Abstract
Proper nomenclature is a major obstacle in understanding and managing vascular anomalies. Often the same term is used for totally different types of lesions or, conversely, the same lesion may be labeled with different terms. Although in recent times there has been a greater understanding of the problems concerning vascular anomalies, episodes of improper use of terminology still remain. The aim of this article, starting from the most recent classification of vascular anomalies, is to provide a clinical and instrumental approach to identifying these lesions and to converge towards a clear and unambiguous terminology that must become univocal among the various operators to avoid diagnostic misunderstandings and therapeutic errors.
Keywords: Vascular anomalies, Hemangioma, Ultrasound, Doppler, Children
Sommario
L’esatta terminologia è stata il maggior ostacolo alla comprensione e alla gestione delle anomalie vascolari. Spesso lo stesso termine è stato usato per lesioni totalmente diverse o, viceversa, la medesima lesione è stata etichettata con termini diversi. Nonostante in tempi recenti vi sia stata una maggiore comprensione delle problematiche riguardanti le anomalie vascolari, ancora permangono episodi di uso improprio della terminologia. Scopo di questo articolo, partendo dalla più recente classificazione delle anomalie vascolari, è fornire un approccio clinico-strumentale a queste lesioni e convergere verso una terminologia chiara ed univoca che deve diventare comune fra i vari operatori per evitare fraintendimenti diagnostici e errori terapeutici.
Classification and nomenclature of vascular anomalies
Vascular anomalies may be present at birth or arise shortly thereafter. Since they are often very apparent, they have always made a great impression among the observers who have tried to classify them. Obviously, attempts at classification have been conditioned by the historical context and the methodological trends in vogue at the time of observation. This has led to a continuous revision and redefinition of terms and caused increasing confusion, sometimes with fatal results.
From the initial descriptive classification—based above all on the external appearance of those lesions which dazzled the first observers—some very suggestive terms emerged, some of which have extended their influence to the present day. For example, a French dermatologist during the early nineteenth century used the generic term “angioses” (i.e., affection of the vascular system) to describe a variety of acquired and congenital lesions, as well as the term “haematoncus tuberosus” (“tumor of the blood similar to a potato”) [1, 2].
Next came anatomopathological classification, under which Virchow, considered the father of the cellular pathology, in 1863 generally classified all vascular anomalies as “angiomas”, labeling them according to vascular architecture (e.g., “angioma simplex”, “angioma racemosum”, and “angioma cavernosus”) [1, 3].
Beginning in the early 1900s, embryological classification became widespread. One of the leading figures of this system was the Italian Edmondo Malan. In his monumental monograph, Malan divides angiomas into “venous,” “truncular”, “arteriovenous”, and “capillary” [1, 4]. Although conceptually interesting, classification on an embryological basis does not appear clinically useful, as it fails to differentiate between involutive lesions and non-involutive ones.
Finally, on the basis of clinical-histological studies, in 1982 Mulliken and Glowacki proposed a biological classification that distinguishes lesions based on the behavior of endothelial cells, with lesions broadly classified as hemangiomas (lesions in which endothelial hyperproliferation is present) and malformations (lesions presenting normal turnover of endothelial cells) [5].
This fundamental distinction remains the basis of modern classification today. In fact, it was adopted and appropriately modified by the International Society for the Study of Vascular Anomalies (ISSVA) in 1996 to provide the medical community with a universal scientific language that would prevent both misdiagnosis and inaccurate management. Following adoption, the classification was reviewed in 2014 and updated in 2018 [6]. Under this classification, the vascular anomalies are divided into two main groups: vascular tumors, characterized by a clonal proliferation of endothelial cells, and vascular malformations, determined by errors in different developmental stages of embryogenesis but with a normal endothelial cell turnover. Vascular tumors are divided into three groups in relation to their neoplastic aggressiveness. Vascular malformations are divided into four groups in relation to the vascular structures involved.
Imaging techniques
The first level examination of pediatric vascular anomalies is ultrasound (US). The predominantly superficial location allows a morphological evaluation of the lesions and a dynamic examination of the vascularization. The subsequent integration with magnetic resonance is reserved for selected cases and is always a next step compared to the ultrasound examination.
However, the instrumental investigation cannot be separated from a first clinical evaluation. Some elements must be known by the radiologist or sonographer who performs the examination to better interpret the findings of the investigation:
When was the lesion first noticed?
Was it already present at birth or did it develop later?
What was its growth rate?
The role of ultrasound imaging is fundamental in diagnostic orientation and to address the subsequent therapeutic phase. The main elements that the ultrasound examination must look for are:
the anatomical localization of the lesion by determining the involved layers (e.g., skin, subcutis, muscular layer).
degree of compressibility of the lesion (a hard, non-compressible lesion must always be suspicious of possible different nature).
distribution and density of vascularization of the lesion, including recognition of the type of vessels (arterial or venous) involved.
assessment whether the clinically detectable lesion is single or consisting of multifocal elements.
The ultrasound examination, however, has intrinsic limits. For example, it cannot clearly define the limits in the case of very extensive and particularly deep lesions, and it presents difficulties in exploring some areas such as those near bony and air-filled structures.
Despite the numerous information that the ultrasound examination can provide, it is not always possible to arrive at a precise diagnosis with the color Doppler exam alone. The great semeiological overlap does not always allow for a differential diagnosis, not only between different vascular anomalies but also with other benign or malignant lesions of the soft tissues. In these cases, it is mandatory to use the MRI and, if necessary, the biopsy examination.
Vascular tumors
Vascular tumors are divided into three main categories according to their nature: benign, locally aggressive, and malignant.
Malignant tumors are, fortunately, infrequent among pediatric patients. Given the large number of recognized lesions, we will cover in particular those most frequently encountered in clinical practice. Benign tumors include:
Infantile hemangioma.
Congenital hemangioma (RICH, NICH, PICH).
