Abstract
Multiple sclerosis (MS) is an autoimmune disease characterized by progressive neuronal loss, neuroinflammation, axonal degeneration, and demyelination. Previous studies have reported that 6-shogaol, a major constituent of ginger (Zingiber officinale rhizome), and its biological metabolite, 6-paradol, have anti-inflammatory and anti-oxidative properties in the central nervous system (CNS). In the present study, we investigated whether 6-shogaol and 6-paradol could ameliorate against experimental autoimmune encephalomyelitis (EAE), a mouse model of MS elicited by myelin oligodendrocyte glycoprotein (MOG35-55) peptide immunization with injection of pertussis toxin. Once-daily administration of 6-shogaol and 6-paradol (5 mg/kg/day, p.o.) to symptomatic EAE mice significantly alleviated clinical signs of the disease along with remyelination and reduced cell accumulation in the white matter of spinal cord. Administration of 6-shogaol and 6-paradol into EAE mice markedly reduced astrogliosis and microglial activation as key features of immune responses inside the CNS. Furthermore, administration of these two molecules significantly suppressed expression level of tumor necrosis factor-α, a major proinflammatory cytokine, in EAE spinal cord. Collectively, these results demonstrate therapeutic efficacy of 6-shogaol or 6-paradol for EAE by reducing neuroinflammatory responses, further indicating the therapeutic potential of these two active ingredients of ginger for MS.
Keywords: Multiple sclerosis, Experimental autoimmune encephalomyelitis, 6-Shogaol, 6-Paradol, Astrogliosis, Microglia
INTRODUCTION
Multiple sclerosis (MS) is a chronic neuroinflammatory disease of the brain and spinal cord in which focal lymphocytic infiltration leads to damage of myelin and axons (Compston and Coles, 2008). Experimental autoimmune encephalomyelitis (EAE) has been developed as a mouse model to investigate clinical, immunological, and histopathological features of MS (Lassmann and Bradl, 2017). Accumulating evidence suggests that both MS and EAE are processed by infiltrated immune cells (especially activated T cells) across the blood-brain barrier (BBB) to promote neuroinflammation, demyelination, gliosis, and axonal degeneration (Frischer et al., 2009; Goverman, 2009). The key morphological feature of MS and EAE is primary demyelination of nerve axons which results in a blockade of signal conduction or a decline of conduction at the site of demyelination (Fletcher et al., 2010). In addition, activated microglia and astrocytes can induce neuroinflammation, axonal damage, and demyelination through increasing the production of proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) (Raivich and Banati, 2004; Wang et al., 2005; Lee et al., 2018). Therefore, all approved medications for MS exert anti-inflammatory properties, and they are more effective when applied at the early phases of disease development (Hauser et al., 2013; Torkildsen et al., 2016).
Ginger (Zingiber officinale rhizome) has been commonly used as a traditional medicine for food ingredient as well as a medicinal herb for common cold, nausea, asthma, cough, bleeding, and muscle pain (Mascolo et al., 1989). Previous studies have reported that ginger has anti-inflammatory, anti-oxidative, and neuroprotective activities in the central nervous system (CNS) (Choi et al., 2018). In particular, administration of ginger extract prior to EAE onset can reduce clinical symptoms of EAE via regulation of inflammatory cytokines and chemokines such as IL-17, IL-33, IFN-γ, CCL20, and CCL22 (Jafarzadeh et al., 2014, 2017). Interestingly, 6-shogaol [1-(4-hydroxy-methoxyphenyl)-4-decen-one] is a major active ingredient in dried ginger and 6-paradol [1-(4-hydroxy-methoxyphenyl)-3decen-one] is produced from 6-shogaol through biotransformation process (Dugasani et al., 2010; Ok and Jeong, 2012). Neuroprotective effects of ginger extract seem to be due to pharmacological actions of both 6-shogaol and 6-paradol because they exert anti-neuroinflammatory and anti-oxidative activities in the CNS. It has been shown that 6-shogaol can suppress the activation of glial cells, such as astrogliosis and microglial activation, and the expression of proinflammatory cytokines in both in vitro and in vivo models of transient global ischemia and Parkinson’s disease (Ha et al., 2012; Park et al., 2013). It also protects against secondary damage and motor dysfunction in traumatic spinal cord injury in rats (Kyung et al., 2006) and lipopolysaccharide (LPS)-induced toxicity in astrocyte (Shim et al., 2012). Additionally, 6-paradol possesses anti-oxidant and anti-inflammatory activities, similar to 6-shogaol (Nagendra chari et al., 2013). We have previously demonstrated that 6-paradol can suppress the expression of proinflammatory cytokines in LPS-treated BV2 microglia and reduce brain damage in transient focal cerebral ischemia by inhibiting microglial activation (Gaire et al., 2015).
