Abstract
Colorectal cancer is one of the leading causes of cancer related death due to a poor prognosis. In this study, we investigated the effect of Gomisin G on colon cancer growth and examined the underlying mechanism of action. We found that Gomisin G significantly suppressed the viability and colony formation of LoVo cells. Gomisin G reduced the phosphorylation level of AKT implying that Gomisin G suppressed the PI3K-AKT signaling pathway. Gomisin G also induced apoptosis shown by Annexin V staining and an increased level of cleaved poly-ADP ribose polymerase (PARP) and Caspase-3 proteins. Furthermore, Gomisin G remarkably triggered the accumulation of cells at the sub-G1 phase which represents apoptotic cells. In addition, the level of cyclin D1 and phosphorylated retinoblastoma tumor suppressor protein (Rb) was also reduced by the treatment with Gomisin G thus curtailing cell cycle progression. These findings show the suppressive effect of Gomisin G by inhibiting proliferation and inducing apoptosis in LoVo cells. Taken together, these results suggest Gomisin G could be developed as a potential therapeutic compound against colon cancer.
Keywords: Gomisin G, Colon cancer, AKT, Apoptosis, PARP, Cell cycle
INTRODUCTION
Colorectal cancer is the fourth leading cause of cancer-related deaths and is the third most commonly diagnosed malignancy of the gastrointestinal tract worldwide (Arnold et al., 2017; Navarro et al., 2017). In 2012, around 1.4 million cases of colorectal cancer were diagnosed, out of which approximately 700,000 deaths were reported (Arnold et al., 2017). Regardless of the implementation of a screening program and the promotion of community health facilities, the global burden of colorectal cancer by 2030 is predicted to rise by 60% (Arnold et al., 2017). The prognosis of colorectal cancer patients depends on the stage of metastasis, and the 5-year survival is around 65.2%. Despite the use of conventional therapies such as surgical resection, chemotherapy, targeting molecules, drugs and certain promising combinations of therapeutic antibodies and drugs, the lack of a curative treatment in patients with advanced metastasis still prevails (Moriarity et al., 2016). The extent of this drawback demands the development of promising therapeutic molecular targets.
Numerous studies have shown the various therapeutic potentials of Gomisins with their anti-inflammatory, anti-obesity, antioxidant, and anti-cancer activities as well as acting as a liver protectant (Choi et al., 2006; Min et al., 2008; Oh et al., 2010; Park et al., 2014; Jang et al., 2017). Gomisins, which are phytoestrogens, extracted from the fruits of Schisandra chinesis have traditionally been used in herbal medicine in Korea, China, Japan and Russia (Opletal et al., 2004; Choi et al., 2006; Min et al., 2008; Park et al., 2014; Jang et al., 2017). Gomisin G is a lignan from S. chinesis and was reported to have anti-HIV, anti-liver cancer and anti-inflammatory activities (Chen et al., 1997; Ryu et al., 2011; Xiaoyang et al., 2015). In a previous study, we found that Gomisin G has promise as a therapeutic agent against triple-negative breast cancer cells via an AKT-cyclin D1 dependent mechanism (Maharjan et al., 2018).
Apoptosis is an important cellular process characterized by regulated cell death. Collective evidence has shown that apoptotic cell death is a major target in the prevention and treatment of cancer (Ichim and Tait, 2016). The phosphatidylinositol 3-kinase (PI3K) /AKT signaling pathway is vital for intracellular signal transduction processes such as proliferation, apoptosis, survival, cell cycle progression and differentiation (Fresno Vara et al., 2004; Osaki et al., 2004). It has often been associated with the development and metastasis of various human cancers (Fresno Vara et al., 2004; Osaki et al., 2004). The expression of phospho-AKT and AKT has been found to be higher in colorectal cancer tissues than healthy tissues ones (Johnson et al., 2010).
The aim of the present study was to investigate the inhibitory effect of Gomisin G on growth, apoptosis, and cell cycle progression in a colon cancer cell line LoVo. We also examined the underlying mechanisms of action.
