Abstract
We evaluated long-term outcomes of three protocols of intensity-modulated radiation therapy (IMRT) for localized prostate cancer. Between 2005 and 2014, 348 patients were treated with 5-field IMRT. The first 74 patients were treated with a daily fraction of 2.0 Gy to 74 Gy (low-risk prostate cancer) or 78 Gy (intermediate- or high-risk prostate cancer); then 101 patients were treated with 2.1-Gy daily fractions to 73.5 or 77.7 Gy. More recently, 173 patients were treated with 2.2-Gy fractions to 72.6 or 74.8 Gy. The median age of all patients was 70 years and the median follow-up period was 82 months. The median follow-up periods were 124 months in the 2.0-Gy group, 98 months in the 2.1-Gy group, and 69 months in the 2.2-Gy group. The overall and prostate-specific antigen (PSA) failure-free survival (PSA-FFS) rates were, respectively, 89 and 68% at 10 years for the 2.0-Gy group, 91 and 84% at 8 years for the 2.1-Gy group, and 93 and 92% at 6 years for the 2.2-Gy group. The PSA-FFS rate for high-risk patients in all groups was 80% at 7 years. The cumulative incidences of Grade ≥2 late genitourinary (GU) and gastrointestinal (GI) toxicity were, respectively, 7.2 and 12.4% at 10 years for the 2.0-Gy group, 7.4 and 14.1% at 8 years for the 2.1-Gy group, and 7.1 and 7.9% at 6 years for the 2.2-Gy group. All three fractionation schedules yielded good tumor control with acceptable toxicities.
Keywords: prostate cancer, intensity-modulated radiotherapy, long-term outcomes, late toxicity
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) has been fairly well established as a definitive treatment for prostate cancer in Japanese patients. Accordingly, the proportion of patients undergoing radiation therapy as an initial therapy has increased in Japan [1]. Another study reported that definitive IMRT using helical tomotherapy appeared to be a valuable treatment option for patients with localized and locally advanced prostate cancer, even in extremely elderly patients [2]. However, IMRT with conventional regimens (1.8–2 Gy per daily fraction) takes nearly 2 months or longer, and this long treatment period may be disadvantageous compared with brachytherapy and recently developed stereotactic body radiotherapy. Several studies have suggested a low α/β ratio for prostate adenocarcinoma (1–1.8 Gy) [3–5], even lower than the ratios of late-responding normal tissues such as the bladder and rectum [6, 7]. Therefore, shorter treatment periods using a higher dose per fraction would be expected to improve therapeutic outcomes and make IMRT economically attractive [8].
Due to its relatively short history, reports of long-term results of IMRT for prostate cancer in the Japanese population are fewer than those in Western populations. In our institution, we started IMRT with 2.0-Gy daily fractions for localized prostate cancer using five static beams. Thereafter, the number of patients waiting for the treatment steadily increased because the 2.0-Gy daily fractionation schedule took a long time (~8 weeks). So, shortening the overall treatment time was desirable. At that time, however, the existing data were relatively short-term and insufficient to support the safety and usefulness of hypofractionated regimens, so we attempted to increase the daily dose in a step-by-step manner. After evaluating middle-term toxicities of the 2.0-Gy regimen, we investigated a 2.1-Gy daily fractionation schedule and then a 2.2-Gy regimen to shorten the treatment period in a stepwise fashion. After increasing the dose to 2.2 Gy per day, the protocol was continued until recently, when the number of the patients became stable due to the introduction of IMRT in nearby facilities.
In 2012, tomotherapy was introduced, and volumetric-modulated arc therapy also became available from 2015; so, 5-field IMRT is no longer used at our institution. Moreover, we have used a 2.5-Gy daily fraction since April 2018, because the use of hypofractionated regimens has become the worldwide trend based on the favorable clinical results of hypofractionation [9–14] and higher medical fees are now allocated to the hypofractionation schedule (≥2.5 Gy/day) in Japan. Therefore, the purpose of this study was to evaluate the long-term clinical outcomes of 5-field IMRT for localized prostate cancer and our three dose-fractionation regimens by updating the results reported in our previous publication [15]. We included new patients, extended follow-up periods from the previous study, and evaluated 10-year results for the 2.0-Gy protocol, 8-year results for the 2.1-Gy protocol and 6-year results for the 2.2-Gy protocol in this study.
