Abstract
Background: Impairment in episodic memory is one of the most robust findings in schizophrenia. Disruptions of fronto‐temporal functional connectivity that could explain some aspects of these deficits have been reported. Recent work has identified abnormal hippocampal function in unmedicated patients with schizophrenia (SZ), such as increased metabolism and glutamate content that are not always seen in medicated SZ. For these reasons, we hypothesized that altered fronto‐temporal connectivity might originate from the hippocampus and might be partially restored by antipsychotic medication. Methods: Granger causality methods were used to evaluate the effective connectivity between frontal and temporal regions in 21 unmedicated SZ and 20 matched healthy controls (HC) during performance of an episodic memory retrieval task. In 16 SZ, effective connectivity between these regions was evaluated before and after 1‐week of antipsychotic treatment. Results: In HC, significant effective connectivity originating from the right hippocampus to frontal regions was identified. Compared to HC, unmedicated SZ showed significant altered fronto‐temporal effective connectivity, including reduced right hippocampal to right medial frontal connectivity. After 1‐week of antipsychotic treatment, connectivity more closely resembled the patterns observed in HC, including increased effective connectivity from the right hippocampus to frontal regions. Conclusions: These results support the notion that memory disruption in schizophrenia might originate from hippocampal dysfunction and that medication restores some aspects of fronto‐temporal dysconnectivity. Patterns of fronto‐temporal connectivity could provide valuable biomarkers to identify new treatments for the symptoms of schizophrenia, including memory deficits. Hum Brain Mapp 36:1442–1457, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: schizophrenia, functional connectivity, effective connectivity, episodic memory, memory retrieval, memory encoding, hippocampus, prefrontal cortex, unmedicated, antipsychotic medication
INTRODUCTION
Schizophrenia is a chronic psychiatric illness with a world‐wide prevalence of approximately 1% (Jablensky, et al., 1992) that is characterized by positive, negative, and cognitive symptoms. Importantly, the cognitive deficits (Tandon, et al., 2009) are most predictive of poor functional outcome (Green, 1996; Liddle, 1987), but there currently are no effective treatments for this symptom complex (Deserno, et al., 2012). Thus, there is a critical need to better understand the dysfunction that characterizes cognitive deficits to identify therapeutic targets.
Impairments in working and episodic memory are amongst the most replicated and robust abnormalities in schizophrenia (Aleman, et al., 1999; Heinrichs and Zakzanis, 1998; Kraguljac, et al., 2013aa). They are often seen at the initial diagnosis and may already be present in the prodromal phase of the illness (Tandon, et al., 2009). Functional MRI (fMRI) can reliably map episodic memory processes using appropriate tasks (Hutcheson, et al., 2012; Ragland, et al., 2009). Early work investigating episodic memory in schizophrenia focused on abnormal activation of specific brain regions such as the hippocampus (Heckers, et al., 1999) and prefrontal cortex (Ragland, et al., 2001). More recently, several studies have used functional connectivity, a measure of the temporal coherence of the blood‐oxygen level dependent (BOLD) fluctuations in distinct brain regions, and reported disruption of fronto‐temporal connections in schizophrenia (Benetti, et al., 2009; Lawrie, et al., 2002; Meyer‐Lindenberg, et al., 2005; Wolf, et al., 2009).
In our previous work, we reported that, in drug free patients with schizophrenia, regional cerebral blood flow (rCBF) in hippocampus is elevated (Medoff, et al., 2001) and is correlated with the severity of positive symptoms (Lahti, et al., 2006). In addition, we found that the reduction in hippocampal rCBF with antipsychotic medication is associated with good treatment outcome (Lahti, et al., 2009). Furthermore, we recently reported increased hippocampal glutamate levels in unmedicated patients (Kraguljac, et al., 2013bb), but not in medicated patients (Kraguljac, et al., 2012). For these reasons, we hypothesized that fronto‐temporal disruption during memory processing in drug‐free schizophrenia patients might originate from abnormal hippocampal function and could be partially restored by antipsychotic medication.
Effective connectivity is a more recent data analysis method that describes the influence one brain region exerts over another through the analysis of time‐lagged BOLD time series (Büchel, et al., 1999) and thereby helps clarify how brain areas communicate (Friston, 1994b; Friston, et al., 1993, 1994aa). Understanding the transfer of neural information in memory and how it may be disrupted in schizophrenia may help uncover the neural bases of the memory deficits seen in the illness. In first‐episode schizophrenia, a decrease in effective connectivity originating from the hippocampus to the frontal cortex was identified during a working memory task using dynamic causal modeling (DCM) (Benetti, et al., 2009). DCM and structural equation modeling (SEM) are useful techniques that impose restrictions on the number of permissible regions of interest (ROIs) in the model. They require prior assumptions about the underlying connections and as such are confirmatory methods. In contrast, effective connectivity accessed with multivariate autoregressive (MVAR) Granger causality does not require prior assumptions about the underlying connectional architecture, has less restrictions on the number of ROIs that can be included in the model and is therefore more exploratory in its nature (Abler, et al., 2006; Deshpande, et al., 2008, 2013; Roebroeck, et al., 2005; Sathian, et al., 2011). To our knowledge, no studies have used effective connectivity accessed with MVAR Granger to investigate episodic memory abnormalities in unmedicated patients with schizophrenia and to examine the effect of antipsychotic medication on effective connectivity patterns.
In the present study, we evaluated the degree of functional impairments between regions of a memory network, including fronto‐temporal connections, in patients with schizophrenia during performance of an episodic memory task. We evaluated these impairments when patients were off medication and after one‐week of treatment with risperidone, a second‐generation antipsychotic medication. We hypothesized that unmedicated patients with schizophrenia would show significant reductions in directional connectivity from the hippocampus to prefrontal regions that would be partially restored with antipsychotic treatment.
METHODS
Subjects
Thirty‐six participants with a diagnosis of schizophrenia or schizoaffective disorder (SZ), not currently taking antipsychotic medication (for at least 10 days), and seeking treatment at the University of Alabama at Birmingham (UAB) were recruited for this study (Table 1). Thirty healthy controls (HC), matched on age, gender, and parental occupation, were recruited by advertisements in flyers and the University's newspaper. The exclusion criteria were major medical or neurological conditions, substance abuse within past 6 months, previous serious head injury, history of loss of consciousness, and pregnancy. The UAB IRB approved this study, and all participants provided written informed consent and the SZ performed an evaluation to provide consent (Carpenter, et al., 2000). Diagnoses were established using participants' medical records and the Diagnostic Interview for Genetic Studies (Nurnberger, et al., 1994). The Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph, et al., 1998) were used to characterize symptom severity, response to antipsychotic treatment and general cognitive function.
Table 1.
Group demographics (mean ± standard deviation)
| SZ (n = 21) | HC (n = 20) | |
|---|---|---|
| Age, years | 31.2 ± 10.2 | 36.5 ± 13.4 |
| Gender, M/F | 16/5 | 13/7 |
| Parental occupationa | 8.8 ± 6.1 | 7.0 ± 5.3 |
| RBANSb Total index | 82.5 ± 16.6 | 96.0 ± 8.7f |
| First episode (yes/ no) | 7/21 | – |
| Duration of illness, (years) | 9.5 ± 9.2 | – |
| Medication status (previously medicaited/ naïve) | 12/9 | – |
| Average medication dosage (mg of resperidone) | ||
| Week 1 | 2.60 | – |
| Week 6 | 4.76 | – |
| BPRSc | ||
| Baseline total | 48.8 ± 10.9 | – |
| Baseline positive | 13.6 ± 3.5 | – |
| Baseline negative | 7.1 ± 2.5 | – |
| Treatment response totald | 61.7% ± 24.2% | – |
| Treatment response positivee | 76.8% ± 29.1% | – |
| Treatment response negativee | 55.5% ± 39.9% | – |
HC, healthy control; F, female; M, male; SES, Socioeconomic status; SZ, patients with schizophrenia.
Ranks determined from Diagnostic Interview for Genetic Studies (1–18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status. Data missing for five patients with schizophrenia.
Repeatable battery for the assessment of neuropsychological status.
