Abstract
Background:
Prenatal cocaine exposure (PCE) has been linked to child/adolescent behavior problems and substance use in several longitudinal cohort studies. It is unclear whether these effects extend into adulthood and influence young adult behavior problems and substance use and, if so, whether they are mediated by childhood and adolescent experiences.
Methods:
These data are from an ongoing longitudinal study of individuals born to women who were recruited early in pregnancy. Trimester-specific data on prenatal drug exposure were obtained. Caregivers and offspring were assessed at delivery and at 1, 3, 7, 10, 15, and 21 years postpartum. This report is from age 21, when 225 offspring (52% females; 54% African American, 46% Caucasian) reported on behavior problems, emotion regulation, and substance use.
Results:
There were significant direct associations between PCE and early initiation of marijuana, 21-year emotion regulation problems, arrest history, and Conduct Disorder. The relation between PCE and young adult internalizing behavior was mediated by adolescent mood symptoms. The association between PCE and 21-year marijuana use was mediated by early initiation of marijuana use.
Conclusions:
PCE has both direct and indirect long-term associations with young adult development. Using statistical models that considered the complex interrelationships among PCE and adult outcomes, we demonstrated that the direct effects of PCE on young adult emotion regulation problems, arrest history, and Conduct Disorder are not completely explained by earlier adolescent behavior. Moreover, the analyses suggesting mediated pathways from PCE to young adult problems identify crucial variables to target interventions for exposed children and adolescents.
Keywords: Prenatal Cocaine, Young Adult, Substance Use, Behavior Problems
1. Introduction
Emerging or young adulthood is an important and distinct developmental life stage with increased stress and demands as young adults transition into adult roles (Arnett, 2000). This developmental stage may prove particularly challenging for those with prenatal cocaine exposure (PCE) because of the child/adolescent deficits associated with PCE. In our longitudinal study of PCE, we have found persistent associations between PCE and three domains of child and adolescent outcomes that may make the transition into young adulthood difficult. First, PCE predicted externalizing behavior problems (aggression and delinquency) at ages 1, 3, 7, 10, and 15 years (Richardson et al., 2009, 2011, 2015; Richardson et al., 2013a, 2013b), a finding replicated by many others (Bada et al., 2012; Bennett et al., 2013; Delaney-Black et al., 2000; Lambert et al., 2013; Min et al., 2014a, 2016; Minnes et al., 2010). Second, PCE predicted fussy and difficult infant temperament at 1 and 3 years (Richardson et al., 2008, 2009) and mood problems including internalizing behavior problems (Richardson et al., 2009, 2011) and depressive symptoms (Richardson et al., 2013a, 2015) through age 15. Most other studies have not found effects of PCE on mood or internalizing behavior problems (Accornero et al., 2006; Bada et al., 2011; Bennett et al., 2002; Delaney-Black et al., 2000; Min et al., 2014b; Minnes et al., 2010). Third, we and others found that PCE increases risk for early initiation of alcohol and/or marijuana use (Frank et al., 2011; Min et al., 2014a; Minnes et al., 2014, 2017; Richardson et al., 2013b) and cocaine use (Delaney-Black et al., 2011). What is not known is whether PCE will be associated with these same domains in young adulthood and, if so, whether the relations will be mediated by earlier effects.
We know that adult behaviors are affected by child/adolescent behaviors in each of these three domains. First, previous research has established that the externalizing behavior problems of adult aggression and criminal behaviors are associated with difficult temperament and conduct symptoms during childhood (Fergusson et al., 2000; Moffitt et al., 1996) and with lower socioeconomic status (SES), exposure to violence and child abuse, negative life events, and earlier aggression, delinquency, and substance use (Copeland et al., 2007; Eitle and Turner, 2002; Leschied et al., 2008; Mason et al., 2010). Second, predictors of adult internalizing problems (such as depression and anxiety) and temperament include lower SES, exposure to violence and child abuse, substance use, and child/adolescent mood symptoms such as depression and anxiety (Copeland et al., 2009; Kessler et al., 2010; Klein et al., 2011; Najman et al., 2010; Reinherz et al., 2003). Third, substance use is particularly salient in emerging adulthood when levels of substance use and abuse peak and then generally decline as individuals take on adult roles such as employment and parenthood (Bachman et al., 1997; Chen and Jacobson, 2012; Jessor et al., 1991). However, for some, substance use continues to escalate and interferes with their transition into adult roles (Ellickson et al., 2004; Green et al., 2010; Oesterle et al., 2011; Schulenberg et al., 2005; Slade et al., 2008; Staff et al., 2010). Previous research has found that emerging adult substance abuse is associated with child/adolescent behavior problems (Doherty et al., 2008; Fergusson et al., 2007; Hayatbakhsh et al., 2009) as well as with early initiation of substance use (Wells et al., 2004).
To date, there have been no investigations of the association of PCE with young adult behavior problems, temperament, and substance use and no consideration of earlier behaviors that may mediate this association. The relations between PCE and child/adolescent behavior problems and the associations of child/adolescent behavior with young adult behavior problems raise the question of whether the linkage between PCE and young adult behavior problems is mediated by earlier behavior problems. Similarly, the relation between PCE and young adult substance use may be mediated through adolescent substance use. To accurately understand these relations, we must also consider that individuals with PCE are more likely to be exposed prenatally to other drugs and to maternal substance use, lower SES, and violence and/or abuse during childhood/adolescence (Alati et al., 2006; Bada et al., 2011; Baer et al., 2003; Frank et al., 1988; Lester et al., 2002; Min et al., 2017; Minnes et al., 2017; Porath and Fried, 2005; Schwab-Stone et al., 2013; Sonon et al., 2015). Additional risk factors for poor young adult outcomes, such as negative life events, poor social support, depression, and family history of alcohol/drug problems must also be considered (Stone et al., 2012; Tucker et al., 2005).
This program of research is informed by multiple theoretical approaches. Aspects of the teratologic model that have informed our research include the importance of the specific developmental period of exposure, the range of developmental domains that can be affected, and the emergence of effects later in development (Vorhees, 1989). As we evaluate developmental pathways to adult functioning, early risk factors need to be examined when investigating the relation between PCE and young adult outcomes, particularly in low-income samples (Glantz and Chambers, 2006; Stone et al., 2012). We must consider both early (distal) consequences and correlates of PCE and current (proximal) environmental risk factors as illustrated by theories of developmental transition such as the Transition Overload model and the Increased Heterogeneity model (Schulenberg et al., 2002, 2004). These models predict increased vulnerability of the PCE-exposed offspring in their transition to adulthood (Transition Overload) and an increasing divergence from their non-exposed peers (Increased Heterogeneity).
The purpose of this report is to investigate whether PCE is related to adult development and whether any relations are mediated by earlier PCE-associated deficits using data from the 21-year follow-up of individuals whose mothers were recruited early in pregnancy. Based on previous findings (cited above), we investigated the roles of: adolescent delinquency on adult externalizing problems, adolescent mood on adult temperament and internalizing problems, and adolescent substance use on adult substance use. We used prospectively collected data from childhood, adolescence, and young adulthood to understand the direct and indirect associations between PCE and adult outcomes considering covariates associated with PCE and/or the outcomes (cited above).
2. Methods
2.1. Study design
This sample was recruited from the Magee-Womens Hospital prenatal clinic from 1988 to 1992. Pregnant women, at least 18 years of age, were approached by research staff. Informed consent was obtained prior to interviewing, and 90% agreed to participate. Medical chart reviews conducted on a random sample of those who refused revealed only 5% with a history of prenatal drug use. This research was approved by the University of Pittsburgh’s Institutional Review Board. A Certificate of Confidentiality from the Department of Health and Human Services assured participants that their responses could not be subpoenaed.
During the initial interview (4th or 5th prenatal month), women were asked about cocaine/crack, alcohol, tobacco, marijuana, and other illicit drug use in the year prior to pregnancy and the first trimester. Any woman who reported any cocaine/crack use during the first trimester was enrolled. The next woman interviewed for recruitment purposes who reported no cocaine/crack use during both the year prior to pregnancy and the first trimester was also enrolled in the study. Women selected for the study (N = 320) were interviewed during the 7th prenatal month about their 2nd trimester substance use and in their hospital room after delivery about their 3rd trimester substance use. Between enrollment and delivery, there were 25 women who were not seen (e.g., moved, refused, fetal loss). Offspring were examined at birth by research staff, and follow-up assessments were conducted at 1, 3, 7, 10, 15, and 21 years postpartum. At all follow-up phases, mothers (or caregivers) were interviewed about their past-year substance use, sociodemographic and psychosocial characteristics, and psychiatric symptoms. We report here on the age 21 phase (conducted from 2009 to 2014). At this phase, 13% of the women interviewed were non-maternal caregivers; for ease of presentation, we refer to maternal variables.
