Abstract
This study aimed to determine influence of corn inclusion on glutathion peroxidase (GPx) activity, selected minerals concentration, and gene expression in sheep-fed palm kernel cake (PKC) and urea-treated rice straw. Twenty-seven of Dorper sheep were divided into three groups and fed a basal diet of (20% rice straw and 80% concentrate) with addition of ground corn at either 0% (T1), 5% (T2), or 10% (T3), respectively. After 120 days feeding trial, all animals were slaughtered and tissue samples of kidney, liver, and muscles were taken for enzyme and mineral analyses. The results showed that Cu concentration in the liver was lower treatment T3 compared to the control and T2. The serum activity of GPx was higher in T2 than in T3 at day 120 of experiment. Serum malondialdehyde (MDA) concentrations decreased at day 80 in sheep on T3, whereas MDA of liver increased linearly with increasing corn supplementation. The qRT-PCR analyses revealed significant up-regulation of ATP7A and MIa genes in T3, while hepatic Cu/Zn SOD, GPx1, and GPx4 mRNA showed a higher expression in lamb hepatocytes in T3 compared to those on T1. Present study results suggest that feeding PKC as basal diet can increase antioxidant activity, but cause liver dysfunction in sheep. Inclusion corn was found to regulate transcriptional levels of the GPx family and metallothionein genes. These genes may play a role in the antioxidant protection response and reduce incidence of toxicity associated with Cu.
Keywords: Gene expression, Toxicity, Corn, Antioxidant enzymes, Dorper sheep, Palm kernel cake
Introduction
Availability of feed leads to the usage of costly supplementary feeding sources which has prompted much research on the physiological effects of nutritional restrictions and complementary feeding (Prescott et al. 2008). In many tropical countries, under-nutrition of livestock is fairly common owing to the inadequate pasture conditions and the type of feedstuff used. Such insufficient nutritional intake leads to the utilisation of body fat and protein reserves to support the energy needs of the animals. Hence, it is important to assess the physiological indicators of nutritional status and its adaptation mechanisms arising from insufficient nutrient intake.
Palm kernel cake (PKC) is a major byproduct from the extraction of oil from the kernels of the palm fruit and is available in many tropical countries, such as Malaysia, Indonesia, and Nigeria (Jahromi et al. 2017). Palm kernel cake is a good source of energy for ruminants and also provides considerable amount of crude protein. However, it contains high amounts of copper (Cu) (11–55 mg/kg dry) matter, which is critical to the sheep (Rahman et al. 1989). Nevertheless, the previous studies have shown that adding chelating agents such as ammonium molybdate, zinc sulphate, and sodium sulphate in feed formulations resulted decrease of Cu solubility in rumen and Cu accumulation in the liver of sheep (Abdullah et al. 1995; Ivan et al. 1999). Thus, dietary manipulation is critical to reduce Cu absorption and tissue accumulation in sheep fed with PKC.
In developed countries, corn is the widely grown grain with large production annually. It is the main grain for feeding poultry and to some extent ruminants. Corn grain has a potent antioxidant that protects the body against harming by free radicals responsible for cellular damage (Hall et al. 2009). Some of the known antioxidants found in corn grain are ferulic acid, anthocyanins, zeaxanthins, and lutein. Inclusion of corn as energy source into PKC-based diet may enhance the antioxidant defense system and protect the cells against Cu toxicity.
Ruminants have an antioxidant defense system that protects the functional and structural integrity of the cells. Constituents of this system include glutathione peroxidase (GPx) and thiobarbituric acid-reacting substances (TBARS), which are considered to be a multifunctional antioxidants linking the different scavenger systems (Mayne 2003; Yue et al. 2009; Singh et al. 2009). The previous studies showed that these two systems are sensitive markers of oxidative stress, as their levels may change in response to reactive oxygen species (ROS) primarily GPx which is a selenoenzyme responsible for the elimination of reactive oxygen species (Craig et al. 2007; BV et al. 2011). Furthermore, copper stress has led to the expression of a panel of genes that play a role in the cellular and molecular events leading to toxicity in the liver cells (Merle and Weiskirchen). The incorporation of Cu into metallothionein (MT) is an example of the cells’ defense mechanisms to protect its structure from Cu toxicity and to prevent oxidative damage. Pal and Prasad (2015) showed that hepatic MT-Ia gene expression increased significantly but that of hepatic ATP7B remained unaltered due to chronic Cu-intoxication in the Wistar rat model for non-Wilsonian brain Cu toxicosis. However, there has been limited study examining the effects of Cu toxicity on the regulation of genes related to antioxidant defense systems in sheep (Juszczuk-Kubiak et al. 2016). The effects of corn inclusion into PKC diet on the Cu toxicity and antioxidant systems in Dorper sheep are still unknown. Hence, this study was conducted to determine the effect of corn supplementation into PKC–urea-treated rice straw (PKC-UTS)-based diet on selected mineral concentration (Cu, Se, Fe, and Zn), antioxidant enzymes (GPx and MDA) in the blood serum, liver, and kidney, and the expression pattern of genes related to antioxidant protection response and copper toxicity expression (ATP7A, MIa, Cu/Zn SOD, GPx1, and GPx4) of Dorper sheep.
Materials and methods
Experimental design and feeding trial
The animal experimental procedures were conducted in compliance to the Animal Utilisation Protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Universiti Putra Malaysia (Reference no.: UPM/IACUC/AUP-R064/2016). Twenty-seven 6-month-old male Dorper lambs (average live weight of 15.00 ± 0.59 kg) were randomly assigned to three treatment groups of nine animals each and fed a basal diet of PKC-UTS supplemented with either 0% (control, T1), 5% (T2), or 10% (T3) ground corn. The basal diet consisted of UTS (20%) and PKC ranging from 65.3 to 70.3%, with the corn substituting the PKC (Table 1). The three diets were formulated be iso-nitrogenously and iso-caloric. The rice straw was treated with urea according to method described by Wanapat et al. (2009). The ingredients and chemical composition of treatment groups are presented in Table 1. The lambs were placed in individual pens provided with feeders and water. The animals were fed daily between 9.00 and 11.00 a.m. Water was provided ad libitum. The feeding trial lasted 120 days.
Table 1.
