Abstract
Objective: Early life trauma (ELT) is a significant risk factor for the onset of depression. Emerging findings indicate ELT is associated with enhanced amygdala reactivity to aversive stimuli in never‐depressed healthy controls as well as those with acute depression but may be absent in non‐ELT exposed depressed. The precise mechanism mediating these differences in amygdala reactivity remains unclear. Method: The authors used Granger causality methods to evaluate task‐based directional connectivity between medial or lateral prefrontal cortex (PFC) and amygdala in 20 unmedicated patients with current major depressive disorder (MDD) and 19 healthy matched controls while participants engaged in an affective variant of the flanker task comparing response to sad and neutral faces. These data were correlated with childhood trauma history. Results: Exposure to ELT was associated with failure of inhibition within the MDD group based on medial PFC–amygdala connectivity. In contrast, non‐ELT exposed MDD was associated with a negative causal pathway from medial prefrontal cortex to amygdala, despite reduced dorsolateral PFC input in comparison to healthy controls. Neither MDD group demonstrated significant lateral PFC–amygdala connectivity in comparison to healthy controls. Conclusions: Failure of the circuit implicated in emotion regulation was associated with a significant history of ELT but not with MDD more broadly. Non‐ELT related depression was associated with intact regulation of emotion despite the absence of difference in severity of illness. These findings indicate opposing system‐level differences within depression relative to ELT are expressed as differential amygdala reactivity. Hum Brain Mapp 35:4815–4826, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: imaging, major depressive disorder, early life trauma, amygdala, medial prefrontal cortex, dorsal lateral prefrontal cortex
INTRODUCTION
Enhanced amygdala response is posited to be a key aspect of the underlying pathophysiology of depression [Price and Drevets, 2010]. Imaging studies of amygdala activity in major depressive disorder (MDD) using both positron emission tomography and functional magnetic resonance imaging (fMRI) methods have mostly demonstrated increased glucose metabolism and cerebral blood flow in response to aversive stimuli [Drevets et al., 1992; Surguladze et al., 2005; Suslow et al., 2010; Victor et al., 2010] in comparison to healthy volunteers; although see Townsend et al. [2010] for contradictory findings. This pattern of response is consistent with the increased negative affect associated with MDD, as well as the subjective experience of self‐induced sadness in healthy volunteers [Abercrombie et al., 1998; Posse et al., 2006]. Yet recent investigations indicate amygdala hyperreactivity may be a consequence of early life trauma (ELT) such as sexual and physical abuse or neglect, evident among healthy volunteers with no psychiatric history, as well as unmedicated depressed patients [Dannlowski et al., 2012; Grant et al., 2011]. In contrast, MDD in the absence of ELT does not appear to be linked to amygdala hyperreactivity [Grant et al., 2011]. This study sought to determine whether these differences are attributable to opposing connectivity patterns in the circuit implicated in implicit regulation of emotion.
Investigations of regulation of emotion in healthy volunteers have reliably demonstrated a role for dorsal lateral prefrontal cortex (DLPFC) in the modulation of amygdala response [Banks et al., 2007; Meyer‐Lindenberg et al., 2005; Stein et al., 2007]. Despite sparse, unidirectional, ascending projections from amygdala to DLPFC observed in trace studies [Ghashghaei and Barbas, 2002], functional connectivity between these structures is theorized to underlie cortical inhibition of amygdala output, though indirectly. A proposed route for this indirect influence is via medial prefrontal cortex (mPFC) which has direct anatomical connections with both lateral PFC and amygdala [Price, 2005]. Subsequent stimulation of neurons within the mPFC has been found to underlie decreased responsiveness of output neurons in the amygdala [Quirk et al., 2003].
Altered lateral and medial prefrontal cortical activity has been observed relative to MDD. Specifically, decreased DLPFC activity [Siegle et al., 2007] and reduced DLPFC–amygdala connectivity have been observed [Dannlowski et al., 2009; Erk et al., 2010]. Likewise, altered rostral and subgenual cingulate activity and reduced mPFC–amygdala connectivity have both been observed in MDD [Johnstone et al., 2007; Mayberg et al., 1997; Pizzagalli et al., 2001].
However, recent studies denote an underlying heterogeneity within unipolar depression based on trauma history associated with a cluster of key neurobiological features that include altered HPA and autonomic output [Heim et al., 2000], altered brain morphology (e.g., atrophy of hippocampus and mPFC/anterior cingulate [Treadway et al., 2009; Vythilingam et al., 2002]), and genetic mechanisms (CRHR1; Bradley et al. [2008]; Rogers et al. [2013]), as well as exaggerated localized amygdala reactivity [Grant et al., 2011]. However, recent reviews indicate the effects of cumulative stress are more mixed in humans than animal models [Frodl and O'Keane, 2013]. Yet these findings parallel rodent and non‐human primate findings following chronic variable stress and ELT [Mirescu et al., 2004; Radley et al., 2008; Vyas et al., 2003] demonstrating glucocorticoid and CRF‐mediated transformations in brain morphology and neuronal hyperexcitability [Duvarci and Pare, 2007; Radley et al., 2004, 2008].
Thus, based on evidence of divergent brain structure and physiology of key aspects of the circuit that typically underlie the modulation of emotion, we anticipated (1) replication of prior connectivity differences between MDD and controls [Dannlowski et al., 2009; Erk et al., 2010] but with causal directions from lateral PFC to amygdala via mPFC among controls and (2) within the MDD group, differential and opposing connectivity patterns for mPFC–amygdala inhibition based on trauma history (i.e., reduced or absent mPFC–amygdala connectivity within the ELT‐exposed MDD group but intact mPFC–amygdala connectivity with the non‐ELT exposed MDD group). Given the ongoing search for neuroimaging biomarkers to improve the classification of depression and treatment effectiveness [McGrath et al., 2013], we believe findings from the current investigation will help reduce heterogeneity of findings relative to unipolar depression.
