Abstract
Purpose
Vitamin D deficiency has been proposed to be a risk factor in the pathogenesis of uterine fibroid in few recently published studies conducted in Europe and Africa. Nevertheless, no study has ever addressed similar query in Indian women where hypovitaminosis is very common.
Methods
A total of 144 women of age group 20–50 years belonging to Jamshedpur, Jharkhand, India, were included in the study. Out of which, 72 women had uterine fibroids and rest healthy women without fibroids served as controls. All women were subjected to ultrasound examination of uterus followed by measurement of serum FSH level (on 3rd day of menstruation) and serum vitamin D3.
Results
The mean serum concentration of vitamin D3 was significantly lower in women with uterine fibroids compared to controls (10.81 ± 6.18 vs. 22.91 ± 16.18, p < 0.0001). On further analysis, 62.5% of cases were found to be severely deficient (vitamin D3 < 10 ng/mL) as compared to 26.39% of controls (p < 0.0001). Besides that, only 2.77% of cases had sufficient vitamin D level as compared to 23.61% of controls (p = 0.0002). The odds ratio (OR) of occurrence of fibroid with serum vitamin D3 level of < 10 ng/dl compared to that of level > 10 ng/dl was 4.64 (95% confidence interval [CI] 2.28–9.44) (p = 0.0001).
Conclusion
Serum vitamin D3 level inversely correlated with burden of uterine fibroid and possibly its deficiency is a risk factor for uterine fibroid occurrence in eastern part of India.
Keywords: Vitamin D deficiency, Risk factor, Uterine fibroid
Background
Uterine fibroid is a benign tumour of uterine smooth muscle and a common aetiology for menorrhagia and dysmenorrhoea in women of reproductive age group [1]. In India, the incidence is high and it inflicts a heavy burden on women’s health and health care system.
The etiopathogenesis of fibroid is multifactorial. Hormonal factors play a role in pathogenesis, but other factors that lead to the development of fibroids are still elusive [2].
Vitamin D3 deficiency has been suggested to be a risk factor in many chronic conditions like cardiovascular disease, autoimmune disease and also in several types of cancers [3]. Interestingly, it is also implicated in the pathogenesis of uterine fibroid in few recently conducted studies in Europe and Africa [4–6].
The biological effects of vitamin D3 have been extensively studied. Essentially, it exerts its effects via activation of its cellular receptor (VDR), which in turn alters transcription rate of target genes responsible for various biological responses. This includes reduction in cell proliferation and regulation of biological processes including angiogenesis, extracellular matrix production and immune response [7, 8]. Not surprisingly, vitamin D3 receptor (VDR) is present in wide range of tissues including both myometrium and endometrium of human uterus and also expressed in uterine fibroid tissue [9, 10]. It has been suggested that uterine fibroids develop because of aberrant response to tissue repair leading to altered extracellular matrix production and vitamin D might suppress this abnormal response by regulating this response [11]. This was confirmed by a study on Eker rat, which has demonstrated significant reduction in fibroid size after treatment with 1,25-dihydroxyvitamin D3 [12].
Hypovitaminosis including vitamin D deficiency is very common in Indian women [13]. However, there is no such study in Indian population to find out this potential factor as aetiology in the development of uterine fibroid. Hence, we took up this study to assess serum levels of vitamin D3 in women with fibroids in our population taking healthy women as control.
Methodology
This study was designed as a cross-sectional observational study after approval of the institutional ethics committee of Tata Main Hospital, Jamshedpur, India. The study population included women between 20 and 50 years of age visiting outpatient department (Obstetrics and Gynaecology), Tata Main Hospital from 1 November 2014 to 31 May 2016. Women with at least one uterine fibroid of 2 cm3 in volume or larger in transvaginal ultrasound (TVS) along with serum follicle stimulating hormone level < 10 mIU/mL measured on day 3 of their menstrual cycle were eligible as cases. Control subjects were recruited from women of similar age group as cases with normal uterus on ultrasound examination. Women with history of pregnancy or miscarriage within last 6 months, and women currently on hormonal therapy or vitamin supplements were not included in the study. Patients with chronic diseases like hypertension, diabetes, autoimmune disorders, coronary, hepatic, or renal diseases were also excluded from both study groups. A written informed consent was obtained from all patients.