Ingredients and chemical compositions of different inclusion of corn into palm kernel cake—urea rice straw treated (DM basis)
| Item | Levels of corn (%) | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| Rice straw urea treated | 20 | 20 | 20 |
| PKC | 75.3 | 70.3 | 65.3 |
| Protected fat (Megalac) | 3 | 3 | 3 |
| Corn | 0 | 5 | 10 |
| CaCO3 | 1 | 1 | 1 |
| NaCl | 0.5 | 0.5 | 0.5 |
| Vitamin premix | 0.2 | 0.2 | 0.2 |
| Total | 100 | 100 | 100 |
| Chemical composition | |||
| DM | 91.78 | 91.66 | 91.55 |
| Ash | 13.80 | 12.72 | 12.74 |
| OM | 86.19 | 87.27 | 87.26 |
| CP | 15.42 | 14.88 | 14.09 |
| EE | 5.3 | 5.1 | 4.33 |
| CF | 26.6 | 24.50 | 20.83 |
| NDF | 62.36 | 60.06 | 55.66 |
| ADF | 45.60 | 40.96 | 37.30 |
| ADL | 6.56 | 6.10 | 5.43 |
| Hemicellulose | 16.76 | 19.10 | 18.36 |
| Cellulose | 39.03 | 34.86 | 31.86 |
| NFE | 40.44 | 41.11 | 48.39 |
| ME, MJ/kg DM | 7.36 | 8.23 | 8.92 |
Vitamin premix; vitamin A: 10,000,000 IU; vitamin E: 70,000 IU; vitamin D: 1,600,000 IU
DM dry matter, OM organic matter, CP crude protein, EE ether extract, CF crude fiber, NDF neutral detergent fiber, ADF acid detergent fiber, ADL acid detergent lignin, NFE nitrogen free extract, ME metabolizable energy
Blood sampling
Blood samples (5 mL) were collected from the jugular vein using non-heparinized vacutainer tubes from each sheep before feeding at days 0 (beginning of the trial), 40, 80, and 120. The serum tubes were kept slanted for 1 h and centrifuged at 3000g for 10 min. Serum was collected and kept at − 20 °C.
Animals slaughtering and tissues sampling
After 120 days of feeding trial, five lambs were chosen randomly from each treatment and were slaughtered according to the standard protocol outlined in the MS 1500:2004 (Department of Standards Malaysia 2009) at the Slaughter House of Department of Animal Science, Faculty of Agriculture, Universiti Putra Malaysia. Muscles [supraspinatus (SS), semitendinosus (ST), and longissimus lumborum (LL)], liver and kidney tissues were collected, deep-frozen in liquid nitrogen and kept at − 80 °C until minerals content and gene expression analysis.
Determination of mineral content
Liver and kidney tissues were digested according to the protocol described by Kolmer et al. (1951). The tissues (1 g) were wet-ashed in 10 mL of concentrated HNO3 (98%) and 5 mL of HClO4. The concentrations of Cu, zinc (Zn), and iron (Fe) were determined using an Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-OES) (model Optima 8300). The concentration of selenium (Se) was measured using an ICP Mass Spectrometer ELAN DRC-e Axial Field Technology (Perkin Elmer SCIEX, Waltham).
Determination of antioxidant enzymes
Glutathione peroxidase (GPx) concentration in blood serum, SS, ST, LL muscles, and liver tissue were determined with Glutathione Peroxidase Assay Kit according to the manufacturer’s protocol (Cayman Chemical Company, Ann Arbor, MI, USA). The malondialdehyde (MDA) content in these tissues was determined using thiobarbituric acid reactive substance (TBARS) assay (Pareek et al. 2013).
Gene expression analysis
For RNA extraction, 30 mg of liver (9 samples/treatment), with each sample duplicated three times, were used. Total RNA was extracted from liver tissue with RNeasy® tissue mini kit (Qiagen, USA) according to the manufacturer’s protocol. The extracted RNA was quantified with ND-1000 NanoDrop spectrophotometer (NanoDrop Technologies, USA). The cDNA was synthesised from 1.5 µg extracted total RNA using Transcription First-Strand cDNA Synthesis Kit (Qiagen, USA). Aliquots of cDNA were used as the template for quantitative real-time polymerase chain reaction (qRT-PCR) in accordance with Bustin et al. (2009).
Quantative real-time PCR (qRT-PCR) was conducted to examine the transcriptional level of two selenoprotein genes (glutathione peroxidase 1, GPx1 and glutathione peroxidase 4, GPx4) and three genes associated with metallothionein (ATP7A; Metallothionein-Ia, MIa, and Cu/Zn Superoxide Dismutase, Cu/Zn SOD). Both of the β-actin (ACTB) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes were used as reference genes. The primers of these genes were designed using Primer 3 Plus (NHK Bioscience Solutions Sdn. Bhd) based on Ovis aries sequences in the GenBank. The primers were synthesised by BioNeer Corporation (South Korea). The information of the primers is presented in Table 2.
Table 2.
Genes and primers used for relative quantification by qRT-PCR in the liver of lambs
| Target genea | Primer sequences (5′–3′) | Product length (bp) | Annealing temperature, °C | Accession number |
|---|---|---|---|---|
| GAPDH | F: ACCACTTTGGCATCGTGGAG R: GGGCCATCCACAGTCTTCTG |
75 | 61 | NM_002046 |
| β actin | F: CTTGATGTCACGGACGATTT R: CACGGCATTGTCACCAACT |
380 | 61 | NM_001101.4 |
| ATP7A | F: TGATGATGAGCTGTGCGGTT R: CATTGCTACCCGTTTCCCCT |
241 | 61 | NM_000052.6 |
| MIa | F: CACTGACCACACTTCCCTACA R: AGGACTCTGGAGGGTTCACAA |
226 | 63 | NM_177389.3 |
| Cu/Zn SOD | F: GACTTGGGCAGAGGTGGAAA R: CAGGGAATGTTTACGGGGCA |
100 | 61 | NM_000454.4 |
| GPx1 | F: CCTGGTCGTACTCGGCTTC R: CCTTCTCGCCATTCACCTC |
154 | 61 | NM_000581.3 |
| GPX4 | F: GGGAGTAATGCGGAGATCAA R: CATACCGCTTCACCACACAG |
210 | 61 | NM_001039847.2 |
a GAPDH glyceraldehyde 3-phosphate dehydrogenase, ACTB β-actin, MIa metallothionein-Ia, SOD Cu/Zn superoxide dismutase, GPx1 glutathione peroxidase 1, GPx4 glutathione peroxidase 4
The qRT-PCR was performed using Light Cycler 96 System CFX96 Touch Real-Time PCR Detection System (BioRad, USA), in 96-well optical reaction plates with a total reaction volume of 25 µL. The reaction mixture consisted of 1 µL of each primer, 12.5 µL of SYBR Green Master Mix (Qiagen, USA), 9.5 µL of PCR-grade water, and 1 µL of cDNA. The amplification program involved 10 min of initial denaturation at 95 °C, 40 cycles of denaturation (10 s, 95 °C), annealing (9 s, 59–61 °C), and elongation (20 s, 72 °C). The comparative CT method (ΔΔCT) expression of the examined genes was normalized with the endogenous control hypoxanthine phosphoribosyltransferase 1. CT values are means of duplicate measurements. Comparative CT quantification was determined by the ΔΔCT method.
