Abstract
Urinary tract dilatation is identified sonographically in 1–2% of fetuses and reflects a spectrum of possible nephro-uropathies. There is significant variability in the clinical management of individuals with prenatal urinary tract dilatation to postnatal urinary pathologies, because of a lack of consensus and uniformity in defining and classifying urinary tract dilation. Ultrasonography is the first step to screen and diagnose kidneys and the urinary tract diseases of the children. The need for a correct ultrasound approach led to the realization of algorithms aimed at standardizing the procedures, the parameters and the classifications. Our objective was to highlight the strengths of the Classification of Urinary Tract Dilation (UTD) suggested by the Consensus Conference which took place in 2014 with the participation of eight Scientific Societies and was subsequently published on the Journal of Pediatric Urology. Before its spread out, the definition of UTD was not uniform and the ultrasonographic measurements were not clearly defined, leading to misunderstandings between physicians. The Classification by the Consensus Conference of 2014 represents a revolutionary tool for the diagnosis and management of UTD. Furthermore, the parameters suggested by the classification proposed are applicable for both prenatal and postnatal classification, ensuring a correct follow-up in children with UTD whose diagnosis had been already made during pregnancy.
Keywords: Urinary tract dilatation, Ultrasound, Contrast-enhanced ultrasound, Kidney
Sommario
Dilatazioni a carico delle vie urinarie vengono identificate nell’ 1–2% dei feti e riflettono uno spettro molto variabile di possibilinefro-uropatie. A causa dell’assenza di un chiaro consenso ed uniformità nella definizione e classificazione di dilatazione a carico delle vie urinarie esiste una notevole variabilità nella gestione clinica di soggetti con questa diagnosi prenatale. L’ecografia rappresenta certamente la metodica di primo livello per l’iniziale individuazione e diagnosi di malattie dei reni e delle vie urinarie. L’esigenza di un corretto approccio ecografico ha portato ad una standardizzazione delle procedure, dei parametri e delle classificazioni. L’obiettivo di questo lavoro è quello di evidenziare i punti di forza della Classificazione della dilatazione delle vie urinarie (Urinary Tract Dilation—UTD) suggerita dalla Consensus Conference tenutasi nel 2014 con la partecipazione di 8 società scientifiche e pubblicata successivamente sul Journal of Pediatric Urology. Prima della sua diffusione, infatti, la definizione di UTD non era uniforme e le misurazioni ecografiche non erano chiaramente definite, dando luogo a incomprensioni o diverse interpretazioni tra i medici coinvolti. La classificazione della Consensus Conference del 2014 rappresenta uno strumento rivoluzionario per la diagnosi e la gestione dell’ UTD. I parametri suggeriti da tale classificazione, inoltre, sono applicabili sia al periodo prenatale che a quello postnatale, assicurando una corretta classificazione e follow-up dei bambini con UTD la cui diagnosi sia stata già posta durante la gravidanza.
Introduction
In the past decades, ultrasonography received a growing attention in most fields of medicine. Ultrasound has the advantages of being a radiation-free procedure, less expensive than computed tomography and can be performed directly bedside [1]. This remarkable expansion has involved pediatric nephrology too. The contrast-enhanced ultrasound (CEUS) is the application of ultrasound contrast agents (UCAs) to traditional medical sonography. The development of UCAs allowed to overcome some of the limitations of B-mode and Doppler ultrasound techniques and to make a more appropriate diagnosis of kidneys disease [2].
Until 2014 the nomenclature used to identify different types of UTD was confusing and redundant due to the partial overlapping of terms such as hydronephrosis, pelvisectasis, pyelectasis, etc.
The need for a correct ultrasound approach led to the realization of algorithms aimed at standardizing the procedures, the parameters and the classifications [3–8]. The extension of these procedures to the standard ultrasound survey and to the new applications based on the use of contrast agents (contrast enhanced voiding urosonography) has allowed to standardize and compare the results obtained.
The aim of this paper was to illustrate the problems concerning the Classification of UTD which subsisted before Classification published after the Consensus Conference in 2014 [3] and the advantages entailed by its adjustments in the categorization, and, therefore, in their follow-up, of patients affected by UTD.
