Abstract
Parkinson’s disease is a neurodegenerative disease characterized by the progressive loss of dopaminergic neurons within the substantia nigra pars compacta. In the present study, we investigated whether β-Lapachone (β-LAP), a natural naphthoquinone compound isolated from the lapacho tree (Tabebuia avellanedae), elicits neuroprotective effects in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease mouse model. β-LAP reduced the tyrosine hydroxylase (TH)-immuno-reactive fiber loss induced by MPTP in the dorsolateral striatum, and alleviated motor dysfunction as determined by the rotarod test. In addition, β-LAP protected against MPTP-induced loss of TH positive neurons, and upregulated B-cell lymphoma 2 protein (Bcl-2) expression in the substantia nigra. Based on previous reports on the neuroprotective role of nuclear factor-E2-related factor-2 (Nrf2) in neurodegenerative diseases, we investigated whether β-LAP induces upregulation of the Nrf2-hemeoxygenae-1 (HO-1) signaling pathway molecules in MPTP-injected mouse brains. Western blot and immunohistochemical analyses indicated that β-LAP increased HO-1 expression in glial fibrillary acidic protein-positive astrocytes. Moreover, β-LAP increased the nuclear translocation and DNA binding activity of Nrf2, and the phosphorylation of upstream adenosine monophosphate-activated protein kinase (AMPK). β-LAP also increased the localization of p-AMPK and Nrf2 in astrocytes. Collectively, our data suggest that β-LAP exerts neuroprotective effect in MPTP-injected mice by upregulating the p-AMPK/Nrf2/HO-1 signaling pathways in astrocytes.
Keywords: β-Lapachone, Parkinson’s disease, Neuroprotection, Astrocyte, Nrf2/HO-1 signaling
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease (AD). PD is characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and depletion of dopamine in nerve terminals projecting to the striatum. The major symptoms of PD are characterized by impaired movement such as rigidity, resting tremor, bradykinesia, and postural instability (Przedborski, 2017). Among the many pathological factors of PD, mitochondrial dysfunction and oxidative stress have been reported to play an important role in dopaminergic neuron damage (Poewe et al., 2017; Puspita et al., 2017). Environmental toxins such as pesticides and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induce mitochondrial complex I defects and lead to adenosine triphosphate (ATP) deficiency and reactive oxygen species (ROS) generation, which subsequently induce cell death (Tieu, 2011). Because oxidative stress plays a key role in dopaminergic neuronal cell death, controlling oxidative stress has been suggested as a promising target for PD treatment.
Astrocytes are the most abundant cells in the brain and play the critical role of supporting neuronal survival. In particular, astrocytes are enriched with antioxidant enzymes and afford protection of surrounding neuronal cells against oxidative stress (Vargas and Johnson, 2009). Phase II antioxidant enzymes such as hemeoxygenae-1 (HO-1) and NAD(P) H:quinone oxidoreductase 1 (NQO1) are mainly involved in the detoxification and cytoprotection mechanisms of astrocytes, the expression of which is under the control of nuclear factor-E2-related factor-2 (Nrf2)/antioxidant response element (ARE) signaling (Zhang et al., 2013). Previous studies have reported that astrocyte-specific overexpression of Nrf2 protects against neurodegeneration in mouse models of AD, PD, Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS), suggesting that Nrf2 is a crucial therapeutic target for the treatment of many neurodegenerative diseases (Vargas et al., 2008; Chen et al., 2009; Johnson and Johnson, 2015). In the case of PD, the neuroprotective role of Nrf2 signaling has been reported in MPTP- or 6-hydroxydopamine (6-OHDA)-injected mice, and alpha-synuclein mutant (A53T) mice (Jakel et al., 2007; Chen et al., 2009; Gan et al., 2012). Based on these studies, the development of agents that can activate Nrf2 signaling in astrocytes has been suggested as a promising therapeutic approach in the treatment of PD.