Although they are identified with the same term (hemangioma), these two lesions have two substantial differences, one from the clinical point of view and the other from the histopathological point of view:
Infantile hemangioma occurs and develops only after birth, while congenital hemangioma (as the name suggests) is already fully developed at birth.
From the histopathological point of view, the lesions are characterized by different behavior of the glucose transporter 1 (GLUT 1) protein. Infantile hemangioma is GLUT 1 + , while congenital hemangioma is GLUT 1 − (this behavior is common with vascular malformations) [7, 8].
Infantile hemangioma
Infantile hemangioma is the most common vascular tumor in children up to 1 year old [9]. Diagnosis is generally clinical, and ultrasound examination is often unnecessary. In any case, US may be required for extensive and deep lesions and during pharmacological follow-up [7, 9, 10].
It should be noted that this lesion has a characteristic clinical history. Mass is never present at birth, but there may be flat prodromal signs on the skin that subsequently increase in volume during the proliferative phase of hemangioma [9]. Infantile hemangioma, in fact, characteristically has two phases: a proliferative phase, in which it appears in the first days/weeks of life and grows rapidly for several months (Fig. 1), and an involutive phase, lasting up to age 8–9 years, in which there is a progressive reduction in volume with an increase in adipose tissue compared to the vascular tissue [7, 11–13].
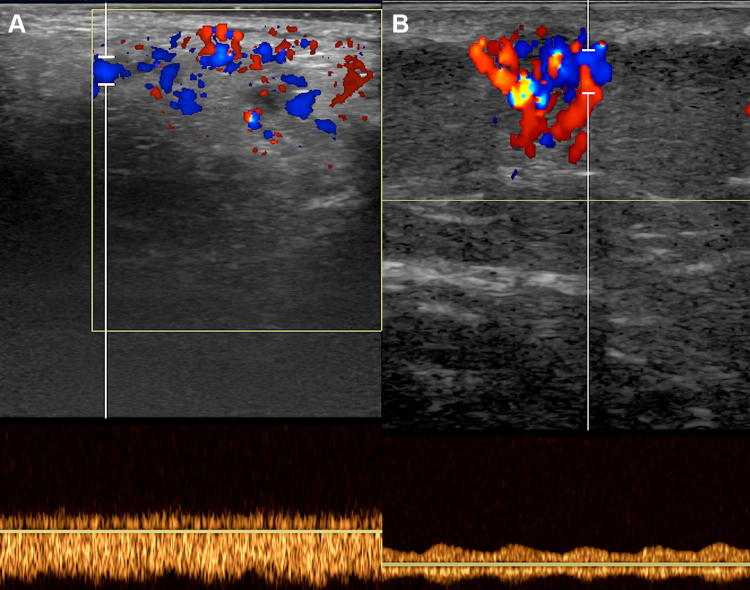
The US aspect of hemangioma also depends on the phase in which it is examined. In the proliferative phase, the hemangioma is generally a well-defined mass with a non-homogeneous echostructure and may be predominantly hyperechoic, hypoechoic, or with a lobulated appearance (Fig. 2). Of these patterns, it is difficult to say which is the prevailing one, and the results in the literature are rather discordant. In some cases, the hyperechoic aspect prevails [9], while in others the hypoechoic aspect prevails [14]. At the color Doppler (ECD) exam, infantile hemangioma appears richly vascularized with high vascular density and uniform vessel distribution within the lesion [7, 9, 13, 15] (Fig. 3). Regarding vascular density, some authors have determined the vascular density in relation to the number of vessels per square centimeter, classifying as highly vascularized lesions with more than five vessels per square centimeter [16]. However, this quantitative approach is not very significant at present due to the enormous improvement of modern ultrasound devices (Fig. 4) and is not very useful from the practical point of view, presupposing a technical uniformity of examination usually not obtainable. Spectral analysis detects prevalent presence of arterial flows that show low resistance and relatively high velocities [7, 17–19] (Fig. 5). It is also possible to identify venous vessels that show slow flow and are generally poorly modulated [14].
Fig. 1.
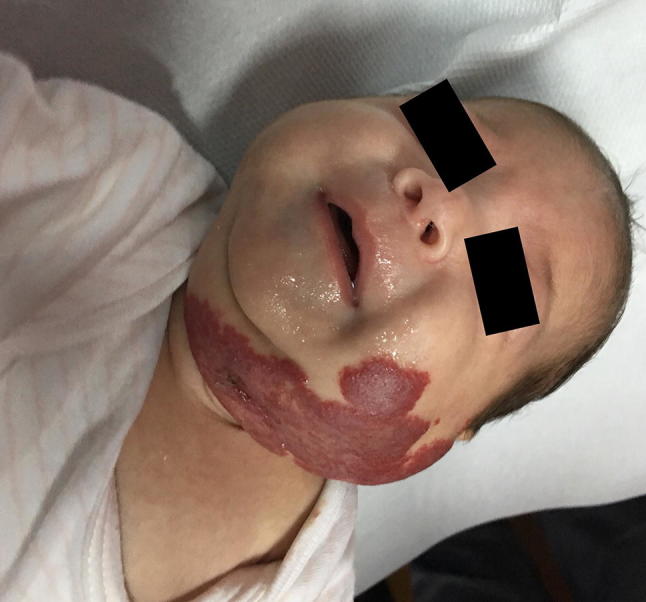
Infantile hemangioma: proliferative phase in a 5-week-old boy. The lesion healed after treatment with propanol
Fig. 2.
