Abstract
Background
Systemic lupus erythematosus is prone to recurrent attacks, and its treatment is related to disease activities. It is important to accurately assess the patient's disease activity. So, the purpose of this study was to investigate the relation between neutrophil‐to‐C3 ratio (NC 3R), neutrophil‐to‐lymphocyte ratio (NLR), and disease activity in patients with Systemic lupus erythematosus (SLE).
Methods
This was a retrospective study. One hundred and ninety‐four patients with SLE and 71 healthy controls were included in this study. We divided the patients into two groups according to the SLE disease activity (SLEDAI). Group 1 included patients with a score of >9 (patients with severe disease activity), and Group 2 included patients with a score of 9 and lower (patients with mild disease activity). Correlations between NC 3R, NLR, and disease activity were analyzed.
Results
NC 3R and NLR in patients with SLE were obviously higher compared to healthy controls (P < 0.05). There was an obviously significant difference in NC 3R and NLR between Group 1 and Group 2 (P < 0.05). SLEDAI scores were positively correlated with NC 3R (r = 0.353, P < 0.01) and NLR (r = 0.237, P = 0.01). Receiver operating characteristic (ROC) curve analysis showed that the cutoff value of NC 3R to identify SLE with high disease activity was 5.935, with sensitivity and specificity being 75.9% and 67.0%, while that of NLR was 2.293, with sensitivity being 68.9% and specificity being 82.8%.
Conclusion
NC 3R and NLR are two useful inflammatory markers for evaluating disease activity in patients with SLE.
Keywords: disease activity, neutrophil‐to‐C3 ratio, neutrophil‐to‐lymphocyte ratio, systemic lupus erythematosus
1. INTRODUCTION
Systemic lupus erythematosus (SLE) is a clinically common autoimmune disease characterized by abnormal immune response to autologous tissue, eventually resulting in systemic disorders and diverse clinical manifestations of patients.1 The prevalence of women is significantly higher than that of men.1 The pathogenesis of SLE remains unclear, but environmental triggers and genetic factors have been reported to contribute to the destruction of immune tolerance systems and the production of immunological lymphocytes, antibodies, and inflammatory cytokines, damaging tissues and organs.1, 2 Components of lymphocytes, antibodies, inflammatory cytokines, and complements in peripheral circulation vary among different active stages of SLE.3, 4, 5 Patients with higher disease activity often present severer damage of tissues and organs, many of which even threaten the patients’ life.6 It is of great significance in SLE management to early and accurately determine the disease activity of patients. In this study, we determined neutrophil‐to‐C3 ratio (NC3R), neutrophil‐to‐lymphocytes ratio (NLR), neutrophil‐to‐C4 ratio (NC4R), CRP‐to‐C4 ratio (CC4R), and other inflammatory markers of 194 patients with SLE, to investigate their correlation with disease activity.
2. MATERIALS AND METHODS
2.1. Participants
A total of 220 patients with SLE from the Second Affiliated Hospital between January 2016 and June 2017 were enrolled. One hundred and ninety‐four of them were finally included in this study as SLE group, who were diagnosed by 2 attending rheumatologists. All patients with SLE were diagnosed based on the criteria established by Systemic Lupus International Collaborating Clinics.7 Patients who had the following diseases were excluded from the study: (a) Secondary infection such as fever and sputum‐positive, (b) Hematological disease (granulocytic leukemia, macroglobulinemia, multiple myeloma, infectious mononucleosis, etc.), (c) HIV infection, (d) complicated with other autoimmune diseases (sicca syndrome, rheumatoid arthritis, mixed connective tissue disease, etc.), (e) chronic hepatic diseases, and (f) resent antibiotic taking, and 71 healthy individuals being in fine basic examination were enrolled as control group. All participants signed the informed consent, and this study obtained ethical approval from the ethics committee of the Second Affiliated Hospital of Nanchang University. Blood samples were taken in the morning, 2.0 mL was placed in the coagulation tube, and another 2.0 mL was placed in the anticoagulant of EDTA‐K2 tube.
2.2. Specimen processing
Beckman Immage 800 (Beckman Coulter, Inc., Brea, CA, USA) and matching detection reagent were utilized to detect serum levels of IgG, IgM, C3, C4, and CRP. Neutrophilic, lymphocyte and monocyte counts in the peripheral blood were analyzed with automatic blood fluid module analyzer (XN‐20[AI], Sysmex Corporation, Kobe, Japan) and matching reagents.