Brief psychiatric rating scale, scored on a 1–7 scale; positive subscale (conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content); negative subscale (emotional withdrawal, motor retardation, and blunted affect).
Treatment response total = (B 0–B LOCF)/ (B 0−20). B 0 is BPRS score at baseline, and B LOCF is the BPRS last observation carried forward.
Treatment response positive = (B 0–B LOCF)/ (B 0−4).
Treatment response negative = (B 0–B LOCF)/ (B 0−3).
P < 0.005 significant difference between HC and SZ in independent samples t‐test.
Experimental Design
HC were scanned once and SZ were scanned while unmedicated (baseline scan), and then entered into a 6‐week trial with risperidone (flexible dosing regimen). At the baseline time point 15 SZ and 10 HC were excluded from analyses because of movement, failure to correctly perform the memory task, and in SZ, failure to complete their medication regimen after the end of 6 weeks. This left us with a combined group of 21 SZ and 20 HC at baseline. To evaluate the short‐term effects of antipsychotic medication on effective connectivity, a second scan was performed after one week of treatment. Sixteen 16 SZ had usable imaging data at baseline and week 1. Treatment response was assessed calculating the percentage change in Brief Psychiatric Rating Scale (BRPRS) total score (Leucht, et al., 2007) from baseline to week 6 for each participant using the formula: (B 0 – B LOCF)/ (B 0 – 20), where B 0 = BPRS total score at baseline, B LOCF = the last observation carried forward, and 20 being the minimum score on the 20 item BPRS scale.
Image Acquisition
All imaging was performed on a 3T head‐only scanner (Siemens Allegra, Erlangen, Germany), equipped with a circularly polarized transmit/receive head coil. fMRI data were acquired using the gradient recalled echo‐planar imaging sequence (repetition time/echo time [TR/TE] = 2100/30 msec, 70° flip angle, 24 × 24 cm2 field of view, 64 × 64 matrix, 4‐mm slice thickness, 1‐mm gap, 26 axial slices). A high‐resolution structural scan was acquired using the T1‐weighted magnetization prepared rapid acquisition gradient‐echo sequence (TR/TE/inversion time [TI] =2300/3.93/1100 msec, 12° flip angle, 256 × 256 matrix, 1‐mm isotropic voxels). An IFIS‐SA system (In Vivo, Orlando, FL) running E‐Prime software (version 1.2; Psychology Software Tools, Pittsburgh, PA) controlled stimulus delivery and recorded responses and reaction times.
fMRI Task
This previously validated episodic memory task (Hutcheson, et al., 2012) consists of an intentional encoding phase, followed by a recognition memory phase after a 15‐min delay. To maximize retrieval performance, a deep encoding paradigm utilizing an animacy decision was used. During encoding, participants saw a series of 60 words, presented one at a time for 300 msec followed by a fixation screen. A 2‐second prestimulus cue (“Alive?”) indicated that the participant had to answer by button press whether the upcoming word was alive or not alive. Following a 15‐min distraction task, participants performed the retrieval task, where they saw 60 words, including 30 words previously seen during the encoding phase (old words) and 30 new words, presented one at a time for 300 msec. A 2‐sec warning stimulus (“Ready?”) indicated that the participant had to answer by button press whether the upcoming word was “old” or “new.” The events of interest were the correctly identified old words, Retrieve Old Correct (ROC), and the correctly identified new words, Retrieve New Correct (RNC). For each task, the interstimulus interval (fixation screen) was jittered, ranging from 3 to 5 sec. Behavioral measures were compared across groups using a one‐way ANOVA, and the alpha level was set at 0.05.
fMRI Analysis and Design
Data analyses were performed in SPM8 running in MATLAB (version R2010b). Preprocessing of the fMRI data included slice timing correction, realignment and reslicing to the mean functional volume, artifact/motion correction using ArtRepair (Mazaika PK and Reiss, 2007), coregistraton to the structural scan, and normalization to Montreal Neurological Institute (MNI) space using DARTEL (Ashburner, 2007) with 4‐mm Full Width Half Maximum (FWHM) Gaussian kernel smoothing. Participants were excluded from further analyses if 33% or more of their data were repaired during artifact and motion correction.
The subject‐level statistical analysis consisted of an event‐related general linear model. We included the following regressors: ROC and RNC (Ragland, et al., 2001, 2004). All events were modeled using a canonical hemodynamic response function, and data were high‐pass filtered (cutoff = 256 sec). Statistical parametric maps were generated for the following contrast: ROC (Hits) > RNC (Correct Rejects), which is referred in the literature as the “old/new effect” or “retrieval success effect” (Vincent, et al., 2006). At the group level, the contrasts (ROC > RNC) for each individual were entered as conditions in a diagnosis‐by‐condition factorial design (Achim and Lepage, 2005). An independent sample t‐test was used to compare the SZ and HC groups during the baseline time point. To compare the SZ group before and after 1‐week of medication, a paired‐sample t‐test was performed. Whole‐brain results were corrected for multiple comparisons using a cluster‐level false discovery rate (FDR) correction, P < 0.05 (Chumbley and Friston, 2009).
Seed Region Selection
We chose to investigate 11 ROIs that are part of the memory network (5 bilateral and 1 central): bilateral inferior frontal gyrus (IFG) centered at MNI coordinates, ±35, 20, −5; medial frontal gyrus (medial FG), ±13, 39, 32; middle frontal gyrus (MFG), ±31, 6, 47; posterior hippocampus, ±30, −30, −10; superior temporal gyrus (STG), ±45, −53, 18; and central precuneus, 0, −65, 27 (Fig. 1 ). The locations for all seeds were defined based on four criteria as follows: (1) seeds must be part of the memory network reported in the literature (Achim and Lepage, 2005; Spaniol, et al., 2009), (2) seeds must lie within the functional activation generated by our retrieval task, (3) the seed area must be activated in each subject, and (4) the coordinates of the seed was chosen as the locations of brain regions within the memory network reported in the literature, which also satisfied #2 and #3 above.
Figure 1.
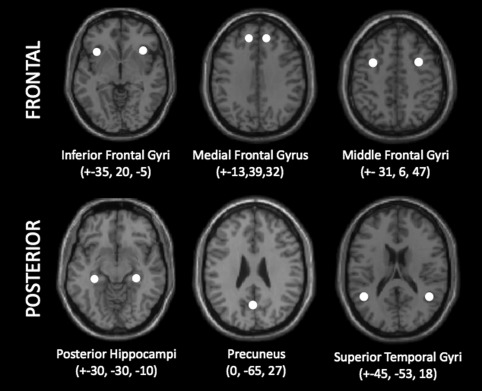
Locations of the 11 spherical ROIs used for effective connectivity analyses, overlaid on a single subject T1 anatomic image in the axial plane. All spheres have a radius of 5 mm and coordinates are x, y, and z in MNI space and are mirrored on the left and right hemisphere except for the precuneus.
Effective Connectivity Terminology
The following terminology was adopted from the field of functional and effective connectivity (Rubinov and Sporns, 2010). The term node refers to the 11 ROIs which made up our memory network (Fig. 1). The terms paths and edges indicate the unidirectional projections of information flow from one node to another. All the paths and edges shown in figures are significant when considering our contrast of interest (ROC > RNC) and have greater connectivity than 0. The degree of a node refers to the sum of the in‐degree and out‐degree, which is the sum of the number of paths the node has projecting from or projecting to itself. The subset of nodes that have the highest degree are referred to as hubs.