Statistical analysis
All statistical analyses were performed using one-way analysis of variance (ANOVA) procedure in Completely Randomized Design (SAS 9.4) software (SAS Inst. Inc., USA) at the 95% of confidence level. Significant differences among treatment means were compared using Duncan’s new multiple range test. The collected data of minerals tissues concentration and antioxidant enzymes were analyzed by employing GLM procedure applicable for SAS.
Results
Minerals concentration in liver and kidney tissues
The concentrations of Cu, Fe, and Zn in the liver (n = 9) and kidney (n = 9) of sheep are presented in Table 3. The amount of Cu in the liver (P < 0.01) was significantly lower in lambs on diet T3 compared to those on diet T1 and diet T2. The concentration of Se in the liver of lambs was not significantly different among all treatment groups. The concentrations of Zn and Fe in the liver of lambs on diet T2 were significantly higher (P < 0.01) than those of T1 and T3.
Table 3.
The mineral concentration (ppm) of soft tissue (liver and kidney) of sheep fed on different levels of corn
| Tissue | T1 | T2 | T3 | SEM | Lev of sig. |
|---|---|---|---|---|---|
| Liver (n = 9) | |||||
| Cu | 18.73a | 18.70a | 12.85b | 1.15 | * |
| Se | 0.74 | 0.58 | 0.47 | 0.05 | NS |
| Zn | 8.40b | 13.86a | 8.33b | 1.14 | * |
| Fe | 33.55ab | 41.78a | 26.36b | 2.57 | ** |
| Kidney (n = 9) | |||||
| Cu | 1.30b | 1.76a | 1.51ab | 0.08 | * |
| Se | 2.06a | 1.82a | 1.31b | 0.13 | * |
| Zn | 8.95b | 13.26a | 16.78a | 1.27 | ** |
| Fe | 16.21 | 18.86 | 19.58 | 1.02 | NS |
T1: (control, 0% corn), T2: (5% corn), and T3: (10% corn)
NS not significant statistically (P > 0.05), *P < 0.05, **P < 0.01
a,b,cMeans in the same row with different superscripts are significantly different
In the kidney, Cu concentration was significantly greater in lambs on treatment T2 compared to those of T1, but was not significantly different to that of T3. The concentration of Se was observed to be lower (P < 0.05) in T3 than either T1 or T2. The Zn concentration in the kidney of lambs in treatment T2 and T3 was significantly higher than those in T1. There was no significant difference in Fe concentrations in the kidney of the lambs (P > 0.05) for all treatment groups in the present study.
Glutathione peroxidase (GPx) and malondialdehyde (MDA) in serum, muscles, liver, and kidney of sheep
The mean GPx enzyme of the SS, ST, and the LL muscles are summarized in Table 4. No significant differences (P > 0.05) were observed on the concentration of GPx in blood serum on days 0, 40, and 80 of the feeding trial among all treatment groups (Table 4). However, the serum GPx at day 120 was significantly higher in lambs on treatment T2 compared to those on treatments T1 and T3 (Table 4). The GPx concentration in the SS muscle was not significantly affected by the dietary treatments. On the other hand, the GPx of the ST muscle was significantly higher (P < 0.05) in T1 and T3 compared to those on treatment T2. The concentration of GPx in the LL muscle decreased with increasing corn supplementation. There were significant differences (P < 0.001) among treatment groups with T1 being higher than T2 and T3 at 37.05, 5.29, and 11.42 U/L respectively. In the liver, GPx was not significantly affected (P > 0.05) in sheep-fed corn dietary among all groups. The other essential antioxidant measured was MDA concentrations in the blood serum, muscles, and liver of sheep, and is shown in Table 5. Serum MDA concentrations increased dramatically at day 80 for all treatments and then declined at day 120 compared to the initial values, such that there were no significant differences among all treatment. The concentration of MDA at day 80 was significantly (P < 0.001) lower in treatment T2 and T3 compared to the control group. In addition, meat lipid oxidation stability was affected by the inclusion of energy to the PKC diet. The most lipid oxidation stability in the SS muscle was almost similar among the groups in the study (P > 0.05). Lower (P < 0.01) MDA values were detected in the ST muscles of those in the T1 and T2 group compared to the T3 group. Higher (P < 0.05) MDA values of LL muscle were found in the T2 treatment than in the T1 and T3 diet groups. Liver MDA concentrations were significantly higher in the T3 group than in the other groups (P < 0.01).
Table 4.
Activity of glutathione peroxidase in the serum, muscles, and liver among different groups fed on experiment diets
| Parameters | T1 | T2 | T3 | SEM | Level of sig. |
|---|---|---|---|---|---|
| Serum (U/L)A | |||||
| 0 day | 68.39 | 87.36 | 74.93 | 18.26 | NS |
| 40 day | 177.89 | 191.65 | 170.38 | 16.44 | NS |
| 80 day | 67.13 | 91.58 | 104.73 | 7.38 | NS |
| 120 day | 81.95b | 143.37a | 70.13b | 11.47 | *** |
| Muscles (U/L) | |||||
| SS | 38.65 | 15.74 | 27.44 | 8.57 | NS |
| ST | 14.62a | 3.55b | 17.89a | 2.85 | ** |
| LL | 37.05a | 5.29b | 11.42b | 6.21 | *** |
| Liver | 444.01 | 433.54 | 462.51 | 34.22 | NS |
T1: (control, 0% corn), T2: (5% corn), T3: (10% corn)
a,bMeans in the same row with different superscripts are significantly different. NS not significant statistically (P > 0.05), **P < 0.01, ***P < 0.001
AU: is the amount of GPx that produces 1 µM of glutathione disulfide (GS–SG) per minute at pH 7.6 in room temperature. Muscles SS: supraspinatus muscle, ST: semitendinosus muscle, LL: longissimus lumborum muscle
Table 5.