Accumulating findings on CNS diseases implicate that 6-shogaol and/or 6-paradol might be effective for other types of CNS diseases including MS based on their anti-inflammatory properties. In fact, it has been found that ginger extract can prevent disease onset in EAE mouse model (Jafarzadeh et al., 2014). However, it is currently unknown whether 6-shogaol and 6-paradol are effective for EAE. Furthermore, no study has reported whether these two active ingredients of ginger are effective for EAE as therapeutic regimen. In the current study, we determined whether 6-shogaol and 6-paradol were therapeutically effective in a mouse EAE model. We also assessed their effects on histopathological sequelae, including demyelination and increased cell density in injured spinal cord. In particular, we determined whether these ingredients reduced glial activation such as astrogliosis and microglial activation. In addition, we examined the link between their neuroprotective effects and suppression of TNF-α expression in EAE spinal cord because they could suppress its expression in other neuroinflammatory diseases such as cerebral ischemia (Gaire et al., 2015).
MATERIALS AND METHODS
Mice
Female C57BL/6 mice (7 weeks old) were purchased from Orient Co., Ltd. (Seongnam, Korea) and acclimated for a week under pathogen-free conditions with freely access to water and food on a 12-hour light/dark cycle. All experiments were performed under controlled conditions at temperature of 22°C ± 2°C and relative humidity of 60 ± 10% according to the Center of Animal Care and Use (CACU) guidelines of Lee Gil Ya Cancer and Diabetes Institute (LCDI) at Gachon University (approved animal protocol number: LCDI-2015-0028).
Materials
6-Shogaol and 6-paradol were provided by Dr. Dong Yoon Shin (Gachon University). A recombinant myelin oligodendrocyte glycoprotein (MOG35-55, MEVGWYRSPFSRVVH-LYRNGK) was purchased from Peptron (Daejeon, Republic of Korea). Complete Freund’s adjuvant (CFA) containing Mycobacterium tuberculosis H-37 RA (5 mg/mL) was purchased from Chondrex (Redmond, WA, USA). Bordetella pertussis toxin was purchased from List Biological Laboratories (Campbell, CA, USA). FluoroMyelin was purchased from Invitrogen (Waltham, MA, USA). Primary antibodies for GFAP and Iba1 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibody for TNF-α was purchased from Abcam (Cambridge, MA, USA). Alexa-Fluor® 488 secondary antibody was purchased from Jackson ImmunoResearch (West Grove, PA, USA). All other materials were of the highest grade available. They were obtained from normal commercial sources.
EAE induction and treatment
Mice were immunized with MOG35-55 as described previously (Lee et al., 2018). Briefly, MOG35-55 was emulsified in an equal amount of CFA. Mice were anesthetized with isoflurane and 200 µg of emulsion MOG35-55 in CFA was injected subcutaneously at the start day of immunization (day 1). In addition, 400 ng of Bordetella pertussis toxin (PTX) per mouse was injected intraperitoneally on the start day of MOG immunization and 2 days later. Mice were weighed and monitored daily for clinical symptoms of EAE as follows: 0, no clinical signs of EAE; 0.5, some lack of tone, however, some strength at the base of tail; 1.0, total loss of tail tonicity and flaccid tail; 2.0, hind limb weakness; 2.5, incomplete paralysis of one or both hind limbs; 3.0, total paralysis of one or both hind limbs; 4.0, hind and fore limbs paralysis; 5.0, death from disease (Lee et al., 2018). For drug administration, symptomatic EAE mice were divided into three groups; (1) the vehicle-treated EAE group, (2) the 6-shogaol-treated EAE group, and (3) the 6-paradol-treated EAE group. Vehicle (10% Tween 80), 6-shogaol (5 mg/kg), or 6-paradol (5 mg/kg) was orally administered daily into symptomatic EAE mice from day 29 to day 42 (for 13 days) post immunization. Dosage of 6-shogaol and 6-paradol was set according to a previous report (Gaire et al., 2015).
Tissue preparation
At the end of experiments, spinal cord was quickly removed from each mouse after CO2 exposure. Removed spinal cord was cut into 5–6 pieces, embedded in Tissue-Tek Optimal Cutting Temperature (OCT) compound, and frozen using powdered dry ice. For histological analyses, frozen spinal cord samples were cut into 20 µm sections on a cryostat (J4800AMNZ, Thermo, Dreieich, Germany) and tissue sections were fixed in 4% paraformaldehyde (PFA) for 10 min.
Histological and immunohistochemical analysis
Cresyl violet staining was performed to determine increased cell density. Sections were blocked with chloroform/ethanol (1:1), rehydrated, stained with 0.5% cresyl violet acetate, dehydrated with increasing ethanol series and xylene, and mounted with Entellan medium.