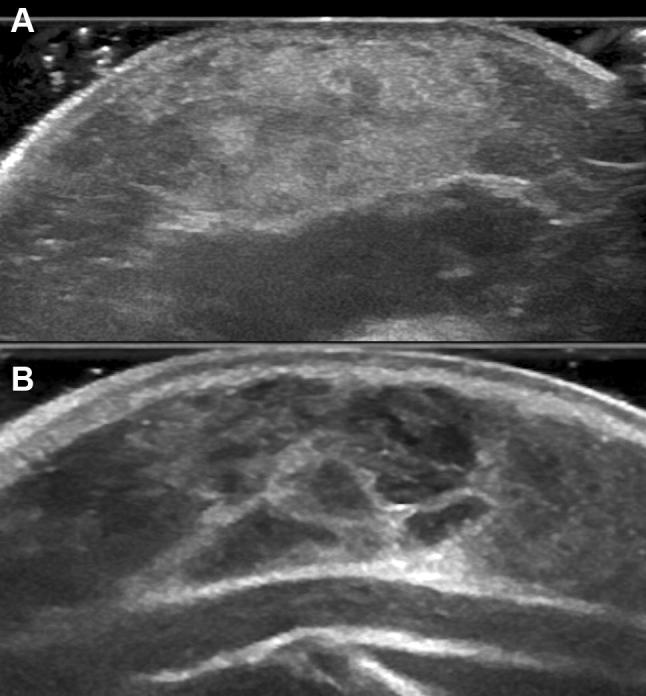
Infantile hemangioma. a Sonogram shows hyperechoic pattern, 8-month-old girl; b sonogram shows hypoechoic lobulated pattern, 5-month-old girl
Fig. 3.
Color Doppler sonogram shows high vascular density, characteristic of infantile hemangioma
Fig. 4.

Infantile hemangioma in 4-month-old girl. B-flow technology shows the very high vascular density
Fig. 5.

Infantile hemangioma. Spectral analysis shows arterial flow with low resistance and high velocities
Normally, infantile hemangioma is not often examined in the involutive phase. In this stage, the echogenicity of the lesion increases progressively, due to the increase in adipose tissue and at the same time decreases the vascular density with arterial flows which show a progressive increase in the Resistive Index (RI) [7, 9, 20, 21].
Congenital hemangioma
Congenital hemangioma is by definition already present at birth. Introduced for the first time in 1996 [22, 23], congenital hemangioma, although presenting vascular characteristics similar to infantile hemangioma, is differentiated by clinical history and histopathological aspects [12, 24, 25]. These lesions grow rapidly in the uterus, so they are very evident at birth (Fig. 6). Based on their evolution they are distinguished in rapidly involuting congenital hemangioma (RICH) and in non-involuting congenital hemangioma (NICH). The RICH shows regression by about 14 months, unlike infantile hemangioma whose regression is slower and can even occur over several years [22].
Fig. 6.
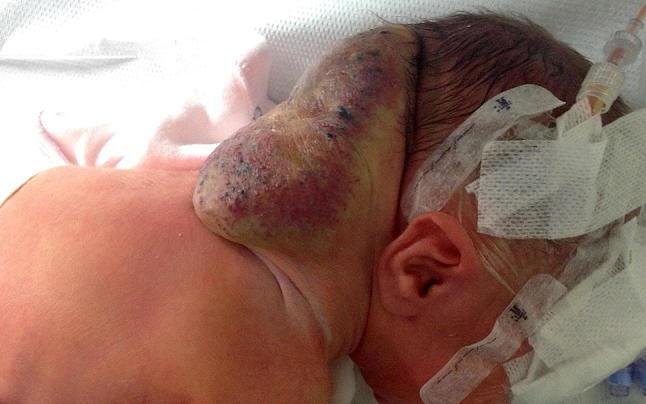
Rapidly involuting congenital hemangioma (RICH) in 2-day-old boy (same case as of Fig. 8). Newborn with congenital vascular tumor in the occipital region. Biopsy confirmed the diagnosis
On the contrary, the NICH does not show involution and tends to grow with the child [23, 26, 27]. This behavior is very similar to the natural history of vascular malformations.
More recently, a third category has been hypothesized called partially involutive congenital hemangioma (PICH) [12], as it is easy to imagine these lesions showing a first involutive phase, similar to the RICH, but at some point the involution stops and these lesions assume the same behavior of the NICH [9, 28].
Congenital hemangioma is much less common than infantile hemangioma. It is generally solitary and occurs with more frequency at the level of the head and limbs near the joints [29].
Sometimes it is not possible, on the basis of clinical history alone and of the instrumental finding, to make a differential diagnosis between infantile hemangioma and congenital hemangioma. If a diagnosis is necessary, it is possible to distinguish the two lesions on the basis of immunohistochemistry with the GLUT 1 research (the infant hemangioma is positive for GLUT 1, while the congenital hemangioma is negative for GLUT 1). The ultrasound and color Doppler findings can be very similar to what is found in infantile hemangioma; however, it is possible to identify some elements more often present in the congenital form [7, 12, 24, 26, 27, 30, 31].
Congenital hemangioma B-mode ultrasound is more inhomogeneous than infantile hemangioma. It is often possible to identify vascular structures of relatively high diameter that well distinguishable in the lesion (Fig. 7) in comparison to the infantile hemangioma whose vascular structures are of smaller caliber and difficult to distinguish in grayscale. The calcifications are not common but when present they aid in distinguishing the congenital hemangioma from the infantile hemangioma, where the calcifications are very rare (Fig. 8).
Fig. 7.

Rapidly involuting congenital hemangioma (RICH) in 1-month-old boy. Sonogram shows heterogeneous subcutaneous mass that contains large visible vessel (arrow)
Fig. 8.

Rapidly involuting congenital hemangioma (RICH) in 2-day-old boy. Sonogram shows heterogeneous subcutaneous mass with intralesional calcification (arrow) and large visible vessel (arrowhead)
The color Doppler vascular density is very high for congenital hemangioma, similar to that of infantile hemangioma, with arterial flows at high velocities and low resistance (Fig. 9). However, the venous vascular signal is more frequently seen as compared to the infantile form. In some cases, the venous flow tends to be predominant and often corresponds to the larger vessels seen at the B-mode that represent dysplastic veins that cross the lesion transversely. Moreover, it is possible to detect the presence of arteriovenous MicroShunt with turbulent flow on the spectral examination, particularly frequent in the NICH. Finally, the possible presence of thrombi in the larger venous vessels has been described, and this characteristic is never present in infantile hemangioma.