β-Lapachone (β-LAP) is a natural naphthoquinone compound isolated from the South American lapacho tree (Tabebuia avellanedae) (Schaffner-Sabba et al., 1984). β-LAP is known as a substrate and activator of NQO1, and thereby increases NAD+ and sirtuin 1 levels (Oh et al., 2014). β-LAP has many beneficial activities such as anti-bacterial, antifungal, anti-inflammatory, anti-angiogenic, and anti-carcinogenic effects. In addition, β-LAP exhibits therapeutic effects against rheumatoid arthritis, metabolic syndromes, infection, and cancer (Gómez Castellanos et al., 2009; Hussain and Green, 2017). Recently, the neuroprotective effects of β-LAP have been reported in cerebral ischemia, MS, and HD mouse models (Xu et al., 2013; Kim et al., 2017; Lee et al., 2018). Moreover, our group recently reported that β-LAP suppressed neuroinflammation and elicited antioxidant effects in microglia and astrocytes (Lee et al., 2015; Park et al., 2016). Although many papers have reported the beneficial effects of β-LAP in various disease models, the effect in PD models has not been demonstrated. Therefore, in the present study, we investigated whether β-LAP elicits a neuroprotective effect in MPTP-injected mouse brains.
MATERIALS AND METHODS
Reagents
β-LAP (3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione) and anti-β-actin antibody were purchased from Sigma-Aldrich (St Louis, MO, USA). MPTP was obtained from Tokyo Chemical Industry Co (Tokyo, Japan). Anti-HO-1, anti-Nrf2 and anti-TH antibodies were purchased from Santa Cruz (Santa Cruz Biotechnology Inc., CA, USA). Antibodies against p-AMPK and Bcl-2 were purchased from Cell Signaling Technology (Danvers, MA, USA) and Abcam (Cambridge, UK), respectively.
Animals and drug administration
Male C57BL/6 mice were purchased from the Orient Bio Incorporation (Seongnam, Korea), and housed in standard conditions (under a 12 h light/dark cycle) at 22 ± 2°C with water and food available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Ewha Womans University Medical School (#EMS 17-0371) and conformed to international guidelines for the ethical use of experimental animals. β-LAP (5 mg/kg) was dissolved in a vehicle solution (1% dimethyl sulfoxide and 0.9% sodium chloride) and administered daily via intraperitoneal (i.p.) injection for 3 days before MPTP stimulation. A schematic of this procedure is provided in Fig. 1A.
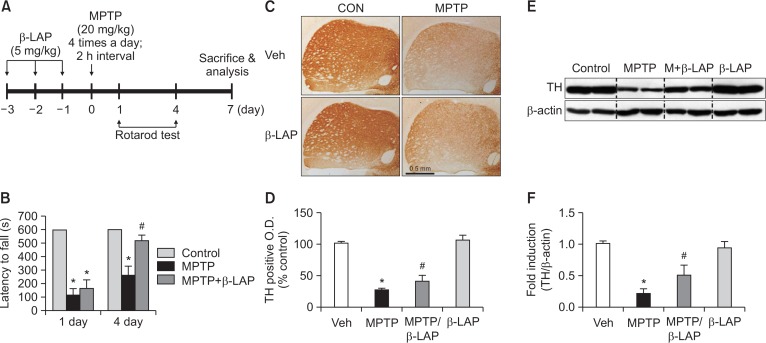
Fig. 1.
β-LAP rescued motor dysfunction and led to upregulation of TH expression in the striatum of MPTP-injected mice. (A) A schematic of the experimental procedure. Mice were injected with β-LAP (5 mg/kg, i.p.) or vehicles every day for 3 days before MPTP injection (20 mg/kg, every 2 h for a total of four injections). Mice were sacrificed at 7 day of MPTP injection, and histological and biochemical analyses were performed. (B) The rotarod performance test was performed 1 and 4 days after the final MPTP injection (n=10 per group). (C) Striatum sections (n=3 per brain) from each group (n=5 per group) were immunostained with TH antibodies and the resulting images are provided. (D) A histogram of the optical density of TH-positive cells. (E) The protein extracts from the striatum of each group were subjected to western blot analyses using TH antibodies (n=5 per group), and representative blots are provided. (F) Quantification of the western blot data. *p<0.05 vs. the control group; #p<0.05 vs. the MPTP group.
