Abstract
Objective
The purpose of this systematic review and meta-analysis was to assess the sensitivity and specificity of contrast-enhanced ultrasound (CEUS) compared to computed tomography angiography (CTA) for the detection of endoleaks within endovascular aortic aneurysm repair (EVAR) surveillance at time of follow up.
Methods
A comprehensive literature search was undertaken among the four major databases (PubMed, Embase, Scopus and Ovid) to identify all articles assessing diagnostic specificity and accuracy with comparative modality (CEUS vs CTA) for endoleaks in adult patients at time of follow-up following EVAR. Databases where evaluated and assessed to October 2018.
Results
A total of 1773 patients were analysed from across 18 included studies in the quantitative analysis of the parameters of interest. There was no significant difference in detection rate of endoleak type I with detection rate 4.3% for both groups OR 1.09, 95% CI [0.78, 1.53], p = 0.62; type II endoleak detection rate was 22% in the CEUS group vs 23% in the CTA group OR 1.16, 95% CI [0.75–1.79], p = 0.50; while type III detection rate was 1.8% in CEUS group vs 2% in CTA group OR 0.85, 95% CI [0.43, 1.68], p = 0.64. However, the sensitivity rate for endoleak detection was higher in CEUS (p = 0.001) while no difference in specificity rate was noted (p = 0.28). There was higher rate of missed endoleaks in CTA groups (n = 12 vs n = 20).
Conclusion
Evidences from this study suggest that contrast-enhanced ultrasound scan post-EVAR can be utilised as safe and effective method in screening for endoleaks during post-EVAR surveillance without exposing the patient for additional risk of radiation and contrast. CEUS conveys no inferiority to CTA in detecting endoleaks.
Keywords: Ultrasound, Angiography, Contrast study, EVAR, Computerized tomography
Introduction
Endovascular aortic aneurysm repair (EVAR), described for the first time in 1991 [1], is currently considered a valid and safe alternative in suitable patients with abdominal aortic aneurysm to open surgical repair. EVAR is associated with significantly reduced perioperative mortality and morbidity as well as shorter hospital stay [2, 3]. However, this technique is associated with risk of postoperative complications such as persistent aneurysmal growth, new aneurysm formation, endoleaks, potential rupture, device thrombosis, migration or kinking [4].
Endoleaks, defined as presence of blood within the sac, but outside of the stented device, are the most common complications post-EVAR with an incidence between 10 and 20% [5]. The most common among all endoleaks is the type II endoleak with an incidence reported around 20–44% of all endoleak types [6].
Long-term follow-up post-endovascular repair is necessary to have early detect of any complications; as well as identify the type of endoleak and aneurysmal growth and allow appropriate intervention to prevent rupture.
Currently, contrast-enhanced computed tomography angiography (CTA) is considered the gold standard for endoleak detection and classification and also long-term surveillance [7]. However, this modality of imaging is limited by lifelong patient exposure to nephrotoxic intravenous contrast [8], ionising radiation [9] and by high costs [10]
Contrast-enhanced ultrasound (CEUS) has been increasingly used in post-EVAR surveillance with the benefits of not using any nephrotoxic contrast and no radiation exposure [11, 12]. Some of the radiological appearances of type II endoleak on CTA and CEUS is briefly shown in (Figs. 1, 2, 3) [13].
Fig. 1.
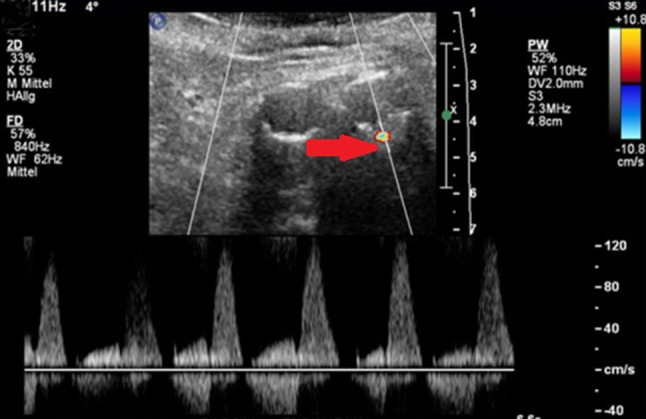
Cross-sectional view of the infrarenal aorta. Detection of an endoleak (arrow) using color and pulse-wave ultrasonics [13]
Fig. 2.