Effective Connectivity Model
Directional causal influence from time series A to time series B can be inferred if past values of time series A help predict the present and future values of the time series B (Granger, 1969). Based on this principle of Granger causality, many earlier studies have used MVAR models to obtain the predictive relationship between time series from different regions of the brain (Abler, et al., 2006; Deshpande and Hu, 2012a; Deshpande, et al., 2008, 2009, 2010aa, 2011, 2012bb; Hampstead, et al., 2011; Krueger, et al., 2011; Lacey, et al., 2011; Preusse, et al., 2011; Roebroeck, et al., 2005; Sathian, et al., 2011; Strenziok, et al., 2011). However, other reports have shown that Granger causal metrics obtained from raw fMRI time series could be confounded due to the variability of the hemodynamic response (David, et al., 2008; Deshpande, et al., 2010bb). Subsequently, hemodynamic deconvolution has been proposed as a preprocessing step before Granger causality analysis (Sathian, et al., 2013). Therefore, in this study, to obtain accurate directional connectivity information, the effect of hemodynamic response (HRF) was removed by blind hemodynamic deconvolution (Havlicek, et al., 2011). Hemodynamic deconvolution removes the intersubject and inter‐regional variability of the HRF (Handwerker, et al., 2004) as well as its smoothing effect, thus increasing the effective resolution of the signal. By applying hemodynamic deconvolution, the underlying neuronal variables were obtained, which were then input into a dynamic MVAR model to obtain condition‐specific connectivity values.
Let b fMRI time series be represented as X(t) = [x 1 (t) x 2 (t) … xb(t)]. A dynamic state‐space model can be described as follows.
Where n, u, p are the hidden neuronal state variables, the exogenous input, and the HRF parameter variables, respectively. The function that links the current neuronal state to the previous neuronal states is given by Δ. The subscript τ and superscript b indicate continuous time and number of time series in the model, respectively. Q, L, and M are zero mean Gaussian state noise vectors. The observation equation, which links the state to observed variables (i.e., fMRI time series), is as follows.
Here Ψ represents the measurement function that links the state variables to the measurement variables, and t and g represent discrete time and measurement noise, respectively. The inputs to the model are exogenous inputs u (experimental boxcar function) and fMRI time series xb(t). The hidden neuronal state variables and HRF parameters can be jointly estimated very efficiently by the cubature Kalman filter (Havlicek, et al., 2011). In addition to this, the neuronal variables can be successfully estimated at an effective temporal resolution up to 10 times smaller than TR using smaller step size in the estimation. The neuronal state variables nb(t) were input into the MVAR as follows
Where ϑ is the order of the model determined by the Akaike/Bayesian information criterion (Deshpande, et al., 2009), α are the model coefficients, and ξ is the model error. Note that both instantaneous influences and causal influences between the time series have been included in the model, which are represented by α(0) and α (k), k=1 … ϑ, respectively. By including both these terms in a single model, the effect of instantaneous correlation on causality was minimized (Deshpande, et al., 2010cc). A dynamic MVAR model was obtained by varying the model coefficients (α) as a function of time as shown below
Using the algorithm proposed by Arnold et al (Arnold, et al., 1998) the model coefficients αij(k,t) were taken as the state vector of a Kalman filter and were adaptively estimated. The Dynamic Granger causality was then obtained as follows
Effective Connectivity Analysis
For all the participants, mean time series were extracted from the 11 ROIs. A boxcar function was derived such that it had a value of 1 from the time the subject saw the word to the time he/she pressed the button (Supporting Information Fig. 1) and was used as the exogenous input to the deconvolution model along with normalized fMRI time series. The outputs from this model were the hidden neuronal variables, which were then input into the dynamic Granger causality model to obtain time‐varying connectivity metrics for each of the HC and SZ (both before and after medication). The causal connectivity values obtained were then populated into different samples corresponding to ROC and RNC conditions for HC and SZ (before and after medication) groups. Separate t‐tests were performed between these samples in each of the following groups: HC, SZ before medication, and SZ after medication. Only the paths that were significantly greater (P < 0.05, FDR corrected) in the ROC condition as compared to RNC condition were considered for further statistical comparisons. This ROC – RNC contrast was done to analyze functional activation associated with correct retrieval of successfully encoded information (ROC) and to remove factors of no interest such as reading words and button presses (Fig. 2).
Figure 2.
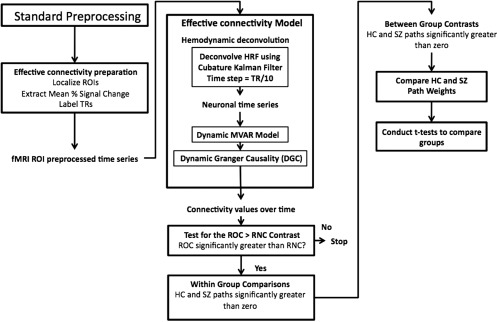
Schematic of steps used to analyze effective connectivity data. Shown are both the preprocessing steps and the statistical methods used to generate within‐ and between‐group circle plots. Abbreviations: DARTEL, diffeomorphic anatomical registration through exponentiated lie algebra; HC, Healthy Control; HRF, hemodynamic response function; MVAR, multivariate autoregressive; RNC, retrieve new correct; ROC, retrieve old correct; ROI, region of interest; SPM, statistical parametric mapping; SZ, Schizophrenia; TR, repetition time.
For the paths identified above, we performed 2 two‐sample t‐tests and 5 one‐sample t‐tests using the samples derived from ROC and RNC connectivity values from the different participant groups (HC, SZ before medication, and SZ after medication). The 2 two‐sample t‐tests were done to obtain the difference in connectivity between the following groups: (1) HC and SZ before medication and (2) SZ before medication and SZ after medication.
The 5 one sample t‐tests were performed to obtain the paths that were significantly greater than zero in the following groups: (1) HC, n = 20; (2) a subset of the larger HC group n=16, which was done to compare to the SZ group with data before and after medication; (3) SZ before‐medication, n=21; (4) a subsample of the larger SZ group that had data before medication, n=16; and (5) SZ after medication, n=16.
Effective Connectivity–Accounting for Differences in the Number of Memory Trials
To control for task performance, we only considered correct responses during the ROC and RNC trials (Hutcheson, et al., 2012). Despite this approach, the HC group had a significantly greater number of correct trials (ROC and RNC) compared to the SZ group. Also, unmedicated SZ had a significantly greater number of correct trials than medicated SZ. To account for this, Welch's two‐sample t‐tests were utilized for between‐group analyses (P < 0.05, FDR corrected). Welch's two sample t‐tests are robust to unequal sample size and unequal variance (Satterthwaite, 1946).
However, Welch's t‐test is not appropriate for one‐sample t‐tests. Therefore, for the HC group, which had larger number of TRs labeled as trials of interest (e.g. ROC and RNC), a resampling procedure was used such that the sample size of the HC group was made comparable to the smaller sample size in the SZ group (Supporting Information Fig. 4). This resampling procedure entailed randomly down‐sampling the connectivity distribution of the HC group 1,000 times, and in each of these 1000 instances, using a one sample t‐test to test whether the resulting connectivity distribution of smaller size for each path was significantly greater than zero. This resulted in a distribution of 1000 t‐values for each path, which was then tested, using a one sample one‐sided t‐test, for mean greater than zero. Paths which passed this test were deemed to be significant within the HC group. For the schizophrenia samples with the lowest number of ROC and RNC [Baseline SZ (n = 21) and medicated SZ (n = 16), down sampling was unnecessary and a conventional one sample t‐test was performed to obtain paths that were significantly (P < 0.05, FDR corrected) greater than zero.
Leave‐One‐Out Resampling of Unmedicated Schizophrenia Group
A leave‐one‐out resampling method was used to assess the consistency of the paths identified in the unmedicated schizophrenia group (n = 21). To perform this resampling 21 separate samples of size n−1 (e.g., 21 – 1 = 20) were generated and the paths that were significant in a proportion of these 21 separate samples were then plotted in a summary figure (Supporting Information Fig. 5).
Correlations Using Effective Connectivity Path Weights
To explore the association between the length of illness, BPRS subscores, RBANS total scores, medication dosages, and effective connectivity, correlations were performed. We sought to reduce the number of correlations by restricting our correlations to fronto‐temporal paths connecting the bilateral hippocampus and medial frontal gyri.
Displaying Effective Connectivity Networks
An open source software, Gephi (Version 0.8.2 beta), was used to display the memory network (Bastian, et al., 2009; https://gephi.org/users/download/). The frontal brain regions were plotted in red and the temporal‐parietal seeds were plotted in blue. The hierarchical mode was used to plot the two layers and show all connections between them. For each connection, its color correspond to which layer it originated from (e.g., frontal‐ blue and posterior‐ red), and the sizes of nodes and text labels were associated with the node's degree. The degree was set to total degree, which is the total number of connections projecting to or leaving a node.
