Abstract
Objective
This study was conducted at Swapna Health Care (a tertiary centre for endoscopy), to analyse the outcome of hysteroscopic resection of submucous fibroids in 125 symptomatic women.
Materials and Methods
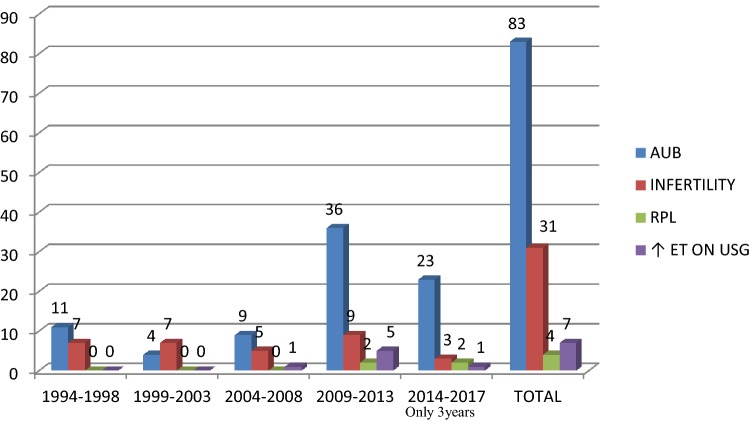
A total of 125 women were diagnosed with submucous fibroids between 1994 and 2017, 83 presented with AUB, 31 with infertility and four with RPL. Seven postmenopausal women had thickened endometrium on routine transvaginal ultrasonography, and hysteroscopy showed submucous fibroids.
Results
Out of 83 with AUB, 68 were premenopausal and 15 were postmenopausal. All these patients underwent submucous fibroid resection by hysteroscopy. Out of 83, 75 (90.4%) had total relief from AUB and did not need any further management. Only eight patients needed hysterectomy after submucous fibroid resection. Thirty-one patients came for infertility. Twenty-five patients followed infertility treatment with us after SMFR. 32% of our infertile patients conceived (8/25).
Conclusions
Submucous fibroid resection is a safe procedure, giving good results in symptomatic women with AUB and infertility. Hence, all gynaecologists should be trained in diagnostic and operative hysteroscopy and should be able to offer this option to their patients in the spectrum of choices available.
Keywords: Hysteroscopic resection of submucous fibroid, Abnormal uterine bleeding, Postmenopausal bleeding, Infertility
Introduction
Uterine fibroids are a common concern in women, and submucous fibroids are especially known to cause multiple symptoms like menorrhagia, infertility, recurrent pregnancy loss and pain. Submucous fibroid resection by hysteroscopy is a safe procedure with low morbidity and is a good option to treat women with abnormal uterine bleeding and infertility.
Material and Methods
This study was conducted to analyse the outcome of resection of submucous fibroids (SMFs) at a tertiary care endoscopy training centre specialized in operative hysteroscopy and operative laparoscopy. Retrospective analysis was done in 125 symptomatic women who underwent submucous fibroid resection (SMFR) by hysteroscopy between 1994 and 2017 (Fig. 1).
Fig. 1.
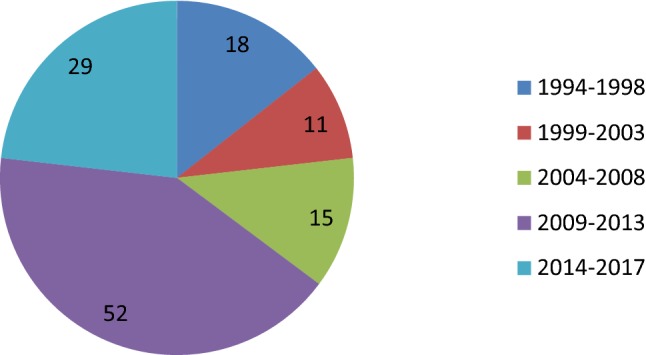
Four-yearly distribution of cases of SMFR
They presented with different symptoms, like abnormal uterine bleeding (AUB), infertility and recurrent pregnancy loss (RPL). A few postmenopausal women with abnormal thickening of endometrium on routine ultrasonography turned out to be SMF on hysteroscopy were included.