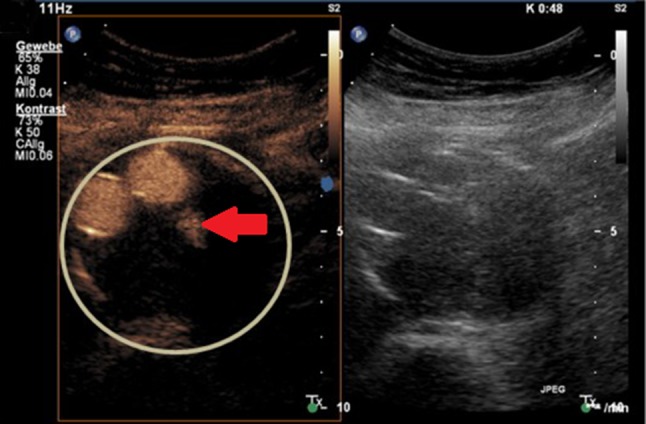
Type II endoleak from a lumbar artery following endovascular aortic repair (EVAR). Contrast mode (left) and conventional B-mode (right) simultaneously; after bolus injection of contrast agent extravascular enhancement is shown (arrow) within the aneurysm sack [13]
Fig. 3.
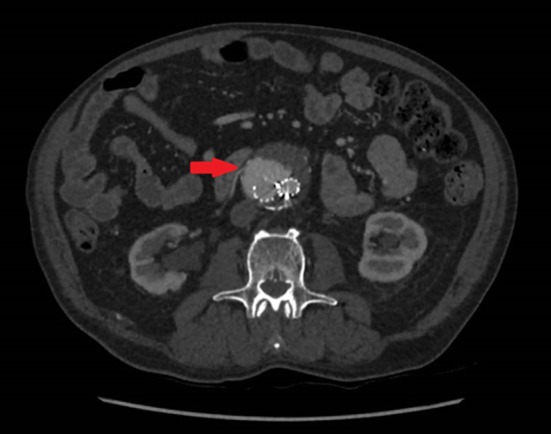
CT- angiogram Type II endoleak from an accessory right renal artery following endovascular aortic repair (EVAR) [13]
Our objective was to assess the sensitivity and specificity of CEUS vs CTA for the detection of endoleaks within EVAR surveillance at time of follow up.
Materials and methods
Literature search strategy and inclusion criteria
Electronic database searches were performed utilising PubMed, Ovid Medline, Scopus, and Embase to identify all randomised and nonrandomized controlled trials comparing the use of contrast-enhanced ultrasound (CEUS) or computed tomography angiography (CTA) at the time of follow up for endovascular abdominal aorta repair (EVAR). The studies were performed either as paired study, having CEUS and CTA for the same patient, or it was unpaired studies by comparing CTA and CEUS from different patients in the same cohort and time period. Limits were placed on articles that were comparative studies between both scanning types. Search terms included key words as ‘ultrasound’ ‘contrast enhanced’ ‘EVAR’ ‘CEUS’ ‘computed tomography’ ‘CTA’ ‘endoleak’ ‘follow up’ or ‘aortic aneurysm’. All search terms were combined with Boolean operators and searched as both key words and MeSH terms to ensure maximal sensitivity. Reference lists of papers found in the literature search were manually searched to assess suitability for inclusion in this review. Articles were first screened by three reviewers (AH, EZ and LJ) based on their titles and abstracts. All identified articles were systematically assessed using the inclusion and exclusion criteria for further study.
Selection criteria
Articles were deemed eligible for inclusion if the authors compared the sensitivity and specificity utilising CEUS and/or CTA at time of follow up post-successful EVAR. Articles were excluded if they were non-comparative studies or contained only CEUS or CTA. The target of the studies must have outlined the look for presence or absence of endoleaks including all of its subtypes. If the study reported the cohort in two different time periods, the latest study and the most updated one has been included in the analysis. Case reports, editorial, consensus documents, and expert reviews were the main exclusion criteria.
Methodological quality assessment of included studies
Qualitative assessment of included studies was performed using the Newcastle–Ottawa scale (NOS; Table 1). The NOS was devised specifically to assess the quality of non-randomised studies included in meta-analyses. This scale assesses bias of each study using a star-based rating system, with a maximum score of 9 indicating lowest risk of bias, and a minimum of 0 indicating highest risk. Scores ≥ 7 generally represent the lack of substantial bias. Quality of included studies was rated by two reviewers (GS, MJ). There were no major discrepancies. Minor discrepancies were resolved by consensus and arbitration by a third independent reviewer (AH).
Table 1.