2.2. Participants
Seventy-six percent of the birth cohort of 295 mother/infant pairs (Richardson et al., 1999) completed the age 21 phase (N = 225). Seventy offspring were not included in the current analyses for the following reasons: died, placed for adoption and could not be located, incarcerated or in a rehabilitation facility, refused, moved out of the area, lost to follow-up. There were no differences in PCE, maternal sociodemographic (education, income, work, marital status), or newborn (gestational age, weight, length) characteristics between those who were and were not included in these analyses. The two groups differed only on gender (48% versus 73%, respectively, were male, p < 0.001) and maternal Center for Epidemiological Studies—Depression Scale (CES-D) (Radloff, 1977) depressive symptoms at delivery (mean 41.4 versus 44.2, respectively, p < 0.05; items scored 1 – 4).
2.3. Measures
2.3.1. Maternal measures.
2.3.1.1. Maternal substance use.
Maternal cocaine/crack, alcohol, tobacco, marijuana, and other illicit drug use were assessed during confidential interviews at each phase. Usual, maximum, and minimum quantity and frequency of cocaine and crack use were reported in lines, rocks, or grams (see Richardson et al., 2008 for details). For the analyses, first, second, and third trimester cocaine use were analyzed as any versus no use during each trimester. The alcohol, tobacco, and marijuana variables were average number of reported drinks, cigarettes, or joints per day, respectively. Alcohol, tobacco, and marijuana use for each trimester of pregnancy and at the age 21 follow-up were used as continuous variables, with one exception: at the age 21 phase, too few mothers reported marijuana or cocaine use for them to be analyzed as continuous variables: maternal marijuana use was defined as any versus no use, and other illicit drug use was defined as any reported use of cocaine or other illicit drugs versus no use.
2.3.1.2. Other maternal measures.
At the age 21 follow-up, mothers were asked structured questions about current sociodemographic, social support (how many people to turn to; how often see/talk to relatives/friends), and psychosocial characteristics. The CES-D (Radloff, 1977) was used to assess depressive symptoms (item scores ranged from 1 – 4), and the Spielberger State-Trait Personality Inventory (STPI) anger subscale (Spielberger, 1979) was used to measure hostility symptoms (e.g., I am quick tempered; I am a hotheaded person).
2.3.2. Offspring measures.
2.3.2.1. Offspring substance use.
At the 15- and 21-year phases, offspring were asked about their age of initiation of alcohol (beer, wine, liquor), tobacco, and marijuana. To minimize recall bias, age of initiation as reported at the 15-year follow-up was used in the analyses unless initiation had not yet occurred, in which case age of initiation as reported at age 21 was used. At ages 15 and 21, offspring past-year alcohol, tobacco, marijuana, and other illicit drug use were assessed using questions parallel to those described above. At age 21, we collected a urine sample to test for marijuana: 98% of those with positive screens reported current use.
2.3.2.2. Offspring behavior:
Adult Self-Report (ASR) (Achenbach and Rescorla, 2003). At the age 21 phase, offspring completed the ASR, an extension of the Child Behavior Checklist (CBCL) (Achenbach, 1991). The following standardized measures of behavior problems were used: total, internalizing, and externalizing scores as well as the individual syndrome scales: anxious/depressed, withdrawn, and aggression. Reliability and validity are well-established (Achenbach and Rescorla, 2003). In addition, we asked the offspring about their arrest history, which was dichotomized as no versus any arrests.
2.3.2.3. Adult Temperament Questionnaire (ATQ)
(Evans and Rothbart, 2007; Rothbart et al., 2000). We used the 19-item Effortful Control (EC) subscale from the ATQ as a measure of the capacity for self-regulation, given earlier findings on the relation between EC and alcohol/drug use (Piehler et al., 2012; Wong et al., 2006). The EC subscale consists of items regarding Activation (“I can keep performing a task even when I would rather not do it”), Attentional (“It is very hard for me to focus my attention when I am distressed” – reverse coded), and Inhibitory Control (“I usually have trouble resisting my cravings for food, drink, etc.” – reverse coded). Higher scores indicate more control. Very good reliability and validity have been reported (Evans and Rothbart, 2007).
2.3.2.4. Difficulties in Emotion Regulation Scale (DERS)
(Gratz and Roemer, 2004). This is a 36-item assessment of regulation of negative emotional states including the following four subscales: lack of emotional awareness (“I pay attention to how I feel” – reverse coded); lack of emotional clarity (“I have no idea how I’m feeling”); difficulty engaging in goal-directed behavior while upset (“When I’m upset, I have difficulty concentrating”); and difficulty with impulse control (“When I’m upset, I lose control over my behaviors”). Higher scores indicate more difficulties. The scale has good test-retest reliability and high internal consistency (Gratz and Roemer, 2004).
Conduct Disorder was diagnosed using the Diagnostic Interview Schedule (DIS-IV; Robins et al., 1981, 2000), a widely used structured diagnostic interview that assesses current (12-month) and lifetime prevalence of DSM-IV disorders. Interviewers were trained to administer the computerized DIS-IV by a trained and experienced clinician. Reliability standards were met, and regular reliability checks were conducted throughout data collection.
2.4. Other offspring measures
The Self-Reported Delinquency Scale (SRD)
(Loeber et al., 1998) was completed by the offspring at the age 15 follow-up. The SRD assesses antisocial behaviors such as purposefully breaking or damaging things, stealing, hitting, fighting, and running away from home. Higher scores indicate more delinquent behaviors. The status offenses subscale was used in analyses because it had the best internal consistency (Loeber et al., 1998). The Revised Dimensions of Temperament Survey (DOTS-R) (Windle and Lerner, 1986), also completed by offspring at 15 years, is a self-assessment of temperament or behavioral style. The mood subscale consisted of seven questions about happiness (laughing, smiling, cheerful) and was dichotomized to ≤ 25th (coded as 1) versus > 25th (coded as 0) percentile due to its asymmetric distribution.
The Childhood Trauma Questionnaire (CTQ) (Bernstein and Fink, 1998) was completed by the offspring at the age 21 follow-up. The CTQ has been used to measure childhood and adolescence physical and emotional abuse and neglect and sexual abuse in a variety of populations, and good reliability and validity have been reported (Cammack et al., 2016). The 21-year-old offspring were also interviewed about their sociodemographic characteristics (education, income, work/school status), life events during the past year (Recent Life Changes Questionnaire) (Miller and Rahe, 1997), and their immediate family history (biological parents or siblings) of serious alcohol and/or drug problems.
2.5. Statistical analyses
The following outcome variables were transformed prior to the analyses due to their asymmetric distributions: young adult offspring’s use of alcohol (none, < 1 drink/day, ≥ 1 drink/day); tobacco (none, < 5 cigarettes/day, ≥ 5 cigarettes/day); and marijuana (none, < 1 joint/day, ≥ 1 joint/day). Multiple stepwise regression was used for the continuous outcome variables, ordinal logistic regression for the trichotomous variables, and Cox proportional hazards regression analysis for the age of initiation variables. Analyses were conducted separately by trimester to assess the effects of exposure during each gestational period.
Table 1 presents maternal and offspring characteristics, including the covariates that were considered for inclusion in the analyses based on the literature and on their associations with the outcomes or PCE in initial bivariate analyses. In addition to carefully selecting variables for consideration based on our theoretical framework and the relevant literature, we used the bivariate analyses to select the most parsimonious set of covariates to include in the final multivariate models. Covariates were included if they were significantly related to either PCE or the outcome at p ≤ .05. We also tested race by PCE interactions for the outcome variables where PCE was significant in the regression. None of the interactions were significant.
Table 1.