Urinary tract dilation
Urinary tract dilation (UTD) could be the expression of congenital or acquired pathologies [9–12]. The first occurs in approximately 1–2% of pregnancies. Although an etiological framing is often complicated, the identification of congenital alterations avoids the development of complications (infections, renal dysfunction) [3]. Therefore, ultrasound investigation has progressively assumed a predominant role in the diagnosis and follow-up of the UTD [1].
The evolution of ultrasound classification
To define the clinical relevance of UTD different classifications have been proposed. A first attempt suggested by Grignon in 1986 was aimed at achieving a quantitative morphological classification that included 5° of ectasia [4].
| Grade I | < 10 mm |
| Grade II | 10–15 mm |
| Grade III | > 15 mm − slight calyceal dilation |
| Grade IV | > 15 mm − moderate calyceal dilation |
| Grade V | > 15 mm − severe calyceal dilation + atrophic cortex |
In 1993 the American Society of Fetal Urology proposed a qualitative classification. It is based on the presence or absence of the dilation of renal pelvis and calyces (major or minor) and reduction of renal parenchymal thickness. They are distinguished in 5° starting from grade I [5].
The quantitative and qualitative classification is the first expression of the need to classify UTD, but a clear therapeutic indication was not yet available.
In the attempt to evaluate which patients needed pyeloplasty surgery, in 2007 Onen proposed an alternative classification that implemented SFU with renal function evaluation, based on renography test [6].The different classes of UTD were categorized as follows: 0, no hydronephrosis; 1, dilatation of renal pelvis alone; 2, plus caliceal dilation; 3, plus < ½ renal parenchymal loss; 4 plus > ½ renal parenchymal loss. Surgical intervention was performed when the progression of hydronephrosis ranged from grade 1 to 3, from grade 2 to 3 with some degree of persistence of grade 3 until 3 years of age and persistence of grade 4 for more than 1 month [6].This classification was proposed on the basis of well-known tight association between severity of hydronephrosis and prognosis: non-remediable renal damage could occur in severe hydronephrosis not readily treated [6–10].
Various studies have shown that the grade of the fetal UTD correlates with the risk of a kidney and UT disease in the post-natal period [9–12]. In 1998, Dhillon suggested that the pyelic A–P diameter can predict the functional evolution of the affected kidney [8].
In conclusion, the classifications preceding 2014 had been shown to be unsuitable for standardizing the ultrasound approach to UTDs, due to the difference of the measurement and evaluation criteria, the parameters examined and the terminology used, and the need for a correct and complete classification was clear.
Therefore, on March 2014, a Consensus meeting was convened to unify the descriptions of UTD and to create a standardized scheme for perinatal and post-natal ultrasound evaluation of infant [3].
Particularly, the Consensus underlines a series of diagnostic and clinical misunderstanding of problems due to the incompatibility between the classifications of prenatal UTD and of postnatal urological diseases:
the lack of correlation between the prenatal and postnatal period in terms of definition and classification of UTD;
the inability to communicate between clinician and sonographer due to different terminology used (for example the different conception of the term “hydronephrosis”, which indicates even mild degrees of UTD for sonographers while representing a distension of the renal pelvis and calyces secondary to the obstruction of urine flow for clinicians) and methodical approach;
the variability of the UTD ultrasonographic features, often consequent of paraphysiological variables (patient position, degree of bladder filling, state of the child hydration).
Therefore, the main objective of the Consensus was to create a classification system that included sonographic UT parameters applicable both fetus and children, which can standardize the language of the sonographer and the clinician and the methods of evaluation and measurement of kidneys and urinary tracts, in order to describe a disease in a unique way. The Consensus suggested using the general term “urinary tract dilatation” to indicate the ultrasound finding, with the aim to avoid the partially overlapping terms hydronephrosis, pelviectasis, pyelectasis, etc.
The proposed UTD classification system is based on following ultrasound features (Table 1):
Table 1.
Ultrasound parameters included in the UTD classification system
| US parameters | Measurement/findings |
|---|---|
| Anterior-PosteriorRenalPelvicDiameter (APDPD) | (mm) |
| Cayiceal dilation | |
| Central (major calyces) | Yes/no |
| Peripheral (minor calyces) | Yes/no |
| Parenchymal | |
| Thickness | Normal/abnormal |
| Appearance | Normal/abnormal |
| Ureter | Normal/abnormal |
| Bladder | Normal/abnormal |
Furthermore, Nguyen et al. [3]. define in detail the methods of execution of US evaluation and the parameters to be evaluated.