MATERIALS AND METHODS
Gomisins
Gomisin G, D, J, N and O were purchased from Biopurify Phytochemicals Ltd (Sichuan, China). The purity of the Gomisins were measured using an Agilent 1100 series high performance liquid chromatography (HPLC) system fitted with a RP-C18 column (Gemini, 5 μm, 4.6×250 mm; Phenomenex, Torrance, CA, USA) at room temperature as previously described (Maharjan et al., 2018). A UV/VIS detector (Agilent Technologies, Santa Clara, CA, USA) was used to measure the absorbance at 220 nm. The mobile phase was 65% aqueous acetonitrile, and the flow rate was 3.0 mL/min. Its chromatogram showed 98% purity; thus, it was used without further purification.
Cell culture
The human colon cancer cell line LoVo was purchased from the Korean Cell Line Bank (Seoul, Korea). The LoVo cells were originated from a fragment of a metastatic tumor nodule. The LoVo cells were maintained in RPMI1640 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific), 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in an atmosphere of 5% CO2.
MTT assay
The MTT assay was performed as previously described (Li et al., 2017). LoVo cells were treated with Gomisin D, G, J, N and O each at a concentration of 0, 1, 5, and 10 μM, or with dimethyl sulfoxide (DMSO) as a control for 3 and 5 days. After the designated time, the cells were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO, USA) for 4 h at 37°C. The resultant formazan crystals were dissolved in DMSO, and the absorbance was detected at 570 nm using spectrophotometer (Molecular Devices, Orleans, CA, USA).
Colony formation assay
Five hundred cells per well were seeded in a 6 well-plate. After a 24 h incubation, the cells were treated with DMSO, Gomisin G (10 μM) or Gomisin O (10 μM) and incubated for a further ten days at 37°C. The cells were then washed with PBS and stained with 0.4% crystal violet in methanol for 1 h. Images of the colonies were captured using ChemiDoc (BioRad, Hercules, CA, USA).
Western blot analysis
Western blot was done as previously described (Shin et al., 2017). Briefly, the cell lysates were centrifuged at 14,000 rpm and 4°C. The proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane were blocked and probed with primary antibodies followed by incubation with HRP-conjugated secondary antibodies. The antibodies against PARP, caspase-3, ERK, phospho-ERK (pERK), AKT, pAKT, p38, pp38, Rb (retinoblastoma protein), pRb, and cyclin D1 were purchased from Cell Signaling Technology (Danvers, MA, USA), and antibody against β-actin was purchased from Sigma-Aldrich.
Annexin V and propidium iodide (PI) staining
To detect cell apoptosis, LoVo cells were treated with DMSO, Gomisin G (10 μM) or Gomisin O (10 μM) for 24 h and detached and washed with FACS buffer (1% FBS in PBS). The collected cells were resuspended in buffer containing annexin V (eBioscience, San Diego, CA, USA) for 15 min. at room temperature in the dark. The cells were washed and then incubated with PI (eBioscience) followed by analysis using a FACSCalibur (BD Biosciences, San Jose, CA, USA), and the data were analyzed with the Flowing software (Turku Centre for Biotechnology, Turun Yliopisto, Finland) (Sung et al., 2016).
Cell cycle analysis
LoVo cells treated with DMSO, Gomisin G (10 μM) or Gomisin O (10 μM) for 72 h were detached, collected and washed with FACS buffer (1% FBS in PBS). The cells were fixed with ice-cold 70% ethanol in PBS at 4°C overnight. After fixation, the cells were washed and resuspended in buffer containing RNase (Sigma-Aldrich) for 30 min. at 37°C. The cells were then incubated with PI and immediately analyzed with FACSCalibur (BD Biosciences).
