Abstract
Methamphetamine (METH) acts strongly on the nervous system and damages neurons and is known to cause neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Flavonoids, polyphenolic compounds present in green tea, red wine and several fruits exhibit antioxidant properties that protect neurons from oxidative damage and promote neuronal survival. Especially, epicatechin (EC) is a powerful flavonoid with antibacterial, antiviral, antitumor and antimutagenic effects as well as antioxidant effects. We therefore investigated whether EC could prevent METH-induced neurotoxicity using HT22 hippocampal neuronal cells. EC reduced METH-induced cell death of HT22 cells. In addition, we observed that EC abrogated the activation of ERK, p38 and inhibited the expression of CHOP and DR4. EC also reduced METH-induced ROS accumulation and MMP. These results suggest that EC may protect HT22 hippocampal neurons against METH-induced cell death by reducing ER stress and mitochondrial damage.
Keywords: Epicatechin, Methamphetamine, Neuroprotection
INTRODUCTION
Methamphetamine (METH) is an illegal and potent mental stimulant that acts strongly on the central nervous system. METH is abused by more than 35 million people worldwide (Hanson et al., 2004; Talloczy et al., 2008). The serious neuro-psychological consequences of METH abuse include memory and cognitive impairment that persist long after withdrawal (Meredith et al., 2005). Repeated METH administration to rats results in long-term damage to sensorimotor cortical neurons, which has been correlated with impaired motor function or cognitive memory (Walsh and Wagner, 1992; Marshall et al., 2007). Since METH has relatively high lipid solubility, the drug permeates through the blood-brain barrier. When METH enters the monoamine reactive end via dopamine or serotonin transporter, it is oxidized instead of vesicular and intracellular dopamine and serotonin, leading to the production of reactive oxygen species and neuronal death (Davidson et al., 2001; Barr et al., 2006).
Recent studies suggest that oxidative stress, mitochondrial apoptosis and excitotoxicity pathways play an important role in METH-induced neurotoxic damage. Superoxide radicals (Jayanthi et al., 1998) and hydroxyl radicals (Giovanni et al., 1995) were found to mediate the neurotoxicity of METH. Thus, excessive production of free radicals by repeated administration of METH may contribute to oxidative damage (Jayanthi et al., 1998). However, the mechanism associated with METH toxicity has yet to be fully elucidated.
It is well known that METH increases glutamate levels in mammalian brain. High levels of glutamate contribute to neurotoxicity (Eisch et al., 1996; O’Dell and Marshall, 2005). Glutamate induces neuronal apoptosis via interaction between endoplasmic reticulum stress and mitochondrial death pathways upon administration of METH to rodents (Jayanthi et al., 2001, 2004).
Hippocampal neuronal cell death plays an important role in memory disorders associated with various brain diseases (Lee et al., 2012; Debette, 2013). Therefore, preventing hippocampal neuronal cell death represents a new therapeutic strategy to improve memory and cognitive impairment in various neurological disorders. The HT22 hippocampal cell line is functionally deficient in glutamate receptors, and has been used to study glutamate-mediated molecular mechanisms (Lee et al., 2012; Kempf et al., 2014). Further, HT22 cells exposed to glutamate exhibit neurotoxicity via oxidative stress such as decreased glutathione, changes in intracellular cysteine homeostasis, inhibition of cysteine uptake, and ultimately activation of ROS resulting in neuronal cell death (Murphy et al., 1989; Stanciu et al., 2000). Oxidative stress and accumulation of ROS eventually lead to hippocampal cell death and affect learning and memory impairment (Yang et al., 2013).
Flavonoids are polyphenolic compounds present in green tea, red wine and several fruits (Cuevas et al., 2009). Flavonoids exhibit antioxidant properties that protect neurons from oxidative damage and promote neuronal survival (Blount et al., 2012). Epicatechin (EC) is a flavonoid with antibacterial (Bhattacharyya et al., 2004), antiviral (Apostolides and Weisburger, 1995), antitumor (Geetha et al., 2004) and antimutagenic effects (Hanasaki et al., 1994) as well as antioxidant effects (Nakagawa and Yokozawa, 2002). It has also been shown to effectively remove nitric oxide and O2 (Katiyar et al., 1994). In the present study, we investigated the protective effects of EC on METH-induced cytotoxicity using HT22 hippocampal cells. We further investigated whether the cytoprotective effect of EC is involved in oxidative stress regulation.
