Abstract
Exposure to chronic stress such as living in disadvantaged neighborhoods has been related to cardiovascular disease (CVD). Chronic stress may increase the risk for CVD by increasing levels of systemic inflammation (e.g., higher levels of pro-inflammatory cytokines). Differential DNA methylation of inflammation-related candidate genes is also related to higher risk for CVD. Thus, the purpose of this review was to examine the association of neighborhood disadvantage with DNA methylation. A search of literature was conducted using Scopus, CINAHL, PubMed, Medline, and Embase databases. The keywords neighborhood, neighborhood disorder, neighborhood crime, neighborhood violence, neighborhood safety, built environment, and housing vacancy were combined with the keywords DNA methylation and epigenetics. Five studies were included in this review (n = 3 adult blood samples and n = 2 fetal blood samples). Four of the five studies reported an association of neighborhood socioeconomic status, social environment, and crime with either global or gene-specific DNA methylation. Only two studies examined the association of neighborhood disadvantage with inflammation-related candidate genes. One of these studies found a significant association of neighborhood socioeconomic disadvantage and social environment with DNA methylation in inflammation-related candidate genes. Thus, data are limited on the association between neighborhood disadvantage and DNA methylation of inflammation-related candidate genes, as well as genes in other potential mechanistic pathways including psychosocial stress, toxin response, and adiposity. Future studies should examine these associations and the potential epigenetic mechanisms by which neighborhood disadvantage increases the risk for CVD.
Keywords: Neighborhood, DNA methylation, Stress, Depression, Inflammation
Introduction
Cardiovascular disease (CVD) is a growing concern in the USA. In 2016, heart disease was the number one cause of death, accounting for 23% of all deaths [1]. Furthermore, heart disease and stroke accounted for 28% of all deaths among women compared with 21% of all deaths among women due to cancer [1]. Although older age is a risk factor for CVD, including myocardial infarction, stroke, heart failure, and cardiac arrhythmias [2], in the past two decades there has been an increase in CVD among younger women (ages 35–54 years) [3]. Hypertension, high cholesterol, and diabetes increase the risk of CVD as do behaviors (e.g., unhealthy diet, obesity, and physical inactivity) [4]. Race is also a risk factor for CVD. In a recent study with 1527 participants from New York City ages 20 or older (58% women), Black women had a significantly higher risk of having hypertension and diabetes, and being overweight or obese, compared with White women, Black men, and White men (p values < 0.05) [5].
Disadvantaged neighborhoods characterized by poverty, violence, and physical and social disorder have been related to CVD. For example, using data from the Multi-Ethnic Study of Atherosclerosis (MESA), Mujahid et al. found that neighborhood-level stressors (e.g., disorder) were positively associated with hypertension after adjustment for age and gender (p < 0.001) [6]. Similarly, Claudel et al. found that among a Dallas, TX sample of 1174 participants aged 18–65 years, those living in areas of high neighborhood deprivation (e.g., poor housing conditions, poverty) had 1.69 times greater odds of developing hypertension after adjustment for covariates (OR = 1.69, 95% CI 1.02, 2.02) [7]. These results suggest that neighborhood disadvantage is related to CVD.
Neighborhood disadvantage has been related to psychological stress and depressive symptoms [8–11] which may increase the risk for CVD [12]. Vaccarino et al. found significant differences in susceptibility to myocardial ischemia triggered by psychological stress, with women ≤ 50 years showing more evidence of psychological stress-induced myocardial ischemia than men of similar ages [13]. Among a sample of young and middle-aged Black women (30–64 years old), higher levels of depressive symptoms were related to higher systolic blood pressure and waist-to-hip ratio and lower levels of high-density lipoprotein cholesterol (HDL-C) [14]. Thus, psychological stress and depressive symptoms are an emerging risk factor for CVD in young and middle-aged women which may explain the association of neighborhood disadvantage with CVD.
According to the theory of allostatic load, chronic psychosocial stress induces a maladaptive process that increases a person’s allostatic load resulting in dysregulation of the adaptive physiological systems and can result in development of disease and poor health outcomes [15–17]. The hypothalamic-pituitary-adrenal (HPA) axis produces cortisol which, under normal conditions, downregulates the body’s inflammatory responses [18, 19]. However, during chronic stress, the HPA axis becomes dysregulated and cortisol becomes less effective at suppressing inflammation, resulting in higher levels of pro-inflammatory cytokines [e.g., interleukin (IL)-6] and C-reactive protein (CRP) [20–25], which can have adverse effects on cardiovascular health and increase the risk for CVD [12, 26]. High-sensitivity CRP (hsCRP) was associated with coronary artery calcification after adjustment for CVD risk factors [e.g., body mass index (BMI)] among a sample of healthy Black women (ages 42–52), suggesting that hsCRP may play a role in development of coronary heart disease [27]. Neighborhood disadvantage is a chronic stressor that may result in allostatic overload and greater systemic inflammation. For example, low neighborhood socioeconomic status was associated with less favorable levels of CRP in a geographically diverse sample of US Black women [28]. The age-adjusted mean of CRP was 1.24 mg/dL greater in the lowest quintile of neighborhood socioeconomic status than in the highest quintile [28]. Saban et al. proposed a model based on allostatic load theory in which social context and psychological stress are associated with genome-wide as well as gene-specific epigenetic modifications that confer a pro-inflammatory epigenetic signature, which drives a heightened inflammatory state that increases risk for CVD [29].