Sample characteristics by first trimester cocaine exposure (unadjusted values).
| No cocaine use 1st trimester | Cocaine use 1st trimester | p valuea | |
|---|---|---|---|
| n = 133 | N = 92 | ||
| First trimester maternal characteristics | |||
| African American (%) | 42.1 | 55.4 | <.05 |
| Age (yrs) (mean, SD) | 24.1 (5.0) | 26.5 (5.0) | < .001 |
| Education (yrs) (mean, SD) | 12.1 (1.4) | 11.9 (1.2) | ns |
| Single (%) | 70 | 89 | < .001 |
| Family income ($/mo) (mean, SD) | 782 (614) | 568 (683) | < .05 |
| Drinks/day (mean, SD) | 0.3 (0.6) | 2.3 (3.0) | <.001 |
| Cigarettes/day (mean, SD) | 5.7 (8.9) | 10.7 (9.0) | <.001 |
| Joints/day (mean, SD) | 0.06 (0.2) | 0.5 (1.3) | <.001 |
| Other illicit drugs (except cocaine) (%) | 2.3 | 9.8 | <.05 |
| 21-year maternal characteristics | |||
| Education (yrs) (mean, SD) | 13.0 (1.7) | 13.2 (1.9) | ns |
| Family income ($/mo) (mean, SD) | 2927 (2155) | 2138 (2176) | < .05 |
| Single (%) | 59 | 70 | ns |
| Current depressive symptoms (CES-Db) | 38.3 (10.3) | 41.3 (9.1) | < .05 |
| Current hostility (STPIc) | 14.9 (4.2) | 15.7 (4.6) | ns |
| People to turn to (#) | 4.8 (2.4) | 4.4 (2.2) | ns |
| How often see/talk to relatives/friendsd | 4.3 (0.6) | 4.2 (0.7) | ns |
| Drinks/day (mean, SD) | 0.7 (2.4) | 1.8 (3.5) | < .05 |
| Cigarettes/day (mean, SD) | 5.5 (9.1) | 6.4 (7.4) | ns |
| Marijuana (% any) | 9.3 | 17.4 | ns |
| Illicit drugs (cocaine & other) (%) | 1.7 | 9.3 | < .05 |
| 21-year offspring characteristics | |||
| Age (yrs) (mean, SD) | 21.3 (0.7) | 21.3 (0.6) | ns |
| Male (%) | 48.1 | 46.7 | ns |
| African American (%) | 47.4 | 63.0 | < .05 |
| Education (yrs) (mean, SD) | 12.8 (1.5) | 12.6 (1.4) | ns |
| Working (% yes) | 56.4 | 59.8 | ns |
| Attend school (% yes) | 40.6 | 43.5 | ns |
| Personal income ($/mo) (mean, SD) | 661(739) | 914 (1213) | ns |
| Receive public assistance (% yes) | 15.8 | 12.0 | ns |
| Live with partner (%) | 19.6 | 19.6 | ns |
| ≥ 1 child (%) | 22.6 | 28.3 | ns |
| Current life events (#) | 5.5 (3.2) | 6.3 (3.3) | ns |
| Drink alcohol (%) | 90.2 | 95.7 | ns |
| Drinks/day (mean, SD) | 1.6 (3.3) | 1.9 (3.1) | ns |
| Use ≥ 1 drink/day (%) | 30.8 | 40.2 | ns |
| Smoke cigarettes (%) | 36.1 | 46.7 | ns |
| Cigarettes/day (mean, SD) | 2.8 (5.3) | 3.5 (5.4) | ns |
| Smoke ≥ 1/4 pack/day (%) | 24.0 | 33.7 | ns |
| Use marijuana (%) | 49.6 | 68.5 | < .01 |
| Joints/day (mean, SD) | 1.3 (5.7) | 1.7 (4.0) | ns |
| Use ≥ 1 joint/day (%) | 16.5 | 31.5 | < .01 |
| Use other illicit drugs (includes cocaine) (%) | 15.0 | 20.7 | ns |
| Family history alcohol problemse (%) | 27 | 50 | < .001 |
| Family history drug problemse (%) | 23 | 50 | < .001 |
| CTQf | 2.2 (0.8) | 2.5 (1.0) | < .05 |
| Ever arrested (% yes) | 24 | 47 | < .001 |
| DISg Conduct Disorder (%) | 9 | 21 | < .01 |
| ASRh externalizing problems (t-score) | 53.1 (10.8) | 55.4 (10.1) | ns |
| ASR aggression (raw score) | 6.2 (4.9) | 6.8 (5.4) | ns |
| ASR internalizing problems (t-score) | 52.7 (12.2) | 53.1 (11.7) | ns |
| ASR withdrawn (raw score) | 3.2 (3.2) | 4.0 (3.2) | < .05 |
| ASR anxious/depressed (raw score) | 7.6 (6.7) | 8.0 (7.0) | ns |
| ATQi inhibitory control | 3.5 (0.6) | 3.3 (0.6) | < .05 |
| ATQ attention control | 3.5 (0.7) | 3.4 (0.6) | ns |
| ATQ activational control | 3.6 (0.6) | 3.6 (0.6) | ns |
| DERSj lack of clarity | 9.7 (3.7) | 10.4 (4.3) | ns |
| DERS lack of awareness | 13.7 (4.9) | 15.4 (5.8) | < .05 |
| 15-year offspring mediators | |||
| Early marijuana use (% < 15 years) | 18.0 | 43.5 | < .001 |
| Status offenses | 1.5 (1.4) | 2.3 (1.5) | < .001 |
| DOTS-Rk mood (% ≤25th percentile) | 18.8 | 31.5 | < .05 |
Based on t-test or Mann-Whitney for continuous variables and on Chi-square test for dichotomous variables
Center for Epidemiological Studies - Depression scale (Radloff, 1977) (items scored 1–4)
State Trait Personality Inventory (Spielberger, 1979)
1 = Never to 5 = Very often
Any biological parent or sibling serious problem
Childhood Trauma Questionnaire (Bernstein and Fink, 1998)
Diagnostic Interview Schedule (Robins et al., 2000)
Adult Self-Report (Achenbach and Rescorla, 2003)
Adult Temperament Questionnaire (Rothbart et al., 2000)
Difficulties in Emotion Regulation Scale (Gratz and Roemer, 2004)
Dimensions of Temperament Scale - Revised (Windle and Lerner, 1986)
In the final models, the tolerance of each covariate was examined to assure that the estimated regression slopes were not unstable because of multicollinearity. Residuals and the modified Cook’s statistic (Cook and Weisberg, 1982) were used to identify possible outliers and influential points. There were no outliers for any of the outcome variables. There was one influential case each for the ASR, ATQ, and DERS. The significant relations with PCE reported here did not change with removal of these cases.
For those outcomes for which PCE was a significant predictor, mediating analyses were conducted to determine whether age 15 behaviors (SRD for adult externalizing problems, DOTS-R mood for adult temperament and internalizing problems, and early marijuana use (<15 years) for adult substance use) were part of the pathway between PCE and the age 21 outcomes. These variables were chosen because they were significantly associated with PCE at age 15 (Richardson et al., 2013b, 2015). For the logistic and multiple regression models, mediation was evaluated with path analysis using the product of coefficients, and MacKinnon’s z’ distribution was applied to determine significance of the mediator (z’ ≥ 0.97 corresponds to p ≤ 0.05; MacKinnon et al., 2002). Partial mediation was attributed when the direct effect of PCE remained significant after including the mediator in the model; complete mediation was attributed when the direct effect of PCE became non-significant after including the mediator.
3. Results
3.1. Sample characteristics
At delivery, mothers were, on average, 24.8 years old (range: 18–41), had 11.9 years of education (range: 9–16), 25% were primigravidous, 23% were married, and 52% were Caucasian (Richardson et al., 1999). In the first trimester, 41% reported using cocaine. Use decreased over pregnancy with 8% and 11% reporting cocaine use during the second and third trimesters, respectively. In those who used first trimester, 50% reported snorting powder cocaine only; the rest smoked crack. In those who continued to use in the third trimester, 20% reported snorting powder cocaine only, and 80% smoked crack.
The median age of the offspring at the age 21 assessment was 21.3 years (range: 20–24). Forty-eight percent were male, 46% were Caucasian, and 54% were African American. The mean educational level was 12.7 years (range: 8–16 years). The majority (85%) had graduated high school, half attended some college, less than 2% had graduated college, 42% were attending school, 58% were working, and 3% served in the military. Median personal income was $500/month (range: $0–8,000), and 14% reported receiving public assistance. Most lived with their parents or relatives, and only 35% lived on their own. Three percent were married, and 25% had at least one child (range: 0–3). At 21 years, 92% drank alcohol, 40% smoked cigarettes, and 56% used marijuana; 36% drank ≥1 drink/day, 5% smoked ≥1 pack/day, and 25% used ≥1 joint/day. Thirty-six percent, 41%, and 28% reported using alcohol, tobacco, and marijuana, respectively, prior to age 15.
3.2. Bivariate relations with PCE
As seen in Table 1, mothers who used cocaine during the first trimester were significantly more likely to be African-American, older, single, have lower family incomes, and to use more alcohol, tobacco, marijuana, and other illicit drugs than were first trimester non-users. At the age 21 phase, maternal income was lower, depressive symptoms were higher, and current use of alcohol and illicit drugs was greater in first trimester cocaine users compared to non-users. At age 21, there were no significant demographic differences between exposed and non-exposed offspring except for race: exposed offspring were more likely to be African American. Offspring exposed during the first trimester were significantly more likely to use marijuana and at heavier levels, to have immediate family members with a history of alcohol or drug problems, and to report more childhood trauma than non-exposed offspring. In unadjusted analyses, exposed offspring were more likely to have been arrested, be diagnosed with Conduct Disorder (CD), and to report more ASR withdrawn symptoms, less ATQ inhibitory control, and greater DERS lack of awareness than non-exposed offspring.
