Abstract
The interface of biological components with semiconductors is a growing field with numerous applications. For example, the interfaces can be used to sense and modulate the electrical activity of single cells and tissues. From the materials point of view, silicon is the ideal option for such studies due to its controlled chemical synthesis, scalable lithography for functional devices, excellent electronic and optical properties, biocompatibility and biodegradability. Recent advances in this area are pushing the bio-interfaces from the tissue and organ level to the single cell and sub-cellular regimes. In this progress report, we will describe some fundamental studies focusing on miniaturizing the bioelectric and biomechanical interfaces. Additionally, many of our highlighted examples involve freestanding silicon-based nanoscale systems, in addition to substrate-bound structures or devices; the former offers new promise for basic research and clinical application. In this report, we will describe recent developments in the interfacing of neuronal and cardiac cells and their networks. Moreover, we will briefly discuss the incorporation of semiconductor nanostructures for interfacing non-excitable cells in applications such as probing intracellular force dynamics and drug delivery. Finally, we will suggest several directions for future exploration.
Keywords: silicon, nanowires, bioelectronics, optical, mechanical
1. Introduction
Semiconductor-based bio-interfaces represent a domain of intense research effort where biological components, i.e. cells or tissues, are interfaced with semiconducting materials or devices[1–6]. This emerging field has grown exponentially over the past decade. The discovery of new materials, the improved fundamental understanding of signal transduction at the bio-interfaces, and the development of novel biomedical applications are changing the research paradigms. In particular, new bioelectronic tools and methodologies have significantly expanded our capability to understand and manipulate bioelectric activities across multiple length scales. For example, bioengineers and material scientists are now collaborating with neuroscientists and clinicians to broaden the toolkit available for the complicated and technically demanding research in brain modulation.
Traditional inorganic materials-based bio-interfaces were established by, e.g., contacting a single electrode or an electrode array with the cells or tissues. With the advances in top-down lithography and the miniaturization of electronic and mechanical components, the spatial and temporal resolutions of the bio-interfaces have both increased dramatically. This progress, however, has been limited by the mismatches between the rigid and static inorganic materials and the soft and dynamic biological components. In recent years, flexible and stretchable electronics, which contain one- or two-dimensional active device components[7, 8] with thicknesses in the sub-micron range, have shown promise to become the key platform for the next generation bioelectronic or biomechanical devices[9, 10–14]. These flexible and stretchable devices have allowed for the integration of active components with mechanical properties comparable to those found in living tissues; this important topic will not be covered intensively in this progress report given many other excellent reviews on the subject[2, 8, 15]. In this report, we will primarily focus on the use of nanotechnologies for cellular and subcellular scale studies[16, 17]. We will describe a few new tools developed in this field, both for sensing and stimulation; thereafter, we will discuss the current limitations and challenges, as well as future opportunities that may lead to the next generation pathways of interfacing biological matter and electronics.
The discussion will be divided into several sub-groups, based on the application targets, i.e. neuronal, cardiac, and non-excitable cells or tissues. We will focus on silicon (Si)-based materials, but several other materials will be mentioned briefly as well. Moreover, we will highlight wireless and freestanding material and device configurations, as opposed to interconnected approaches, in the context of their pros and cons and future challenges. Finally, we will limit our discussion to mammalian cells only; for microbial systems, please refer to other excellent reviews[5].
2. Traditional bioelectric interface
Here, we will discuss two aspects of the bioelectric interface, namely recording and stimulation. Although their functioning is somewhat opposite, they are naturally coupled in signal transduction. In biological systems, the charged carriers are ions and charged molecules, while they exist as electrons and holes in electronic components. To establish communications between them, we need to identify certain biophysical links. Electrical potential is one of these links, and it can be in the form of extracellular field potential or intracellular action potential (AP). For example, the dynamic flow of ions in biological tissues yields spatially defined field potentials, which can be understood with Poisson’s equation. When crossing a plasma membrane, a molecule would experience an electrical field due to the differing ion concentrations at the two sides of the membrane. These potentials, generated by either dynamic ion flow or ion concentration difference, can then be sensed by electronic devices, such as a nanowire field-effect transistor (FET), where the sensitivity is inversely proportional to the FET channel length[18], suggesting one advantage of using nanoscale device element. Before describing how nanotechnology may address the limitations of the more traditional interfacing tools, we would like to highlight some key technologies that are broadly used.
2.1. Recording or sensing
Parallel recording of extracellular electrical activity was demonstrated four decades ago with microelectronic systems where cells were cultured and recorded over an array of planer microelectrodes. The integration of microfabricated electrodes with cells was pioneered by Gross et al.[19] and further improved later on[20, 21]. Wise and colleagues were the first to push this microelectrode array to the realm of in-vivo application by designing a 3D microelectrode array[22]. This approach of using a 3D microelectrode array for in-vivo investigation was mastered by Norman and colleagues, who developed the Utah array[23], which later became one of the most broadly used bio-electric interfacing device in this field. The design of multi-electrode arrays (MEA) enabled the investigation of neuronal networks in an unprecedented way. In the past decades, microfabrication techniques improved exponentially and the feature sizes in these devices decreased dramatically. Significantly, a recently developed Neuropixels probe has 384 recording channels that can programmatically address 960 low-impedance recording sites in a single shank[24]. The fully integrated functions and small size of the Neuropixels probes allowed for the large-scale recording of single neural activities from several brain regions in freely moving rodents (Figure 1A). The main shortcomings of many of these extracellular devices, however, lies in the limited signal-to-noise ratio and the ‘modified’ shapes of the endogenous biological signals. This has meant that researchers are constrained solely to the recording of extracellular forms of APs[1, 21, 25]. More sophisticated signals, such as subthreshold synaptic activities, cannot be easily detected by planar or shank-based extracellular devices[26]. Therefore, the development of intracellular recording methodologies led to groundbreaking advances in this field.
Figure 1. Semiconductor-enabled advances in traditional electrophysiology tools.
(A) The Neuropixels probe, showing checkerboard site layout (left), a Scanning electron microscopy (SEM) of a probe tip (upper right), and one approximate probe location overlaid on the Allen Mouse Brain Atlas (lower right). (B) Quantum dot-assisted dendritic patching. A QD 625–coated pipette was used to record the electrical activities from the apical trunk of an in vitro CA1 pyramidal cell (labeled with Alexa Fluor 488). Sequential images i–iii (upper right) showed the patch formation. With this approach, uncaging-evoked excitatory postsynaptic potentials can be recorded from a dendrite (lower right). (A) Reproduced with permission.[24] Copyright 2017, Springer Nature. (B) Reproduced with permission.44 Copyright 2014, Springer Nature.
Patch clamping was first demonstrated by Neher and Sakmann[27], who were granted the Nobel Prize in Physiology or Medicine for their contribution. In this technique, a glass micropipette establishes an intracellular interface with single cells, and is used to record the membrane voltage or current. The high membrane impedance during a patch clamp experiment enables extremely high signal-to-noise ratio, enabling the recording of subthreshold events, unraveling intracellular electrical activities with sub-millisecond temporal resolution. Despite many modifications and adjustments, this technique continues to have several drawbacks. For example, because the pipette solution has direct contact with cytosol, this approach is inherently associated with diluting and dialyzing the intracellular composition of the cells[27]. Although the use of sharp microelectrodes[28], as well as the modification of the pipette pore[29] minimized this effect, the limitation remains. Moreover, the precise targeting of subcellular parts of neurons for electrophysiological recording can be affected by the poor visualization of pipette locations, especially with tissue scattering. However, recent work has demonstrated that glass pipettes coated with quantum dots [30] can provide strong two-photon contrast. This simple modification of pipettes allowed for the targeted patch-clamp recording as well as the single-cell electroporation, both in vitro and in vivo (Figure 1B). Finally, patching cells is still technically demanding and tedious, and requires skilled personnel; as it involves the use of a bulky micromanipulator, the recording of multiple cells in parallel is limited. In addition to patch clamps or other microelectrodes, voltage and calcium sensitive dyes are frequently used tools[31, 32]. The ease of use and minimal invasiveness make them key tools for investigating electrical activities in cells and tissues. However, these dyes have issues such as certain phototoxicity, and low signal-to-noise ratio, limiting them to short-term study of suprathreshold events.
2.2. Stimulation
In terms of stimulation, some of the aforementioned electrical tools can be readily used. Planner MEA were used for dual excitation and recording application[33]. Intracellular currents, injected via micropipettes in the patch clamp apparatus, were used to extract essential biophysical parameters at the cellular and subcellular scales. The invention and development of optogenetics has opened many new avenues of research. This method relies on genetic modifications involving the introduction of light-sensitive microbial ion channels into mammalian cells[34], which decouples the optical stimuli from the electrical recordings[35]. Moreover, genetically targeting specific cell populations in vivo enables the investigation of cell-specific optical stimulation of brain activities[36]. As for therapeutic applications, many ways of integrating this technology were proposed, such as recapitulating the lost image-receiving function of malfunctioning photoreceptors[37], and optical-pacing of the heart for resynchronization therapies[38]. Despite the promising and exciting progress achieved with optogenetics, its association with viral infection during gene delivery stands as a major disadvantage in terms of therapeutic applications.