We tested our hypotheses using a between‐group comparison of directional connectivity employing Granger causality mapping based on a priori defined regions of interest (ROIs) garnered from the existing literature in relation to depression and emotion processing. We subsequently tested our second hypothesis that strength and direction of connectivity within MDD would be driven by trauma history using within‐group correlations between trauma history and connectivity coefficients. On the basis of our prior finding of discrete amygdala reactivity differences [Grant et al., 2011] and mPFC atrophy relative to trauma history among moderately depressed outpatients [Treadway et al., 2009], we predicted a failure of inhibition among ELT‐exposed MDD but intact inhibition among non‐ELT exposed MDD.
METHODS
Twenty patients with current depression and 19 healthy control subjects completed this study. The Vanderbilt University Institutional Review Board approved the experimental protocol. A complete description of the study was provided to all participants, and all subjects provided written informed consent. Subjects were recruited through the Vanderbilt University Medical Center Outpatient Psychiatry Clinic and through television advertisements in the community.
All subjects were evaluated using the Structured Clinical Interview for DSM‐IV (SCID; First et al. [2002]). Clinical evaluations were performed by master's level and doctoral level therapists in the department of psychiatry. Supervision and review were provided by authors MMG and RCS. Participants were between 18 and 55 years of age with no significant history of neurological disease or lifetime history of brain injury. Patients were diagnosed with unipolar depression and met full criteria for one or more episodes of MDD as determined by the SCID. Patients were excluded if they met criteria for specific Comorbid Axis I disorders that included obsessive‐compulsive disorder, schizophrenia and other psychotic disorders, bipolar disorder, substance dependence, or substance abuse. A score of 16 or higher on the 17‐item Hamilton depression rating scale (HDRS; Hamilton [1967]) was required for inclusion. Participants who were currently taking antidepressants were excluded from the study. Never‐depressed control subjects were free of (1) neurological disease and head injury (2) either current or past mood disorders, as well as (3) current or past history of Axis I disorders with the exception of one subject who was diagnosed with mild agoraphobia without panic disorder. Control subjects were required to have a score of six or less on the HDRS‐17. All participants who met criteria were then scheduled for a scan session.
Additional Measures
Groups were matched on age, education, and IQ and all participants were administered the childhood trauma questionnaire (CTQ‐SF; Bernstein and Fink [1998]). The CTQ is a self‐report measure of childhood maltreatment comprised of five factors that assess emotional, physical, or sexual abuse, emotional neglect, and physical neglect. Cutoff scores were based on the work of Scher et al. [2004]. Additional clinical measures included assessments of severity of illness (HRSD‐17) and anxiety (Beck Anxiety Inventory) [Hamilton, 1967; Piotrowski, 1999].
Stimuli and Paradigm
An affective paradigm described previously in more detail in our prior work related to ELT and amygdala reactivity was performed [Grant et al., 2011]. Participants completed a gender identification variant of the Eriksen flanker task of selective attention [Eriksen and Eriksen, 1974] using selected Ekman faces as stimuli [Ekman and Friesen, 1974]. The task was designed to identify the influence of valence on the efficiency of selective attention (positive, sad, and neutral) and level of task difficulty (nonconflict, congruent, and incongruent). Participants were instructed to respond with a predetermined button press (index or middle key) to identify either male or female centralized target faces. In this study, only findings for the nonconflict level of task difficulty are presented as increased task difficulty has been shown to elicit decreased limbic activity. Subjects performed 12 blocks of 9 trials. Each trial had a total duration of 3,000 ms. Trials were counterbalanced across subjects and trial order randomized.
Image Acquisition
Scans were acquired on a 3T Philips Intera Achieva scanner at the Vanderbilt University Institute of Imaging Sciences. High‐resolution structural images were acquired in the axial plane to facilitate spatial normalization using a 3‐D T1‐weighted IR Prepped 3DFFE sequence (TR = 10.1, TE = 4.2, slice thickness = l.2 mm) and T2‐weighted [TR = 450 ms, TE = 17 ms, FOV = 24 cm, and slice thickness = 4 mm]. Twenty‐eight axial interleaved 4.0 mm functional slices (0.5 mm skip) were acquired parallel to the AC‐PC line using a gradient echo‐pulse sequence providing whole brain coverage (T2*‐weighted images sensitive to BOLD signal changes; TR = 3,000 ms, TE = 28 ms, FOV = 24 cm, flip = 90, and slice thickness = 4 mm).
Functional imaging data were analyzed using statistical parametric mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm). All participants were scanned while viewing sad and neutral faces. Echo‐planar time series data were subsequently slice time and motion corrected (aligned to the first slice), coregistered and spatially normalized to standard Montreal Neurological Institute space and smoothed with an 8‐mm Gaussian kernal. A first‐level model was used that included movement parameters from the realignment stage as covariates of no interest in the regression model. Regressors representing trial type (negative and neutral valenced faces) were modeled with a canonical hemodynamic response function (HRF). Second‐level analysis was used for group comparisons of the contrast negative > neutral for a priori defined ROIs.
Region Selection
Six bilateral regions were selected based on findings from trace studies in non‐human primates as well as connectivity in nonclinical studies of regulation of emotion [Banks et al., 2007; Ghashghaei and Barbas, 2002; Stein et al., 2007]. The selected regions included two rostral cingulate regions (BA 32 and BA 24), subgenual cingulate (BA 25), lateral prefrontal cortex (BA 46/9), amygdala, and hippocampus. Amygdala and hippocampus ROIs were selected using the automated anatomical labeling system based on the WFU PickAtlas toolbox [Maldjian et al., 2003]. Brodmann's areas were defined using the Talaraich Daemon within the PickAtlas toolbox. Functionally defined regions within these ROIs were then used in the connectivity analysis. Peak voxel values within each ROI are included in Table 2. The mean time series for each ROI per subject was subsequently extracted. All comparisons were reported using a FDR corrected P‐value of 0.05.