Quality assessment of the included studies using Newcastle Ottawa Scale
| References | Selection | Comparability | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|
| Representation of patients followed up with CTA and/or CEUS post-EVAR | Selection of consecutive patients for follow up with CEUS and CTA post-EVAR | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Study controls for patient age, sex* Study controls for demographic and AAA diameter** |
Assessment of outcomes | Rate of detection of endoleaks | Follow-up long enough for outcomes to occur | |
| Abbas et al. [15] | * | * | * | ** | * | * | * | |
| Bendick et al. [11] | * | * | * | * | ** | * | * | * |
| Bredahl et al. [16] | * | * | * | ** | * | * | * | |
| Cantisani et al. [17] | * | * | * | * | ** | * | * | * |
| Clevert et al. [18] | * | * | ** | * | * | * | ||
| Giannoni et al. [20] | * | * | * | ** | * | * | * | |
| Gurtler et al. [21] | * | * | * | * | * | * | * | * |
| Clevert et al. [19] | * | * | * | * | * | * | * | |
| Henao et al. [12] | * | * | * | * | ** | * | * | * |
| Houdek et al. [22] | * | * | * | * | ** | * | * | * |
| Iezzi et al. [23] | * | * | * | * | * | * | * | * |
| McWilliams et al. [24] | * | * | * | * | ** | * | * | * |
| Millen et al. [25] | * | * | * | * | ** | * | * | * |
| Motta et al. [26] | * | * | * | * | * | * | * | * |
| Perini et al. [28] | * | * | * | * | ** | * | * | * |
| Perini et al. [27] | * | * | * | ** | * | * | * | |
| Shaeffer et al. [29] | * | * | * | * | ** | * | * | * |
| Ten Bosh et al. [30] | * | * | * | * | ** | * | * | * |
Each * indicates one score, a paper with a score of 7 or more has less chance of bias
Data extraction and critical appraisal
The main outcome measures extracted included the rate of detection of endoleaks at the time of follow up. Four different categories were assessed in the form of false positive, false negative, true positive and true negative findings between both study techniques. Rate of missed endoleak detections between each cohort were reviewed and assessed accordingly.
Statistical analysis
This meta-analysis was performed in-line with recommendations from Quality of Reporting of Meta-analysis (QUOROM) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [14]. The reported outcomes were assessed using standard and cumulative meta-analysis techniques, with odd ratios (OR) or weighted mean differences (WMD) used as summary statistics for raw data extracted from each included study. Both fixed- and random-effects models were tested. χ2 Tests were used to study heterogeneity and the I2 statistic was used to estimate the proportion of total variation across studies, owing to heterogeneity rather than chance. A cut-off threshold of 50% was chosen and values exceeding this were considered to signify substantial heterogeneity. All p values were two-sided, p value of 0.05 or less is considered as significant. Statistical analyses were performed using Review Manager V0.5.2.1 (Cochrane Collaboration, Oxford, UK).
Results
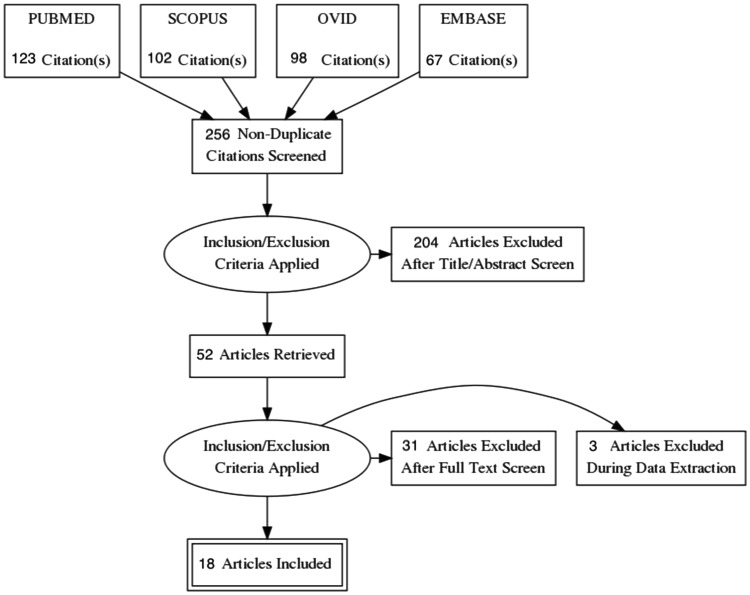
The initial electronic database search yielded 256 non-duplicate articles, of which 52 articles were retrieved for assessment in full-text. After detailed evaluation of these articles and assessment according to inclusion and exclusion criteria, only 18 comparative studies satisfied our selection criteria and were included in the qualitative and quantitative meta-analysis as shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) chart (Fig. 4). A total of 499 of paired scans were performed in 1773 patients. Table 2 shows the summary of the study characteristics.
Fig. 4.
PRSIMA flow chart of article selection for this systematic review and meta-analysis
Table 2.
