Abstract
We identified in-frame fusion transcripts of KIF5B (the kinesin family 5B gene) and the RET oncogene, which are present in 1–2% of lung adenocarcinomas (LADCs) from people from Japan and the United States, using whole-transcriptome sequencing. The KIF5B-RET fusion leads to aberrant activation of RET kinase and is considered to be a new driver mutation of LADC because it segregates from mutations or fusions in EGFR, KRAS, HER2 and ALK, and a RET tyrosine kinase inhibitor, vandetanib, suppresses the fusion-induced anchorage-independent growth activity of NIH3T3 cells.
A considerable proportion of LADCs, the most common histological type of lung cancer that comprises ~40% of the total cases, develops through activation of oncogenes, for example, somatic mutations in EGFR (10–50% of cases) or KRAS (10–30% of cases) or fusion of ALK (5% of cases), in a mutually exclusive manner1–4. Tyrosine kinase inhibitors (TKIs) targeting the EGFR and ALK proteins are effective in the treatment of LADCs that carry EGFR mutations and ALK fusions1–3, respectively.
We performed whole-transcriptome sequencing (RNA sequencing)5 of 30 LADC specimens from Japanese individuals to identify new chimeric fusion transcripts that could be targets for therapy3,5,6. These LADCs were 2 carcinomas with EML4-ALK fusions, 4 with EGFR or KRAS mutations and 24 without these fusions or mutations (Supplementary Table 1). Identifying candidate fusions represented by >20 paired-end reads and validation by Sanger sequencing of the RT-PCR products (Supplementary Methods) led to the identification of seven fusion transcripts, including EML4-ALK (Supplementary Table 1). We detected one of these fusions between KIF5B on chromosome 10p11.2 and RET on chromosome 10q11.2 in subject BR0020 (Fig. 1 and Supplementary Fig. 1a). We then further investigated this fusion, as fusions between RET and genes other than KIF5B have previously been shown to drive papillary thyroid tumor formation6,7.
Figure 1.
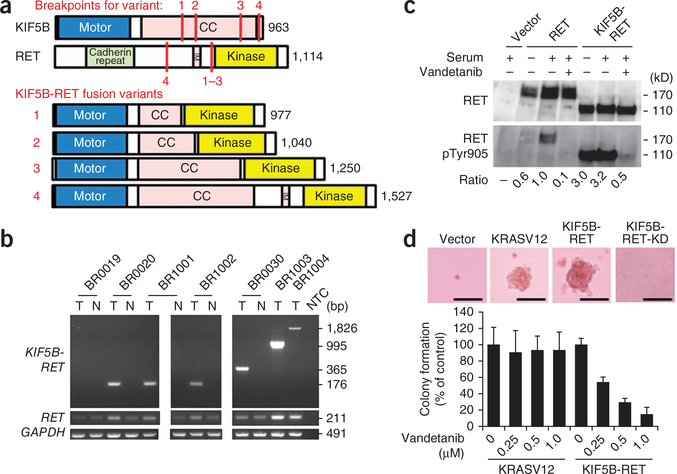
KIF5B-RET fusions in LADC. (a) Schematic representations of the wild-type KIF5B and RET proteins as well as the four fusion variants identified in this study. The breakpoints for each variant are indicated with red lines. CC, coiled coil; TM, transmembrane. (c) Detection of KIF5B-RET fusions by RT-PCR. RT-PCR products for the RET kinase domain (exons 12 and 13) and GAPDH are shown below. Six LADCs positive for KIF5B-RET fusions (T) are shown, with four corresponding non-cancerous lung tissues (N), a no-template control (NTC) and one LADC that was negative for the fusion (BR0019). (c) Activation of RET kinase activity in the KIF5B-RET protein and the suppression of this activity by vandetanib. H1299 lung cancer cells were transfected with an empty vector, wild-type RET (RET) or KIF5B-RET expression plasmids and treated either with DMSO (serum) or vandetanib, as indicated. The ratios of phosphorylated Tyr905 (pTyr905) RET to total RET signals with respect to wild-type RET after the serum treatment are listed below the gels. (d) Anchorage-independent growth of NIH3T3 cells expressing KIF5B-RET protein and the suppression of this growth by vandetanib. Representative pictures of colonies without vandetanib treatment (top). Scale bars, 50 μm. Bar graph showing the percentage (± s.d.) of colonies formed after treatment with the indicated amounts of vandetanib (average results of three independent experiments) with respect to those formed by DMSO-treated cells. The study was approved by the institutional review boards of institutions participating in this study.
RT-PCR and a Sanger sequencing analysis of 319 LADC specimens from Japanese individuals (Supplementary Table 2), including 30 that had been subjected to whole-transcriptome sequencing, revealed that 1.9% (6 out of 319) expressed KIF5B-RET fusion transcripts (Fig. 1b and Supplementary Fig. 1b). We identified four variants in these six tumors, and all of these variants were in frame (Fig. 1a).