Table 2.
Between Between‐group differences in directional brain connectivity
| ROI | Peak voxel coordinates | t‐value | P‐value |
|---|---|---|---|
| (A) Patients > Controls Patths originating from mPFC | |||
| Amygdala_L | −22, −2, −16 | 1.93 | 0.02 |
| Amygdala_R | 20, 2, −16 | 1.67 | 0.04 |
| BA25_L | −2, 2, −6 | 1.72 | 0.04 |
| BA25_R | 2, 4, −10 | 2.47 | 0.01 |
| BA32_L | −14, 24, 34 | 2.26 | 0.01 |
| BA32_R | 14, 8, 38 | 2.05 | 0.02 |
| DLPFC_L | −32, 20, 34 | 2.01 | 0.02 |
| DLPFC_R | 32, 24, 40 | 2.33 | 0.01 |
| B. Controls > Patients Paths originating from DLPFC | |||
| Amygdala_L | −22, −2, −16 | 2.10 | 0.01 |
| Amygdala_R | 20, 2, −16 | 1.76 | 0.03 |
| BA24_L | −6, −8, 38 | 1.88 | 0.02 |
| BA25_R | 2, 4, −10 | 3.40 | 0.001 |
| BA32_R | 14, 8, 38 | 3.22 | 0.001 |
Note. The t‐values are derived from between group comparison of connectivity values obtained for each path. Region of Interest; ROI, BA; Brodmann's Area, DLPFC; dorsal lateral prefrontal cortex, mPFC; medial prefrontal cortex; Right; R and Left; L.
Voxel‐Based Morphometry
Voxel‐based morphometry (VBM) was used to investigate what role atrophy of a priori defined regions central to the regulation of emotion (bilateral BAs 24 and 32) may have in explaining subsequent differences in connectivity relative to trauma history. We selected these ROIs based on our prior VBM findings in MDD with and without a history of ELT [Treadway et al., 2009]. All structural images were examined for artifacts and then reoriented to a center point located on the anterior commissure. A customized anatomical template was created from the reoriented structural MRI images. Template creation included spatial normalization of all the images to MNI space. Spatially normalized images were resliced with a final voxel size of 1.5 × 1.5 × 1.5 mm3, and subsequently segmented into gray matter, white matter, and CSF images. After segmentation, the segmented gray matter images were modulated by multiplication of the Jacobian determinant of the spatial normalization function to estimate volumetric differences between groups. All subsequent statistical analyses were performed on the normalized, segmented, modulated, and smoothed gray matter images.
Directional Connectivity Analysis
The principle of Granger causality asserts that the directional causal influence from time series A to time series B can be inferred if past values of time series A help predict the present and future values of the time series B [Granger, 1969]. The prediction framework is realized using a multivariate autoregressive (MVAR) model. In this study, the HRF was deconvolved [Havlicek et al., 2011] to obtain the hidden neuronal variables to which the MVAR model was applied (Fig. 1). Instead of using traditional techniques where the time series are sliced corresponding to respective conditions, the MVAR model coefficients were made to vary as function of time. Subsequently, the time‐varying connectivity measures were then populated into different condition‐specific samples, which were statistically compared for forming inferences.
Figure 1.
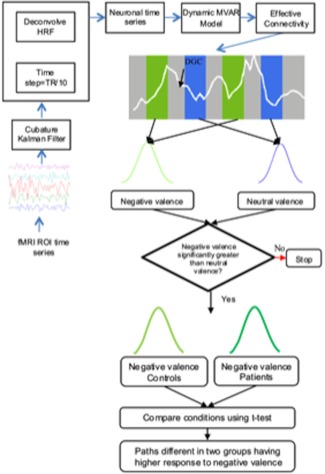
Schematic illustrating the directional connectivity analysis pipeline. The time series extracted from different ROIs were first deconvolved using a cubature Kalman filter without any assumptions about the shape of the underlying hemodynamic response. As this is a continuous time model, a time step of TR/10 was used to discretize it. The resulting latent neuronal variables were input into the dynamic multivariate autoregressive model to obtain time‐varying directional connectivity between the ROI time series. The connectivity values corresponding to negative and neural valence conditions were populated into different samples to find the paths which were greater during negative valence. Among these paths, the connectivity values for negative valence were compared across the two groups to determine those which differed between groups. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Granger Causality Modeling
In previous studies, the predictive relationship between the fMRI time series from different regions of the brain has been characterized using MVAR [Deshpande et al., 2008, 2009; Roebroeck et al., 2005]. Several of the earlier studies demonstrated that the spatial variability of the HRF can have a vascular origin and can confound the Granger causality metrics obtained from the fMRI time series. One way to address this confound is hemodynamic deconvolution, which not only increases the effective temporal resolution of the signal by removing the smoothing effect of the HRF but also removes the intersubject and interregional variability of HRF [Handwerker et al., 2004].
