Abstract
Purpose
Typhlitis, also known as neutropenic colitis, is a rare inflammatory condition and a potentially life-threatening disease process that typically involves the cecum. Delay in diagnosis may lead to a fatal prognosis with a death rate of 21–48%. Ultrasound evaluation of right lower quadrant may lead to an accurate and rapid diagnosis.
Methods
We describe the case of a 59-year-old female with advanced Churg–Strauss syndrome treated with cyclophosphamide, with acute right lower quadrant pain.
Results
Ultrasound was the first diagnostic step in the diagnosis of typhlitis. Sonographic findings were comparable to CT imaging.
Conclusions
Bowel bedside ultrasound evaluation in emergency settings may lead to a prompt and definitive diagnosis. Although CT is considered the gold standard in the diagnosis and staging of neutropenic colitis, ultrasound was able to identify the pathology accurately. Ultrasound findings of typhlitis are highly characteristic, showing circumferential wall thickening with predominant submucosa.
Keywords: Bedside ultrasound, Typhlitis, Bedside, Acute colitis, Emergency ultrasound
SOMMARIO
La tiflite, nota anche come colite neutropenica, è una rara forma di colite infiammatoria potenzialmente mortale (21–48%) che coinvolge il cieco, correlata ad alta mortalità se diagnosticata tardivamente. La tipica sintomatologia e localizzazione a livello cecale, rendono la valutazione ecografica e la conoscenza della patologia fondamentale, consentendo una diagnosi differenziale rapida ed efficace. Descriviamo il caso di una donna di 59 anni affetta da sindrome di Churg Strauss, in trattamento con ciclofosfamide che presentava dolore addominale localizzato al quadrante inferiore destro. L’ecografia della fossa iliaca destra ha dimostrato un quadro caratteristico e sovrapponibile ai reperti TC, con presenza di ispessimento e stratificazione parietale del ceco e dell’ultima ansa con prevalenza della sottomucosa. L’esame ecografico dell’intestino in emergenza è in grado di diagnosticare rapidamente ed efficacemente molte patologie intestinali acute, consentendo di differenziare tra patologie chirurgiche non chirurgiche. Nel caso della colite neutropenica, anche se La TC è ancora considerata il gold standard diagnostico, l’esame ecografico può identificare correttamente il quadro patologico ed essere una valido strumento diagnostico per il monitoraggio. I segni ecografici di tifilite sono estremamente caratteristici mostrando un incremento di spessore di parete circonferenziale con prevalenza ed iperecogenicità della sottomucosa.
Introduction
Typhlitis, also known as neutropenic colitis, is a rare inflammatory condition and a potentially life-threatening disease process that typically involves the cecum; sometimes, the inflammatory process can spread to the ascending colon or to the terminal ileum. Although it is rare in adults, it should be considered in the differential diagnosis of acute lower quadrant pain in immunosuppressed patients undergoing chemotherapy for hematological malignancies and solid tumors (breast, lung, colorectal and ovarian cancer). The exact pathogenesis of typhlitis was not completely understood; probably, it involves a combination of factors including direct mucosal damage by cytotoxic drugs, severe neutropenia, and impaired host defense. Typhlitis is characterized by bowel wall edema, engorged vessels, disrupted mucosal surface and ulceration which become more vulnerable to bacterial intramural invasion, translocation and hemorrhage. Typhlitis can rapidly progress to intestinal perforation, multisystem organ failure, and sepsis. Presenting signs and symptoms may include fever, abdominal pain, nausea, vomiting, and diarrhea. Rapid identification and timely aggressive medical and/or surgical interventions are the cornerstones for the survival of these patients [1–4].