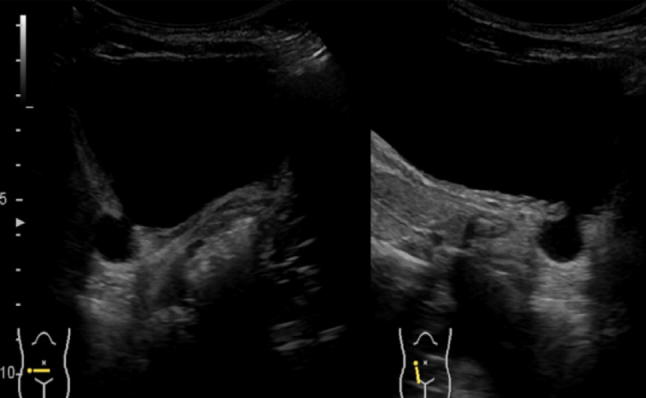
anterior–posterior diameter: measurement of the pelvis at the intrarenal level is recommended by transversal scanning. The patient must be evaluated in both the prone and the supine position (Fig. 1).
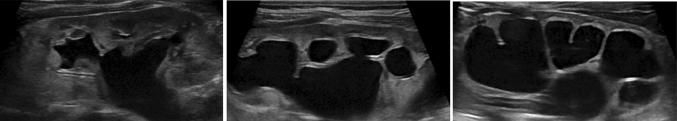
calyceal dilation: it is important to distinguish between the involvement of central calyceal from an involvement of both central and peripheral ones (Fig. 2).
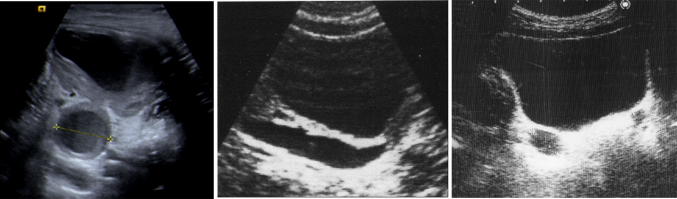
parenchymal thickness and appearance: in the first case the evaluation is subjective. The Classification by the Society of Fetal Urology suggest to consider reduced the thickness of the kidney if the parenchymal thickness of the pathological kidney is reduced of more than half the parenchymal thickness of the normal kidney or measures less than 4 mm [6]. Appearance is assessed by comparison with the adjacent parenchymatous organ (liver on the right, spleen on the left) (Fig. 3).
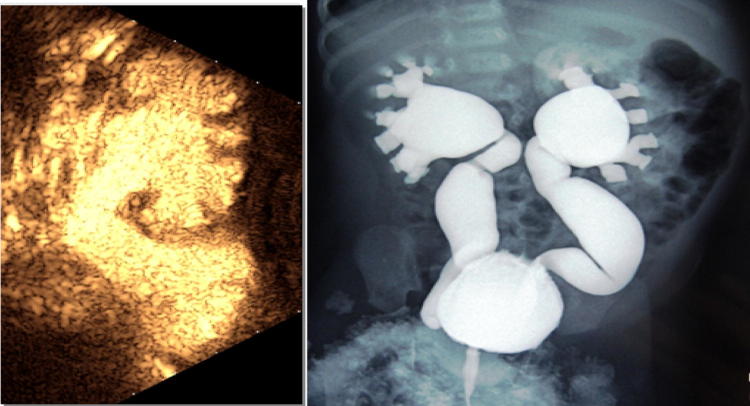
ureteral dilation: a transitory dilation is to be considered physiological postnatally (Fig. 4).
bladder: consider wall thickness, dilations of the posterior urethra, ureteroceles, diverticula and other anomalies (Figs. 5, 6).
Fig. 1.