MPTP injection and motor performance assessment
The C57BL/6 mice (8–10 weeks) received four i.p. injections of 20 mg/kg body weight of MPTP dissolved in saline at 2 h intervals, and were euthanized 7 days after the final injection. Mice receiving vehicle-only injections served as controls. All procedures involving MPTP were conducted in strict accordance with published safety and handling guidelines (Jackson-Lewis and Przedborski, 2007). Motor performance was assessed using the rotarod test. MPTP injections commenced at 1 day after pre-training, and motor performance was assessed at 1, 4, and 7 days after the final MPTP injection. Mice were subjected to the rotarod test (three trials/mouse, 10 min/trial) and the longest latency was recorded. The cut-off latency was set at 600 s.
Tissue preparation
For histological analysis, the mice were anesthetized with sodium pentobarbital (80 mg/kg body weight, i.p. injection), transcardially perfused with 0.9% saline followed by 4% para-formaldehyde for tissue fixation, and stored in 30% sucrose solution at 4°C for cryoprotection. For biochemical analysis, the mice were transcardially perfused with saline. Each brain was cut into 1 mm thick coronal sections using a mouse brain slicer matrix (RBM-2000C, ASI Instruments, Warren, MI, USA), dissected using the mouse brain atlas coordinates, and immediately frozen in liquid nitrogen until used in the western blot and real-time polymerase chain reaction (PCR) analyses.
Immunohistochemistry and immunofluorescence analysis
Using a cryotome (CM1860; Leica, Mannheim, Germany), 30 µm coronal sections were collected and stored in anti-freezing solution (30% ethylene glycol, 30% glycerol in phosphate-buffered saline) at −20°C for immunohistochemistry (IHC) or immunofluorescence (IF) analyses. The IHC and IF analyses were performed with primary antibodies specific to Bcl-2, glial fibrillary acidic protein (GFAP), HO-1, Nrf2, p-AMPK, and tyrosine hydroxylase (TH). For IHC, the sections were incubated with biotinylated secondary antibodies for 1 h at 22 ± 2°C, and subsequently incubated with avidin-biotin-horseradish peroxidase (HRP) complex reagent solution (Vector Laboratories, Burlingame, CA, USA). The peroxidase reaction was then performed using diaminobenzidine tetrahydrochloride (Vector Laboratories). Digital images of IHC staining were captured using a Leica DM750 microscope and images of IF staining were captured with a Zeiss LSM780 confocal microscope (Carl Zeiss, Oberkochen, Germany) and a Leica DMi8 fluorescence microscope (Leica).
Western blot analysis
Tissues were lysed in RIPA buffer (10 mM Tris-Cl [pH 7.4], 300 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 0.1% sodium deoxycholate, 1 mM ethylenediaminetet-raacetic acid) containing a protease inhibitor cocktail (Complete Mini, Roche, Mannheim, Germany). The proteins (20–50 µg) were separated by SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% bovine serum albumin in 10 mM Tris-HCl containing 150 mM NaCl and 0.05% Tween-20 (TBST), then incubated with primary antibodies (1:1,000) that recognized HO-1, Lamin A, Nrf2, p-AMPK, or TH. After being thoroughly washed with TBST, HRP-conjugated secondary antibodies (1:2,000 dilution in TBST; Amersham, Little Chalfont, UK) were applied, and the blots were developed using an enhanced chemiluminescence detection kit (Thermo Scientific, Waltham, MA, USA).
Real-time RT-PCR
Brain tissue was homogenized using a bead-beater (MO BIO Laboratories, Carlsbad, CA, USA), and the total RNA was extracted using the TRI reagent. For RT-PCR, the total RNA (1 µg) was reverse-transcribed in a reaction mixture containing 1 unit of RNase inhibitor, 500 ng of random primers, 3 mM MgCl2, 0.5 mM dNTP, 1× RT buffer, and 10 units of reverse transcriptase (Promega, Madison, WI, USA). The synthesized cDNA was amplified with SYBR Green PCR Master Mix (Bioline, Taunton, MA, USA), and PCR was performed on an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using the following primers: 5′-AGGCTTTAAGCTGGTGAT GG-3′ (sense) and 5′-GGCATAGACTGGGTTCTGCT-3′ (anti-sense) for HO-1, and 5′-GGCATGGACTGTGGTCATGA-3′ (sense) and 5′-TTCACCACCATGGAGAAGGC-3′ (anti-sense) for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The expression levels of the target genes were normalized against that of GADPH using the following formula: 2(Ct, test gene - Ct, GAPDH).
