Abstract
Delayed presentation of breast cancer is a common theme in most low and middle income countries. This study evaluates barriers to mammography screening in two Nigerian communities with different geographic access to screening facilities. A 35 item questionnaire was administered to women, 40 years and older, 1169(52.6%) in Ife Central Local Government where mammography services are offered and 1053(47.4%) in Iwo Local Government where there are no mammography units. Information on breast cancer screening practices and barriers to mammography screening were compared between the two communities. Most women had heard of breast cancer (Ife 94%, Iwo 97%), but few were aware of mammography (Ife 11.8%, Iwo 11.4%). Mammography uptake in Ife Central was 2.8% and 1.8% in Iwo, despite the former offering mammography services. Knowledge and practice of mammography were not statistically different between the two communities (p= 0.74, 0.1). Lack of awareness was the commonest reason cited for not having mammography in both communities. Others include, lack of perceived need and cost. Awareness creation to ensure optimal utilization of existing facilities as well as innovative measures to address the barrier of cost are required to improve breast cancer screening uptake in Nigeria.
Keywords: Breast, Cancer, Mammography, Barriers, Screening, Access
Introduction
Low and middle income countries (LMICs) are experiencing increasing incidence and mortality from breast cancer, a trend projected to continue over the next few decades (Ferlay, Shin et al. 2010). Over the past two decades, there has been a remarkable increase in the incidence of breast cancer from 33.6 per 100,000 in the 1990s to 64.6 per 100,000 after about two decades making breast cancer the most common female malignancy in Nigeria (Adebamowo and Adekunle 1999, Jedy-Agba, Curado et al. 2012). The majority of women present with advanced disease (Adesunkanmi, Lawal et al. 2006), making breast cancer one of the leading causes of cancer death.(Akinde, Phillips et al. 2015).
While outcomes remain poor in most LMICs, developed nations have demonstrated remarkable improvements in breast cancer survival. In Nigeria, breast cancer five year survival rates range from 11– 25% compared to 90% in the United States of America (Arowolo, Akinkuolie et al. 2010) (Edwards, Noone et al. 2014, Makanjuola, Popoola et al. 2014). This difference has been partly attributed to early detection as well as widely available adjuvant treatment modalities in developed countries like the USA (Berry, Cronin et al. 2005). In Nigeria, as well as most LMICs, adjuvant treatments such as radiation therapy and targeted therapy are not widely available or are unaffordable. Surgical treatment therefore remains the most common treatment with chemotherapy, when affordable. Although improving adjuvant treatment would be ideal, maximizing the benefit of surgical treatment through early diagnosis should be the initial focus, surgery being cheaper and more widely available (Groot, Baltussen et al. 2006). Mammography is the gold standard for breast cancer screening in developed countries with some evidence showing improved survival particularly among women 50–69 years in some randomized controlled trials in developed countries where breast cancer prevalence is high (Fletcher and Elmore 2003, Nystrom, Bjurstam et al. 2017). Whether the same benefits will be recorded in parts of the world with lower prevalence of breast cancer however remains contentious. In the United States, Women with average risk of breast cancer are recommended to undergo annual screening mammography starting at age 45years up to age 54 years after which they should transition to biennial screening or continue screening annually (Smith, Andrews et al. 2016). It is also recommended that women between ages 40 and 44 years should have the opportunity to begin annual screening. Nigeria currently has no National breast cancer screening guideline, as such screening recommendations are made based on international guidelines. Reports from various parts of Nigeria show very low mammography screening uptake (Okobia, Bunker et al. 2006, Akhigbe and Omuemu 2009, Obajimi, Adeniji-Sofoluwe et al. 2011, Akande, Olafimihan et al. 2015). While lack of geographic access may be suggested as a factor limiting uptake in areas with no mammography services, reasons for poor uptake in places with geographic access are not well understood. A possible explanation is lack of financial access, given that health costs are largely out of pocket with less than 5% of the population covered by the National Health Insurance Scheme (Okoronkwo, Ejike-Okoye et al. 2015) (Okebukola and Brieger 2016). There are reasons however, to believe that other factors aside cost contribute significantly to poor mammography uptake given that other forms of inexpensive or free screening similarly demonstrate poor uptake (Gharoro and Ikeanyi 2006, Okobia, Bunker et al. 2006). Understanding the drivers of poor mammography screening is therefore key for planning an effective breast cancer screening and interventional programme. The purpose of this study is to identify barriers to mammography screening uptake in Nigeria by surveying two communities with different geographical access to screening facilities.