MATERIALS AND METHODS
Materials and reagents
Methamphetamine (METH) was purchased from the Ministry of Food and Drug Safety (Cheongju, Korea). Epicatechin (EC) was purified and received from Dr. Gil-Saeng Jeong, a professor of the College of Pharmacy, Keimyung University (Deagu, Korea). Methamphetamine (METH) was dissolved in dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA) as a 1 M stock solution and stored at 4°C. Further dilution was done in cell culture medium. Antibodies against CHOP, BAX were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against phospho-ERK, ERK, phospho-JNK, JNK, phospho-p38, p38, DR4, DR5, cleaved caspase-3, cleaved caspase-8, cleaved caspase-9, cleaved PARP, and β-actin were obtained from Cell Signaling Technology (Beverly, MA, USA). β-actin was used as a loading control.
Cell line and cell culture
HT22 murine hippocampal neuronal cells (The Salk Institute, La Jolla, CA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics at 37°C in an atmosphere of 5% CO2-95% air. Cells were passaged at 80% confluence in 0.25% trypsin-EDTA (ethylenediaminetetraacetic acid) for 2 min. DMEM, FBS, antibiotic and trypsin-EDTA were purchased from Gibco (Grand Island, NY, USA).
Cell viability assay
HT22 cells were incubated in 24-well plates at a density of 3×105 cells per well. Media were pretreated with different concentration of Epicatechin (10, 20 μM) for 1 h, followed by stimulation with Methamphetamine (1, 2, 5 or 10 mM) for 24 h. After treatment, 3-(4, 5-dimethylthiazol-2-yl)-2,5 diphenylterazolium bromide (MTT, 5 mg/ml; Sigma) was added to each well (2.5 mg/mL), following incubation for 4 h at 37°C in a 5% CO2. The supernatant was removed, and the formazan precipitate was dissolved in dimethyl sulfoxide (DMSO). The absorbance was measured at 540 nm using a microplate reader (Tecan Austria GmbH, Salzburg, Austria).
Live/Dead assay
To measure apoptosis, we used the Live/Dead assay kit (Invitrogen, Gaithersburg, MD, USA), which determines intracellular esterase activity and plasma membrane integrity. Calcein-AM, a non-fluorescent polyanionic dye, is retained by live cells, in which it produces green fluorescence through enzymatic (esterase) conversion. In addition, the ethidium homodimer enters cells with damaged membranes and binds to nucleic acids, thereby producing a red fluorescence in dead cells. Briefly, the cells were seeded in a chamber slide (Nalge Nunc International, Naperville, IL, USA), incubated with 20 μM Epicatechin for 1 h, and then treated with 5 mM Methamphetamine for 24 h at 37°C. The cells were stained with the Live/Dead reagent (5 μM ethidium homodimer, 5 μM calcein-AM) and then incubated at 37°C for 30 min. The cells were analyzed under a Labophot-2 fluorescence microscope (Nikon, Tokyo, Japan).
Apoptosis analysis
Proportions of apoptotic cells were detected by flow cytometry using the Annexin V-FITC Apoptosis Detection kit I (BD PharmingenTM, BD Biosciences, NJ, USA). The experiment was performed according to the manufacturer’s protocol. To investigate the neurotoxic effect of Epicatechin on Methamphetamine, cells were pretreated with 20 μM Epicatechin for 1 h and then exposed to 5 mM Methamphetamine for 24 h. Floating cells were collected, and the attached cells were trypsinized and washed twice with ice-cold PBS at the indicated time. Then cell suspension (1×105 cells/ml) was incubated with Annexin V-FITC for 15 min in the dark. Finally, 400 μl of 1X binding buffer was added to each sample. The samples were counted using a BD FACS VerseTM (BD Biosciences) and analyzed by Flowing 2.5 version software.
Measurement of intracellular ROS
Intracellular ROS level was measured using the fluorescent dye, DCF-DA. Cells were pretreated with EC (10, 20 μM) for 1 h, and treated with 1mM METH for 24 h. Cells were then treated with 5 μM of DCF-DA for 15 min at 37°C, and the cells were imaged using fluorescence microscopy.
Measurement of MMP
To determine MMP, cells were pretreated with EC for 1 h, followed by treatment with 5 mM METH for 24 h. Cells were washed with warm PBS, resuspended in warm PBS containing 100 nM TMRM, and then incubated at 37°C for 30 min. Cells were washed with warm PBS, resuspended in PBS, and then MMP level was measured by flow cytometry. TMRM fluorescence excited at 488 nm and emitted at 588 nm was measured by FL2 channel using a BD FACS VerseTM (BD Biosciences) and analyzed with Flowjo software. Apoptotic cells showed decreased intensity of TMRM staining. At least ten thousand events were analyzed per sample and the experiment was repeated at least twice.