Electrophoretic mobility shift assay
Nuclear extracts were prepared from brain tissue using an NE-PER nuclear extraction kit (Thermo Fisher Scientific). The double-stranded DNA oligonucleotides containing the ARE consensus sequence (Sigma-Aldrich) were end-labeled using T4 polynucleotide kinase (New England Biolabs, Beverly, MA, USA) in the presence of [γ-32P] ATP. Nuclear proteins (5 µg) were incubated with a 32P-labeled ARE probe on ice for 30 min, resolved on a 5% acrylamide gel, and visualized by autoradiography.
Statistical analysis
All values are reported as the mean ± standard error. Significant differences between groups were determined using one-way analyses of variance (ANOVAs; SPSS for windows, version 23, Chicago, IL, USA). Post-hoc comparisons were performed using the Scheffe test. Statistical significance was accepted for p-values of < 0.05.
RESULTS
β-LAP improved the impaired movement in MPTP-injected mice via recovery of striatal dopaminergic neurons
To determine the effect of β-LAP on motor function, the latency to fall on the rotarod apparatus was analyzed at 1 and 4 days after the final MPTP injection (Fig. 1A). Remarkable motor dysfunction was observed in MPTP-injected mice, as determined by the decreased sustained rotarod time in the MPTP group compared to that in the control group. With β-LAP treatment, the latency to fall was significantly increased at 1 and 4 days, which indicated behavioral rescue (Fig. 1B). The IHC analysis of mouse brains using dopaminergic neuron-specific TH antibodies revealed MPTP-induced neurotoxicity in TH-positive fibers in the striatum at 7 days in the MPTP group compared to that in the control group, and β-LAP treatment led to recovery of TH-positive fibers (Fig. 1C, 1D). In accordance with this data, the western blot analysis revealed that β-LAP recovered the TH protein expression in the striatum of MPTP-injected mice (Fig. 1E, 1F).
β-LAP rescued nigral dopaminergic neurons and Bcl-2 expression in MPTP-injected mice
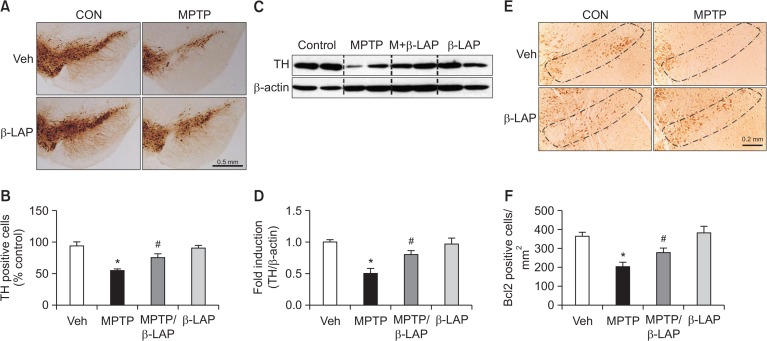
The IHC analysis revealed that MPTP induced significant neurotoxicity in TH-positive cell bodies in the SNpc in MPTP-injected mice compared to that in control mice, and β-LAP restored TH-positive dopaminergic neurons (Fig. 2A, 2B). In addition, β-LAP recovered the TH protein expression in the SN of MPTP-injected mice, as indicated by the western blot analysis (Fig. 2C, 2D). Previous studies demonstrated that MPTP causes apoptotic cell death and Bcl-2 protein can protect against the cell death of dopaminergic neurons in PD (Yamada et al., 1999; Chen et al., 2017). In this study, we found that application of MPTP reduced the expression of Bcl-2 in the SNpc region of MPTP-injected mice, and β-LAP treatment led to recovery of this expression (Fig. 2E, 2F). Collectively, these data provide evidence for the neuroprotective effect of β-LAP against dopaminergic neuronal cell death in MPTP-injected mouse brains.
Fig. 2.
