirschbaum, 2005), as well as our desire to minimize any emergent effects of craving on the response to social exclusion. While a large body of literature suggests that acute stress is associated with elevated craving (Back et al 2005, 2010), future studies are needed to determine whether the brain response to social stress is associated with elevated craving.
4.7. Summary and future directions
The results of this investigation demonstrate that chronic cocaine users have a significantly larger neural response to social ostracism than non-drug using controls, in brain areas involved in processing negative affect and physical pain. These findings are consistent with a large body of evidence that suggests substance dependent individuals may be more vulnerable to social exclusion or ostracism from their peers. These data also demonstrate however that there is likely a strong relationship between baseline depressive symptoms as well as gender in predicting the response to a social stressor such as social exclusion. Considered together these observations suggest that cocaine users are more vulnerable to the effects of social exclusion than their non-drug using counterparts. These data provide a window into potential treatment targets (e.g. targeting depression may ameliorate the negative effects of social stress) as well as raise the awareness that there may be important drug and gender related disparities in the effects of social exclusion on the brain. Future studies with higher temporal resolution imaging approaches (such as multiband imaging) in populations with some of the same comorbidities as these cocaine users (e.g heavy alcohol users, smokers, individuals with high depressive symptomology) may help determine the temporal profile of these brain responses to exclusion and the specificity that these emergent feelings have to cocaine craving or drug-cue reactivity at large.
Financial disclosures
The authors declare no financial conflicts of interest. This research was supported by NIH grants R01DA036617 (Hanlon), K01DA027756 (Hanlon), R01DA009085 (Porrino).
Acknowledgements
The authors would like to acknowledge Mack D. Miller, Anna Lack, Ph.D., Marla Torrence, and William DeVries for their assistance with Cyberball fMRI task development, data organization, patient recruitment, and manuscript preparation respectively.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2018.10.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Babor T.F., Higgins-Biddle J.C., Saunders J.B., Monteiro M.G. World Health Organization; Geneva, Switzerland: 2001. The Alcohol Use Disorders Identification Test (AUDIT) Manual. [Google Scholar]
- Back S.E., Brady K.T., Jackson J.L., Salstrom S., Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berlin) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Back S.E., Hartwell K., DeSantis S.M., Saladin M., McRae-Clark A.L., Price K.L. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., Delgado M.R., Hikosaka O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatr. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bolla K., Ernst M., Kiehl K., Mouratidis M., Eldreth D., Contoreggi C., Matochik J., Kurian V., Cadet J., Kimes A., Funderburk F., London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J. Neuropsychiatry Clin. Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H.C., Gollub R.L., Weisskoff R.M., Kennedy D.N., Makris N., Berke J.D. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Cacioppo J.T., Hawkley L.C. Perceived social isolation and cognition. Trends Cognit. Sci. 2009;13(10):447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin T.M., Hong K., Fox H.C., Siedlarz K.M., Bergquist K., Sinha R. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Hum. Psychopharmacol. 2010;25:368–376. doi: 10.1002/hup.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K.E., Schacht J.P., Hutchison K., Roche D.J., Ray L.A. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict. Biol. 2016;21(1):3–22. doi: 10.1111/adb.12314. 2016 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha P.J., Bechara A., de Andrade A.G., Nicastri S. Decision-making deficits linked to real-life social dysfunction in crack cocaine-dependent individuals. Am. J. Addict. 2011;20:78–86. doi: 10.1111/j.1521-0391.2010.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N.D., O'Doherty J.P., Dayan P., Seymour B., Dolan R.J. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortical responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Farb N.A., Anderson A.K., Bloch R.T., Segal Z.V. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biol. Psychiatry. 2011;70:366–372. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Hong K.I., Siedlarz K., Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Pankiewicz J., Bloom A., Cho J.K., Sperry L., Ross T.J. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garrison K.A., Potenza M.N. Neuroimaging and biomarkers in addiction treatment. Curr Psychiatry Rep. Dec. 2014;16(12):513. doi: 10.1007/s11920-014-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Salmeron B.J., Ross T.J., Geng X., Zhan W., Stein E.A. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C.A., Dowdle L.T., Jones J.L. Biomarkers for success: using neuroimaging to predict relapse and develop brain stimulation treatments for cocaine-dependent individuals. Int. Rev. Neurobiol. 2016;129:125–156. doi: 10.1016/bs.irn.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgerink C.H., van Beest I., Wicherts J.M., Williams K.D. The ordinal effects of ostracism: a meta-analysis of 120 Cyberball studies. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0127002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks P.S., Ditre J.W., Drobes D.J., Brandon T.H. The early time course of smoking withdrawal effects. Psychopharmacology. Aug. 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Kajantie E., Phillips D.I. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kilts C.D., Gross R.E., Ely T.D., Drexler K.P. The neural correlates of cue-induced craving in cocaine-dependent women. Am. J. Psychiatry. 2004;161(2):233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Wust S., Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Koechlin E., Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kreek M.J., Koob G.F. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kross E., Berman M.G., Mischel W., Smith E.E., Wager T.D. Social rejection shares somatosensory representations with physical pain. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kufahl P.R., Li Z., Risinger R.C., Rainey C.J., Wu G., Bloom A.S. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Okuda J., Fujii T., Ohtake H., Tsukiura T., Yamadori A., Frith C.D. Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time- and event-based prospective memory. Int. J. Psychophysiol. 2007;64:233–246. doi: 10.1016/j.ijpsycho.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J. Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M.N., Hong K.I., Lacadie C.M., Fulbright R.K., Tuit K.L., Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am. J. Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N., Owen A.M. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Risinger R.C., Salmeron B.J., Ross T.J., Amen S.L., Sanfilipo M., Hoffmann R.G. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26(4):1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Catapano D., O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R., Fuse T., Aubin L.R., O'Malley S.S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R., Lacadie C., Skudlarski P., Fulbright R.K., Rounsaville B.J., Kosten T.R. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R., Talih M., Malison R., Cooney N., Anderson G.M., Kreek M.J. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology. 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sinha R., Garcia M., Paliwal P., Kreek M.J., Rounsaville B.J. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatr. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. 1984. State-trait Anxiety Inventory for Adults (STAI-Y). Mind Garden. Menlo Park, CA USA. [Google Scholar]
- Waldrop A.E., Price K.L., Desantis S.M., Simpson A.N., Back S.E., McRae A.L. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 2010;35:798–806. doi: 10.1016/j.psyneuen.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Braun C., Enck P. How the brain reacts to social stress (exclusion) - a scoping review. Neurosci. Biobehav. Rev. 2017;80:80–88. doi: 10.1016/j.neubiorev.2017.05.012. 2017 Sep. [DOI] [PubMed] [Google Scholar]
- Wang Z., Faith M., Patterson F., Tang K., Kerrin K., Wileyto E.P. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J. Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K.D., Cheung C.K., Choi W. Cyberostracism: effects of being ignored over the Internet. J. Pers. Soc. Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Xu J., Mendrek A., Cohen M.S., Monterosso J., Simon S., Jarvik M. Effect of cigarette smoking on prefrontal cortical function in nondeprived smokers performing the Stroop Task. Neuropsychopharmacology. 2007;32:1421–1428. doi: 10.1038/sj.npp.1301272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.