After a brief history and physical examination, all recruited patients underwent transvaginal ultrasound (TVS) using Hitachi ALOKA IPF 1506 ultrasound machine equipped with a 5-MHz transvaginal probe. Parameters like uterine size (in three perpendicular planes), number of fibroid lesion, volume of all fibroid lesions (by Prolate Ellipse formula = a × b × c × 0.523 where a is height, b is width and c is depth) were noted down. Patients in whom TVS was not sufficient to evaluate fibroid lesion in their entirety, especially in case of large fundal fibroid, transabdominal sonography (TAS) was performed. Blood sample was collected from all patients to measure serum FSH and serum vitamin D 25 (OH) D3 level. Both these parameters were measured by automated chemiluminescent immunoassay (CLIA) technology.
Statistical analysis was carried out using “Medcalc” software. Numerical data were expressed as mean ± standard deviation, and categorical data were expressed as relative frequency and percentage. Statistically significant differences were determined using Chi-square test, Fisher’s exact test, or Student’s t test as appropriate. The Pearson correlation coefficient was used to measure the strength of a linear association between two variables. A p value < .05 was considered statistically significant.
The sample size was calculated from serum vitamin D3 level in controls as 21.7 ± 8.56 based on data from a previous study [14], and it was hypothesized that a decrease of 20% of this value in women with uterine fibroid would be clinically significant. Considering alpha error of 5% and power of study 80%, a calculated sample would have been a minimum of 122 patients with 61 patients in each group. However, we included all eligible 72 patients visited to our hospital during the study period along with 72 matching controls.
Results
Baseline parameters of both case and control groups were comparable except a statistically significant higher BMI was noticed in women with fibroid (Table 1). Most of the patients were multiparous in both groups. Menorrhagia (47.22%) was the most common presenting complaint followed by pain in abdomen (26.38%) and dysmenorrhea (13.88%) in women with fibroid.
Table 1.
Baseline parameters of cases with fibroid and control
| Parameters | Cases of fibroid (n = 72) | Controls (n = 72) | p value |
|---|---|---|---|
| Age (years) | 41.79 ± 4.91 | 42.18 ± 5.37 | 0.649 |
| BMI (kg/m2) | 27.18 ± 3.68 | 25.92 ± 3.51 | 0.037 |
| Parity (%) | |||
| P0 | 6.94% | 6.94% | 0.93 |
| P1 | 19.44% | 13.89% | |
| P2 | 38.89% | 43.06% | |
| ≥ P3 | 34.72% | 36.11% | |
| Age at menarche (years) | 12.40 ± 1.03 | 12.64 ± 1.03 | 0.164 |
| Day 3 serum FSH (IU/mL) | 6.06 ± 2.12 | 6.28 ± 1.98 | 0.521 |
| Demographic distribution | |||
| Urban (%) | 70.83% | 68.06% | 0.856 |
| Rural (%) | 29.17% | 31.94% |
Serum levels of 25-hydroxyvitamin D3 were significantly lower in women with fibroids than in controls (Table 2). Severe deficiency of vitamin D3 (< 10 ng/dl) was noticed in 62.5% of women with fibroids and 26.39% in controls. Moreover, only 2.77% of women with fibroids had sufficient (> 30 ng/dl) serum levels of vitamin D3 as compared to 23.61% in controls. The odds ratio (OR) of occurrence of fibroid with serum vitamin D3 level of < 10 ng/dl compared to that of serum vitamin D3 level > 10 ng/dl was 4.64 (95% confidence interval [CI] 2.28–9.44) (p = 0.0001).
Table 2.
Serum vitamin D3 level in cases with fibroid and control
| Parameters | Cases of fibroid (n = 72) | Controls (n = 72) | p value |
|---|---|---|---|
| Serum level (ng/mL) | 10.81 ± 6.18 | 22.91 ± 16.18 | < 0.0001 |
| Serum vitamin D level categories n (%) | |||
| Severe deficiency (< 10) | 45 (62.5) | 19 (26.39) | < 0.0001 |
| Deficiency (10–20) | 22 (30.56) | 19 (26.39) | 0.579 |
| Insufficient (20–30) | 3 (4.16) | 17 (23.61) | 0.007 |
| Sufficient (> 30) | 2 (2.77) | 17 (23.61) | 0.0002 |
Further analysis of cases in terms of fibroid number and size was performed in relation to serum vitamin D3 level to explore possible association. In women with number of fibroids more than three, the serum vitamin D3 level was lower in comparison with women with fibroids less than three. However, the result was not statistically significant (9.36 ± 5.77 vs. 11.24 ± 6.22; p = 0.26). We failed to find out any correlation between volume of the largest fibroid and serum vitamin D3 level (Table 3; Fig. 1).