FluoroMyelin staining was carried out to determine demyelination and remyelination. Sections were stained with FluoroMyelin solution (1:300) for 2 h, labeled with 4′,6-diamidino-2-phenylindole (DAPI) solution (1:10,000), and mounted with VECTASHIELD® mounting media.
For immunohistochemical study, sections were blocked with 1% fetal bovine serum containing 0.5% Triton X-100 and incubated with primary antibodies against glial fibrillary acidic protein (GFAP, 1:500, for astrogliosis), ionized calcium-binding adapter molecule 1 (Iba1, 1:500, for microglial activation), or TNF-α (1:100) overnight at 4°C. In case of signal development with 3, 3′-diaminobenzidinetetrahydrochloride hydrate (DAB) staining, sections were treated with H2O2 prior to blocking. To develop GFAP signals, sections were incubated with Alexa Fluor 488 donkey anti-rabbit (1:1000) as a secondary anti-body, counterstained with DAPI, and mounted with VECTA-SHIELD® mounting media. To develop signals for Iba1, sections were labeled with secondary anti-rabbit antibody (1:200) and further labeled with avidin/biotin complex (1:100). Sections were developed using 0.02% DAB solution containing 0.01% H2O2, rinsed with PBS, dehydrated with ethanol, kept in xylene, and mounted with Entellan medium. To detect TNF-α in spinal cords, sections were exposed to Tris-EDTA solution at 90 to 100°C for 30 min before H2O2 exposure and labeled with a primary antibody for TNF-α (1:100) followed by labeling with secondary anti-rabbit antibody (1:200) and avidin/biotin complex. Signals were developed with DAB.
Colored images were collected using DP72 camera (Olympus Co., Tokyo, Japan). Fluorescent images were obtained with a laser scanning confocal microscope (Eclipse A1 Plus, Nikon, Tokyo, Japan). Quantification of FluoroMyelin and GFAP staining was carried out by measuring fluorescence intensity which was quantified using ImageJ program (National Institute of Mental Health, Bethesda, MD, USA). Quantification of CV staining, Iba1 immunohistochemistry, and TNF-α immunohistochemistry was done by counting the number of stained or immunopositive cells in the white matter region of the spinal cord. Data are expressed as total number of cells/mm2 of the white matter region.
Statistical analysis
All data analyses were done using GraphPad Prism Version 5.02 (GraphPad, La Jolla, CA, USA). Date are shown as mean ± SEM. Statistical significance among groups were analyzed using Student’s t-test between two groups and one-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc test for multiple comparisons. Statistical significance was set at p<0.05.
RESULTS
Effects of 6-shogaol and 6-paradol on clinical symptoms in EAE-challenged mice
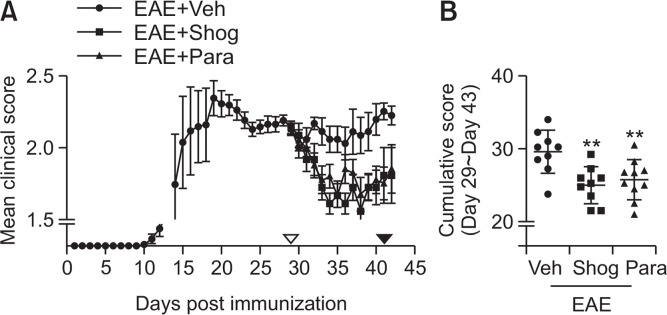
We determined neuroprotective effects of 6-shogaol and 6-paradol in EAE mice under therapeutic regimen. EAE-symptomatic mice were treated with 6-shogaol or 6-paradaol (5 mg/kg, p.o.) once daily for 14 days (between days 29 and 42 after immunization). Both 6-shogaol and 6-paradol significantly improved EAE-relevant symptoms from the fourth day after drug administration (between days 32 and 42) except days 36 and 37 in the 6-paradol-treated EAE group compared to the vehicle-treated EAE group (Fig. 1A, Supplementary Table 1). The effectiveness of 6-shogaol and 6-paradol was also clearly shown from cumulative clinical scores from days 30 to 43. Administration of 6-paradol or 6-shogaol significantly reduced the cumulative clinical score (6-shogaol, 25.25%; 6-paradol, 25.75%) compared to the vehicle-treated EAE group (Fig. 1B). These results demonstrate that 6-shogaol and its metabolite, 6-paradaol, exert neuroprotective effects against EAE. Moreover, these molecules are therapeutically effective for EAE.
Fig. 1.
Administration of 6-shogaol or 6-paradol improves clinical symptoms of EAE. 6-Shogaol (Shog, 5 mg/kg), 6-paradol (Para, 5 mg/kg), or vehicle (Veh) was orally given daily into symptomatic EAE mice, from days 29 to 42 after immunization. On day 43, the experiment was completed. (A) Mean clinical score of each group. ▿ or ▾ indicate the start or stop of a daily drug administration. Clinical scores of experimental groups were statistically analyzed by Mann-Whitney’s test and actual p values were documented (Supplementary Table 1). (B) Cumulative score of each mouse was calculated as the sum of all scores from days 30 to 43 in each mouse. The mean value was then obtained for each group. n=9–10 per group. **p<0.01 vs. the vehicle-treated group (Newman-Keuls multiple range test).