3. Nanoscale semiconductor tools for neuronal applications
In all likelihood, the brain is the most complicated biosystem. As such, brain research should ideally be associated with the most state-of-the-art, innovative, and multidisciplinary technologies available. Neurons, the main electrically active part of the brain, are the most extensively studied cells in the bioelectric arena. Given that other topics have been extensively addressed in the literature [6, 16, 39], in this section we will focus solely on neural sensing and stimulation, exploring new bio-interface geometries and new signal transduction mechanisms at the bio-interfaces. Some of the examples shown below are relevant to metals or magnetic materials; however, they may be incorporated into semiconductor systems in order to expand the materials repertoire.
3.1. Material design for sensing or recording
As described above, and as extensively reviewed previously[40], multiplex recording of electrical activity in vitro was traditionally limited to suprathreshold APs. To overcome these limitations, 3D electrodes were fabricated to increase the contact area of the cell with the electrode. For instance, nanopillars were shown to double the recorded amplitude as compared to planer electrodes, but the sensing capabilities were still limited to suprathreshold APs[41]. The first demonstration of the arrayed recording of subthreshold activity was done by Spira and colleagues [42]. Using an elegant design, the researchers created gold (Au) mushroom-shaped microelectrodes with size and surface chemistry that activated endocytotic-like mechanisms. These Au microelectrodes, which were connected to semiconductor-based signal processing circuit, were engulfed by cultured neurons (Figures 2A and2B). This enfoldment simultaneously increased the area of the junctional membrane and dramatically improved the seal. These converging contributions increased the neuron–microelectrode electrical coupling coefficient by more than two orders of magnitude. Another important approach used to address this challenge was demonstrated by Park and colleagues (Figures 2C and2D). By utilizing an array of vertically aligned Si nanowires, they recorded and stimulated neuronal activity intracellularly. They showed that electroporation can be used to facilitate internalization[43]. This device was able to record intracellular electrical activity with exceptional clarity, as evidenced by the recording of positive monophasic AP. However, at this stage it was ‘blind’ to subthreshold synaptic potentials. Electrode size is a key factor that contributes to junction impedance; in this case, the smaller surface area of individual nanowires reduced the signal-to-noise ratio, and overall performance of the device. An interesting attempt to overcome this limitation was performed using plasmonic nanoelectrodes[44], but the limitation remains for most applications. Finally, it is important to note that Cui and colleagues have recently performed a series of careful biophysical and structural characterizations of the bio-interfaces established from various materials[45]. The information gleaned from these studies can be readily used to improve the performance of future neural recording devices that are based on semiconductors.
Figure 2. Microelectrode arrays for electrical recording from neurons.
(A) SEM image of Au mushroom-shaped microelectrode, and (B) Transmission electron microscopy image of Au mushroom-shaped microelectrode engulfed by a cell. The engulfment of the electrode dramatically improves the cellular-electrode electrical coupling. (C) False-colored SEM image of a nanowire array, showing metal tip (gray) and the insulating silicon dioxide stem (blue). The array was used for intracellular recording from neurons. (D) The nanowires penetrated the rat cortical cell (false-colored yellow), while the insolation layer of silicon dioxide allows the electrode to record electrical activity from the intracellular region alone, maximizing the signal-to-noise ratio. (A) Reproduced under the terms of the Creative Commons Attribution 4.0 International License. Copyright 2015, Springer Nature. Reproduced with permission.[43] Copyright 2012, Springer Nature.
In the context of neuronal networks research, one of the most substantial challenges is to allow neuronal recording in vivo. Traditionally, this was performed by incorporating an MEA into the brain, with the most broadly used tools being Utah arrays[23] and Michigan probes[46]. Despite their long-standing status as the gold standard for studying cortical circuits in non-human primates, these probes are large and bulky, and have limitations in the context of their mechanical integration. The inherent mismatch between the soft and dynamic tissue to the rigid, bulky probe results in the formation of glial scar tissue on the electrodes, impairing their ability to perform chronic recordings[47]. One of the most promising strategies to resolve this mismatch is the design of sub-micron, ultrathin, flexible Si structures[48]. By decreasing the thickness of the Si substrate, the mechanical properties greatly differ from the bulk material; in this case, the thin Si substrate is flexible, while maintaining its electrical properties (even when bent and stretched). This quality of Si nanostructures and their unprecedented implications are reviewed extensively[2, 6, 49], and thus will not be highlighted in this report.
3.2. Material design for modulation or stimulation
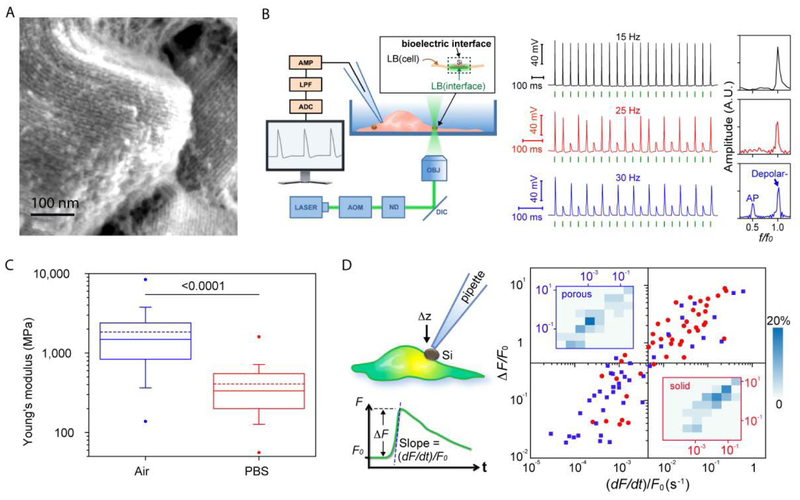
Thus far, we have discussed certain considerations in designing the electrode-cell interface, and highlighted some key contributions in this field. However, as these elements are broadly discussed and reviewed elsewhere[4, 40, 50], we would like to extend our focus to nontraditional stimulating approaches. In traditional stimulation approaches, the electrode interfacing with the cells may be used in a dual fashion: recording and stimulation. The advantages of such a dual approach are evident, but this method also possesses several inherent complications. As previously reviewed in depth[39], the incorporation of conventional electronics and their associated leads are inherently mismatched to the neural tissue. Despite tremendous progress in the field of flexible electronics[15], the challenges of their seamless integration with living tissues still remain. Additionally, although wireless technologies are developing rapidly, they are still associated with leads from electrodes to a processor, which may lead to chronic irritation between that lead and the tissue. Thus, in cases that can benefit from stimulation alone, it may be possible to use freestanding, leadless approaches for the bioelectric interface. Si is the most extensively used material in the field of bioelectric interfacing. However, most of its applications as a biomaterial have focused on Si’s tunable electrical properties. Thus, most biomedical applications rely on single-crystalline and doped Si, with other forms receiving little attention. Recently, our group has designed a new class of porous Si mesostructure that has an amorphous framework and forms more compliant to the natural, complex order of biological cellular entities. Instead of using top-down fabrication, this material was chemically synthesized by a nano-casting approach, utilizing mesoporous silica as a template. Using chemical vapor deposition (CVD), our lab deposited Si onto this template, thus forming a nanowire array, which was supported by ‘cross-linking’ micro-bridges[51] (Figure 3A). Thereafter, subsequent wet chemical etching removed the template and yielded a porous mesostructure with random micrometer- and sub-micrometer-scale voids.
Figure 3. Mesostructured and deformable Si particles for optically controlled neuromodulation.
(A) SEM image of the nanoporous Si, showing periodic arrangement of Si nanowire assembly. (B) Schematic diagram of the experimental setup (left) for neuromodulation. AMP, amplifier; LPF, low-pass filter; ADC, analog-to-digital converter; AOM, acousto-optic modulator; ND, neutral density filters; DIC, dichroic mirror; OBJ, microscope objective. Patch clamp recordings (right) of a DRG neuron that was subjected to stimulation at different frequencies (5.32 μJ for each pulse), with corresponding FFTs (right). f0 and f are input and output frequencies, respectively. Green bars show the laser pulse locations. (C) A Box-and-whisker plot of the Young’s moduli of nanoporous Si. The boxes include half of the data points, while the whiskers contain 80% of the points. Solid and dashed lines denote the medians and means, respectively. The dots are the maximum and minimum values. The p-value (the number above the bar) is from the Mann-Whitney test. (D) A schematic diagram for the single-cell calcium assay (upper left), with (∆F/F0) and ((dF/dt)/F0) defined below. A scattered plot (right) for the amplitude and slope values of the calcium dynamics shows that porous Si is mechanically less invasive. Insets show the 2D distribution histograms. See original paper for details. Reproduced with permission.[51] Copyright 2016, Springer Nature.