β-LAP recovered the TH and Bcl-2 positive cells in the SN of MPTP-treated mice. (A) Images of the immunostaining results showing TH expression in the SN (n=3 per brain and n=5 per group). (B) Quantification of TH-positive cells in the SNpc. (C) The protein extracts from the SN of each group were subjected to western blot analysis using TH antibodies (n=5 per group), and representative blots are provided. (D) Quantification of the western blot data. (E) Immunostaining images showing the Bcl-2 expression in the SNpc (n=3 per brain and n=5 per group). (F) Quantification of the Bcl-2 positive cells in the SNpc of the mice. *p<0.05 vs. the control group; #p<0.05 vs. the MPTP group.
β-LAP upregulated HO-1 expression in the striatal astrocytes of MPTP-injected mice
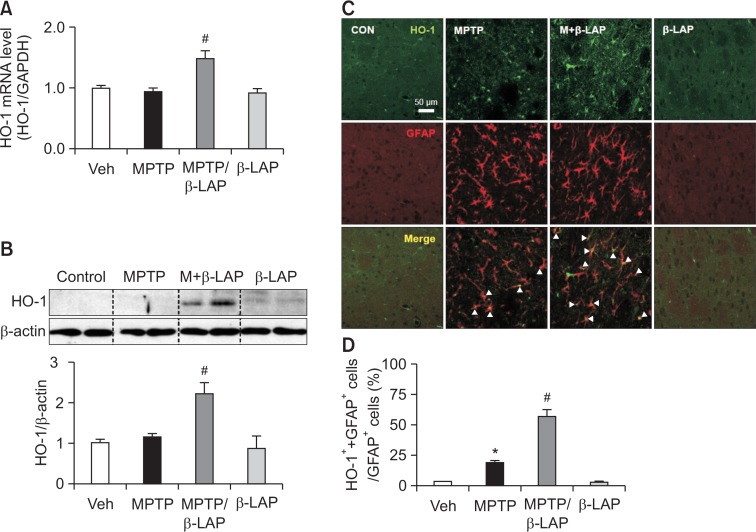
Previous studies have reported that the Nrf2/HO-1 pathway plays a critical role in neuroprotection in PD models (Liddell, 2017; Jo et al., 2018). Therefore, we examined the effects of β-LAP on HO-1 expression in the MPTP-injected mouse brains. We found that the mRNA and protein expression levels of HO-1 were significantly increased by β-LAP in the striatum of MPTP-injected mice (Fig. 3A, 3B). However, β-LAP did not significantly increase HO-1 expression in the SN region (data not shown). To investigate whether β-LAP increased HO-1 expression is astrocytes, we performed double IF staining for HO-1 and GFAP, an astrocyte-specific marker. We found that β-LAP significantly increased the expression of HO-1 in striatal astrocytes (Fig. 3C, 3D).
Fig. 3.
β-LAP upregulated the expression of HO-1 in striatal astrocytes. (A) Quantitative real-time PCR data showing the mRNA expression of HO-1 in striatum of MPTP-injected mice (n=5 per group). (B) Western blot analysis showing the expression of HO-1 protein in the striatum of MPTP-injected mice (n=5 per group). The quantification data are provided in the right panel. (C) The double IF staining results indicate that the HO-1 protein (green) was upregulated by MPTP in striatal astrocytes that were labeled with GFAP (red), and combined treatment of β-LAP with MPTP increased the HO-1 expression level. The white arrows indicate the cells that are positive for both GFAP and HO-1. (D) The quantification data showing the proportion of GFAP/HO-1-positive cells to GFAP only-positive cells. *p<0.05 vs. the control group; #p<0.05 vs. the MPTP group.