Effects of 6-shogaol and 6-paradol on damage of spinal cords in EAE-challenged mice
Demyelination and cell accumulation are known to be key histopathological features of spinal cords in EAE mice (Lee et al., 2018). Since 6-shogaol and 6-paradol were therapeutically effective for EAE, we next investigated whether these therapeutic agents could reduce demyelination and cell accumulation in spinal cords of EAE mice. In the vehicle-treated EAE group, demyelination of myelin sheath (Fig. 2A, 2B) and cell accumulation (Fig. 2C, 2D) occurred in the white matter of the spinal cord, showing reduced FluoroMyelin staining (Fig. 2A, 2B) and increased number of cresyl violet-stained cells (Fig. 2C, 2D). These histological features were significantly reversed by 6-shogaol or 6-paradol administration (Fig. 2).
Fig. 2.
Administration of 6-shogaol or 6-paradol reduces demyelination and cell accumulation in spinal cords of EAE mice. (A, B) Effects of 6-shogaol (Shog) and 6-paradol (Para) on demyelination were examined by FluoroMyelin staining (green). Sections were counterstained with DAPI (blue). Representative images of FluoroMyelin-stained lumbar spinal cord of each group (A) and quantification of FluoroMyelin intensity (B; a.u., artificial unit). (C, D) Effects of 6-shogaol and 6-paradol on increased cell density were examined by cresyl violet (CV) staining. Representative images of CV-stained lumbar spinal cord of each group (C) and quantification by counting the number of CV-stained cells (D). Scale bars, 100 μm. n=4 per group. ***p<0.001 vs. naïve. ##p<0.01 and ###p<0.001 vs. the vehicle-treated group. Newman-Keuls multiple range test.
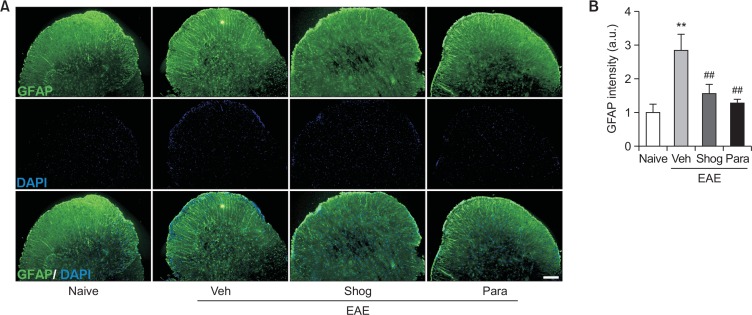
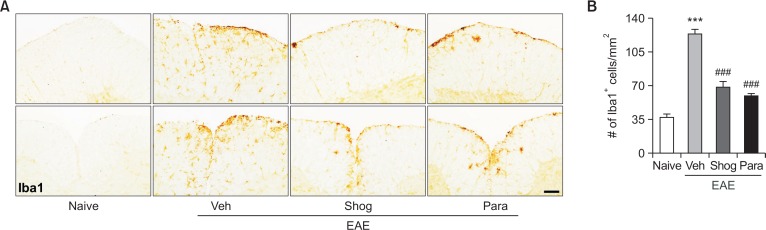
In addition to demyelination and cell accumulation, glial activation such as astrogliosis microglial activation is also a pathological feature of EAE. It occurs in the white matter of the spinal cord, leading to neuroinflammation and neuronal damage (Lee et al., 2018). We then examined whether the therapeutic efficacy of 6-shogaol and 6-paradol was associated with reduced astrogliosis and microglial activation in spinal cords of EAE mice by immunolabeling with GFAP and Iba1, respectively. In the vehicle-treated EAE mice, astrogliosis (Fig. 3) and microglial activation (Fig. 4) were observed in the white matter of the spinal cord. These were markedly reduced by administration of 6-shogaol or 6-paradol (Fig. 3, 4).
Fig. 3.
Administration of 6-shogaol or 6-paradol reduces astrogliosis in spinal cords of EAE mice. Effects of 6-shogaol (Shog) and 6-paradol (Para) on astrogliosis were examined by GFAP immunolabeling (green). Sections were counterstained with DAPI (blue). (A) Representative images of GFAP immunoreactivity in the lumbar spinal cord of each group. (B) Quantification of fluorescence intensity (a.u., artificial unit). Scale bar, 100 μm. n=4 per group. **p<0.01 and ##p<0.01 vs. naïve and the vehicle-treated group, respectively, by Newman-Keuls multiple range test.