This 3D architecture was inspired by common natural biomaterials [52] [53] with an ordered and anisotropic fibril-based architecture. Moreover, using amorphous Si[54] improves the photothermal reactivity attributed to its enhanced light absorptivity (compared to crystalline Si), and the porous architecture reduces the thermal conductivity and capacity[55], which further enhances this photothermal effect. This feature can be exploited as a bioelectric modulator, as abrupt temperature variations were found to trigger transient capacitive currents across biological membranes[56, 57]. This hypothesis was tested on a synthetic phospholipid bilayer which was interfaced with a Si mesostructure and remotely modulated using laser pulses[51]. These pulses increased the bilayer capacitance, which resulted in a reduction of impedance; consequently, a photo-induced transient depolarizing membrane current was repeatedly demonstrated. Although this technique is associated with elevated temperatures, thermal ablation is avoided by limiting the temperature burst. Thereafter, we showed that these light-induced transient membrane currents can be used to depolarize the membrane potential of dorsal root ganglia (DRG) neurons and elicit single APs (Figure 3B). Laser pulses of 5.32 μJ were found to be the average threshold for actuating DRG neurons, a value 30 times lower than that of Au nanoparticles (NP)[57]. This threshold decrease is crucial for future applications, as light penetration through tissue may hamper such neuromodulation in the organ and in-vivo settings. To show its modulation capability, and to verify the absence of thermal ablation of the cells, this modulation was performed in trains of photo-induced APs of up to 15Hz.
The mechanical properties of this biomaterial are also attractive, as they are a key factor affecting its biocompatibility[3, 58] (where soft flexible materials are minimally invasive). Our Si mesostructure was found to have Young’s modulus of ~1.84 GPa (Figure 3C). This value is about two orders of magnitude lower than that of single crystalline Si, and one order of magnitude lower than porous Si made by electrochemical etching. Immersing it in phosphate-buffered saline (PBS) solution resulted in a further reduction of the Young’s modulus at the particle surface, making it comparable to that of hydrated collagen fibers[59]. To demonstrate the advantage of using mesostructured Si, our lab developed a calcium imaging assay (Figure 3D) to assess mechanically induced calcium dynamics in a single cell. It was shown that using a glass micropipette to press these particles against the membrane of human umbilical vein endothelial cell (HUVEC) was less invasive as compared to a single-crystalline microparticle (as expected from soft deformable materials)[2]. As for chronic biocompatibility, subcutaneous administration did not elicit adverse effects, and degradation of the mesostructured Si was observed within a period of three weeks.
In addition to the photothermal reactivity of Si discussed above[54, 55], Si possess photoelectric and photoelectrochemical properties(Figure 4). These properties have been exploited for biomedical applications, such as neuronal photoactivation[60] and photoreceptor prosthesis[61]. In a series of papers published by Palanker’s group, a class of photovoltaic subretinal prosthesis devices was developed, where near-infrared illumination pulsed over Si-based photodiodes yield photocurrents for neuron stimulation in retina. The group has showed the device efficacy in degenerate rat retinas, with stimulation threshold peak irradiances several orders of magnitude below the ocular safety limit. These technologies currently cannot be easily administered at the subcellular level. Si nanostructures, however, may address this issue given that their length scales are on the order of subcellular components. They can be grown with well-controlled doping profiles that are needed for photoelectric or photoelectrochemical response. For example, coaxial p-type/intrinsic/n-type (PIN) Si nanowires can be synthesized by dopant modulation during a CVD process and were used intensively for photovoltaic studies at single nanostructure level[62]. Our lab recently used coaxial Si nanowires and other Si-based heterostructures to wirelessly and photoelectrochemically modulate primary DRG neurons[63]. Light illumination could induce carrier separation, such that electrons migrate to the n-type region while holes to the p-type region. The electrons at the n-type shell surface could yield a cathodic process that can locally depolarize a target neuron[64].
Figure 4. Photodiode devices for optically-controlled neuromodulation.
(A) SEM image of a hexagonal array of three-diode pixels used for subretinal implant. (B) Zoom-in view of one of the pixels. (C and D) SEM images of coaxial p-type/intrinsic/n-type Si nanowires, used for nanoscale photovoltaic studies. The cross-sectional image (D) shows the coaxial structure with dopant modulation. This structure has the potential for subcellular-level neuromodulation. (A and B) Reproduced with permission.91 Copyright 2012, Springer Nature. (C and D) Reproduced with permission.[62] Copyright 2007, Springer Nature.
Besides photothermal and photoelectrochemical actuation, remote mechanical control may also be exploited to perform leadless modulation of neurons, which could be either done by a piezoelectric mechanism or triggered by a magnetic field. In the latter process, magnetic nanoparticles instead of semiconductors are the typical materials of choice; they have already been used to mechanically actuate neuronal circuits[65]. By using magnetic nanoparticles, the mechanical impact may be localized to sub-micrometer scale, which allows for precise control and high spatial resolution. The mechanical effect may be further localized by using magnetic needles, which focuses the magnetic gradient to the target cell[66]. As magnetic fields easily penetrate tissues, this approach may also be utilized for therapeutic applications. Indeed, this leadless mechanical stimulation has already been demonstrated for organ-level modulation in rats and pigs[67]. These types of magnetic materials offer a unique mechanical modulation pathway, and can potentially be integrated with other semiconductor sensor systems via, e.g., surface coating or layer-by-layer stacking.
Our ability to use nanoscale semiconductors as neuromodulators in a freestanding fashion, while avoiding gene transfection associated with optogenetics, holds many opportunities for future clinical applications. In particular, they can be simply administered, their nanoscale sizes enable high spatial specificity, and their surfaces can be easily modified to interact with specific cell types. This can be achieved by conjugating them to antibodies, which can be achieved by functionalizing them with streptavidin[68], biotin[69] or glutaraldehyde linker[70]. Additionally, we believe that remote intracellular neuromodulation is possible given that many semiconductor nanostructures can be internalized by mammalian cells, including neurons[71, 72]. As opposed to what is possible with substrate-anchored nanomaterials, moving the bioelectric interfaces into the neurons or neuron-associated cells holds great promise.
4. Nanoscale tools for cardiac applications
In this section, we will discuss applications that involve the interfaces with individual cardiomyocytes and engineered cardiac constructs. The most apparent difference between interfacing with traditional planar cell culture and engineered tissues is the 2D vs. 3D nature of these arenas. As tissue engineering involves 3D tissue constructs, addressing the interior of these constructs raises challenges that are fundamentally different from that involved in planar cell culture. Thus, the discussion in this section will be divided into 2D and 3D bio-interfaces.
4.1. Recording from two-dimensional cell culture
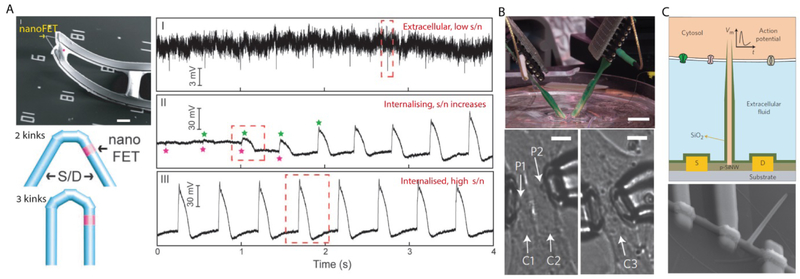
The cell-electronic interfaces have an inherent requirement for signal transduction between ionic and electronic currents[1, 73] at the electrode-cleft interface. Here, we would like to present a different approach for interfacing cells, one which overcomes this inherent limitation. In traditional pipette and metal electrodes, the signal-to-noise ratio greatly depends on the impedance of the sensing electrode[27, 40, 74, 75]. Increasing the electrode size, however, augments the invasiveness of the sensing probe, limiting the size of the electrode to the micrometer scale. Field-effect transistors (FETs), however, do not rely on charge transfer between the probe and the measured specimen, thus impedance-related limitations do not apply. Consequently, these probes can record electric potentials inside cells[11] without the aforementioned size limitations[13, 76], potentially minimizing the probe’s invasiveness. Another key advantage of FET, as oppose to a micropipette, is the absence of a cytosol dilution effect[27, 74, 77], as FETs do not rely on probe-cytosol electrolyte interaction. Thus, intracellular electrical recording may be maintained for longer durations without altering the cellular integrity. In a study performed by Lieber’s group[11], a 3D probe was designed using a kinked SiNW (Figure 5A, left). Based on previous work from the same group[78], they used a variation of reactant pressure to introduce reproducible 120° kinks. Connecting two 120° kinks in a cis- configuration resulted in a 60° kink (Figure 5A). Moreover, this junction region could be doped to create localized FET or p-n diode with a 3D geometry, which allows it to be introduced into cells. Then, remote electrical interconnects to the S/D nanowire arms were performed on ultrathin SU-8 polymer ribbons. The mechanical mismatch between the SU-8 substrate and the metal interconnect layers resulted in an interfacial stress that was used to bend the probe upward. Repetitive bending of the probe up and down did not result in any apparent degradation in its function.
Figure 5. 3D Si nanowire FETs for cardiac recording.
(A) Kinked nanowire probe for intracellular recording, showing an SEM image of a device (upper left), schematic diagrams of the material design (lower left), and representative electrical recordings (right). During the experiment, extracellular recording (I) was obtained, followed by the transitions (II) into internal recording until it maintained steady-state intracellular recording (III). Scale bar, 5 µm. (B) Multiplexed recording is possible by using two independent nanoFETs mounted over micromanipulators (upper). Two probes, P1 and P2, were positioned at two adjacent cardiomyocytes (lower left). Alternatively, they were placed in two different subcellular locations of the same cardiomyocyte (lower right). Scale bars, 1 cm (upper) and 10 µm (lower). (C) A schematic illustration (upper) and an SEM image (lower) of a branched nanotube-enabled nanoFET intracellular recording device, where the intracellular fluid can be introduced to the nanoFET by a silicon dioxide nanotube. (A) Reproduced with permission.[11] Copyright 2010, AAAS. (B) Reproduced with permission.[12] Copyright 2014, Springer Nature. (C) Reproduced with permission.[13] Copyright 2012, Springer Nature.