RESULTS
Demographics and Behavioral Performance
HC and SZ were not significantly different on age, sex, or parental occupation (Table 1). At baseline, SZ performed significantly worse compared to HC on all behavioral measures in the episodic memory task (Table 2). In SZ, no improvement in performance was observed after 1 week (Table 3). The demographic data on the SZ subsample, which had imaging data at the baseline and 1‐week time point, and its matched HC sample (n = 16) are in the Supporting Information Table 1.
Table 2.
Memory task performance in HC and SZa
| HC (n = 20) | Baseline SZ (n = 21) | |
|---|---|---|
| Retrieve | ||
| Hit Rate, HRb | 0.81 ± 0.14h | 0.65 ± 0.19h |
| Percent Correctc | 0.86 ± 0.09h | 0.72 ± 0.11h |
| False Alarm Rate, FARd | 0.19 ± 0.14h | 0.33 ± 0.19h |
| Pr Indexe | 0.64 ± 0.25h | 0.31 ± 0.35h |
| Response Bias, Brf | 0.50 ± 0.01g | 0.49 ± 0.02g |
HC, healthy control; RT, Reaction Time; SZ, schizophrenia.
Mean ± SD unless indicated otherwise.
Retrieve hit rate, HR = [number of correctly identified old words(Hits)]/30.
Retrieve percentage correct = (hits + correct rejections)/60.
False alarm rate, FAR = number false alarms/30.
Index of discriminability, Pr = (HR–FAR).
Response bias, Br = [FAR/(1–Pr)].
P < 0.05 corrected significant difference between HC and baseline SZ in independent samples t‐test.
P < 0.005 significant difference between HC and baseline SZ in independent samples t‐test.
Table 3.
Memory task performance‐repeated measuresa
| Baseline SZ (n = 16) | 1‐week SZ (n = 16) | |
|---|---|---|
| Retrieve | ||
| Hit Rate, HRb | 0.65 ± 0.05 | 0.60 ± 0.07 |
| Percent Correctc | 0.75 ± 0.02 | 0.71 ± 0.04 |
| False Alarm Rate, FARd | 0.33 ± 0.05 | 0.40 ± 0.07 |
| Pr Indexe | 0.32 ± 0.10 | 0.21 ± 0.13 |
| Response Bias, Brf | 0.49 ± 0.01 | 0.50 ± 0.00 |
HC, healthy control; RT, Reaction Time; SZ, schizophrenia.
Mean ± Standard error unless indicated otherwise.
Retrieve hit rate, HR = [number of correctly identified old words(Hits)]/30.
Retrieve percentage correct = (hits + correct rejections)/60.
False alarm rate, FAR = number false alarms/30.
Index of discriminability, Pr = (HR–FAR).
Response bias, Br = [FAR/(1–Pr)].
fMRI Activation Analysis
In the between‐group comparison at baseline, SZ showed significantly increased BOLD signal in the middle cingulate gyrus during ROC > RNC (Supporting Information Table 2 and Supporting Information Fig. 2). In the SZ over time comparison, we found that SZ before medication showed significantly higher BOLD signal during ROC > RNC in regions including the precuneus, MFG, postcentral gyrus, superior parietal lobule, angular gyrus, and the caudate (Supporting Information Table III and Supporting Information Fig. 3). The within‐group results for HC, unmedicated SZ, and medicated SZ can be found in the Supplement (Supporting Information Tables IV, V, and VI, respectively).
Effective Connectivity Results in HC and Unmedicated SZ
Both HC and SZ groups showed paths whose connectivity was significantly greater during the ROC as compared to the RNC in within‐group comparisons (Fig. 3) (Supporting Information Table VII, VIII). The HC group (n = 20) had 25 significant paths and within this graph, the two hub regions were both in posterior regions‐ the right hippocampus (11 connections) and the right STG (10 connections). The right hippocampus sent projections to every other node in the network but received projections only from the right STG. The right STG sent nine projections to all nodes except the right IFG and received projections originating from the right hippocampus. Overall, there were 3 projections from frontal to posterior nodes and 11 from posterior to frontal nodes.
Figure 3.
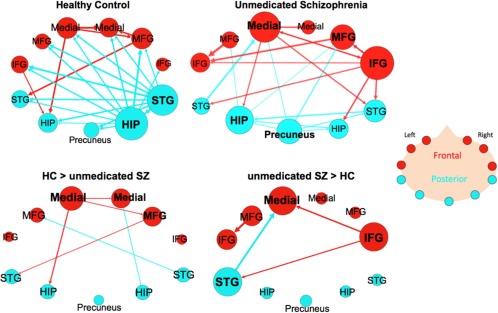
Effective connectivity paths among the 11 ROI within the memory network in Healthy Controls (HC) and unmedicated patients with schizophrenia (SZ). The top row has within‐group results for the unmedicated SZ group (n = 21) and matched HC group (n = 20). The bottom row has the between‐group results showing paths that were significantly greater in HC than unmedicated SZ (HC > SZ) or paths that were significantly greater in SZ compared to HC (SZ > HC). Frontal brain regions are depicted in red and the posterior brain regions in blue. The sizes of the ROI circle and its label correspond to that region's degree (number of in and out paths). Paths with greater strength (t values) are depicted as having thicker lines‐ t values range: 3.35–7.31. ROIs are arranged as if looking on top of head from above with left on left and right on right. Abbreviations: HIP, posterior hippocampus; IFG, inferior frontal gyrus; Medial, medial frontal gyrus; MFG, middle frontal gyrus; STG, superior temporal gyrus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The unmedicated SZ group (n = 21) had 23 significant paths that made up the graph (Fig. 3) (Supporting Information Table VIII); the two hub nodes with the largest degree were the right IFG and left medial FG. The right IFG sent out six projections to both frontal and posterior nodes and received projections from the left medial FG and right MFG. The left medial FG was a source for four paths, which projected out to frontal (right medial FG and right IFG) and posterior nodes (left hippocampus and right STG), and was the target for three paths, which originated from both frontal and posterior nodes. The right hippocampus and right STG, which were the hubs in HC, were of low degree‐ 3 and 4, respectively in the SZ group. Within this graph there were an equal number of projections from frontal to posterior nodes and vice versa (five connections).
Between‐group comparative analysis of HC and unmedicated SZ showed that HC has six paths that had significantly increased connectivity during ROC > RNC compared to unmedicated SZ (Fig. 3) (Supporting Information Table XII). Two of the paths were among regions in the frontal nodes (left medial FG → right medial FG and left medial FG → right MFG). Two of these paths were frontal to temporal projections (Left medial FG → left hippocampus and right MFG → left STG) and two were posterior to frontal projections (right hippocampus → right medial FG and right STG → left MFG). Unmedicated SZ showed four paths with significantly increased connectivity compared to HC group.
Consistency of Paths in the Unmedicated Schizophrenia Group Measured Using a Leave‐One‐Out Resampling Technique
Many of the paths present in the original unmedicated schizophrenia group (Fig. 3) were also present when the leave‐one‐out resampling procedure was performed (Fig. 4 and Supporting Information Fig. 4) (Supporting Information Table VIII).Using the most stringent criteria, 9 paths were found to be present in all 21 subsamples. Four of these paths originated from the right IFG, three originated from the precuneus, one originated from the left hippocampus, and one originated from the left medial FG. In 18 out of 21 samples, a total of 12 paths were present‐ the three additional paths were the left MFG → left IFG, left hippocampus → left MFG, and left medial FG → right STG. By measuring approximately half of the subsamples, 10 out of 21, we observed a pattern of connectivity nearly identical to the original result (see Fig. 3 ). The only path present in this plot that was not present in the original plot is the projection from the precuneus → right STG.
Figure 4.