Materials and Methods
Study design and study population
From February to April 2016, women 40 years and older were surveyed in Ife central and Iwo local districts. The two communities are located in the South-western part of Nigeria about 32 miles apart. Ife central local district has a population of 167,254 people (2006 census figures). It has a University with an affiliated Teaching Hospital offering surgical and radiological services including mammography. The Teaching Hospital is easily accessible from every part of Ife Central Local district with an estimated travel time of about 15 minutes from the farthest part of the Local district. Iwo Local district on the other hand has a population of 191,348 people, also has a University, but only district hospitals without mammography services. Travel distance from Iwo to the Teaching Hospital in Ife Central Local district is about an hour and thirty minutes.
The minimum required sample size of 1152 was calculated based on an estimated 3% mammography screening rate from a previous study at a confidence level at 95% and a margin of error set at 1% (0.01). Participants were selected from all the 11 wards in Ife central local district and 14 wards in Iwo Local district. The number of women sampled from each ward was proportional to the fraction of the total population the ward represented. Within each ward, eligible women were identified from door to door starting with the first street in each ward until the desired sampled size was obtained.
Instrument
A 35 item study specific interviewer administered questionnaire was utilized, first designed in English and later translated to Yoruba, the local language in both local districts, by a Yoruba language education expert. Pre-testing of 20 market women in Ife was done in both languages with subsequent survey revision before final adoption. Ten minute interviews were conducted by trained undergraduate and graduate students. Interviewers had the questionnaires available in both English and Yoruba languages with participants interviewed in their preferred language. The questionnaire gathered demographic characteristics, knowledge and experience about breast cancer, knowledge and practice of mammography, reasons for not undergoing mammography, practice of clinical breast examination, and general health behavior. The majority of the items were multiple-choice questions while few, such as barriers to mammography were open ended. Multiple responses were allowed for open ended questions such as reasons for not having mammography. In addition they were asked to indicate the most important reason for not having had mammography.
Key variables
Study key variables were awareness of mammography, practice of mammography and reasons for not having mammography. Awareness of mammography was defined as knowledge of its existence as a breast cancer screening modality while practice of mammography was defined as having had a mammogram in the past. Reason for not having mammography was stated as ‘The most important why you have not had a mammogram’.
Statistical analysis
Data was stratified based on location (Iwo and Ife) and descriptive statistics used to present socio-demographic characteristics, knowledge of breast cancer, practice of breast cancer screening and other key variables. Comparisons were made between Ife and Iwo based on the key outcome variables- awareness and practice of mammography using chi-square test.
Factors associated with mammography uptake were assessed using simple and multivariate Poisson regression with robust variance estimation used to derive prevalence ratios with 95% confidence interval. Item selection for the multivariate regression was by backward elimination method in which a p-value of 0.25 was set as the level for removal from the model.
Results
Socio-demographics
A total of 2,222 women were interviewed, 1169 (52.6%) from Ife central and 1053 (47.4%) from Iwo. Twenty six women declined to be interviewed, for reasons such as avoiding painful memories from loss of relatives to breast cancer, fear of contracting cancer, lack of concern about it, or lack of time.
Most participants were in their forties, with women sampled from Ife Central slightly older than those from Iwo (Mean 49.13±10.45 years versus 47.95±8.79, Table 1). The majority in both communities had some form of education, with a large percentage completing secondary education (742/1169, 63.47% Ife central, 612/1053, 58.1%- Iwo) with a significant difference between those with primary and secondary education (p=0.01). Occupational distribution differed, however petty trading was the predominant occupation in both communities.