Average values of MDA in the serum, muscles, and liver among different groups fed on experiment dietary
| Parameters | T1 | T2 | T3 | SEM | Lev of sig |
|---|---|---|---|---|---|
| Serum (µM) MDA/mL | |||||
| 0 day | 4.30 | 4.84 | 3.31 | 0.38 | NS |
| 40 days | 5.38 | 5.54 | 5.23 | 0.37 | NS |
| 80 days | 9.00a | 7.19b | 5.38c | 0.68 | ** |
| 120 days | 2.61 | 2.82 | 2.60 | 0.12 | NS |
| MusclesA (µM) MDA/g | |||||
| SS | 12.77 | 16.46 | 14.26 | 0.77 | NS |
| ST | 8.10b | 10.20b | 14.97a | 1.16 | ** |
| LL | 9.54ab | 12.36a | 7.92b | 0.83 | * |
| Liver | 21.84b | 24.41ab | 27.79a | 0.99 | ** |
T1: (control, 0% corn), T2: (5% corn), T3: (10% corn)
a,b,cMeans in the same row with different superscripts are significantly different. NS not significant statistically (P > 0.05), *P < 0.05, **P < 0.01
AMuscles SS: supraspinatus muscle, ST: semitendinosus muscle LL: longissimus lumborum muscle
Gene expression analysis
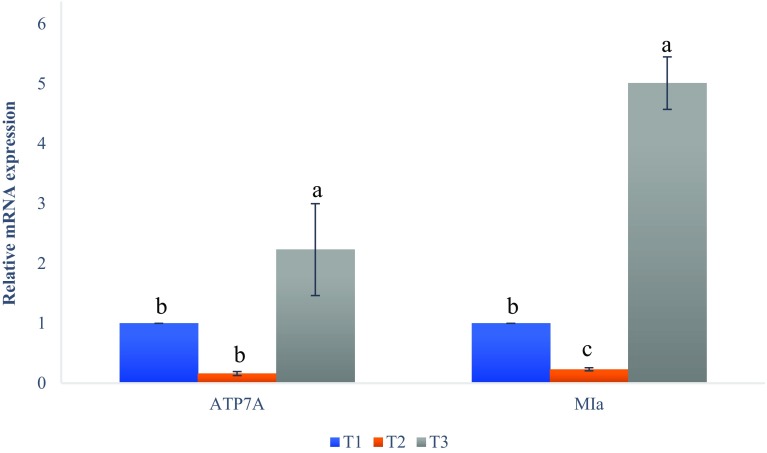
The results in Fig. 1 show a proportional increase in ATP7A and MIa in the liver of sheep fed with different levels of inclusion of corn. The supplementation of corn significantly (P < 0.01) increased the expression of ATP7A in the liver of sheep on treatment T3 compared to those on T1. Moreover, the MIa expression in the liver was significantly (P < 0.001) higher in T3 than in the others. T2 (5% corn) was lower in ATP7A and MIa expression compared with the control group of T1.
Fig. 1.
Expression of copper toxicity genes ATP7A, and MIa in hepatic cells. The expressions of ATP7A and MIa at the mRNA level were measured by qReal-Time-PCR, and β actin and GAPDH were used for normalization. T1: (control 0% corn), T2: (5% corn), and T3: (10% corn). a,b,cBars with common letter differ significantly (P < 0.05)
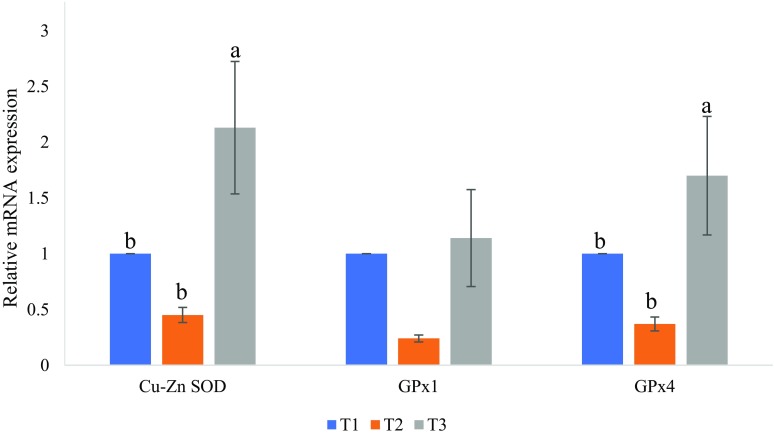
Hepatic Cu/Zn SOD, GPx1, and GPx4 mRNA expression levels between treatment groups are depicted in Fig. 2. There were significantly (P < 0.01) increased expressions of Cu/Zn SOD and GPx4 genes in the liver tissues of the T3 with the 10% corn compared to the T1. However, the results show that there were no changes in the expressions levels of the hepatic GPx1 gene among the T1, T2, and T3. Significantly lower (P < 0.01) Cu/Zn SOD and GPx4 concentrations were seen in the liver tissues of the T2 lambs compared to the control group.
Fig. 2.
Expression of antioxidant genes Cu/Zn SOD, GPx1, and GPx4 in hepatic cells. The expressions of Cu/Zn SOD, GPx1, and GPx4 at the mRNA level were measured by qReal-Time-PCR, and β actin and GAPDH were used for normalization. T1: (control 0% corn), T2: (5% corn), and T3: (10% corn). a,b,cBars with common letter differ significantly (P < 0.05)
Discussion
Minerals concentration in liver and kidney tissues
The use of PKC as main ingredients in the main ingredients in diets of ruminants has received attention by nutritionist throughout the world. Although it has fair amount of crude protein (14–18%) for ruminant, the high concentration of copper (Cu) (11–55 mg/kg dry matter) has particularly discouraged farmers and feed millers from using it (Alimon 2004) for fear of copper toxicity. The livers of young ruminants generally contain high concentrations of Cu (> 200 mg of Cu/kg liver DM) (Hidiroglou and Williams 1982; Branum et al. 1998) that are affected by maternal Cu status. In sheep affected with copper toxicity, the liver Cu concentration in most of the hemolytic crises reaches 1000–3000 ppm compared to 70–200 ppm in the kidney (Bostwick 1982). It is interesting to note that this study showed no clinical symptoms associated with copper toxicity due to feeding high level of PKC throughout the experimental period and this is in line with the findings of Al-Kirshi et al. (2011). Our results show that liver Cu concentration decreased in sheep on T3, but the concentration in kidney for the same group did not change and stayed within the normal range (6–279 ppm) (Zantopoulos et al. 1996) which suggested that Cu levels in the kidney may provide diagnostic values in cases of a hemolytic crisis rather than the liver. The reduction of Cu concentration in the liver in treatment T3 might be due to rumen solubility, and the biological availability of Cu in PKC may be quite high in T1 (control group) (Al-Kirshi et al. 2011). It has been hypothesized that Cu could be bound to denature the microbial protein or is present in another form that is insoluble in low pHs, such as CuS (Ward and Spears 1997). The concentration of Cu in the kidneys of sheep in groups T2 and T3 were higher than that in the T1. From the data obtained in this study, it is concluded that corn inclusion diet increased the loss of Cu from the body through the kidneys.