MATERIALS AND METHODS
Patient characteristics
This was a retrospective study of protocol-based treatments; 348 patients with biopsy-confirmed prostate cancer treated with a 5-field IMRT technique at Nagoya City University Hospital between January 2005 and June 2014 were analyzed. Protocols were approved by the institutional review board (No. 273), and written informed consent was obtained from all patients. Dose-fractionation protocols were revised twice, as reported in our previous study [15]. The first 74 patients were treated with a daily fraction of 2.0 Gy to a total of 74 Gy (low-risk prostate cancer) or 78 Gy (intermediate- or high-risk prostate cancer), and then 101 patients were treated with 2.1-Gy daily fractions to 73.5 or 77.7 Gy. More recently, 173 patients were treated with 2.2-Gy fractions to 72.6 or 74.8 Gy. The median age of all patients was 70 years (range, 54–83) and the median follow-up period was 82 months (range, 18–157). The median follow-up periods were 124 months in the 2.0-Gy group, 98 months in the 2.1-Gy group, and 69 months in the 2.2-Gy group. The patient characteristics are summarized in Table 1. The average age of the 2.0-Gy group was slightly lower (P = 0.01) and the 2.2-Gy group had a lower rate of low-risk patients than the other groups (P < 0.01). All patients were staged according to the 7th edition of TNM staging and D’Amico Risk Categories [16], using computed tomography (CT), magnetic resonance imaging (MRI) and bone scintigraphy.
Table 1.
Patient characteristics
| Group | All patients | 2.0 Gy/day | 2.1 Gy/day | 2.2 Gy/day | P |
|---|---|---|---|---|---|
| Total dose (Gy) | 72.6–74/74.8–78a | 74/78a | 73.5/77.7a | 72.6/74.8a | |
| No. of patients | 348 | 74 | 101 | 173 | |
| Age (years) | 54–83 | 54–80 | 56–80 | 56–83 | 0.01b |
| (median) | 70 | 68 | 70 | 70 | |
| Initial PSA (ng/ml) | 2.6–283 | 3.2–283 | 4.6–241 | 2.6–248 | 0.95b |
| (median) | 11.3 | 11.1 | 11.5 | 10.8 | |
| Risk Low/intermediate/high | 27/92/156 | 10/20/44 | 14/31/56 | 6/64/103 | 0.01c |
| T stage 1/2/3 | 81/114/80 | 24/30/20 | 27/43/31 | 40/81/52 | 0.64c |
| ADT | 240 (87%) | 51 (69%) | 91 (90%) | 171 (99%) | <0.01c |
| Use of anticoagulant | 49 (18%) | 15 (20%) | 15 (15%) | 29 (17%) | 0.63c |
| Coexistent DM | 47 (17%) | 16 (22%) | 11 (11%) | 27 (16%) | 0.15c |
| Follow-up (months) | 18–157 | 25–157 | 20–123 | 18–94 | |
| (median) | 82 | 124 | 98 | 69 |
PSA = prostate-specific antigen, ADT = androgen deprivation therapy, DM = diabetes mellitus. aFor low-risk/intermediate- or high-risk patients. bExamined by one-factor analysis of variance. cExamined by chi-squared test.
IMRT and androgen deprivation therapy
We reported details of the IMRT methods in our previous studies [15, 17]. Patients were immobilized in a supine position with a whole-body vacuum bag system, and the CT scans were performed at 3.2-mm and reconstructed to 2.5-mm thickness. The contouring was completed by reference to MRI images. The clinical target volume included the entire prostate and seminal vesicles depending on the T stage of the patient. The dose constraints, including the dose to the rectum and bladder, for all groups are provided in detail in our previous publication [15]. Patients were treated with 18-MV X-rays from five static ports using an optically guided 3D-ultrasound target localization system.