Let j fMRI time series be represented as X(t) = [x 1(t) x 2(t) … xj(t)]. A dynamic state‐space model can be described as follows.
where n is the neuronal state variable, u is the exogenous input, and θ are the parameter variables. The current neuronal state is linked to the previous neuronal states, exogenous inputs, and parameters by function f. The subscript τ indicates continuous time and the superscript j indicates the number of time series in the model. X, Y, and Z are zero mean Gaussian state noise vectors. The observation equation, which links the state to observation variables, is as follows.
where h is the measurement function which links the state variables to measurement variables, t is discrete time, and η is the measurement noise. The inputs to the model are exogenous inputs u, which is the experimental boxcar function, and xj(t) is the observed fMRI signal. As shown earlier, blind hemodynamic deconvolution using cubature Kalman filter is very efficient in performing a joint estimation of the hidden neuronal variables and parameters [Havlicek et al., 2011]. In addition, by using a time step up to 10 times smaller than the TR while discretizing the continuous time model, higher effective temporal resolution can be obtained. As a result, the efficiency of the connectivity analysis is improved. The neuronal state variables nj(t) can be input into the MVAR as follows:
where ρ is the order of the model determined by the Akaike/Bayesian information criterion, e is the model error, and a are the model coefficients. Here a (0) represents the instantaneous influences between time series while a(m), m = 1, …, j represent the causal influences between time series. As shown previously, the effect of instantaneous correlation on the causal metrics can be minimized by modeling both causal and instantaneous terms in a single model [Deshpande et al., 2010]. The MVAR model can be made dynamic by allowing the model coefficients to vary as a function of time as given below.
The model coefficients a(m, t) were taken as the state vector of a Kalman filter and adaptively estimated using the algorithm proposed by Arnold et al. (1998). Dynamic Granger causality was then obtained as follows:
Statistical Analysis
We tested our initial hypothesis that our MDD and healthy control groups would utilize divergent causal pathways for the regulation of emotion by obtaining connectivity coefficients for each a priori defined path per group. Unless otherwise specified, subsequent analysis was based on analysis of variance with group as the between subjects factor (healthy controls vs. MDD) and path strength as the dependent factor of comparison. To address potential concerns that illness severity or anxiety may better explain the observed group differences, follow‐up analysis included self‐report and clinician evaluated measures of anxiety and depression as covariates. In addition, based specifically on our prior finding of reduced rostral medial cortical volume among ELT‐exposed depressed [Treadway et al., 2009], brain morphology (bilateral rostral BA 24 and BA 32) was also included as a covariate. To address our second hypothesis regarding differential connectivity within MDD based on trauma history, follow‐up comparisons within the depressed group were subsequently performed, with analysis restricted to the pathways in which MDD > healthy controls. Correlations were performed to identify individual differences in the relationship between CTQ subscale scores and connectivity coefficients for each group.
RESULTS
Demographics and Clinical Measures
The depressed group exceeded matched controls in severity on all clinical measures, as well as measures of ELT (Table 1). While there were no differences between patients and controls for age, group differences for age were observed between the MDD groups (ELT‐exposed MDD > non‐ELT exposed MDD), thus, age was included as a covariate for within‐group comparisons of patients. Trauma scores within the patient group ranged for PA ranged between 5 and 21; SA ranged between 5 and 25; median scores for PA; 8.5 and SA; 5.0).
Table 1.
Demographic data and CTQ scores
| Variable | Healthy controls | MDD | Non‐ELT MDD | MDD + ELT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Sample size | 19 | 20 | 10 | 10 | ||||||||
| Number of female participants | 10 | 11 | 7 | 4 | ||||||||
| Agec | 19 | 31.2 | 9.2 | 20 | 34.5 | 10.7 | 10 | 29.2 | 9.3 | 10 | 39.3 | 9.5 |
| Estimated IQ (Shipley | 19 | 109.4 | 8.8 | 20 | 106.3 | 8.2 | 10 | 103.4 | 8.1 | 10 | 109.2 | 7.7 |
| Hamilton rating scale of depressionb | 19 | 0.84 | 1.3 | 20 | 21.4 | 4.1 | 10 | 21.4 | 4.3 | 10 | 21.4 | 4 |
| BDIb | 19 | 0.79 | 1.1 | 20 | 31.6 | 9.5 | 10 | 29.2 | 8.9 | 10 | 34 | 9.9 |
| BAIb, c | 19 | 1.47 | 2.1 | 20 | 15.4 | 10.7 | 10 | 10.4 | 6.0 | 10 | 20.4 | 12.2 |
| Onset ageb, c | 20 | 34. | 24.9 | 10 | 24.6 | 9.9 | 10 | 48.8 | 33.2 | |||
| Number of previous episodes | 20 | 2.6 | 0.9 | 10 | 2.7 | 1 | 9 | 2.3 | 0.5 | |||
| Average duration of illness (years) | 20 | 11.8 | 13.2 | 10 | 5.13 | 4.4 | 10 | 17.9 | 15.6 | |||
| Past alcohol abuse | 3 | 1 | 2 | |||||||||
| Co‐morbid/ anxiety disorder | 10 | 2 | 8 | |||||||||
| Past anxiety disorder | 1 | 3 | 2 | 1 | ||||||||
| CTQ emotional abuse scaleb, c | 19 | 6.1 | 1.5 | 20 | 12.1 | 6.1 | 10 | 9.3 | 5.3 | 10 | 14.9 | 5.6 |
| CTQ physical abuse scaleb, c | 19 | 5.4 | 0.6 | 20 | 9.9 | 4.5 | 10 | 6.7 | 1.1 | 10 | 13.1 | 4.3 |
| CTQ sexual abuse scalea, c | 19 | 5.6 | 2.5 | 20 | 9.8 | 6.8 | 10 | 5.5 | 1.2 | 10 | 14.0 | 7.6 |
| CTQ emotional neglectb | 19 | 7.2 | 2.8 | 20 | 12.6 | 4.4 | 10 | 11.0 | 4.4 | 10 | 14.9 | 3.8 |
| CTQ physical neglecta | 19 | 5.1 | 0.3 | 20 | 8.5 | 4 | 10 | 9.1 | 2.6 | 10 | 7.8 | 5.1 |
P < 0.05 in the comparison between Healthy Controls and MDD.
P < 0.001 in the comparison between Healthy Controls and MDD.
P < 0.05 in the comparison between MDD Only and MDD + Trauma.,
P < 0.001 in the comparison between MDD only and MDD + Trauma.