Fig. 9.
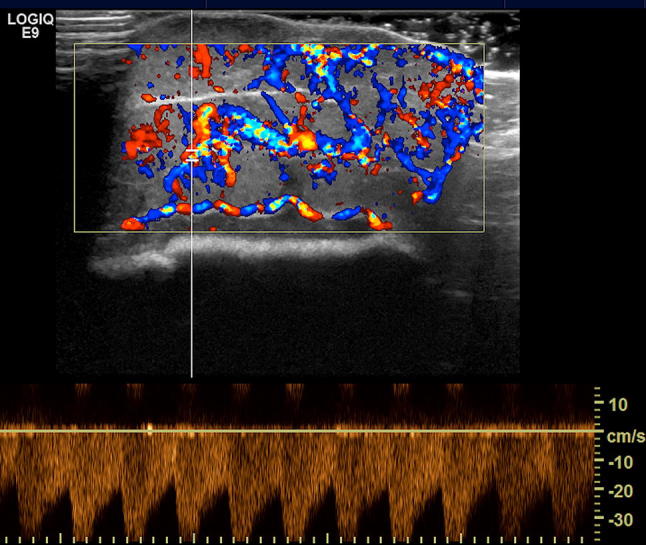
Rapidly involuting congenital hemangioma (RICH) in 1-month-old boy. Color Doppler shows high vascular density, and spectral analysis shows the presence of low resistance arterial flow
Locally aggressive tumors
Kaposiform Hemangioendothelioma (and Tufted Angioma)
Although Tufted angioma is categorized as a benign tumor while the Kaposiform hemangioendothelioma is considered a locally aggressive tumor [32], the two lesions are treated together because they have many similar aspects.
Both forms may be associated with systemic complications such as the Kasabach–Merritt phenomenon (characterized by thrombocytopenia, microangiopathic hemolytic anemia, and consumption of coagulation factors). This common behavior led to the hypothesis that the two lesions may represent a spectrum of the same neoplasm [13]. The finding of the Kasabach–Merritt phenomenon is indicative of the presence of one of these two tumors [9].
On the US examination these lesions tend to be inhomogeneous with poorly defined margins compared to the surrounding tissues [11, 33–37] (Fig. 10). Tufted angioma is generally more superficial, usually surrounded by subcutaneous fat, with a maximum thickness of 1 cm [9, 33], while the Kaposiform hemangioendothelioma is generally deeper, involving the underlying soft tissues. In addition, there are sometimes calcifications that are not present in the Tufted angioma. Although some authors report that the Kaposiform hemangioendothelioma shows a lower vascular density than infantile hemangioma [33], others argue that the color Doppler shows a high vascular density with the presence of vessels already recognizable at B-mode [9, 38] (Fig. 11).
Fig. 10.

Kaposiform hemangioendothelioma in a 2-day-old girl. The “extended view” sonogram shows a large subcutaneous mass of the abdominal wall (arrows). The mass is heterogeneous with some vessels visible on the gray-scale image. L liver, S spleen
Fig. 11.

Kaposiform hemangioendothelioma in a 2-day-old girl. Color Doppler sonogram confirms the high vascular density of lesion
Malignant tumors
Angiosarcoma
The angiosarcoma is an extremely rare tumor. Only 2% of vascular tumors are malignant, and angiosarcoma accounts for 0.5% of all pediatric sarcomas [13]. It is a lesion more frequently localized to the limbs. At the moment there are no pediatric ultrasound reports of soft tissue angiosarcoma [9].
Vascular malformations
Vascular malformations are the second large class of ISSVA classification [6]. They are a heterogeneous group of congenital lesions present at birth but sometimes not clinically evident [39]. They tend to grow slowly in proportion to the patient, although some conditions—trauma, hormonal changes, internal bleeding, and pregnancy—can lead to sudden and tumultuous growth [1, 39]. The revision of the ISSVA classification of 2018, confirming when already proposed in 2014, divides vascular malformations into four categories: simple, combined, of a major vessel, and associated with other anomalies. These categories are based on the presence of one or more types of vessels combined with each other in the same lesion.
Here we will deal mainly with simple malformations, which are in turn divided into further categories according to the vessels involved: capillaries, venous, lymphatic, and arteriovenous.
From a practical point of view, it is useful to distinguish vascular malformations on the basis of Mulliken’s proposal [1, 5] according to their behavior to the spectral analysis in high-flow lesions and in slow-flow lesions. The former always provide for the presence of arteries and often can be difficult to distinguish from vascular tumors such as infantile hemangioma [14].
Capillary malformation
This category includes various forms of lesions, some common and others very rare. In general, being very superficial, clinically they do not present many problems with the exception of esthetic implications. However, their importance lies in the fact that they can often be associated with other diseases, including, for example, Sturge–Weber syndrome, Parkers–Weber syndrome, or to forms of overgrowth [40, 41].
With normal ultrasound probes, the capillary malformation is not visible in a direct way but only as a nonspecific thickening of the skin and subcutaneous tissue compared with the contralateral healthy side. Only with dedicated dermatological probes of 20 MHz is it possible to detect an increase in vascularization [40, 42, 43].
Venous malformation
Venous malformations are the most frequent form of vascular malformations with a prevalence of about 1% [44, 45]. Their presentation may be varied; they may appear as a group of ectatic and dysplastic superficial veins or, more frequently, be deeper and appear as a real mass in soft tissues with a bluish appearance of the superficial skin [1, 39]. These masses are always soft and compressible (Fig. 12). Only in rare cases can they increase in consistency due to the formation of internal clots. Like all vascular malformations, they can rapidly grow and become symptomatic with puberty or pregnancy.
Fig. 12.