Epigenetic changes are subject to environmental influences. DNA methylation, the most frequently studied epigenetic change, occurs in the context of sequences of nucleotide bases cytosine (C) and guanine (G) linked through a phosphodiester bond (CpG). Concentrated areas of CpGs are often though not exclusively located near transcription start sites in gene promoters and are known as CpG islands, with other CpG-rich island-flanking areas known as shores and shelves. These concentrated CpG regions in close proximity to gene promoters provide a target for epigenetic modification through the addition or removal of methyl (CH3) groups to cytosine nucleotide bases, with potential effects of attenuating or enhancing gene expression, respectively [30]. Global and gene-specific changes in DNA methylation have been correlated with the timing and severity of essential hypertension, pulmonary arterial hypertension, and atherosclerotic lesions [31]. A recent systematic review reported that differential DNA methylation at specific genes, including inflammation-related genes, is associated with coronary heart disease and atherosclerosis [32]. We propose that DNA methylation partially mediates the association of neighborhood environment with CVD (see Fig. 1). As noted above, neighborhood disadvantage has been related to higher levels of psychological stress, systemic inflammation, and risk for CVD. Further, DNA methylation has been related to risk for CVD [32]. Thus, the purpose of this review was to examine associations of neighborhood disadvantage with DNA methylation.
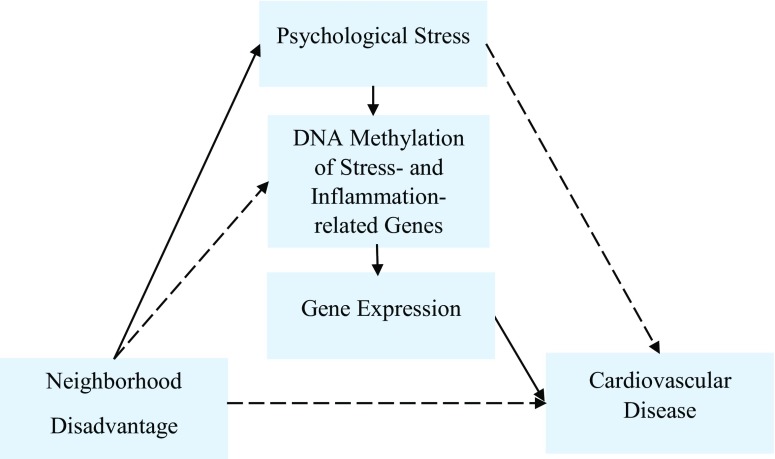
Fig. 1.
Proposed pathways between neighborhood disadvantage and cardiovascular disease. We propose that DNA methylation of stress- and inflammation-related genes may mediate the associations of neighborhood environment and psychological stress with cardiovascular disease (CVD). Continuous lines represent hypothesized pathways between neighborhood disadvantage and CVD. Dotted lines represent other potential pathways between neighborhood disadvantage and CVD (e.g., air pollution, smoking)
Search Strategy
Using Scopus, CINAHL, PubMed, Medline, and Embase databases, the first two authors searched for research studies that examined the association of neighborhood disadvantage with DNA methylation (see Fig. 2). We used the keywords neighborhood, neighborhood disorder, neighborhood crime, neighborhood violence, neighborhood safety, built environment, and housing vacancy. We combined each of these keywords with the keywords DNA methylation and epigenetics. We selected studies that measured non-chemical neighborhood stressors given that chemical neighborhood stressors (e.g., air pollution, metals) may have different pathways by which DNA methylation is influenced. We considered the socioeconomic status (e.g., poverty) and physical (e.g., vacant housing) and social (e.g., violence) neighborhood environment as potential sources of stress that may influence DNA methylation through stress- and inflammation-related pathways. We did not place any limits on years of publication in order to survey the state of science. We eliminated review articles. Five research studies examined the association of neighborhood environment with DNA methylation (see Table 1).
Fig. 2.
Search strategy. *DNAm = DNA methylation
Table 1.