Fig. 4.
Administration of 6-shogaol or 6-paradol suppresses microglial activation in spinal cords of EAE mice. Effects of 6-shogaol (Shog) and 6-paradol (Para) on microglial activation were examined by Iba1 immunolabeling. (A) Representative images of Iba1 immunoreactivity in the lumbar spinal cord of each group. (B) Quantification of the number of Iba1-immunopositive cells. Scale bar, 50 μm. n=4 per group. ***p<0.001 and ###p<0.001 vs. naïve and the vehicle-treated group, respectively, by Newman-Keuls multiple range test.
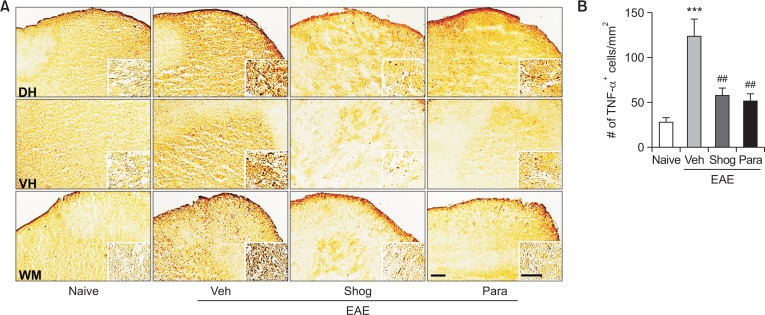
Proinflammatory cytokines such as TNF-α from activated microglia have been considered as major pathological mediators in EAE and MS (Sharief and Hentges, 1991; Renno et al., 1995). Previously, we have reported that 6-shogaol or 6-paradol can influence TNF-α expression in injured brain (Gaire et al., 2015). Thus, we determined whether the therapeutic efficacy of 6-shogaol and 6-paradol was also associated with downregulation of TNF-α expression in spinal cords of EAE mice using immunohistochemical analysis. In the vehicle-treated EAE group, the number of TNF-α-immunopositive cells was increased in spinal cords of EAE mice, which was markedly attenuated by administration of 6-shogaol or 6-paradol (Fig. 5).
Fig. 5.
Administration of 6-shogaol or 6-paradol suppresses TNF-α expression in spinal cords of EAE mice. Effects of 6-shogaol (Shog) and 6-paradol (Para) on TNF-α expression were examined by TNF-α immunolabeling. (A) Representative images of TNF-α immunoreactivity in the lumbar spinal cord of each group. (Insets) High magnification image of TNF-α-immunopositive cells from the dorsal horn (DH), the ventral horn (VH), and the white matter (WM) of the spinal cord. (B) Quantification of the number of TNF-α-immunopositive cells. Scale bars, 100 μm (inset, 50 μm). n=4 per group. ***p<0.001 and ##p<0.01 vs. naïve and the vehicle-treated group, respectively, by Newman-Keuls multiple range test.
Taken together, these histological analyses on neuroinflammatory components demonstrate that 6-shogaol and 6-paradol exert therapeutic efficacy by alleviating neuroinflammatory responses, including reduced astrogliosis and microglial activation and downregulated TNF-α expression in injured spinal cords of EAE mice.
DISCUSSION
In the present study, we demonstrated that 6-shogaol and its biotransformed metabolite, 6-paradol, were therapeutically effective for EAE by reducing EAE clinical symptoms with histopathological sequelae including demyelination, cell accumulation, and astrogliosis in injured spinal cords of EAE mice. We also provided evidence that therapeutic effects of 6-shogaol and 6-paradol on EAE were associated with attenuating microglial activation and TNF-α upregulation in injured spinal cords of EAE mice. These aggregate findings implicate that 6-shogaol or 6-paradol might have therapeutic potential for alleviating MS symptoms by suppressing neuroinflammatory responses.
Ginger is known to possess anti-inflammatory and neuroprotective effects on diverse CNS diseases, including 3,4-methylenedioxymethamphetamine-induced neurotoxicity (Mehdizadeh et al., 2012), Alzheimer’s disease (Zeng et al., 2013), focal cerebral ischemia (Wattanathorn et al., 2011), and EAE (Jafarzadeh et al., 2014). In addition, there have been many efforts to identify active ingredients in ginger extract in view of drug discovery for CNS diseases. Among constituents of ginger, pungent non-volatile compounds such as gingerols, shogaols, and zingerone and non-pungent biotransformed metabolites of shogaols, paradols, have been identified as active ingredients that exert pharmacological actions in the CNS (Choi et al., 2018). 6-Gingerol, a major pungent compound in fresh ginger, is converted to 6-shogaol through dehydration and hydrogenation of 6-shogaol can lead to the formation of 6-paradol (Wohlmuth et al., 2005). Interestingly, anti-inflammatory and anti-oxidative properties of 6-shogaol and 6-paradol are higher than those of 6-gingerol (Dugasani et al., 2010), implicating that these two compounds might be active ingredients of ginger that exert neuroprotective effects on diverse CNS diseases. In case of EAE, one group has demonstrated neuroprotective effects of ginger extract in EAE under prophylactic regimen, in which ginger extract is given to immunized mice prior to disease onset (from day 3 post immunization) (Jafarzadeh et al., 2014). This administration has prevented disease onset of immunized mice, along with suppressed expression of immune response-relevant cytokines and chemokines (Jafarzadeh et al., 2014, 2017). Considering these previous findings along with our data, 6-shogaol and 6-paradol might be active ingredients responsible for the previously reported efficacy of ginger extract. It is notable in this study that these two ingredients are therapeutically effective because they could improve clinical symptoms of EAE mice even under therapeutic regimen.