Generally, neoadjuvant androgen deprivation therapy (ADT) was used for 6 months in intermediate- or high-risk patients, and adjuvant ADT for 2–3 years in high-risk patients. In the 2.0-Gy group, the proportion of patients undergoing ADT was lower (69%) than in the other groups (90 and 98% in the 2.1-Gy and 2.2-Gy groups, respectively, P < 0.01).
Follow-up and data collection
We performed follow-up evaluations at 1- to 3-month intervals until 1 year, and every 3–6 months thereafter. All end points were calculated from the start of IMRT. Prostate-specific antigen (PSA) failure was defined as a PSA rise of ≥2 ng/ml above the nadir according to the Phoenix definition [18]. One patient who developed radiographic evidence of bone metastasis at low PSA levels was counted as a PSA-failure case. Toxicities were evaluated with the Common Terminology Criteria for Adverse Events version 4.0. Late toxicities were defined as those occurring later than 3 months after starting IMRT. In the present study, the toxicities for some of the patients were re-evaluated, so the toxicity data in the 2.0-Gy and 2.1-Gy groups is slightly different from those reported previously [15].
Statistical analysis
Differences in patient characteristics and incidences of acute genitourinary (GU)/gastrointestinal (GI) toxicities between groups were examined by one-factor analysis of variance and the chi-squared test. Overall survival rates, PSA-failure-free survival (PSA-FFS) rates and cumulative incidences of Grade ≥2 late toxicity were calculated by the Kaplan–Meier method, and differences between groups were examined by the log-rank test. We used univariate and multivariate Cox proportional hazards model to investigate the associations of patient characteristics (age, risk classification, ADT, use of anticoagulants and presence of diabetes mellitus) with outcomes and with toxicities. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics [19].
RESULTS
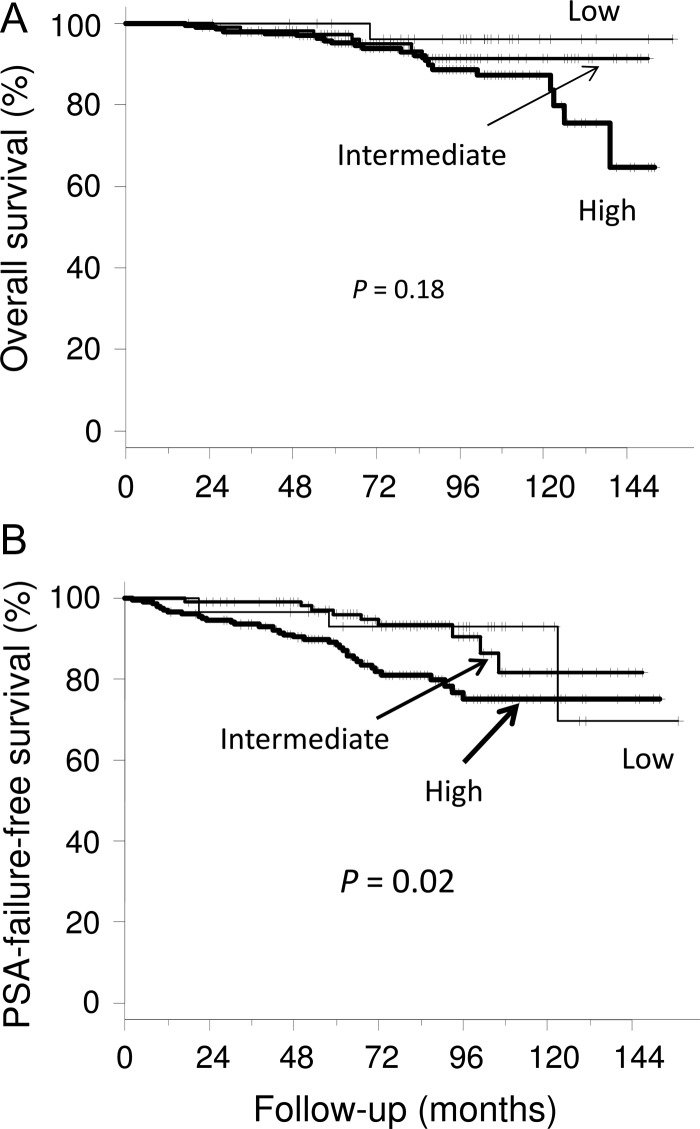
Overall survival and PSA-FFS rates were, respectively, 89 and 68% at 10 years for the 2.0-Gy group, 91 and 84% at 8 years for the 2.1-Gy group, and 93 and 92% at 6 years for the 2.2-Gy group (Fig. 1). Six patients died of prostate cancer and 23 patients died from intercurrent diseases. No significant difference was found in overall survival between the three dose groups (P = 0.88), but a difference was found in PSA-FFS (P = 0.01). When comparing pairs of dose groups, there was a difference only between