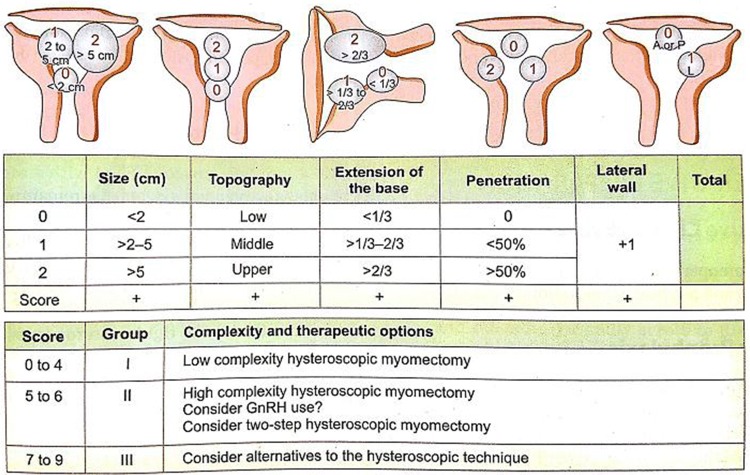
Pre-operative evaluation was done mostly by transvaginal ultrasonography (TVUS). Location, size, extent of myometrial penetration and distance from serosa were noted by mapping the fibroids. MRI was needed only in a few cases where there were multiple fibroids and high vascularity. They were classified according to sizes and myometrial penetration (ESGE classification).
Another more detailed system of evaluation of submucous fibroids (STEPW classification) is the one proposed by Lasmar in 2005. This system includes the depth of penetration of the myoma, largest myoma diameter, extension of myoma to endometrial cavity surface and location along the uterine wall (Fig. 2).
Fig. 2.
STEP W classification of submucous fibroid
This is important since the volume of tissue to be resected increases exponentially with increase in diameter of the myoma from 0.52 cc for a 1-cm myoma to 65 cc for a 5-cm myoma. This in addition to factors such as increased myometrial penetration increases the time of surgery and fluid absorption along with risk of complication such as fluid overload, electrolyte disturbances and uterine perforation (Fig. 3).
Fig. 3.
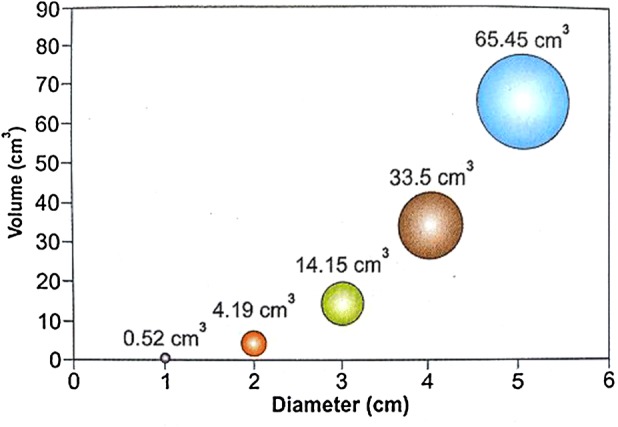
Volume–diameter relationship of fibroids
They all underwent SMFR by hysteroscopy. Sixteen of them had simultaneous laparoscopy for pelvic evaluation before ART, or removal of co-existing subserous and intramural fibroids. Concomitant laparoscopy was done when there were associated big intramural and/or subserous fibroids, when other pelvic pathologies like chocolate cysts, endometriosis, pelvic adhesions, PCOS requiring ovarian drilling were present. Also done as a part of pelvic evaluation in infertility, it was omitted in all other cases where SMF was type 0 and there was no other pelvic pathology.