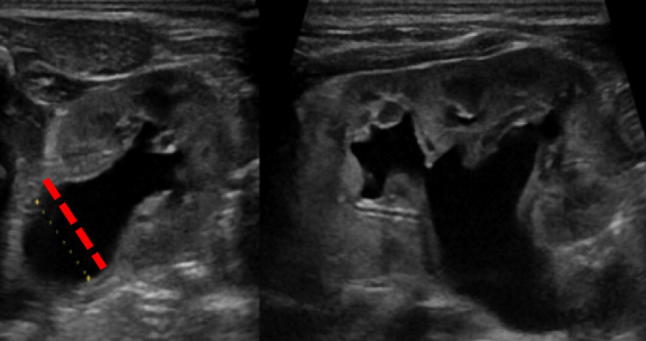
Correct measurement of A–P kidney pelvis diameter
Fig. 2.
a Central calyceal dilation of renal pelvis. b Central and peripheral dilation of calyces
Fig. 3.
Measurement of the renal parenchyma thickness: normal (left figure), slightly reduced (central figure) and severely reduced (right figure)
Fig. 4.

Ostructive megaureter: aperistaltic distal tract
Fig. 5.
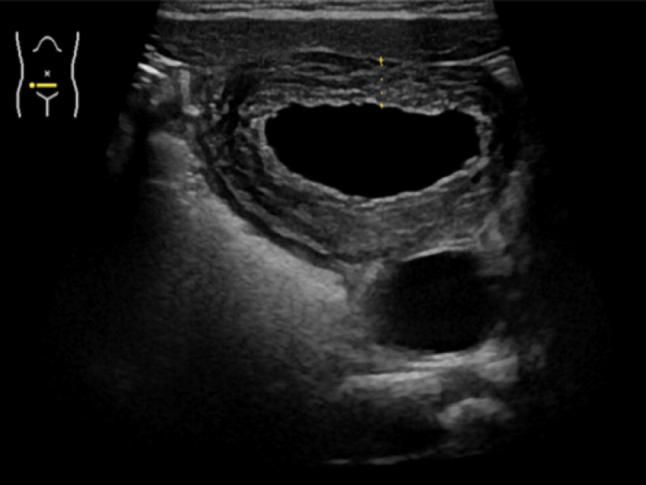
US allows the evaluation of the bladder wall thickness, which may appear in a diffused (the image shown) or partial thickening in case of cystitis
Fig. 6.
Para-ureteral diverticulum. Diverticulum of Hutch
The definition of an US parameter as “pathological” changes during the gestational age. Normal values at the time at presentation are shown in Table 2.
Table 2.
The kidneys and the urinary tract change during the gestational age
| US findings | Time at presentation | ||
|---|---|---|---|
| 16–27 weeks | ≥ 28 weeks | Postnatal (> 48 h) | |
| Anterior-PosteriorRenalPelvicDiameter (APDPD) | < 4 mm | < 7 mm | < 10 mm |
| Cayiceale dilation | |||
| Central | No | No | No |
| Peripheral | No | No | No |
| Parenchima | |||
| Thickness | Normal | Normal | Normal |
| Appearance | Normal | Normal | Normal |
| Ureter | Normal | Normal | Normal |
| Bladder | Normal | Normal | Normal |
| UnexplainedOligohydramnios | No | No | No |
This table shows the threshold values considered normal according to the gestational age
According to the Consensus, the values for the diagnosis of UT dilation based on sonographic imaging are stratified based on gestational age at presentation. The renal pelvis is considered not to be dilated when the APRPD measures < 4 mm at < 28 weeks gestation, < 7 mm at ≥ 28 weeks, and < 10 mm postnatally. In the normal fetus, calyceal dilation is absent, the renal parenchyma has normal thickness and appearance, the ureter is not seen, and the bladder is normal. Additionally, there is no unexplained oligohydramnios. Additional sonographic features that should be evaluated include:calyceal dilation, parenchymal thickness, parenchymal appearance with respect to echogenicity, the presence or absence of cortical cysts and corticomedullary differentiation, bladder abnormalities, the presence of ureterocele or dilated posterior urethra and the presence of otherwise unexplained oligohydramnios on prenatal imaging. The prenatal UTD identification allows to stratify into different risk groups based on the possibility of developing uropathy.