the 2.0-Gy and 2.2-Gy groups (P = 0.02). No significant differences were found in overall survival between the three risk groups (P = 0.18) (Fig. 2). The PSA-FFS rate for high-risk patients in all groups was 80% at 7 years, while the rate was 93% for both intermediate- and low-risk patients (P = 0.02 for the three groups). Three low-risk patients (10%) had PSA failure, and one of them relapsed at 123 months.
Fig. 1.
Overall and prostate-specific antigen (PSA)-failure-free survival curves for the three dose groups.
Fig. 2.
Overall and prostate-specific antigen (PSA)-failure-free survival curves for the three risk groups.
The incidences of Grade 2 acute GU and GI toxicity were, respectively, 9.5 and 1.4% for the 2.0-Gy group, 19.8 and 2.0% for the 2.1-Gy group, and 20.8 and 1.2% for the 2.2-Gy group (P = 0.09 and 0.74, respectively). No Grade ≥3 acute toxicity was observed. The cumulative incidences of Grade ≥2 late GU and GI toxicity were, respectively, 7.2 and 12.4% at 10 years for the 2.0-Gy group, 7.4 and 14.1% at 8 years for the 2.1-Gy group, and 7.1 and 7.9% at 6 years for the 2.2-Gy group (P = 0.98 and 0.29, respectively) (Table 2). Four (1.1% of all patients) developed Grade 3 GU toxicities at 28, 41, 53 and 64 months, respectively: two developed severe hematuria requiring endoscopic coagulation and blood infusion, one each developed urinary retention, and one developed hydronephrosis. Improvement was achieved by conservative treatment including urethral catheterization. Argon plasma coagulation (APC) was administered to 16 patients (4.6% of all patients) for rectal hemorrhage. Three patients (0.9% of all patients) had Grade 3 GI toxicity: patient A at 12 months, patient B at 27 months and patient C at 28 months, respectively. Blood transfusion and APC were performed. No patients had Grade ≥4 toxicity.
Table 2.
Grade ≥2 late toxicities
| Grade 2/3 toxicity | P | |||
|---|---|---|---|---|
| 2.0 Gy/day | 2.1 Gy/day | 2.2 Gy/day | ||
| Genitourinary | ||||
| Urinary frequency | 1/0 | 1/0 | 5/0 | |
| Hematuria | 2/0 | 2/2 | 0/1 | |
| Urinary retention | 0/1 | 2/0 | 1/0 | |
| Urinary incontinence | 1/0 | 0/0 | 4/0 | |
| Totala | 7.2%b | 7.4%c | 7.1%d | 0.98e |
| Gastrointestinal | ||||
| Rectal hemorrhage | 9/0 | 11/3 | 12/0 | |
| Totala | 12.4%b | 14.1%c | 7.9%d | 0.29e |
aCumulative incidence of Grade ≥2 late genitourinary/gastrointestinal toxicity. bIncidence at 10 years. cIncidence at 8 years. dIncidence at 6 years. eExamined by logrank test.