Table 1:
Socio-demographic characteristics
| Variable | Ife Central (N =1169) Freq. (%) |
Iwo (N = 1053) Freq. (%) |
Total (N = 2222) Freq. (%) |
Chi-square test |
|---|---|---|---|---|
| Age group | ||||
| 40 – 49 | 687 (58.8) | 652 (61.9) | 1339 (60.3) | |
| 50 – 59 | 266 (22.8) | 261 (24.8) | 527 (23.7) | |
| 60 – 69 | 140 (12) | 100 (9.5) | 240 (10.8) | p = 0.03 |
| >69 | 76 (6.5) | 40 (3.8) | 116 (5.2) | |
| Marital status | ||||
| Married | 980 (83.8) | 870 (82.6) | 1850 (83.3) | |
| Single | 15 (1.3) | 15 (1.4) | 30 (1.4) | |
| Widowed | 150 (12.8) | 131 (12.4) | 281 (12.6) | p = 0.141 |
| Separated | 10 (0.9) | 23 (2.2) | 33 (1.5) | |
| Undisclosed | 14 (1.2) | 14 (1.3) | 28 (1.3) | |
| Highest level of education | ||||
| None | 182 (15.6) | 180 (17.1) | 362 (16.3) | |
| Primary | 230 (19.7) | 279 (26.5) | 509 (22.9) | |
| Secondary | 486 (41.6) | 333 (31.6) | 819 (36.9) | p < 0.001 |
| College | 256 (21.9) | 255 (24.2) | 511 (23) | |
| Not disclosed | 15 (1.3) | 6 (0.6) | 21 (0.9) | |
| Occupation | ||||
| Artisans | 74 (6.3) | 46 (4.4) | 120 (5.4) | |
| Traders | 832 (71.2) | 654 (62.1) | 1486 (66.9) | |
| Farmers | 45 (3.8) | 57 (5.4) | 102 (4.6) | p <0.001 |
| Civil servants | 148 (12.6) | 238 (22.6) | 160 (17.4) | |
| Others | 70 (6) | 58 (5.6) | 128 (5.8) | |
Awareness of breast cancer
In both communities most women had heard of breast cancer (Table 2), slightly more in Iwo (97.2%) than Ife Central (94%). Knowledge of someone with breast cancer was similar in both and the majority stated the persons they knew received medical treatment and had died (Table 2).
Table 2:
Knowledge and experience of breast cancer
| Ife No (%) |
Iwo No (%) |
Total No (%) |
Chi-square | |
|---|---|---|---|---|
| Have you ever heard of breast cancers | ||||
| Yes | 1099 (94) | 1024 (97.2) | 2123 (95.5) | p = 0.002 |
| No | 70(6) | 29 (2.8) | 99 (4.5) | |
| Do you know anyone who has had breast cancer? | ||||
| Yes | 319 (27.3) | 306 (29.1) | 625 (28.1) | p = 0.2 |
| No | 850 (72.7) | 747 (70.9) | 1597 (71.9) | |
| Did the person receive medical treatment? | Ife (N=319) | Iwo (N=306) | Total (N=625) | |
| Yes | 265(83.1) | 246(80.4) | 511 (81.8) | p = 0.24 |
| No | 15 (4.7) | 14 (4.6) | 29 (4.6) | |
| Don’t know | 39 (12.2) | 46 (15) | 85 (13.6) | |
| What was the outcome in that person? | ||||
| Alive and well | 60 (18.7) | 67 (21.9) | 127 (20.3) | p = 0.05 |
| Alive but sick | 32 (10) | 38 (12.4) | 70 (11.2) | |
| Dead | 207 (64.5) | 158 (51.6) | 365 (58.2) | |
| Don’t know | 22 (6.9) | 43 (14.1) | 65 (10.4) | |
Knowledge and practice of mammography screening
Breast cancer screening of any type had been recommended to 827 women (37.2%), from both communities, 441 (37.7%) of Ife Central and 386 (36.6%) of Iwo respondents respectively. Of these, screening was recommended by health workers in 48.2% of cases, while 38.3% were from the media, 10% obtained information about screening through friends and family members, while 2.7% got their information from Church seminars. Only 138 women (11.4%) from Ife Central and 120 women (11.6%) from Iwo were however aware of mammography. Only 33 women (2.8%) from Ife Central and 19 women (1.8%) from Iwo had undergone mammography at any time, the majority not within the last year. Knowledge and practice of mammography screening did not significantly differ between the two communities (Table 3).