All SMFRs were performed under GA, using Karl-Storz resectoscope. In the initial 50 cases, monopolar wire loop was used with glycine as distension medium, and in remaining 75 cases, bipolar loop was used with normal saline as distension medium. As soon as the bipolar electrosurgical unit was made available in our institution, we switched over from monopolar to bipolar instruments for resection and from glycine to normal saline as distension medium. The bipolar energy is quoted to have an advantage over monopolar as the electric arc is formed only in the loop and does not pass through the patient’s tissues to reach the neutral electrode. In our series, there was no difference in the complication rate or surgical outcome between the two modes. The duration of operation was 20 min to 3 h 20 min with a mean of 1 h 20 min. Longer durations were when big fibroids or multiple fibroids were removed by simultaneous laparoscopy. Average hospital stay was 12–24 h. The mean follow-up was for a period of 36 ± 12 months.
Results
The age groups of patients in our study ranged from 18 to 76 years. Only one patient was under 20 years (0.8%), 31 between 21 and 30 years (24.8%), 48 between 31 and 40 years (38.4%), 25 between 41 and 50 years (20%), 18 patients were > 50 years (16.0%). The maximum number of patients (48) was in the age group of 31–40 years.
Eighty-three women presented with AUB, [including 15 with postmenopausal bleeding (PMB)], 31 with infertility, four with RPL and seven postmenopausal women with routine screening TVUS findings of thickened endometrium (Fig. 4).
Fig. 4.
Distribution of cases based on symptoms—4-yearly study
The SMFs were classified according to their sizes and myometrial penetration (Table 1).
Table 1.
Grading according to the size of SMF
| Size of SMF | No. of cases | % |
|---|---|---|
| 1.0–2.9 cm | 82 | 65.6 |
| 3.0–4.9 cm | 31 | 24.8 |
| ≥ 5 cm | 6 | 4.8 |
| Multiple SMFs (1–5 cm) | 6 | 4.8 |
Grading was also done according to ESGE classification. In 91 patients, the fibroids were completely intracavitary (72.8%), in 25, the myometrial penetration was less than 50% (20%) and in 9, it was more than 50% (7.2%).
Seventy-one of the patients had abdominal scars, including caesarean scars (27), laparoscopy (17), laparotomy (4), tubectomy (22) and cholecystectomy (1).
A second sitting of hysteroscopy for resection of remnant fibroid was required in three out of six patients when fibroids were bigger than 5 cm. We found co-existing pathologies like polyps (21), septum (3) and polypoidal endometrium (8).
Out of 83 patients with AUB, 75 had good relief (90.4%); out of these 75, 72 did not require any further treatment and three had Mirena insertions. Only eight out of 83 underwent hysterectomy (9.6%). Out of eight hysterectomies, five were from premenopausal and three from PMB group. The seven postmenopausal women with abnormal TVUS, who had SMFR, did not need any further treatment after resection. One of them had hysterectomy for prolapse after several years.
Thirty-one patients came with infertility. Out of them, six were referred back to the doctor who sent them for hysteroscopic surgery. The rest of 25 patients followed treatment for infertility with us. In 25 patients, 17 were primary infertility and eight were secondary infertility. In primary infertility group, seven conceived (41%). Five delivered at term, one had termination of pregnancy in view of chickenpox in first trimester and one had embryonal demise. Eight patients came for secondary infertility, and one of them conceived (12.5%) and delivered at term gestation. Out of four patients with RPL, two conceived (50%), one delivered at term and one had embryonal demise.
Of the 16 cases in whom concomitant laparoscopy was done, four were diagnostic, seven had big, or type I and II, or multiple fibroids; three had chocolate cysts and five had polycystic ovaries. Seven had laparoscopic myomectomy, three had chocolate cystectomy and five had ovarian drilling. These included cases of AUB as well as infertility.