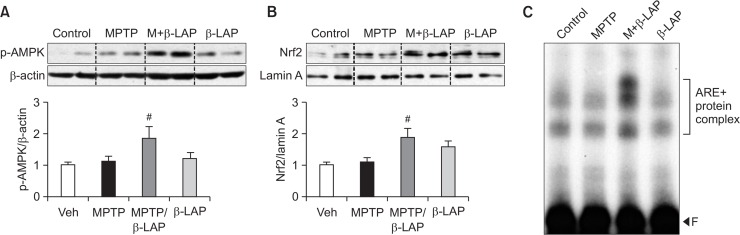
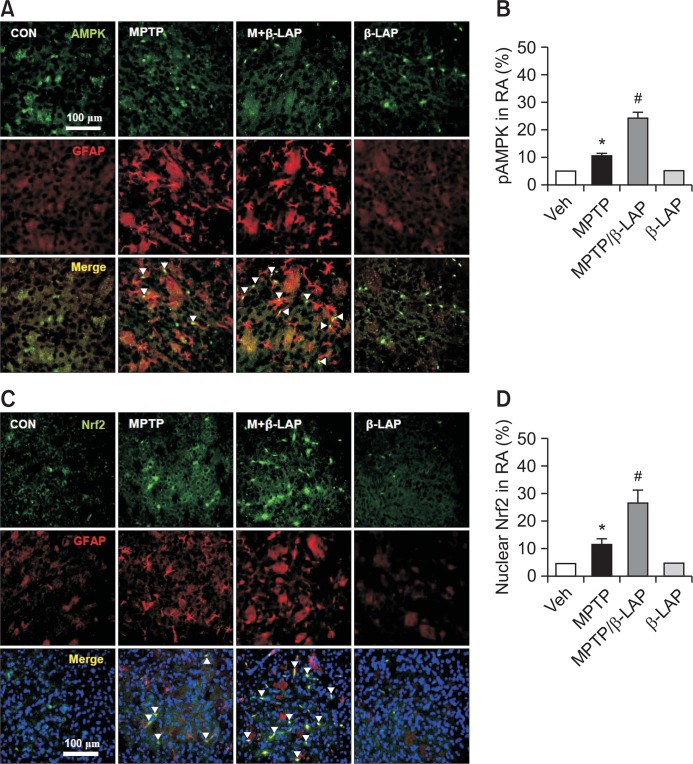
β-LAP increased the phosphorylation of AMPK and nuclear translocation of Nrf2 in the striatal astrocytes of MPTP-injected mice
We recently reported that β-LAP upregulated the expression of phase II antioxidant enzymes via p-AMPK and Nrf2 signaling pathways in rat primary astrocytes (Park et al., 2016). To investigate whether the same signaling pathway is involved in in vivo PD models, we examined the effect of β-LAP on p-AMPK and Nrf2 in the striatum of MPTP-injected mice. We found that β-LAP significantly increased AMPK phosphorylation and the nuclear translocation of Nrf2 (Fig. 4A, 4B). Moreover, β-LAP increased the DNA binding activity of Nrf2 to ARE as determined by the electrophoretic mobility shift assay (EMSA) analysis (Fig. 4C). To determine the expression of p-AMPK and Nrf2 protein in the striatal astrocytes of MPTP-treated mice, double IF staining for GFAP+p-AMPK or GFAP+Nrf2 was performed. As shown in Fig. 5A and 5B, the p-AMPK level was significantly increased in the striatal astrocytes of the β-LAP+MPTP combined group compared to that in the MPTP only-treated group. Moreover, β-LAP increased the nuclear translocation of Nrf2 in the striatal astrocytes of MPTP-injected mice (Fig. 5C, 5D). The results indicate that β-LAP enhances HO-1 expression via activation of AMPK and Nrf2 in astrocytes, which may contribute to the neuroprotective effects of β-LAP in MPTP-injected mouse brains.
Fig. 4.
β-LAP activated the p-AMPK and Nrf2 signaling pathways in the striatum of MPTP-injected mice. (A) The protein samples from the striatum of each group were subjected to western blot analyses using antibodies against phospho-form of AMPK, and the level of p-AMPK was normalized to that of β-actin (n=5 per group). (B) The level of Nrf2 protein in the nuclear fractions of the striatum was determined by western blot analysis (n=3 per group). The quantification data are provided in the right panel. (C) EMSA was performed using nuclear extracts isolated from the striatum of each group (n=3 per group). The bracket indicates ARE+nuclear protein complex. ‘F’ indicates a free probe. #p<0.05 vs. the MPTP group.
Fig. 5.
β-LAP increased the localization of p-AMPK and Nrf2 in striatal astrocytes. (A) Double IF staining results with GFAP (red) and p-AMPK (green) antibodies in the striatum of each brain. The white arrows indicate the cells that are positive for both GFAP and p-AMPK. (B) The quantification data of p-AMPK in reactive astrocytes (RA). (C) Triple label fluorescence microscopy revealed that Nrf2 (green) was predominantly expressed in the nuclei (blue) of astrocytes (GFAP, red) in the striatum of the MPTP-only group and the MPTP+ β-LAP combined group. The white arrows indicate the cells that are positive for nucleus, GFAP, and Nrf2 translocation. (D) The quantification data of nuclear Nrf2 in reactive astrocytes. *p<0.05 vs. the control group; #p<0.05 vs. the MPTP group.