RESULTS
Gomisin G inhibits the growth of LoVo cells
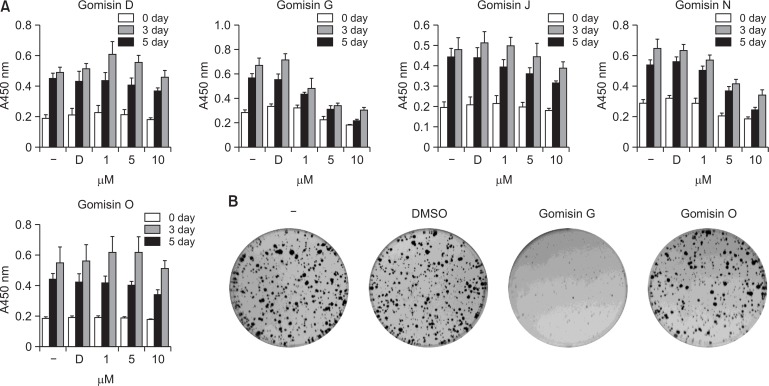
A previous study showed that Gomisin G blocked the proliferation of MDA-MB-231 triple negative breast cancer cells (Maharjan et al., 2018). To determine whether Gomisin G affects colon cancer cells, we investigated its effect on the viability of LoVo cells using the MTT assay. Treatment with Gomisin G significantly inhibited the growth of LoVo cells in a dose and time dependent manner (Fig. 1A). We also performed similar experiments using Gomisin D, J, N and O, which are natural compounds with a backbone structure similar to Gomisin G. While treatment with Gomisin N exhibited a mild suppressive effect, the other compounds failed to inhibit the viability of the LoVo cells. Among these compounds, Gomisin O was further used as a negative control in this study (Fig. 1A). To evaluate the long-term effect of Gomisin G, LoVo cells were grown in the presence of 10 μM Gomisin G for 10 days. As shown in Fig. 1B, there was a significant reduction in the numbers of colonies in the Gomisin G-treated wells. These data suggest that Gomisin G inhibits the growth of LoVo cells.
Fig. 1.
Effect of Gomisin G on the growth of LoVo cells. (A) LoVo cells were untreated (−), or treated with DMSO (D) and 1, 5, or 10 μM of Gomisin D, G, J, N and O for 3 and 5 days followed by the MTT assay. (B) LoVo cells were untreated (−), or treated with DMSO and 10 μM of Gomisin G or Gomisin O for 10 days followed by crystal violet staining.
Gomisin G impairs the AKT signaling pathway in LoVo cells
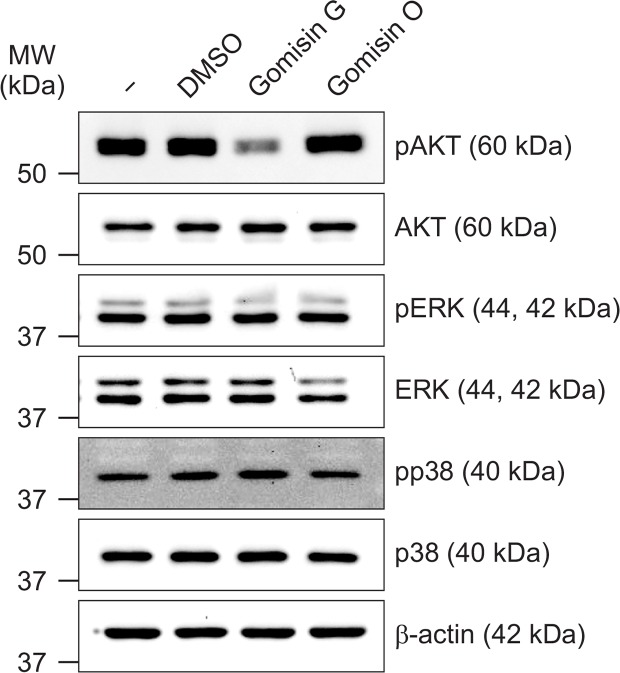
Accumulated studies have shown the critical role of the PI3K/AKT signaling pathway in the proliferation, development, and metastasis of many human cancers (Fresno Vara et al., 2004; Osaki et al., 2004). To evaluate the role of the AKT signaling pathway in the Gomisin G-mediated inhibition of colon cancer cell growth, LoVo cells were treated with or without Gomisin G (10 μM), and western blot analysis was performed. Gomisin G treatment significantly reduced the phosphorylation level of AKT (Fig. 2). However, Gomisin G had no effect on the MAP kinase pathway shown by the unaltered phosphorylation levels of ERK and p38. In contrast, Gomisin O did not affect the phosphorylation level of AKT. These data indicate that Gomisin G might suppress the growth of LoVo cells by inhibiting the AKT signaling pathway.