The complications reported during SMFR in the literature are perforation, haemorrhage, blood transfusion, electrolytic imbalance, anaphylaxis, pulmonary oedema, DIC, infection and unplanned conversion to laparoscopy/laparotomy. In our series, there were no anaesthetic complications and no unplanned conversions. One patient in our series needed one unit of blood transfusion. She had a big SMF occupying the entire cavity, highly vascular, and there was excessive bleeding during the procedure.
Discussion
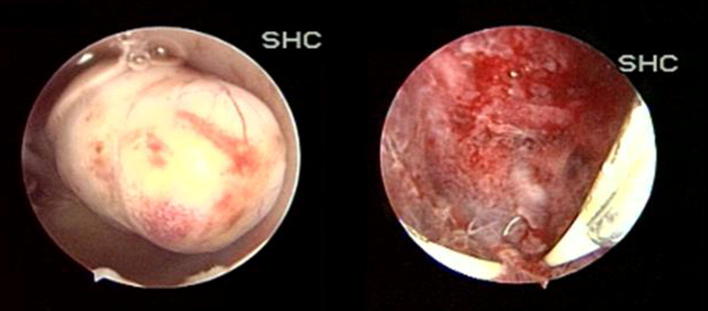
SMFs being adjacent to the endometrium are usually associated with symptoms like AUB, infertility and RPL (Fig. 5).
Fig. 5.
Submucous fibroid and cavity at the end of resection
SMFR by hysteroscopy is the least invasive surgical approach for fibroid removal. It is most effective for type ‘0’ and type ‘1’ fibroids [1].
AUB
The mechanisms by which SMFs cause AUB are unclear. It seems likely that in most instances, mechanical (or) molecular mechanisms involved in endometrial haemostasis are disturbed. In a minority of cases, perimyoma vasculature is likely the source of bleeding [2].
Out of 83 patients with AUB, 75 (90.4%) had total relief and did not need any further management. Only eight (9.6%) patients needed hysterectomy, of which five were from premenopausal and three were from PMB group (Fig. 6).
Fig. 6.
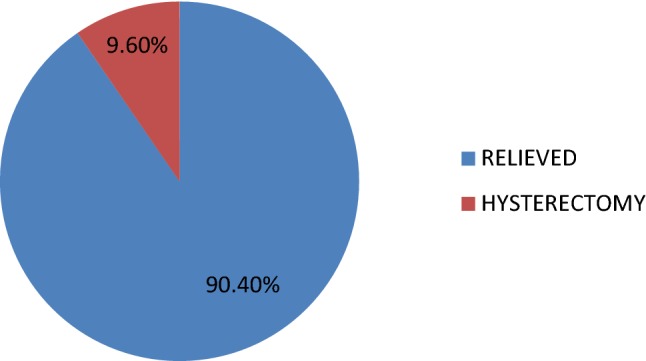
AUB follow-up
A separate analysis of pre- and postmenopausal AUB with SMF followed by SMFR was done (Table 2). Out of 68 patients with premenopausal AUB, only five (7.35%) needed hysterectomy due to failure of conservative management and 63 (92.64%) had total relief. Out of 15 patients with PMB, three (20%) needed hysterectomy. Reasons for hysterectomy were: recurrent PMB in two and endometrioid adenocarcinoma grade III in one. All of them had hysterectomy with bilateral salpingo oophorectomy, and the patient with cancer endometrium had bilateral pelvic lymph nodal dissection in addition. Out of 15 in PMB group, 12 (80%) had total relief. Thus, total relief was very good in 92% of premenopausal and 80% of PMB group.
Table 2.
Reasons for hysterectomy in AUB group
| Group | Number | % | Indications |
|---|---|---|---|
| Premenopausal | 5/68 | 7.35 | Recurrence of AUB (5) (no recurrence of SMF) |
| PMB | 3/15 | 20 | Cancer endometrium (1) Recurrent PMB (2) |
| Total | 8/83 | 9.6 |
Every postmenopausal woman with SMF does not need hysterectomy and 80% still had complete relief with SMFR, and major surgery could be avoided at this age. Total relief was still better in premenopausal women (> 92%).