Western blot analysis
The cells were washed with PBS and lysed for 30 min on ice in RIPA lysis buffer [150 mM NaCl, 10 mM Tris (pH 7.2), 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, 1% deoxycholate and 5 mM ethylene diamine tetra acetic acid (EDTA)] enriched with a complete protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany), and then centrifuged at 14000 rpm for 10 min at 4°C. Protein concentration was determined by using bicinchoninic acid (BCA) protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Total protein (30 μg) was loaded onto 12% SDS-polyacrylamide gel, separated, and transferred onto polyvinyl difluoride (PVDF) membrane (Roche, Diagnostics). The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline with Tween-20 [TBST; 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.05% Tween-20] and incubated with antibody at 4°C. After three washes of 10 min each in TBST, the membranes were incubated with hybridization with horseradish peroxidase conjugated anti-rabbit or anti-mouse secondary antibodies for 2 h and subsequently washed again. The transferred proteins were incubated with super-signal pico-chemiluminescent substrate or dura-luminol substrate (Thermo Scientific, Waltham, MA, USA) for 1 min according to the manufacturer’s instructions and visualized with Image QuantTM LAS 4000 (Fujifilm, Tokyo, Japan; Roche Diagnostics).
Statistical analysis
Experiments were performed at least three times, with consistent results. The results are given as mean ± standard deviation (SD). The p-value was assessed using ANOVA and Student-Newman-Keul tests. Results were considered statistically significant at p<0.01.
RESULTS
EC protects HT22 hippocampal neuronal cells against METH-induced cell death
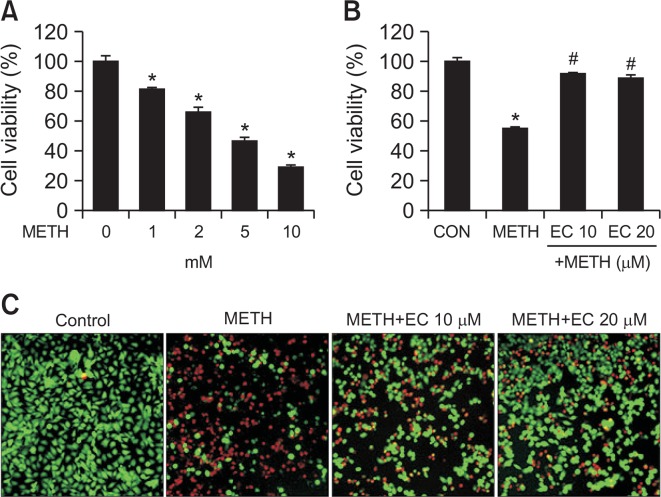
To determine whether EC was cytoprotective, we measured the cell viability after METH treatment of murine hippocampal HT22 cells. When HT22 cells were treated with various concentrations of METH for 24 h, the cell viability was decreased to 50% at 5 mM (Fig. 1A). To determine the protective effect of EC against METH-induced cytotoxicity, cells were pretreated with 10, 20 μM EC for 1 h and incubated with 5 mM METH for 24 h (Fig. 1B). Despite treatment with 5 mM METH, concurrent EC treatment restored the rate of cell survival up to 90%. These results demonstrate that EC protects HT22 hippocampal cells from METH-induced cell death. Further, when we identified live and dead cells using fluorescence dye, the proportion of dead cells was decreased gradually following pretreatment with EC (Fig. 1C).
Fig. 1.
Effects of METH and EC on cell viability in HT22 hippocampal neuronal cells. (A) HT22 cells were treated with various concentrations of METH for 24 h. (B) Cells were pretreated with EC for 1 h, and incubated with 5 mM METH for 24 h. The cell viability was determined by MTT assay and the percent cell viability was plotted as the means ± SEM of at least three experiments. (C) Live/Dead viability/cytotoxicity assay depicts the cytotoxic effects of METH and EC in HT22 cells. Fluorescence images of viable cells were stained with calcein-AM (green) and dead cells were stained with ethidium homodimer 1 (red). Percentage of viable cells was calculated under a fluorescence microscope. A total of five random quadrants were selected from each triplicate for quantification. Data are expressed as mean ± SEM. *p<0.01 when compared with untreated control cells. #p<0.01 when compared with METH-treated cells.
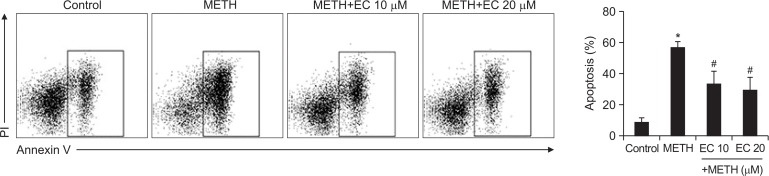
EC prevents METH-mediated apoptosis of HT22 cells
Since HT22 cells underwent apoptotic cell death following treatment with METH, we investigated whether EC prevented apoptosis induced by METH. As shown in Fig. 2, analysis of flow cytometry data revealed that METH induced apoptosis of HT22 cells, and co-treatment with EC significantly reduced it. Based on these results, we suggest that EC has a cytoprotective effect and prevents neuronal toxicity induced by METH.