High Se concentrations were found in the liver of lambs in the T1 (control group) (0.74 ppm), although it is not statistically significant. Large Se concentrations were also present in the kidneys of sheep on control diet (2.06 ppm). The present findings are in agreement with Shi et al. (2011) and Aghwan et al. (2013), who reported that the Se content of the muscles and kidneys of goats that received diets supplemented with Na-selenite were significantly higher than in the control animals. The high Se concentration in animals could be due to the high Se content in PKC. In addition, Lawler et al. (2004) observed that beef steers fed with supra-nutritional sources of Se showed a significant increase in the Se concentration in the kidney and liver. Combs Jr. and Combs (1986) indicated that Se concentrations usually rank highest in the kidney, intermediate in the liver, and least in skeletal muscles. Lawler et al. (2004) and Mateo et al. (2007) reported that animals supplemented with a threefold dietary Se concentration showed non-significant increments in the liver. However, this is in contrast to Yu et al. (2008) who found that selenium supplementation enhanced the concentration of Se in blood and liver of lamb significantly. Ruminal bacteria can transform Se to soluble forms of cysteine to selenocysteine and insoluble forms such as mineral Se and metal selenide (Van Ryssen and Schroeder 2003).
The previous studies have reported that the high intakes of Zn could reduce Cu concentrations in the serum and livers of sheep (Kincaid et al. 1976; Kellogg et al. 1989). In T1 and T2, the mean concentration of Zn in the liver of sheep was in the normal range and was almost the same as that of the kidneys. In contrast, the average liver Zn concentrations of sheep in treatment T3 decreased by approximately 1–2 times the levels found in T2. Concentrations of Zn in the kidney of sheep in T3 were greater than in the liver. These findings on elevated zinc levels in these organs (liver and kidney) support the previous findings (Hair-Bejo et al. 1995). Thus, the elevation of Zn content in the organs in our study could be associated with the induction of metallothionein in the body (Hair-Bejo et al. 1995). This metal-binding protein sequesters Cu and makes it non-toxic to tissues.
Ivan et al. (1986) offered indirect proof of an heightened absorption of Fe, Zn, and, particularly, Cu without protozoa, as liver levels of these minerals and both plasma Cu concentrations rose drastically, while the enzyme of the Cu-dependent ferroxidase was elevated. On the other hand, the impacts of Cu, Fe, and Zn deposits were reduced in the whole liver compared to the impacts on the levels per gram of liver, clearly due to the reduced liver weights in protozoa-free animals. High dietary Fe in sheep (Suttle and Peter 1985) and rats (Yu et al. 1994) decreases Cu absorption. It has been amply publicized that high dietary Fe can significantly lower the Cu status in ruminants (Humphries et al. 1983). However, the present findings showed that average Fe and Zn concentrations in liver were not affected by corn inclusion.
Serum and tissue glutathione peroxidase and lipid oxidation in sheep
Oxidative damage is the result of oxidants and antioxidants imbalance at the cellular level and resulted oxidative changes of cellular macromolecules, cell death by apoptosis or necrosis, as well as structural damage to the tissue (Lykkesfeldt and Svendsen 2007). In analyzed biochemical parameters such as GPx and MDA in the serum, muscles, and liver, there were wide deviations from the reference values for sheep (Hanan et al. 2014). The results of this study show that measures of GPx concentrations in the serum are affected by treatment diets. Corn inclusion in sheep on treatment T2 significantly increased serum GPx at day 120 compared to the controls. The increased concentrations of GPx could be due to the PKC content of Se that helps the body in enhancing GPx creation. Many studies on lambs (Zachara et al. 1993; Juniper et al. 2009; Kumar and Jhariya 2013) documented that dietary Se supplementation significantly increased serum Se concentrations and GPx. Studies by Shi et al. (2011) on growing male Taihang black goats and Pavlata et al. (2012) on white short-haired goats reported that dietary supplementation from different Se sources significantly increased whole blood Se and GPx. However, there were insignificant difference of GPx serum for days 0, 40, and 80 due to the fact that GPx in whole blood, which reside mostly in the erythrocytes (BV et al. 2011), depends mainly on the availability of Se during the formation of the entire erythrocyte population (Hamliri et al. 1990).
Postmortem aging had no effect on GPx in the SS muscle, but affected ST and LL muscles. It could be that the lack of muscle GPx differences among the treatments in this study did not allow to reach the level of oxidation. However, other study suggested that liver GPx may be considered a good predictor of health risk (Ayisi and Zhao 2014). Therefore, in our result, there were no significant differences among treatments, suggesting that corn could contribute a good source of antioxidant in the diet for sheep. BV et al. (2011) reported that elevations in the GPx could represent an adaptive change against potential liver injury and reflect the liver’s capability to scavenge excess reactive oxygen species. Contrary to this, Gunter et al. (2003) observed no difference in concentration of GPx enzyme between sheep given inorganic or organic selenium. The content of MDA in serum in groups T2 and T3 was lower than the controls at day 80. In line with the results of this study, Cu deficiency in rats and the content of MDA decreased with the higher Cu intake and Cu/Zn ratios and was maintained at a relatively low levels (Duan et al. 2010). This could be due to the role of protozoa in minimizing the uptake of Cu. Faixová et al. (2016) reported that Se levels and GPx were significantly higher and MDA concentrations considerably lower in bacterial and protozoal fractions of the rumen fluid in Se-supplemented animals, which confirms the ability of rumen microflora to accumulate dietary energy. Shi et al. (2011) observed that MDA concentrations in the serum of growing male goats supplemented with different sources of Se in their diets were significantly lower than in the control.
The concentration of MDA showed a significant increase in the ST and LL muscles, but was insignificant in the SS muscle which could indicate no effect on lipid peroxidation. This is in agreement with Balogh et al. (2004). The glutathione peroxides in tissues is closely connected to an increase in MDA concentrations. The study by Maraschiello et al. (1999) shows a positive relationship between GPx and TBARS. Li et al. (2012) stated that the inhibition in the formation of MDA by antioxidants during the cooking of meat products might result in reduced concentrations of MDA in plasma and urine. Liver MDA levels were significantly higher in sheep on treatments T2 and T3 than those on the controls. The significant increases of MDA in the liver of sheep fed with corn inclusion indicated that oxidative damage occurred in the liver, but the reason is not clear. On the other hand, Das (2011) reported the rise in MDA depletion in heat-induced oxidative stress in rat liver. These results indicated that the corn inclusion had protective effects on sheep hepatocytes probably by activating the antioxidant mechanism in the cells and preventing the chain reaction of lipid peroxidation (Oskoueian et al. 2014).