Seven patients in our series were postmenopausal women in whom routine screening TVUS showed thickened endometrium. Diagnostic hysteroscopy was done for the assessment of thickened endometrium, and once a SMF was seen, resection was done. None of these patients had a recurrence. One patient had hysterectomy later for a uterine prolapse.
The use of concomitant transcervical endometrial ablation (TCEA) at the time of SMFR has been suggested as a method for improving the chance of success in treating heavy menstrual bleeding for women who do not desire future fertility. A retrospective comparison of outcomes of women undergoing SMFR only with those of women undergoing combined SMFR and TCEA showed a higher success rate when ablation was added to the procedure according to some studies [3].
We did not combine TCEA with SMFR in any of our cases, and SMFR alone gave good relief without concomitant TCEA.
Ahmed Namazov et al. [4] and Pratikshagupta et al. [5] also reported only hysteroscopic resection of SMF without endometrial ablation and subsequent hysterectomy rates. Our study is compared with Namazov et al., Pratikshagupta et al. and Loffer et al. [3], and our results are comparable to both groups (Table 3).
Table 3.
Results of SMFR versus SMFR + TCEA
Hysterectomy rates were more in Loffer’s group in spite of combining SMFR with TCEA.
Benefits of GnRH agonists should be weighed against their cost and side effects for individual patients. A Cochrane Systematic Review [6] to evaluate the role of GnRH agonist prior to either hysterectomy or myomectomy showed that pre- and post-operative haemoglobin and haematocrit were improved significantly by the use of GnRHa prior to surgery. The disadvantages of GnRH agonist include cost, menopausal symptoms, and with prolonged therapy, bone demineralization. In addition, smaller fibroids may be overlooked at the time of surgery, only to recur once GnRH agonist is discontinued (thereby increasing the apparent risk or recurrence of fibroids following myomectomy) [7]. We did not use GnRH agonist in any of our patients pre-operatively. The complications quoted in the literature are perforation, excessive fluid absorption, haemorrhage, thermal burns and adhesions [2].
The incidence of intrauterine adhesions after SMFR by hysteroscopy has been reported in one study as 7.5% [8]. Post-operative adjuvant therapies like oestrogen for 4–8 weeks, insertion of intrauterine device, paediatric Foley’s catheter have all been tried, but there is scant evidence to support these [1]. We did not use any of these post-operative measures in our patients. No intrauterine adhesions were noted in our series. One patient in our series needed one unit of blood transfusion. She had a big SMF occupying the entire cavity, highly vascular, and there was excessive bleeding during the procedure.
Infertility
The mechanisms by which SMF impact fertility are, at the present time, unclear. However, there is evidence that such lesions may contribute to a global molecular impact that inhibits the ‘receptivity’ of the endometrium to implantation as determined by the presence of the transcription factors HOXA-10 and -11. Investigators have demonstrated that there is a reduction in the levels of endometrial HOXA-10 and -11 expression, both over the myoma and remotely in the endometrium overlying normal myometrium that is not seen in the endometrium of women with intramural or subserosal myomas [9].
Other hypotheses are reduced blood flow in fibroids compared to adjacent myometrium and alteration of uterine contractility interfering with sperm and ovum interaction (or) embryo migration [1].
Out of the 31 patients who came for infertility, many had multiple other factors. Four underwent simultaneous laparoscopic myomectomy, three had chocolate cystectomy, five had ovarian drilling, one had septal resection and three had polypectomy along with SMFR. 32% of our infertile patients conceived (eight out of 25). Six of them had only SMF as the cause of infertility and the other two had multiple factors like intramural fibroids, pcos, endometriosis, uterine septum and polyp, etc. (Fig. 7).
Fig. 7.