Fig. 2.
Effects of EC on METH-induced apoptosis in HT22 hippocampal neuronal cells. Cells were pretreated with EC for 1 h, and incubated with 5 mM METH for 24 h. Cells were stained with PI and Annexin V, and then evaluated by flow cytometry (left). Percentage of apoptotic cells was detected by flow cytometry (right). Results are shown as mean ± SEM from three independent experiments. *p<0.01 when compared with untreated control cells. #p<0.01 when compared with METH-treated cells.
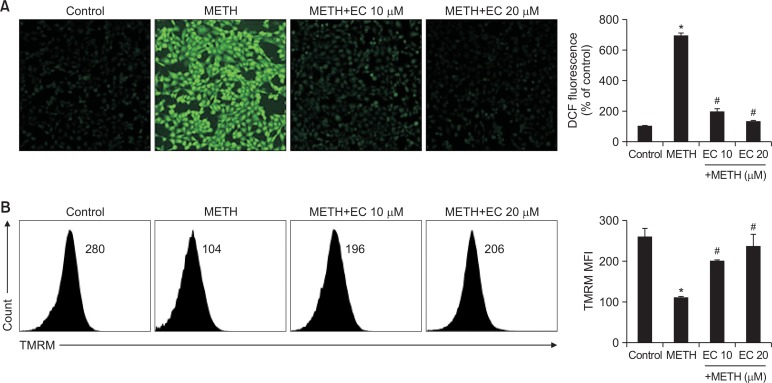
EC reduced ROS accumulation and MMP in HT22 cells
Since oxidative stress including ROS plays an important role in neuronal injury, cell death and neurodegenerative diseases (Simonian and Coyle, 1996), we examined whether ROS mediated METH-induced cytotoxicity. HT22 cells were pretreated with EC for 1 h and exposed to 1 mM METH for 24 h. The ROS levels were analyzed under a microscope via 2′,7′-dichlorofluoresence diacetate (DCF-DA) staining. As shown in Fig. 3A, a large amount of ROS accumulation was detected when cells were treated with 1 mM of METH. However, EC significantly inhibited ROS accumulation suggesting that EC has an antioxidative effect.
Fig. 3.
Effects of EC on METH-induced ROS accumulation and reduction of MMP in HT22 hippocampal neuronal cells. (A) Cells were pretreated with EC for 1 h, and incubated with 1 mM METH for 24 h. Cells were then incubated with 5 μM DCF-DA for 15 min. The intracellular ROS level was determined using a fluorescence microscope. (B) Cells were pretreated with EC for 1 h, and treated with 5 mM METH for 24 h. Cells were stained with TMRM and the fluorescence was measured by flow cytometry (left). Percentage of MMP was calculated and plotted in the graph (right). *p<0.01 when compared with untreated control cells. #p<0.01 when compared with METH-treated cells.
Next, the mitochondrial membrane potential (MMP) was measured to determine whether the mitochondrial pathway was involved in METH-induced neuronal cell death. As an indicator of mitochondrial damage, we monitored the MMP using tetramethylrhodamine ethyl ester (TMRE). When the cells were incubated with 5 mM METH for 24 h, MMP was significantly reduced; however, pretreatment with EC restored METH-induced MMP reduction (Fig. 3B). Overall, we found that neuronal cytotoxicity by METH was associated with ROS accumulation and MMP collapse, which may be attributed to the interaction between ER stress and mitochondrial death pathway, resulting in increased neuronal death. However, EC inhibited ROS accumulation and protected MMP collapse by METH.
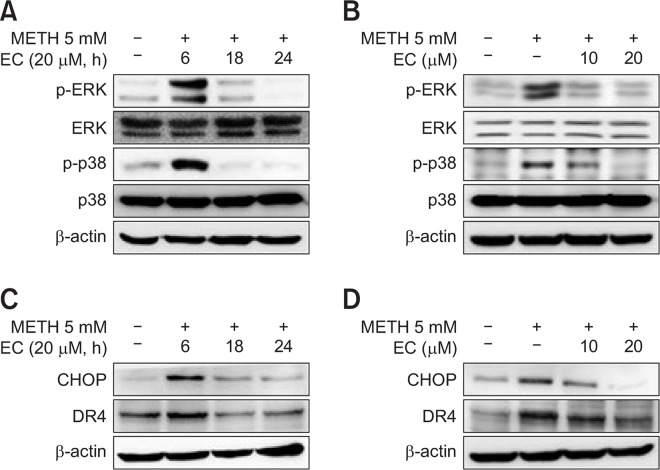
EC inhibits METH-induced MAPK activation
Since MAPKs such as p38, JNK, and ERK mediate cell death by ROS (Ravindran et al., 2011; Son et al., 2013), we examined whether METH activated MAPKs. The activation of MAPKs was confirmed by Western blot analysis. We further explored whether EC inhibited MAPKs activation mediated by METH. As shown in Fig. 4, EC significantly reduced ERK and p38 activation by METH without affecting JNK (data not shown). The activity of MAPKs was robust until 6 h and eventually declined upon EC exposure (Fig. 4A). The inhibition of MAPKs activity was significantly inhibited at 10 μM and 20 μM of EC, respectively, and the inhibition was suppressed further at 20 μM treatment (Fig. 4B).