Study characteristics of the included articles in our analysis
| References | Year | Country | Type of study | Total no. of patients | CEUS+ CT+ | CEUS+ CT− | CEUS− CT+ | CEUS− CT− | CEUS | CTA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity % | Specificity % | Sensitivity % | Specificity % | |||||||||
| Abbas et al. [15] | 2014 | UK | Prospective | 23 | – | 18 | 17 | – | 100 | 92 | – | – |
| Bendick et al. [11] | 2003 | USA | Prospective | 20 | – | – | – | – | 100 | – | – | – |
| Bredahl et al. [16] | 2016 | Denmark | Cross-sectional | 278 | 58 | 11 | 10 | 199 | – | – | 85.3 | 94.8 |
| Cantisani et al. [17] | 2010 | Italy, Finland | Prospective, observational | 108 | – | – | – | – | 96 | 100 | 83 | 100 |
| Clevert et al. [18] | 2009 | Germany | Prospective | 43 | 15 | 2 | – | 26 | 100 | 93 | 100 | 100 |
| Clevert et al. [19] | 2011 | Germany | Retrospective | 35 | – | – | – | – | 100 | 100 | 75 | 100 |
| Giannoni et al. [20] | 2007 | Italy | Prospective | 30 | 7 | 7 | 6 | – | – | – | – | – |
| Gurtler et al. [21] | 2013 | Germany | Retrospective | 171 | 84 | 8 | 3 | 105 | 97 | 93 | – | – |
| Henao et al. [12] | 2006 | USA | Prospective | 20 | 3 | 3 | 0 | – | – | – | – | – |
| Houdek et al. [22] | 2015 | Czech Republic | Prospective | 16 | 8 | – | 1 | – | – | – | – | – |
| Iezzi et al. [23] | 2009 | Italy | Retrospective | 84 | – | – | – | – | 98 | 82 | – | – |
| McWilliams et al. [24] | 1999 | UK | Retrospective | 18 | 3 | 6 | 0 | 11 | 100 | 85 | – | – |
| Millen et al. [25] | 2013 | UK | Prospective | 33 | 19 | – | – | – | – | – | – | – |
| Motta et al. [26] | 2012 | Italy | Prospective | 88 | 105 | 34 | 37 | – | 92 | 100 | 72 | 100 |
| Perini et al. [27] | 2012 | France | Retrospective | 62 | 5 | 1 | 2 | 54 | – | – | – | – |
| Perini et al. [28] | 2011 | France | Retrospective | 395 | 83 | 20 | 16 | 276 | – | – | – | – |
| Schaeffer et al. [29] | 2017 | USA | Retrospective | 266 | 88 | 174 | 4 | 0 | – | – | – | – |
| Ten Bosch et al. [30] | 2010 | Netherland | Prospective | 83 | 22 | 45 | 5 | 55 | – | – | – | – |
In the studies included [11, 12, 15–30], overall sensitivity and specificity were analysed for detection of all types of endoleak in the included 18 studies with a mean follow-up time 8.6 ± 6.4 months in both groups. Data analysis showed higher sensitivity 98% in the CEUS group (p = 0.001) compared with 83% in the CTA group with specificity of 93% in the CEUS group compared with 99% in the CTA group (p = 0.28).
Endoleaks
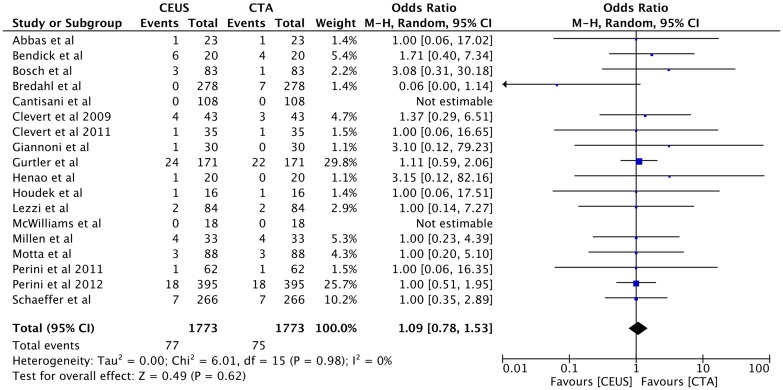
Type I endoleak was detected equally in both techniques of CEUS and CTA as 4.3% (n = 77) vs 4.3% (n = 75), OR 1.09, 95% CI [0.78, 1.53], p = 0.62 (Fig. 5).
Fig. 5.
Forest plot of Type I endoleak detection comparison
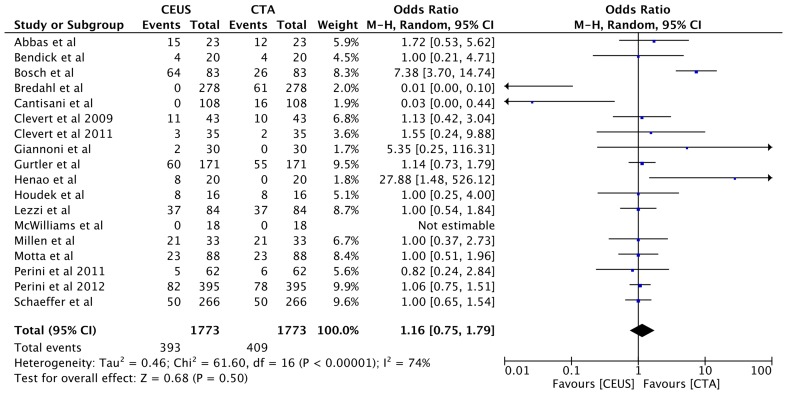
The rate of type II endoleak was detected as 22% (n = 393) using CEUS, while as 23% (n = 409) utilising CTA, but this was not statistically significant OR 1.16, 95% CI [0.75, 1.79], p = 0.50 (Fig. 6).