Thus, if detected in prenatal age, UTD A1 represents the low risk, UTD A2 the intermediate risk and UTD A3 the high risk. Similarly, the consensus suggested UTD P1, UTD P2 and UTD P3 risk group when the UTD are diagnosed in post-natal period. When UT dilation is detected postnatally (denoted as P), the Consensus recommend stratification of risk into three groups: low-risk (UTD P1); intermediate-risk (UTD P2); and high-risk (UTD P3) groups. UTD P1 is considered to be low risk for postnatal uropathies is 10 to < 15 mm. In the low-risk group, central calyceal dilation may be present. The renal parenchyma should have normal thickness and appearance, and the bladder is normal. With UTD P2, which is considered to be intermediate risk for postnatal uropathies, the APRPD is ≥ 15 mm. The calyces may be dilated centrally and peripherally or a dilated ureter is visible, the parenchymal thickness, the appearance and the bladder are normal. In UTD P3 the renal parenchymal is thinned, has increased echogenicity and/or has decreased corticomedullary differentiation, or the bladder is abnormal. When there are parenchymal abnormalities but the APRPD is < 15 mm, are still classified as UTD P3 [3] (Fig. 7).
Fig. 7.
UTD Risk Stratification. In the UT dilation detected postnatally, the Consensus recommends stratification of the risk of developing uropathies in three groups (P1, P2 and P3) based on the ultrasound parameters
Vesicoureteral reflux
Vesicoureteral tract reflux (VUR) is a vesicoureteral junction incompetence with relative urinary reflux from the bladder to upper-urinary tracts. VUR can occasionally and temporarily be found in healthy subjects; nevertheless, its persistence needs to be taken into account as a pathology.
Ethiopathogenesis
Primary VUR is the consequence of a malformation or delayed development of the vesicoureteral junction (VUJ). Secondary VUR happens when the junction begins to lose competence due to pathological or acquired lesions in the bladder, urethral or ureteral area.
Primitive forms are the most frequent and can often be attributed to congenital and anatomical alterations of the VUJ.
Secondary forms are caused by organic or functional lesions (posterior urethral valves, parauretereal diverticulum, pyeloureteral duplication,…).
The term “reflux nephropathy” emphasizes the connection between VURs, kidney damage and renal scars [12].
Epidemiology
The precise incidence of VUR in the general demography is unknown, while for the pediatric population we estimate a value of 0.4–1.8% in asymptomatic patients and about 10–50% in patients affected by UTI [12].
Main differential diagnosis
Pyeloureteral Junction Syndrome
Dysplastic Polycystic Kidney
Obstructive Megaureter
Posterior Urethral Valve
Nowadays, the ultrasound is a technique that provides an important contribution in the diagnostic framework of VUR and urinary tract malformations. In the patient with VUR, standard ultrasound could highlight both calyceal-pyelic ad ureter dilatations but it is not possible to confirm the diagnosis. The contrast-enhanced ultrasound (CEUS) is the application of ultrasound contrast agents (UCAs) to traditional medical sonography. The development of UCAs allowed to overcome some of the limitations of B-mode and Doppler ultrasound [2]. Currently cystourethogram is considered the gold standard to diagnose VUR. Unfortunately, testing for VUR using a voiding cystourethrogram (VCUG) involves bladder catheterisation and exposure to radiation. Radionuclide cystography (RNC) too involves bladder catheterization and intravesical administration of radiopharmaceuticals. The diagnostic performance is comparable to VCUG and the cost is also lower. But, owing to its lower spatial resolution and impaired anatomical delineation of the urethra, RNC is generally used for follow-up of patients with known vesicoureteral reflux. Nowadays, clinicians are interested in finding alternative tests that could replace the VCUG. Contrast-enhanced ultrasound (CEUS) is a technique whereby biocompatible microspheres of inert gas are administered i.v. that reflect ultrasonography sound waves and do not involve radiation. Because the contrast agent is rapidly cleared, contrast images must be obtained within minutes of administration. These agents are not filtered or secreted by the kidneys, and, therefore, allow visualization of the renal parenchyma without interfering with enhancement of the collecting system [13].
Following US contrast agent administration, real-time sonographic evaluation of the urinary tract is performed by scanning the urinary bladder including the retrovesical space to evaluate the distal ureters, and both kidneys alternatively in prone and/or supine positions, during bladder filling, voiding and after voiding [14].