Venous malformation. Compressible mass in the subcutaneous soft tissue (arrow). Note the dysplastic ectatic superficial veins (arrowheads) and the bluish discoloration of the overlying skin
Sonographically, venous malformations appear as well-margined masses with a “spongiform” heterogeneous echostructure (hypoechoic venous spaces separated by hyperechoic septa), which is hypoechoic in comparison to the surrounding tissues. Isoechoic or hyperechogenic echostructure can rarely occur [14, 46]. The mass is always well compressible. Sometimes it is possible to identify anechoic tubular structures that are recognized as vascular channels [47]. Certainly the ultrasound aspect is nonspecific, and it is often not possible to make a differential diagnosis. However, it is a pathognomonic sign that, in addition to the exploration in B-mode, aids in the diagnosis of venous malformation: the presence of a phlebolith (i.e., an intralesional calcification). The presence of phleboliths is extremely rare in the other masses of soft tissues in the pediatric age. Unfortunately, this sign is not frequent in venous malformations being present, depending on the various series, from 9 to 16% of cases [14, 46] (Fig. 13).
Fig. 13.

Venous malformation in 13-year-old girl. Sonogram shows well-margined masses with a “spongiform” echostructure, which is hypoechoic in comparison to the surrounding tissues. The presence of intralesional phlebolith (arrow)
In the color Doppler examination, venous malformations are slow flow lesions. Vascular density is very low. Veins show low-velocity flow with non-modulated spectrum (Fig. 14). Sometimes the flows are so slow that they are not identifiable [46]. In this case, a light compression on the lesion may be useful to reduce the caliber of the vessels and try to increase the velocity of the intravascular flow [34]. The absence of vascularization may also be due to extensive thrombosis of the vessels involved which must therefore be recognized [39]. Venous malformations can often occur near the joints. In this case, it is necessary to identify whether the lesion has a direct involvement with the joint, since the possibility of hemarthrosis and subsequent damage of the articular cartilages are frequent [48]. Given the extreme variety of clinical and ultrasound presentations of vascular malformations, in case of doubt or, as mentioned, in doubt of joint involvement, it is always advisable to perform an MRI examination.
Fig. 14.

Venous malformation in 5-year-old boy. Spectral analysis shows a low-velocity flow with non-modulated spectrum
Lymphatic malformation
The lymphatic malformations are divided into macrocystic, microcystic, and mixed type.
The criterion of distinction between the macrocystic and microcystic form is not univocal. According to some, a 1–2 cm cut-off must be identified to distinguish between the two forms, while according to others, macrocystic lesions should be considered as those that can be reduced by aspiration and sclerotization [8].
The term “lymphatic malformation” has now replaced the old name of “lymphangioma” (whose suffix ‘oma’ should be applied to lesions with cell proliferation) [1, 39]. Clinically, like the previous ones, the lymphatic malformations are slow-growing lesions that can rapidly increase in size in case of intralesional bleeding. In the case of superficial microcystic forms, it is possible to find the presence of micro-vesicles present on the skin or on the mucous membranes.
Lymphatic malformations are more frequently superficial, although they can also arise in deeper regions [49]. The most frequent sites are the neck, the axillary region, and the mediastinum [1, 7, 34, 39].
With US the macrocystic lymphatic malformations, which are the most frequent, appear as lesions containing numerous cystic formations of variable dimensions with liquid content separated by thin hyperechogenic septa (Fig. 15). The lesion is deformable, and compression with the probe alters the shape of the cysts that never collapse completely, unlike the venous malformations [50–52]. At the B-mode, the cystic spaces can be anechoic or have a variable degree of echogenicity in relation to the presence of hemorrhages or infections. In some cases, the formation of fluid–fluid levels is possible (Fig. 16).
Fig. 15.

Macrocystic lymphatic malformation in 1-month-old boy. Sonogram shows multicystic mass in the thoracic subcutaneous tissue. The cystic spaces are anechoic and separated by septa
Fig. 16.

Macrocystic lymphatic malformation in 13-month-old boy. Sonogram shows multicystic mass in the neck subcutaneous tissue. The cystic spaces are hyperechoic from hemorrhage. A fluid-debris level is seen within a cyst (arrow)
The microcystic lymphatic malformations often appear as solid hyperechoic formations in which some scattered cysts may be present. This aspect is due to the presence of numerous cysts that are too small to be displayed with probe resolution [53, 54].
On the color Doppler examination, the malformation is a slow flow lesion. Vascular signals are generally absent. In some cases, it is possible to identify arterial vessels confined in the larger septa. At the spectral examination, the arterial flows show medium–low velocities and, above all, high resistance indices (Fig. 17). This aspect distinguishes them from hemangiomas where arterial resistance is low [14].
Fig. 17.

Macrocystic lymphatic malformation in 1-month-old boy. Doppler sonogram shows flow that is confined to the septa. Spectral analysis shows arterial flow with high resistance
Arteriovenous malformation
Arteriovenous malformations consist of an abnormal network of arterial and venous vessels with scarce or absent presence of capillaries.
They are high-flow lesions and are often in differential diagnosis with hemangiomas. The clinical evolution, from quiescent lesion to hemorrhage and heart failure, is described by the Schobinger classification.1
On ultrasound examination, the arteriovenous malformations can have a very diversified appearance—from a conglomerate of moderate-sized vessels that determine a lesion that can be detected in soft tissues [55], up to a tangle of vessels that do not determine a real “mass effect” and are therefore poorly identifiable at the B-mode exam and can only be highlighted with the color Doppler exam [14]. One element to consider is the presence of adipose tissue around the arteriovenous malformation due to a fibroadipose proliferation [34] that may result in a thin hyperechoic rim that delimits the lesion (Fig. 18).
Fig. 18.