Fig. 6.
Forest plot of Type II endoleak detection comparison
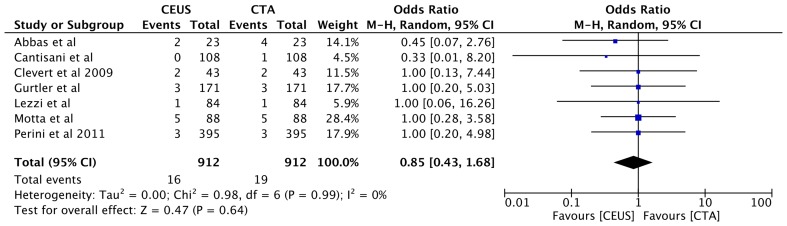
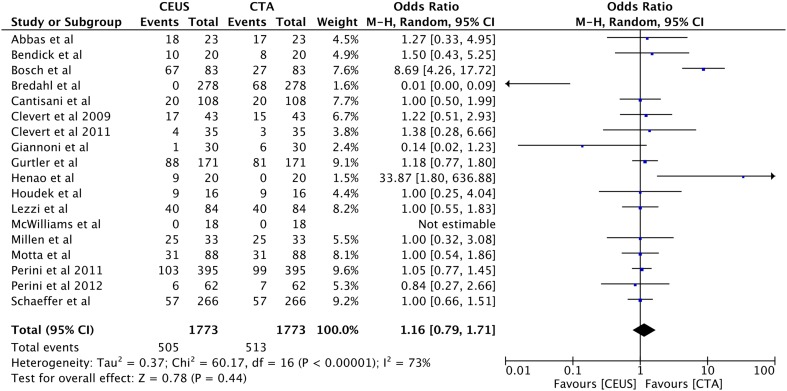
Similarly, no difference in detecting endoleak type III was noted between using CEUS and CTA (1.8%, n = 16 vs 2%, n = 19, OR 0.85, 95% CI [0.43, 1.68], p = 0.64 (Fig. 7). Furthermore, the overall rate of cumulate endoleak rate detection was similar in both CEUS and CTA (28%, n = 505 vs 29%, n = 513, OR 1.16, 95% CI [0.79, 1.71], p = 0.44 (Fig. 8).
Fig. 7.
Forest plot of Type III endoleak detection comparison
Fig. 8.
Forest plot of all type of endoleak detection comparison
Notably, there were higher rates of missed endoleaks in CTA cohort, n = 20 vs n = 12 in CEUS, of which none of these endoleaks required re-intervention in the CEUS cohort; however, 50% of these endoleaks required reinterventions in the CTA cohort. Table 3 shows the summary of endoleak rates found in each study allocated to CEUS and CTA subgroups.
Table 3.