The sensing capabilities of the nanoscale FET (nanoFET) were measured on cultured Embryonic chicken cardiomyocytes (Figure 5A, right). To ease the penetration of the probe into the cells and to minimize its invasiveness, the probe was coated with phospholipid bilayers. Prior to internalization, the probe recorded regularly spaced spikes with an amplitude of ~3 to 5 mV, and low signal-to-noise ratio, comparable to that of extracellular recordings made with nanowire devices on substrates[79]. Briefly, and without further pushing of the probe, the probe was internalized into the cells. This was observed in the form of a decrease in baseline potential, and a dramatic change in the peak shapes. Compared to the extracellular signals, the intracellularly recorded peaks had opposite signs, larger amplitudes, a longer duration and similar frequency. Eventually, the peaks reached a steady state with amplitude and duration of ~80 mV, and ~200 ms, which were similar to those reported for whole-cell patch clamp recordings[80].
In a more recent study[12], the kinked Si nanowire probes with encoded nanoFETs were fabricated over a movable manipulator, whose position could be controlled with high spatial resolution and sub-micron precision (Figure 5B). The designed nanoFET probe was used simultaneously with a patch-clamp probe, which showed the same electrical recording. Moreover, they demonstrated real-time multiplexed recording from adjacent cells, and within the same cell in different subcellular regions, using two independent nanoFET probes. Multiplexing these probes allows for the investigation of intercellular interaction and the electrical communication between cells with spatial precision that may not be performed with a traditional patch clamp approach. Moreover, the ability to internalize two probes into the same cell opens new avenues to investigate sub-cellular structures and organelles. However, although the integration of two independent probes is unique and attractive, it still lacks the mapping capabilities of substrate-integrated arrays. As the kinked nanowire geometry in these studies limits the design to a probe configuration, rather than a multiplexed array like MEA, a different approach may be applied. In another study, the general concept of nanoFET was employed, but with modifications that allowed the FET sensor to be arrayed[13] (Figure 5C). As in more traditional FET applications, the S/D electrodes were integrated into the planar substrate. As opposed to the previous study in which the nanoFET probe was brought into the cytosol, here, the cytosol was brought into the nanoFET. This was performed by branching an insulating silicon dioxide nanotube vertically from the p-type nanowire connecting the S/D (Figure 5C).
4.2. Recording from three-dimensional engineered tissues
When discussing cardiac applications, it is essential to address one of the major aspects of cardiovascular research, namely cardiac tissue engineering. The pressing need to find a substitute for donor dependent organs has resulted in many efforts from multiple disciplines to bring forth a solution from the standpoint of cardiac tissue engineering[81, 82]. It is clear that the ability to perform real-time 3D mapping of the developing cardiac construct may contribute to this effort. Although genetically encoded voltage-sensitive dyes[83] were found to be effective, they suffer from low temporal resolution that is intrinsic to the dynamics of the dyes and the light scattering from tissue layers[84]. Moreover, as they depend on genetic modification, primary cells are hard be used for such applications. Thus, employing electrical recording in the 3D setting is a useful tool for this field. This effort was first demonstrated by Tian et al. in 2012[10]. Subsequently, Dai et al. expanded this approach and used highly multiplexed and flexible bioelectronics mesh that incorporated sensing/stimulating nanoFETs and metal electrodes for 3D tissue engineering[14] (Figure 6). Their ultra-thin designs enabled device mechanical properties similar to that of poly(lactic-co-glycolic acid) (PLGA) electro-spun fibers[81, 85]. The nanoFETs incorporated into the device allowed 3D mapping of the entire cellular construct (Figures 6B and6C). They used the device to monitor the effect of a channel blocker on the construct; administering 1-heptanol resulted in a uniform decrease in the AP conduction velocity. Given the nature of electrical recording, the device array has high temporal resolution, which enabled the monitoring of the diffusion and action of 1-heptanol within the entire cellular construct. Additionally, the AP propagation was controlled by using integrated stimulators, and monitored in situ by the bioelectric scaffold.
Figure 6. Nanoelectronic scaffold for cardiac mapping.
(A) Schematic diagrams of a folded free-standing nanoelectronics scaffold, showing addressable FET sensors distributed over four different layers and engineered cardiac tissue. The inset highlights the individual FETs (blue circles). (B) Electrical recording traces from 16 FETs in the top layer (L1) of the hybrid construct. The (x, y) coordinates of each FET are shown. (C) Zoom-in view of single AP spikes. Reproduced with permission. [14] Copyright 2016, Springer Nature.
In another bioelectric scaffold design, Feiner et al. expanded the modulation capabilities to the next generation[86]. In this study, flexible electronics were rolled into a 3D scaffold, on which cardiomyocytes were cultured. In this study, they integrated 28 small (50μm x 50μm) electrodes that were used for sensing electrical activity and stimulated the cells to enable synchronization. Moreover, they incorporated four larger (150μm x 150μm) electrodes that were imbedded in an electroactive polymer, which was loaded with drugs. The capability of drug delivery with an on/off switch, as well as monitoring and modulating the state of the cultured 3D construct, will enable a controlled microenvironment for engineering artificial tissues.
5. Nanoscale tools for interfacing non-excitable cells
Although the utility of Si-based nanomaterials are mainly due to their electrical and optical properties, we would like to emphasize several different applications that benefit from their other properties.
5.1. Force probes
Si nanowires possess incredible mechanical flexibility due to their 1D geometry and small diameter. Moreover, a recent systematic investigation performed in our lab demonstrated that label-free Si nanowires can be internalized spontaneously in multiple cell lines[72]. When these flexible nanowires were internalized into cells, the forces applied by the cell’s cytoskeletal system bent them as the cells remodeled their intra- or extracellular structures (Figure 7A). These forces are a key factor in regulating physiological processes, in which the mechanical dynamics of the extracellular matrix and cytoskeletal filaments provide physical cues for inter- and intracellular signaling[87, 88]. Our group did the measurements in smooth muscle cells, and the forces they generated during contraction was measured by recording the shape deformation of the internalized nanowires[89]. In this study, we used kinked Si nanowires because the kinked structure serves as a visual and physical anchor (Figure 7A). This anchor limits device rotation, so that the intracellular force is transduced mostly to nanowire strain. By using a combination of scatter (for nanowires) and phase contrast (for cells) microscopy, temporal deformation of the nanowires was extracted. Then, the nanowires were modeled with Euler−Bernoulli beam theory, and the force applied on the nanowires was fitted to the displacement results (Figure 7B). This freestanding mechanical probe, completely independent of the measuring apparatus (a microscope in this case) is unique as compared to other available cellular mechanical probes. In this context, we believe that integrating this freestanding probe with in vivo optical imaging techniques[90] may have unprecedented opportunities. For example, this measurement would allow researchers to investigate cancer metastatic dynamics in vivo, which is difficult to achieve with current techniques[91].
Figure 7. Si nanostructures for mechanical measurements.
(A-B), Si nanowires can be internalized into cells (A), while their nanoscale 1D geometry promotes their flexibility, force applied by cells (C) can be monitored by the mechanical deformation of the nanowires. (C-D), Skeleton-like Si spicules can be synthesized using 3D lithography by controlling atomic Au diffusion during a nanowire growth. These structures can be mounted on an AFM cantilever and used to probe the force applied to them by poking in and out collagen hydrogel, and generating F-D curves (C). Statistical analysis shows that anisotropy at the mesoscale can enhance Si’s interaction with collagen (D). (A and B) Reproduced with permission.132 Copyright 2015, ACS Publications. (C and D) Reproduced (Adapted) with permission.[92] Copyright 2015, AAAS.
Besides force measurements from cells, our lab has recently developed a 3D Si structure for mechanical measurement from the extracellular matrix materials[92] (Figures 7C and7D). In this study, we used iterated deposition-diffusion-incorporation of Au over Si nanowires to achieve 3D mesoscale lithography. This facet-selective process resulted in mesostructured Si spicules with morphologies resembling a skeleton. Then, single mesostructured Si spicules were mounted onto an atomic force microscopy (AFM) cantilever tip, which was used to approach the spicules to and retract them from collagen hydrogel (Figure 7C). During this process, the force and work from the spicule-hydrogel interactions in both directions were measured.
Compared to other Si nanostructures such as nanoporous Si nanowire, the anisotropic spicule requires greater force for detachment from collagen hydrogels (Figure 7D). This observation suggests that interfacial interactions at the mesoscale contribute to the detachment force and work far more than surface area or nanoscale roughness. These findings may unravel the evolutionary role of some natural systems, such as a bee’s stinger, which is intended to root into the skin. Adopting this strategy for building tight junctions between soft and rigid materials, such as in a tissue-bioelectronics interface, may enable better signal transductions and long-lived performance in these devices.