Directional Connectivity
Significant directional connectivity paths were observed for the contrast of negative > neutral. Paths originating from right dorsolateral PFC (R DLPFC; peak voxel, x = 41, y = 19, z = 38) were greater in controls (Fig. 2; Table 2), whereas paths originating from left Brodmann area 24 (BA24L; peak voxel, x = −6, y = 4, z = 34) were significantly greater in MDD (see Fig. 3; Table 2).
Figure 2.
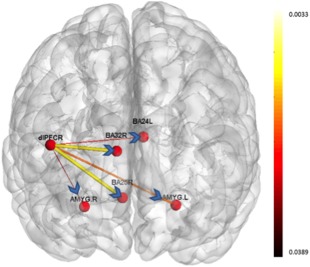
Paths which were significantly greater during the negative valence condition as compared to neutral valence condition in the MDD group. The width and color of the arrows represent the P‐value (thicker lines are associated with more robust connectivity). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
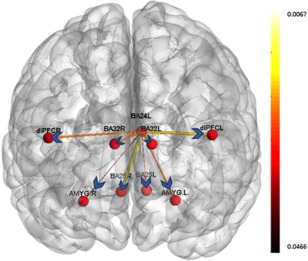
Paths which were significantly greater during the negative valence condition as compared to neutral valence condition in the control group. The width and color of the arrows represent the P‐value (thicker lines are associated with more robust connectivity). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Between‐Group Comparison
Subsequent analysis revealed group differences for paths originating from right DLPFC effecting the following regions: right amygdala [F(1, 34) = 6.73, P = 0.01; left amygdala, F(1, 34) = 8.91, P = 0.005; right BA 32, F(1, 34) = 9.30, P = 0.004; right BA 25, F(1, 34) = 5.74, P < 0.05; left BA 24, F(1, 34) = 6.16, P < 0.05]. In contrast, we did not observe significant group differences for the path originating in mPFC (P‐values ranging between 0.31 and 0.81). Follow‐up analysis without the two participants with a past history of PTSD did not differ greatly from the prior analysis in terms of group differences with right amygdala [F(1, 32) = 4.23, P < 0.05; left amygdala, F(1, 32) = 6.91, P = 0.01; right BA 32, F(1,32) = 8.26, P = 0.007; right BA 25, F(1, 32) = 5.11, P < 0.05; left BA 24, F(1, 32) = 5.16, P < 0.05].
Cingulate Volume as a Covariate
To clarify whether the relationship between ELT and directional brain connectivity is mediated by differences in cingulate morphology, we included brain volumes for BA 32 and 24 derived from VBM analysis as covariates in the model. Neither region contributed significantly to the relationship between trauma history and connectivity patterns in this study (P‐values between 0.24 and 0.85).
Trauma History and Individual Differences in Connectivity
Within‐group correlations were performed for each category of abuse for each pathway. Within the MDD group, significant correlations were observed between physical neglect and left BA 32 → right DLPFC connectivity (Fig. 4; P < 0.05), consistent with intact inhibition among the low trauma participants but weakened inhibition among high trauma participants; physical abuse was also significantly correlated with the left BA32 → right amygdala path, P < 0.05; physical neglect with right BA 32 → right amygdala connectivity, P < 0.01 (Fig. 5); and physical neglect with the right BA 32 → right DLPFC pathway (P < 0.05). No significant correlations were observed within the depressed group for sexual abuse, nor were any significant correlations observed for healthy controls. Follow‐up analysis without the two PTSD participants demonstrated significant relationships between physical neglect and L BA 32 → R DLPFC (P < 0.05) and L BA 32 → R amygdala and physical abuse and L BA 32 → R amygdala path, P < 0.05. The relationship between physical neglect and R BA 32 → R amygdala; and R DLPFC did not remain significant (P‐values between 0.58 and 0.97).
Figure 4.
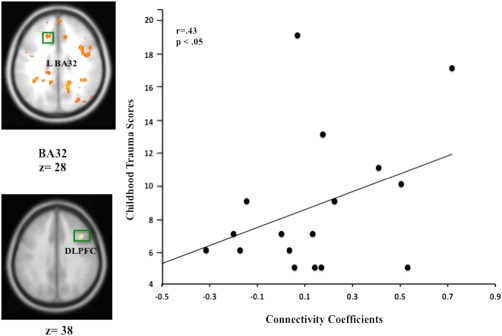
Scatter plot depicting the relationship between trauma history and mPFC‐DLPFC connectivity. Individual differences in the relationship between ELT history and mPFC‐DLPFC indicating a negative causal relationship between mesial and lateral PFC among non‐ELT exposed depressed but a positive causal relationship for ELT‐exposed depressed. N = 20, P < 0.05. Coordinates (BA 32; x = −10, y = 30, z = 28; and x = 41, y = 19, z = 38 Montreal Neurological Institute; MNI coordinate system). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 5.