Based on the literature previously cited and the bivariate analyses, the following variables were selected for inclusion in the multivariate models: offspring race, gender, and current life events; maternal age; prenatal alcohol, marijuana, and tobacco; current maternal family income, alcohol, and other illicit drugs; CTQ; and family history of alcohol/drug problems. Table 2 shows the correlations among the outcomes and variables that were included in the regression models.
Table 2.
Correlations among young adult outcomes and variables used in regression models. a
| Arrests | Conduct Disorder |
Offspring marijuana |
ASRb external |
ASR internal |
ASR anx/dep | ASR withdrawn |
ASR aggression |
DERSc clarity |
DERS awareness |
ATQ ICd |
ATQ ATTe |
ATQ ACf |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st trim. cocaine | 0.24 | 0.18 | 0.21 | 0.11 | 0.02 | 0.03 | 0.12 | 0.06 | 0.09 | 0.15 | −0.15 | −0.04 | −0.06 |
| 3rd trim. cocaine | 0.15 | −0.05 | 0.18 | 0.09 | 0.15 | 0.14 | 0.19 | 0.14 | 0.04 | 0.14 | −0.13 | −0.04 | −0.10 |
| 1st trim. alcohol | 0.07 | 0.16 | 0.16 | 0.07 | 0.03 | 0.03 | 0.11 | 0.02 | 0.02 | 0.09 | −0.10 | 0.02 | 0.01 |
| 1st trim. tobacco | 0.10 | 0.13 | 0.13 | 0.06 | 0.09 | 0.10 | 0.11 | 0.06 | 0.04 | 0.09 | 0.02 | 0.04 | −0.04 |
| 1st trim. marijuana | 0.10 | 0.05 | −0.001 | 0.04 | −0.08 | −0.04 | −0.04 | 0.02 | −0.05 | −0.10 | 0.06 | 0.12 | 0.08 |
| Offspring race | −0.05 | 0.06 | −0.14 | 0.02 | 0.07 | 0.09 | −0.06 | −0.02 | −0.02 | 0.09 | −0.02 | −0.01 | 0.12 |
| Gender | 0.18 | −0.01 | 0.06 | −0.16 | −0.20 | −0.22 | −0.004 | −0.20 | −0.15 | 0.13 | 0.14 | 0.19 | 0.10 |
| Maternal age | −0.03 | 0.01 | −0.05 | −0.20 | −0.19 | −0.23 | −0.10 | −0.21 | −0.25 | −0.11 | 0.07 | 0.05 | 0.02 |
| Current family income | 0.03 | −0.01 | −0.10 | −0.04 | −0.02 | 0.02 | 0.003 | −0.03 | −0.05 | 0.06 | 0.01 | 0.09 | 0.12 |
| Current maternal alcohol | 0.03 | −0.02 | 0.15 | 0.05 | −0.06 | −0.04 | −0.03 | 0.04 | −0.01 | 0.07 | −0.09 | −0.00 | −0.05 |
| Current maternal illicit drugs | 0.17 | 0.32 | 0.17 | 0.14 | 0.02 | −0.03 | 0.04 | 0.09 | 0.06 | 0.06 | −0.10 | −0.07 | −0.05 |
| Offspring life events | 0.22 | 0.18 | 0.29 | 0.37 | 0.29 | 0.25 | 0.18 | 0.31 | 0.28 | −0.02 | −0.27 | −0.22 | −0.23 |
| Family history alcohol problems | 0.17 | 0.15 | 0.13 | 0.15 | 0.15 | 0.15 | 0.08 | 0.12 | 0.14 | 0.06 | −0.14 | −0.07 | −0.07 |
| Family history drug problems | 0.17 | 0.15 | 0.06 | 0.01 | 0.06 | 0.07 | 0.05 | 0.01 | 0.15 | 0.13 | 0.05 | −0.04 | −0.11 |
| CTQg | 0.10 | 0.23 | 0.17 | 0.38 | 0.38 | 0.38 | 0.34 | 0.40 | 0.36 | 0.22 | −0.06 | −0.21 | −0.22 |
| Early marijuana use | 0.41 | 0.40 | 0.34 | 0.22 | 0.12 | 0.11 | 0.17 | 0.16 | 0.12 | 0.09 | −0.12 | −0.10 | −0.13 |
| Status offenses (SRDh) | 0.40 | 0.34 | 0.22 | 0.28 | 0.15 | 0.11 | 0.11 | 0.23 | 0.10 | 0.10 | −0.19 | −0.11 | −0.10 |
| DOTS-Ri Mood | 0.04 | 0.06 | 0.02 | 0.20 | 0.28 | 0.28 | 0.34 | 0.26 | 0.26 | 0.20 | −0.21 | −0.20 | −0.16 |
Correlations indicated in bold are significant at p < .05
Adult Self-Report (Achenbach and Rescorla, 2003)
Difficulties in Emotion Regulation Scale (Gratz and Roemer, 2004)
Adult Temperament Questionnaire Inhibitory Control (Rothbart et al., 2000)
Adult Temperament Questionnaire Attentional Control (Rothbart et al., 2000)
Adult Temperament Questionnaire Activation Control (Rothbart et al., 2000)
Childhood Trauma Questionnaire (Bernstein and Fink, 1998)
Self-Reported Delinquency Scale (Loeber et al., 1998)
Dimensions of Temperament Scale – Revised (Windle and Lerner, 1986) (≤ 25th percentile = 1)
3.3. Regression analyses
As shown in Table 3, first trimester cocaine exposure was a significant predictor of age 21 ATQ inhibitory control and DERS lack of awareness. Third trimester cocaine exposure significantly predicted ASR withdrawn behavior and DERS lack of awareness. Prenatal exposure was associated with more withdrawn behaviors, poorer inhibitory control, and increased difficulty with emotion regulation. There was no significant relation between PCE and ASR externalizing behaviors.
Table 3.
Factors associated with continuous 21-year outcomes, controlling for covariates a (multiple regression analyses).
| Outcome variable | Total R2 | Significant predictors | Raw beta | Standardized regression coefficient | p value |
|---|---|---|---|---|---|
| ASRb withdrawn | 0.13 | CTQc | 1.09 | 0.32 | <.001 |
| 3rd trimester cocained | 1.20 | 0.12 | <.05 | ||
| ATQe inhibitory control | 0.13 | Offspring life events | −0.04 | −0.24 | <.001 |
| 1st trimester cocained | −0.25 | −0.21 | <.001 | ||
| Family history drug problems | 0.20 | 0.16 | <.05 | ||
| Genderf | 0.15 | 0.13 | < .05 | ||
| Maternal age | 0.01 | 0.12 | <.05 | ||
| DERSg lack of awareness | 0.10 | CTQ | 1.15 | 0.19 | < .001 |
| 1st trimester cocaine | 1.80 | 0.17 | <.05 | ||
| 3rd trimester cocaine | 2.64 | 0.15 | <.05 | ||
| Maternal age | −0.15 | −0.15 | <.05 | ||
| Offspring raceh | 1.53 | 0.14 | <.05 |
Covariates included in model: offspring race, gender, current life events; maternal age; prenatal alcohol, marijuana, tobacco; current maternal family income, alcohol, and other illicit drugs; CTQ; family history of alcohol/drug problems. Results are presented only for those outcomes where prenatal cocaine exposure was significant. Predictors are listed in order of standardized regression coefficient, an indication of the magnitude of the effect. Results were run separately by trimester but are shown together for ease of presentation.
Adult Self-Report (Achenbach and Rescorla, 2003)
Childhood Trauma Questionnaire (Bernstein and Fink, 1998)
0 = no use, 1 = use
Adult Temperament Questionnaire (Rothbart et al., 2000)
0 = Female, 1 = Male
Difficulties in Emotion Regulation Scale (Gratz and Roemer, 2004)
0 = Black, 1 = Caucasian
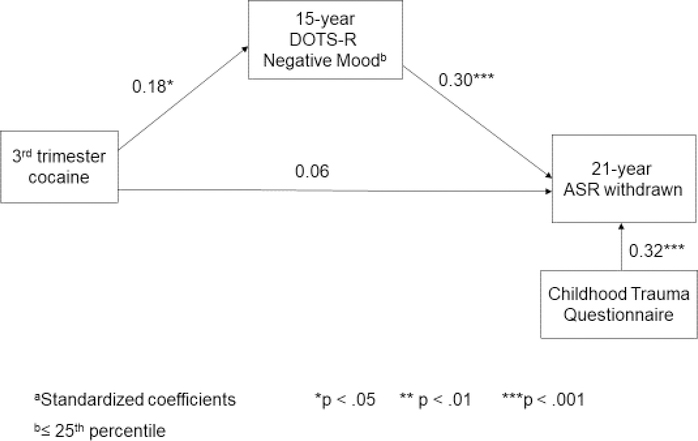
The age 15 DOTS-R mood scale completely mediated the pathway from third trimester exposure to ASR withdrawn (z’ = 1.66) (Figure 1) and the pathway between PCE and ATQ inhibitory control (z’ = −1.92). However, the DOTS-R only partially mediated the relation between PCE and DERS lack of awareness (z’ = 1.38); PCE remained a significant predictor.