2.3. Statistical analysis
Analysis was performed using SPSS software (version 22.0, International Business Machines Corp., Beijing, China). The normality of distribution was checked by Kolmogorov‐Smirnov test, with normal distributions data expressed as mean ± standard deviation and tested by parametric test, whereas non‐normal distributions presented as percentage and tested by nonparametric test. Spearman's correlation coefficient was calculated to examine the association between two continuous variables. Receiver operating characteristic (ROC) curve analysis was performed to determine the sensitivity and specificity of NC3R and NLR in predicting the severe disease activity. Statistical significance was defined as P < 0.05.
3. RESULTS
3.1. The characteristics of SLE patients
A total of 194 eligible patients with SLE were finally included in this study, among which there were 15 males and 179 females, with an average age of 40.61 ± 12.50; among 71 healthy controls, there were 10 males and 61 females and the average age of them was 43.24 ± 13.09. There was no statistically significant difference between the two groups of sex and age. After classifying all patients according to SLE disease activity score (SLEDAI), the number of each score group was illustrated in Table 1. There were 30 patients with SLE in the Group 1 (SLEDAI>9) and 164 patients of Group 2 (SLEDAI ≤ 9). See Table 1 for details.
Table 1.
General clinical characteristics of population
| Variable | SLE (n = 194) | Control (n = 71) |
|---|---|---|
| Age (y) | 40.61 ± 12.50 | 43.24 ± 13.09 |
| Gender (n, %) | ||
| Male | 15 (7.73%) | 10 (14.08%) |
| Female | 179 (92.27%) | 61 (85.92%) |
| SLEDAI (n, %) | ||
| 0 ~ 4 | 120 (61.85%) | |
| 5 ~ 9 | 44 (22.68%) | |
| 10 ~ 14 | 21 (10.82%) | |
| ≥15 | 9 (4.65%) | |
| ANA | 156 (80.41%) | |
| dsDNA | 78 (40.21%) | |
3.2. The differences in NLR, CC4R, NC3R, NC4R, and related laboratory indicators between SLE patients and healthy controls
The levels of IgM, C3, and C4 in patients with SLE were 1.022, 0.618 , and 0.145 g/L, respectively, and lower than those in healthy group (all P < 0.05). The levels of NLR, CC4R, NC3R, and NC4R in patients with SLE were 3.605, 49.236, 6.897, and 34.981, respectively, and higher than those in healthy group (all P < 0.05), as shown in Table 2.
Table 2.
The indicators among SLE and control group
| SLE (n = 194) | Control (n = 71) | P‐value | |
|---|---|---|---|
| Age (y) | 40.61 ± 12.50 | 43.24 ± 13.09 | 0.182 |
| Neutrophils (×109/L) | 3.867 (2.080, 4.838) | 3.559 (3.127, 3.992) | 0.279 |
| Lymphocytes (x109/L) | 1.389 (0.845, 1.708) | 1.538 (1.358, 1.719) | 0.164 |
| Monocyte (x109/L) | 0.362 (0.190, 0.470) | 0.343 (0.293, 0.393) | 0.562 |
| IgA (g/L) | 2.71 (1.770, 3.535) | 2.362 (1.51, 2.79) | 0.102 |
| IgG (g/L) | 13.939 (10.00, 17.60) | 13.451 (12.295, 14.607) | 0.512 |
| IgM (g/L) | 1.022 (0.565, 1.368) | 1.227 (1.06, 1.395) | 0.022 |
| CRP (g/L) | 6.818 (1.543, 6.112) | 5.288 (3.759, 6.817) | 0.320 |
| C3 (g/L) | 0.618 ± 0.22 | 0.891 ± 0.24 | <0.01 |
| C4 (g/L) | 0.145 (0.09, 0.170) | 0.193 (0.176, 0.210) | 0.002 |
| NLR | 3.605 (1.643, 3.878) | 2.80 (2.369, 3.231) | 0.022 |
| CC4R | 49.236 (12.932, 61.712) | 28.049 (20.036, 36.062) | <0.01 |
| NC3R | 6.897 (3.543, 8.693) | 4.545 (3.70, 5.389) | <0.01 |
| NC4R | 34.981 (16.574, 44.771) | 21.709 (17.772, 25.648) | <0.01 |
NLR, Neutrophil‐to‐lymphocytes ratio; CC4R, CRP‐to‐C4 ratio; NC3R, neutrophil‐to‐C3 ratio; NC4R, neutrophil‐to‐C4 ratio.