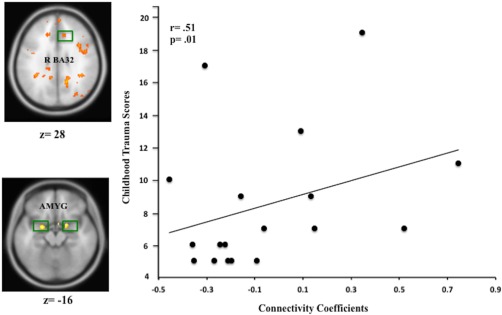
Scatter plot depicting the relationship between trauma history and mPFC–amygdala connectivity. Individual differences in the relationship between ELT history and mPFC–amygdala connectivity indicating a negative causal relationship between medial PFC and amygdala among non‐ELT exposed depressed but a positive causal relationship for ELT‐exposed depressed. N = 20, P < 0.01. Coordinates (BA 32; x = 10, y = 30, z = −6; and x = 32, y = −2, z = −16 Montreal Neurological Institute; MNI coordinate system). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Partial Correlations
Next, we performed follow‐up analysis on the trauma history and connectivity findings within the MDD group using partial correlations to control for severity of illness, anxiety, and sex differences. Findings were mixed, with the relationship between physical neglect and L BA 32 → R DLPFC remaining intact after controlling for illness severity (r = 0.47, P = 0.05), but only a significant trend observed after controlling for sex (r = 0.43, P = 0.080). No significant relationship was observed after controlling for anxiety (r = 0.22, P = 0.39). In contrast, the relationship between physical neglect and R BA 32 → R amygdala remained significant after controlling for anxiety (r = 0.51, P < 0.05), as did the relationship between physical neglect and R BA 32 → R DLPFC (r = 0.48, P = 0.05) but not when severity of illness or sex were used as covariates for either relationship (P‐values between 0.17 and 0.41).
DISCUSSION
Adaptation to change in the environment via implicit or explicit regulation of emotion is posited to facilitate both effective decision making as well as proficient social interactions [Eisenberger and Fabes, 1992; Koenigs and Tranel, 2008]. Investigations of modulation of emotion in individuals with MDD indicate a failure to adapt, originating in part from altered brain morphology as well as ineffective recruitment of the distributed network of brain regions underlying this process [Dannlowski et al., 2009; Erk et al., 2010; Johnstone et al., 2007; Siegle et al., 2007]. Yet recent reviews of the literature indicate these deficits may not be attributable to depression per se, but specifically linked to depression following a history of ELT [Heim et al., 2008; Teicher and Sampson, 2013]. Key neurobiological features differentiating ELT‐exposed MDD from MDD without trauma history have been observed consistent with disinhibited and inhibited phenotypes, respectively [Grant et al., 2011; Treadway et al., 2009; Vythilingam et al., 2002]. In particular, amygdala hyperexcitability has been observed in ELT‐exposed MDD but not non‐ELT exposed MDD [Grant et al., 2011]. Findings from the current investigation indicate, these differences are associated with differential and opposing patterns of connectivity consistent with intact inhibition among the never ELT‐exposed MDD group but failure of inhibition among the ELT‐exposed MDD group. We theorize here that these differences in connectivity likely underlie differences in amygdala reactivity to aversive faces observed in our prior work based on trauma within MDD [Grant et al., 2011].
Between‐Group Differences in Connectivity
Prefrontal cortical regions have been implicated in the modulation of emotion in response to both passive viewing tasks and active regulation and include both medial/anterior cingulate and lateral PFC (dorsal and ventral) [Erk et al., 2010; Johnstone et al., 2007; Lu et al., 2012]. In this study, we replicated prior findings of robust inverse DLPFC amygdala connectivity among healthy controls and the absence thereof in this circuit among the MDD group [Banks et al., 2007; Dannlowski et al., 2007]. Moreover, consistent with recent magnetoencephelography findings [Lu et al., 2012], our Granger causality analysis revealed a directional pattern among controls in which right DLPFC temporally preceded both mPFC and amygdala activity. In contrast, within the MDD group, activity in mPFC (L BA24) preceded activity in either DLPFC or amygdala regions. These findings suggest depressed individuals, in the absence of sufficient DLPFC influence over amygdala, use a compensatory mechanism originating from mPFC.
A recent review [Phillips et al., 2008] has put forward a model of the neurobiological correlates of emotion processing in mood disorders in which voluntary control of affect and automatic regulation of emotion are mediated by distinct circuits comprising DLPFC–amygdala vs. mPFC–amygdala, respectively. In this study, we observed more robust connectivity for the DLPFC–amygdala directional pathway among healthy controls than the MDD group, in contrast to a nonsignificant trend for increased mPFC–amygdala directional connectivity in the MDD group as compared to controls. The latter finding was driven primarily by intact inverse connectivity demonstrated by the non‐ELT exposed MDD group. However, it is unclear based on the current findings whether healthy controls used voluntary control, while patients used automatic inhibitory processes, as the design of the study was not structured to evaluate this hypothesis. An alternative explanation for this finding is that activity in each path may be driven by task‐type in an intact system such as that seen among healthy controls but driven by compensatory mechanisms in a patient population. Specifically, activity in Path A may be optimal for the function of Task A but if there is a loss of integrity in this path (e.g., reduced cingulate volume), such a failure would elicit compensatory activity in pathway B.
Within‐Group Connectivity
Within the depressed group, a significant history of either physical abuse or neglect was associated with failure of inhibition of amygdala indicated by a positive causal pathway from dorsal cingulate to amygdala. Thus, the failure of inhibition among the ELT‐exposed MDD group was linked to increased but ineffective cingulate function and not attenuated cingulate activity. This finding is consistent with prior findings [Johnstone et al., 2007] demonstrating a “counterproductive” increased recruitment of ventromedial PFC activity that failed to inhibit amygdala response during an emotion regulation paradigm among their unmedicated depressed patients. In this investigation, this finding was specific to those depressed individuals with a history of ELT. Together these findings appear to indicate impaired communication between mPFC and amygdala within ELT‐exposed MDD that may elicit increased output from cingulate as a failed compensatory mechanism resulting in a disinhibited emotional subtype.
In contrast, non‐ELT exposed MDD was associated with an intact negative causal pathway from to mPFC–amygdala, consistent with intact inhibitory cortical control of amygdala but reduced DLPFC input. This pattern of connectivity may characterize a specific subtype of depression expressed as an inhibited subtype. Notably, our findings remained significant after controlling for illness severity, anxiety level, and sex differences, consistent with ELT acting as an independent factor in its contributions to amygdala reactivity.