Research studies on the association of neighborhood environment with DNA methylation
| First author (year) | Sample/setting | Neighborhood environment measures | Methylation target | Methylation analysis | Source/tissue | Results |
|---|---|---|---|---|---|---|
| Coker et al. (2018) |
N = 241 maternal/infant pairs who participated in the Center for Health Assessment of Mothers and Children of Salinas (CHAMACOS) Salinas, CA Age: 25.8 + 5.3 years Latina: 232 (96.2%) White: 5 (2.1%) Other: 4 (1.7%) |
2000 U.S. Census Bureau census tract for: -Percentage of homes below poverty line -Median household income -Completion of high school |
LINE-1 and Alu | Bisulfite pyro-sequencing | Umbilical cord blood | Living in the highest poverty neighborhood quartile was associated with higher cord blood LINE-1 methylation compared with living in the lowest poverty neighborhood quartile after adjustment for maternal smoking during pregnancy, maternal age, diet quality during pregnancy, years living in the USA, and prenatal urinary monobenzyl phthalate (MBzP) exposure (β = 0.78; 95% Cl 0.06, 1.50; p = 0.03). No significant differences in LINE-1 methylation by neighborhood income levels or by neighborhood educational attainment in the crude or adjusted models. No significant associations of neighborhood measures with Alu methylation. |
| Janusek et al. (2017) |
N = 34 African American emerging adult males Chicago, IL Age: 25.4 ± 2.3 years |
Survey of Exposure to Community Violence (SECV): 2 subscales [direct (victimization by violence) and indirect (witnessing of violence on another)]; self-report; exposures ranked from 0 (never exposed) to 8 (exposed every day) | IL-6 promoter region (inflammation) | Bisulfite pyro-sequencing | Peripheral blood mononuclear cells | Greater indirect exposure to neighborhood violence was a predictor of steeper rise and slower rate of decline of salivary IL-6 levels after the Trier Social Stress Test (TSST) (p < 0.001). However, indirect exposure to neighborhood violence was not related to DNA methylation at the IL-6 promoter region. |
| King et al. (2016) |
N = 489 Mothers from the Newborn Epigenetic Study (NEST) Durham, NC White: 240 (49.1) Black: 193 (39.5) Asian: 10 (2.0) Other: 36 (7.4) |
Residential addresses geocoded to match 2010 census tracts. Neighborhood disadvantage was assessed as percent non-Hispanic Black, percent families with income below the poverty level, percent of households on public assistance, percent of households with an unmarried female head, percent of population under age 18, and percent of the civilian labor force over age 16 unemployed. | MEG3 (tumor suppressor) | Bisulfite pyro-sequencing | Umbilical cord blood leukocytes | Neighborhood disadvantage was associated with significantly higher MEG3 methylation after controlling for maternal race/ethnicity, gender of newborn, mother’s years of education, maternal household income, pre-pregnancy BMI, cigarette smoking, and use of antibiotics during pregnancy (β = 0.76, p = 0.002). A one standard deviation increase in prenatal neighborhood disadvantage also predicted higher MEG3 methylation (p < 0.05). |
| Lei et al. (2015) |
N = 99 African American women who were primary caregivers. Random subsample from the Family and Community Health Study (FACHS). Age: 48.33 ± 9.30 years |
A summed score of neighborhood crime of two self-report measures: -11-item scale adapted from the Project on Human Development in Chicago Neighborhoods [extent to which various criminal acts were a problem in the neighborhood (e.g., drinking in public, people selling or using drugs, gang violence)] -6-item neighborhood deviance scale (no name provided) focused on the extent to which people in their neighborhoods think it was ok if the kids were drinking alcohol, having sex, smoking cigarettes, and using drugs, and it was easy for young people to get drugs or alcohol; higher scores indicate that respondents perceive their neighborhoods as high in crime and dangerous; Cronbach’s α = 0.83. |
5-HTT (serotonin transporter) 7 CpG sites within the CpG island associated with 5-HTT | Illumina Infinum 450K HumanMethylation Beadchip | Peripheral blood | No differences in neighborhood crime between methylation of 5-HTTLPR. Interaction of 5-HTTLPR × neighborhood crime on depressive symptoms was mediated by5-HTT methylation (indirect effect = 0.062, p < 0.031). There was no indirect effect of neighborhood crime on depressive symptoms through 5-HTT methylation when the respondents carried long 5-HTTLPR allele. However, there was an indirect effect of neighborhood crime on depressive symptoms though 5-HTT methylation when the respondents carried a short 5-HTTLPR allele (indirect effect = 0.064, p = 0.015). |
| Smith et al. (2017) |
N = 1226 Random subsample of longitudinal, population-based Multi-ethnic Study of Atherosclerosis (MESA) Baltimore, Forsyth County, New York, and St. Paul Age in years: N (%) 55–65: 463 (38%) 66–75: 397 (33%) 76–85: 300 (24%) 85–95: 66 (5%) Female: 663 (52%) Male: 593 (5%) White: 581 (47%) African American: 263 (22%) Hispanic: 382 (31%) |