Case report
We reported a 59-year-old female patient, diagnosed with Churg–Strauss syndrome, treated with cyclophosphamide and steroids. She presented at the emergency department with abdominal pain. Clinically, she presented with abdominal distension, tenderness on palpation in the right lower quadrant and fever (38.5 °C). Laboratory investigation showed: white cell count 10.06 (103/mm3), hemoglobin 9.4 g/dL, platelet count 164,000, lymphocytopenia (5.9%), neutrophilia (87.7%), and lactic dehydrogenase 312 IU. Ultrasound was performed as first imaging modality. Abdominal ultrasound showed free fluid in the pelvis, and parenchymatous organs were unremarkable. In the right lower quadrant, cecum was identified and it showed circumferential wall thickening with predominant submucosa, extending to the ascending colon, ileocecal valve, and the terminal ileum. Stratified appearance of bowel wall was observed, and there were no interruption or focal irregularities of the bowel wall (Fig. 1). Terminal ileum appeared hypokinetic, distended and fluid filled with parietal thickening. Color Doppler ultrasound revealed increased vascularity (Fig. 2a, b). Pericecal lymphadenopathies were observed. Appendix was not identified. Computed tomography (CT) with intravenous contrast was performed demonstrating no signs of perforation and regular opacification of arteries. Right lower quadrant CT findings matched completely with the ultrasound findings. The circumferential wall thickening was confirmed and showed edematous submucosa (hypodense) and hyperenhancing mucosa. CT better demonstrated a retrocecal appendix and the presence of mesenteric and anti-mesenteric lymphadenopathies. Fat stranding was observed. Integrity of cecum wall was not surely defined; according to the combined evaluation with ultrasound, no focal wall interruptions were described (Figs. 3, 4). The patient was admitted to the hospital and underwent conservative management with bowel rest, and empirically treated with antibiotics (intravenous cefepime and metronidazole, oral vancomycin), fluids, and steroids. Stool cultures were negative for Salmonella, Shigella, Campylobacter, and Clostridium difficile. During hospitalization, patient improved and underwent colonoscopy after 5 days of conservative treatment. Colonoscopy showed diffuse cecal inflammation and edema, many hyperemic plaques and two large ulcerations (Fig. 5). Ultrasound follow-up was performed. After 12 days, complete regression of symptoms occurred.
Fig. 1.
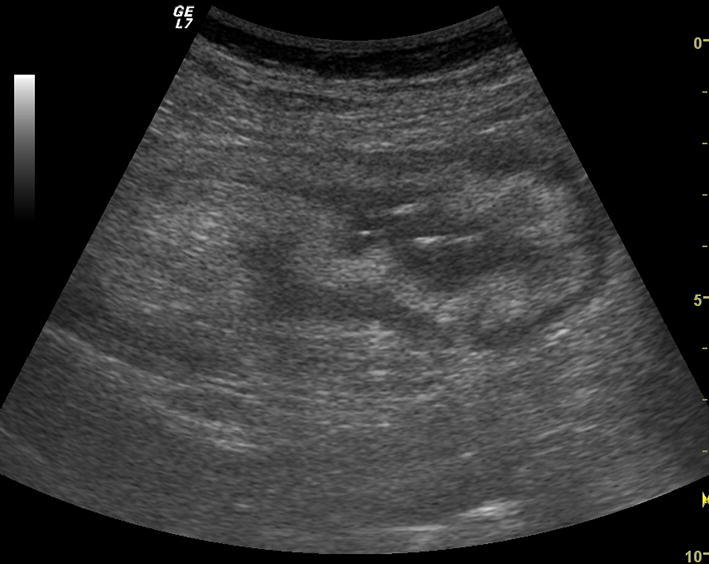
Ultrasound identification of the cecum. Homogenous stratified and circumferential thickening of cecum with preserved and lobulated margins. There is halo sign with predominant submucosa that appears slightly hyperechogenic
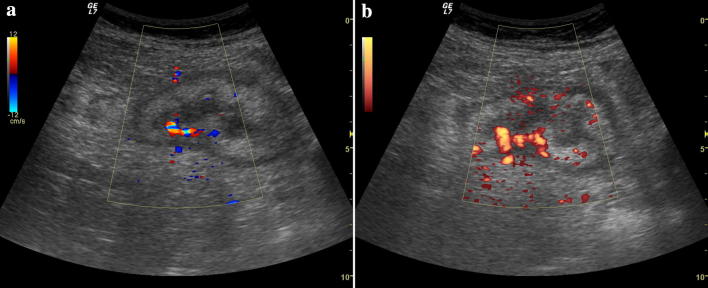
Fig. 2.