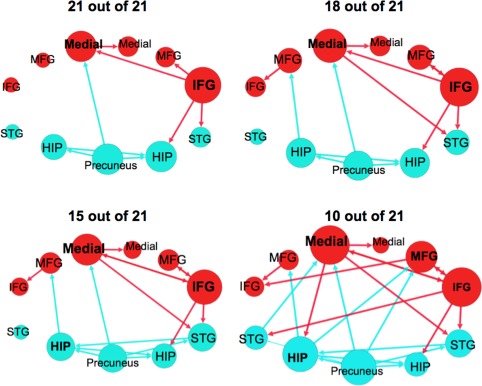
Effective connectivity paths among the 11 regions measured using a leave‐one‐out resampling technique within the sample of unmedicated participants with schizophrenia (n = 21). The plots show paths that were present in a portion of the 21 leave‐one‐out samples. Frontal brain regions are depicted in red and posterior brain regions in blue. The size of the Region of Interest (ROI) circle and its label correspond to that region's degree (sum of the number of in and out paths). Paths with greater strength (t values) are depicted as having thicker lines‐ t values range 7.92–26.67. ROIs are arranged as if looking on top of head from above with left on left and right on right. Abbreviations are as follows: HIP, posterior hippocampus; IFG, inferior frontal gyrus; Medial, medial frontal gyrus; MFG, middle frontal gyrus; STG, superior temporal gyrus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Effective Connectivity Results in Participants with Schizophrenia Before and After 1‐week of Medication
The subsample of the larger unmedicated SZ group (n = 16) had 19 edges that made up its connectivity graph (Fig. 5) (Supporting Information Table IX). The three regions with the largest degree were the right IFG (six connections), the left medial FG (five connections), and right MFG (five connections). The right IFG sent three projections to frontal regions (right MFG, left medial FG, left IFG) and two projections to temporal regions (right hippocampus, and right STG) and received projections from the right middle FG. There were more projections from frontal to posterior nodes (six connections) than in the opposite direction (three connections).
Figure 5.
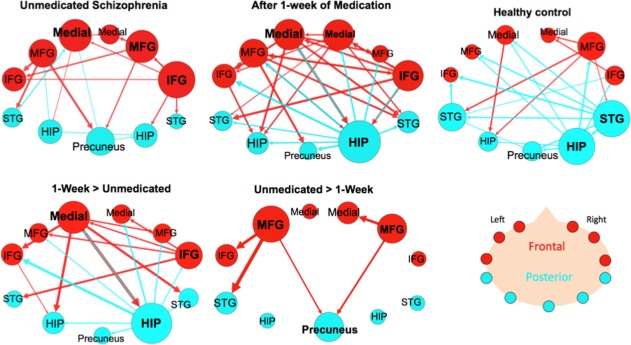
Effective connectivity paths among the 11 regions measured within a sample of patients with schizophrenia (SZ; n = 16) before and after medication and Healthy Controls (HC; n = 16). The top row shows the withingroup results for SZ before and after medication and a matched HC subsample. The bottom row shows paths that were significantly greater in SZ after 1 week of medication compared to unmedicated SZ (Medicated SZ > Unmedicated SZ) and paths that were significantly greater before medication (Unmedicated SZ > Medicated SZ). Frontal brain regions are depicted in red and the posterior brain regions in blue. The size of the Region of Interest (ROI) circle and its label correspond to that region's degree (number of in and out paths). Paths with greater strength (t values) are depicted as having thicker lines‐ t value range 3.10–19.09. ROIs are arranged as if looking on top of head from above with left on left and right on right. Abbreviations: HIP, posterior hippocampus; IFG, inferior frontal gyrus; Medial, medial frontal gyrus; MFG, middle frontal gyrus; STG, superior temporal gyrus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
After 1‐week of medication, the SZ group's (n = 16) connectivity graph consisted of 32 edges. The ROI with the highest degree was the right hippocampus (13 connections), sending projections to every other node (10 outward projections) and only acting as the target for three frontal nodes (left MFG, left medial FG, and right IFG). The three nodes with the second highest degree were all in frontal areas‐ left medial FG, right medial FG, and right IFG (8 connections) (Fig. 5) (Supporting Information Table X). The medicated SZ group exhibited more connections from frontal to posterior nodes (12 connections) than from posterior to frontal nodes (6 connections).
A matched subsample of the larger HC group (n = 16) (Supporting Information Table XI) is also shown here to compare with the smaller SZ group (n = 16). This HC subsample graph had 21 edges, and its two nodes with the highest degree remained the right hippocampus (seven connections) and the right STG (six connections) (Figs. 3 and 4). Both the right hippocampus and STG acted mainly as sources by sending projections (six outward projections each) to both frontal and posterior regions. The right hippocampus only received a projection from the right STG, and the right STG only received a projection from the right MFG. Also, following the same pattern as the larger HC group, this group had more connections that projected from the posterior to frontal network (eight connections) than from the frontal to posterior network (four connections).
Repeated measures analysis of the SZ group showed that unmedicated SZ had five paths with significantly greater connectivity than medicated SZ (Fig. 5) (Supporting Information Table XIII). Two of these connections were among frontal nodes (left MFG → left IFG and right MFG → right medial FG) and three were frontal to posterior projections. After 1‐week of antipsychotic medication, the SZ group showed 19 paths with significantly greater connectivity than unmedicated SZ (Fig. 5) (Supporting Information Table XIII). Two hubs in this graph were the right hippocampus (10 connections) and left medial FG (7).
Effective Connectivity Correlation Results
Correlations between length of illness, BPRS subscores, RBANS total scores, medication dosages, and effective connectivity path strengths in the unmedicated and medicated shcizophrenia group between the bilateral hippocampi and medial frontal gyri did not yield significant findings.
DISCUSSION
To our knowledge, this is the first study to use effective connectivity accessed with MVAR Granger causality to investigate episodic memory abnormalities in unmedicated SZ and to examine the effect of antipsychotic medication on effective connectivity patterns. We observed that unmedicated SZ showed altered connectivity within the episodic memory network, including reduced right hippocampal to right medial frontal effective connectivity, compared to HC. After 1‐week of antipsychotic treatment, connectivity more closely resembled the patterns observed in HC, including increased effective connectivity from the right hippocampus to all frontal regions.
In HC, the two major nodes of connectivity were the right hippocampus and the right STG, which is consistent with prior work implicating these brain regions in episodic memory processes (Spaniol, et al., 2009). The hippocampus is thought to mediate the relational binding of events during encoding as well as memory trace reactivation during retrieval (Miller and D'Esposito, 2012; Tamminga, et al., 2010). Given the nature of our task (word encoding and retrieval) (Hutcheson, et al., 2012), the identification of the STG as a major node is consistent with its role in interpreting and monitoring language (Sun, et al., 2009). In HC, we observed a functional connection between the left and right hippocampus that could be supported by the commissural fibers connecting the two hippocampi (Gloor, et al., 1993). The finding that the nodes were located in the right hippocampus and right STG is perhaps surprising in view of previous work indicating right and left hippocampal involvement for spatial and for context‐dependent memory, respectively (Burgess, et al., 2002; Frisk and Milner, 1990). However, as previously noted, the hippocampi are structurally connected, and findings that the left hippocampus can be activated in navigation tasks suggests that the hippocampal lateralization is not definitive (Burgess, et al., 2002). In HC, the majority of connections were directed from temporal to frontal nodes with only few connecting frontal to temporal. Regions of the prefrontal cortex are important for successful episodic memory by allowing for a strategic search method to be used and memories' source to be verified (Simons and Spiers, 2003; Spaniol, et al., 2009). Our data are consistent with a significant role of the hippocampus in orchestrating fronto‐temporal connectivity. A similar “bottom‐up” direction of influence has been observed between the hippocampus and the prefrontal cortex during the construction phase of autobiographical memory retrieval (McCormick, et al., 2013).