Figure 1.
Pathway from prenatal cocaine exposure to 21-year withdrawn behavior.
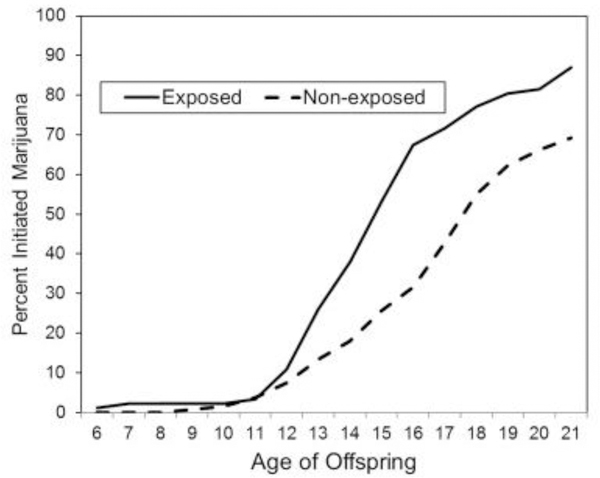
First trimester cocaine exposure was a significant predictor of age of marijuana initiation but not of age of alcohol or tobacco initiation (Table 4). Figure 2 shows that the exposed offspring start to diverge from the non-exposed offspring at about age 12; by age 21, 87% of the exposed and 69% of the non-exposed had initiated marijuana use. The inclusion of family history of alcohol/drug problems and childhood trauma as covariates in the model did not change the significant relation between first trimester cocaine exposure and age of marijuana initiation.
Table 4.
Factors associated with categorical 21-year outcomes, controlling for covariates. a
| Coefficient | Hazard ratio (95% CI) | p value | |
|---|---|---|---|
| Age of marijuana initiationb | |||
| 1st trimester cocainec | 0.49 | 1.62 (1.18–2.24) | < .005 |
| Offspring life events | 0.08 | 1.08 (1.03–1.14) | < .005 |
| CTQd | 0.21 | 1.23 (1.03–1.48) | < .05 |
| Current maternal illicit drug usec | 0.89 | 2.43 (1.09–5.4) | < .05 |
| Coefficient | Cumulative/Adjusted odds ratio (95% CI) | p value | |
| Marijuana use in past yeare,f | |||
| Offspring life events | 0.16 | 1.2 (1.1 – 1.3) | < .001 |
| 1st trimester cocaine | 0.88 | 2.4 (1.4 – 4.2) | < .01 |
| Maternal age | −0.06 | 0.95 (0.9 — 1.0) | < .05 |
| Ever arrestede | |||
| Offspring life events | 0.16 | 1.2 (1.1 – 1.3) | < .001 |
| 1st trimester cocaine | 1.04 | 2.8 (1.5 – 5.2) | < .001 |
| Genderg | 1.02 | 2.8 (1.5 — 5.2) | < .005 |
| 3rd trimester cocainec | 1.1 | 3.0 (1.2 — 7.7) | < .05 |
| Conduct Disordere,h | |||
| CTQ | 0.53 | 1.71 (1.13— 2.58) | < .01 |
| 1st trimester cocaine | 0.87 | 2.39 (1.01 — 5.65) | < .05 |
Covariates included in model: offspring race, gender, current life events; maternal age; prenatal alcohol, marijuana, tobacco; current maternal family income, alcohol, and other illicit drugs; CTQ; family history of alcohol/drug problems. Results are presented only for those outcomes where prenatal cocaine exposure was significant. Predictors are listed in order of p value, an indication of the magnitude of the effect. Results were run separately by trimester but are shown together for ease of presentation.
Cox proportional hazards model
0 = no use, 1 = use
Childhood Trauma Questionnaire (Bernstein and Fink, 1998)
Ordinal logistic regressions
None; < 1 joint/day; ≥ 1 joint/day
0 = Female, 1 = Male
Assessed by the Diagnostic Interview Schedule (Robins et al., 2000)
Figure 2.
Marijuana initiation as a function of prenatal cocaine exposure.
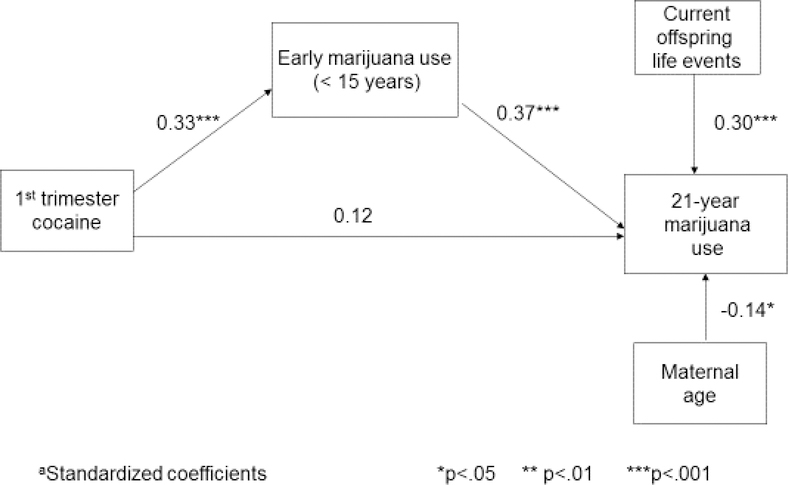
First trimester cocaine exposure was also a significant predictor of past-year marijuana use at age 21 but not of alcohol and tobacco use (Table 4). The percentage of daily marijuana users among the exposed offspring is almost double that of the non-exposed offspring (Table 1). However, as shown in Figure 3, early marijuana use (<15 years of age) completely mediated the relation between PCE and offspring marijuana use at age 21 (z’ = 2.62; 51% of the total effect was due to the indirect effect, 49% was due to the direct effect).
Figure 3.
Pathway from prenatal cocaine exposure to offspring marijuana use.
PCE was also significantly associated with ever being arrested (odds ratios ~3) and with a diagnosis of CD (odds ratio ~2) (Table 4). The relation to arrest history was only partially mediated by age 15 delinquency (SRD) (z’ = 3.14); PCE remained a significant predictor. The SRD was not related to CD so was not tested as a mediator.
4. Discussion
This report is from an ongoing longitudinal study of PCE in a sample enrolled early in pregnancy. Prenatal cocaine use occurred at moderate levels, and most users decreased or discontinued use after the first trimester, which represents the most common pattern of prenatal drug use in non-treatment samples (Day et al., 1989, 1991). Extensive data were collected on maternal characteristics associated with prenatal exposure and on offspring characteristics associated with their substance use and behavior problems, which enabled statistical modeling of their influence on young adult behavior. With control for prenatal and current covariates, PCE was significantly related to withdrawn behavior, inhibitory control, and emotion regulation at age 21. However, the relations of PCE to withdrawn behavior and inhibitory control were mediated by an indirect pathway through adolescent mood. The relation between PCE and emotion regulation was only partially mediated by adolescent mood; the direct pathway remained significant, consistent with the teratologic model. The relations between PCE and young adult arrests and CD were direct and were not mediated by adolescent delinquent behaviors. Those who were prenatally exposed were three times more likely to have been arrested and twice as likely to be diagnosed with CD as those who were not exposed, which is consistent with Schulenberg’s transition models that predict a divergence of exposed from non-exposed individuals during major life transitions. First trimester cocaine exposure was significantly associated with age of marijuana initiation. As shown in Figure 2, those who were prenatally exposed had a different trajectory of marijuana initiation than those who were not exposed, which is also consistent with Schulenburg’s models. However, the relation between PCE and past-year marijuana use was explained by early initiation of marijuana. We did not find direct relations between PCE and young adult’s self-report of planning, attention, or goal-directed behaviors, which are more likely to be associated with cognitive functions rather than emotion regulation.