The presence of echogenic microbubbles within the ureter, the renal pelvis and/or calyces represents retrograde urine flow in keeping with vesicoureteral reflux, which can be classified into five grades (similar to the current grading system used for conventional VCUGs), based on the involved parts of the urinary tract and the degree of the resultant pelvicalyceal or ureteral dilation and tortuosity. Several comparative studies between ceVUS and the conventional ionizing methods for reflux detection and radionuclide cystography have demonstrated that ceVUS is sensitive and accurate in grading VUR and its severity. In fact, studies by Ascenti et al. [15], Ključevšek et al. [16] and Tse et al. [17] found that ce-VUS had 100% sensitivity. Tse et al. compared CEUS and VECUG and reported that ce-VUS did not miss any VUR that was detected on VCUG. Studies by Darge et al. showed a relatively high specificity of Ce-VUS of 92–97%. Moreover, the absence of ionizing radiation permits continuous, real-time scanning of the urinary tract and thus better evaluation of the dynamic nature of the intermittent reflux phenomenon [18, 19].
According to these studies, CEUS has the potential to replace VCUG. It may be introduced as a valid alternative diagnostic modality for detecting vesicoureteral reflux, based on its radiation free, highly efficacious, reliable and safe characteristics (Figs. 8, 9, 10).
Fig. 8.
Distal ureter dilatation and hydronephrosis in a patient with double renal district
Fig. 9.
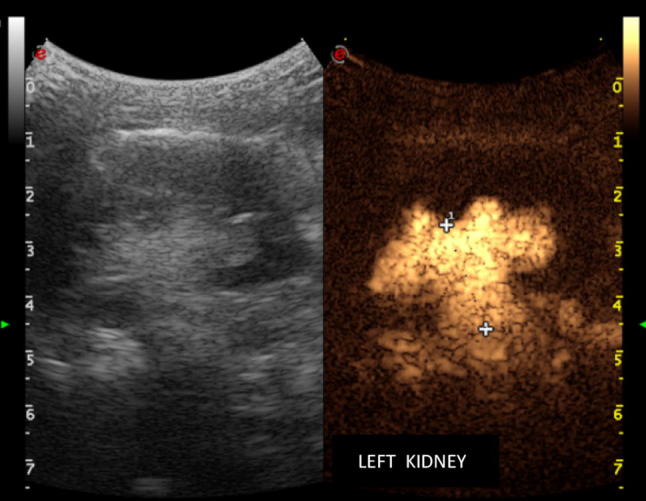
The III-grade renal and ureteral reflux assessed by the cystosonographic method
Fig. 10.
Cystosonography: V-grade renal and ureteral reflux of inferior district
Funding
No external funding was secured for this study.
Conflict of interest
All authors have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
No informed consenti s required.
References
- 1.Sferrazza Papa GF, Mondoni M, Volpicelli G, Carlucci P, Di Marco F, Parazzini EM, Reali F, Pellegrino GM, Fracasso P, Sferrazza Papa S, Colombo L, Centanni S. Point-of-Care lung sonography: an audit of 1150 examinations. J Ultrasound Med. 2017;36(8):1687–1692. doi: 10.7863/ultra.16.09007. [DOI] [PubMed] [Google Scholar]
- 2.Drudi FM, Di Leo N, Maghella F, Malpassini F, Iera J, Rubini A, Orsogna N, D’Ambrosio F. CEUS in the study of bladder, method, administration and evaluation, a technical note. J Ultrasound Med. 2014;17(1):57–63. doi: 10.1007/s40477-013-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen HT, Benson CB, Bromley B, Campbell JB, Chow J, Coleman B, Cooper C, Crino J, Darge K, Herndon CD, Odibo AO, Somers MJ, Stein DR. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system) J Pediatr Urol. 2014;10(6):982–998. doi: 10.1016/j.jpurol.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Grignon A, Filion R, Filiatrault D, Robitaille P, Homsy Y, Boutin H, et al. Urinary tract dilatation in utero: classification and clinical applications. Radiology. 1986;160(3):645–647. doi: 10.1148/radiology.160.3.3526402. [DOI] [PubMed] [Google Scholar]
- 5.Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the society for fetal urology. Pediatr Radiol. 1993;23(6):478–480. doi: 10.1007/BF02012459. [DOI] [PubMed] [Google Scholar]