In contrast, in unmedicated SZ neither the hippocampus nor the STG were identified as major nodes of connectivity; instead multiple nodes were observed in frontal and temporal regions. In addition, the temporal nodes (hippocampus and STG) showed altered connections to frontal nodes: on the right, there was a decreased temporal to frontal connectivity; on the left the pattern was more complex with decreased left medial FG to left hippocampus and right MFG to left STG, but increased left STG to left MFG and right IFG to left STG. Importantly, a similar pattern of decreased effective connectivity from the right hippocampus to the right IFG has been reported in both first‐episode and at risk mental state (ARMS) for psychosis subjects during a delayed matching to sample task (Benetti, et al., 2009). Likewise, altered patterns of coupling between the STG and MFG have been observed in first episode and ARMS subjects (Crossley, et al., 2009). Altered patterns of connectivity (of unspecified direction) between temporal and frontal regions have been observed both in task (Crossley, et al., 2009; Meyer‐Lindenberg, et al., 2005; Wolf, et al., 2009) and during resting‐state analyses in first‐episode (Zhou, et al., 2007) and chronic patients (Zhou, et al., 2008). The pattern of overall altered connectivity between hippocampal and frontal regions is consistent with a recent mouse model of schizophrenia that is characterized by impaired frontal‐hippocampal connectivity during memory performance (Sigurdsson, et al., 2010). Furthermore, healthy individuals, who were carriers of a polymorphism of a gene linked to increased risk for the disease, exhibited alterations in fronto‐temporal coupling (Esslinger, et al., 2009). When considering structural studies, one should note a recent study using SEM, which found significant decreases in the path connecting the entorhinal cortex to the prefrontal cortex in schizophrenia (Corradi‐Dell'Acqua, et al., 2012). In addition, another structural study found that structural abnormalities in a cortical network including medial temporal and frontal cortices, as well as putamen increase the likelihood of experiencing hallucinations (van Tol et al., 2014). In summary our findings along with previous studies underscore the importance of fronto‐temporal dysconnectivity in schizophrenia.
After 1 week of treatment, the hippocampus was restored as the main network hub together with increased connections toward all frontal nodes; frontal (especially left medial) to temporal connections were also significantly increased. Altered fronto‐temporal connectivity could originate from known abnormal hippocampal function in schizophrenia (Medoff, et al., 2001; Schobel, et al., 2009; Tregellas, et al., 2014), and partial restoration of connectivity postmedication may be linked to normalized hippocampal function with treatment. Both hippocampal rCBF (Medoff, et al., 2001) and hippocampal glutamate levels (Kraguljac, et al., 2013bb) are elevated when patients are unmedicated but not when they are medicated (Hutcheson, et al., 2012; Medoff, et al., 2001). Because elevated glutamate levels might result from gamma‐aminobutyric acid interneuron hypofunction (Olney and Farber, 1995) and hippocampal interneurons generate oscillations in the gamma frequency ranges that are thought to synchronize brain activation (Symond, et al., 2005), their dysfunction could affect functional/effective connectivity, including temporal‐frontal connectivity. Schizophrenia is currently conceptualized as a disorder of altered connectivity (Fornito, et al., 2012; Skudlarski, et al., 2010) with symptoms such as hallucinations and delusions emerging as a possible result (Symond, et al., 2005). Changes in functional connectivity with treatment could be a necessary intermediary step (Hadley, et al., 2014; Keshavan, et al., 2008) to symptomatic improvement. In our prior work we found that, after 1 week of treatment, both hippocampal rCBF decrease (Lahti, et al., 2009) and changes in the functional connectivity between the frontal cortex and hippocampus were predictive of treatment response to medication (Bolding, et al., 2012). Animal studies have shown that antipsychotics can be used to restore cortical synchronization and functional connectivity after disruption with hallucinogenic agents (Celada, et al., 2008; Kargieman, et al., 2012). It is also possible that functional connectivity changes are the result of changes in myelination of white matter tracts that could affect the synchronicity of neural transmission. The effect of medications on fractional anisotropy (FA), a measure of white matter tract integrity, is still questionable, with studies in first episode reporting reductions in FA (Szeszko, et al., 2014; Wang, et al., 2013) and one study reporting increase in FA (Reis Marques, et al., 2014) with treatment. Animal data suggest that antipsychotics upregulate dopamine D2 receptors (Lidow and Goldman‐Rakic, 1994) and nerve growth factor (NGF) (Angelucci, et al., 2005) in major network hubs within the frontal, parietal, temporal, and occipital lobes. Specifically, work in NGF and brain‐derived neurotrophic factor (BDNF) suggests that administration of atypical antipsychotics, such as the one used in this study, can cause increases in NGF and decreases in BDNF in the hippocampus. Both of these neurotrophins are involved in neuronal survival and synapse formation and could lead to altered connectivity within the temporal lobe. These mechanisms suggest ways by which antipsychotic medication could affect functional connectivity within neural networks.
Interestingly, after 1 week of treatment, decreased connectivity was observed between bilateral MFG and precuneus nodes. The precuneus has known implications for episodic memory retrieval and shows connectivity with the prefrontal cortex and posterior cingulate cortex during the performance of memory tasks (Cavanna and Trimble, 2006). It is possible that increased precuneus/frontal connectivity when patients were untreated represents a compensatory mechanism.
Although, SZ showed increased connectivity within the memory network after 1‐week and significant improvements in their symptom severity over 6 weeks, they did not show significant improvements in their memory scores. Although it is generally believed that antipsychotics do not improve cognition in schizophrenia (Tandon, et al., 2010), studies that included large numbers of subjects such as CATIE (Keefe, et al., 2007) and EUFEST (Davidson, et al., 2009) have shown that treatment with a wide variety of both first and second generation antipsychotics is associated with moderate improvements on cognitive tests. It will be important to establish whether the changes in connectivity patterns observed in this study are associated with improvement in memory processes, as they could provide biomarkers potentially leading to the identification of novel agents for the treatment of cognition in schizophrenia.
STRENGTHS AND LIMITATIONS
There are a number of strengths to the present study. First, we analyzed effective connectivity within the memory network using an episodic memory task in unmedicated SZ, a population that is difficult to recruit. Therefore, this study will be a significant addition to the body of literature. Second, we chose an exploratory approach by using a MVAR Granger causality model, which does not require a priori specification of the underlying network architecture and allows for data‐driven inferences. Third, since effective connectivity was obtained from latent neural signals estimated from blind deconvolution of fMRI, our results are unlikely to be confounded by hemodynamic variability. A limitation of this study is that the patient group consisted of a mixed group of previously treated and medication‐naïve patients. Also, the addition of a placebo group or scanning a control population at both the baseline and after 1‐weeks of antipsychotics would more clearly show that the increase in connectivity during the memory task following 1‐week of medication was a effect of medication and not repeated exposure to the memory task. In our future longitudinal work we will implement such approaches. Finally, future work in analyzing memory networks with effective connectivity would benefit from the multimodal integration of structural connectivity metrics such as diffusion tensor imaging (Skudlarski, et al., 2010).
CONCLUSION
In conclusion, using Granger causality methods, we found altered connectivity patterns within an episodic memory network, including reduced right hippocampal to medial frontal effective connectivity in unmedicated patients with schizophrenia and the reinstatement of the right hippocampus as a major node of this network after 1 week of antipsychotic treatment. These results support the notion that memory disruption in schizophrenia might originate from hippocampal dysfunction and that medication restores some aspect of fronto‐temporal dysconnectivity. Patterns of fronto‐temporal connectivity could provide valuable biomarkers to identify new treatment for the symptoms of schizophrenia, including memory deficits.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
Medication for this study was donated by Janssen Pharmaceuticals. The authors want to thank all the volunteers with schizophrenia who took part in this project as well as the staff at the UAB Emergency Room Department. In addition, the authors thank Dr. Edward Cook and Dr. Silvia Mrug for statistical assistance. Dr. Kristina Visscher and Dr. David Clark also helped with data analysis suggestions. Muriah Wheelock helped with data visualization and interpretation of effective connectivity theory. Dr. Mark Bolding helped with data management and data interpretation. The authors appreciate comments on this manuscript from Dr. Nina Kraguljac. Nathan Hutcheson analyzed and interpreted the study data and prepared the written paper. Karthik R. Sreenivasan assisted Gopikrishna Deshpande, PhD estimate effective connectivity between relevant brain regions and helped prepare the written paper. Gopikrishna Deshpande developed the effective connectivity multivariate, autoregressive model optimized for event‐related fMRI, which was used to analyze the data and also helped prepare the written paper. Dr. Meredith A. Reid designed Matlab code that allowed for the labeling of subjects responses during the retrieval period, helped with data collection, and helped prepare the written paper. Jennifer Hadley helped with data collection, data preprocessing, and data interpretation. David White was involved in subject recruitment, data collection, and data interpretation. Lawrence Ver Hoef, MD was responsible for reviewing all MRI images for incidental findings. As the P.I. on this project, Adrienne Lahti, MD designed the study and supervised subject recruitment and data collection, analysis, and interpretation.