Table 3:
Knowledge and practice of mammography
| Ife (N =1169) | Iwo (N = 1053) | Total (N = 2222) | p value | |
|---|---|---|---|---|
| Freq. (%) | Freq. (%) | Freq. (%) | ||
| Have you ever heard of mammography? | ||||
| Yes | 138 (11.8) | 120 (11.4) | 258 (11.6) | |
| No | 1031 (88.2) | 933 (88.6) | 1964 (88.4) | p = 0.764 |
| Have you ever had mammography? | ||||
| Yes | 33 (2.8) | 19 (1.8) | 52 (2.3) | |
| No | 1136 (97.2) | 1034 (98.2) | 2170 (97.7) | p = 0.113 |
| When did you last have mammography? | ||||
| N =33 | (N = 19) | (N = 52) | ||
| Less than 1 year | 7 (21.2) | 4 (21.1) | 11 (21.2) | |
| 1 – 2 years | 8 (24.2) | 9 (47.4) | 17 (32.7) | |
| Above 2 years | 18 (54.5) | 6 (31.6) | 24 (46.2) | p = 0.187 |
| Do you know where mammography can be done? | ||||
| (N = 138) | (N = 120) | (N = 258) | ||
| Yes | 96 (69.6) | 33 (27.5) | 129 (50) | |
| No | 42 (30.4) | 87 (72.5) | 129 (50) | p < 0.001 |
| How much does mammography cost? | ||||
| (N=138) | (N=120) | (N=258) | ||
| Underestimated | 18 (13) | 14 (11.7) | 32 (12.4) | |
| Correctly estimated | 21 (15.2) | 6 (5) | 27 (10.5) | |
| Overestimated | 4 (2.9) | 6 (5) | 10 (3.9) | p = 0.05 |
| Don’t know | 95 (68.8) | 94 (78.3) | 189 (73.3) | |
Among those who had heard of mammography, a significantly higher number of women knew where mammography could be done in Ife Central (69.6%) compared to Iwo (27.5%, p<0.001). There were few women in both communities who correctly estimated the cost of mammography, 15.2% in Ife and 6% in Iwo. Poisson regression to determine factors associated with mammography screening showed that, practice of CBE (APR-6.36, p<0.001), knowledge of someone with breast cancer (APR-1.75, p=0.04), Age, 70 years and above (APR-4.6, p< 0.001) were associated with increased uptake.
Barriers to mammography screening
Overall, lack of awareness was the most common reason cited for not having mammography (76.7%) followed by lack of perceived need (15.9%). Other reasons given include challenges locating a mammography facility, obtaining an appointment, cost, fear of test results and embarrassment. The same response pattern was observed in both communities (Figure 1).