Gene expression analysis
The present findings point towards the major role of hepatic ATP7A and MIa expression in liver Cu accumulation due to the feeding of PKC (Fig. 1). Higher expression of ATP7A and MIa genes were upregulated in T3, while the expression for the same genes was depressed in T2 compared to the controls. These results are in agreement with the previous studies (Ravia et al. 2005; Pal and Prasad 2015) who suggested that greater Cu absorption led to larger Cu accumulations in the liver in group T3 which is associated with gene regulation. However, the exposure of hepatic cells to high levels of Cu motivated ATP7A movement from the trans-Golgi network to the plasma membrane to promote Cu efflux (Lutsenko and Petris 2003). Han et al. (2009) identified ATP7A mRNA in bovine liver, a species in which liver Cu can be substantially high. Moreover, the increase in ATP7A in T3 occurred to raise Cu efflux as liver Cu. This is in agreement with the study conducted on rats by Ravia et al. (2005). However, the unexpected findings on the decreased expression of ATP7A at T2 is in contrast with a study by Kim et al. (2010) who reported an increase in serum Cu concentrations which led to suggestions that ATP7A in the liver may have a role in Cu mobilization. The upregulation of MIa shown in the liver of T3 could be due to the adaptive response to rising intracellular Cu concentrations (Dirksen et al. 2017). Kincaid (2000) suggested that the increased levels of MIa protein accounts for most of the increased Zn in the liver. The closely correlation between MIa mRNA and Zn suggests that Metallothionein in the genes is regulated by Zn (Paynter et al. 1990). However, the decrease in MIa expression in T2 was in agreement with the results found in rats as well as in the liver of Wilson disease patients (Delgado et al. 2008). This was also hypothesized in North Ronaldsay sheep, a Cu toxicity susceptible breed, with a decrease in MAT protein levels upon Cu challenge (Simpson et al. 2006). It was also demonstrated that the hepatic expressions of Cu/Zn SOD and GPx4 were highly and significantly upregulated in group T3. This upregulation might be influenced by the high feed intake in the diet which consequently provided a significant amount of minerals such as Cu, Se, Zn, and Fe. The previous findings in bovines indicate that Cu/Zn SOD mRNA was negatively correlated with liver Cu concentrations (Han et al. 2009). Approximately 60% of the Cu in erythrocytes is associated with Cu/Zn SOD (Kincaid 2000). Regardless, Hansen et al. (2008) showed that hepatic Cu/Zn SOD mRNA tended to be down-regulated in severely Cu-deficient cattle. GPx4 is the major antioxidant enzyme that directly reduces phospholipid hydroperoxides within membranes and lipoproteins and provides protection of cells against oxidative stress (Brigelius-Flohé 2006). These results corroborate the ideas of Hugejiletu et al. (2013) who suggested that expressions of GPx4 increased through both Se depletion and supra-nutritional Se-yeast supplementation. The level of the GPx4 in the liver of groups of sheep exhibited a significant response to changes in the level of corn. Our results suggest that GPx1 may be more sensitive to expression than GPx4. Juszczuk-Kubiak et al. (2016) confirmed the association between the response to mRNA expression and dietary Se might exist between the selenoproteins GPx1, GPx2, and GPx4, while our results show that GPx1 transcripts were insignificant in the liver of corn-supplemented group of sheep. Antioxidant can inhibit free radical production by chelating the transition metal catalysts, breaking chain reactions, reducing concentrations of ROS, and scavenging initiating radicals (Liska 1998). Venditti and Di Meo (2006) revealed the antioxidant depletion could be due to and not the cause of oxidative stress. Insignificant differential expression was found in the GPx1 of the experimental animals to reach to oxidation stress. It is generally recognised that Cu/Zn SOD and GPx form a network that provides the first line of cellular defense against oxidative stress, and the evidence shows that manipulating the expression of one enzyme may lead to an imbalance in antioxidant defenses (Omar and McCord 1990).
Conclusion
It can be concluded that inclusion of corn could enhance biological resistance and prevent Cu toxicity in sheep fed with PKC-based diet. Corn inclusion appeared to alter the expression of genes associated with antioxidant and metal regulation and can reduce Cu partly by increasing endogenous losses.
Acknowledgements
The authors extend their appreciation to Universiti Putra Malaysia (UPM) for funding this work and the Higher Education Ministry of Iraq and Al Anbar University for their support.
Author contributions
OAS analyzed and interpreted data regarding carried out laboratory analysis. MF was a major contributor in the part of gene expression and qPCR. OAS, TKL, AQS, HA, MFJ, ARA, and AAS participated in the whole design of the study and performed the statistical analysis and contributed to the preparation of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Abdullah N, Hanita H, Ho Y, Kudo H, Jalaludin S, Ivan M. The effects of bentonite on rumen protozoal population and rumen fluid characteristics of sheep fed palm kernel cake. Asian Australas J Anim Sci. 1995;8(3):249–254. [Google Scholar]
- Aghwan Z, Sazili A, Alimon A, Goh Y, Hilmi M. Blood haematology, serum thyroid hormones and glutathione peroxidase status in Kacang goats fed inorganic iodine and selenium supplemented diets. Asian Australas J Anim Sci. 2013;26(11):1577. doi: 10.5713/ajas.2013.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimon A. The nutritive value of palm kernel cake for animal feed. Palm Oil Dev. 2004;40(1):12–14. [Google Scholar]
- Al-Kirshi R, Alimon A, Ivan M. Effects of dietary molybdenum, sulfur and zinc on the excretion and tissue accumulation of trace minerals in sheep fed palm kernel cake-based diets. Animal. 2011;5(10):1539–1545. doi: 10.1017/S1751731111000693. [DOI] [PubMed] [Google Scholar]
- Ayisi CL, Zhao J-L. Recent developments in the use of palm oil in aquaculture feeds: a review. Int J Sci Technol Res. 2014;3(6):259–264. [Google Scholar]
- Balogh K, Weber M, Erdélyi M, Mezes M. Effect of excess selenium supplementation on the glutathione redox system in broiler chicken. Acta Vet Hung. 2004;52(4):403–411. doi: 10.1556/AVet.52.2004.4.3. [DOI] [PubMed] [Google Scholar]
- Bostwick J. Copper toxicosis in sheep. J Am Vet Med Assoc. 1982;180(4):386–387. [PubMed] [Google Scholar]
- Branum J, Carstens G, McPhail E, McBride K, Johnson A. Effects of prenatal dietary copper level on immune function of calves at birth and 56 days of age. J Anim Sci. 1998;76(Suppl 1):43. [Google Scholar]