Another interesting group of materials that can be used as force probes are piezoelectric materials. Because these materials generate a measurable voltage change upon mechanical stretch and bent, they can be used as an on-line force probe with superior temporal resolution. For instance, in one study, single zinc oxide nanowire was incorporated into a piezoelectric FET to bridge two Ohmic contacts[93]. In this configuration, the source-to-drain current was controlled by the bending of the nanowire. This allowed the researchers to sense forces in the sub-nanonewton range, which is effective for pressure and force sensing. Moreover, piezoelectric nanoribbons were used to measure a 1nm deflection of cellular membrane due to electrical stimulation[94]. Moreover, this configuration allows multiplexing of the probes, which will enable investigators to extend these results to cellular and neural network investigations. Although this technique is essentially substrate-bound and cannot be used in a freestanding manner, it offers superior temporal resolution as well as extremely sensitive sensing capabilities, and are thus very promising tools for biomechanical research.
5.2. Cytosolic drug and gene delivery
The ability of nanostructures to interact with the cell’s cytosol may also be exploited for drug and gene delivery[95]. The capacity of nanowire to penetrate the membrane while avoiding the endocytosis pathway enables direct contact with the interior of the cell[96]. Thus, decorating the nanostructure with small molecules, proteins, or genes, can be used as an efficient intracellular delivery approach[95]. Recently, a number of varying geometries were studied for this purpose. For instance, an array of nanoneedles was placed on cells cultured on substrate with the needles facing down toward the cells[97]. Then centrifugation was used to push the nanoneedle array towards the cells, while suspended in media containing the delivered reagent (fluorescent dye, dextran, antibody, nanoparticle or DNA). By transiently disrupting the integrity of the cell membrane, these large molecules could diffuse into the cytosol. In a similar study, nanoneedle array was used to carry plasmid DNA[98], and the array was next oscillated in three directions while irrigating the cellular membrane. In another study, nanostraws were arrayed on a substrate on which cells were cultured[99]. The top of the nanostraws penetrated the cells, while their bottom was directly connected to a microfluidic channel under the substrate. Thus, the authors established a controllable fluidic conduit into the cell for direct intracellular access.
As an initial effort toward in vivo applications, Stevens, Tasciotti and colleagues employed an array of porous Si nanoneedles for the delivery of pH-sensitive fluorophore and quantum dots[100]. Upon nanoinjection in cultured cells, this sensor mapped intracellular pH with single-cell resolution. Subsequently, in vivo nanoinjection of quantum dots was performed to the muscle and skin. Thereafter, quantum dots were observed in the cytosolic region of cells located at the surface, demonstrating that intracellular injection was feasible in vivo. In another study, the authors successfully demonstrated cytosolic injection of DNA and siRNA into cells in vivo[101]. To demonstrate the functionality of this approach, they used nanoneedles to transfect the VEGF-165 gene. Consequently, neovascularization was substantially enhanced and a localized six-fold increase in blood perfusion was observed.
6. Outlook
As we described in this report, substantial progress with the interface between semiconductors and cellular components has been made in the past several years. In terms of spatial and temporal resolutions, the available tools have long ago exceeded those of biological components. However, the mechanical mismatch, as well as a limited understanding of signal transduction at these interfaces, still presents challenges and opportunities. Additionally, although an ultrathin nanoelectronic framework has been able to overcome the inherent bulkiness of semiconductor-based devices, the leads of these devices are still considered to be the Achilles heel of these systems. Moreover, even though tools for wireless communication between implantable devices and external interfaces are readily available, there remains a need to further miniaturize them to allow for seamless integration with the tissues. Therefore, freestanding and remotely controlled nano-systems seem to be a viable solution. Currently, it appears that most recording devices are inherently associated with interconnects/leads, but stimulating and modulating tools may readily adopt the leadless configuration. In this progress report, we have described several examples of freestanding neuromodulation devices. Unfortunately, they are still associated with extremely high optical power density that may not be applicable for in vivo applications. Therefore, new technologies that solve these issues represent a target of future research.
Next, intracellular dynamics represents a basis for various downstream tissue- or organ-level activities and processes[13, 88, 102]. As mentioned in this progress report, many studies already suggested that cells and their assemblies use mechanical[88, 103] and electrical[31, 40, 104] signals for individual or collective information processing. These electrical and mechanical properties and dynamics, even at the intracellular level, can be rather inhomogeneous. We therefore need to develop tools that can target to individual organelles, are sensitive and minimally invasive, and may be biodegradable on-demand. In this regard, nanostructured semiconductors and their surface chemistry developed over the past few decades [8, 62, 105] should be exploited rigorously to meet these challenges.
Finally, as for integrating semiconductor nanostructures with single cells and subcellular regions, many in vitro reports already exist. However, homing these nanostructure to the site of interest in vivo is far more challenging. Thus, combining the ability to distribute freestanding semiconductor nanostructures in a drug-like fashion with the ability to apply high resolution optical manipulation in vivo, may eventually enable the next generation of single cell studies in vivo.
Acknowledgements
This work was primarily supported by the University of Chicago Materials Research Science and Engineering Center, which is funded by National Science Foundation under award number DMR-1420709. This work is also supported by the Air Force Office of Scientific Research (FA9550–15-1–0285), and the National Science Foundation (NSF CAREER, DMR-1254637).
Biography

Menahem Rotenberg graduated summa cum laude from Ben Gurion University in Israel, with a BSc in biotechnology engineering. He performed his MSc under the supervision of Professor Smadar Cohen, where he graduated cum laude for his research on developing a bioreactor for tissue engineering. As a graduate student, he designed a new modality for leadless heart pacing, using magnetic microparticles and external magnetic forces under the dual mentorship of Professor Smadar Cohen and Dr. Yoram Etzion. Currently, he is working with Professor Bozhi Tian as a postdoctoral scholar, studying new ways to integrate semiconductors with biological entities.

Bozhi Tian received his Ph.D. degree in physical chemistry from Harvard University in 2010. His Ph.D. research with Professor Charles Lieber included new nanowire materials synthesis, the fundamental study of high performance nanowire photovoltaics and the application of novel nanowire devices in cells and tissue. He worked with Professors Robert Langer and Daniel Kohane as a postdoctoral scholar in tissue engineering. He is now an assistant professor at the University of Chicago, working on semiconductor-enabled fundamental studies of subcellular biophysics and soft matter dynamics.
References:
- [1].Fromherz P, Annals of the New York Academy of Sciences 2006, 1093, 143. [DOI] [PubMed] [Google Scholar]