Figure 1:
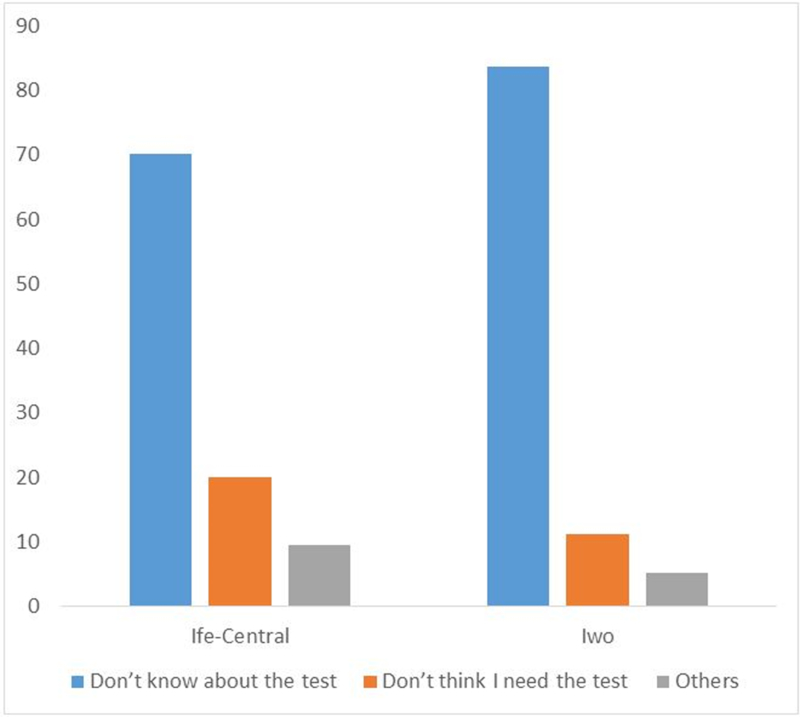
Reasons for not having mammography
Women were asked how much they could afford to pay for annual mammography. Of 1350 responses from both communities, only 20.4% could afford annual mammography at the current price, 12.7% at half the price and 19% less than half the price. Nearly half of the respondents (47.9%) could afford nothing.
Practice of Clinical Breast Exam (CBE)
CBE is a screening modality available to both communities. More Iwo respondents reported having a CBE compared to Ife Central, 27.4% compared to 19.7%, respectively (p<0.001). Among those who had a CBE, 40.8% of Iwo and 30.4% of Ife respondents (p<0.001) had the examination in the last year
Discussion
Low and middle income countries face numerous barriers to quality health care including lack of facilities and resources (O’Donnell 2007, Kingham, Kamara et al. 2009, Grimes, Bowman et al. 2011). While a challenge, the extent inadequate facilities contributes to poor health indices may be exaggerated. To provide a rational basis for planning interventional programmes, as well as help provide a foundation for optimal utilization of available health care facilities, it is imperative to understand factors affecting resource utilization, as examined in this study. This study identifies poor awareness as a major barrier to mammography screening uptake even when there is geographic access. It also identifies other key issues such as cost which must be considered in developing a breast cancer screening programme.
Our study depicts a negative experience of respondents in both communities, with many of those who knew people with breast cancer reporting those they knew had died despite medical care. This may contribute to the belief held in some quarters that medical treatment offers little benefit irrespective of disease extent. This belief may affect patients’ attitudes toward early detection and must be addressed (Anderson, Yip et al. 2006).
It is interesting to note that despite mammography service availability in Ife Central, 88.2% of the respondents were unaware of its existence. It is therefore not surprising that mammography uptake in Ife Central and Iwo were equally poor. This suggests that factors beyond geographic proximity contribute to low uptake. This is further confirmed by the relatively few number of women who had a CBE, which surprisingly was higher in Iwo where specialized services do not exist. Our study did make us aware of a community wide breast cancer awareness and CBE campaign conducted by a non-governmental organization in Iwo General Hospital in June 2013. This campaign may explain the higher CBE rates in Iwo despite Ile-Ife having a teaching hospital with specialized breast care. In resource poor settings, where many needs compete for funds, prioritizing interventions should be the goal. Given our results, we recommend that breast cancer awareness and advocacy be given topmost priority in the effort against breast cancer. Community education and awareness programs should precede interventional programs aimed at increasing the number of existing facilities. Awareness campaigns should emphasize how stage at presentation affects breast cancer outcomes and the role of screening in early stage presentation. Women should also be taught to get more familiar with their breast and how they change through their menstrual cycle in pre-menopausal women. They should learn how to identify breast cancer symptoms and to report promptly for evaluation. Another potential target for education are general practitioners and primary care providers who should be reminded about the importance of prompt referral of all women with suspected malignant breast lesions.
Awareness programmes that feature testimonies from breast cancer survivors may also be an effective approach to correct the erroneous belief that breast cancer is invariably fatal (Koon, Lehman et al. 2013). Additionally, community education programmes must address the lack of perceived breast cancer risk. This may require tailored approaches taking into account religious and cultural factors which underlie such beliefs (Parsa, Kandiah et al. 2006).