Arteriovenous malformation in 3-month-old boy. Hypoechoic subcutaneous lesion (*) with thin hyperechoic rim (arrows)
At the color Doppler examination, the malformation is a high flow lesion. There are numerous vessels showing “high vascular density” with multidirectional flow. The spectral Doppler analysis shows arterial vessels with high-velocity flow and low resistive index (RI) (Fig. 19). Compared to hemangiomas, the venous vessels have a higher average velocity of flow, with an “arterialized” wave for the transmission of the systolic pulse to the venous bed due to the lack of the capillary network [1, 7, 14, 39] (Fig. 20).
Fig. 19.
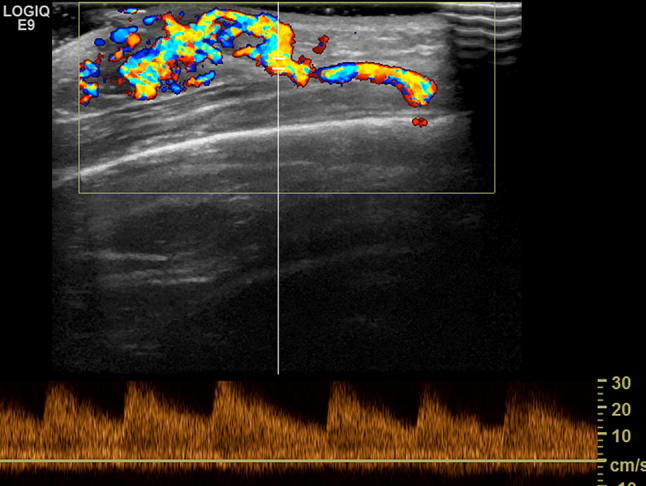
Arteriovenous malformation in 4-year-old boy. Color Doppler shows arterial flow with low resistance and high velocities
Fig. 20.
Spectral venous analysis. a Non-pulsatile venous flow in infantile hemangioma; b pulsatile venous flow in the arteriovenous malformation
Conclusions
Typically, vascular anomalies may appear as isolated lesions, but both the clinician and the radiologist must know that they are often associated with regional or diffuse diseases (Table 1). The treatment of these diseases goes beyond the scope of this brief review. For their study, we refer to more specialized texts.
Table 1.
Summary of association between vascular anomalies with other lesion
| Vascular tumors: association with other lesions | |
| PHACE association/syndrome | Posterior fossa malformations, hemangioma, arterial anomalies, cardiovascular anomalies, eye anomalies, sternal clefting and/or supraumbilical raphe |
| LUMBAR (SACRAL, PELVIS) association/syndrome | Lower body hemangioma, urogenital anomalies, ulceration, myelopathy, bony deformities, anorectal malformations, arterial anomalies, and renal anomalies |
| Vascular malformations: association with other lesions | |
| Klippel–Trenaunay syndrome: CM + VM ± LM + limb overgrowth | |
| Parkes Weber syndrome: CM + AVF + limb overgrowth | |
| Saervelle–Martorell syndrome: limb VM + bone undergrowth | |
| Sturge–Weber syndrome: facial + leptomeningeal CM + eye anomalies ± bone and/or soft tissue overgrowth | |
| Limb CM + congenital non-progressive limb overgrowth | |
| Maffucci syndrome: VM ± spindle-cell hemangioma + enchondroma | |
| Macrocephaly-CM (M-CM/MCAP) | |
| Microcephaly-CM (MICCAP) | |
| CLOVES syndrome: LM + VM + CM ± AVM + lipomatous overgrowth | |
| Proteus syndrome: CM, VM and/or LM + asymmetrical somatic overgrowth | |
| Bannayan–Riley–Ruvalcaba sd: AVM + VM + macrocephaly, lipomatous overgrowth | |
| CLAPO syndrome: lower lip CM + face and neck LM + asymmetry and partial/generalized overgrowth | |
CM capillary malformation, VM venous malformation, LM lymphatic malformation, AVM arteriovenous malformation, M-CM macrocephaly-capillary malformation, MCAP megalencephaly-capillary malformation-polymicrogyria, MICCAP microcephaly-capillary malformation, CLOVES congenital lipomatous overgrowth, vascular malformations, epidermal nevi, skeletal/scoliosis and spinal abnormalities, CLAPO lower lip CM + face and neck LM + asymmetry and partial/generalized overgrowth
Table from Ref. [6]
As repeated several times, the overlap of the ultrasound and color Doppler findings, both among the vascular anomalies and neoplastic lesions of different nature, often makes a safe differential diagnosis difficult if not impossible. However, we must remember that ultrasound is often not asked for a diagnosis of nature but an examination that is scrupulous and that gives information as completely and precisely as possible. For these reasons, we consider it useful to propose a report scheme to standardize the examination, so as to reduce as far as possible the dependency on the operator of the ultrasound and to improve the communication between the radiologist and the clinician.
The scheme is shown in Table 2.
Table 2.
Proposal for report scheme of the eco-color Doppler exam
| B-mode |
| Locate the lesion (subcutaneous tissue, beyond the fascia, in the muscle, etc.) |
| Describe the lesion (hard/soft, margins, echostructure: homogeneous/inhomogeneous) |
| Measure the lesion (three diameters!) |
| Describe the tissues surrounding the lesion (thickened/non-thickened) |
| Color Doppler |
| Non-vascularized lesion |
| Vascularized mass |
| Vascular lesion |
| Spectral analysis: |
| “High” or “low” flow lesion |
| Describe Arterial Resistivity Index (RI) |
| Describe venous spectrum: “flat” or “arterialized” |
Indication of any supplementary investigations and/or follow-up diagnostic hypothesis (if possible)
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its later amendments.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
Additional informed consent was obtained from all the patients for which identifying information is not included in this article.