3.3. The differences in C3, C4, NLR CC4R, NC3R, and NC4R between Group 1 and Group 2
Group 1 (SLEDAI score> 9) had a higher NC3R of 9.532 (7.064, 12.000) and NLR of 5.713 (3.187, 8.239) and lower C3 of 0.521 (0.439, 0.603), while patients in Group 2 (SLEDAI score ≤ 9) had NC3R of 6.273 (5.576, 6.969), C3 of 0.634 (0.600, 0.668), and NLR of 3.108 (2.682, 3.535). There were obviously significant differences in C3, NLR, CC4R, and NC3R between the two groups (all P < 0.05) (Table 3).
Table 3.
The indicators among different activity of SLE
| SLEDAI ≤ 9 (n = 164) | SLEDAI>9 (n = 30) | P‐value | |
|---|---|---|---|
| Age (y) | 40.98 ± 12.07 | 38.52 ± 15.19 | 0.329 |
| C3 (g/L) | 0.634 (0.600, 0.668) | 0.521 (0.439, 0.603) | 0.010 |
| C4 (g/L) | 0.138 (0.1227, 0.149) | 0.129 (0.102, 0.157) | 0.572 |
| NLR | 3.108 (2.682, 3.535) | 5.713 (3.187, 8.239) | 0.046 |
| CC4R | 43.475 (35.427, 51.524) | 74.915 (48.059, 101.77) | 0.028 |
| NC3R | 6.273 (5.576, 6.969) | 9.532 (7.064, 12.00) | 0.014 |
| NC4R | 33.145 (29.193, 37.097) | 42.801 (31.354, 54.248) | 0.070 |
3.4. The relationship between the NLR, NC3R, CC4R, and disease activity in patients with SLE
The relationship between the NLR, NC3R, CC4R, and disease activity was tested by linear regression analysis. SLEDAI scores were positively correlated with NC3R (r = 0.353, P < 0.01), NLR (r = 0.237, P = 0.01), and CC4R (r = 0.263, P < 0.01), negatively associated with lymphocyte counts (r = −0.256, P < 0.01) (Table 4).
Table 4.
Correlations of SLEDAI scores with NLR, NC3R, and CC4R in patients with SLE
| SLEDAI scores | NLR | NC3R | CC4R | |||||
|---|---|---|---|---|---|---|---|---|
| r | P‐value | r | P‐value | r | P‐value | r | P‐value | |
| C3 (g/L) | −0.339 | <0.01 | −0.06 | 0.408 | −0.374 | <0.01 | −0.043 | 0.558 |
| C4 (g/L) | −0.066 | 0.361 | 0.06 | 0.409 | −0.180 | 0.013 | −0.028 | 0.70 |
| CRP (g/L) | 0.120 | 0.096 | 0.157 | 0.03 | 0.01 | 0.004 | 0.794 | <0.01 |
| Neutrophils (×109/L) | 0.068 | 0.349 | 0.460 | <0.01 | 0.659 | <0.01 | 0.091 | 0.207 |
| Lymphocyte (×109/L) | −0.256 | <0.01 | −0.387 | <0.01 | 0.014 | 0.851 | −0.228 | 0.001 |
| SLEDAI scores | ‐ | ‐ | 0.237 | 0.01 | 0.353 | <0.01 | 0.263 | <0.01 |
3.5. The receiver operating characteristic (ROC) curves of NC3R, NLR, and CC4R for the recognition of severe disease activity
ROC curves of NC3R, NLR, and CC4R in identifying higher SLEDAI scores were presented in Table 5 and Figure 1. The optimal threshold for NC3R, NLR, and CC4R in identifying higher SLEDAI scores was 5.935, 2.298, and 37.988, respectively. According to ROC curve analysis, the sensitivity of NC3R, NLR and CC4R in diagnosing the disease activity of SLE patients was 75.9%, 82.8% and 58.6%, and the specificity was 60.7%, 49.7% and 67.5%, respectively.
Table 5.
Receiver operating characteristic curves of the CC4R, NLR, and NC3R for differentiating severe patients with SLE
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Cutoff value | Area | Sensitivity | Specificity | Lower bound | Upper bound | |
| CC4R | 37.988 | 0.644 | 0.586 | 0.675 | 0.530 | 0.757 |
| NLR | 2.298 | 0.689 | 0.828 | 0.497 | 0.578 | 0.800 |
| NC3R | 5.935 | 0.670 | 0.759 | 0.607 | 0.546 | 0.794 |
Figure 1.