Fig. 4.
Effects of EC on METH-induced protein expression in HT22 hippocampal neuronal cells. Cells were pretreated with 20 μM EC for 1 h, treated with 5 mM METH at various time intervals (A, C), or pretreated with 10 μM or 20 μM EC for 1 h and then treated with 5 mM METH for 24 h (B, D). Cell lysates were resolved by SDS-PAGE and then analyzed by Western blot using antibodies against p38, p-p38, ERK, p-ERK, CHOP, DR4 and β-actin. The results shown were obtained from at least three independent experiments.
Next, we evaluated the expression of CHOP, a marker for ER stress, which is associated with the MAPKs-mediated death pathway. The results showed that METH induced CHOP expression, which was significantly abrogated by EC. Since CHOP is also involved in the expression of death receptor (DR) (Gupta et al., 2013b; Refaat et al., 2014), we examined whether EC affects the expression of death receptor. We found that METH increased the expression of DR4, which was also inhibited by EC treatment. Expression of CHOP and DR4 appears to be elevated until 6 h and eventually declined upon EC exposure (Fig. 4C). The blockade of expression was significantly inhibited further at 20 μM treatment (Fig. 4D).
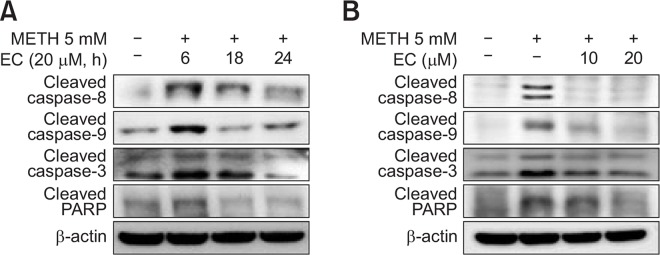
EC inhibits METH-induced apoptotic pathway
DR4/5 is involved in the extrinsic pathway of apoptosis, whereas the mitochondrial pathway is involved in intrinsic apoptosis (Belt et al., 2014). MMP and DR4 mediate METH-induced cell death (Fig. 4B). Therefore, we investigated whether the cytoprotective effect of EC played a role in both intrinsic and extrinsic apoptotic pathways. As shown in Fig. 5, EC decreased the cleavage of caspase-8 and caspase-3, which are extrinsic apoptotic molecules. EC also reduced the cleavage of caspase-9 and PARP, which are signaling molecules in the intrinsic apoptosis. Similar to the results of the previous experiments, METH-induced activation of caspases was decreased 6 h after treatment with EC (Fig. 5A), and the activity was further strongly inhibited at 20 μM (Fig. 5B). Collectively, these results suggest that EC protects HT22 cells by preventing METH-induced mitochondrial damage and DR4 pathway.
Fig. 5.
Effects of EC on METH-induced caspases activation in HT22 hippocampal neuronal cells. Cells were pretreated with 20 μM EC for 1 h, treated with 5 mM METH at various time intervals (A), or pretreated with 10 μM or 20 μM EC for 1 h and then treated with 5 mM METH for 24 h (B). Cell lysates were resolved by SDS-PAGE and then analyzed by Western blot using antibodies against cleaved caspase-8, -9, -3, cleaved PARP and β-actin. The results shown were obtained from at least three independent experiments.
DISCUSSION
Since hippocampus plays a key role in drug addiction, several studies analyzed hippocampus in addiction prevention. In particular, hippocampus is one of the brain regions vulnerable to METH (Shimazu et al., 2006; Raudensky and Yamamoto, 2007). METH mediates the interaction between ER stress and mitochondrial death leading to apoptosis of striatal glutamic acid decarboxylase-containing neurons. In addition to neuronal death, astroglial activation may be triggered by METH toxicity (Pubill et al., 2003; Miyatake et al., 2005). Further, METH induces excessive glutamate release, which damages the cortical neurons via an excitotoxic mechanism (Eisch et al., 1996; O’Dell and Marshall, 2005). The toxicity due to oxidative glutamate occurs due to a sharp decline in intracellular glutathione and increased ROS production in HT22 cells, triggering downstream events such as activation of lipoxygenase, increased Ca2+ concentration, and increased nuclear expression of AIF (Pallast et al., 2009). Recent reports suggest that hypoxia-induced excessive ROS and ROS-based ER stress play an important role in the progression of HT22 cell death. Neurons are particularly susceptible to damage by ROS due to their low levels of endogenous antioxidant enzymes and weak antioxidant defense systems (Jia et al., 2013). Thus, oxidative stress induced by ROS contributes to neuronal cell death in the brain. In this study, we investigated the cytoprotective effects of EC on METH-induced oxidative toxicity in hippocampal HT22 cell line.