Demyelination, increases in cell density, and astrogliosis are hallmarks of MS patients and rodent EAE models (Steinman, 1996; Tani et al., 1996; Malmestrom et al., 2003; Luo et al., 2007; Han et al., 2008). In this study, manifestation of these histopathological hallmarks of EAE was reduced by administration of 6-shogaol or 6-paradol. It has been reported that astrogliosis occurs prior to manifestation of clinical symptoms in EAE (Luo et al., 2007). In addition, astrogliosis and subsequent glial scarring can inhibit central repair processes in MS, including remyelination (Nair et al., 2008). Besides astrogliosis, microglial activation is also critically involved in the pathogenesis of MS and EAE (Benveniste, 1997; Minagar et al., 2002). Such glial activation is generally known to play a pivotal role in immune responses inside the CNS (Duffy et al., 2014). In MS and EAE, glial activation is also closely associated with disease severity. Suppressing gliosis has resulted in improved clinical symptoms of EAE (Reynolds et al., 2011). In a mechanistic view, astrogliosis and microglial activation consequently facilitate production of proinflammatory cytokines such IL-6 and TNF-α, both of which are involved in disease progression (Renno et al., 1995; Choi et al., 2011). These cytokines can also initiate myelin damage in the white matter of the spinal cord through death of oligodendrocytes (Luo et al., 2017). Our results showed that 6-shogaol and 6-paradol could reduce astrogliosis and the number of activated microglia in injured spinal cords of EAE mice. Furthermore, administration of these active ingredients reduced the number of TNF-α expressing cells in injured spinal cords of EAE mice. Therefore, neuroprotective effects of 6-shogaol and 6-paradol in EAE observed in this study might be associated with reduced inflammatory responses in the CNS such as astrogliosis, microglial activation, and TNF-α expression. This notion was supported by previous findings (Shim et al., 2011; Ha et al., 2012; Moon et al., 2014; Gaire et al., 2015). Treatment with 6-shogaol has suppressed secretion of proinflammatory cytokines in LPS-stimulated astrocyte (Shim et al., 2011). In animal studies, exposure to 6-shogaol or 6-paradol can attenuate ischemic injury through inhibiting microglial activation and suppressing expression of inflammatory cytokines (Ha et al., 2012; Gaire et al., 2015). In addition, 6-shogaol can attenuate β-amyloid-induced neuroinflammation and memory impairments through inhibiting glial activation in mice (Moon et al., 2014). Therefore, the neuroprotective efficacy of 6-shogaol and 6-paradol on CNS disorders might be through suppressing immune responses in the CNS.
In this study, 6-shogaol and its biotransformed metabolite, 6-paradol reduced clinical symptoms of EAE and associated histological sequelae to the similar extent, indicating that these two ingredients of ginger have similar pharmacological efficacy. 6-Paradol is a metabolized product which is derived from 6-shogaol by liver enzyme (Surh and Lee, 1992, 1994). These two ingredients exert similar pharmacological activities including anti-oxidative and anti-inflammatory actions (Nagendra chari et al., 2013; Jo et al., 2016). In addition, our previous in vivo study demonstrated that 6-shogaol and 6-paradol were neuroprotective against cerebral ischemia to the same extent (Gaire et al., 2015). Similarly, in the current study, we found that both 6-shogaol and 6-paradol exerted the similar neuro-protective effects in EAE. These in vitro and in vivo studies showing the same efficacy of 6-shogaol and 6-paradol may further indicate that neuroprotective effects of 6-shogaol could be mediated by its metabolite, 6-paradol.
There has been growing evidence for the therapeutic potential of ginger itself as well as its active ingredients such as 6-shogaol and 6-paradol in diverse CNS diseases mainly through their anti-inflammatory properties. In case of MS, the extract can reduce tissue injury in spinal cords of EAE mice. Our in vivo results utilizing a mouse model of MS provided direct evidence that 6-shogaol and 6-paradol were active ingredients that could mediate neuroprotective effects of ginger extract. Furthermore, our current results clearly demonstrated that these active ingredients were therapeutically effective for EAE, further indicating their potential use to treat MS. In particular, 6-paradol could be more useful to avoid unwanted effects such as gastric irritation because it is a non-pungent metabolite unlike ginger or 6-shogaol.