DISCUSSION
In the present study, we demonstrated the neuroprotective effect of β-LAP in MPTP-induced PD model mice. We found that β-LAP protected against MPTP-induced neurotoxicity in the SN and striatum, and improved the impaired motor coordination. Via mechanistic assessment, we demonstrated that β-LAP increased the expression of HO-1 and the upstream Nrf2 and p-AMPK signaling. Interestingly, β-LAP induced the localization of these molecules to astrocytes. Therefore, the results suggest that upregulation of the p-AMPK/Nrf2/HO-1 signaling pathways in astrocytes may be involved in the neuroprotective mechanism of β-LAP in MPTP-induced PD model mice.
Previous studies have reported the neuroprotective role of Nrf2 signaling in mouse models of PD induced by MPTP, 6-OHDA, or an α-synuclein mutation (hSYNA53T) (Jakel et al., 2007; Chen et al., 2009; Gan et al., 2012). In MPTP or 6-OHDA-injected mice, the lack of an Nrf2-mediated transcription in Nrf2−/− mice increased the sensitivity to these neurotoxins (Burton et al., 2006; Jakel et al., 2007; Chen et al., 2009). Consistent with this finding, transgenic mice with Nrf2 under the control of an astrocyte-specific promoter of GFAP were protected against MPTP toxicity, and transplantation of Nrf2-overexpressing astrocytes into the striatum conferred significant resistance to 6-OHDA-induced neuronal damage (Jakel et al., 2007; Chen et al., 2009). Moreover, Nrf2 overexpression in astrocytes resulted in neuroprotection against hSYNA53T-mediated toxicity by promoting the degradation of hSYNA53T via the autophagy-lysosome pathway in a Thy1-hSYNA53T mouse model of PD (Gan et al., 2012). Although the overexpression of Nrf2 in astrocytes elicited neuroprotection in PD and ALS models, Nrf2 overexpression in neurons or muscle cells failed to induce neuroprotection (Johnson and Johnson, 2015; Liddell, 2017). These results suggest that not only the pharmacological target, but also the target cell type may be relevant when an Nrf2-dependent therapeutic approach is considered. In this regard, the astrocyte-targeted upregulation of Nrf2 by β-LAP appears to support the therapeutic potential of β-LAP in the treatment of PD and other neurodegenerative diseases.
Our group recently reported that β-LAP elicits antioxidant effects in rat primary astrocytes (Park et al., 2016). β-LAP inhibited ROS production and cell death in hydrogen peroxide-treated astrocytes. In addition, β-LAP increased the expression of phase II antioxidant enzymes such as HO-1 and NQO1. Via mechanistic assessment, we previously demonstrated that the AMPK/PI3K-Nrf2/ARE signaling pathways are involved in the expression of antioxidant enzymes. In the present study, we found that β-LAP increased the expression levels of HO-1 and the upstream p-AMPK and Nrf2 signaling molecules in the astrocytes of MPTP-injected mice. Thus, the data suggest that the p-AMPK/Nrf2 signaling pathways are commonly involved in β-LAP-induced antioxidant enzyme expression in vitro and in vivo.
Recent studies have reported the therapeutic effects of β-LAP in several animal models of neurological diseases. β-LAP alleviated the neuronal damage induced by in vivo ischemia-reperfusion injury by restoring ATP levels (Kim et al., 2017). β-LAP also ameliorated HD phenotypes by increasing sirtuin 1 expression, cAMP response element binding protein phosphorylation, and peroxisome proliferator-activated receptor-γ coactivator-1α deacetylation (Lee et al., 2018). Moreover, β-LAP was also found to ameliorate the development of experimental autoimmune encephalomyelitis, an animal model of MS, by reducing the production of the interleukin-12 family of cytokines (Xu et al., 2013). In this study, we demonstrated the neuroprotective effect of β-LAP in a PD mouse model. Therefore, the results collectively suggest that β-LAP may be a potential candidate drug for the treatment of various neurological disorders such as PD, HD, and cerebral ischemia.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2010-0027945 & 2018R1A2B6003074 to HK, 2016R1A6A3A11930120 to JP).