Neighborhood-level socioeconomic disadvantage index created from census-tract level variables form the 2000 U.S. Census (e.g., education, income, wealth, poverty, employment, housing); a score was created as the weighted sum of 6 standardized variables (percent in census-tract with a bachelor’s degree; percent with a managerial/professional occupation; percent with a high school education; median home value; median household income; and percent with household income greater than $50,000/year); higher score indicates greater neighborhood socioeconomic disadvantage. Neighborhood social environment index created as the sum of standardized conditional Bayes estimate (CEB) scales for esthetic quality (3 items, e.g., lots of trash and litter on the street), safety (2 items, e.g., violence is a problem in my neighborhood), and social cohesion (4 items, e.g., people in my neighborhood can be trusted); self-report; 5-point scale (1 = strongly agree to 5 = strongly disagree); Cronbach’s α were 0.67 for esthetic quality; 0.64 for safety; and 0.72 for social cohesion |
18 candidate genes related to stress reactivity (AVP, BDNF, CRF, FKBP5, GR, OXTR, SLC6A4) and inflammation (CD1D, CCL1, F8, IL8, KLRG1, LTA4H, NLRP12, PYDC1, SLAMF7, TLR1, TLR3) | Illumina Infinum 450K Human Methylation Beadchip Illumina HumanHT-12 v4 Expression BeadChip to measure gene expression | Monocytes from peripheral blood sample; morning draw after 12 h fast | Neighborhood socioeconomic disadvantage was related to DNA methylation in 2 stress-related genes (CRF, SLC6A4) and 2 inflammation-related genes (F8, TLR1) after adjustment for age, gender, race/ethnicity, childhood SES, adult SES, and enrichment scores for each of the 4 major blood cell types (neutrophils, B cells, T cells, and natural killer cells). However, the results varied across genes and site types (promoter vs. shore/shelf). Neighborhood socioeconomic disadvantage was associated with increased methylation in non-promoter sites of CRF, F8, and TLR1; shore/shelf sites of AVP and SLC6A4; and non-shore sites of F8. Neighborhood socioeconomic disadvantage was associated with decreased methylation in non-shore/shelf sites of CRF (all FDR q-values ranging from 0.01–0.10). Neighborhood social environment was associated with methylation in 4 stress-related genes (AVP, BDNF, AKBP5, SLC6A4) and 7 inflammation-related genes (CCL1, CD1D, F8, KLRG1, NLRP12, SLAMF7, TLR1) after adjustment for age, gender, race/ethnicity, childhood SES, adult SES and enrichment scores for each of the 4 major blood cell types (neutrophils, B cells, T cells, and natural killer cells). Similarly, the results were dependent upon the genes and site type. Neighborhood social environment was associated with increased methylation in promoter sites of AVP, CCL1, CD1D, F8, KLRG1, and SLAMF7; non-promoter sites of AVP, BDNF, NLRP12, and TLR1; shore/shelf sites of AVP, FKBP5; SLC6A4; and non-shore/shelf sites of CD1D, F8, SLAMF7, and TLR1. Neighborhood social environment was associated with decreased methylation in non-promoter sites of CCL1 and KLRG1; shore/shelf sites of F8; and non-shore/shelf sites of FKBP5 (all FDR q-values ≤ 0.10). |
BMI body mass index, SES socioeconomic status
Results
The five studies included in this review had sample sizes ranging from 34 to 1226 participants. Three studies included multiethnic samples [33–35], one study had a sample of Black men only [36], and one study had a sample of Black women only [37]. Two of the studies included pregnant women [34, 35].
The U.S. Census data were used to measure the neighborhood environment in two of the studies [34, 35]. Two studies used self-report measures of the neighborhood [36, 37], and one study used both census data and self-report measures [33]. Three studies measured DNA methylation in the peripheral blood [33, 36, 37], and two studies measured it in umbilical cord blood from women at time of birth, which is of fetal origin [34, 35]. All studies examined DNA methylation targets, including those associated with the global repetitive element DNA in LINE-1 (long interspersed nuclear elements) and Alu transposable elements (Alu) associated with genomic instability [34]. Specific gene targets included IL-6 (interleukin-6) [36], MEG3 (maternally expressed 3) [35], and 5-HTT (serotonin transporter) [37] in addition to 18 candidate genes related to stress reactivity (e.g., AVP) and inflammation (e.g., IL8) [33] (see Table 1).
Coker et al. found that pregnant women living in neighborhoods with the highest levels of poverty gave birth to infants with higher cord blood LINE-1 methylation compared with pregnant women living in the lowest level of poverty after adjusting for covariates (e.g., maternal age, smoking during pregnancy) [34]. Genes with differential DNA methylation that showed statistically significant associations with neighborhood disadvantage are presented in Table 2. King et al. found that prenatal neighborhood disadvantage was associated with significantly higher MEG3 methylation in newborn umbilical cord blood at birth after controlling for maternal race/ethnicity, sex of newborn, maternal education, maternal household income, pre-pregnancy BMI, cigarette smoking, and use of antibiotics during pregnancy [35]. Both studies used the census data to measure neighborhood disadvantage [34, 35]. Lei et al. did not find differences in self-reported neighborhood crime and 5-HTT methylation, which was not influenced by allele variants of HTTLPR [i.e., the repeat element polymorphic region of the 5-HTT with allele variants of short [12] and long [14] repeats] [37]. However, an indirect effect of neighborhood crime on depressive symptoms through 5-HTT methylation was witnessed when the respondents carried a short 5-HTTLPR allele [37].
Table 2.