- 6.Onen A. An alternative grading system to refine the criteria for severity of hydronephrosis and optimal treatment guidelines in neonates with primary UPJ-type hydronephrosis. J Pediatr Urol. 2007;3(3):200–205. doi: 10.1016/j.jpurol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Onen A, Jayanthi VR, Koff SA. Long-term follow-up of prenatally detected severe bilateral newborn hydronephrosis initially managed nonoperatively. J Urol. 2002;168:1118e20. doi: 10.1016/S0022-5347(05)64604-6. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon HK. Prenatally diagnosed hydronephrosis: the Great Ormond Street experience. Br J Urol. 1998;81:39e44. doi: 10.1046/j.1464-410X.1998.0810s2039.x. [DOI] [PubMed] [Google Scholar]
- 9.Chertin B, Rolle U, Farkas A, Puri P. Does delaying pyeloplasty affect renal function in children with a prenatal diagnosis of pelvi-ureteric junction obstruction? BJU Int. 2002;90:72e5. doi: 10.1046/j.1464-410X.2002.02829.x. [DOI] [PubMed] [Google Scholar]
- 10.Peters CA. Lower urinary tract obstruction: clinical and experimental aspects. Br J Urol. 1998;81:22e5. doi: 10.1046/j.1464-410X.1998.0810s2022.x. [DOI] [PubMed] [Google Scholar]
- 11.Garin EH. Primary vesicoureteral reflux; what have we learnt from the recently published randomized, controlled trials? Pediatr Nephrol. 2018 doi: 10.1007/s00467-018-4045-9. [DOI] [PubMed] [Google Scholar]
- 12.Bailey RR. Vesicoureteral reflux in healthy infants and children. In: Hodson J, Kincaid-Smith P, editors. Reflux nephropathy. 1. New York: Masson; 1979. pp. 59–61. [Google Scholar]
- 13.Hains DS, Cohen HL, McCarville MB, Ellison EE, Huffman A, Glass S, Qureshi AH, Pierce KR, Cahill AL, Dixon A, Santos ND. Elucidation of renal scars in children with vesicoureteral reflux using contrast-enhanced ultrasound: a pilot study. Kidney Int Rep. 2017;2(3):420–424. doi: 10.1016/j.ekir.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ntoulia A, Anupindi SA, Darge K, Back SJ. Applications of contrast-enhanced ultrasound in the pediatric abdomen. Abdom Radiol. 2018;43(4):948–959. doi: 10.1007/s00261-017-1315-0. [DOI] [PubMed] [Google Scholar]
- 15.Ascenti G, Zimbaro G, Mazziotti S, Chimenz R, Fede C, Visalli C, Scribano E. Harmonic US imaging of vesicoureteric reflux in children: usefulness of a second generation US contrast agent. Pediatr Radiol. 2004;34(6):481–487. doi: 10.1007/s00247-004-1190-z. [DOI] [PubMed] [Google Scholar]
- 16.Ključevšek D, Battelino N, Tomažič M, Kersnik Levart T. A comparison of echo-enhanced voiding urosonography with X-ray voiding cystourethrography in the first year of life. Acta Paediatr. 2012;101(5):e235–e239. doi: 10.1111/j.1651-2227.2011.02588.x. [DOI] [PubMed] [Google Scholar]
- 17.Tse KS, Wong LS, Lau HY, Fok WS, Chan YH, Tang KW, Chan SC. Paediatric vesicoureteric reflux imaging: where are we? Novel ultrasound-based voiding urosonography. Hong Kong Med J. 2014;20(5):437–443. doi: 10.12809/hkmj144215. [DOI] [PubMed] [Google Scholar]
- 18.Kis E, Nyitrai A, Varkonyi I, et al. Voiding urosonography with second-generation contrast agent versus voiding cystourethrography. Pediatr Nephrol. 2010;25:2289–2293. doi: 10.1007/s00467-010-1618-7. [DOI] [PubMed] [Google Scholar]
- 19.Darge K, Beer M, Gordjani N. Contrast-enhanced voiding urosonography with the use of a 2nd generation US contrast medium: preliminary results. Pediatr Radiol. 2004;34:97. doi: 10.1007/s00247-003-1082-7. [DOI] [PubMed] [Google Scholar]