On univariate analysis, age ≥70 years was associated with worse overall survival (P = 0.01), and high risk was associated with worse PSA-FFS (P = 0.006). Use of anticoagulants was associated with Grade ≥2 late GI toxicity (P = 0.03). Table 3 shows results of multivariate analyses for overall survival, PSA-FFS and late Grade ≥2 toxicities. The use of anticoagulants became insignificant for Grade ≥2 late GI toxicity (P = 0.06). The presence of diabetes mellitus was not a significant factor for Grade ≥2 late GU or GI toxicity.
Table 3.
Multivariate analyses for overall survival, PSA-FFS, Grade ≥2 late GU and GI toxicity
| Overall survival | PSA-FFS | Late GU toxicity | Late GI toxicity | |||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | Hazard ratio | P | Hazard ratio | P | |
| Age | 2.73 | 0.02 | 0.89 | 0.68 | 1.90 | 0.17 | 1.27 | 0.49 |
| (≥ vs <70 years) | (1.19–6.27) | (0.50–1.57) | (1.77–4.71) | (0.65–2.50) | ||||
| Risk | 1.62 | 0.26 | 2.66 | 0.005 | 1.09 | 0.86 | 1.15 | 0.71 |
| (HR vs LR/IR) | (0.70–3.72) | (1.35–5.22) | (0.43–2.70) | (0.56–2.34) | ||||
| ADT | 3.22 | 0.13 | 1.12 | 0.81 | 1.32 | 0.72 | 4.33 | 0.15 |
| (Yes vs no) | (0.71–14.51) | (0.43–2.96) | (0.30–5.81) | (0.59–32.00) | ||||
| Anticoagulant | 1.66 | 0.23 | 0.51 | 0.13 | 1.62 | 0.33 | 2.03 | 0.06 |
| (Yes vs no) | (0.73–3.81) | (0.21–1.22) | (0.61–4.26) | (0.97–4.24) | ||||
| Coexistent DM | 0.86 | 0.76 | 1.43 | 0.32 | 1.42 | 0.49 | 1.71 | 0.17 |
| (Yes vs no) | (0.32–2.29) | (0.71–2.90) | (0.52–3.92) | (0.80–3.66) | ||||
LR, IR, HR = low, intermediate, and high risk, ADT = androgen deprivation therapy, DM = diabetes mellitus.
DISCUSSION
The three dose fractionation regimens yielded similar results in overall survival and toxicities, but the 2.2-Gy group had a higher PSA-FFS rate than the 2.0-Gy group. There were differences in the follow-up duration: the median follow-up period for the 2.2-Gy group was 69 months, but several patients in the 2.0-Gy group developed PSA failure after 6 years. The proportion of patients undergoing ADT was lower in the 2.0-Gy group; this was in part due to the higher proportion of low-risk patients; in addition, a proportion of the intermediate- and high-risk patients in the 2.0-Gy group did not undergo ADT. Also, a learning curve effect might have developed in our treatment planning and actual treatment, which would lead to better outcomes in more recent patients (i.e. the 2.2-Gy group patients). These facts may account for the difference in the PSA-FFS rate. Further investigations are necessary to evaluate whether shortening the treatment period contributes to therapeutic gains. Nevertheless, all groups obtained favorable overall survival and PSA-FFS rates.
The rates of acute Grade ≥2 GU toxicities (9.5–20.8%) and late Grade 3 GU toxicities (1.1%) for all patients in this study seemed to be comparable with or compare favorably with those in previous studies [9, 10, 12, 20]. Arcangeli et al. [21] reported that urinary toxicity continued to increase beyond 4 years, whereas rectal toxicity plateaued at 20–26 months. In this study, the cumulative incidence of Grade ≥2 GU toxicities in the three dose groups increased until 66–72 months. Longer follow-up is necessary to evaluate late Grade ≥2 GU toxicity. Grade ≥2 late GI toxicities (10.8% at 7 years in all patients) were also comparable with those reported in previous studies [20, 22]. As we previously reported, our treatment strategy and outcome for patients with late rectal bleeding mean that this adverse event may not be so troublesome [23]. Some risk factors such as the use of anticoagulants, diabetes mellitus and the high-dose-irradiated volume of the rectum have been reported [24–27]. In this study, the use of anticoagulants and diabetes mellitus were not significant risk factors for late Grade ≥2 GI toxicity in multivariate analysis. This might have been due to the relatively small patient number.