Genes related to neighborhood disadvantage and their functions
| Gene name | Function | |
|---|---|---|
| AVP [33] | Arginine vasopressin | Member of the vasopressin/oxytocin family; plays a role in glucocorticoid signaling, antidiuretic action on the kidney, and vasoconstriction of the peripheral vessels |
| BDNF [33] | Brain-derived neurotrophic factor | Plays a role in the regulation of the stress response and in the biology of mood disorders |
| CCL1 [33] | C-C motif chemokine ligand 1 | Small glycoprotein secreted by activated T cells that belongs to a family of inflammatory cytokines known as chemokines; has a role in cytokine and chemokine activity |
| CD1D [33] | CD1d molecule | Role in cell adhesion molecule binding |
| CRF [33] | Corticotropin-releasing factor | Major regulator of homeostasis, mediating the autonomic, behavioral, and neuroendocrine responses to stress |
| F8 [33] | Coagulation factor VIII | Acts as a cofactor for F9/factor IXa when it converts F10/factor X to the activated form, factor Xa |
| FKBP5 [33] | FK506 binding protein 5 | Member of the immunophilin protein family; plays a role in immunoregulation and basic cellular processes involving protein folding and trafficking |
| KLRG1 [33] | Killer cell lectin-like receptor G1 | Plays an inhibitory role on natural killer (NK) cells and T cell functions upon binding to their non-MHC ligands |
| MEG3 [35] | Maternally expressed 3 | RNA gene affiliated with the non-coding RNA class; inhibits tumor cell proliferation in vitro |
| NLRP12 [33] | NLR family pyrin domain containing 12 | NLRP12 is implicated in the activation of pro-inflammatory caspases (e.g., CASP1 = cysteine-type endopeptidase activator activity involved in apoptotic process). |
| SLAMF7 [33] | SLAM family member 7 | SLAM receptors triggered by homo- or heterotypic cell-cell interactions are modulating the activation and differentiation of a wide variety of immune cells and are involved in the regulation and interconnection of both innate and adaptive immune responses. |
| SLC6A4 [33, 37] | Solute carrier family 6 member 4 | Sodium- and chloride-dependent members of the solute carrier family 6 encode an integral membrane protein, 5-HTT, that transports the neurotransmitter serotonin from synaptic spaces into presynaptic neurons. The encoded protein terminates the action of serotonin and recycles it in a sodium-dependent manner |
| TLR1 [33] | Toll-like receptor 1 | Single transmembrane cell-surface receptors; participates in the innate immune response to microbial agents; mediates the production of cytokines necessary for the development of effective immunity; leads to NF-kappa-B activation, cytokine secretion, and the inflammatory response |
Source: GeneCards. Human gene database retrieved from https://www.genecards.org
One study examined the association of neighborhood environment with DNA methylation of stress-related genes [33]. Specifically, Smith et al. found that neighborhood socioeconomic disadvantage was related to DNA methylation in two stress-related genes, CRF and SLC6A4, after adjustment for age, sex, race/ethnicity, childhood socioeconomic status, adult socioeconomic status, and enrichment scores for each of the four white blood cell types (neutrophils, B cells, T cells, and natural killer cells) (all FDR q-values 0.01–0.10) [33]. Neighborhood social environment was associated with methylation in four stress-related genes (AVP, BDNF, FKBP5, SLC6A4) after adjustment for covariates [33]. However, the direction of methylation varied by gene and location (promoter or shore/shelf) [33]. Of differentially methylated genes associated with neighborhood socioeconomic disadvantage and social environment, expression analysis was limited to FKBP5 indicating significant association of methylation with gene expression in shore/shelf sites, promoter and non-promoter regions.
Two studies measured inflammation-related DNA methylation using candidate gene and genome-wide approaches [33, 36]. Janusek et al. found that greater indirect exposure to neighborhood violence (i.e., witnessing of violence on another) was a predictor of a steeper rise and slower rate of decline of salivary IL-6 levels after the Trier Social Stress Test (TSST); however, indirect exposure to neighborhood violence was not related to DNA methylation at the IL-6 promoter region [36]. Finally, Smith et al. found that neighborhood socioeconomic disadvantage was related to DNA methylation in two inflammation-related genes (F8, TLR1) after adjustment for covariates [33]. Neighborhood social environment, measured by an index that included neighborhood aesthetic quality and safety, was associated with methylation in seven inflammation-related genes (CCL1, CD1D, F8, KLRG1, NLRP12, SLAMF7, TLR1) after adjustment for covariates [33]. Similarly, the direction of methylation varied based on the gene and site types [33].
Discussion
Neighborhood disadvantage has been related to CVD. However, research on the epigenetic pathways between neighborhood disadvantage and CVD is sparse. In this review, we examined the association of neighborhood disadvantage with DNA methylation, a potential pathway between neighborhood disadvantage and risk for CVD. Only two studies examined the association of neighborhood disadvantage with inflammation-related candidate genes [33, 36]. Systemic inflammation is a risk factor for CVD. However, data are lacking on the relationships among neighborhood disadvantage, methylation of inflammation-related genes, and CVD.
Neighborhood disadvantage has been related to psychological stress and depressive symptoms. Table 2 shows the genes that were related to neighborhood disadvantage and their functions. AVP, BDNF, CRF, FKBP5, and SLC6A4 are stress-related genes. Smith et al. found associations of neighborhood socioeconomic disadvantage and social environment with DNA methylation in these genes [33]. LINE-1 methylation has been implicated in neuropsychiatric conditions such as depression [38]. Coker et al. found that neighborhood poverty was related to higher cord blood LINE-1 methylation [34]. Lei et al. also found an indirect effect of neighborhood crime on depressive symptoms through methylation of 5-HTT [37]. These results suggest that neighborhood disadvantage is related to methylation of genes related to psychological stress and depressive symptoms.