Ultrasound color and power Doppler evaluation of the cecum. There is increased vascular signal, indicating active inflammation
Fig. 3.
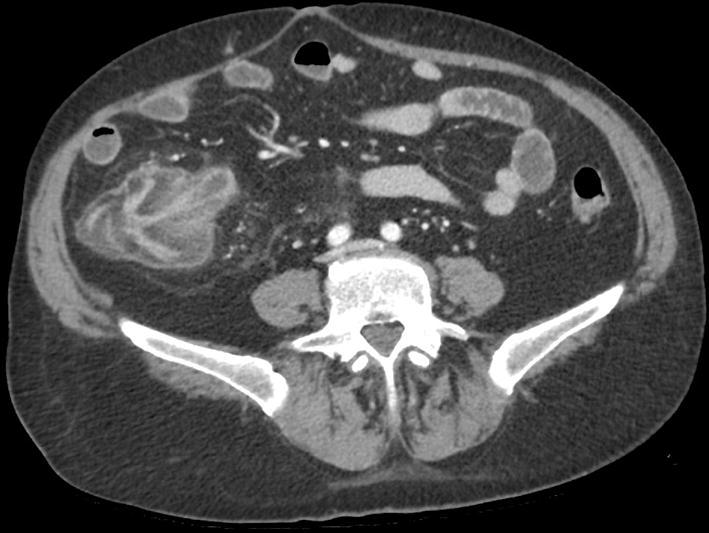
CT with intravenous contrast. Axial image at the ileocecal valve level showing marked and circumferential thickening of the cecum and terminal ileum. The submucosa appears hypodense due to edema; the mucosa is hyperenhancing due the inflammation process. Fat stranding is present
Fig. 4.
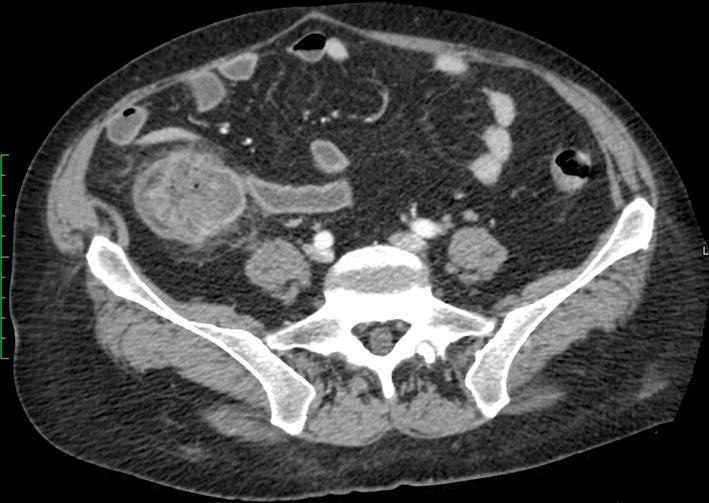
CT with intravenous contrast. Axial image at the level of cecal fundus, stratified and thickened appearance of cecum, terminal ileum appears distended and fluid filled. There is pericecal fat stranding. The anterior margin of cecum appears slightly irregular. Focal perforation was not excluded. The previous ultrasound examination showing parietal integrity permitted to consider the pathology as non-complicated, so the patient underwent conservative therapy
Fig. 5.