- Brigelius-Flohé R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387(10/11):1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- BV SK, Kumar A, Kataria M. Effect of heat stress in tropical livestock and different strategies for its amelioration. J Stress Physiol Biochem. 2011;7(1):45–54. [Google Scholar]
- Combs GF, Jr, Combs SB. The role of selenium in nutrition. Ithaca, New York: Academic Press, Inc.; 1986. [Google Scholar]
- Craig PM, Wood CM, McClelland GB. Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio) Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R1882–R1892. doi: 10.1152/ajpregu.00383.2007. [DOI] [PubMed] [Google Scholar]
- Das A. Heat stress-induced hepatotoxicity and its prevention by resveratrol in rats. Toxicol Mech Methods. 2011;21(5):393–399. doi: 10.3109/15376516.2010.550016. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pérez-Miguelsanz J, Garrido F, Rodríguez-Tarduchy G, Pérez-Sala D, Pajares MA. Early effects of copper accumulation on methionine metabolism. Cell Mol Life Sci. 2008;65(13):2080–2090. doi: 10.1007/s00018-008-8201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of standards, Malaysia . MS1500:2009: Halal food production, preparation, handling and storage-General guidelines (1st revision) Cyberjaya, Selangor: Department of Standards Malaysia; 2009. [Google Scholar]
- Dirksen K, Spee B, Penning LC, van den Ingh TS, Burgener IA, Watson AL, Koerkamp MG, Rothuizen J, van Steenbeek FG, Fieten H. Gene expression patterns in the progression of canine copper-associated chronic hepatitis. PloS One. 2017;12(5):e0176826. doi: 10.1371/journal.pone.0176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Cheng Y, Jin Y. Effect of copper intake and copper-zinc ratio on rat lipid peroxidation in copper deficiency. Wei Sheng Yan Jiu. 2010;39(1):25–28. [PubMed] [Google Scholar]
- Faixová Z, Piešová E, Maková Z, Čobanová K, Faix Š. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on ruminal enzyme activities and blood chemistry in sheep. Acta Vet Brno. 2016;85(2):185–194. [Google Scholar]
- Gunter S, Beck P, Phillips J. Effects of supplementary selenium source on the performance and blood measurements in beef cows and their calves. J Anim Sci. 2003;81(4):856–864. doi: 10.2527/2003.814856x. [DOI] [PubMed] [Google Scholar]
- Hair-Bejo M, Davis MP, Alimon AR, Moonafizad M (1995) Chronic copper toxicosis: utilization of palm kernel cake in sheep fed solely on concentrate diets. In: Proceedings of the first symposium on integration of livestock to palm oil production. MSAP, 1995, pp 155–159
- Hall J, Seay W, Baker S (2009) Nutrition and feeding of the cow-calf herd: essential nutrients, feed classification and nutrient content of feeds. Publication 400-011
- Hamliri A, Johnson D, Kessabi M, Olson W. The evaluation of selenium status of sheep from the major production areas of Morocco. Annales de Recherches Vétérinaires. 1990;2:137–142. [PubMed] [Google Scholar]
- Han H, Archibeque SL, Engle TE. Characterization and identification of hepatic mRNA related to copper metabolism and homeostasis in cattle. Biol Trace Elem Res. 2009;129(1–3):130–136. doi: 10.1007/s12011-008-8293-6. [DOI] [PubMed] [Google Scholar]
- Hanan Z, Ibrahim N, Donia G, Younis F, Shaker Y. Scrutinizing of trace minerals and antioxidant enzymes changes in Barki ewes fed salt-tolerant plants under South Sinai conditions. J Am Sci. 2014;10(2):241–249. [Google Scholar]
- Hansen S, Schlegel P, Legleiter L, Lloyd K, Spears J. Bioavailability of copper from copper glycinate in steers fed high dietary sulfur and molybdenum. J Anim Sci. 2008;86:173–179. doi: 10.2527/jas.2006-814. [DOI] [PubMed] [Google Scholar]
- Hidiroglou M, Williams C. Trace minerals status of fetuses from ewes fed a copper-deficient ration. Am J Vet Res. 1982;43(2):310–313. [PubMed] [Google Scholar]
- Hugejiletu H, Bobe G, Vorachek WR, Gorman ME, Mosher WD, Pirelli GJ, Hall JA. Selenium supplementation alters gene expression profiles associated with innate immunity in whole-blood neutrophils of sheep. Biol Trace Elem Res. 2013;154(1):28–44. doi: 10.1007/s12011-013-9716-6. [DOI] [PubMed] [Google Scholar]
- Humphries W, Phillippo M, Young B, Bremner I. The influence of dietary iron and molybdenum on copper metabolism in calves. Br J Nutr. 1983;49(1):77–86. doi: 10.1079/bjn19830013. [DOI] [PubMed] [Google Scholar]
- Ivan M, Veira D, Kelleher C. The alleviation of chronic copper toxicity in sheep by ciliate protozoa. Br J Nutr. 1986;55(2):361–367. doi: 10.1079/bjn19860042. [DOI] [PubMed] [Google Scholar]
- Ivan M, Rusihan M, Alimon A, Hair-Bejo M, Jelan Z, Jalaludin S. The efficacy of dietary supplements of bentonite and sulphur plus molybdenum to alleviate chronic copper toxicity in sheep fed palm kernel cake. Czech J Anim Sci. 1999;44(3):125–130. [Google Scholar]
- Jahromi MF, Shokryazdan P, Idrus Z, Ebrahimi R, Liang JB. In Ovo and dietary administration of oligosaccharides extracted from palm kernel cake influence general health of pre-and neonatal broiler chicks. PloS One. 2017;12(9):e0184553. doi: 10.1371/journal.pone.0184553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper DT, Phipps RH, Ramos-Morales E, Bertin G. Effect of high dose selenium enriched yeast diets on the distribution of total selenium and selenium species within lamb tissues. Livest Sci. 2009;122(1):63–67. [Google Scholar]
- Juszczuk-Kubiak E, Bujko K, Cymer M, Wicińska K, Gabryszuk M, Pierzchała M. Effect of inorganic dietary selenium supplementation on selenoprotein and lipid metabolism gene expression patterns in liver and loin muscle of growing lambs. Biol Trace Elem Res. 2016;172(2):336–345. doi: 10.1007/s12011-015-0592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D, Rakes J, Gliedt D. Effect of zinc methionine supplementation on performance and selected blood parameters of lactating dairy cows. Nutr Rep Int. 1989;40(6):1049–1057. [Google Scholar]
- Kim B-E, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab. 2010;11(5):353–363. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. Assessment of trace mineral status of ruminants: a review. J Anim Sci. 2000;77(E-Suppl):1–10. [Google Scholar]
- Kincaid R, Miller W, Fowler P, Gentry R, Hampton D, Neathery M. Effect of high dietary zinc upon zinc metabolism and intracellular distribution in cows and calves. J Dairy Sci. 1976;59(9):1580–1584. doi: 10.3168/jds.s0022-0302(76)84408-6. [DOI] [PubMed] [Google Scholar]
- Kolmer JA, Spaulding EH, Robinson HW. Approved laboratory techniques. New York: Appleton Century Crafts; 1951. p. 1091. [Google Scholar]
- Kumar D, Jhariya AN. Nutritional, medicinal and economical importance of corn: a mini review. Res J Pharm Sci. 2013;2319:555X. [Google Scholar]