Endoleak detection rate between using CEUS and CTA among included studies
| References | Cohort size (n) | CEUS | CTA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endoleak detected | Missed | Endoleak detected | Missed | ||||||||||||
| Type I (n) | Type II (n) | Type III (n) | Type IV (n) | Type V (n) | All types | All (n) | Type I (n) | Type II (n) | Type III (n) | Type IV (n) | Type V (n) | All types | All (n) | ||
| Abbas et al. [15] | 23 | 1 | 15 | 2 | – | – | 18 | – | 1 | 12 | 4 | – | – | 17 | 1 |
| Bendick et al. [11] | 20 | 6 | 4 | – | – | – | 10 | – | 4 | 4 | – | – | – | 8 | – |
| Bredahl et al. [16] | 278 | – | – | – | – | – | – | 10 | 7 | 61 | – | – | – | 68 | 11 |
| Cantisani et al. [17] | 108 | – | – | – | – | – | 20 | 1 | – | 16 | 1 | – | – | 20 | 4 |
| Clevert et al. [18] | 43 | 4 | 11 | 2 | – | – | 17 | – | 3 | 10 | 2 | – | – | 15 | – |
| Clevert et al. [19] | 35 | 1 | 3 | – | – | – | 4 | – | 1 | 2 | – | – | – | 3 | 1 |
| Giannoni et al. [20] | 30 | 1 | – | – | – | – | 1 | – | – | – | – | – | – | 6 | – |
| Gurtler et al. [21] | 171 | 24 | 60 | 3 | 1 | – | 88 | – | 22 | 55 | 3 | 1 | – | 81 | – |
| Henao et al. [12] | 20 | 1 | 8 | – | – | – | 9 | – | – | – | – | – | – | 0 | 3 |
| Houdek et al. [22] | 16 | 1 | 8 | – | – | – | 9 | – | 1 | 8 | – | – | – | 9 | – |
| Iezzi et al. [23] | 84 | 2 | 37 | 1 | – | – | 40 | – | 2 | 37 | 1 | – | – | 40 | – |
| McWilliams et al. [24] | 18 | – | – | – | – | – | 0 | – | – | – | – | – | – | 0 | – |
| Millen et al. [25] | 33 | 4 | 21 | – | – | – | 25 | – | 4 | 21 | – | – | – | 25 | – |
| Motta et al.[26] | 88 | 3 | 23 | 5 | – | – | 31 | 1 | 3 | 23 | 5 | – | – | 31 | – |
| Perini et al. [27] | 62 | 1 | 5 | – | – | – | 6 | – | 1 | 6 | – | – | – | 7 | – |
| Perini et al. [28] | 395 | 18 | 82 | 3 | – | – | 103 | – | 18 | 78 | 3 | – | – | 99 | – |
| Schaeffer et al. [29] | 266 | 7 | 50 | – | – | – | 57 | – | 7 | 50 | – | – | – | 57 | – |
| Ten Bosch et al. [30] | 83 | 3 | 64 | – | – | – | 67 | – | 1 | 26 | – | – | – | 27 | – |
CEUS contrast-enhanced ultrasound, CTA computed tomography angiogram
Discussion
Following EVAR, long-term surveillance aims to identify complications that may otherwise lead to aneurysm-related death [31]. There are various imaging modalities that can be used for surveillance screening including CTA, duplex ultrasonography (DUS), CEUS, magnetic resonance angiography (MRA) [32].
Given the advancements with ultrasound imaging, it is important to assess the diagnostic accuracy and sensitivity of CEUS in comparison to the already-established CTA. Moreover, given the limitations associated with CTA, there is a need to identify alternative techniques that provides the same level of diagnostic accuracy, but also have minimal side-effects.
Our results highlighted no difference between CEUS and CTA in detection rate of type I, type II and type III endoleaks as well as in detection rate of all type of endoleaks as cumulative.
CEUS demonstrated to have a higher sensitivity (98%) in detecting endoleaks when compared with CTA, although there was no significant difference in specificity rate between the two imaging modalities. In comparison with previous studies, our review included a larger number of studies (n = 18) as well as paired scans (n = 499).
Mirza et al. [33] were the first to undertake a systematic review and bivariate meta-analysis to compare sensitivity and specificity of CEUS against CTA. The analysis of 285 paired scans from seven studies demonstrated a pooled sensitivity of 0.98 and specificity of 0.88 although the study was limited the heterogeneity of analysed trials.
Subsequently, Karthikesalingam et al. [34] analysed further 961 paired scans from eleven studies comparing CEUS to CTA in detection of all endoleaks. The pooled sensitivity and specificity were respectively 0.96 and 0.85.
Currently, CTA remains the gold-standard imaging technique for post-EVAR surveillance because of its high sensitivity and specificity in detection of endoleaks of 70% and 98%, respectively. However, due to the long-term follow-up recommendations; continual exposure to ionising radiation, nephrotoxic contrast agents [8, 9] and high-cost implications [10], make CTA a less-preferred option in several centres and certain cohorts of patients [12].
CEUS is a novel alternative technique used in the detection of post-EVAR complications [15]. This imaging modality combines newer generation, non-toxic contrast agents and advanced ultrasound techniques [20]. Early studies suggested that CEUS improves sensitivity and accuracy of detecting endoleaks and classifying them into subtypes. This is based on the CEUS technique’s ability to detect velocity and direction of blood flow [21, 23].
Compared with CTA, CEUS is a cheaper alternative that is easier to perform and interpret, whilst also being minimally invasive. Furthermore, CEUS also limits exposure to ionising radiation [9] and nephrotoxic contrast agents, hence, reducing the possibility of developing contrast-induced nephrotoxicity [8].
However, CEUS imaging presents also some limitations. First of all, it remains an operator-dependent imaging modality requiring specific equipment and training. The imaging can result suboptimal by patient-related factors including high BMI, bowel gas, aortic wall calcification or ascites. CEUS is not able to visualise stent migration or fracture requiring additional plain x-rays. Furthermore, CTA may be necessary to further characterise detected endoleaks and planning further intervention.