Financial Disclosures: Over the past four years, ACL has received research funds from the National Institute of Mental Health (NIMH) (R01 MH081014 and R01 MH102951), and an investigator initiated grant from Pfizer. LVH works as a neurology subspecialist in epilepsy (25% effort), including formal interpretation of MRI scans in his clinical practice (5% effort), and is funded by NIH/NIBIB K23EB008452. All other authors reported no biomedical financial interests.
Conflict of interest: All authors declare that they have no conflicts of interest.
REFERENCES
- Abler B, Roebroeck A, Goebel R, Höse A, Schönfeldt‐Lecuona C, Hole G, Walter H (2006): Investigating directed influences between activated brain areas in a motor‐response task using fMRI. Magn Reson Imaging 24:181–185. [DOI] [PubMed] [Google Scholar]
- Achim AM, Lepage M (2005): Episodic memory‐related activation in schizophrenia: Meta‐analysis. Br J Psychiatry 187:500–509. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EHF, Kahn RS (1999): Memory impairment in schizophrenia: A meta‐analysis. Am J Psychiatry 156:1358–1366. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Aloe L, Iannitelli A, Gruber SHM, Mathé AA (2005): Effect of chronic olanzapine treatment on nerve growth factor and brain‐derived neurotrophic factor in the rat brain. Eur Neuropsychopharmacol 15:311–317. [DOI] [PubMed] [Google Scholar]
- Arnold M, Milner XHR, Witte H, Bauer R, Braun C (1998): Adaptive AR modeling of nonstationary time series by means of Kalman filtering. IEEE Trans Biomed Eng 45:553–562. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Bastian M, Heymann S, Jacomy M (2009): Gephi an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media.
- Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P (2009): Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain 132:2426–2436. [DOI] [PubMed] [Google Scholar]
- Bolding MS, White DM, Hadley JA, Weiler M, Holcomb HH, Lahti AC (2012): Antipsychotic drugs alter functional connectivity between medial frontal cortex, hippocampus, and nucleus accumbens as measured by H215O PET. Front Psychiatry 3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Coull JT, Friston KJ (1999): The predictive value of changes in effective connectivity for human learning. Science 283:1538–1541. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J (2002): The human hippocampus and spatial and episodic memory. Neuron 35:625–641. [DOI] [PubMed] [Google Scholar]
- Carpenter William T Jr, Gold JM, Lahti AC, et al. (2000): Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry 57:533–538. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Díaz‐Mataix L, Artigas F (2008): The hallucinogen DOI reduces low‐frequency oscillations in rat prefrontal cortex: Reversal by antipsychotic drugs. Biol Psychiatry 64:392–400. [DOI] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ (2009): False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage 44:62–70. [DOI] [PubMed] [Google Scholar]
- Corradi‐Dell'Acqua C, Tomelleri L, Bellani M, Rambaldelli G, Cerini R, Pozzi‐Mucelli R, Balestrieri M, Tansella M, Brambilla P (2012): Thalamic‐insular dysconnectivity in schizophrenia: Evidence from structural equation modeling. Hum Brain Mapp 33:740–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Fusar‐Poli P, Broome MR, Matthiasson P, Johns LC, Bramon E, Valmaggia L, Williams SCR, McGuire PK (2009): Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first‐episode psychosis. Hum Brain Mapp 30:4129–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A (2008): Identifying neural drivers with functional MRI: An electrophysiological validation. PLoS Biol 6:e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker W, Keefe R, Boter H, Keet MDI, Prelipceanu D, Rybakowski J, Libiger J, Hummer M, Dollfus S, López‐Ibor J, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher‐Rössler A, Kahn R (2009): Cognitive effects of antipsychotic drugs in first‐episode schizophrenia and schizophreniform disorder: A randomized, open‐label clinical trial (EUFEST). Am J Psychiatry 166:675–682. [DOI] [PubMed] [Google Scholar]
- Deserno L, Sterzer P, Wüstenberg T, Heinz A, Schlagenhauf F (2012): Reduced prefrontal‐parietal effective connectivity and working memory deficits in schizophrenia. J Neurosci 32:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Hu X (2012a): Investigating effective brain connectivity from fMRI data: Past findings and current issues with reference to granger causality analysis. Brain Connect 2:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Hu X, Stilla R, Sathian K (2008): Effective connectivity during haptic perception: A study using Granger causality analysis of functional magnetic resonance imaging data. Neuroimage 40:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, LaConte S, James GA, Peltier S, Hu X (2009): Multivariate granger causality analysis of fMRI data. Hum Brain Mapp 30:1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Hu X, Lacey S, Stilla R, Sathian K (2010a): Object familiarity modulates effective connectivity during haptic shape perception. Neuroimage 49:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Sathian K, Hu X (2010b): Effect of hemodynamic variability on Granger causality analysis of fMRI. Neuroimage 52:884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Sathian K, Xiaoping H (2010c): Assessing and compensating for zero‐lag correlation effects in time‐lagged granger causality analysis of fMRI. IEEE Trans Biomed Eng 57:1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Santhanam P, Hu X (2011): Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage 54:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Sathian K, Hu X, Buckhalt JA (2012b): A rigorous approach for testing the constructionist hypotheses of brain function. Behav Brain Sci 35:148–149. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Libero L, Sreenivasan KR, Deshpande H, Kana RK (2013): Identification of neural connectivity signatures of autism using machine learning. Front Hum Neurosci 7:670. doi: 10.3389/fnhum.2013.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt SH, Rietschel M, Cichon S, Meyer‐Lindenberg A (2009): Neural mechanisms of a genome‐wide supported psychosis variant. Science 324:605. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET (2012): Schizophrenia, neuroimaging and connectomics. Neuroimage 62:2296–2314. [DOI] [PubMed] [Google Scholar]
- Frisk V, Milner B (1990): The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia 28:349–359. [DOI] [PubMed] [Google Scholar]
- Friston KJ (1994b): Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp 2:56–78. [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RSJ (1993): Time‐dependent changes in effective connectivity measured with PET. Hum Brain Mapp 1:69–79. [Google Scholar]
- Friston KJ, Tononi G, Reeke GN Jr, Sporns O, Edelman GM (1994a): Value‐dependent selection in the brain: Simulation in a synthetic neural model. Neuroscience 59:229–243. [DOI] [PubMed] [Google Scholar]
- Gloor P, Salanova V, Olivier A, Quesney LF (1993): The human dorsal hippocampal commissure: An anatomically identifiable and functional pathway. Brain 116:1249–1273. [DOI] [PubMed] [Google Scholar]
- Granger CWJ (1969): Investigating causal relations by econometric models and cross‐spectral methods. Econometrica 37:424–438. [Google Scholar]
- Green MF (1996): What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 153:321–330. [DOI] [PubMed] [Google Scholar]
- Hadley JA, Nenert R, Kraguljac NV, Bolding MS, White DM, Skidmore FM, Visscher KM, Lahti AC (2014): Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 39:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Deshpande G, Hu X, Moore AB, Sathian K (2011): Activation and effective connectivity changes following explicit‐memory training for face–name pairs in patients with mild cognitive impairment: A pilot study. Neurorehabil Neural Repair 25:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M (2004): Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21:1639–1651. [DOI] [PubMed] [Google Scholar]
- Havlicek M, Friston KJ, Jan J, Brazdil M, Calhoun VD (2011): Dynamic modeling of neuronal responses in fMRI using cubature Kalman filtering. Neuroimage 56:2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Goff D, Schacter DL, Savage C, Fischman A, Alpert N, Rauch S (1999): Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch Gen Psychiatry 56:1117–1123. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK (1998): Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 12:426–445. [DOI] [PubMed] [Google Scholar]
- Hutcheson NL, Reid MA, White DM, Kraguljac NV, Avsar KB, Bolding MS, Knowlton RC, den Hollander JA, Lahti AC (2012): Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophr Res 140:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A (1992): Schizophrenia manifestations, incidence and course in different cultures. A World Health Organization ten‐country study. Psychol Med Monogr Suppl 22:1–97. [DOI] [PubMed] [Google Scholar]