- [2].Kim D-H, Ghaffari R, Lu N, Rogers JA, Annual review of biomedical engineering 2012, 14, 113. [DOI] [PubMed] [Google Scholar]
- [3].Lanzani G, Nature materials 2014, 13, 775. [DOI] [PubMed] [Google Scholar]
- [4].Tian B, Lieber CM, Annual review of analytical chemistry 2013, 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saldrnoto KK, Kornienko N, Yang PD, Accounts of Chemical Research 2017, 50, 476. [DOI] [PubMed] [Google Scholar]
- [6].Zhang A, Lieber CM, Chemical reviews 2015, 116, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang YH, Zhang F, Yan Z, Ma Q, Li XL, Huang YG, Rogers JA, Nature Reviews Materials 2017, 2. [Google Scholar]
- [8].Rogers JA, Lagally MG, Nuzzo RG, Nature 2011, 477, 45. [DOI] [PubMed] [Google Scholar]
- [9].Fu TM, Hong GS, Zhou T, Schuhmann TG, Viveros RD, Lieber CM, Nature Methods 2016, 13, 875; [DOI] [PubMed] [Google Scholar]; Kang SK, Murphy RKJ, Hwang SW, Lee SM, Harburg DV, Krueger NA, Shin JH, Gamble P, Cheng HY, Yu S, Liu ZJ, McCall JG, Stephen M, Ying HZ, Kim J, Park G, Webb RC, Lee CH, Chung SJ, Wie DS, Gujar AD, Vemulapalli B, Kim AH, Lee KM, Cheng JJ, Huang YG, Lee SH, Braun PV, Ray WZ, Rogers JA, Nature 2016, 530, 71; [DOI] [PubMed] [Google Scholar]; Liu J, Fu TM, Cheng ZG, Hong GS, Zhou T, Jin LH, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo ZG, Fang Y, Lieber CM, Nature Nanotechnology 2015, 10, 629; [DOI] [PMC free article] [PubMed] [Google Scholar]; Xie C, Liu J, Fu TM, Dai XC, Zhou W, Lieber CM, Nature Materials 2015, 14, 1286; [DOI] [PubMed] [Google Scholar]; Yu KJ, Kuzum D, Hwang SW, Kim BH, Juul H, Kim NH, Won SM, Chiang K, Trumpis M, Richardson AG, Cheng HY, Fang H, Thompson M, Bink H, Talos D, Seo KJ, Lee HN, Kang SK, Kim JH, Lee JY, Huang YG, Jensen FE, Dichter MA, Lucas TH, Viventi J, Litt B, Rogers JA, Nature Materials 2016, 15, 782; [DOI] [PMC free article] [PubMed] [Google Scholar]; Webb RC, Bonifas AP, Behnaz A, Zhang YH, Yu KJ, Cheng HY, Shi MX, Bian ZG, Liu ZJ, Kim YS, Yeo WH, Park JS, Song JZ, Li YH, Huang YG, Gorbach AM, Rogers JA, Nature Materials 2013, 12, 938; [DOI] [PMC free article] [PubMed] [Google Scholar]; Xu S, Yan Z, Jang KI, Huang W, Fu HR, Kim J, Wei Z, Flavin M, McCracken J, Wang R, Badea A, Liu Y, Xiao DQ, Zhou GY, Lee J, Chung HU, Cheng HY, Ren W, Banks A, Li XL, Paik U, Nuzzo RG, Huang YG, Zhang YH, Rogers JA, Science 2015, 347, 154; [DOI] [PubMed] [Google Scholar]; Hwang SW, Tao H, Kim DH, Cheng HY, Song JK, Rill E, Brenckle MA, Panilaitis B, Won SM, Kim YS, Song YM, Yu KJ, Ameen A, Li R, Su YW, Yang MM, Kaplan DL, Zakin MR, Slepian MJ, Huang YG, Omenetto FG, Rogers JA, Science 2012, 337, 1640; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim DH, Lu NS, Ma R, Kim YS, Kim RH, Wang SD, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim TI, Chowdhury R, Ying M, Xu LZ, Li M, Chung HJ, Keum H, McCormick M, Liu P, Zhang YW, Omenetto FG, Huang YG, Coleman T, Rogers JA, Science 2011, 333, 838; [DOI] [PubMed] [Google Scholar]; Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao JL, Huang YG, Contreras D, Rogers JA, Litt B, Nature Neuroscience 2011, 14, 1599; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim DH, Lu NS, Ghaffari R, Kim YS, Lee SP, Xu LZ, Wu JA, Kim RH, Song JZ, Liu ZJ, Viventi J, de Graff B, Elolampi B, Mansour M, Slepian MJ, Hwang S, Moss JD, Won SM, Huang YG, Litt B, Rogers JA, Nature Materials 2011, 10, 316; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim DH, Viventi J, Amsden JJ, Xiao JL, Vigeland L, Kim YS, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang YG, Hwang KC, Zakin MR, Litt B, Rogers JA, Nature Materials 2010, 9, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tian BZ, Liu J, Dvir T, Jin LH, Tsui JH, Qing Q, Suo ZG, Langer R, Kohane DS, Lieber CM, Nature Materials 2012, 11, 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tian B, Cohen-Karni T, Qing Q, Duan X, Xie P, Lieber CM, Science 2010, 329, 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Qing Q, Jiang Z, Xu L, Gao R, Mai L, Lieber CM, Nature nanotechnology 2014, 9, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duan X, Gao R, Xie P, Cohen-Karni T, Qing Q, Choe HS, Tian B, Jiang X, Lieber CM, Nature nanotechnology 2012, 7, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dai X, Zhou W, Gao T, Liu J, Lieber CM, Nature nanotechnology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim D-H, Lu N, Ghaffari R, Rogers JA, NPG Asia Materials 2012, 4, e15; [Google Scholar]; Rogers JA, Someya T, Huang Y, Science 2010, 327, 1603. [DOI] [PubMed] [Google Scholar]
- [16].Zimmerman J, Parameswaran R, Tian BZ, Biomaterials Science 2014, 2, 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ledesma HA, Tian BZ, Journal of Materials Chemistry B 2017, 5, 4276. [DOI] [PubMed] [Google Scholar]
- [18].Stutzmann N, Friend RH, Sirringhaus H, Science 2003, 299, 1881; [DOI] [PubMed] [Google Scholar]; Javey A, Guo J, Wang Q, Lundstrom M, Dai H, nature 2003, 424, 654. [DOI] [PubMed] [Google Scholar]
- [19].Gross G, Rieske E, Kreutzberg G, Meyer A, Neuroscience Letters 1977, 6, 101; [DOI] [PubMed] [Google Scholar]; Gross GW, Williams AN, Lucas JH, Journal of neuroscience methods 1982, 5, 13. [DOI] [PubMed] [Google Scholar]
- [20].Eversmann B, Jenkner M, Hofmann F, Paulus C, Brederlow R, Holzapfl B, Fromherz P, Merz M, Brenner M, Schreiter M, IEEE Journal of Solid-State Circuits 2003, 38, 2306. [Google Scholar]
- [21].Pine J, Journal of neuroscience methods 1980, 2, 19. [DOI] [PubMed] [Google Scholar]
- [22].Najafi K, Wise KD, IEEE Journal of Solid-State Circuits 1986, 21, 1035; [Google Scholar]; Hoogerwerf AC, Wise KD, IEEE Transactions on Biomedical Engineering 1994, 41, 1136. [DOI] [PubMed] [Google Scholar]
- [23].Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA, IEEE Transactions on Biomedical Engineering 1991, 38, 758. [DOI] [PubMed] [Google Scholar]
- [24].Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, Lee AK, Anastassiou CA, Andrei A, Aydin C, Barbic M, Blanche TJ, Bonin V, Couto J, Dutta B, Gratiy SL, Gutnisky DA, Hausser M, Karsh B, Ledochowitsch P, Lopez CM, Mitelut C, Musa S, Okun M, Pachitariu M, Putzeys J, Rich PD, Rossant C, Sun WL, Svoboda K, Carandini M, Harris KD, Koch C, O’Keefe J, Harris TD, Nature 2017, 551, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fromherz P, Offenhausser A, Science 1991, 252, 1290. [DOI] [PubMed] [Google Scholar]
- [26].Chorev E, Epsztein J, Houweling AR, Lee AK, Brecht M, Current opinion in neurobiology 2009, 19, 513. [DOI] [PubMed] [Google Scholar]
- [27].Sakmann B, Neher E, Annual review of physiology 1984, 46, 455. [DOI] [PubMed] [Google Scholar]
- [28].Li W-C, Soffe SR, Roberts A, Journal of neurophysiology 2004, 92, 380. [DOI] [PubMed] [Google Scholar]
- [29].Akaike N, Harata N, The Japanese journal of physiology 1994, 44, 433. [DOI] [PubMed] [Google Scholar]
- [30].Jayant K, Hirtz JJ, Jen-La Plante I, Tsai DM, De Boer W, Semonche A, Peterka DS, Owen JS, Sahin O, Shepard KL, Yuste R, Nature Nanotechnology 2017, 12, 335; Andrasfalvy BK, Galinanes GL, Huber D, Barbic M, Macklin JJ, Susumu K, Delehanty JB, Huston AL, Makara JK, Medintz IL, Nature Methods 2014, 11, 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Scanziani M, Häusser M, Nature 2009, 461, 930. [DOI] [PubMed] [Google Scholar]
- [32].Grinvald A, Hildesheim R, Nature Reviews Neuroscience 2004, 5, 874. [DOI] [PubMed] [Google Scholar]
- [33].Novak JL, Wheeler BC, Journal of neuroscience methods 1988, 23, 149. [DOI] [PubMed] [Google Scholar]
- [34].Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K, Nature neuroscience 2005, 8, 1263. [DOI] [PubMed] [Google Scholar]
- [35].Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K, Nature neuroscience 2012, 15, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, De Lecea L, Nature 2007, 450, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bi A, Cui J, Ma Y-P, Olshevskaya E, Pu M, Dizhoor AM, Pan Z-H, Neuron 2006, 50, 23; [DOI] [PMC free article] [PubMed] [Google Scholar]; Lagali PS, Balya D, Awatramani GB, Münch TA, Kim DS, Busskamp V, Cepko CL, Roska B, Nature neuroscience 2008, 11, 667. [DOI] [PubMed] [Google Scholar]