Limitations
Our meta-analysis included 18 studies of which 7 were retrospective. Data analysis showed that CEUS is equal if not superior to CTA in detection of all type of endoleaks; CEUS, however, remains operator-dependent and this can create bias.
It has also to be taken in consideration the fact that the studies included use different types of CT scan and different US scanning protocols that may affect overall sensitivity and specificity of the study.
Conclusion
Our study demonstrated that contrast-enhanced ultrasound scan has a higher sensitivity and comparable specificity to computed tomography angiography for detection of endoleaks post-EVAR; CEUS can be utilised as safe and effective method in long-term screening during post-EVAR surveillance without exposing the patient for risk of radiation and contrast.
Funding
No funding obtained for this study.
Conflict of interest
All authors wish to disclose no conflict of interest.
Ethical approval
This article does not contain any direct studies with human participants performed by any of the authors of this paper.
Informed consent
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(6):491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 2.Elkouri S, Gloviczki P, McKusick MA, et al. Perioperative complications and early outcome after endovascular and open surgical repair of abdominal aortic aneurysms. J Vasc Surg. 2004;39:497–505. doi: 10.1016/j.jvs.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DM, Hulten EA, Ellis ST, et al. Open versus endovascular repair of abdominal aortic aneurysm in the elective and emergent setting in a pooled population of 37,781 patients: a systematic review and meta-analysis. ISRN Cardiol. 2014;2014:149243. doi: 10.1155/2014/149243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maleux G, Koolen M, Heye S. Complications after endovascular aneurysm repair. Semin Interv Radiol. 2009;26:003–009. doi: 10.1055/s-0029-1208377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choke E, Thompson M. Endoleak after endovascular aneurysm repair: current concepts. J Cardiovasc Surg. 2004;45:349. [PubMed] [Google Scholar]
- 6.Brown A, Saggu GK, Bown MJ, Sayers RD, Sidloff DA. Type II endoleaks: challenges and solutions. Vasc Health Risk Manag. 2016;12:53–63. doi: 10.2147/VHRM.S81275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fillinger MF. Postoperative imaging after endovascular AAA repair. Semin Vasc Surg. 1999;12:327–338. [PubMed] [Google Scholar]
- 8.Walsh SR, Tang TY, Boyle JR. Renal consequences of endovascular abdominal aortic aneurysm repair. J Endovasc Ther. 2008;15(1):73–82. doi: 10.1583/07-2299.1. [DOI] [PubMed] [Google Scholar]
- 9.Weerakkody RA, Walsh SR, Cousins C, Goldstone KE, Gaunt ME. Radiation exposure during endovascular aneurysm repair. Br J Surg. 2008;95:699–702. doi: 10.1002/bjs.6229. [DOI] [PubMed] [Google Scholar]
- 10.Michaels JA, Drury D, Thomas SM. Cost-effectiveness of endovascular abdominal aortic aneurysm repair. Br J Surg. 2005;92:960–967. doi: 10.1002/bjs.5119. [DOI] [PubMed] [Google Scholar]
- 11.Bendick PJ, Bove PG, Long GW, Zelenock GB, Brown OW, Shanley CJ. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg. 2003;37:381–385. doi: 10.1067/mva.2003.17. [DOI] [PubMed] [Google Scholar]
- 12.Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Charles H, Lin PH, Lumsden AB, Bush L. Contrast-enhanced duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg. 2006;43:259–264. doi: 10.1016/j.jvs.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 13.Partovi S, Kaspar M, Aschwanden M, Lopresti C, Madan S, Uthoff H, Imfeld S, Staub D. Contrast-enhanced ultrasound after endovascular aortic repair—current status and future perspectives. Cardiovasc Diagn Ther. 2015;5(6):454–463. doi: 10.3978/j.issn.2223-3652.2015.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/S0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 15.Abbas A, Hansrani V, Sedgwick N, Ghosh J, McCollum CN. 3D contrast enhanced ultrasound for detecting endoleak following endovascular aneurysm repair (EVAR) Eur J Vasc Endovasc Surg. 2014;47:487–492. doi: 10.1016/j.ejvs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Bredahl KK, Taudorf M, Lönn L, Vogt KC, Sillesen H, Eiberg JP. Contrast enhanced ultrasound can replace computed to-mography angiography for surveillance after endovascular aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2016;52:729–734. doi: 10.1016/j.ejvs.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Cantisani V, Ricci P, Grazhdani H, et al. Prospective comparative analysis of colour-doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2010;2011(41):186–192. doi: 10.1016/j.ejvs.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Clevert D-A, Minaifar N, Kopp R, Stickel M, Meimarakis G, Sommer W, et al. Imaging of endoleaks after endovascular aneurysm repair (EVAR) with contrast-enhanced ultrasound (CEUS). A pictorial comparison with CTA. Clin Hemorheol Microcirc. 2009;41(3):151–168. doi: 10.3233/CH-2009-1160. [DOI] [PubMed] [Google Scholar]