While awareness creation and community outreach programmes should be the starting point, sustainability for continued practice depends on the development of breast cancer screening programmes. As in most LMIC, such programs do not exist in Nigeria (Dey 2014). Currently, there are no national or regional screening guidelines for breast cancer screening in Nigeria. This deficiency contributes to poor screening rates and poor breast cancer outcomes. In developing a breast cancer screening programme, various expert recommendations have been made which are applicable in LMIC depending on available resources (Anderson, Yip et al. 2008). These recommendations, particularly at the basic and limited levels, can serve as a template to develop programmes adaptable to each locality. In the development of a programme, the issue of cost is however a key factor that must be considered. It is of no benefit creating awareness when financial access is not guaranteed. In this study, we assessed the potential effect of cost on mammography uptake by asking how much each respondent could afford for annual mammography. Although there were more (20%) who could afford screening than have been screened (2.8%), it is clear that a screening programme based solely on mammography will exclude the majority of women who are unable to afford mammography at the present cost of about 50 US Dollars. This is not surprising given that more than two-thirds of the Nigerian population live below 1 US dollar per day (Ucha 2010). Designing a comprehensive breast cancer screening programme therefore calls for a lot of innovation to ensure maximum coverage. A biennial screening program for instance may improve affordability by decreasing overall cost; however there will remain many women requiring financial assistance or government subsidy to have mammography. Given the prevailing economic situation and other competing health needs in most LMIC, this may not happen soon. As such there may be a need to selectively recommend mammography to high risk women who are likely to benefit the most, while CBE is recommended for the majority of women. An additional advantage of a selective mammography screening program of this nature is that it will create a demand more commensurate with the screening facilities available to avoid overwhelming limited personnel and facilities.
We conclude from this study that geographic access without awareness creation and community mobilization does not guarantee utilization of breast cancer screening services. Much more can therefore be achieved with the limited resources and facilities available provided an action plan to improve awareness and ensure optimal utilization of available resources is designed. This, in addition to a cost effective breast cancer screening programme are urgently required.
Table 4:
Multivariate Poisson regression analysis to determine factors associated with mammography uptake
| Variable | Adjusted Prevalence Ratio with 95% Confidence interval |
p-value |
|---|---|---|
| Town | ||
| Iwo | ||
| Ife | 1.60 (0.89–2.88) | 0.12 |
| Age | ||
| 40 – 49 years | ||
| 50 – 59 years | 1.49 (0.83–2.65) | 0.18 |
| 60 – 69 years | 0.36 (0.05–2.81) | 0.33 |
| 70 years and above | 4.60 (1.63–12.97) | <0.001 |
| Marital Status | ||
| Others | ||
| Married | 1.73 (0.78–3.85) | 0.18 |
| Education | ||
| None | ||
| Primary | 2.32 (0.38–14.31) | 0.37 |
| Secondary | 2.39 (0.45–12.65) | 0.30 |
| College graduate | 5.07 (0.94–27.20) | 0.06 |
| Ever had CBE | ||
| No | ||
| Yes | 6.36 (3.18–12.70) | <0.001 |
| Knowledge of someone with Breast Cancer | ||
| No | ||
| Yes | 1.75 (1.04–2.94) | 0.04 |
Acknowledgements
We are immensely grateful to the Thompson Family Foundation, The MSKCC Global Cancer Disparity Initiative, and the African Research Group for Oncology for funding the project. This work was also supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. We specially thank Prof Murray Brennan for his guidance and mentorship. We thank Prof OO Lawal and Prof OA Sowande for their advisory role. Our appreciations go to the Faculties and students of the MS program of the Division of Clinical Epidemiology and Evaluative Sciences Research, Weill Cornell Medicine for their comments and suggestions and encouragement towards the successful completion of the project.
Financial support: The Thompson Family Foundation, MSKCC Global Cancer Disparity Initiative, and the African Research Group for Oncology
Footnotes
Ethics
Approval from the ethical committee of the Institute of public health of the Obafemi Awolowo University Ile-Ife and from Ife Central and Iwo Local government area authorities was obtained before survey administration.