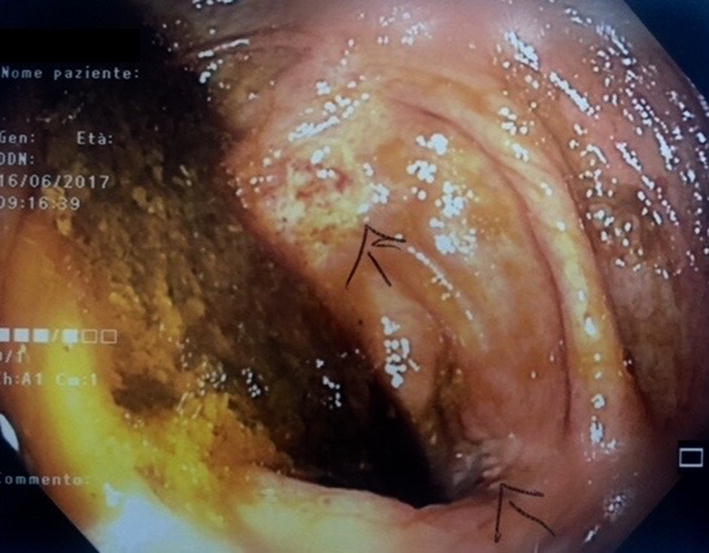
Colonoscopy was performed after patient’s relief of symptoms during the hospitalization. It showed multiple mucosal plaques and diffuse hyperemia. Mucosa was diffusely friable and hemorrhagic with superficial ulceration associated with loss of haustration and loss of normal vascular pattern
Image findings
Ultrasound findings of typhlitis matched CT findings as previously described. Circumferential wall thickening involving the cecum was present; it extended to the ascending colon and terminal ileum. Stratified appearance with a predominant hyperechogenic submucosa was well demonstrated. Terminal ileum was distended and fluid filled. Integrity of bowel wall was investigated and perforation was excluded. Ultrasound imaging revealed a diffuse thickened submucosa with circumferential aspect that extended for a long segment including the ileocecal valve and the terminal ileum which was not suggestive of malignant process or appendicitis, a regular margin and homogeneously stratified aspect of the bowel layer, and a significant increase of bowel vascularity. CT confirmed the initial ultrasound for the suspected diagnosis; although the integrity of bowel layer was undetermined at cecum level, cecum parietal integrity was better identified on ultrasound.
Discussion
Typhlitis is a rare life-threatening condition that occurs in neutropenic patients due to compromised integrity of the bowel wall. Due to its nonspecific presentation, typhlitis can mimic many other diagnoses. Delay in diagnosis may lead to a fatal prognosis with a death rate of 21–48%. Typhlitis diagnosis is based on imaging and clinical findings. Surgical treatment has a high-mortality rate; therefore, surgical management should be prompted in complicated cases such as bowel perforations, intractable gastrointestinal bleeding or generalized peritonitis [5]. Conservative management may be successful, especially if started early [4, 6]. Diagnosis generally involves fever, abdominal pain, neutropenia and thickening of abdominal wall (cecum and ascending colon) [2]. Abdominal X-rays can show dilated atonic cecum, ascending colon filled with liquid or gas, and small bowel dilatation; however, this technique has limited value due to its poor sensitivity and specificity [2, 7]. Computed tomography is considered the gold standard technique in typhlitis diagnosis, delineating circumferential wall thickening of cecum, ascending colon and terminal ileum [8], dilated cecum, inflammatory mass, pericolonic inflammation, pneumatosis, and perforation; it can also help visualize other organs and make a differential diagnosis [4, 7, 9, 10]. Ultrasonography is considered a useful imaging modality for children with suspected typhlitis [9]. Ultrasound typhlitis findings in adults are not commonly described, because adult bowel evaluation in the emergency settings is still not widely performed. The careful evaluation of bowel wall thickening combined with ancillary findings such as fluid collection, increased echogenicity of bowel layer or the mesenteric fat tissue, enlarged lymph nodes, hyperemic bowel changes, and abnormal bowel peristalsis can increase the diagnostic capability of US [11–14]. At US, typhlitis presented bowel wall thickening that produced a target or halo sign, with hyperechogenic submucosa suggestive of colitis. In our case, we demonstrated that bowel ultrasound evaluation lead to a rapid suspected diagnosis and the ultrasound findings matched completely with CT [4, 8]. The routinely use of ultrasound in acute abdominal pain evaluation may reveal unsuspected diagnosis rapidly; moreover, ultrasound appearance of bowel wall may represent an important parameter for patient follow-up, avoiding additional scans [15]. Ultrasound may play an important role in acute bowel emergencies also in adult population, and its role should be emphasized: ultrasound is readily available; the exam can be performed bedside and repeated for follow-up; the ultrasound accurately determines bowel wall thickness that represents an important parameter related to mortality rate [7]. Early ultrasound bowel examination, despite the specificity of the echographic imaging may lead to a final diagnosis in case of typhlitis, confirmed by the full correspondence between the echographic and CT imaging.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
It was a retrospective study. For this type of study, formal consent is not required, and all sensitive data were collected and protected in respect of present privacy statements.