DNA methylation may be an important biological mechanism underlying neighborhood disadvantage-associated adverse health outcomes (e.g., CVD), as mediated by differential gene transcription and expression [33, 39, 40]. Among the reviewed studies, only one included concurrent measurement of DNA methylation and gene expression, with Smith et al. finding that neighborhood disadvantage was associated with increased DNA methylation in the non-promoter sites in F8 which, in turn, was associated with decreased gene expression [33]. Furthermore, in this study, social environment was associated with both DNA methylation and gene expression for five genes (promoter region of CD1D, shore/shelf regions of FKBP5 and F8, non-promoter regions of NLRP12, and non-promoter regions of KLRG1) [33]. The direction of these associations varied based on the individual gene and site type (see Table 3) [33]. As noted in Table 2, these genes are part of the stress and inflammation pathways. Thus, it is plausible that DNA methylation of stress- and inflammation-related genes and the expression of these genes may be mediators of the association of neighborhood environment with CVD (see Fig. 1).
Table 3.
The associations among neighborhood environment, DNA methylation, and gene expression
| Gene | Neighborhood environment | DNA methylation | Gene expression |
|---|---|---|---|
| CD1D promoter sites | Worse social environment | Increased | Decreased |
| FKBP5 shore/shelf sites | Worse social environment | Increased | Decreased |
| F8 shore/shelf sites | Worse social environment | Decreased | Increased |
| F8 non-promoter sites | More neighborhood disadvantage | Increased | Decreased |
| NLRP12 non-promoter sites | Worse social environment | Increased | Decreased |
| KLRG1 non-promoter sites | Worse social environment | Decreased | Decreased |
The studies presented in this review have several important limitations. Even though the authors excluded chemical neighborhood stressors, some of the results may still reflect these stressors (e.g., chemical stressors may be present in neighborhoods with high levels of disorder). Four of the studies had sample sizes of 99 or more participants; however, the study by Janusek et al. included only 34 participants. The researchers measured DNA methylation across a wide variety of candidate genes, making it difficult to identify consistent methylation targets associated with neighborhood disadvantage. As presented in Table 2, these genes have varied functions. However, it is plausible that stress- and inflammation-related candidate genes may be differentially methylated due to the chronic stress of living in disadvantaged neighborhoods. Black women are more likely to live in disadvantaged neighborhoods [6, 41] and to experience psychological stress and depressive symptoms [42–45] compared with White women. However, only one study included a sample of Black women exclusively [37]. One study included Black men only [36] and three studies included multiethnic samples [33–35]. Thus, research is needed on the relationship between neighborhood environment and DNA methylation of stress- and inflammation-related candidate genes among Black women. Future research should include measures of gene expression of differentially methylated genes in order to understand the biologic mechanisms by which neighborhood environment is related to DNA methylation and adverse health outcomes (e.g., CVD). All of the studies presented in this review focused on candidate gene-based or measured global DNA methylation. Future research needs to measure epigenome-wide DNA methylation in order to identify other potential genes related to neighborhood disadvantage.
The National Institute of Minority Health and Health Disparities (NIMHD) Minority Health and Health Disparities Research Framework presents factors that influence minority health and health disparities [46]. The model displays five domains of influence across the lifespan (biological, behavioral, physical/built environment, sociocultural environment, and healthcare system) and four levels of influence (individual, interpersonal, community, and societal). This model illustrates the biologic-environmental interactions across the lifecourse which may outline the source for epigenetic changes, toward development of disease. For example, the interaction between a Black woman’s physical environment across her lifecourse and her biological vulnerabilities and mechanisms (e.g., DNA methylation of inflammation-related genes) may increase her susceptibility for CVD. Indeed, DNA methylation of inflammation-related genes has been related to risk for CVD. We propose that DNA methylation of stress- and inflammation-related candidate genes partially mediates the associations of neighborhood environment and psychological stress with CVD (see Fig. 1). Future research needs to address the interaction of neighborhood environment and epigenetic markers of CVD risk among Black women. Future research should also incorporate measures of neighborhood disadvantage across the lifespan. Incorporating residential histories into studies for CVD risk will allow the creation of a dynamic, neighborhood-level typology of residential history and an examination of its association with DNA methylation and risk for CVD. Furthermore, examining residential mobility and its role on risk for CVD will further our understanding of the association of neighborhood disadvantage with risk for CVD, increasing our understanding of bio-environmental interactions. Given the identification of DNA methylation and CVD risk as well as its potential relationship with neighborhood disadvantage, more work to develop targeted interventions is also needed. Finally, studies that examine differences in the association between neighborhood environment and DNA methylation across age groups (adults vs. children), racial/ethnic groups, and timing of stressors (adult vs. childhood neighborhood environment) are needed. These studies will provide data to conduct meta-analyses across multiple large cohorts in order to have a better understanding of the association between neighborhood environment and DNA methylation.