Conceptions in infertility following SMFR
We have compared our study with Jayakrishnan et al. who analysed effects of SMFR and factors influencing the outcome of infertility. When the SMF was the only reason for infertility, conception rates were 33% in our group and 40% in Jayakrishnan et al. group. When the infertility was multifactorial, the conception rates were 28.6% in our group and 22.7% in Jayakrishnan et al. [10] group. Conception rates were better when the reason for infertility was SMF alone in both groups. The presence of other factors for infertility adversely affects the reproductive outcome.
We have compared our pregnancy rates after the procedure with those reported by various other authors. 32% of our infertile patients conceived after SMFR (eight out of 25), which is comparable with the results of other authors (Table 4).
Table 4.
Reproductive outcome following SMFR in comparison with other authors
All these studies show that SMFs are associated with infertility and their resection definitely improves the chances of pregnancy.
Regarding conceptions, we analysed our conception rates according to the ESGE classification and compared them with study by Jayakrishnan et al. [10].
There were no conceptions in type ‘2’ in both groups, more in type ‘1’ in our study and more in type ‘0’ in other study. Six out of eight (75%) pregnancies continued till term and delivered. One (12.5%) had embryonal demise at 8 weeks gestational age, and medical termination of pregnancy was done in one (12.5%) due to chickenpox in first trimester (Table 5).
Table 5.
Conceptions according to the type of fibroids (ESGE classification)
Recurrent Pregnancy Loss
In the meta-analysis of Pritts et al. [14], there were significantly higher spontaneous abortion rates in women with SMF that seemed to vanish after SMFR.
We had only four patients with RPL in our series, and of these, two conceived after SMFR. One had a term delivery, and the other had embryonal demise. The number is too small for analysis and conclusions.
Twenty-seven patients in our series had LSCS scar in the uterus, and 71 had an adnominal scar. Concomitant laparoscopy was done in 16 patients. There is no need to perform laparoscopy as control for every case of SMFR, provided the pre-operative classification is done correctly. Only a fraction of them need to have laparoscopy when there are other issues that need to be addressed. There were no uterine scar perforations or injuries during laparoscopic entry.
Conclusion
SMFR is a safe procedure, giving good results in symptomatic SMFs associated with AUB and infertility. In women with AUB, it helps to conserve the uterus and replaces a major surgery like hysterectomy with a day care procedure, with minimum complications. In postmenopausal women with SMF with or without PMB, when previously hysterectomy was the only solution available, SMFR effectively avoids a major surgery at an advanced age. In women with infertility, the conception rates were good when the reason was SMF alone.
Many of our patients had uterine and abdominal scars, and there were no incidents of uterine perforation or organ injury during laparoscopic entry.
Hence, all gynaecologists should be trained in diagnostic and operative hysteroscopy and should be able to offer this option to their patients, in the spectrum of choices available.
Dr. K. S. Yendru
D.N.B., D.G.O., Dip. in Gyn End. Surg (Germany), F.I.C.O.G, is a Senior Consultant Obstetrician, Gynaecologist and Laparoscopic Surgeon, Director of Units of Laparoscopy, Hysteroscopy and Colposcopy, Clinical director of Department of Obstetrics and High-Risk Pregnancy, Swapna Health Care, Hyderabad, Telangana. She is also secretary of Indian Society of Perinatology and Reproductive Biology, Hyderabad Chapter, since 4 years. She has published several papers, and has been faculty in several conferences and organized several CMEs and workshops, including workshops in hysteroscopy, laparoscopy and colposcopy. She is faculty for the FOGSI recognized endoscopy training centre.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was taken from the patients.