Fig. 2.
Gomisin G mediated alteration of AKT phosphorylation in LoVo cells. LoVo cells were treated with 10 μM Gomisin G or Gomisin O for 24 h and lysed. Equal concentrations of proteins were separated by SDS-PAGE and transferred to membrane blots. The blots were detected with pAKT, AKT, pERK, ERK, pp38, and p38 antibodies. β-actin was used as a loading control.
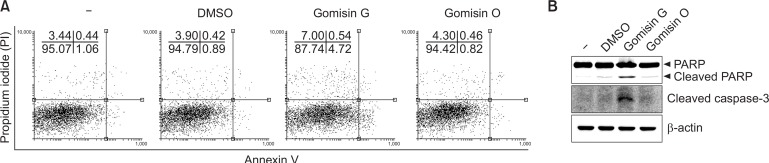
Gomisin G induces apoptosis in LoVo cells
To investigate whether apoptosis is involved in the growth inhibition of Gomisin G in LoVo cells, the cells were treated with or without Gomisin G (10 μM) for 24 h. FACS analysis was conducted to detect annexin V-positive cells. Fig. 3A shows that the apoptotic cell population increased by more than four fold in the Gomisin G-treated LoVo cells compared with the untreated, DMSO- or Gomisin O-treated cells. Caspases have a critical role in the implementation of programmed cell death (apoptosis) (Boatright and Salvesen, 2003; Parrish et al., 2013). An increased level of cleaved Caspase-3 was seen in Gomisin G-treated cells whereas no effect was seen in the Gomisin O-treated cells (Fig. 3B). Cleavage of PARP is also regarded as a hallmark of apoptosis (Soldani and Scovassi, 2002). Treatment with 10 μM Gomisin G for 24 h elicited PARP cleavage (Fig. 3B). These results taken together demonstrate that Gomisin G effectively induces apoptosis in LoVo cells.
Fig. 3.
Apoptosis analysis of LoVo cells. (A) Cells were treated with 10 μM of Gomisin G or Gomisin O for 24 h. The Annexin V and PI detection kit was used for the detection of apoptosis and analyzed with FACSCalibur. (B) LoVo cells were treated with 10 μM of Gomisin G for 24 h and analyzed with immunoblotting. Blots were detected using PARP, cleaved Caspase-3, and β-actin antibodies.
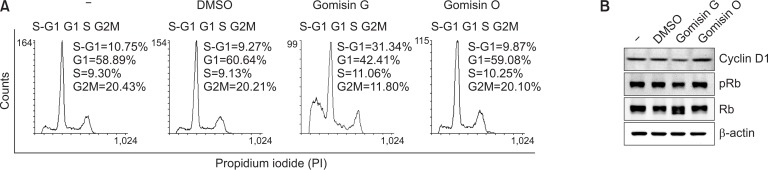
Effect of Gomisin G on the cell cycle progression of LoVo cells
FACS analysis of LoVo cells treated with or without Gomisin G showed DNA patterns representing sub-G1, G1, S, and G2M phases of the cell cycle. Gomisin G-treated LoVo cells showed a significantly higher population at the sub-G1 (S-G1) phase compared with the untreated, DMSO- or Gomisin O-treated cells. The higher percentage of the S-G1 phase implies induction of apoptosis by Gomisin G. Treatment with Gomisin G led to a decrease in the population of cells in the G1 and G2M phase of the cell cycle. The G1 to G2/M ratio was higher in the Gomisin G-treated sample which implies moderate cell cycle arrest at the G0-G1 phase (Fig. 4A). Overexpression of cyclin D1 has been associated with the maintenance and development of cancer (Qie and Diehl, 2016). To illustrate the mechanism of cell death, we observed the expression of cyclin D1 and Rb, important cell cycle regulatory proteins (VanArsdale et al., 2015). Following the treatment of Gomisin G (10 μM), the levels of cyclin D1 and phosphorylated Rb were slightly decreased (Fig. 4B). Therefore, these results imply that cell cycle progression in the LoVo cells is impaired by the treatment with Gomisin G.