Several Japanese groups have reported long-term outcomes of conventionally fractionated regimens [28–30]. Hypofractionated regimens have also been studied to increase therapeutic gain. A few institutions have reported 5-year or longer outcomes of slightly or moderately hypofractionated regimens using 2.2–3-Gy daily fractions with satisfactory disease controls and acceptable toxicities [31, 32]. Hypofractionated radiation therapy regimens have recently been compared with conventional radiation therapy in randomized trials [9–13]. According to a systematic review and meta-analysis of these trials [14], biochemical failure, biochemical and/or clinical failure, overall mortality, prostate cancer–specific mortality, acute GU toxicity, and late GU and GI toxicities were all similar. Nevertheless, the incidence of acute GI toxicity was 9.1% lower with the conventional regimen. Several studies in Japan have reported toxicities of hypofractionated regimens, with high incidences of late rectal toxicities observed in some of the studies [33–35]. Further investigations are necessary to determine the optimal fractionation schedule.
Optimal daily and total doses should be determined carefully. The LQ model is often used to estimate the equivalence between different fractionation regimens, but it does not take reoxygenation into consideration. Recent laboratory studies suggest that the LQ model overestimates the effect of a high fractional dose of radiation [36–38]. Since the α/β ratio represents the dose at which cell killing from linear (α) and quadratic (β) components of the LQ formula is equal, this model is considered appropriate when used for daily doses around the α/β ratio [36]. With increase in the fractional doses, however, the β cell kill component dominates in the LQ model; thus, actual data would deviate. Hence, it was proposed that the LQ model might only be applicable for fractional doses up to twice the α/β ratio [36]. Based on these considerations, our approach for stepwise shortening of the overall treatment time based on the LQ model seems reasonable, and so far it has yielded the expected outcomes. We are now using a daily dose of 2.5 Gy. In this study, we reported the culmination of our 2.0–2.2-Gy daily fractionation regimens.
Our study has a few limitations. This was not a well-controlled study, and there were imbalances between the three groups in terms of patient characteristics, especially risk-group distributions, proportions of patients undergoing hormone therapy, and follow-up periods. Comparison between the three dose-fractionation groups might reflect these biases, and may not be so useful. In future, randomized studies on the optimal fractionation schedules are warranted.
In conclusion, tumor control was good and toxicities were acceptable in all dose groups after long-term follow-up periods, suggesting that our stepwise shortening of treatment periods has been successful.
ACKNOWLEDGEMENTS
We are grateful to Fumiya Baba, Rumi Murata, Shinya Otsuka, Akifumi Miyakawa, Hiroyuki Ogino, Michio Iwabuchi, Aiko Nagai, Natsuo Tomita and Dr Shiho Ayakawa, and Hiroshi Fukuma, Yasujiro Hirose, Makoto Higuchi, Harumasa Kasai, Yuta Eguchi, Fujio Kawamura, Takahiro Tsuchiya, Yuji Mekata, and Messrs Takamine Esaka for their valuable help in this research. This study was presented in part at the 58th Annual Meeting of the American Society for Radiation Oncology.
CONFLICT OF INTEREST
The authors state that they have no conflicts to declare.
FUNDING
None.