These findings are consistent with earlier reports of significant associations between PCE and adolescent substance use and externalizing behavior problems (Delaney-Black et al., 2000; Lambert et al., 2013; Min et al., 2014a; Minnes et al., 2017; Richardson et al., 2013b, 2015) and a lack of association with global cognitive functions (Buckingham-Howes et al., 2013; Ross et al., 2015). This is the first report to show that some of these associations persist into young adulthood and that young adult outcomes are not merely a result of earlier problems. We have shown the importance of both distal and proximal influences on young adult development as emphasized by Schulenberg’s transition models of development. There is only one other report from the adult follow-up of a longitudinal study of PCE, in which there was no significant relation between PCE and a composite measure of adaptive function (Forman et al., 2017). Our reported associations between PCE and young adult emotion regulation and externalizing behavior are also consistent with findings from animal studies (Behnke et al., 2013; Ross et al., 2015), in which PCE has been shown to affect the development and function of reward circuitry in the brain by increasing the reward potency of cocaine in operant conditioning tasks such as drug self-administration (Keller et al., 1996; Lin and Kellogg, 1996; Rocha et al., 2002) and causing long-term changes in D1-dopamine receptor signaling (Tropea et al., 2008). Our findings of direct associations with PCE are consistent with the teratologic model, which states that an exposure can act directly on specific mechanisms of development, particularly functional abnormalities (Vorhees, 1989).
We found that emotion regulation problems and arrests were associated with both first and third trimester exposure, while Conduct Disorder was associated with first trimester exposure. Weese-Mayer et al. (1993) reported that prenatal cocaine exposure early in gestation in rabbits was associated with a reduction in striatal dopamine. Howard et al. (1997) found that postnatal cocaine administration in rats (equivalent to third trimester in the human) was associated with changes in dopamine concentrations. These alterations in the monoaminergic system are thought to be potential mechanisms for the PCE-associated behaviors that are seen in humans (Dow-Edwards, 2011; Mayes, 2002; Ross et al., 2015). However, most research, both with humans and animals, reports PCE as an across-pregnancy average, so it is not possible to make further comparisons. In fact, both Buckingham-Howes et al. (2013) and Ross et al. (2015) recommend that more work is needed to investigate the timing of prenatal exposure.
In addition to the consistency with the PCE literature, we also found that childhood maltreatment and current life events were strong predictors of adult behaviors, consistent with many previous studies (as cited in the Introduction). Our finding that adolescent mood mediated the relationship between PCE and adult inhibitory control and withdrawn behaviors is indicative of a pathway showing the continuity of adolescent and adult internalizing symptoms (Hofstra et al., 2000).
There are potential limitations of this work. One, this is a sample of women who sought prenatal care early in pregnancy at a clinic that served low-income women. Thus, the findings may not apply to samples with different health care or socioeconomic characteristics. Two, we did not have biological verification of prenatal substance use, which might lead to misclassification. However, with careful interviewer selection and training and attention to question format, our maternal self-report substance use measures identified a higher percentage of users than did hospital urine screening (Richardson et al., 1999, 2006), a finding reported by others (Ashling et al., 1994; Fendrich et al., 2004; Lester et al., 2001; Rutherford et al., 2000). We did collect biological samples from the 21-year-old offspring, and those data also support this point: 98% of offspring with positive urine screens for marijuana reported current use. However, 40% of the offspring who reported marijuana use had negative urine screens and thus would not have been detected if we had used only biological measures of marijuana use.
There are also strengths of this research. One, follow-up rates have been consistently good across phases, with 76% of the birth cohort assessed at age 21. There were no SES or prenatal drug exposure differences between those who were and were not seen at 21 years. Two, this is one of the only samples with an equal representation of African American and Caucasian women, which reflects the prenatal clinic from which they were recruited. Three, we have detailed assessments of all types of drug use both during pregnancy and in the postpartum. Four, we carefully measured the sociodemographic and environmental characteristics of both mothers and offspring, allowing control for associations with these characteristics. Five, this work with a predominantly non-college bound, diverse sample has added to our understanding of the transition into young adulthood. Six, by detecting adolescent mediators of the effects of PCE on common problems faced by young adults, we have identified crucial variables, such as early marijuana use and emotion dysregulation, to target interventions for exposed children and adolescents.
5. Conclusions
We found a consistent pattern of direct associations between PCE and young adult emotion regulation, arrest history, and Conduct Disorder. Of particular concern is the association of PCE with early initiation of marijuana use: 43.5% of the exposed and 18% of the non-exposed had initiated marijuana use prior to 15 years of age, and by 21 years 69% of the exposed and 50% of the non-exposed had used marijuana in the past year. These rates are higher than the most recent NSDUH data, which show that ~35% of 18- to 25-year-olds reported use in the past year (SAMHSA, 2017). These associations with PCE were not explained by family history of alcohol/drug use, childhood environment, or adolescent behavior. These findings have implications for the continuity of risk among prenatally exposed individuals and for intergenerational patterns of illicit drug use. The results of this study support the need for interventions to prevent initiation of marijuana use targeted at children who have been prenatally exposed to cocaine. These findings also suggest a pathway from PCE to problems with emotion regulation, mood, and internalizing behavior problems that are apparent in adolescence and may continue unabated into young adulthood if not addressed at an earlier developmental phase. For example, both school- and parent-based programs designed to support and strengthen the development of emotional self-regulation in vulnerable youth through mentoring and skill development have been shown to be effective (Kohlhoff et al., 2016; Wyman et al., 2010) and could be used with children who have been prenatally exposed.
Highlights.
Prospective study of prenatal cocaine exposure (PCE) and adult behavior problems.
Women assessed prenatally, at delivery, 1, 3, 7, 10, 15, and 21 years postpartum.
Direct associations found between PCE and early initiation of marijuana.
PCE directly predicted 21-year emotion regulation, arrests, and Conduct Disorder.
PCE has direct and indirect long-term associations with young adult development.
Acknowledgments
Role of the Funding Source
This work was supported by the National Institute on Drug Abuse grants DA05460, DA06839, DA08916, and DA12401 (G. Richardson, Principal Investigator). The study sponsor had no role in study design, collection, analysis, and interpretation of data, writing the report, or the decision to submit the report for publication. The content does not necessarily represent the official views of NIDA.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accornero V, Anthony JC, Morrow CE, Xue L, Bandstra ES, 2006. Prenatal cocaine exposure: An examination of childhood externalizing and internalizing behavior problems at age 7 years. Epidemiol. Psychiatr. Soc 15, 20–29. [PMC free article] [PubMed] [Google Scholar]
- Achenbach T, 1991. Manual for the child behavior checklist/4–18 and 1991 profile. University of Vermont Department of Psychiatry, Burlington, VT. [Google Scholar]
- Achenbach TM, Rescorla LA, 2003. Manual for the ASEBA adult forms and profiles. University of Vermont, Research Center for Children, Youth, and Families, Burlington, VT. [Google Scholar]
- Alati R, Ak Mamun A, Williams GM, O’Callahan M, Najman JM, Bor W, 2006. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: A birth cohort study. Arch. Gen. Psychiatry 63, 1009–1016. [DOI] [PubMed] [Google Scholar]
- Arnett JJ, 2000. Emerging adulthood: A theory of development from the late teens through the twenties. Am. Psychol 55, 469–480. [PubMed] [Google Scholar]
- Ashling K, Gross AH, Coghlin DT, Sweeney PJ, 1994. Prevalence of positive urine drug screens in a prenatal clinic: Correlation with patients’ self-report of drug use. R. I. Med 77, 371–373. [PubMed] [Google Scholar]
- Bachman JG, Wadsworth KN, O’Malley PM, Schulenberg JE, Johnston LD, 1997. Marriage, divorce, and parenthood during the transition to young adulthood: Impacts on drug use and abuse In: Schulenberg J, Maggs JL, Hurrelmann K (Eds.). Health risks and developmental transitions during adolescence. Cambridge University Press, Cambridge, UK, pp. 246–279. [Google Scholar]