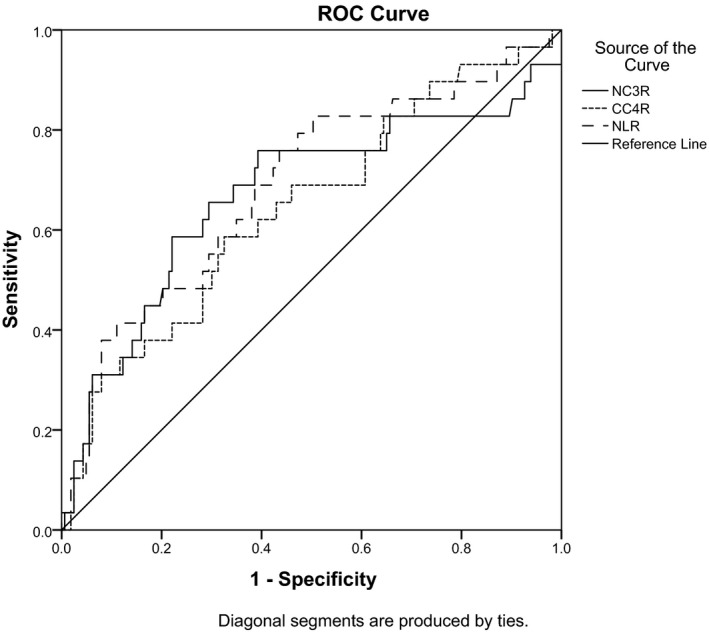
Receiver operating characteristic curves of the CC4R, NLR, and NC3R for differentiating severe patients with SLE
4. DISCUSSION
Systemic lupus erythematosus is a chronic autoimmune disease characterized by a broad spectrum of clinical manifestations, but its course and organ involvement are unpredictable.8, 9 Timely treatment adjustment according to disease activity is of great importance in the management of SLE.6 SLEDAI, which containing 21 scoring items, is clinically used to evaluate SLE disease activity.10 However, disease activity evaluated via SLEDAI score is of partial subjectivity, such as feeling disorder, insomnia, or daytime sleepiness. To quickly and correctly determine the activity level and get patients treated timely and effectively, therefore, we design the current study to explore new correlation indicators to well reflect patients’ disease activity degree.
Neutrophil‐to‐lymphocyte ratio (NLR), calculated as neutrophil counts divided by lymphocyte counts, is considered as a marker for general immune responses to various stress stimuli.11 It was considered as a good diagnostic marker with autoimmune diseases, such as adult‐onset Still's disease.12 It was associated with disease activity in patients with systemic lupus erythematosus.13 Complement system activation, production and partial deposition of complement fragments, and subsequent inflammation all play critical roles in the pathogenesis of SLE, and during the complement activation pathway, C3 and C4 were at the core position.9 Besides, the compounds and antimicrobial peptides released from neutrophils of patients with SLE caused inflammation and damaged tissues and organs.14 Inflammatory cells (such as neutrophils, lymphocytes, and monocytes) play the same important role as complement systems in the progression of SLE disease.4, 8, 9, 15 It reported that patients with SLE had lower complement levels than healthy people,6, 9, 16 with inflammatory cells higher than which in healthy people.14, 15 So, NLR, CC4R, NC3R, NC4R, and related inflammatory markers were selected in the study to study its correlation with SLE activity.
There were significant differences in IgM, C3, C4, NLR, CC4R, NC3R, and NC4R between SLE and healthy population: Levels of IgM, C3, and C4 were lower, and NLR, CC4R, NC3R, and NC4R were higher compared to healthy controls. The immune and complement systems were more active compared to healthy population, which formed circulating immune complexes (ICs) depositing in tissues and organs and causing corresponding damage. The complement system was activated by a classical approach, which causing C3 and C4 degradation9, 16 and decreased levels of C3 and C4 in SLE population. NC3R and NC4R in SLE patients were higher than those in normal patients, because the number of immune‐related cells increased and the levels of C3 and C4 decreased. Previous study has shown that mature B lymphocytes were generated in mouse model of systemic lupus erythematosus, which did not secrete antibodies yet.17 Therefore, the quantities of IgM in patient with SLE were lower than controls.
Levels of C3, NLR, CC4R, and NC3R were obviously different between SLE and healthy groups (both P < 0.05). The complement system of patients with SLE in Group 1 was more active, and tissue damaged was more serious compared with Group 2. In addition, the degradation of C3 was faster and the number of inflammatory cells was higher in Group 1. Therefore, C3, NLR, CC4R, and NC3R may clearly distinguish the degree of SLE activity. Linear regression analysis showed that SLEDAI scores were correlated with CC4R (r = 0.236), NC3R (r = 0.353), and NLR (r = 0.237) (P values < 0.05). It was reported that CRP, C3, and C4 were indicators for autoimmune disease diagnosis and related closely to disease activity.18, 19 Our study found that CC4R, NC3R, and NLR correlated with CRP, C3, C4, and inflammatory cells. Therefore, CC4R, NC3R, and NLR can be served as new indicators of inflammation to evaluate SLE activity.