- [38].Nussinovitch U, Gepstein L, Nature biotechnology 2015, 33, 750. [DOI] [PubMed] [Google Scholar]
- [39].Chen R, Canales A, Anikeeva P, Nature Reviews Materials 2017, 2, natrevmats201693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Spira ME, Hai A, Nature Nanotechnology 2013, 8, 83. [DOI] [PubMed] [Google Scholar]
- [41].Brüggemann D, Wolfrum B, Maybeck V, Mourzina Y, Jansen M, Offenhäusser A, Nanotechnology 2011, 22, 265104. [DOI] [PubMed] [Google Scholar]
- [42].Hai A, Shappir J, Spira ME, Journal of neurophysiology 2010, 104, 559; [DOI] [PubMed] [Google Scholar]; Hai A, Dormann A, Shappir J, Yitzchaik S, Bartic C, Borghs G, Langedijk J, Spira ME, Journal of The Royal Society Interface 2009, 6, 1153; [DOI] [PMC free article] [PubMed] [Google Scholar]; Ojovan SM, Rabieh N, Shmoel N, Erez H, Maydan E, Cohen A, Spira ME, Scientific reports 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Robinson JT, Jorgolli M, Shalek AK, Yoon M-H, Gertner RS, Park H, Nature nanotechnology 2012, 7, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dipalo M, Amin H, Lovato L, Moia F, Caprettini V, Messina GC, Tantussi F, Berdondini L, De Angelis F, Nano Letters 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hanson L, Zhao WT, Lou HY, Lin ZC, Lee SW, Chowdary P, Cui Y, Cui BX, Nature Nanotechnology 2015, 10, 554; [DOI] [PMC free article] [PubMed] [Google Scholar]; Lin ZLC, Xie C, Osakada Y, Cui Y, Cui BX, Nature Communications 2014, 5; [DOI] [PMC free article] [PubMed] [Google Scholar]; Santoro F, Zhao WT, Joubert LM, Duan LT, Schnitker J, van de Burgt Y, Lou HY, Liu BF, Salleo A, Cui LF, Cui Y, Cui BX, Acs Nano 2017, 11, 8320; [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhao WT, Hanson L, Lou HY, Akamatsu M, Chowdary PD, Santoro F, Marks JR, Grassart A, Drubin DG, Cui Y, Cui BX, Nature Nanotechnology 2017, 12, 750; [DOI] [PMC free article] [PubMed] [Google Scholar]; Hanson L, Lin ZC, Xie C, Cui Y, Cui BX, Nano Letters 2012, 12, 5815; [DOI] [PMC free article] [PubMed] [Google Scholar]; Xie C, Lin ZL, Hanson L, Cui Y, Cui BX, Nature Nanotechnology 2012, 7, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Drake KL, Wise K, Farraye J, Anderson D, BeMent S, IEEE Transactions on Biomedical Engineering 1988, 35, 719. [DOI] [PubMed] [Google Scholar]
- [47].Polikov VS, Tresco PA, Reichert WM, Journal of neuroscience methods 2005, 148, 1; [DOI] [PubMed] [Google Scholar]; Kozai TD, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT, ACS chemical neuroscience 2015, 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fu T-M, Hong G, Viveros RD, Zhou T, Lieber CM, Proceedings of the National Academy of Sciences 2017, 114, E10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Duan X, Lieber CM, Chemistry–An Asian Journal 2013, 8, 2304; [DOI] [PMC free article] [PubMed] [Google Scholar]; Jeong J-W, Shin G, Park SI, Yu KJ, Xu L, Rogers JA, Neuron 2015, 86, 175. [DOI] [PubMed] [Google Scholar]
- [50].Robinson JT, Jorgolli M, Park H, Frontiers in neural circuits 2013, 7; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angle MR, Cui B, Melosh NA, Current opinion in neurobiology 2015, 32, 132. [DOI] [PubMed] [Google Scholar]
- [51].Jiang Y, Carvalho-de-Souza JL, Wong RC, Luo Z, Isheim D, Zuo X, Nicholls AW, Jung IW, Yue J, Liu D-J, Nature materials 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wegst UG, Bai H, Saiz E, Tomsia AP, Ritchie RO, Nature materials 2015, 14, 23. [DOI] [PubMed] [Google Scholar]
- [53].Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA, Nature medicine 2008, 14, 213. [DOI] [PubMed] [Google Scholar]
- [54].Tanaka K, Amorphous silicon, Wiley, Chichester; New York: 1999. [Google Scholar]
- [55].Canham LT, 2014. [Google Scholar]
- [56].Shapiro MG, Homma K, Villarreal S, Richter C-P, Bezanilla F, Nature communications 2012, 3, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Carvalho-de-Souza JL, Treger JS, Dang B, Kent SB, Pepperberg DR, Bezanilla F, Neuron 2015, 86, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Minev IR, Musienko P, Hirsch A, Barraud Q, Wenger N, Moraud EM, Gandar J, Capogrosso M, Milekovic T, Asboth L, Science 2015, 347, 159. [DOI] [PubMed] [Google Scholar]
- [59].Gautieri A, Vesentini S, Redaelli A, Buehler MJ, Nano letters 2011, 11, 757. [DOI] [PubMed] [Google Scholar]
- [60].Ghezzi D, Antognazza MR, Dal Maschio M, Lanzarini E, Benfenati F, Lanzani G, Nature Communications 2011, 2, 166. [DOI] [PubMed] [Google Scholar]
- [61].Ghezzi D, Antognazza MR, Maccarone R, Bellani S, Lanzarini E, Martino N, Mete M, Pertile G, Bisti S, Lanzani G, Nature Photonics 2013, 7, 400; [DOI] [PMC free article] [PubMed] [Google Scholar]; Mathieson K, Loudin J, Goetz G, Huie P, Wang L, Kamins TI, Galambos L, Smith R, Harris JS, Sher A, Nature photonics 2012, 6, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tian B, Zheng X, Kempa TJ, Fang Y, Yu N, Yu G, Huang J, Lieber CM, nature 2007, 449, 885. [DOI] [PubMed] [Google Scholar]
- [63].Parameswaran R, Carvalho-de-Souza JL, Jiang YW, Burke MJ, Zimmerman JF, Koehler K, Phillips A, Yi J, Adams EJ, Bezanilla F, Tian BZ, Nature Nanotechnology DOI: 10.1038/s41565-017-0041-7, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Su Y, Liu C, Brittman S, Tang J, Fu A, Kornienko N, Kong Q, Yang P, Nature nanotechnology 2016, 11, 609. [DOI] [PubMed] [Google Scholar]
- [65].Tay A, Di Carlo D, Nano letters 2017, 17, 886. [DOI] [PubMed] [Google Scholar]
- [66].Matthews BD, LaVan DA, Overby DR, Karavitis J, Ingber DE, Applied Physics Letters 2004, 85, 2968. [Google Scholar]
- [67].Rotenberg MY, Gabay H, Etzion Y, Cohen S, Scientific reports 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kim ST, Kim D-J, Kim T-J, Seo D-W, Kim T-H, Lee S-Y, Kim K, Lee K-M, Lee S-K, Nano letters 2010, 10, 2877. [DOI] [PubMed] [Google Scholar]
- [69].Masood M, Chen S, Carlen E, Berg A. v. d., ACS applied materials & interfaces 2010, 2, 3422. [DOI] [PubMed] [Google Scholar]
- [70].Pui T-S, Agarwal A, Ye F, Huang Y, Chen P, Biosensors and Bioelectronics 2011, 26, 2746. [DOI] [PubMed] [Google Scholar]
- [71].Lee J-H, Zhang A, You SS, Lieber CM, Nano letters 2016, 16, 1509. [DOI] [PubMed] [Google Scholar]
- [72].Zimmerman JF, Parameswaran R, Murray G, Wang Y, Burke M, Tian B, Science advances 2016, 2, e1601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fromherz P, Physica E: low-dimensional systems and nanostructures 2003, 16, 24. [Google Scholar]
- [74].Molleman A, Patch clamping: an introductory guide to patch clamp electrophysiology, John Wiley & Sons, 2003. [Google Scholar]
- [75].Kado RT, Experimental Physiology 1982, 67, 510; [Google Scholar]; Hai A, Shappir J, Spira ME, Nature methods 2010, 7, 200; [DOI] [PubMed] [Google Scholar]; de Asis ED Jr, Leung J, Wood S, Nguyen CV, Applied Physics Letters 2009, 95, 153701. [Google Scholar]
- [76].Patolsky F, Zheng G, Lieber CM, ACS Publications, 2006; [Google Scholar]; Sze SM, Ng KK, Physics of semiconductor devices, John wiley & sons, 2006. [Google Scholar]
- [77].Purves R, Microelectrode methods for intracellular recording and ionophoresis, Academic Press, 1981. [Google Scholar]
- [78].Tian B, Xie P, Kempa TJ, Bell DC, Lieber CM, Nature nanotechnology 2009, 4, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cohen-Karni T, Timko BP, Weiss LE, Lieber CM, Proceedings of the National Academy of Sciences 2009, 106, 7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bers DM, Nature 2002, 415, 198; [DOI] [PubMed] [Google Scholar]; Zipes DP, JalitTe J, 1989. [Google Scholar]
- [81].Eschenhagen T, Zimmermann WH, Circulation research 2005, 97, 1220; [DOI] [PubMed] [Google Scholar]; Dvir T, Timko BP, Kohane DS, Langer R, Nature nanotechnology 2011, 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T, Circulation research 2002, 90, e40; [DOI] [PubMed] [Google Scholar]; Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed L, American Journal of Physiology-Heart and Circulatory Physiology 2001, 280, H168; [DOI] [PubMed] [Google Scholar]; Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L, Nature materials 2016, 15, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].St-Pierre F, Marshall JD, Yang Y, Gong Y, Schnitzer MJ, Lin MZ, Nature neuroscience 2014, 17, 884; [DOI] [PMC free article] [PubMed] [Google Scholar]; Herron TJ, Lee P, Jalife J, Circulation research 2012, 110, 609; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE, Nature methods 2012, 9, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH, Science 2004, 305, 1007. [DOI] [PubMed] [Google Scholar]