- 19.Clevert DA, Helck A, D'Anastasi M, Gürtler V, Sommer WH, Meimarakis G, Weidenhagen R, Reiser M. Improving the follow up after EVAR by using ultrasound image fusion of CEUS and MS-CT. Clin Hemorheol Microcirc. 2011;49(1–4):91–104. doi: 10.3233/CH-2011-1460. [DOI] [PubMed] [Google Scholar]
- 20.Giannoni MF, Palombo G, Sbarigia E, Speziale F, Zaccaria A, Fiorani P. Contrast-enhanced ultrasound imaging for aortic stent-graft surveillance. J Endovasc Ther. 2003;10:208–217. doi: 10.1177/152660280301000208. [DOI] [PubMed] [Google Scholar]
- 21.Gürtler V, Sommer W, Meimarakis G, Kopp R, Weidenhagen R, Reiser M, Clevert D. A comparison between contrast-enhanced ultrasound imaging and multislice computed tomography in detecting and classifying endoleaks in the follow-up after endovascular aneurysm repair. J Vasc Surg. 2013;58:340–345. doi: 10.1016/j.jvs.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Houdek K, Třeška V, Čertík B, et al. Initial experience of follow up of patients after the endovascular treatment of abdominal aortic aneurysms using contrast-enhanced ultrasound. Cor et Vasa. 2015;57:e121–e126. doi: 10.1016/j.crvasa.2015.03.005. [DOI] [Google Scholar]
- 23.Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2009;49:552–560. doi: 10.1016/j.jvs.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 24.McWilliams RG, Martin J, White D, Gould DA, Rowlands PC, Haycox A, Brennan J, Gilling-Smith GL, Harris PL. Detection of endoleak with enhanced ultrasound imaging: comparison with biphasic computed tomography. J Endovasc Ther. 2002;9:170–179. doi: 10.1177/152660280200900206. [DOI] [PubMed] [Google Scholar]
- 25.Millen A, Canavati R, Harrison G, et al. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg. 2013;58:18–23. doi: 10.1016/j.jvs.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 26.Motta R, Rubaltelli L, Vezzaro R, et al. Role of multidetector CT angiography and contrast-enhanced ultrasound in redefining follow-up protocols after endovascular abdominal aortic aneurysm repair. Radiol Med (Torino) 2012;117:1079–1092. doi: 10.1007/s11547-012-0809-x. [DOI] [PubMed] [Google Scholar]
- 27.Perini P, Sediri I, Midulla M, Delsart P, Gautier C, Haulon S. Contrast-enhanced ultrasound vs. CT angiography in fenestrated EVAR surveillance: a single-center comparison. J Endovasc Ther. 2012;19:648–655. doi: 10.1583/JEVT-12-3909R.1. [DOI] [PubMed] [Google Scholar]
- 28.Perini P, Sediri I, Midulla M, et al. Single-centre prospective comparison between contrast-enhanced ultrasound and computed tomography angiography after EVAR. Eur J Vasc Endovasc Surg. 2011;42:797–802. doi: 10.1016/j.ejvs.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffer JS, Shakhnovich I, Sieck KN, Kallies KJ, Davis CA, Cogbill TH. Duplex ultrasound surveillance after uncomplicated endovascular abdominal aortic aneurysm repair. Vasc Endovasc Surg. 2017;51:295–300. doi: 10.1177/1538574417708131. [DOI] [PubMed] [Google Scholar]
- 30.Ten Bosch JA, Rouwet EV, Peters CTH, Jansen L, Verhagen HJM, Prins MH, Teijink JAW. Contrast-enhanced ultrasound versus computed tomographic angiography for surveillance of endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol. 2010;21:638–643. doi: 10.1016/j.jvir.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Bargellini I, Napoli V, Petruzzi P, et al. Type II lumbar endoleaks: Hemodynamic differentiation by contrast-enhanced ultrasound scanning and influence on aneurysm enlargement after endovascular aneurysm repair. J Vasc Surg. 2005;41:10–18. doi: 10.1016/j.jvs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 32.Harky A, Khan D, Singh VP, Sajid MM, Zywicka E. What is the optimal imaging modality to detect endoleaks following elective endovascular repair of abdominal aortic aneurysm. Ann Vasc Med Res. 2018;5(3):1094. [Google Scholar]
- 33.Mirza TA, Karthikesalingam A, Jackson D, et al. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis. Eur J Vasc Endovasc Surg. 2010;39:418–428. doi: 10.1016/j.ejvs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Karthikesalingam A, Al-Jundi W, Jackson D, et al. Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. Br J Surg. 2012;99:1514–1523. doi: 10.1002/bjs.8873. [DOI] [PubMed] [Google Scholar]