Fig. 4.
Effect of Gomisin G on cell cycle progression. (A) LoVo cells were treated with 10 μM Gomisin G or Gomisin O for 72 h, stained with PI, and analyzed with FACSCalibur. (B) LoVo cells were treated with 10 μM of Gomisin G or Gomisin O for 24 h, and the expression levels of cyclin D1, pRb and Rb were determined with immunoblotting. β-actin was used as a loading control.
DISCUSSION
Natural products from a vast range of natural sources such as plants, micro-organisms, marine organisms, and animals have excellent therapeutic significance since ancient times (Newman and Cragg, 2016). There have been numerous anticancer drugs derived from natural sources which have been identified and approved for use against various cancers (Newman and Cragg, 2016). The lignans in the fruit of Schisandra chinesis have been extensively used as oriental medicine. These natural compounds have been reported to possess a wide range of therapeutic activities such as hepatoprotective, antioxidant, anticancer, and chemoprotective (Opletal et al., 2004; Choi et al., 2006; Min et al., 2008; Park et al., 2014). In particular, Gomisin G showed an anti-tumor effect by inducing cell cycle arrest and the inhibition of AKT-cyclin D1 signaling in MDA-MB-231 triple negative breast cancer cells (Maharjan et al., 2018). Given the high death rate of colon cancer patients, the relapse of cancer even after surgery, and the prevailing toxicity and inadequate response rate to current therapeutic regimens (Arnold et al., 2017), there has been immense pressure to identify promising natural anticancer agents that have low toxicity. Here, we show that Gomisin G has potential as a therapeutic agent against colon cancer.
The fundamental mechanism by which tumor cells oppose death is gaining resistance to apoptosis, and thus, it is a crucial point in the development of anticancer drugs (Fresno Vara et al., 2004; Danielsen et al., 2015). In this study, we found that Gomisin G significantly suppressed the viability of the colon cancer cell line LoVo. In addition, colony formation was sharply reduced in Gomisin G-treated cells, which further supports the growth-inhibitory action of Gomisin G (Maharjan et al., 2018). Moreover, the results of Annexin V and PI staining indicate that Gomisin G induced apoptosis in the LoVo cells.
There are two ways by which the apoptotic signaling exerts oncogenic effects: one is through the intrinsic (the mitochondrial) pathway and the other through the extrinsic (death receptor) pathway (Ichim and Tait, 2016). Caspases are the fundamental proteins known to be involved in apoptotic cell death (Boatright and Salvesen, 2003; Parrish et al., 2013). In both pathways, Caspase-3 is the vital effector protein of apoptosis, which is activated by signals from initiator caspases like Caspase-8 and Caspase-9 (Boatright and Salvesen, 2003; Parrish et al., 2013). One of the regulatory cellular substrates of caspase is PARP, and its cleavage is regarded as a characteristic of apoptosis (Kaufmann et al., 1993; Soldani and Scovassi, 2002; Chaitanya et al., 2010). In this study, we found an increased level of cleaved Caspase-3 in Gomisin G-treated cells. Furthermore, we also observed a prominent increase of cleaved PARP when the LoVo cells were treated with Gomisin G. These data suggest that Gomisin G induces apoptosis by regulating PARP and Caspase-3.