Table 3.
Serum vitamin D3 and number of fibroids
| No. of fibroids | No. of patients | Level of serum vitamin D (ng/mL) (mean ± SD) | p value |
|---|---|---|---|
| < 3 | 55 | 11.24 ± 6.22 | 0.26 |
| ≥ 3 | 17 | 9.36 ± 5.77 |
Fig. 1.
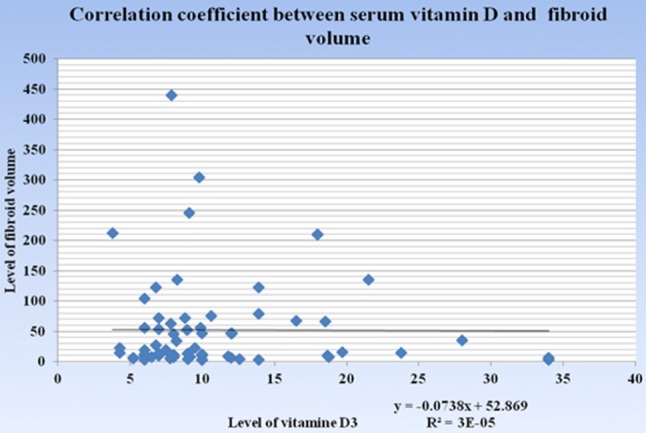
For correlation “Karl Pearson correlation coefficient” was used. r = − 0022: there is no correlation
Discussion
Our study demonstrated significantly lower serum vitamin D3 level in women with fibroid as compared to control population (10.81 ± 6.18 vs. 22.91 ± 16.18, p ≤ 0.0001). Furthermore, the relative odd of the presence of fibroid in a women with vitamin D3 level < 10 ng/dl was 4.64 (95% confidence interval [CI] 2.28–9.44) (p = 0.0001). This finding connotes a possible inverse correlation between serum vitamin D3 and uterine fibroid in the present study population which corroborates the results of the studies conducted on different populations outside India.
A study by Paffoni et al. [6] who investigated similar research query in 126 women with fibroid and 256 controls visiting two infertility clinics in Italy. They found lower mean serum concentration of 25-hydroxyvitamin D3 in women with fibroid compared to controls (18.0 ± 7.7 vs. 20.8 ± 11.1 ng/mL, respectively, p = .010). Moreover, they reported that the crude odds ratio (OR) for the presence of fibroids in women with serum levels of 25-hydroxyvitamin D3 below 10 ng/mL compared with those with 25-hydroxyvitamin D3 > 10 ng/mL was 2.2 (95% confidence interval [CI] 1.1–4.3) (p = .022). Another cross-sectional study conducted in Egypt by Sabry et al. [4] inducted 104 women with fibroid and 50 controls without the disease. They similarly reported lower mean serum vitamin D3 concentration among cases with fibroid (19.7 ± 11.8 vs 22.3 ± 6.5 ng/mL, p = .01). The National Institute of Environmental Health Sciences (NIEHS) Uterine Fibroid Study enrolled 1036 women from Washington DC who belonged to the age group of 35–49 from 1996 to 1998. A retrospective analysis of the data by Baird and colleagues reported that women with sufficient vitamin D3 (> 20 ng/mL) had an estimated 32% lower odds of fibroids compared with those with vitamin D insufficiency (adjusted odds ratio [aOR] 0.68, 95% confidence interval [CI] 0.48–0.96) [5]. So, all these studies conducted in different geographical locations confirm our study hypothesis. However, in contrast to most of these studies, serum vitamin D3 level is significantly lower in women with fibroid in our study.