- Bada HS, Bann CM, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Whitaker T, Das A, Tan S, Higgins R, 2011. Preadolescent behavior problems after prenatal cocaine exposure: Relationship between teacher and caretaker ratings (Maternal Lifestyle Study). Neurotoxicol. Teratol 33, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada H, Bann C, Whitaker T, Bauer C, Shankaran S, LaGasse L, Lester B, Hammond J, Higgins R, 2012. Protective factors can mitigate behavior problems after prenatal cocaine and other drug exposures. Pediatrics 130, e1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP, 2003. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch. Gen. Psychiatry 60, 377–385. [DOI] [PubMed] [Google Scholar]
- Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn, 2013. Prenatal substance abuse: Short- and long-term effects on the exposed fetus. Pediatrics 131, e1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Bendersky M, Lewis M, 2002. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev. Psychol 38, 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Marini V, Berzenski S, Carmody DP, Lewis M, 2013. Externalizing problems in late childhood as a function of prenatal cocaine exposure and environmental risk. J. Pediatr. Psychol 38, 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D, Fink L, 1998. Childhood Trauma Questionnaire: A retrospective self-report. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Buckingham-Howes S, Berger SS, Scaletti LA, Black MM 2013. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics 131, e1917–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack AL, Hogue CJ, Drews-Botsch CD, Kramer MR, Pearce BD, Knight BT, Stowe ZN, Newport DJ, 2016. Test-retest reliability of retrospective self-reported maternal exposure to childhood abuse and neglect. Arch. Womens Ment. Health 19, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Jacobson K, 2012. Developmental trajectories of substance use from early adolescence to young adulthood: Gender and racial/ethnic differences. J. Adolescent Health 50, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RD, Weisberg S, 1982. Residuals and influence in regression. Chapman and Hall, New York, NY. [Google Scholar]
- Copeland WE, Miller-Johnson S, Keeler G, Angold A, Costello EJ, 2007. Childhood psychiatric disorders and young adult crime: A prospective, population-based study. Am. J. Psychiatry 164, 1668–1675. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Costello J, Angold A, 2009. Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Arch. Gen. Psychiatry 66, 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M, 1989. Prenatal exposure to alcohol: Effect on infant growth and morphologic characteristics. Pediatrics 84, 536–541. [PubMed] [Google Scholar]
- Day N, Sambamoorthi U, Taylor P, Richardson G, Robles N, Jhon Y, Scher M, Stoffer D, Cornelius M, Jasperse D, 1991. Prenatal marijuana use and neonatal outcome. Neurotoxicol. Teratol 13, 329–334. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Partridge RT, Ager J, Sokol RJ, 2011. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol. Teratol 33, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Ager J, Nordstrom-Klee B, Martier S, Leddick L, Czerwinski RH, Sokol RJ, 2000. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics 106, 782–791. [DOI] [PubMed] [Google Scholar]
- Doherty EE, Green KM, Ensminger ME, 2008. Investigating the long-term influence of adolescent delinquency on drug use initiation. Drug Alcohol Depend. 93, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D, 2011. Translational issues for prenatal cocaine studies and the role of environment. Neurotoxicol. Teratol 33, 9–16. [DOI] [PubMed] [Google Scholar]
- Eitle D, Turner RJ, 2002. Exposure to community violence and young adult crime: The effects of witnessing violence, traumatic victimization, and other stressful life events. J. Res. Crime Delinq 39, 214–237. [Google Scholar]
- Ellickson P, Martino S, Collins R, 2004. Marijuana use from adolescence to young adulthood: Multiple developmental trajectories and their associated outcomes. Health Psychol. 23, 299–307. [DOI] [PubMed] [Google Scholar]
- Evans DE, Rothbart MK, 2007. Developing a model for adult temperament. J. Res. Pers 41, 868–888. [Google Scholar]
- Fendrich M, Johnson TP, Wislar JS, Hubbell A, Spiehler V, 2004. The utility of drug testing in epidemiological research: Results from a general population survey. Addiction 99, 197–208. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Nagin DS, 2000. Offending trajectories in a New Zealand birth cohort. Criminology 38, 525–551. [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM, 2007. Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence. Drug Alcohol Depend. 88S, S14–S26. [DOI] [PubMed] [Google Scholar]
- Forman LS, Liebschutz JM, Rose-Jacobs R, Richardson MA, Cabral HJ, Heeren TC, Frank DA, 2017. Urban young adults’ adaptive functioning: Is there an association with history of prenatal exposure to cocaine and other substances? J. Drug Issues 47, 261–276. [Google Scholar]
- Frank DA, Rose-Jacobs R, Crooks D, Cabral HJ, Gerteis J, Hacker KA, Martin B, Weinstein ZB, Heeren T, 2011. Adolescent initiation of licit and illicit substance use: Impact of intrauterine exposures and post-natal exposure to violence. Neurotoxicol. Teratol 33, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Zuckerman BS, Amaro H, Aboagye K, Bauchner H, Cabral H, Fried L, Hingson R, Kayne H, Levenson SM, Parker S, Reece H, Vinci R, 1988. Cocaine use during pregnancy: Prevalence and correlates. Pediatrics 82, 888–895. [PubMed] [Google Scholar]
- Glantz MD, Chambers JC, 2006. Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Dev. Psychopathol 18, 893–922. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Roemer L, 2004. Multidimensional assessment of emotion regulation and dysregulation: Factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. J. Psychopathol. Behav 26, 41–54. [Google Scholar]
- Green K, Doherty E, Stuart E, Ensminger M, 2010. Does heavy adolescent marijuana use lead to criminal involvement in adulthood? Evidence from a multiwave longitudinal study of urban African Americans. Drug Alcohol Depend. 112, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Najman JM, Bor W, O’Callahan MJ, Williams GM, 2009. Multiple risk factor model predicting cannabis use and use disorders: A longitudinal study. Am. J. Drug Alcohol Abuse 35, 399–407. [DOI] [PubMed] [Google Scholar]
- Hofstra MB, der Ende JV, Verhulst FC, 2000. Continuity and change of psychopathology from childhood into adulthood: A 14-year follow-up study. J. Am. Acad. Child Adolesc. Psychiatry 39, 850–858. [DOI] [PubMed] [Google Scholar]
- Howard SG, Fisher R, Landry CF, 1997. Alterations in the spontaneous release of dopamine and the density of the DA D2 receptor mRNA after chronic postnatal exposure to cocaine. Brain Res. Bull 43, 101–106. [DOI] [PubMed] [Google Scholar]
- Jessor R, Donovan JE, Costa FM, 1991. Beyond adolescence: Problem behavior and young adult development. Cambridge University Press, New York. [Google Scholar]
- Keller RW Jr., LeFevre R, Raucci J, Carlson JN, Glick SD, 1996. Enhanced cocaine self-administration in adult rats prenatally exposed to cocaine. Neurosci. Letter 205, 153–156. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, Benjet C, Bromet E, Chatterji S, de Girolamo G, Demyttenaere K, Fayyad J, Florescu S, Gal G, Gureje O, Haro JM, Hu CY, Karam EG, Kawakami N, Lee S, Lépine JP, Ormel J, Posada-Villa J, Sagar R, Tsang A, Ustün TB, Vassilev S, Viana MC, Williams DR, 2010. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Brit. J. Psychiatry 197, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ, 2011. Personality and depression: Explanatory models and review of the evidence. Ann. Rev. Clin. Psychol 7, 269–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhoff J, Hawes DJ, Mence M, Russell AMT, Wedgwood L, Morgan S, 2016. Emotion regulation strategies and parenting practices among parents of children with clinic-referred conduct problems. Parent. Sci. Pract 16, 302–319. [Google Scholar]
- Lambert B, Bann C, Bauer C, Shankaran S, Bada H, Lester B, Whitaker TM, LaGasse LL, Hammond J, Higgins RD, 2013. Risk-taking behavior among adolescents with prenatal drug exposure and extrauterine environmental adversity. J. Dev. Behav. Pediatr 34, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschied A, Chiodo D, Nowicki E, Rodger S, 2008. Childhood predictors of adult criminality: A meta-analysis drawn from the prospective longitudinal literature. Can. J. Criminol. Crim 50, 435–467. [Google Scholar]
- Lester B, ElSohly M, Wright L, Smeriglio V, Verter J, Bauer C, Shankaran S, Bada H, Walls H, Huestis M, Finnegan L, Maza P, 2001. The maternal lifestyle study: Drug use by meconium toxicology and maternal self-report. Pediatrics 107, 309–317. [DOI] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, Finnegan LP, 2002. The Maternal Lifestyle Study: Effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics 110, 1182–1192. [DOI] [PubMed] [Google Scholar]
- Lin D, Kellogg CK, 1996. Neonatal exposure to cocaine enhances the reward-potentiating properties of the drug in young adult animals. Behav. Neurosci 110, 791–801. [DOI] [PubMed] [Google Scholar]
- Loeber R, Farrington DP, Stouthamer-Loeber M, Van Kammen WB, 1998. Antisocial behavior and mental health problems Explanatory factors in childhood and adolescence. Lawrence Erlbaum Associates, Mahwah, NJ. [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V, 2002. A comparison of methods to test mediation and other intervening variable effects. Psychol. Methods 7, 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Hitch JE, Kosterman R, McCarty CA, Herrenkohl TI, Hawkins JD, 2010. Growth in adolescent delinquency and alcohol use in relation to young adult crime, alcohol use disorders, and risky sex: A comparison of youth from low- versus middle-income backgrounds. J. Child Psychol. Psychiatry 51, 1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes LC, 2002. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol. Teratol. 24, 385–395. [DOI] [PubMed] [Google Scholar]