PI3K/AKT is one of the important intracellular signaling pathways regulating cell growth, proliferation, differentiation, metabolism, survival and apoptosis (Fresno Vara et al., 2004; Engelman, 2009; Danielsen et al., 2015). Several studies have reported frequent aberrant expression of PI3K/AKT signaling in cancer pathogenesis (Vivanco and Sawyers, 2002). Furthermore, PI3K/AKT signaling has a vital role in the development, maintenance, and metastasis of colorectal cancer (Engelman, 2009; Malinowsky et al., 2014; Danielsen et al., 2015). Therapeutic molecules inhibiting PI3K/AKT signaling have been recommended for use against colon cancer. (Engelman, 2009; Malinowsky et al., 2014; Danielsen et al., 2015). Previously, we found that Gomisin G diminished AKT phosphorylation in MDA-MB-231 breast cancer cells (Maharjan et al., 2018). Accordingly, the treatment of LoVo cells with Gomisin G also effectively inhibited the phosphorylation of AKT while the treatment with Gomisin O did not induce any changes in AKT phosphorylation. A very well-known protein that regulates cell cycle progression is cyclin D1, which is found to be overexpressed in human colorectal cancer cells (Ogino et al., 2009). Targeting cyclin D1 is considered to be an important factor in colorectal cancer prevention (Alao, 2007; Ogino et al., 2009). Another key regulator of cell cycle progression is the Rb protein, which binds and secludes transcription factor E2F. As a result, the synthesis of cell cycle genes are blocked (VanArsdale et al., 2015). Our data show that Gomisin G treatment lead to a noticeable reduction of cyclin D1 and phosphorylated Rb protein. In addition, prolonged treatment of the LoVo cells with Gomisin G for 72 h led to a remarkable accumulation of the cells at the sub-G1 phase which represents apoptotic cells (Ormerod, 1998; Darzynkiewicz et al., 2010). Although Gomisin G induced cell cycle arrest without involving the process of apoptosis in MDA-MB-231 triple negative breast cancer cells, it efficiently triggered cell cycle arrest and apoptosis in the LoVo cells.
In conclusion, our current study provides insight into the suppressive effect of Gomisin G in colon cancer cells through an increment of apoptosis and a reduction of proliferation involving AKT and cyclin D1. These findings show that Gomisin G has potential as a therapeutic agent for colon cancer; however, further studies are required to further verify its antitumor activity.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT in the Republic of Korea (2009-0093812) and by the Agri-Bio Industry Technology Development Program (316028-3, Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries). Byoung Kwon Park was supported by the Hallym University Postdoctoral Fellowship Program of 2017 (HLM-PF-2017-0001).
REFERENCES
- Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DF, Zhang SX, Xie L, Xie JX, Chen K, Kashiwada Y, Zhou BN, Wang P, Cosentino LM, Lee KH. Anti-AIDS agents--XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg Med Chem. 1997;5:1715–1723. doi: 10.1016/S0968-0896(97)00118-1. [DOI] [PubMed] [Google Scholar]
- Choi YW, Takamatsu S, Khan SI, Srinivas PV, Ferreira D, Zhao J, Khan IA. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J Nat Prod. 2006;69:356–359. doi: 10.1021/np0503707. [DOI] [PubMed] [Google Scholar]
- Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 2015;1855:104–121. doi: 10.1016/j.bbcan.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Halicka HD, Zhao H. Analysis of cellular DNA content by flow and laser scanning cytometry. Adv Exp Med Biol. 2010;676:137–147. doi: 10.1007/978-1-4419-6199-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Ichim G, Tait SW. A fate worse than death: apoptosis as an oncogenic process. Nat Rev Cancer. 2016;16:539–548. doi: 10.1038/nrc.2016.58. [DOI] [PubMed] [Google Scholar]