A genomic PCR analysis of the six tumors that were positive for RET fusions revealed somatic fusions of the KIF5B introns 15, 16, 23 or 24 at chromosome 10p11.2 with the RET introns 7 or 11 at 10q11.2 (Supplementary Fig. 1c,d), indicating that a chromosomal inversion had occurred between the long and short arms in the centromeric region of chromosome 10 (Supplementary Figs. 1e and 2). We verified this chromosomal inversion using fluorescence in situ hybridization, which revealed a split in the signals for the probes that flank the RET translocation sites in tumors positive for the KIF5B-RET fusion (Supplementary Fig. 2).
The tumors positive for the KIF5B-RET fusion were all well or moderately differentiated (Table 1 and Supplementary Fig. 3). None of the subjects with these tumors had a history of thyroid cancer, and none showed abnormal findings in their thyroid tissues as determined by computed tomography or positron emission tomography before surgery for LADC. All five examined tumors with the KIF5B-RET fusion were positive for thyroid transcription factor 1 (TTF-1) and napsin A aspartic proteinase (Napsin A)8 but were negative for thyroglobulin9, indicating that they were of pulmonary origin (Table 1 and Supplementary Fig. 3). The LADCs that were positive for the KIF5B-RET fusion showed twofold to 30-fold higher RET expression than non-cancerous lung tissues (Fig. 1b and Supplementary Figs. 4 and 5). An immunohistochemical analysis using an antibody against the C-terminal region of the RET protein detected positive cytoplasmic staining in the tumor cells of the fusion-positive LADCs (Table 1 and Supplementary Fig. 3b) but did not detect this staining in any of the non-cancerous lung cells. A western blot analysis confirmed the expression of the fusion proteins in the LADCs (Supplementary Fig. 6).
Table 1.
Characteristics of lung adenocarcinomas with the KIF5B-RET fusion
| Sample | Country | Sex | Agea | Smoking | KIF5B-RET fusionb | Pathological stage | Pathological findings | RET staining | TTF-1 staining | Napsin A staining | Thyrogloblin staining |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BR0020 | Japan | Male | 57 | Never | K15; R12 (variant 1) | IIB | Moderately differentiated ADC | + | + | + | − |
| BR1001 | Japan | Female | 65 | Never | K15; R12 (variant 1) | IB | Well differentiated ADC | + | + | + | − |
| BR1002 | Japan | Female | 64 | Never | K15; R12 (variant 1) | IB | Well differentiated ADC | + | + | + | − |
| BR0030 | Japan | Male | 57 | Never | K16; R12 (variant 2) | IA | Well differentiated ADC | + | + | + | − |
| BR1003 | Japan | Male | 28 | Never | K23; R12 (variant 3) | IA | Well differentiated ADC | + | + | + | − |
| BR1004 | Japan | Female | 71 | Never | K24; R8 (variant 4) | IA | Moderately differentiated ADC | NT | NT | NT | NT |
| NCI1580 | USA | Male | 63 | Everc | K15; R12 (variant 1) | II | Moderately differentiated ADC | NT | NT | NT | NT |
Age in years.
Fused exon numbers of KIF5B (K) and RET (R); and variant types (in parentheses) are shown. None of the subjects had oncogenic EGFR, KRAS, HER2 or ALK mutations or fusions.
The number of pack years smoked for this subject is not known. NT, not tested.
To address the prevalence of KIF5B-RET fusions in LADCs from individuals of non-Asian ancestry, we examined LADCs in cohorts from the United States and Norway (Supplementary Table 2). We detected a fusion transcript in 1 of the 80 (1.3%) subjects from the United States (an individual of European ancestry) (Supplementary Fig. 7), but we detected no fusion transcripts in the 34 subjects from Norway (Supplementary Table 3); KIF5B-RET fusions occurred in 1–2% of LADCs in both Asians and non-Asians. The individual from the United States with the RET fusion was classified as an ‘ever smoker’, whereas the six individuals from Japan with the RET fusion were ‘never smokers’ (Table 1). Therefore, prevalence of LADC with regard to smoking status is unclear. We did not detect the KIF5B-RET fusion in other major subtypes of lung cancer, including 234 squamous-cell, 17 large-cell and 20 small-cell lung carcinomas (Supplementary Table 3). The fusion was also not present in other types of adenocarcinomas, including those of the ovary (n = 100) and colon (n = 200) (data not shown), suggesting that it is specific to LADC.