- Miller MA, Rahe RH, 1997. Life changes scaling for the 1990s. J. Psychosom. Res, 43, 279–292. [DOI] [PubMed] [Google Scholar]
- Min MO, Minnes S, Kim J-Y, Yoon M, Singer LT, 2017. Association of prenatal cocaine exposure, childhood maltreatment, and responses to stress in adolescence. Drug Alcohol Depend. 177, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Lang A, Albert JM, Kim J-Y, 2016. Pathways to adolescent sexual risk behaviors: Effects of prenatal cocaine exposure. Drug Alcohol Depend. 161, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Lang A, Weishampel P, Short EJ, Yoon S, Singer LT, 2014a. Externalizing behavior and substance use related problems at 15 years in prenatally cocaine exposed adolescents. J. Adolesc. Health 37, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Yoon S, Short EJ, Singer LT, 2014b. Self-reported adolescent behavioral adjustment: Effects of prenatal cocaine exposure. J. Adolesc. Health 55, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Min MO, Kim J-Y, Francis MW, Lang A, Wu M, Singer LT, 2017. The association of prenatal cocaine exposure, externalizing behavior and adolescent substance use. Drug Alcohol Depend. 176, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, Quehl D, 2010. The effects of prenatal cocaine exposure on problem behavior in children 4–10 years. Neurotoxicol. Teratol 32, 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer L, Min MO, Wu M, Lang A, Yoon S, 2014. Effects of prenatal cocaine/polydrug exposure on substance use by age 15. Drug Alcohol Depend. 134, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Dickson N, Silva PA, Stanton W, 1996. Childhood-onset versus adolescent-onset antisocial conduct in males: Natural history from age 3 to 18. Dev. Psychopathol 8, 399–424. [Google Scholar]
- Najman JM, Hayatbakhsh MR, Clavarino A, Bor W, O’Callaghan MJ, Williams GM, 2010. Family poverty over the early life course and recurrent adolescent and young adult anxiety and depression: A longitudinal study. Am. J. Public Health 100, 1719–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle S, Hawkins J, Hill K, 2011. Men’s and women’s pathways to adulthood and associated substance misuse. J. Stud. Alcohol Drugs 72, 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehler TF, Véronneau MH, Dishion TJ, 2012. Substance use progression from adolescence to early adulthood: Effortful control in the context of friendship influence and early-onset use. J. Abnorm. Child Psychol 40, 1045–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath AJ, Fried PA, 2005. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol. Teratol 27, 267–277. [DOI] [PubMed] [Google Scholar]
- Radloff L, 1977. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas 1, 385–401. [Google Scholar]
- Reinherz HZ, Paradis AD, Giaconia RM, Stashwick CK, Fitzmaurice G, 2003. Childhood and adolescent predictors of major depression in the transition to adulthood. Am. J. Psychiatry 160, 2141–2147. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Larkby C, Day NL, 2015. Effects of prenatal cocaine exposure on adolescent development. Neurotoxicol. Teratol 49, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Larkby C, Day NL, 2013a. Effects of prenatal cocaine exposure on child behavior and growth at 10 years of age. Neurotoxicol. Teratol 40, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Larkby C, Goldschmidt L, Day NL, 2013b. Adolescent initiation of drug use: Effects of prenatal cocaine exposure. J. Am. Acad. Child. Adolesc. Psychiatry 52, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Leech S, Willford J, 2011. Prenatal cocaine exposure: Effects on mother- and teacher-rated behavior problems and growth in school-age children. Neurotoxicol. Teratol 33, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J, 2009. Continued effects of prenatal cocaine use: Preschool development. Neurotoxicol. Teratol 31, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J, 2008. The effects of prenatal cocaine use on infant development. Neurotoxicol. Teratol 30, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Hamel SC, Goldschmidt L, Day NL, 1999. Growth of infants prenatally exposed to cocaine/crack: Comparison of a prenatal care and a no prenatal care sample. Pediatrics. 104, e18. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Huestis MA, Day NL, 2006. Assessing in utero exposure to cannabis and cocaine In: Bellinger DC (Ed.). Human developmental neurotoxicology. Taylor and Francis Group, New York, NY: pp. 287–302. [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM, 2000. Diagnostic interview schedule for DSM-IV. Washington University School of Medicine, Department of Psychiatry, St. Louis, MO. [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS, 1981. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch. Gen. Psychiatry 38, 381–389. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Mead AN, Kosofsky BE, 2002. Increased vulnerability to administer cocaine in mice prenatally exposed to cocaine. Psychopharmacology 163, 221–229. [DOI] [PubMed] [Google Scholar]
- Ross EJ, Graham DL, Money KM, Stanwood GD, 2015. Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacol. Rev 40, 61–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE, 2000. Temperament and personality: Origins and outcomes. J. Pers. Soc. Psychol 78, 122–135. [DOI] [PubMed] [Google Scholar]
- Rutherford MJ, Cacciola JS, Alterman AI, McKay JR, Cook TG, 2000. Contrasts between admitters and deniers of drug use. J. Subst. Abuse Treat 18, 343–348. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2017. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Schulenberg J, Maggs JL, 2002. A developmental perspective on alcohol use and heavy drinking during adolescence and the transition to young adulthood. J. Stud. Alcohol Suppl 14, 54–70. [DOI] [PubMed] [Google Scholar]
- Schulenberg J, Merline A, Johnston L, O’Malley P, Bachman J, Laetz V, 2005. Trajectories of marijuana use during the transition to adulthood: The big picture based on national panel data. J. Drug Issues 35, 255–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg J, Sameroff A, Cicchetti D, 2004. The transition to adulthood as a critical juncture in the course of psychopathology and mental health. Dev. Psychopathol 16, 799–806. [DOI] [PubMed] [Google Scholar]
- Schwab-Stone M, Koposov R, Vermeiren R, Ruchkin V, 2013. Cross-cultural findings on community violence exposure and internalizing psychopathology: Comparing adolescents in the United States, Russia, and Belgium. Child Psychiatry Hum. Dev 44, 516–524. [DOI] [PubMed] [Google Scholar]
- Slade E, Stuart E, Salkever D, Karakus M, Green K, Ialongo N, 2008. Impacts of age of onset of substance use disorders on risk of adult incarceration among disadvantaged urban youth: A propensity score matching approach. Drug Alcohol Depend. 95, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonon KE, Richardson GA, Cornelius JR, Kim KH, Day NL, 2015. Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol. Teratol 47, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, 1979. Preliminary manual for the state-trait personality inventory Center for Research in Behavioral Medicine and Health Psychology, University of South Florida, Tampa FL. [Google Scholar]
- Staff J, Schulenberg J, Maslowsky J, Bachman J, O’Malley P, Maggs J, Johnston LD, 2010. Substance use changes and social role transitions: Proximal developmental effects on ongoing trajectories from late adolescence through early adulthood. Dev. Psychopathol 22, 917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A, Becker L, Huber A, Catalano R, 2012. Review of risk and protective factors of substance use and problem use in emerging adulthood. Addict. Behav 37, 747–775. [DOI] [PubMed] [Google Scholar]
- Tropea TF, Guerriero RM, Willuhn I, Unterwald EM, Ehrlich ME, Steiner H, Kosofsky BE, 2008. Augmented D-sub-1 dopamine receptor signaling and immediate-early gene induction in adult striatum after prenatal cocaine. Biol. Psychiatry 63, 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker J, Ellickson P, Orlando M, Martino S, Klein D, 2005. Substance use trajectories from early adolescence to emerging adulthood: A comparison of smoking, binge drinking, and marijuana use. J. Drug Issues 35, 307–331. [Google Scholar]
- Vorhees C, 1989. Concepts in teratology and developmental toxicology derived from animal research. Ann. N.Y. Acad. Sci 562, 31–41. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Silvestri JM, Lin D, Buhrfiend CM, Lo ES, Carvey PM, 1993. Effect of cocaine in early gestation on striatal dopamine and neurotrophic activity. Pediatr. Res 34, 389–392. [DOI] [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, Fergusson DM, 2004. Drinking patterns in mid-adolescence and psychosocial outcomes in late adolescence and early adulthood. Addiction 99, 1529–1541. [DOI] [PubMed] [Google Scholar]
- Windle M, Lerner R, 1986. Reassessing the dimension of temperament individuality across the life span: The Revised Dimensions of Temperament Survey (DOTS-R). J. Adolesc. Res 1, 213–30. [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Glass JM, Adams K 2006. Behavioral control and resiliency in the onset of alcohol and illicit drug use: A prospective study from preschool to adolescence. Child Dev. 77, 1016–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman PA, Cross W, Brown CH, Yu Q, Tu X, Eberly S, 2010. Intervention to strengthen emotional self-regulation in children with emerging mental health problems: Proximal impact on school behavior. J. Abnorm. Child Psychol 38, 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]