- Kargieman L, Riga MS, Artigas F, Celada P (2012): Clozapine reverses phencyclidine‐induced desynchronization of prefrontal cortex through a 5‐HT1A receptor‐dependent mechanism. Neuropsychopharmacology 37:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe Richard SE, Bilder RM, Davis SM, et al. (2007): Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the catie trial. Arch Gen Psychiatry 64:633–647. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Tandon R, Boutros NN, Nasrallah HA (2008): Schizophrenia, “just the facts”: What we know in 2008: Part 3: Neurobiology. Schizophr Res 106:89–107. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC (2012): Regional decoupling of N‐acetyl‐aspartate and glutamate in schizophrenia. Neuropsychopharmacology 37:2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac N, Srivastava A, Lahti A (2013a): Memory deficits in schizophrenia: A selective review of functional magnetic resonance imaging (fMRI) studies. Behav Sci 3:330–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Reid MA, Lahti AC (2013b): Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry 70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Landgraf S, van der Meer E, Deshpande G, Hu X (2011): Effective connectivity of the multiplication network: A functional MRI and multivariate granger causality mapping study. Hum Brain Mapp 32:1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey S, Hagtvedt H, Patrick VM, Anderson A, Stilla R, Deshpande G, Hu X, Sato JR, Reddy S, Sathian K (2011): Art for reward's sake: Visual art recruits the ventral striatum. Neuroimage 55:420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT Jr, McMahon R (2006): Correlations between rCBF and symptoms in two independent cohorts of drug‐free patients with schizophrenia. Neuropsychopharmacology 31:221–230. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL (2009): Modulation of limbic circuitry predicts treatment response to antipsychotic medication: A functional imaging study in schizophrenia. Neuropsychopharmacology 34:2675–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC (2002): Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51:1008–1011. [DOI] [PubMed] [Google Scholar]
- Leucht S, Davis JM, Engel RR, Kane JM, Wagenpfeil S (2007): Defining /'response/' in antipsychotic drug trials: Recommendations for the use of scale‐derived cutoffs. Neuropsychopharmacology 32:1903–1910. [DOI] [PubMed] [Google Scholar]
- Liddle PF (1987): Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med 17:49–57. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman‐Rakic PS (1994): A common action of clozapine, haloperidol, and remoxipride on D1‐ and D2‐dopaminergic receptors in the primate cerebral cortex. Proc Natl Acad Sci 91:4353–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika PK, Whitfield‐Gabrieli S, Reiss A (2007): Artifact repair for fMRI data from high motion clinical subjects. Human Brain Mapping Conference. Chicago, IL.
- McCormick C, St‐Laurent M, Ty A, Valiante TA, McAndrews MP (2013): Functional and effective hippocampal–neocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cereb Cortex. doi: 10.1093/cercor/bht324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA (2001): Probing the human hippocampus using rCBF: Contrasts in schizophrenia. Hippocampus 11:543–550. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF (2005): Regionally specific disturbance of dorsolateral prefrontal–hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62:379–386. [DOI] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M (2012): Spatial and temporal dynamics of cortical networks engaged in memory encoding and retrieval. Front Hum Neurosci 6:109. doi: 10.3389/fnhum.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York‐Cooler C, Simpson S, Harkavy‐Friedman J, Severe J, Malaspina D, Reich T (1994): Diagnostic interview for genetic studies: Rationale, unique features, and training. Arch Gen Psychiatry 51:849–859. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB (1995): GLutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52:998–1007. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962): The brief psychiatric rating scale. Psychol Rep 10:799–812. [Google Scholar]
- Preusse F, Van Der Meer E, Deshpande G, Krueger F, Wartenburger I. (2011): Fluid intelligence allows flexible recruitment of the parieto‐frontal network in analogical reasoning. Front Hum Neurosci 5:22. doi: 10.3389/fnhum.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE (2001): Effect of schizophrenia on frontotemporal activity during word encoding and recognition: A PET cerebral blood flow study. Am J Psychiatry 158:1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE (2004): Event‐related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry 161:1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J, Laird A, Ranganath C, Blumenfeld R, Gonzales S, Glahn D (2009): Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry 166:863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN (1998): The repeatable battery for the assessment of neuropsychological status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol 20:310–319. [DOI] [PubMed] [Google Scholar]
- Reis Marques T, Taylor H, Chaddock C, Dell'Acqua F, Handley R, Reinders AATS, Mondelli V, Bonaccorso S, DiForti M, Simmons A, David AS, Murray RM, Pariante CM, Kapur S, Dazzan P (2014): White matter integrity as a predictor of response to treatment in first episode psychosis. Brain 137:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R (2005): Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage 25:230–242. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Sathian K, Lacey S, Stilla R, Gibson GO, Deshpande G, Hu X, LaConte S, Glielmi C (2011): Dual pathways for haptic and visual perception of spatial and texture information. Neuroimage 57:462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Deshpande G, Stilla R (2013): Neural changes with tactile learning reflect decision‐level reweighting of perceptual readout. J Neurosci 33:5387–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite FE (1946): An approximate distribution of estimates of variance components. Biometrics 2:110. [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA (2009): Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 66:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA (2010): Impaired hippocampal‐prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464:763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ (2003): Prefrontal and medial temporal lobe interactions in long‐term memory. Nat Rev Neurosci 4:637–648. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G (2010): Brain connectivity is not only lower but different in schizophrenia: A combined anatomical and functional approach. Biol Psychiatry 68:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL (2009): Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimation. Neuropsychologia 47:1765–1779. [DOI] [PubMed] [Google Scholar]
- Strenziok M, Krueger F, Deshpande G, Lenroot RK, van der Meer E, Grafman J (2011): Fronto‐parietal regulation of media violence exposure in adolescents: A multi‐method study. Soc Cogn Affect Neurosci 6:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Maller JJ, Guo L, Fitzgerald PB (2009): Superior temporal gyrus volume change in schizophrenia: A review on region of interest volumetric studies. Brain Res Rev 61:14–32. [DOI] [PubMed] [Google Scholar]
- Symond MB, Harris AWF, Gordon E, Williams LM (2005): “Gamma Synchrony” in first‐episode schizophrenia: A disorder of temporal connectivity? Am J Psychiatry 162:459–465. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, Malhotra AK (2014): White matter changes associated with antipsychotic treatment in first‐episode psychosis. Neuropsychopharmacology 39:1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD (2010): The hippocampal formation in schizophrenia. Am J Psychiatry 167:1178–1193. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS (2009): Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res 110:1–23. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS (2010): Schizophrenia, “Just the Facts” 5. Treatment and prevention Past, present, and future. Schizophr Res 122:1–23. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, Eichman LC, Lyons E, Freedman R (2014): Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry 171:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol MJ, van der Meer L, Bruggeman R, Modinos G, Knegtering H, Aleman A (2014): Voxel‐based gray and white matter morphometry correlates of hallucination in schiozphrenia: The superior temporal gyrus does not stand alone. Neuroimage Clin 4:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL (2006): Coherent spontaneous activity identifies a hippocampal‐parietal memory network. J Neurophysiol 96:3517–3531. [DOI] [PubMed] [Google Scholar]
- Wang Q, Cheung C, Deng W, Li M, Huang C, Ma X, Wang Y, Jiang L, Sham PC, Collier DA, Gong Q, Chua SE, McAlonan GM, Li T (2013): White‐matter microstructure in previously drug‐naive patients with schizophrenia after 6 weeks of treatment. Psychol Med 43:2301–2309. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Sambataro F, Höse A, Frasch K, Schmid M, Walter H (2009): Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33:1464–1473. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F (2007): Functional dysconnectivity of the dorsolateral prefrontal cortex in first‐episode schizophrenia using resting‐state fMRI. Neurosci Lett 417:297–302. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, Yu C, Liu Z, Jiang T (2008): Altered resting‐state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res 100:120–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