On categorical analysis of vitamin D levels, we found that 62.5% of women with fibroid are associated with severe deficiency (< 10 ng/dl) as compared to 26.9% in controls. Moreover, only 6.94% of women with fibroid as compared to 47.22% of controls have vitamin D level > 20 ng/dl. Similar analysis performed by Paffoni et al. [6] in their study revealed that 15% of women with fibroid had severe deficiency as compared to 7% in controls and sufficient vitamin D level was found in 37% of cases as compared to 45% in controls. So the correlation appears to be much stronger in our study as compared to the study by Paffoni et al. as more number of women with fibroid in our study have severe vitamin D3 deficiency compared to controls.
Like previous studies, we measured 25-hydroxyvitamin D3 to monitor serum vitamin D level. The advantage is that it represents the total body vitamin D from dietary intake, sunlight exposure and peripheral conversion of vitamin D. On the flip side, it has a shorter half life of 15 days [15]. Therefore, its level as a causative factor in the development of fibroid can be erroneous. However, studies found that baseline serum vitamin D3 level remains stable over long period of time (i.e. person with a particular serum level tends to remain constant during multiple years of follow-up) [16, 17].
Cross-sectional designed studies like ours are prone to have confounders which can be the biggest deterrent in drawing firm inferences. We tried to address some of the risk factors associated with the development of fibroid like age, parity and BMI by carefully choosing controls. The possibility of reverse causation is also an issue to be dealt with. However, uterine fibroid causing deficiency of vitamin D is hard to explain.
Another pertinent question, which needs to be answered, is whether vitamin D deficiency is implicated in the development or growth of the fibroid. Our data did not reveal any significant correlation between the fibroid number and size with serum vitamin D3 level, which indirectly suggested its role in both development and growth of fibroid. In contrast, Paffoni reported that the vitamin D deficiency correlated more with the number than the size of the fibroid, suggesting its implication more with the development than the growth of fibroid [6].
The best way to confirm a definite causal association would have been to identify women with vitamin D deficiency and follow them regularly to see how many of them actually develop uterine fibroid. This type of exhaustive analytical study may take many years and difficult to achieve.
Moreover, it is also vital to find out whether or not vitamin D supplementation will help in regression of fibroid. In this regard, Halder and colleague reported reduction in the size of uterine fibroid in the Eker rat model after vitamin D3 supplementation [12]. Some in vitro studies have also found a dose-dependent inhibitory effect of Vitamin D on human fibroid cell growth [18, 19]. However, we did not find any in vivo human studies testing this hypothesis.
The pathophysiological explanation of effect of vitamin D is based on the fact that the active form (1,25 dihydroxy vitamin D3) is a strong growth inhibitor [20]. It suppresses tissue proliferation, induces cell differentiation and apoptosis by regulating biological processes like angiogenesis, extracellular matrix production and immune response. Its effect is exerted via activation of cellular receptor (VDR), present in wide variety of tissues including both myometrium and endometrium of human uterus [9]. Not surprisingly, it is also expressed in uterine fibroid tissues [10]. One of the plausible explanations towards fibroid development is altered extracellular matrix production due to aberrant response to tissue repair. Vitamin D might suppress this abnormal response by regulating the extracellular matrix production. In addition, studies have speculated that it inhibits catechol-O-methyl transferase an enzyme supposed to be overexpressed in human uterine fibroid leading to suppression of growth of fibroid cells [18].
In India, vitamin D deficiency is in epidemic proportion and females suffer the most. Dietary deficiency appears to be the primary aetiology [13]. Moreover, the incidence of uterine fibroid in reproductive age group is around 37% as reported by a study in South India [21]. This number in all probabilities might be higher as many more are undetected. So implication of vitamin D deficiency in uterine fibroid will bring in a whole new therapeutic aspect and it will impart a huge impact in the outcome.
Our study showed a definite indirect association of vitamin D deficiency and uterine fibroid in this part of India. This opens up an interesting facet in prevention as well as treatment of uterine fibroid in women of child bearing age. However, further studies are warranted in this regard.
Dr. Vinita Singh
is currently working as HOD and Head Consultant in the Department of Obstetrics and Gynaecology at Tata Main Hospital, Jamshedpur, Jharkhand. She did her M.D. in Obstetrics and Gynaecology from Ranchi University in 1993 and MRCOG from UK in 1997. She attained her FRCOG (London) in 2010. Her special interest lies in Gynaecological endoscopy. Her other passion lies in teaching, training and guiding young doctors. She was the president of Jamshedpur Obstetrics and Gynaecology Society (JOGS) in 2007–2008. She did her fellowship in Day Care Gynaecological Endoscopy in 2013.