- Lawler T, Taylor J, Finley J, Caton J. Effect of supranutritional and organically bound selenium on performance, carcass characteristics, and selenium distribution in finishing beef steers. J Anim Sci. 2004;82(5):1488–1493. doi: 10.2527/2004.8251488x. [DOI] [PubMed] [Google Scholar]
- Li Q, Mair C, Schedle K, Hammerl S, Schodl K, Windisch W. Effect of iodine source and dose on growth and iodine content in tissue and plasma thyroid hormones in fattening pigs. Eur J Nutr. 2012;51(6):685–691. doi: 10.1007/s00394-011-0247-7. [DOI] [PubMed] [Google Scholar]
- Liska DJ. The detoxification enzyme systems. Altern Med Rev. 1998;3(3):187–198. [PubMed] [Google Scholar]
- Lutsenko S, Petris M. Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J Membr Biol. 2003;191(1):1–12. doi: 10.1007/s00232-002-1040-6. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Svendsen O. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. 2007;173(3):502–511. doi: 10.1016/j.tvjl.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Maraschiello C, Sárraga C, Garcia Regueiro J. Glutathione peroxidase activity, TBARS, and α-tocopherol in meat from chickens fed different diets. J Agric Food Chem. 1999;47(3):867–872. doi: 10.1021/jf980824o. [DOI] [PubMed] [Google Scholar]
- Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol. 2007;40(6):1451–1463. [Google Scholar]
- Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133(3):933S–940S. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- Merle U, Weiskirchen R Wilson’s disease: an inherited, silent, copper intoxication disease. EMJ Neurol 4(1):74–83
- Omar BA, McCord JM. The cardioprotective effect of Mn-superoxide dismutase is lost at high doses in the postischemic isolated rabbit heart. Free Radic Biol Med. 1990;9(6):473–478. doi: 10.1016/0891-5849(90)90124-2. [DOI] [PubMed] [Google Scholar]
- Oskoueian E, Abdullah N, Idrus Z, Ebrahimi M, Goh YM, Shakeri M, Oskoueian A. Palm kernel cake extract exerts hepatoprotective activity in heat-induced oxidative stress in chicken hepatocytes. BMC Complement Altern Med. 2014;14(1):368. doi: 10.1186/1472-6882-14-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Prasad R. Expression profile of hepatic metallothionein-I and ATP7B, and brain metallothionein-III and acetyl cholinesterase genes in wistar rat model for non-wilsonian brain copper toxicosis. J Neurol Neurol Disord. 2015;2(1):102. [Google Scholar]
- Pareek A, Godavarthi A, Issarani R, Nagori BP. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J Ethnopharmacol. 2013;150(3):973–981. doi: 10.1016/j.jep.2013.09.048. [DOI] [PubMed] [Google Scholar]
- Pavlata L, Misurova L, Pechova A, Husakova T, Dvorak R. Direct and indirect assessment of selenium status in sheep—a comparison. Vet Med. 2012;57(5):219–223. [Google Scholar]
- Paynter JA, Camakaris J, Mercer JF. Analysis of hepatic copper, zinc, metallothionein and metallothionein-Ia mRNA in developing sheep. FEBS J. 1990;190(1):149–154. doi: 10.1111/j.1432-1033.1990.tb15558.x. [DOI] [PubMed] [Google Scholar]
- Prescott SL, Smith P, Tang M, Palmer DJ, Sinn J, Huntley SJ, Cormack B, Heine RG, Gibson RA, Makrides M. The importance of early complementary feeding in the development of oral tolerance: concerns and controversies. Pediatr Allergy Immunol. 2008;19(5):375–380. doi: 10.1111/j.1399-3038.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Rahman A, Wong H, Zaini H, Sharif H (1989) Preliminary observation on the alleviation of copper in sheep fed with palm kernel meal based diet. In: Proceedings of 12th Malaysian society of animal production conference, Malaysia, 1989, pp 75–78
- Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem. 2005;280(43):36221–36227. doi: 10.1074/jbc.M506727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Xun W, Yue W, Zhang C, Ren Y, Shi L, Wang Q, Yang R, Lei F. Effect of sodium selenite, Se-yeast and nano-mineralal selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin Res. 2011;96(1):49–52. [Google Scholar]
- Simpson DM, Mobasheri A, Haywood S, Beynon RJ. A proteomics study of the response of North Ronaldsay sheep to copper challenge. BMC Vet Res. 2006;2(1):36. doi: 10.1186/1746-6148-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Kaur S, Kumari K, Singh G, Kaur A. Alterations in lipid peroxidation and certain antioxidant enzymes in different age groups under physiological conditions. J Hum Ecol. 2009;27(2):143–147. [Google Scholar]
- Suttle N, Peter D. Rumen sulphide metabolism as a major determinant of copper availability in the diets of sheep. Farnham Royal: Commonwealth Agricultural Bureaux; 1985. [Google Scholar]
- Van Ryssen J, Schroeder G. Effect of heat processing of protein sources on the disappearance of their selenium from mobile bags in the digestive tract of dairy cows. Anim Feed Sci Technol. 2003;107(1):15–27. [Google Scholar]
- Venditti P, Di Meo S. Thyroid hormone-induced oxidative stress. Cell Mol Life Sci. 2006;63(4):414–434. doi: 10.1007/s00018-005-5457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanapat M, Polyorach S, Boonnop K, Mapato C, Cherdthong A. Effects of treating rice straw with urea or urea and calcium hydroxide upon intake, digestibility, rumen fermentation and milk yield of dairy cows. Livest Sci. 2009;125(2):238–243. [Google Scholar]
- Ward J, Spears J. Long-term effects of consumption of low-copper diets with or without supplemental molybdenum on copper status, performance, and carcass characteristics of cattle. J Anim Sci. 1997;75(11):3057–3065. doi: 10.2527/1997.75113057x. [DOI] [PubMed] [Google Scholar]
- Yu S, West CE, Beynen AC. Increasing intakes of iron reduce status, absorption and biliary excretion of copper in rats. Br J Nutr. 1994;71(6):887–895. doi: 10.1079/bjn19940194. [DOI] [PubMed] [Google Scholar]
- Yu Z, García-González R, Schanbacher FL, Morrison M. Evaluations of different hypervariable regions of archaeal 16S rRNA genes in profiling of methanogens by Archaea-specific PCR and denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2008;74(3):889–893. doi: 10.1128/AEM.00684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Zhang C, Shi L, Ren Y, Jiang Y, Kleemann D. Effect of supplemental selenomethionine on growth performance and serum antioxidant status in Taihang black goats. Asian Australas J Anim Sci. 2009;22(3):365. [Google Scholar]
- Zachara B, Trafikowska U, Labedzka H, Mikolajczak J. Effect of dietary Se intake on blood Se levels and glutathione peroxidase activities in lambs. Small Rumin Res. 1993;9(4):331–340. doi: 10.1111/j.1439-0442.1993.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Zantopoulos N, Antoniou V, Petsaga V, Zdragas A. Copper concentrations in sheep liver and kidney in Greece. Vet Hum Toxicol. 1996;38(3):184–185. [PubMed] [Google Scholar]