Contributor Information
Olalekan Olasehinde, Department of Surgery, Obafemi Awolowo University, Ile-Ife.
Olusegun I Alatise, Department of Surgery, Obafemi Awolowo University, Ile-Ife.
Olukayode A Arowolo, Department of Surgery, Obafemi Awolowo University, Ile-Ife.
Victoria L Mango, Department of Radiology, Memorial Sloan-Kettering Cancer center, New-York.
Olalere S Olajide S, AIDS Prevention Initiative in Nigeria, Ibadan.
Adeleye D Omisore, Department of Radiology, Obafemi Awolowo University, Ile-Ife.
Carla Boutin-Foster, State University of New York, Downstate Medical Center.
Thomas P Kingham, Department of Surgery, Memorial Sloan-Kettering Cancer center, New-York.
References
- Adebamowo CA and Adekunle OO (1999). “Case-controlled study of the epidemiological risk factors for breast cancer in Nigeria.” Br J Surg 86(5): 665–668. [DOI] [PubMed] [Google Scholar]
- Adesunkanmi AR, Lawal OO, Adelusola KA and Durosimi MA (2006). “The severity, outcome and challenges of breast cancer in Nigeria.” Breast 15(3): 399–409. [DOI] [PubMed] [Google Scholar]
- Akande HJ, Olafimihan BB and Oyinloye OI (2015). “A five year audit of mammography in a tertiary hospital, North Central Nigeria.” Niger Med J 56(3): 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhigbe AO and Omuemu VO (2009). “Knowledge, attitudes and practice of breast cancer screening among female health workers in a Nigerian urban city.” BMC Cancer 9: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinde OR, Phillips AA, Oguntunde OA and Afolayan OM (2015). “Cancer mortality pattern in lagos university teaching hospital, lagos, Nigeria.” J Cancer Epidemiol 2015: 842032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BO, Yip CH, Ramsey SD, Bengoa R, Braun S, Fitch M, Groot M, Sancho-Garnier H and Tsu VD (2006). “Breast cancer in limited-resource countries: health care systems and public policy.” Breast J 12 Suppl 1: S54–69. [DOI] [PubMed] [Google Scholar]
- Anderson BO, Yip CH, Smith RA, Shyyan R, Sener SF, Eniu A, Carlson RW, Azavedo E and Harford J (2008). “Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007.” Cancer 113(8 Suppl): 2221–2243. [DOI] [PubMed] [Google Scholar]
- Arowolo OA, Akinkuolie AA, Lawal OO, Alatise OI, Salako AA and Adisa AO (2010). “The impact of neoadjuvant chemotherapy on patients with locally advanced breast cancer in a Nigerian semiurban teaching hospital: a single-center descriptive study.” World J Surg 34(8): 1771–1778. [DOI] [PubMed] [Google Scholar]
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD and Feuer EJ (2005). “Effect of screening and adjuvant therapy on mortality from breast cancer.” N Engl J Med 353(17): 1784–1792. [DOI] [PubMed] [Google Scholar]
- Dey S (2014). “Preventing breast cancer in LMICs via screening and/or early detection: The real and the surreal.” World J Clin Oncol 5(3): 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR and Ward EM (2014). “Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer.” Cancer 120(9): 1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM (2010). “Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008.” Int J Cancer 127(12): 2893–2917. [DOI] [PubMed] [Google Scholar]
- Fletcher SW and Elmore JG (2003). “Clinical practice. Mammographic screening for breast cancer.” N Engl J Med 348(17): 1672–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharoro EP and Ikeanyi EN (2006). “An appraisal of the level of awareness and utilization of the Pap smear as a cervical cancer screening test among female health workers in a tertiary health institution.” Int J Gynecol Cancer 16(3): 1063–1068. [DOI] [PubMed] [Google Scholar]