REFERENCES
- 1. Onozawa M, Hinotsu S, Tsukamoto T et al. . Recent trends in the initial therapy for newly diagnosed prostate cancer in Japan. Jpn J Clin Oncol 2014;44:969–81. [DOI] [PubMed] [Google Scholar]
- 2. Okonogi N, Katoh H, Kawamura H et al. . Clinical outcomes of helical tomotherapy for super-elderly patients with localized and locally advanced prostate cancer: comparison with patients under 80 years of age. J Radiat Res 2015;56:889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miralbell R, Roberts SA, Zubizarreta E et al. . Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 2012;82:e17–24. [DOI] [PubMed] [Google Scholar]
- 4. Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys 2001;50:1021–31. [DOI] [PubMed] [Google Scholar]
- 5. Brenner DJ, Martinez AA, Edmundson GK et al. . Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6–13. [DOI] [PubMed] [Google Scholar]
- 6. Tucker SL, Thames HD, Michalski JM et al. . Estimation of α/β for late rectal toxicity based on RTOG 94–06. Int J Radiat Oncol Biol Phys 2011;81:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys 2004;60:1013–5. [DOI] [PubMed] [Google Scholar]
- 8. Zemplenyi AT, Kalo Z, Kovacs G et al. (19 January 2018) Cost–effectiveness analysis of intensity modulated radiation therapy with normal and hypofractionated schemes for the treatment of localized prostate cancer. Eur J Cancer Care 2018;27 10.1111/ecc.12430. [DOI] [PubMed] [Google Scholar]
- 9. Catton CN, Lukka H, Gu CS et al. . Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017;35:1884–90. [DOI] [PubMed] [Google Scholar]
- 10. Arcangeli G, Saracino B, Arcangeli S et al. . Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol 2017;35:1891–7. [DOI] [PubMed] [Google Scholar]
- 11. Lee WR, Dignam JJ, Amin MB et al. . Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 2016;34:2325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dearnaley D, Syndikus I, Mossop H. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomized, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Incrocci L, Wortel RC, Alemayehu WG et al. . Hypofractionated versus conventionally fractionated radiotherapy for patients with localized prostate cancer (HYPRO): final efficacy results from a randomized, multicentre, open-label, phase 3 trial. Lancet Oncol 2016;17:1061–9. [DOI] [PubMed] [Google Scholar]
- 14. Datta NR, Stutz S, Rogers S et al. . Conventional versus hypofractionated radiation therapy for localized or locally advanced prostate cancer: a systematic review and meta-analysis along with therapeutic implications. Int J Radiat Oncol Biol Phys 2017;99:573–89. [DOI] [PubMed] [Google Scholar]
- 15. Manabe Y, Shibamoto Y, Sugie C et al. . Toxicity and efficacy of three dose-fractionation regimens of intensity-modulated radiation therapy for localized prostate cancer. J Radiat Res 2014;55:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Amico AV, Whittington R, Malkowicz SB et al. . Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969–74. [DOI] [PubMed] [Google Scholar]
- 17. Hayashi A, Shibamoto Y, Hattori Y et al. . Dose–volume histogram comparison between static 5-field IMRT with 18-MV X-rays and helical tomotherapy with 6-MV X-rays. J Radiat Res 2015;56:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roach M III, Hanks G, Thames H Jr et al. . Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–74. [DOI] [PubMed] [Google Scholar]
- 19. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ (Easy R) for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zelefsky MJ, Levin EJ, Hunt M et al. . Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9. [DOI] [PubMed] [Google Scholar]
- 21. Arcangeli G, Fowler J, Gomellini S et al. . Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2011;79:1013–21. [DOI] [PubMed] [Google Scholar]