ER stress induces the release of ROS, which is central to the activation of multiple signaling pathways. METH induces ER stress, and therefore, we determined whether METH exposure generated ROS. The results suggested that METH significantly increased ROS production, which was inhibited by pretreatment with EC (Fig. 3). In addition, we confirmed that EC inhibited the activation of ERK and p38 MAPK and the expression of CHOP and DR4 by METH (Fig. 4). These results indicate that EC inhibits glutamate-induced ER stress by METH. In addition to glutamate, a variety of ER stress inducers, such as tunicamycin, increase CHOP expression and induce cell death by ER stress in HT22 cells (Ono et al., 2012). CHOP is a crucial mediator of ER stress-related apoptosis and is expressed highly in neurodegenerative diseases such as Alzheimer’s disease (Ito et al., 2009; Salminen et al., 2009). These studies suggest that the increased expression of CHOP in neurons induces apoptosis associated with ER stress. Therefore, the inhibition of CHOP expression by EC can prevent the effect of ER stress caused by METH (Lauro et al., 2010; Chhunchha et al., 2013). Our study suggests that EC acts as a potent antioxidant against ROS and ER stress-induced apoptosis in HT22 cells.
ER-induced apoptosis increases the expression of DR4 and DR5 via increased expression of CHOP, and ROS is involved in the upregulation of DR4 and DR5. The expression of CHOP and DR4 is upregulated via ERK and p38 MAPK signaling (Park et al., 2010; Sung et al., 2010; Prasad et al., 2011; Gupta et al., 2013a; Yoon et al., 2013). In the present study, we showed that ERK and p38 activities are upregulated by ROS and ER stress induced by METH, resulting in the upregulation of CHOP and DR4 expression. EC significantly abrogated the upregulation.
In general, it is well known that apoptosis occurs via two pathways: the extrinsic pathway mediated via death receptor and the intrinsic pathway via mitochondria. ROS induce apoptosis via both the extrinsic apoptotic receptor and the intrinsic mitochondrial apoptotic pathway. Our study showed that EC inhibited METH-induced activation of caspase-3, -8, -9 and PARP (Fig. 4C). It was also confirmed that EC restored MMP reduced by METH (Fig. 3B). These results suggest that the extrinsic pathway via DR4 and the intrinsic pathway via mitochondria mediate the METH-induced apoptotic pathway, and EC significantly abrogated both apoptotic pathways.
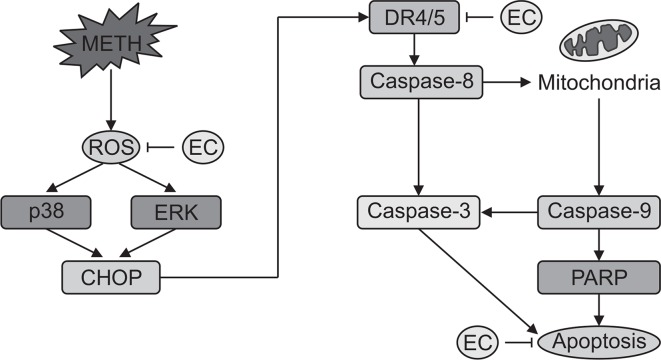
In this study, we have demonstrated the protective effect of EC against neuronal cell death via oxidative stress and ER stress induced by METH. EC inhibited ROS generation as well as MAPK activity, and CHOP and DR4 expression during METH-mediated apoptosis. In addition, EC inhibits apoptosis via inhibition of caspase activity (Fig. 6). Overall, our results support the hypothesis that EC has a protective effect against METH-induced cytotoxicity in HT22 hippocampal cells. These results provide insight into the etiology of neurodegenerative diseases associated with METH abuse.
Fig. 6.
A schematic diagram for cytoprotective effect of EC against METH-induced neuronal cell death.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1A6A1A03011325), and by the Ministry of Science, ICT & Future Planning (NRF-2016R1A1A1A05921696).