Acknowledgments
This work was supported by grants from the National Research Foundation (NRF) funded by the Korean Government to JWC [NRF-2014M3A9B6069339].
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interests.
REFERENCES
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med. 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Choi JG, Kim SY, Jeong M, Oh MS. Pharmaco-therapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Ther. 2018;182:56–69. doi: 10.1016/j.pharmthera.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl. Acad. Sci. U.S.A. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Duffy SS, Lees JG, Moalem-Taylor G. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult. Scler. Int. 20142014:285245. doi: 10.1155/2014/285245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurode-generation in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaire BP, Kwon OW, Park SH, Chun KH, Kim SY, Shin DY, Choi JW. Neuroprotective effect of 6-paradol in focal cerebral ischemia involves the attenuation of neuroinflammatory responses in activated microglia. PLoS ONE. 2015;10:e0120203. doi: 10.1371/journal.pone.0120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh MS, Kim SY. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology. 2012;63:211–223. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Han MH, Hwang SI, Roy DB, Lundgren DH, Price JV, Ousman SS, Fernald GH, Gerlitz B, Robinson WH, Baranzini SE, Grinnell BW, Raine CS, Sobel RA, Han DK, Steinman L. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Chan JR, Oksenberg JR. Multiple sclerosis: prospects and promise. Ann. Neurol. 2013;74:317–327. doi: 10.1002/ana.24009. [DOI] [PubMed] [Google Scholar]
- Jafarzadeh A, Arabi Z, Ahangar-Parvin R, Mohammadi-Kordkhayli M, Nemati M. Ginger extract modulates the expression of chemokines CCL20 and CCL22 and their receptors (CCR6 and CCR4) in the central nervous system of mice with experimental autoimmune encephalomyelitis. Drug Res. (Stuttg) 2017;67:632–639. doi: 10.1055/s-0043-113455. [DOI] [PubMed] [Google Scholar]
- Jafarzadeh A, Mohammadi-Kordkhayli M, Ahangar-Parvin R, Azizi V, Khoramdel-Azad H, Shamsizadeh A, Ayoobi A, Nemati M, Hassan ZM, Moazeni SM, Khaksari M. Ginger extracts influence the expression of IL-27 and IL-33 in the central nervous system in experimental autoimmune encephalomyelitis and ameliorates the clinical symptoms of disease. J. Neuroimmunol. 2014;276:80–88. doi: 10.1016/j.jneuroim.2014.08.614. [DOI] [PubMed] [Google Scholar]
- Jo SK, Kim IS, Rehman SU, Ha SK, Park HY, Park YK, Yoo HH. Characterization of metabolites produced from the biotransformation of 6-shogaol formed by Aspergillus niger. Eur. Food Res. Technol. 2016;242:137–142. doi: 10.1007/s00217-015-2519-6. [DOI] [Google Scholar]
- Kyung KS, Gon JH, Geun KY, Sup JJ, Suk WJ, Ho KJ. 6-Shogaol, a natural product, reduces cell death and restores motor function in rat spinal cord injury. Eur. J. Neurosci. 2006;24:1042–1052. doi: 10.1111/j.1460-9568.2006.04908.x. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2017;133:223–244. doi: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Jeon SJ, Cho KS, Moon E, Sapkota A, Jun HS, Ryu JH, Choi JW. Activation of glucagon-like peptide-1 receptor promotes neuroprotection in experimental autoimmune encephalomyelitis by reducing neuroinflammatory responses. Mol. Neurobiol. 2018;55:3007–3020. doi: 10.1007/s12035-017-0550-2. [DOI] [PubMed] [Google Scholar]
- Luo C, Jian C, Liao Y, Huang Q, Wu Y, Liu X, Zou D. The role of microglia in multiple sclerosis. Neuropsychiatr. Dis. Treat. 2017;13:1661–1667. doi: 10.2147/NDT.S140634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Ho PP, Buckwalter MS, Hsu T, Lee LY, Zhang H, Kim DK, Kim SJ, Gambhir SS, Steinman L, Wyss-Coray T. Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J. Clin. Invest. 2007;117:3306–3315. doi: 10.1172/JCI31763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmestrom C, Haghighi S, Rosengren L, Andersen O, Lycke J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology. 2003;61:1720–1725. doi: 10.1212/01.WNL.0000098880.19793.B6. [DOI] [PubMed] [Google Scholar]
- Mascolo N, Jain R, Jain SC, Capasso F. Ethnopharmacologic investigation of ginger (Zingiber officinale) J. Ethnopharmacol. 1989;27:129–140. doi: 10.1016/0378-8741(89)90085-8. [DOI] [PubMed] [Google Scholar]