References
- 1.Cloutier RL. Neutropenic enterocolitis. Hematol Oncol Clin N Am. 2010;3:577–584. doi: 10.1016/j.hoc.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues FG, Dasilva G, Wexner SD. Neutropenic enterocolitis. World J Gastroenterol. 2017;23(1):42–47. doi: 10.3748/wjg.v23.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo S, Bellomi M. Typhlitis in a post-chemotherapy lymphoma patient; images in clinical medicine. Ecancermedicalscience. 2010;4(1):193. doi: 10.3332/ecancer.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado NO. Neutropenic enterocolitis: a continuing medical and surgical challenge. N Am J Med Sci. 2010;2(7):293–300. [PMC free article] [PubMed] [Google Scholar]
- 5.Junpaparp P, et al. Concomitant typhlitis and Clostridium difficile colitis developed after first R-CHOP chemotherapy in a non-Hodgkin lymphoma patient. BMJ Case Rep 2013. 2013 doi: 10.1136/bcr-2013-008894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloutier RL. Neutropenic enterocolitis. Emerg Med Clin N Am. 2009;27(3):415–422. doi: 10.1016/j.emc.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Cartoni C, et al. Neutropenic enterocolitis in patients with acute leukemia: prognostic significance of bowel wall thickening detected by ultrasonography. J Clin Oncol. 2001;19(3):756–761. doi: 10.1200/JCO.2001.19.3.756. [DOI] [PubMed] [Google Scholar]
- 8.Gootenberg JE, Abbondanzo SL. Rapid diagnosis of neutropenic enterocolitis (typhlitis) by ultrasonography. Am J Pediatr Hematol Oncol. 1987;9(3):222–227. [PubMed] [Google Scholar]
- 9.McCarville MB. Evaluation of typhlitis in children: CT versus US. Pediatr Radiol. 2006;36(8):890–891. doi: 10.1007/s00247-006-0221-3. [DOI] [PubMed] [Google Scholar]
- 10.McCarville MB, et al. Typhlitis in childhood cancer. Cancer. 2005;104(2):380–387. doi: 10.1002/cncr.21134. [DOI] [PubMed] [Google Scholar]
- 11.Carter D, Eliakim R. Feasibility of bedside bowel ultrasound performed by a gastroenterologist for detection and follow-up of inflammatory bowel disease. Isr Med Assoc J. 2017;19(3):139–142. [PubMed] [Google Scholar]
- 12.Hwang JY. Emergency ultrasonography of the gastrointestinal tract of children. Ultrasonography. 2017;36(3):204–221. doi: 10.14366/usg.16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maturen KE, et al. Ultrasound imaging of bowel pathology: technique and keys to diagnosis in the acute abdomen. AJR Am J Roentgenol. 2011;197(6):W1067–W1075. doi: 10.2214/AJR.11.6594. [DOI] [PubMed] [Google Scholar]
- 14.Anupindi SA, et al. Common and uncommon applications of bowel ultrasound with pathologic correlation in children. AJR Am J Roentgenol. 2014;202(5):946–959. doi: 10.2214/AJR.13.11661. [DOI] [PubMed] [Google Scholar]
- 15.Wale A, Pilcher J. Current role of ultrasound in small bowel imaging. Semin Ultrasound CT MR. 2016;37(4):301–312. doi: 10.1053/j.sult.2016.03.001. [DOI] [PubMed] [Google Scholar]