- Grimes CE, Bowman KG, Dodgion CM and Lavy CB (2011). “Systematic review of barriers to surgical care in low-income and middle-income countries.” World journal of surgery 35(5): 941–950. [DOI] [PubMed] [Google Scholar]
- Groot MT, Baltussen R, Uyl-de Groot CA, Anderson BO and Hortobagyi GN (2006). “Costs and health effects of breast cancer interventions in epidemiologically different regions of Africa, North America, and Asia.” Breast J 12 Suppl 1: S81–90. [DOI] [PubMed] [Google Scholar]
- Jedy-Agba E, Curado MP, Ogunbiyi O, Oga E, Fabowale T, Igbinoba F, Osubor G, Otu T, Kumai H, Koechlin A, Osinubi P, Dakum P, Blattner W and Adebamowo CA (2012). “Cancer incidence in Nigeria: a report from population-based cancer registries.” Cancer Epidemiol 36(5): e271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham TP, Kamara TB, Cherian MN, Gosselin RA, Simkins M, Meissner C, Foray-Rahall L, Daoh KS, Kabia SA and Kushner AL (2009). “Quantifying surgical capacity in Sierra Leone: a guide for improving surgical care.” Arch Surg 144(2): 122–127; discussion 128. [DOI] [PubMed] [Google Scholar]
- Koon KP, Lehman CD and Gralow JR (2013). “The importance of survivors and partners in improving breast cancer outcomes in Uganda.” Breast 22(2): 138–141. [DOI] [PubMed] [Google Scholar]
- Makanjuola SB, Popoola AO and Oludara MA (2014). “Radiation therapy: a major factor in the five-year survival analysis of women with breast cancer in Lagos, Nigeria.” Radiother Oncol 111(2): 321–326. [DOI] [PubMed] [Google Scholar]
- Nystrom L, Bjurstam N, Jonsson H, Zackrisson S and Frisell J (2017). “Reduced breast cancer mortality after 20+ years of follow-up in the Swedish randomized controlled mammography trials in Malmo, Stockholm, and Goteborg.” J Med Screen 24(1): 34–42. [DOI] [PubMed] [Google Scholar]
- O’Donnell O (2007). “Access to health care in developing countries: breaking down demand side barriers.” Cad Saude Publica 23(12): 2820–2834. [DOI] [PubMed] [Google Scholar]
- Obajimi MO, Adeniji-Sofoluwe AT, Oluwasola AO, Adedokun BO, Soyemi TO, Olopade F and Newstead G (2011). “Mammographic breast pattern in Nigerian women in Ibadan, Nigeria.” Breast Dis 33(1): 9–15. [DOI] [PubMed] [Google Scholar]
- Okebukola PO and Brieger WR (2016). “Providing Universal Health Insurance Coverage in Nigeria.” Int Q Community Health Educ. [DOI] [PubMed] [Google Scholar]
- Okobia MN, Bunker CH, Okonofua FE and Osime U (2006). “Knowledge, attitude and practice of Nigerian women towards breast cancer: a cross-sectional study.” World J Surg Oncol 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoronkwo IL, Ejike-Okoye P, Chinweuba AU and Nwaneri AC (2015). “Financial barriers to utilization of screening and treatment services for breast cancer: an equity analysis in Nigeria.” Niger J Clin Pract 18(2): 287–291. [DOI] [PubMed] [Google Scholar]
- Parsa P, Kandiah M, Abdul Rahman H. and Zulkefli NM (2006). “Barriers for breast cancer screening among Asian women: a mini literature review.” Asian Pacific journal of cancer prevention 7(4): 509. [PubMed] [Google Scholar]
- Smith RA, Andrews K, Brooks D, DeSantis CE, Fedewa SA, Lortet-Tieulent J, Manassaram-Baptiste D, Brawley OW and Wender RC (2016). “Cancer screening in the United States, 2016: A review of current American Cancer Society guidelines and current issues in cancer screening.” CA Cancer J Clin 66(2): 95–114. [DOI] [PubMed] [Google Scholar]
- Ucha C (2010). “Poverty in Nigeria: Some dimensions and contributing factors.” Global Majority E-Journal 1(1): 46–56. [Google Scholar]