- Mehdizadeh M, Dabaghian F, Nejhadi A, Fallah-Huseini H, Choopani S, Shekarriz N, Molavi N, Basirat A, Mohammadzadeh Kazorgah F, Samzadeh-Kermani A, Soleimani Asl S. Zingiber officinale alters 3,4-methylenedioxymethamphetamine-induced neurotoxicity in rat brain. Cell J. 2012;14:177–184. [PMC free article] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 2002;202:13–23. doi: 10.1016/S0022-510X(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Moon M, Kim HG, Choi JG, Oh H, Lee PK, Ha SK, Kim SY, Park Y, Huh Y, Oh MS. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem. Biophys. Res. Commun. 2014;449:8–13. doi: 10.1016/j.bbrc.2014.04.121. [DOI] [PubMed] [Google Scholar]
- Nagendra chari KL, Manasa D, Srinivas P, Sowbhagya HB. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe) Food Chem. 2013;139:509–514. doi: 10.1016/j.foodchem.2013.01.099. [DOI] [PubMed] [Google Scholar]
- Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell. Mol. Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ok S, Jeong WS. Optimization of extraction conditions for the 6-shogaol-rich extract from ginger (Zingiber officinale Roscoe) Prev. Nutr. Food Sci. 2012;17:166–171. doi: 10.3746/pnf.2012.17.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G, Kim HG, Ju MS, Ha SK, Park Y, Kim SY, Oh MS. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson’s disease models via anti-neuroinflammation. Acta Pharmacol. Sin. 2013;34:1131–1139. doi: 10.1038/aps.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res. Brain Res. Rev. 2004;46:261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J. Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- Reynolds R, Roncaroli F, Nicholas R, Radotra B, Gveric D, Howell O. The neuropathological basis of clinical progression in multiple sclerosis. Acta Neuropathol. 2011;122:155–170. doi: 10.1007/s00401-011-0840-0. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N. Engl. J. Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Shim S, Kim S, Choi DS, Kwon YB, Kwon J. Anti-inflammatory effects of [6]-shogaol: potential roles of HDAC inhibition and HSP70 induction. Food Chem. Toxicol. 2011;49:2734–2740. doi: 10.1016/j.fct.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Shim S, Kim S, Kwon YB, Kwon J. Protection by [6]-shogaol against lipopolysaccharide-induced toxicity in murine astrocytes is related to production of brain-derived neurotrophic factor. Food Chem. Toxicol. 2012;50:597–602. doi: 10.1016/j.fct.2011.11.042. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/S0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Lee SS. Enzymatic reduction of shogaol: a novel biotransformation pathway for the alpha,beta-unsaturated ketone system. Biochem. Int. 1992;27:179–187. [PubMed] [Google Scholar]
- Surh YJ, Lee SS. Enzymatic reduction of xenobiotic alpha,beta-unsaturated ketones: formation of allyl alcohol metabolites from shogaol and dehydroparadol. Res. Commun. Chem. Pathol. Pharmacol. 1994;84:53–61. [PubMed] [Google Scholar]
- Tani M, Glabinski AR, Tuohy VK, Stoler MH, Estes ML, Ransohoff RM. In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental autoimmune encephalomyelitis. Am. J. Pathol. 1996;148:889–896. [PMC free article] [PubMed] [Google Scholar]
- Torkildsen O, Myhr KM, Bo L. Disease-modifying treatments for multiple sclerosis - a review of approved medications. Eur. J. Neurol. 2016;23(Suppl 1):18–27. doi: 10.1111/ene.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Ayers MM, Catmull DV, Hazelwood LJ, Bernard CC, Orian JM. Astrocyte-associated axonal damage in pre-onset stages of experimental autoimmune encephalomyelitis. Glia. 2005;51:235–240. doi: 10.1002/glia.20199. [DOI] [PubMed] [Google Scholar]
- Wattanathorn J, Jittiwat J, Tongun T, Muchimapura S, Ingkaninan K. Zingiber officinale mitigates brain damage and improves memory impairment in focal cerebral ischemic rat. Evid. Based Complement. Alternat. Med. 2011;2011:429505. doi: 10.1155/2011/429505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlmuth H, Leach DN, Smith MK, Myers SP. Gingerol content of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe) J. Agric. Food Chem. 2005;53:5772–5778. doi: 10.1021/jf050435b. [DOI] [PubMed] [Google Scholar]
- Zeng GF, Zhang ZY, Lu L, Xiao DQ, Zong SH, He JM. Protective effects of ginger root extract on Alzheimer disease-induced behavioral dysfunction in rats. Rejuvenation Res. 2013;16:124–133. doi: 10.1089/rej.2012.1389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.