REFERENCES
- Apostolides Z, Weisburger JH. Screening of tea clones for inhibition of PhIP mutagenicity. Mutat. Res. 1995;326:219–225. doi: 10.1016/0027-5107(94)00175-5. [DOI] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J. Psychiatry. Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Belt EJ, Stockmann HB, Delis-van Diemen PM, Bril H, Tijssen M, van Essen HF, Heymans MW, Belien JA, Carvalho B, Cillessen SA, Meijer GA. Expression of apoptosis regulating proteins identifies stage II and III colon cancer patients with high risk of recurrence. J. Surg. Oncol. 2014;109:255–265. doi: 10.1002/jso.23495. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Mandal D, Lahiry L, Sa G, Das T. Black tea protects immunocytes from tumor-induced apoptosis by changing Bcl-2/Bax ratio. Cancer Lett. 2004;209:147–154. doi: 10.1016/j.canlet.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Blount JW, Ferruzzi M, Raftery D, Pasinetti GM, Dixon RA. Enzymatic synthesis of substituted epicatechins for bioactivity studies in neurological disorders. Biochem. Biophys. Res. Commun. 2012;417:457–461. doi: 10.1016/j.bbrc.2011.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhunchha B, Fatma N, Kubo E, Rai P, Singh SP, Singh DP. Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-κB regulation. Am. J. Physiol. Cell Physiol. 2013;304:C636–C655. doi: 10.1152/ajpcell.00345.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas E, Limon D, Perez-Severiano F, Diaz A, Ortega L, Zenteno E, Guevara J. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25–35 in rats. Eur. J. Pharmacol. 2009;616:122–127. doi: 10.1016/j.ejphar.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res. Brain Res. Rev. 2001;36:1–22. doi: 10.1016/S0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Debette S. Vascular risk factors and cognitive disorders. Rev. Neurol (Paris) 2013;169:757–764. doi: 10.1016/j.neurol.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, O’Dell SJ, Marshall JF. Striatal and cortical NMDA receptors are altered by a neurotoxic regimen of methamphetamine. Synapse. 1996;22:217–225. doi: 10.1002/(SICI)1098-2396(199603)22:3<217::AID-SYN3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Geetha T, Garg A, Chopra K, Pal Kaur I. Delineation of antimutagenic activity of catechin, epicatechin and green tea extract. Mutat. Res. 2004;556:65–74. doi: 10.1016/j.mrfmmm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Giovanni A, Liang LP, Hastings TG, Zigmond MJ. Estimating hydroxyl radical content in rat brain using systemic and intraventricular salicylate: impact of methamphetamine. J. Neurochem. 1995;64:1819–1825. doi: 10.1046/j.1471-4159.1995.64041819.x. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Francis SK, Nair MS, Mo YY, Aggarwal BB. Azadirone, a limonoid tetranortriterpene, induces death receptors and sensitizes human cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) through a p53 protein-independent mechanism: evidence for the role of the ROS-ERK-CHOP-death receptor pathway. J. Biol. Chem. 2013a;288:32343–32356. doi: 10.1074/jbc.M113.455188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Francis SK, Nair MS, Mo YY, Aggarwal BB. Azadirone, a limonoid tetranortriterpene, induces death receptors and sensitizes human cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) through a p53 protein-independent mechanism: evidence for the role of the ROS-ERK-CHOP-death receptor pathway. J. Biol. Chem. 2013b;288:32343–32356. doi: 10.1074/jbc.M113.455188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Rau KS, Fleckenstein AE. The metham-phetamine experience: a NIDA partnership. Neuropharmacology. 2004;47(Suppl 1):92–100. doi: 10.1016/j.neuropharm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yamada M, Tanaka H, Aida K, Tsuruma K, Shimazawa M, Hozumi I, Inuzuka T, Takahashi H, Hara H. Involvement of CHOP, an ER-stress apoptotic mediator, in both human sporadic ALS and ALS model mice. Neurobiol. Dis. 2009;36:470–476. doi: 10.1016/j.nbd.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Ladenheim B, Cadet JL. Methamphetamine-induced changes in antioxidant enzymes and lipid peroxidation in copper/zinc-superoxide dismutase transgenic mice. Ann. N. Y. Acad. Sci. 1998;844:92–102. doi: 10.1111/j.1749-6632.1998.tb08224.x. [DOI] [PubMed] [Google Scholar]