SUMMARY
Differential and opposing amygdala reactivity patterns have been observed in depressed individuals with and without a history of ELT. To date, the underlying pathophysiology of these differences has been unclear. This study identified reduced mPFC–amygdala causal connectivity among ELT‐exposed depressed but intact causal connectivity among non‐ELT exposed depressed. The failed connectivity among ELT‐exposed MDD may underlie the increased treatment resistance and recurrence of illness associated with the phenotype [Nanni et al., 2012].
LIMITATIONS
Some limitations of this study merit consideration. We did not observe a significant relationship between trauma history and directional brain connectivity within the control group although prior work has demonstrated a relationship between ELT and amygdala reactivity among healthy controls [Dannlowski et al., 2012; Maheu et al., 2010; McMcrory et al., 2011; Tottenham et al., 2011; van Harmelen et al., 2013]. Although ELT did occur in the healthy control group, it was infrequent in this study. We are limited in the interpretation of our findings in the absence of this group as we cannot state with certainty whether our findings are driven by ELT alone or the interaction of ELT and depression. Future investigations may consider oversampling for trauma history among healthy controls or a larger sample size to assure a range of trauma severity within both the patient and control groups. Although we did obtain significant differences between our MDD groups with and without a history of ELT, future studies should consider a larger sample size to provide sufficient power to disentangle the relationship between ELT, illness severity, and anxiety where we observed mixed findings.
Another limitation of this study is that the analysis did not include response to positive stimuli. Given differences in both valence and arousal associated positive and negative stimuli it is important to disentangle differences associated with valence (response to negative stimuli) and arousal. However, prior work regarding valence and arousal has demonstrated findings consistent with our current work including enhanced response to increasingly negative and positive words in anterior and subgenual cingulate and enhanced left amygdala response in response to increased arousal for both positive and negative stimuli [Lewis et al., 2007].
A more minor limitation was the use of a retrospective self‐report measure to evaluate childhood trauma. Prior work on mood state and memory has demonstrated enhanced recall of negative events during sad mood states, and thus, it is possible this may have contributed to greater reporting of ELT among the MDD group [Ucros, 1989]. However, the non‐ELT group was equally as depressed as the MDD with ELT group on the clinical measure of depression in our study, and yet reported less trauma severity. Moreover, prior work has demonstrated a prospective relationship between ELT and risk for MDD [Widom et al., 2007].
Finally, measures of current life stress were not evaluated in this study. This assessment will be important for future investigations. Prior work in both MDD and healthy controls with a history of ELT have observed that neither current life stress, nor adult onset stressors were better predictors of amygdala reactivity to aversive stimuli than ELT [Dannlowski et al., 2012; Grant et al., 2011].
ACKNOWLEDGMENTS
The authors thank Lauren Johnson, Morgan Shields, and Rob Hilton for their efforts with patient recruitment, technical assistance with data collection, and management in the completion of this research. Dr. Shelton has served as a consultant to Bristol‐Myers Squibb Company; Cerecor, Inc; Cyberonics, Inc.; Eli Lilly and Company; Janssen Pharmaceutica; Medtronic, Inc.; Pamlab, Inc.; Pfizer, Inc.; Ridge Diagnostics; Shire Plc; and Takeda Pharmaceuticals The other authors report no financial relationships with commercial interests. The NIMH had no role in study design; in the collection, analysis or interpretation of data; in the writing of the report; in the decision to submit the paper for publication.
REFERENCES
- Abercrombie HC, Schafer S, Larson C, Oakes T, Lindgren K, Holden J, Perlman S, Turski P, Krahn D, Benca R, Davidson R (1998): Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport 9:3301–3307. [DOI] [PubMed] [Google Scholar]
- Arnold M, Miltner W, Witte H, Bauer R, Braun C (1998): Adaptive AR modeling of nonstationary time series by means of Kalman filtering. IEEE Trans Biomed Eng 45:553–562. [DOI] [PubMed] [Google Scholar]
- Banks S, Eddy K, Angstadt M, Nathan P, Phan KL (2007): Amygdala‐frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D, Fink LA (1998): Manual for the Childhood Trauma Questionnaire. New York: The Psychological Corporation. [Google Scholar]
- Bradley R, Binder E, Epstein M, Tang Y, Nair H, Lui W, Gillespie C, Berg T, Evces M, Newport DJ, Stowe Z, Heim C, Nemeroff C, Schwartz A, Cubells J, Ressler K (2008): Influence of child abuse on adult depression: Moderation by the corticotropin‐releasing hormone receptor gene. Arch Gen Psychiatry 65:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J (2007): Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res 154:13–20. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, Hohoff C, Schoning S, Kerstling A, Bernhard B, Mortensen LS, Arolt V, Zwitserlood P, Deckert J, Heindel W, Suslow T (2009): Reduced amygdala‐prefrontal coupling in major depression: Association with MAOA genotype and illness severity. Int J Neuropsychopharmacol 12:11–22. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H (2012): Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71:286–293. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Hu X, Stilla R, Sathian K (2008): Effective connectivity during haptic perception: A study using granger causality analysis of functional magnetic resonance imaging data. NeuroImage 40:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, LaConte S, James G, Peltier S, Hu X (2009): Multivariate granger causality analysis of brain networks. Hum Brain Mapp 30:1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Sathian K, Hu X (2010): Assessing and compensating for zero‐lag correlation effects in time‐lagged Granger causality analysis of FMRI. IEEE transactions on biomedical engineering 57:1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Videe TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME (1992): A functional anatomical study of unipolar depression. J Neurosci 12:3628–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D (2007): Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci 27:4482–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N, Fabes R (1992): Emotion, regulation, and the development of social competence In: Clark M, editor. Emotion and Social Behavior: Review of Personality and Social Psychology, Vol. 14 Newbury Park, CA: Sage: pp 119–150. [Google Scholar]
- Ekman P, Friesen W (1974): Detecting deception from the body or face. J Pers Soc Psychol 29:288–298. [Google Scholar]
- Eriksen H, Eriksen C (1974): Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 16:143–149. [Google Scholar]
- Erk S, Mikschi A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H (2010): Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci 30:15726–15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J (2002): Structured clinical interview for DSM‐IV‐TR axis disorders, research version, non‐patient edition (SCID‐I/NP). New York, NY: Biometrics Research. [Google Scholar]