Footnotes
Dr. Katyayani Swapna Yendru, D.N.B, D.G.O., F.I.C.O.G, Senior Consultant Obstetrician and Gynaecologist, Swapna Health Care, 6-3-1111/19, Nishathbagh, Begumpet, Hyderabad, Telangana, 500 016, India; Dr. Savitha Devi Yelamanchi, M.D., D.G.O.,FIMSA.,F.I.C.O.G, Senior Consultant Obstetrician, Gynaecologist and Laparoscopic Surgeon, Swapna Health Care, 6-3-1111/19, Nishathbagh, Begumpet, Hyderabad, Telangana, 500 016, India; Dr. Ganga Bhavani Vaddiraju, M.S (OBG), Senior Registrar, Department of Obstetrics and Gynaecology, Swapna Health Care, 6-3-1111/19, Nishathbagh, Begumpet, Hyderabad, Telangana, 500 016, India.
Contributor Information
Katyayani Swapna Yendru, Phone: 040-23402417, Phone: 040-47270007, Email: swapnahealthcare@gmail.com, http://www.swapnahealthcare.com/.
Savitha Devi Yelamanchi, Phone: 040-23402417, Phone: 040-47270007, Email: drsavithay@gmail.com.
Ganga Bhavani Vaddiraju, Phone: 040-23402417, Phone: 040-47270007.
References
- 1.Carranza-Mamane B, Sherbrooke QC, Havelock J, et al. The management of uterine fibroids in women with otherwise unexplained infertility. J Obstet Gynaecol Can. 2015;37(3):277–285. doi: 10.1016/S1701-2163(15)30318-2. [DOI] [PubMed] [Google Scholar]
- 2.American Association of Gynecologic Laparoscopists (AAGL): Advancing Minimally Invasive Gynecology Worldwide AAGL practice report: practice guidelines for the diagnosis and management of submucous Leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171. doi: 10.1016/j.jmig.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Loffer FD. Improving results of hysteroscopic submucosal Myomectomy for menorrhagia by concomitant endometrial ablation. J Minim Invasive Gynecol. 2005;12:254–260. doi: 10.1016/j.jmig.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed Namazov MD, Resul Karakus MD, et al. Do submucous myoma characteristics affect fertility and menstrual outcomes in patients underwent hysteroscopic myomectomy? Iran J Reprod Med. 2015;13(6):367–372. [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta P. Symptomatic submucous uterine fibroid: outcome of conservative surgery in a tertiary care hospital. Int J Reprod Contracept Obstet Gynecol. 2016;5(4):1010–1013. doi: 10.18203/2320-1770.ijrcog20160677. [DOI] [Google Scholar]
- 6.Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2000;2:CD000547. doi: 10.1002/14651858.CD000547. [DOI] [PubMed] [Google Scholar]
- 7.Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Women’s Health. 2014;6:95–114. doi: 10.2147/IJWH.S51083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touboul C, Fernandez H, Deffieux X, Berry R, Frydman R, Gervaise A. Uternine syndechiae after bipolar hysteroscopic resection of submucosal myomas in patients with infertility. Fertil Steril. 2009;92:1690–1693. doi: 10.1016/j.fertnstert.2008.08.108. [DOI] [PubMed] [Google Scholar]
- 9.Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93:2027–2034. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayakrishnan K, Menon V, Nambiar D. Submucous fibroids and infertility: effect of hysteroscopic myomectomy and factors influencing outcome. J. Hum Reprod Sci. 2013;6(1):35–39. doi: 10.4103/0974-1208.112379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vercellini P, et al. Hysteroscopic myomectomy: long term effects on menstrual pattern and fertility. Obstet Gynecol. 1999;94:341–347. doi: 10.1016/s0029-7844(99)00346-4. [DOI] [PubMed] [Google Scholar]
- 12.Bernard G, Darai E, et al. Fertility after hysteroscopic myomectomy: effect of intramural fibroids associated. Eur J Obstetr Gynaecol Reprod Biol. 2000;88:85–90. doi: 10.1016/S0301-2115(99)00123-2. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez H, et al. Hysteroscopic resection of submucosal myomas in patients with infertility. Hum Reprod. 2001;16:1489–1492. doi: 10.1093/humrep/16.7.1489. [DOI] [PubMed] [Google Scholar]
- 14.Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91:1215–1223. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]