Footnotes
References
- 1.Mulliken JB, Burrows PE, Fishman SJ. Mulliken and Young’s vascular anomalies—hemangiomas and malformation. Oxford Med Online. 2013 [Google Scholar]
- 2.Alibert JL. Nosologie naturelle ou les maladies du corp humain distribuees par familless. Paris: Caille and Ravier; 1817. pp. 349–351. [Google Scholar]
- 3.Virchow R. Die krankhaften Geswulsthe. Berlin: Hirschwald; 1863. Angiome; pp. 306–425. [Google Scholar]
- 4.Malan E. Vascular malformation (Angiodysplasias) Milan: Carlo Erba; 1974. [Google Scholar]
- 5.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 6.International Society for the Study of Vascular Anomalies (2018) ISSVA classification for vascular anomalies—2018. ISSVA
- 7.Kollipara R, Odhav A, Rentas KE, et al. Vascular anomalies in pediatric patients: updated classification, imaging, and therapy. Radiol Clin N Am. 2013;51:659–672. doi: 10.1016/j.rcl.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Wassef M, Blei F, Adams D, et al. Vascular Anomalies Classification: recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:e203–e214. doi: 10.1542/peds.2014-3673. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CM, Navarro OM. Clinical and sonographic features of pediatric soft-tissue vascular anomalies part 1: classification, sonographic approach and vascular tumors. Pediatr Radiol. 2017;47:1–12. doi: 10.1007/s00247-017-3885-y. [DOI] [PubMed] [Google Scholar]
- 10.Caprio MG, Di Serafino M, Pontillo G, et al. Paediatric neck ultrasonography: a pictorial essay. J Ultrasound. 2018 doi: 10.1007/s40477-018-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kollipara R, Dinneen L, Rentas KE, et al. Current classification and terminology of pediatric vascular anomalies. Am J Roentgenol. 2013;201:1124–1135. doi: 10.2214/AJR.12.10517. [DOI] [PubMed] [Google Scholar]
- 12.Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol. 2004;50:875–882. doi: 10.1016/j.jaad.2003.10.670. [DOI] [PubMed] [Google Scholar]
- 13.Merrow AC, Gupta A, Patel MN, Adams DM. 2014 Revised Classification of Vascular Lesions from the International Society for the Study of Vascular Anomalies: radiologic–pathologic update. RadioGraphics. 2016;36:1494–1516. doi: 10.1148/rg.2016150197. [DOI] [PubMed] [Google Scholar]
- 14.Paltiel HJ, Burrows PE, Kozakewich HP, et al. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology. 2000;214:747–754. doi: 10.1148/radiology.214.3.r00mr21747. [DOI] [PubMed] [Google Scholar]
- 15.Razek AA, Huang BY. Soft tissue tumors of the head and neck: imaging-based review of the WHO classification. Radiographics. 2011;31:1923–1954. doi: 10.1148/rg.317115095. [DOI] [PubMed] [Google Scholar]
- 16.Dubois J, Patriquin HB, Garel L, et al. Soft-tissue hemangiomas in infants and children: diagnosis using Doppler sonography. Am J Roentgenol. 1998;171:247–252. doi: 10.2214/ajr.171.1.9648798. [DOI] [PubMed] [Google Scholar]
- 17.Behr GG, Johnson C. Vascular anomalies: hemangiomas and beyond—Part I, fast-flow lesions. Am J Roentgenol. 2013;200:414–422. doi: 10.2214/AJR.11.7852. [DOI] [PubMed] [Google Scholar]
- 18.Manaster BJ. Soft-tissue masses: optimal imaging protocol and reporting. Am J Roentgenol. 2013;201:505–514. doi: 10.2214/AJR.13.10660. [DOI] [PubMed] [Google Scholar]
- 19.Restrepo R, Francavilla ML, Mas R, Lee EY. Up-to-date practical imaging evaluation of neonatal soft-tissue tumors: what radiologists need to know. Am J Roentgenol. 2017;2017:1–10. doi: 10.2214/AJR.16.17576. [DOI] [PubMed] [Google Scholar]
- 20.Wu JS, Hochman MG. Soft-tissue tumors and tumorlike lesions: a systematic imaging approach 1. Radiology. 2009;253:297–316. doi: 10.1148/radiol.2532081199. [DOI] [PubMed] [Google Scholar]
- 21.Kassarjian A, Dubois J, Paltiel HJ, et al. Infantile hepatic hemangiomas: clinical and imaging findings and their correlation with therapy. Am J Roentgenol. 2004;182:785–795. doi: 10.2214/ajr.182.3.1820785. [DOI] [PubMed] [Google Scholar]
- 22.Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329–335. doi: 10.1016/S0022-3476(96)70276-7. [DOI] [PubMed] [Google Scholar]
- 23.Dubois J, Rypens F. Vascular anomalies. ultrasound clin. 2009;4:471–495. doi: 10.1016/j.cult.2009.11.002. [DOI] [Google Scholar]
- 24.Krol A, MacArthur CJ. Congenital hemangiomas: rapidly involuting and noninvoluting congenital hemangiomas. Arch Facial Plast Surg. 2005;7:307–311. doi: 10.1001/archfaci.7.5.307. [DOI] [PubMed] [Google Scholar]
- 25.Berlien HP. Hemangiomas Vasc. Malformations. Milan: Springer; 2009. Classification of infantile hemangiomas and other congenital vascular tumors BT—hemangiomas and vascular malformations; pp. 23–34. [Google Scholar]
- 26.Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647–1654. doi: 10.1097/00006534-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 27.A.E. K-B Congenital hemangioma: a diagnostic challenge. Pediatr Dermatol. 2017;34:S203–S204. doi: 10.1111/pde.13142. [DOI] [Google Scholar]
- 28.Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75–79. doi: 10.1016/j.jaad.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Lowe LH, Marchant TC, Rivard DC, Scherbel AJ. Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol. 2012;47:106–117. doi: 10.1053/j.ro.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Gorincour G, Kokta V, Rypens F, et al. Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol. 2005;35:1178–1185. doi: 10.1007/s00247-005-1557-9. [DOI] [PubMed] [Google Scholar]