Conclusion
The results of this review suggest that neighborhood environment is related to DNA methylation within close proximity to stress- and inflammation-related candidate genes. The differential methylation of these genes may be a pathway by which neighborhood disadvantage increases risk for CVD. Future research should examine the potential mediating effect of DNA methylation of stress- and inflammation-related candidate genes as well as novel gene targets identified through epigenome-wide interrogation on the association of neighborhood disadvantage with risk for CVD among Black women. This investigation will also provide a template for replication in other chronic conditions. DNA methylation may be a pathway through which disadvantaged neighborhoods influence multiple health outcomes.
Acknowledgments
There was no funding for this review article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heron M. Deaths: Leading causes for 2016. Hyattsville, MD: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 2.Smykiewicz P, Segiet A, Keag M, Żera T. Proinflammatory cytokines and ageing of the cardiovascular-renal system. Mech Ageing Dev. 2018;175:35–45. doi: 10.1016/j.mad.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Wilbur J, Braun LT, Arslanian-Engoren C, Lauver DR, Halloway S. Assessing and addressing cardiovascular risk in young women. Nurs Outlook. 2018;66(3):325–328. doi: 10.1016/j.outlook.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Fryar CD, Chen T, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS data brief, no 103. Hyattsville: National Center for Health Statistics; 2012. [PubMed]
- 5.Kanchi R, Perlman SE, Chernov C, et al. Gender and race disparities in cardiovascular disease risk factors among New York City adults: New York City Health and Nutrition Examination Survey (NYC HANES) 2013–2014. J Urban Health. 2018;95(6):801–12. doi: 10.1007/s11524-018-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the multi-ethnic study of atherosclerosis) Am J Hypertens. 2011;24(2):187–193. doi: 10.1038/ajh.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claudel SE, Adu-Brimpong J, Banks A, Ayers C, Albert MA, Das SR, de Lemos JA, Leonard T, Neeland IJ, Rivers JP, Powell-Wiley TM. Association between neighborhood-level socioeconomic deprivation and incident hypertension: a longitudinal analysis of data from the Dallas heart study. Am Heart J. 2018;204:109–118. doi: 10.1016/j.ahj.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giurgescu C, Zenk SN, Dancy BL, Park CG, Dieber W, Block R. Relationships among neighborhood environment, racial discrimination, psychological distress, and preterm birth in African American women. JOGNN J Obstet Gynecol Neonatal Nurs. 2012;41(6):E51–E61. doi: 10.1111/j.1552-6909.2012.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giurgescu C, Misra DP, Sealy-Jefferson S, Caldwell CH, Templin TN, Slaughter- Acey JC, Osypuk TL. The impact of neighborhood quality, perceived stress, and social support on depressive symptoms during pregnancy in African American women. Soc Sci Med. 2015;130:172–180. doi: 10.1016/j.socscimed.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giurgescu C, Zenk SN, Templin TN, Engeland CG, Dancy BL, Park CG, Kavanaugh K, Dieber W, Misra DP. The impact of neighborhood environment, social support and avoidance coping on depressive symptoms of pregnant African American women. Womens Health Issues. 2015;25(3):294–302. doi: 10.1016/j.whi.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giurgescu C, Zenk SN, Templin TN, Engeland CG, Kavanaugh K, Misra DP. The impact of neighborhood conditions and psychological distress on preterm birth in African-American women. Public Health Nurs. 2017;34:256–266. doi: 10.1111/phn.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baghai TC, Varallo-Bedarida G, Born C, et al. Classical risk factors and inflammatory biomarkers: One of the missing biological links between cardiovascular disease and major depressive disorder. Int J Mol Sci. 2018;19(6). 10.3390/ijms19061740. [DOI] [PMC free article] [PubMed]
- 13.Vaccarino V, Wilmot K, Mheid IA, Ramadan R, Pimple P, Shah AJ, et al. Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Heart Assoc. 2016;5(9). 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed]
- 14.Cooper DC, Trivedi RB, Nelson KM, Reiber GE, Zonderman AB, Evans MK, Waldstein SR. Sex differences in associations of depressive symptoms with cardiovascular risk factors and metabolic syndrome among African Americans. Cardiovasc Psychiatry Neurol. 2013;2013:1–10. doi: 10.1155/2013/979185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen BS. Stressed or stressed out: what is the difference? J Psychiatry Neurosci. 2005;30(5):315–318. [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;109(SUPPL.2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters A, BS ME. Introduction for the allostatic load special issue. Physiol Behav. 2012;106(1):1–4. doi: 10.1016/j.physbeh.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Witek-Janusek L, Mathews HL. Stress and Coping. Virginia: Sage; 2000. Stress, immunity and health outcomes. [Google Scholar]
- 19.Giurgescu C, Engeland CG, Zenk SN, Kavanaugh K. Stress, inflammation and preterm birth in African American women. Newborn Infant Nurs Rev. 2013;13(4):171–177. doi: 10.1053/j.nainr.2013.09.004. [DOI] [Google Scholar]