- Jang MK, Yun YR, Kim JH, Park MH, Jung MH. Gomisin N inhibits adipogenesis and prevents high-fat diet-induced obesity. Sci Rep. 2017;7:40345. doi: 10.1038/srep40345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Gulhati P, Rampy BA, Han Y, Rychahou PG, Doan HQ, Weiss HL, Evers BM. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J Am Coll Surg. 2010;210:767–768. doi: 10.1016/j.jamcollsurg.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- Li XQ, Liu XX, Wang XY, Xie YH, Yang Q, Ding YY, Cao W, Wang SW. Cinnamaldehyde derivatives inhibit coxsackievirus B3-induced viral myocarditis. Biomol Ther (Seoul) 2017;25:279–287. doi: 10.4062/biomolther.2016.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowsky K, Nitsche U, Janssen KP, Bader FG, Spath C, Drecoll E, Keller G, Hofler H, Slotta-Huspenina J, Becker KF. Activation of the PI3K/AKT pathway correlates with prognosis in stage II colon cancer. Br J Cancer. 2014;110:2081–2089. doi: 10.1038/bjc.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan S, Park BK, Lee SI, Lim Y, Lee K, Kwon HJ. Gomisin G inhibits the growth of triple-negative breast cancer cells by suppressing AKT phosphorylation and decreasing cyclin D1. Biomol Ther (Seoul) 2018;26:322–327. doi: 10.4062/biomolther.2017.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HY, Park EJ, Hong JY, Kang YJ, Kim SJ, Chung HJ, Woo ER, Hung TM, Youn UJ, Kim YS, Kang SS, Bae K, Lee SK. Antiproliferative effects of dibenzocyclooctadiene lignans isolated from Schisandra chinensis in human cancer cells. Bioorg Med Chem Lett. 2008;18:523–526. doi: 10.1016/j.bmcl.2007.11.082. [DOI] [PubMed] [Google Scholar]
- Moriarity A, O’Sullivan J, Kennedy J, Mehigan B, McCormick P. Current targeted therapies in the treatment of advanced colorectal cancer: a review. Ther Adv Med Oncol. 2016;8:276–293. doi: 10.1177/1758834016646734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23:3632–3642. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Oh SY, Kim YH, Bae DS, Um BH, Pan CH, Kim CY, Lee HJ, Lee JK. Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci Biotechnol Biochem. 2010;74:285–291. doi: 10.1271/bbb.90597. [DOI] [PubMed] [Google Scholar]
- Ogino S, Nosho K, Irahara N, Kure S, Shima K, Baba Y, Toyoda S, Chen L, Giovannucci EL, Meyerhardt JA, Fuchs CS. A cohort study of cyclin D1 expression and prognosis in 602 colon cancer cases. Clin Cancer Res. 2009;15:4431–4438. doi: 10.1158/1078-0432.CCR-08-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opletal L, Sovova H, Bartlova M. Dibenzo[a, c]cyclooctadiene lignans of the genus Schisandra: importance, isolation and determination. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:357–371. doi: 10.1016/S1570-0232(04)00646-4. [DOI] [PubMed] [Google Scholar]
- Ormerod MG. The study of apoptotic cells by flow cytometry. Leukemia. 1998;12:1013–1025. doi: 10.1038/sj.leu.2401061. [DOI] [PubMed] [Google Scholar]
- Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee SJ, Song Y, Jang SH, Ko YG, Kang SN, Chung BY, Kim HD, Kim GS, Cho JH. Schisandra chinensis prevents alcohol-induced fatty liver disease in rats. J Med Food. 2014;17:103–110. doi: 10.1089/jmf.2013.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol. 2013;5:a008672. doi: 10.1101/cshperspect.a008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med. 2016;94:1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu EY, Park SY, Kim SG, Park DJ, Kang JS, Kim YH, Seetharaman R, Choi YW, Lee SJ. Anti-inflammatory effect of heme oxygenase-1 toward Porphyromonas gingivalis lipopolysaccharide in macrophages exposed to gomisins A, G, and J. J Med Food. 2011;14:1519–1526. doi: 10.1089/jmf.2011.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–328. doi: 10.1023/A:1016119328968. [DOI] [PubMed] [Google Scholar]
- Shin DW, Kwon YJ, Ye DJ, Baek HS, Lee JE, Chun YJ. Auranofin suppresses plasminogen activator inhibitor-2 expression through annexin A5 induction in human prostate cancer cells. Biomol Ther (Seoul) 2017;25:177–185. doi: 10.4062/biomolther.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung NY, Kim SC, Kim YH, Kim G, Lee Y, Sung GH, Kim JH, Yang WS, Kim MS, Baek KS, Cho JY. Anti-proliferative and pro-apoptotic activities of 4-methyl-2, 6-bis(1-phenylethyl)phenol in cancer cells. Biomol Ther (Seoul) 2016;24:402–409. doi: 10.4062/biomolther.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanArsdale T, Boshoff C, Arndt KT, Abraham RT. Molecular pathways: targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin Cancer Res. 2015;21:2905–2910. doi: 10.1158/1078-0432.CCR-14-0816. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Xiaoyang L, Chenming N, Chengqing L, Tao L. Drug-drug interation prediction between ketoconazole and anti-liver cancer drug Gomisin G. Afr Health Sci. 2015;15:590–593. doi: 10.4314/ahs.v15i2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]