- 22. Tomita N, Soga N, Ogura Y et al. . Preliminary analysis of risk factors for late rectal toxicity after helical tomotherapy for prostate cancer. J Radiat Res 2013;54:919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takemoto S, Shibamoto Y, Ayakawa S et al. . Treatment and prognosis of patients with late rectal bleeding after intensity-modulated radiation therapy for prostate cancer. Radiat Oncol 2012:7;87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Someya M, Hori M, Tateoka K et al. . Results and DVH analysis of late rectal bleeding in patients treated with 3D-CRT or IMRT for localized prostate cancer. J Radiat Res 2015;56:122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tucker SL, Dong L, Michalski JM et al. . Do intermediate radiation doses contribute to late rectal toxicity? An analysis of data from radiation therapy oncology group protocol 94–06. Int J Radiat Oncol Biol Phys 2012;84:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chennupati SK, Pelizzari CA, Kunnavakkam R et al. . Late toxicity and quality of life after definitive treatment of prostate cancer: redefining optimal rectal sparing constraints for intensity-modulated radiation therapy. Cancer Med 2014;3:954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalakota K, Liauw SL. Toxicity after external beam radiotherapy for prostate cancer: an analysis of late morbidity in men with diabetes mellitus. Urology 2013;81:1196–201. [DOI] [PubMed] [Google Scholar]
- 28. Mizowaki T, Norihisa Y, Takayama K et al. . Ten-year outcomes of intensity-modulated radiation therapy combined with neoadjuvant hormonal therapy for intermediate- and high-risk patients with T1c–T2N0M0 prostate cancer. Int J Clin Oncol 2016;21:783–90. [DOI] [PubMed] [Google Scholar]
- 29. Mizowaki T, Norihisa Y, Takayama K et al. . Long-term outcomes of intensity-modulated radiation therapy combined with neoadjuvant androgen deprivation therapy under an early salvage policy for patients with T3–T4N0M0 prostate cancer. Int J Clin Oncol 2016;21:148–55. [DOI] [PubMed] [Google Scholar]
- 30. Tomita N, Soga N, Ogura Y et al. . High-dose radiotherapy with helical tomotherapy and long-term androgen deprivation therapy for prostate cancer: 5-year outcomes. J Cancer Res Clin Oncol 2016;142:1609–19. [DOI] [PubMed] [Google Scholar]
- 31. Shimizu D, Yamazaki H, Nishimura T et al. . Long-term tumor control and late toxicity in patients with prostate cancer receiving hypofractionated (2.2 Gy) soft-tissue–matched image-guided intensity-modulated radiotherapy. Anticancer Res 2017;37:5829–35. [DOI] [PubMed] [Google Scholar]
- 32. Hashimoto Y, Motegi A, Akimoto T et al. . The 5-year outcomes of moderately hypofractionated radiotherapy (66 Gy in 22 fractions, 3 fractions per week) for localized prostate cancer: a retrospective study. Int J Clin Oncol 2018;23:165–72. [DOI] [PubMed] [Google Scholar]
- 33. Kozuka T, Nakano M, Hashimoto M et al. . Acute and late complications after hypofractionated intensity modulated radiotherapy in prostate cancer. Jpn J Radiol 2017;35:269–78 [DOI] [PubMed] [Google Scholar]
- 34. Nishimura T, Yamazaki H, Aibe N et al. . Exceptionally high incidence of grade 2–3 late rectal toxicity in patients with prostate cancer receiving hypofractionated (2.2 Gy) soft tissue–matched image-guided intensity-modulated radiotherapy. Anticancer Res 2013;33:5507–10. [PubMed] [Google Scholar]
- 35. Akimoto T, Muramatsu H, Takahashi M et al. . Rectal bleeding after hypofractionated radiotherapy for prostate cancer: correlation between clinical and dosimetric parameters and the incidence of grade 2 or worse rectal bleeding. Int J Radiat Oncol Biol Phys 2004;60:1033–9. [DOI] [PubMed] [Google Scholar]
- 36. Shibamoto Y, Otsuka S, Iwata H et al. . Radiobiological evaluation of the radiation dose as used in high-precision radiotherapy: effect of prolonged delivery time and applicability of the linear-quadratic model. J Radiat Res 2012;53:1–9. [DOI] [PubMed] [Google Scholar]
- 37. Iwata H, Matsufuji N, Toshito T et al. . Compatibility of the repairable–conditionally repairable, multi-target and linear quadratic models in converting hypofractionated radiation doses to single doses. J Radiat Res 2013;54:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shibamoto Y, Miyakawa A, Otsuka S et al. . Radiobiology of hypofractionated stereotactic radiotherapy: what are the optimal fractionation schedules? J Radiat Res 2016;57:i76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]