Conflict of interest
Dr Vinita Singh, Dr Archana Barik and Dr Nadia Imam declare that they have no conflict of interest.
Informed Consent in Studies with Human Subjects
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Footnotes
Vinita Singh MD FRCOG is currently working as HOD and Head Consultant in the Department of Obstetrics and Gynaecology at Tata Main Hospital, Jamshedpur, Jharkhand; Archana Barik DNB Associate Specialist in the Department of Obstetrics and Gynaecology at Tata Main Hospital, Jamshedpur, Jharkhand; Nadia Imam DNB Resident at Tata Main Hospital, Jamshedpur, Jharkhand.
References
- 1.Khyade RL. A study of menstrual disturbance in cases of fibroid uterus. Int J Reprod Contracept Obstet Gynecol. 2017;6:2494–2497. doi: 10.18203/2320-1770.ijrcog20172338. [DOI] [Google Scholar]
- 2.Schwartz SM, Marshall LM, Baird DD. Epidemiologic contributions to understanding the etiology of uterine leiomyomata. Environ Health Perspect. 2000;108(suppl 5):821–827. doi: 10.1289/ehp.00108s5821. [DOI] [PubMed] [Google Scholar]
- 3.Makariou S, Liberopoulos EN, Elisaf M, et al. Novel roles of vitamin D in disease: what is new in 2011? Eur J Intern Med. 2011;22:355–362. doi: 10.1016/j.ejim.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Sabry M, Halder SK, Allah AS, et al. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health. 2013;5:93–100. doi: 10.2147/IJWH.S38800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird DD, Hill MC, Schectman JM, et al. Vitamin D and the risk of uterine fibroids. Epidemiology. 2013;24:447–453. doi: 10.1097/EDE.0b013e31828acca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paffoni A, Somigliana E, Vigano’ P, et al. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab. 2013;98(8):E1374–E1378. doi: 10.1210/jc.2013-1777. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Fleet JC, DeSmet M, Johnson R, et al. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vienonen A, Miettinen S, Blauer M, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11(2):104–112. doi: 10.1016/j.jsgi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Feng L, Jayes FL, Jung SH, et al. Vitamin D receptor (VDR) is over-expressed in the center of uterine fibroids. Fertil Steril. 2010;94(4):S75. doi: 10.1016/j.fertnstert.2010.07.291. [DOI] [Google Scholar]
- 11.Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod. 2012;86:116. doi: 10.1095/biolreprod.111.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A. Vitamin D deficiency in India: prevalence, causalities and interventions. Nutrients. 2014;6(2):729–775. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta D, Maisnam I, Shrivastava A, et al. Serum vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J Med Res. 2013;138(6):853–860. [PMC free article] [PubMed] [Google Scholar]
- 15.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582–586. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 16.Jorde R, Sneve M, Hutchinson M, et al. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 17.Kluczynski MA, Wactawski-Wende J, Platek ME, et al. Changes in vitamin D supplement use and baseline plasma 25-hydroxyvitamin D concentration predict 5-year change in concentration in postmenopausal women. J Nutr. 2012;142:1705–1712. doi: 10.3945/jn.112.159988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharan C, Halder SK, Thota C, et al. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-omethyl transferase. Fertil Steril. 2011;95:247–253. doi: 10.1016/j.fertnstert.2010.07.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bläuer M, Rovio PH, Ylikomi T, et al. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril. 2009;91:1919–1925. doi: 10.1016/j.fertnstert.2008.02.136. [DOI] [PubMed] [Google Scholar]
- 20.Ylikomi T, Laaksi I, Lou YR, et al. Antiproliferative action of vitamin D. Vitam Horm. 2002;64:357–406. doi: 10.1016/S0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 21.Munusamy MM, Sheelaa WG, Lakshmi VP. Clinical presentation and prevalence of uterine fibroids: a 3-year study in 3-decade rural South Indian women. Int J Reprod Contracept Obstet Gynecol. 2017;6:5596–5601. doi: 10.18203/2320-1770.ijrcog20175288. [DOI] [Google Scholar]