- [85].Place ES, George JH, Williams CK, Stevens MM, Chemical Society Reviews 2009, 38, 1139. [DOI] [PubMed] [Google Scholar]
- [86].Feiner R, Engel L, Fleischer S, Malki M, Gal I, Shapira A, Shacham-Diamand Y, Dvir T, Nature Materials 2016, 15, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wang N, Butler JP, Ingber DE, Science 1993, 260, 1124. [DOI] [PubMed] [Google Scholar]
- [88].Fletcher DA, Mullins RD, Nature 2010, 463, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zimmerman JF, Murray GF, Wang Y, Jumper JM, Austin JR, Tian B, Nano letters 2015, 15, 5492; [DOI] [PubMed] [Google Scholar]; Zimmerman JF, Murray GF, Tian BZ, Journal of Physical Chemistry C 2015, 119, 29105. [Google Scholar]
- [90].Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan W-B, Nature protocols 2010, 5, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Legant WR, Miller JS, Blakely BL, Cohen DM, Genin GM, Chen CS, Nature methods 2010, 7, 969; [DOI] [PMC free article] [PubMed] [Google Scholar]; Fischer RS, Myers KA, Gardel ML, Waterman CM, Nature protocols 2012, 7, 2056; [DOI] [PMC free article] [PubMed] [Google Scholar]; Li Z, Song J, Mantini G, Lu M-Y, Fang H, Falconi C, Chen L-J, Wang ZL, Nano letters 2009, 9, 3575; [DOI] [PubMed] [Google Scholar]; Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Nature 2010, 466, 263; [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang X, Ha T, Science 2013, 340, 991; [DOI] [PMC free article] [PubMed] [Google Scholar]; Oddershede LB, Nature chemical biology 2012, 8, 879. [DOI] [PubMed] [Google Scholar]
- [92].Luo Z, Jiang Y, Myers BD, Isheim D, Wu J, Zimmerman JF, Wang Z, Li Q, Wang Y, Chen X, Science 2015, 348, 1451. [DOI] [PubMed] [Google Scholar]
- [93].Wang X, Zhou J, Song J, Liu J, Xu N, Wang ZL, Nano letters 2006, 6, 2768. [DOI] [PubMed] [Google Scholar]
- [94].Nguyen TD, Deshmukh N, Nagarah JM, Kramer T, Purohit PK, Berry MJ, McAlpine MC, Nature nanotechnology 2012, 7, 587. [DOI] [PubMed] [Google Scholar]
- [95].Shalek AK, Robinson JT, Karp ES, Lee JS, Ahn DR, Yoon MH, Sutton A, Jorgolli M, Gertner RS, Gujral TS, MacBeath G, Yang EG, Park H, Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 1870; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim W, Ng JK, Kunitake ME, Conklin BR, Yang PD, Journal of the American Chemical Society 2007, 129, 7228; [DOI] [PubMed] [Google Scholar]; Yan RX, Park JH, Choi Y, Heo CJ, Yang SM, Lee LP, Yang PD, Nature Nanotechnology 2012, 7, 191. [DOI] [PubMed] [Google Scholar]
- [96].Delehanty JB, Bradburne CE, Boeneman K, Susumu K, Farrell D, Mei BC, Blanco-Canosa JB, Dawson G, Dawson PE, Mattoussi H, Integrative Biology 2010, 2, 265. [DOI] [PubMed] [Google Scholar]
- [97].Wang Y, Yang Y, Yan L, Kwok SY, Li W, Wang Z, Zhu X, Zhu G, Zhang W, Chen X, Nature communications 2014, 5, ncomms5466. [DOI] [PubMed] [Google Scholar]
- [98].Matsumoto D, Sathuluri RR, Kato Y, Silberberg YR, Kawamura R, Iwata F, Kobayashi T, Nakamura C, Scientific reports 2015, 5, 15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].VanDersarl JJ, Xu AM, Melosh NA, Nano letters 2011, 12, 3881; [DOI] [PubMed] [Google Scholar]; Cao YH, Hjort M, Chen HD, Birey F, Leal-Ortiz SA, Han CM, Santiago JG, Pasca SP, Wu JC, Melosh NA, Proceedings of the National Academy of Sciences of the United States of America 2017, 114, E1866; [DOI] [PMC free article] [PubMed] [Google Scholar]; Xu AM, Aalipour A, Leal-Ortiz S, Mekhdjian AH, Xie X, Dunn AR, Garner CC, Melosh NA, Nature Communications 2014, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chiappini C, Martinez JO, De Rosa E, Almeida CS, Tasciotti E, Stevens MM, ACS nano 2015, 9, 5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chiappini C, De Rosa E, Martinez J, Liu X, Steele J, Stevens M, Tasciotti E, Nature materials 2015, 14, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E, Lippincott-Schwartz J, Nature 2017, 546, 162; [DOI] [PMC free article] [PubMed] [Google Scholar]; Rubinsztein DC, Nature 2006, 443, 780; [DOI] [PubMed] [Google Scholar]; Vale RD, Cell 2003, 112, 467. [DOI] [PubMed] [Google Scholar]
- [103].Browne WR, Feringa BL, Nature Nanotechnology 2006, 1, 25; [DOI] [PubMed] [Google Scholar]; Discher DE, Mooney DJ, Zandstra PW, Science 2009, 324, 1673; [DOI] [PMC free article] [PubMed] [Google Scholar]; Geiger B, Spatz JP, Bershadsky AD, Nature Reviews Molecular Cell Biology 2009, 10, 21; [DOI] [PubMed] [Google Scholar]; Gu Y, Sun W, Wang GF, Jeftinija K, Jeftinija S, Fang N, Nature Communications 2012, 3; [DOI] [PubMed] [Google Scholar]; Hirokawa N, Takemura R, Nature Reviews Neuroscience 2005, 6, 201. [DOI] [PubMed] [Google Scholar]
- [104].Beane WS, Morokuma J, Lemire JM, Levin M, Development 2013, 140, 313; [DOI] [PMC free article] [PubMed] [Google Scholar]; Bezanilla F, Physiological Reviews 2000, 80, 555; [DOI] [PubMed] [Google Scholar]; Bezanilla F, Nature Reviews Molecular Cell Biology 2008, 9, 323; [DOI] [PubMed] [Google Scholar]; Blackiston DJ, McLaughlin KA, Levin M, Cell Cycle 2009, 8, 3527; [DOI] [PMC free article] [PubMed] [Google Scholar]; Cifra M, Biosystems 2012, 109, 356; [DOI] [PubMed] [Google Scholar]; Cifra M, Fields JZ, Farhadi A, Progress in Biophysics & Molecular Biology 2011, 105, 223; [DOI] [PubMed] [Google Scholar]; Cifra M, Pokorny J, Havelka D, Kucera O, Biosystems 2010, 100, 122; [DOI] [PubMed] [Google Scholar]; Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M, Nature 2013, 496, 159; [DOI] [PMC free article] [PubMed] [Google Scholar]; Havelka D, Kucera O, Deriu MA, Cifra M, Plos One 2014, 9; [DOI] [PMC free article] [PubMed] [Google Scholar]; Pokorny J, Hasek J, Jelinek F, Journal of Biological Physics 2005, 31, 501; [DOI] [PMC free article] [PubMed] [Google Scholar]; Reardon S, Nature 2014, 511, 18; [DOI] [PubMed] [Google Scholar]; Sekulic DL, Sataric BM, Tuszynski JA, Sataric MV, European Physical Journal E 2011, 34; [DOI] [PubMed] [Google Scholar]; Sundelacruz S, Li CM, Choi YJ, Levin M, Kaplan DL, Biomaterials 2013, 34, 6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Duan XJ, Fu TM, Liu J, Lieber CM, Nano Today 2013, 8, 351; [DOI] [PMC free article] [PubMed] [Google Scholar]; Jiang XC, Tian BZ, Xiang J, Qian F, Zheng GF, Wang HT, Mai LQ, Lieber CM, Proceedings of the National Academy of Sciences of the United States of America 2011, 108, 12212; [DOI] [PMC free article] [PubMed] [Google Scholar]; Qian Y. Li, F., Xiang J, Lieber CM, Materials Today 2006, 9, 18; [Google Scholar]; Lu W, Lieber CM, Nature Materials 2007, 6, 841; [DOI] [PubMed] [Google Scholar]; Lu W, Xie P, Lieber CM, Ieee Transactions on Electron Devices 2008, 55, 2859; [Google Scholar]; Fiori G, Bonaccorso F, Iannaccone G, Palacios T, Neumaier D, Seabaugh A, Banerjee SK, Colombo L, Nature Nanotechnology 2014, 9, 768; [DOI] [PubMed] [Google Scholar]; Segawa Y, Ito H, Itami K, Nature Reviews Materials 2016, 1; [Google Scholar]; Aida T, Meijer EW, Stupp SI, Science 2012, 335, 813; [DOI] [PMC free article] [PubMed] [Google Scholar]; De Volder MFL, Tawfick SH, Baughman RH, Hart AJ, Science 2013, 339, 535; [DOI] [PubMed] [Google Scholar]; Novoselov KS, Fal’ko VI, Colombo L, Gellert PR, Schwab MG, Kim K, Nature 2012, 490, 192; [DOI] [PubMed] [Google Scholar]; Fang Y, Jiang YW, Cherukara MJ, Shi FY, Koehler K, Freyermuth G, Isheim D, Narayanan B, Nicholls AW, Seidman DN, Sankaranarayanan S, Tian BZ, Nature Communications 2017, 8; [DOI] [PMC free article] [PubMed] [Google Scholar]; Yi J, Wang YC, Jiang YW, Jung IW, Liu WJ, De Andrade V, Xu RQ, Parameswaran R, Peters IR, Divan R, Xiao XH, Sun T, Lee Y, Park WI, Tian B, Nature Communications 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]