- Jia J, Xiao Y, Wang W, Qing L, Xu Y, Song H, Zhen X, Ao G, Alkayed NJ, Cheng J. Differential mechanisms underlying neuroprotection of hydrogen sulfide donors against oxidative stress. Neurochem. Int. 2013;62:1072–1078. doi: 10.1016/j.neuint.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Agarwal R, Mukhtar H. Inhibition of spontaneous and photo-enhanced lipid peroxidation in mouse epidermal microsomes by epicatechin derivatives from green tea. Cancer Lett. 1994;79:61–66. doi: 10.1016/0304-3835(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Kempf SJ, Buratovic S, von Toerne C, Moertl S, Stenerlow B, Hauck SM, Atkinson MJ, Eriksson P, Tapio S. Ionising radiation immediately impairs synaptic plasticity-associated cytoskeletal signalling pathways in HT22 cells and in mouse brain: an in vitro/in vivo comparison study. PLoS ONE. 2014;9:e110464. doi: 10.1371/journal.pone.0110464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro C, Cipriani R, Catalano M, Trettel F, Chece G, Brusadin V, Antonilli L, van Rooijen N, Eusebi F, Fredholm BB, Limatola C. Adenosine A1 receptors and microglial cells mediate CX3CL1-induced protection of hippocampal neurons against Glu-induced death. Neuropsychopharmacology. 2010;35:1550–1559. doi: 10.1038/npp.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Jeong EJ, Huh J, Cho N, Kim TB, Jeon BJ, Kim SH, Kim HP, Sung SH. Cognition-enhancing and neuroprotective activities of the standardized extract of Betula platyphylla bark and its major diarylheptanoids. Phytomedicine. 2012;19:1315–1320. doi: 10.1016/j.phymed.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Belcher AM, Feinstein EM, O’Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2007;102(Suppl 1):61–69. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv. Rev. Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Miyatake M, Narita M, Shibasaki M, Nakamura A, Suzuki T. Glutamatergic neurotransmission and protein kinase C play a role in neuron-glia communication during the development of methamphetamine-induced psychological dependence. Eur. J. Neurosci. 2005;22:1476–1488. doi: 10.1111/j.1460-9568.2005.04325.x. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem. Toxicol. 2002;40:1745–1750. doi: 10.1016/S0278-6915(02)00169-2. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Marshall JF. Neurotoxic regimens of methamphetamine induce persistent expression of phospho-c-Jun in somatosensory cortex and substantia nigra. Synapse. 2005;55:137–147. doi: 10.1002/syn.20098. [DOI] [PubMed] [Google Scholar]
- Ono Y, Shimazawa M, Ishisaka M, Oyagi A, Tsuruma K, Hara H. Imipramine protects mouse hippocampus against tunicamycin-induced cell death. Eur. J. Pharmacol. 2012;696:83–88. doi: 10.1016/j.ejphar.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J. Neurochem. 2009;111:882–889. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Sanders BG, Kline K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Res. Treat. 2010;124:361–375. doi: 10.1007/s10549-010-0786-2. [DOI] [PubMed] [Google Scholar]
- Prasad S, Yadav VR, Ravindran J, Aggarwal BB. ROS and CHOP are critical for dibenzylideneacetone to sensitize tumor cells to TRAIL through induction of death receptors and downregulation of cell survival proteins. Cancer Res. 2011;71:538–549. doi: 10.1158/0008-5472.CAN-10-3121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn Schmiedebergs Arch. Pharmacol. 2003;367:490–499. doi: 10.1007/s00210-003-0747-y. [DOI] [PubMed] [Google Scholar]
- Raudensky J, Yamamoto BK. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain Res. 2007;1135:129–135. doi: 10.1016/j.brainres.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran J, Gupta N, Agrawal M, Bala Bhaskar AS, Lakshmana Rao PV. Modulation of ROS/MAPK signaling pathways by okadaic acid leads to cell death via, mitochondrial mediated caspase-dependent mechanism. Apoptosis. 2011;16:145–161. doi: 10.1007/s10495-010-0554-0. [DOI] [PubMed] [Google Scholar]
- Refaat A, Abd-Rabou A, Reda A. TRAIL combinations: the new ‘trail’ for cancer therapy (review) Oncol. Lett. 2014;7:1327–1332. doi: 10.3892/ol.2014.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. JNeuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn. Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Sung B, Ravindran J, Prasad S, Pandey MK, Aggarwal BB. Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. J. Biol. Chem. 2010;285:35418–35427. doi: 10.1074/jbc.M110.172767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Talloczy Z, Martinez J, Joset D, Ray Y, Gacser A, Toussi S, Mizushima N, Nosanchuk JD, Goldstein H, Loike J, Sulzer D, Santambrogio L. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008;4:e28. doi: 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Wagner GC. Motor impairments after methamphetamine-induced neurotoxicity in the rat. J. Pharmacol. Exp. Ther. 1992;263:617–626. [PubMed] [Google Scholar]
- Yang EJ, Park GH, Song KS. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology. 2013;39:114–123. doi: 10.1016/j.neuro.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA, Lim JH, Kwon TK, Choi KS. Monensin, a polyether ionophore antibiotic, overcomes TRAIL resistance in glioma cells via endoplasmic reticulum stress, DR5 upregulation and c-FLIP downregulation. Carcinogenesis. 2013;34:1918–1928. doi: 10.1093/carcin/bgt137. [DOI] [PubMed] [Google Scholar]