- Frodl T, O'Keane V (2013): How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis 52:24–37. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H (2002): Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115:1261–1279. [DOI] [PubMed] [Google Scholar]
- Granger C (1969): Investigating causal relations by econometric models and cross‐spectral methods. Econometrica 37:424–438. [Google Scholar]
- Grant M, Cannistraci C, Hollon S, Gore J, Shelton R (2011): Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatry Res 45:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1967): Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6:278–296. [DOI] [PubMed] [Google Scholar]
- Handwerker D, Ollinger J, D'Esposito M (2004): Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage 21:1639–1651. [DOI] [PubMed] [Google Scholar]
- Havlicek M, Friston K, Jan J, Brazdil M, Calhoun V (2011): Dynamic modeling of neuronal responses in fMRI using cubature Kalman filtering. Neuroimage 56:2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB (2000): Pituitary‐adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284:592–597. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008): The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 33:693–710. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ (2007): Failure to regulate: Counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. J Neurosci 27:8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Tranel D (2008): Prefrontal cortex damage abolishes brand‐cued changes in cola preference. Soc Cogn Affect Neurosci 3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ (2007): Neural correlates of processing valence and arousal in affective words. Cereb Cortex 17:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Li H, Luo G, Wang Y, Tang H, Han L, Yao Z (2012): Impaired prefrontal‐amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: A dynamic causal modeling study on MEG. Neurosci Lett 523:125–130. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JY, Ackerman JP, Pine DS, Ernst M (2010): A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci 10:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Mayberg H, Brannan S, Mahurin R, Jerabek P, Brickman J, Tekell J, Silva J, McGinnis S, Glass T, Martin C, Fox P (1997): Cingulate function in depression: A potential predictor of treatment response. Neuroreport 8:1057–1061. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, … Mayberg HS (2013): Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry 70:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMcrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E (2011): Heightened neural reactivity to threat in child victims of family violence. Curr Biol 21:R947–R948. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Olsen R, Kohn P, Brown T, Egan MF, Weinberger DR, Berman K (2005): Regionally specific disturbance of dorsolateral prefrontal‐hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62:379–386. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E (2004): Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A (2012): Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta‐analysis. Am J Psychiatry 169:141–151. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC (2008): A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13:829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski C (1999): The status of the Beck Anxiety Inventory in contemporary research. Psychol Rep 85:261–262. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Pascual‐Marqui R, Nitschke J, Oakes T, Larson C, Abercrombie HC, Schaefer SM, Koger J, Benca R, Davidson RJ (2001): Anterior cingulate activity as a predictor of degree of treatment response in major depression: Evidence from brain electrical tomography analysis. Am J Psychiatry 158:405–415. [DOI] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore G, Schneider F (2006): Real‐time fMRI of temporolimbic regions detects amygdala activation during single‐trial self‐induced sadness. Neuroimage 18:760–768. [DOI] [PubMed] [Google Scholar]
- Price JL (2005): Free will versus survival: Brain systems that underlie intrinsic constraints on behavior. J Comp Neurol 493:132–139. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC (2010): Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G, Likhtik E, Pelletier J, Pare D (2003): Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23:8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, … Hof PR (2008): Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. [Research Support, N.I.H., Extramural Research Support, Non‐U.S. Gov't]. J Comp Neurol 507:1141–1150. doi: 10.1002/cne.21588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, … Morrison JH (2004): Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125:1–6. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R (2005): Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage 25:230–242. [DOI] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, Cheverud J, Muzny DM, Gibbs RA, Davidson RJ, Kalin NH (2013): CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol Psychiatry 18:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Forde DR, McQuaid JR, Stein MB (2004): Prevalence and demographic correlates of childhood maltreatment in an adult community sample. Child Abuse Negl 28:167–180. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME (2007): Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry 61:198–209. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer‐Lindenberg A (2007): A validated network of effective amygdala connectivity. [Research Support, N.I.H., Intramural]. Neuroimage 36:736–745. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer M, Keedwell P, Giampietro V, Young AW, Travis M, Williams SCR, Phillips ML (2005): A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 57:201–209. [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, Ohrmann P, Bauer J, Pyks M, Kersting A, Arolt V, Heindel W, Dannlowski U (2010): Autonomic mood‐congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry 67:115–160. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Sampson JA (2013): Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry 170:1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Milner A, Gilhooly T, Zevin JD, Casey BJ (2011): Elevated amygdala response to faces following early deprivation. Dev Sci 14:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Eberhart N, Bookheumer S, Eisenberger N, Folan‐Ross L, Cook I, Sugar C, Altshuler L (2010): fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Res 183:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC (2009): Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One 4:e4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucros C (1989): Mood state‐dependent memory: A meta‐analysis. Cogn Emot 3:132–169. [Google Scholar]
- van Harmelen A‐L, van Tol M‐J, Demenescu LR, van der Wee NJA, Veltman DJ, Aleman A, van Buchem MA, Spinhoven P, Penninx, BWJH , Elzinga BM (2013): Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci 8:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T, Furey M, Fromm S, Ohman A, Drevets WC (2010): Relationship between amygdala responses to masked faces and mood state treatment in major depressive disorder. Arch Gen Psychiatry 67:1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S (2003): Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res 965:290–294. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, … Bremner JD (2002): Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 159:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ (2007): A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry 64:49–56. [DOI] [PubMed] [Google Scholar]