- 20.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines and autoimmunity. Ann N Y Acad Sci. 2002;996:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 21.Kunz-Ebrecht SR, Mohamed-Ali V, Fledman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav Immun. 2003;17(5):373–383. doi: 10.1016/S0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 22.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Ho RCM, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Valkanova V, Ebmeier KP, Allan CLCRP. IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-α, and IL-6 in women with PTSD. J Trauma Stress. 2008;21(6):530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Wang NC, Matthews KA, Barinas-Mitchell EJM, Chang CCH, El Khoudary SR. Inflammatory/hemostatic biomarkers and coronary artery calcification in midlife women of African-American and white race/ethnicity: the Study of Women’s Health Across the Nation (SWAN) heart study. Menopause. 2016;23(6):653–661. doi: 10.1097/GME.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cozier YC, Albert MA, Castro-Webb N, Coogan PF, Ridker P, Kaufman HW, Palmer JR, Rosenberg L. Neighborhood socioeconomic status in relation to serum biomarkers in the black women’s health study. J Urban Health. 2016;93(2):279–291. doi: 10.1007/s11524-016-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saban KL, Mathews HL, de Von HA, Janusek LW. Epigenetics and social context: implications for disparity in cardiovascular disease. Aging Dis. 2014;5(5):346–355. doi: 10.14336/AD.2014.0500346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 31.Schiattarella GG, Madonna R, Van Linthout S, et al. Epigenetic modulation of vascular diseases: assessing the evidence and exploring the opportunities. Vasc Pharmacol. 2018;107:43–52. doi: 10.1016/j.vph.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Fernández-Sanlés A, Sayols-Baixeras S, Subirana I, Degano IR, Elosua R. Association between DNA methylation and coronary heart disease or other atherosclerotic events: a systematic review. Atherosclerosis. 2017;263:325–333. doi: 10.1016/j.atherosclerosis.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Smith JA, Zhao W, Wang X, Ratliff SM, Mukherjee B, Kardia SLR, Liu Y, Roux AVD, Needham BL. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: the multi-ethnic study of atherosclerosis. Epigenetics. 2017;12(8):662–673. doi: 10.1080/15592294.2017.1341026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coker ES, Gunier R, Huen K, Holland N, Eskenazi B. DNA methylation and socioeconomic status in a Mexican-American birth cohort. Clin Epigenetics. 2018;10(1). 10.1186/s13148-018-0494-z. [DOI] [PMC free article] [PubMed]
- 35.King KE, Kane JB, Scarbrough P, Hoyo C, Murphy SK. Neighborhood and family environment of expectant mothers may influence prenatal programming of adult cancer risk: discussion and an illustrative DNA methylation example. Biodemography Soc Biol. 2016;62(1):87–104. doi: 10.1080/19485565.2015.1126501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janusek LW, Tell D, Gaylord-Harden N, Mathews HL. Relationship of childhood adversity and neighborhood violence to a proinflammatory phenotype in emerging adult African American men: an epigenetic link. Brain Behav Immun. 2017;60:126–135. doi: 10.1016/j.bbi.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Lei MK, Beach SRH, Simons RL, Philibert RA. Neighborhood crime and depressive symptoms among African American women: genetic moderation and epigenetic mediation of effects. Soc Sci Med. 2015;146:120–128. doi: 10.1016/j.socscimed.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn JP, Savage AL, Bubb VJ. Non-coding genetic variation shaping mental health. Curr Opin Psychol. 2019;27:18–24. doi: 10.1016/j.copsyc.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garfield L, Mathews HL, Inflammatory JLW. Epigenetic pathways for perinatal depression. Biol Res Nurs. 2015;18(3):331–343. doi: 10.1177/1099800415614892. [DOI] [PubMed] [Google Scholar]
- 40.Mathews HL, Janusek LW. Epigenetics and psychoneuroimmunology: mechanisms and models. Brain Behav Immun. 2011;25(1):25–39. doi: 10.1016/j.bbi.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laraia BA, Messer L, Kaufman JS, Dole N, Caughy M, O'Campo P, Savitz DA. Direct observation of neighborhood attributes in an urban area of the US south: characterizing the social context of pregnancy. Int J Health Geogr. 2006;5:11. doi: 10.1186/1476-072X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giurgescu C. The Impact of Uncertainty, Social Support, and Prenatal Coping on the Psychological Well-Being of Women with High-Risk Pregnancy. Chicago: Loyola University of Chicago; 2004. [DOI] [PubMed] [Google Scholar]
- 43.Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and black-white differences in preterm and low-birthweight deliveries: the CARDIA study. Am J Public Health. 2004;94(12):2125–2131. doi: 10.2105/AJPH.94.12.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holzman C, Eyster J, Tiedje LB, Roman LA, Seagull E, Rahbar MH. A life course perspective on depressive symptoms in mid-pregnancy. Matern Child Health J. 2006;10(2):127–138. doi: 10.1007/s10995-005-0044-0. [DOI] [PubMed] [Google Scholar]
- 45.Seng JS, Kohn-Wood LP, McPherson MD, Sperlich M. Disparity in posttraumatic stress disorder diagnosis among African American pregnant women. Arch Womens Ment Health. 2011;14(4):295–306. doi: 10.1007/s00737-011-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, Lurie N, Rebbeck T, Goodwin J, Flack J, Srinivasan S, Kerner J, Heurtin-Roberts S, Abeles R, Tyson FL, Patmios G, Hiatt RA. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]