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WF, Wu L, Du ZR, Chen L, Xu AL, Chen XH, Teng JJ, Wong MS. Neuroprotective properties of icariin in MPTP-induced mouse model of Parkinson’s disease: involvement of PI3K/Akt and MEK/ERK signaling pathways. Phytomedicine. 2017;25:93–99. doi: 10.1016/j.phymed.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Gan L, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J. Neurosci. 2012;32:17775–17787. doi: 10.1523/JNEUROSCI.3049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez Castellanos JR, Prieto JM, Heinrich M. Red Lapacho (Tabebuiaimpetiginosa)-a global ethnopharmacological commodity? J. Ethnopharmacol. 2009;121:1–13. doi: 10.1016/j.jep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo MG, Ikram M, Jo MH, Yoo L, Chung KC, Nah SY, Hwang H, Rhim H, Kim MO. Gintoin mitigates MPTP-induced loss of nigrostriatal dopaminergic neurons and accumulation of α-synuclein via the Nrf2/HO-1 pathway. Mol. Neurobiol. 2019;56:39–55. doi: 10.1007/s12035-018-1020-1. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Johnson JA. Nrf2-a therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain H, Green IR. Lapachol and lapachone analogs: a journey of two decades of patent research (1997–2016) Expert. Opin. Ther. Pat. 2017;27:1111–1121. doi: 10.1080/13543776.2017.1339792. [DOI] [PubMed] [Google Scholar]
- Kim AY, Jeong KH, Lee JH, Kang Y, Lee SH, Baik EJ. Glutamate dehydrogenase as a neuroprotective target against brain ischemia and reperfusion. Neuroscience. 2017;340:487–500. doi: 10.1016/j.neuroscience.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Ko HM, Jeong YH, Park EM, Kim HS. β-Lapachone suppresses neuroinflammation by modulating the expression of cytokines and matrix metalloproteinases in activated microglia. J. Neuroinflammation. 2015;12:133. doi: 10.1186/s12974-015-0355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Ban JJ, Chung JY, Im W, Kim M. Amelioration of Huntington’s disease phenotypes by β-lapachone is associated with increases in Sirt1 expression, CREB phosphorylation and PGC-1α deacetylation. PLoS ONE. 2018;9:e0195968. doi: 10.1371/journal.pone.0195968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell JR. Are astrocytes the predominant cell type for activation of Nrf2 in aging and neurodegeneration? Antioxidants. 2017;6:65. doi: 10.3390/antiox6030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh GS, Kim HJ, Choi JH, Shen A, Choe SK, Karna A, Lee SH, Jo HJ, Yang SH, Kwak TH, Lee CH, Par R, So HS. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014;85:547–560. doi: 10.1038/ki.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Lee YY, Kim J, Seo H, Kim HS. β-Lapachone increases phase II antioxidant enzyme expression via NQO1-AMPK/PI3K-Nrf2/ARE signaling in rat primary astrocytes. Free Radic. Biol. Med. 2016;97:168–178. doi: 10.1016/j.freeradbiomed.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Przedborski S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017;18:251–259. doi: 10.1038/nrn.2017.25. [DOI] [PubMed] [Google Scholar]
- Puspita L, Chung SY, Shim JW. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain. 2017;10:53. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner-Sabba K, Schmidt-Ruppin KH, Wehrli W, Schuerch AR, Wasley JW. β-Lapachone: synthesis of derivatives and activities in tumor models. J. Med. Chem. 1984;27:990–994. doi: 10.1021/jm00374a010. [DOI] [PubMed] [Google Scholar]
- Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011;1:a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev. Mol. Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wagoner G, Douglas JC, Drew PD. β-Lapachone ameliorization of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2013;254:46–54. doi: 10.1016/j.jneuroim.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Oligino T, Mata M, Goss JR, Glorioso JC, Fink DJ. Herpes simplex virus vector-mediated expression of Bcl-2 prevents 6-hydroxydopamine-induced degeneration of neurons in the substantia nigra in vivo. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4078–4083. doi: 10.1073/pnas.96.7.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]