- 31.Rogers M, Lam A, Fischer G. Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH) Pediatr Dermatol. 2002;19:5–11. doi: 10.1046/j.1525-1470.2002.00011.x. [DOI] [PubMed] [Google Scholar]
- 32.International Society for the Study of Vascular Anomalies (2014) ISSVA classification for vascular anomalies—2014. ISSVA. 10.1542/peds.2014-3673
- 33.Dubois J, Garel L, David M, Powell J. Vascular soft-tissue tumors in infancy: distinguishing features on doppler sonography. Am J Roentgenol. 2002;178:1541–1545. doi: 10.2214/ajr.178.6.1781541. [DOI] [PubMed] [Google Scholar]
- 34.Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol. 2010;40:895–905. doi: 10.1007/s00247-010-1621-y. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Jiang L, Wu D, et al. Clinicopathological features of Kaposiform hemangioendothelioma. Int J Clin Exp Pathol. 2015;8:13711–13718. [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández Y, Bernabeu-Wittel M, García-Morillo JS. Kaposiform hemangioendothelioma. Eur J Intern Med. 2009;20:106–113. doi: 10.1016/j.ejim.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Vanliooteghem O, André J, Bruderer P, et al. Tufted angioma, a particular form of angioma. Dev Neurosci. 1996;194:402–404. doi: 10.1159/000246161. [DOI] [PubMed] [Google Scholar]
- 38.Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German soft tissue sarcoma cooperative group. Med Pediatr Oncol. 2002;39:109–114. doi: 10.1002/mpo.10078. [DOI] [PubMed] [Google Scholar]
- 39.Johnson CM, Navarro OM. Clinical and sonographic features of pediatric soft-tissue vascular anomalies part 2: vascular malformations. Pediatr Radiol. 2017;47:1196–1208. doi: 10.1007/s00247-017-3906-x. [DOI] [PubMed] [Google Scholar]
- 40.Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589–594. doi: 10.1016/j.jaad.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 41.Peterman CM, Vadeboncoeur S, Mulliken JB, et al. Wilms tumor screening in diffuse capillary malformation with overgrowth and macrocephaly–capillary malformation: a retrospective study. J Am Acad Dermatol. 2017;77:874–878. doi: 10.1016/j.jaad.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Kim C, Ko CJ, Baker KE, Antaya RJ. Histopathologic and ultrasound characteristics of cutaneous capillary malformations in a patient with capillary malformation–arteriovenous malformation syndrome. Pediatr Dermatol. 2015;32:128–131. doi: 10.1111/pde.12188. [DOI] [PubMed] [Google Scholar]
- 43.Alfageme Roldán F, Salgüero Fernández I, Zamanta Muñoz Garza F, Roustán Gullón G. Update on the use of ultrasound in vascular anomalies. Actas Dermosifiliogr. 2016;107:284–293. doi: 10.1016/j.ad.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Puig S, Aref H, Chigot V, et al. Classification of venous malformations in children and implications for sclerotherapy. Pediatr Radiol. 2003;33:99–103. doi: 10.1007/s00247-002-0838-9. [DOI] [PubMed] [Google Scholar]
- 45.Legiehn GM, Heran MKS. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin N Am. 2008;46:545–597. doi: 10.1016/j.rcl.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Trop I, Dubois J, Guibaud L, et al. Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology. 1999;212:841–845. doi: 10.1148/radiology.212.3.r99au11841. [DOI] [PubMed] [Google Scholar]
- 47.Behr GG, Johnson CM. Vascular anomalies: hemangiomas and beyond—Part 2, slow-flow lesions. Am J Roentgenol. 2013;200:423–436. doi: 10.2214/AJR.11.7853. [DOI] [PubMed] [Google Scholar]
- 48.Dalmonte P, Granata C, Fulcheri E, et al. Intra-articular venous malformations of the knee. J Pediatr Orthop. 2012;32:394–398. doi: 10.1097/BPO.0b013e31824b29ef. [DOI] [PubMed] [Google Scholar]
- 49.Lin JI, Fisher J, Caty MG. Newborn intraabdominal cystic lymphatic malformations. Semin Pediatr Surg. 2000;9:141–145. doi: 10.1053/spsu.2000.7563. [DOI] [PubMed] [Google Scholar]
- 50.Morrow MS, Oliveira AM. Imaging of lumps and bumps in pediatric patients: an algorithm for appropriate imaging and pictorial review. Semin Ultrasound CT MRI. 2014;35:415–429. doi: 10.1053/j.sult.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Wagner JM, Lamprich BK. Ultrasonography of lumps and bumps. Ultrasound Clin. 2014;9:373–390. doi: 10.1016/j.cult.2014.02.004. [DOI] [Google Scholar]
- 52.Bansal AG, Rosenberg HK. Sonography of pediatric superficial lumps and bumps: illustrative examples from head to toe. Pediatr Radiol. 2017;47:1171–1183. doi: 10.1007/s00247-017-3859-0. [DOI] [PubMed] [Google Scholar]
- 53.Dubois J, Garel L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol. 1999;29:879–893. doi: 10.1007/s002470050718. [DOI] [PubMed] [Google Scholar]
- 54.Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178–185. doi: 10.1053/j.sempedsurg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Dunham GM, Ingraham CR, Maki JH, Vaidya SS. Finding the nidus: detection and workup of non-central nervous system arteriovenous malformations. RadioGraphics. 2016;36:891–903. doi: 10.1148/rg.2016150177. [DOI] [PubMed] [Google Scholar]
- 56.Mulliken JB (2007) Vascular anomalies. Grabb Smith’s Plast Surg. 10.1016/0007-1226(91)90114-y
- 57.Mulliken JB. Color atlas of vascular tumors and vascular malformations. Plast Reconstr Surg. 2008;121:333. doi: 10.1097/01.prs.0000302375.97376.7f. [DOI] [Google Scholar]
- 58.Enjolras O, Wassef M, et al (2006) Color atlas of vascular tumors and vascular malformations. http://books.google.com