All seven subjects with LADC harboring the KIF5B-RET fusion were negative for EGFR, KRAS and ALK mutations or fusions and were negative for mutations in HER2, which is an additional driver mutation in LADC10 (Table 1 and Supplementary Table 4). The mutually exclusive nature of the RET fusions and other oncogenic alterations1,2,11 suggests that the KIF5B-RET fusion is a driver mutation. All proteins encoded by the four KIF5B-RET fusion variants contained the KIF5B coiled-coil domain, which functions in protein dimerization12, and retained the full RET kinase domain, similar to other types of oncogenic RET fusions observed in thyroid tumors (Fig. 1a)13. The KIF5B-RET proteins are likely to form a homodimer through the coiled-coil domain of KIF5B, causing an aberrant activation of the kinase function of RET in a manner similar to the PTC-RET and KIF5B-ALK fusions7,14. In fact, the N-terminal portion of the KIF5B coiled-coil region, which is retained in all variants, has been predicted to have the ability to dimerize through two coiled-coil structures15. Consistently, when the KIF5B-RET variant 1 was exogenously expressed in H1299 human lung cancer cells without wild-type or fusion RET expression, Tyr905, which is located in the activation loop of the RET kinase site15,16, was phosphorylated in the absence of serum stimulation, indicating an aberrant activation of RET kinase16,17 by fusion with KIF5B (Fig. 1c). This phosphorylation was suppressed by vandetanib, a TKI against RET (as well as other tyrosine kinases, including EGFR and VEGFR)18 (Fig. 1c and Supplementary Fig. 8).
Expression of exogenous KIF5B-RET, but not KIF5B-RET-KD (a kinase-dead mutant corresponding to S765P in wild-type RET17), induced morphological transformation (Supplementary Fig. 9) and anchorage-independent growth of NIH3T3 fibroblasts in a way that was analogous to the induction caused by mutant KRAS (KRASV12) (Fig. 1d). Consistently, phosphorylation of Tyr905 was higher in the KIF5B-RET protein than in the KIF5B-RET-KD protein. The anchorage-independent growth induced by KIF5B-RET was suppressed by treatment with vandetanib (<1 μM), whereas the growth induced by mutant KRAS was not (Fig. 1d). These results are similar to those observed for RET fusions in thyroid cancer19. We also detected phosphorylation of the KIF5B-RET protein at Tyr905 in fusion-positive LADC specimens (Supplementary Fig. 6). These results suggest that the RET fusions are a previously unidentified LADC driver mutation and a potential target for existing TKIs, including vandetanib, which has been recently approved by the US Food and Drug Administration for the treatment of thyroid cancer18. Further studies are warranted to promote molecular subtype diagnoses and personalized therapy options for LADC. For this purpose, both the clinical and biological features of this fusion are being investigated. For further information, please see the Supplementary Note and Supplementary Tables 5 and 6.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the program for promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NiBio), Grants-in-Aid from the Ministry of Health, Labour and Welfare for the 3rd-term Comprehensive 10-year Strategy for Cancer Control, the National Cancer Center Research and Development Fund and the Norwegian Cancer Society. National Cancer Center Biobank is supported by the National Cancer Center Research and Development Fund, Japan. We thank T. Urushidate, S. Ohashi, S. Mitani, K. Yokozawa, S. Wakai, C. Otsubo and H. Isomura of the National Cancer Center and D. Suzuki and K. Nagase of the National Center for Global Health and Medicine for technical assistance. We also thank J.D. Minna and L. Girard of the University of Texas Southwestern Medical Center, K. Kumamoto of Saitama Medical University and A. Okamoto of Jikei University for RET fusion screening, N. Morii of the National Institute of Advanced Industrial Science and Technology (AIST) for thermodynamic characterization of the KIF5B protein and M. Maekawa of the GSP laboratory for rapid preparation of the FISH probes.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Note: Supplementary information is available on the Nature Medicine website.
References
- 1.Janku F, Stewart DJ & Kurzrock R Nat. Rev. Clin. Oncol 7, 401–414 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Gerber DE & Minna JD Cancer Cell 18, 548–551 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovly CM & Carbone DP Nat. Rev. Clin. Oncol 8, 68–70 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Soda M et al. Nature 448, 561–566 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Meyerson M, Gabriel S & Getz G Nat. Rev. Genet 11, 685–696 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Mani RS & Chinnaiyan AM Nat. Rev. Genet 11, 819–829 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Wells SA Jr. & Santoro M Clin. Cancer Res 15, 7119–7123 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Bishop JA, Sharma R & Illei PB Hum. Pathol 41, 20–25 (2010). [DOI] [PubMed] [Google Scholar]
- 9.DeLellis RA, Shin SJ & Treaba DO Immunohistology of Endocrine Tumors (Saunders, Philadelphia, Pennsylvania, USA, 2010). [Google Scholar]
- 10.Shigematsu H et al. Cancer Res. 65, 1642–1646 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Herbst RS, Heymach JV & Lippman SM N. Engl. J. Med 359, 1367–1380 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirokawa N, Noda Y, Tanaka Y & Niwa S Nat. Rev. Mol. Cell Biol 10, 682–696 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Jhiang SM Oncogene 19, 5590–5597 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi K et al. Clin. Cancer Res 15, 3143–3149 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Morii H, Takenawa T, Arisaka F & Shimizu T Biochemistry 36, 1933–1942 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Vitagliano D et al. Endocr. Relat. Cancer 18, 1–11 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Croyle M et al. Cancer Res 68, 4183–4191 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Commander H, Whiteside G & Perry C Drugs 71, 1355–1365 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Carlomagno F et al. Cancer Res 62, 7284–7290 (2002). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.