Furthermore, CC4R, NC3R, and NLR had good sensitivity and specificity to evaluate the activity of SLE. The area under the curve of CC4R, NC3R, and NLR, respectively, was 0.644, 0.670, and 0.689 by ROC curve analysis. CC4R was excluded, because this study was focused on the diagnosis of systemic lupus erythematosus with high disease activity, while the sensitivity of it was 0.586. The diagnostic performance of NC3R and NLR for severe SLE patients was higher than other indicators, and the best NC3R cutoff value was 5.935, with 75.9% sensitivity and 67.0% specificity, while the best NLR cutoff value was 2.293, with 68.9% sensitivity and 82.8% specificity. Therefore, NC3R and NLR, as the new inflammatory markers of SLE, can be used to reflect the activity of SLE, which can simplify the clinical workflow, allow patients with SLE to get reasonable treatment in time and more importantly, and reduce patients’ additional cost for medical checkups.
Yu J, Zeng T, Wu Y, et al. Neutrophil‐to‐C3 ratio and neutrophil‐to‐lymphocyte ratio were associated with disease activity in patients with systemic lupus erythematosus. J Clin Lab Anal. 2019;33:e22633 10.1002/jcla.22633
Funding infromation
The National Natural Science Foundation Grants Program (81760382), Jiangxi Natural Science Foundation Grants Project (2017BAB205076), Jiangxi provincial education department science and technology research project (160157).
Jianlin Yu, and Tingting Zeng are contributed equally to this work and should be considered cofirst authors
REFERENCES
- 1. La Paglia GMC, Leone MC, Lepri G, et al. One year in review 2017: systemic lupus erythematosus. Clin Exp Rheumatol. 2017;35(4):551‐561. [PubMed] [Google Scholar]
- 2. Su F, Xiao W, Yang P, Chen Q, Sun X, Li T. Anti‐neutrophil cytoplasmic antibodies in new‐onset systemic lupus erythematosus. An Bras Dermatol. 2017;92(4):466‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kluger MA, Melderis S, Nosko A, et al. Treg17 cells are programmed by Stat3 to suppress Th17 responses in systemic lupus. Kidney Int. 2016;89(1):158‐166. [DOI] [PubMed] [Google Scholar]
- 4. Wiener A, Schippers A, Wagner N, et al. CXCR5 is critically involved in progression of lupus through regulation of B cell and double‐negative T cell trafficking. Clin Exp Immunol. 2016;185(1):22‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monteith AJ, Kang S, Scott E, et al. Defects in lysosomal maturation facilitate the activation of innate sensors in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2016;113(15):E2142‐E2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon C, Amissah‐Arthur MB, Gayed M, et al. The British society for rheumatology guidelin e for the management of systemic lupus erythematosus in adults: executive Summary. Rheumatology (Oxford). 2018;57(1):14‐18. [DOI] [PubMed] [Google Scholar]
- 7. Petri M, Orbai A‐M, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677‐2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szabo K, Papp G, Szanto A, Tarr T, Zeher M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjogren's syndrome and systemic lupus erythematosus. Clin Exp Immunol. 2016;183(1):76‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barilla‐Labarca ML, Toder K, Furie R. Targeting the complement system in systemic lupus erythematosus and other diseases. Clin Immunol. 2013;148(3):313‐321. [DOI] [PubMed] [Google Scholar]
- 10. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630‐640. [DOI] [PubMed] [Google Scholar]
- 11. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5‐14. [PubMed] [Google Scholar]
- 12. Seo JY, Suh CH, Jung JY, Kim AR, Yang JW, Kim HA. The neutrophil‐to‐lymphocyte ratio could be a good diagnostic marker and predictor of relapse in patients with adult‐onset Still's disease: a STROBE‐compliant retrospective observational analysis. Medicine (Baltimore). 2017;96(29):e7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Y, Chen Y, Yang X, Chen L, Yang Y. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol. 2016;36:94‐99. [DOI] [PubMed] [Google Scholar]
- 14. Bosch X. Systemic lupus erythematosus and the neutrophil. N Engl J Med. 2011;365(8):758‐760. [DOI] [PubMed] [Google Scholar]
- 15. Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol. 2015;27(5):448‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Birmingham DJ, Bitter JE, Ndukwe EG, et al. Relationship of Circulating Anti‐C3b and Anti‐C1q IgG to Lupus Nephritis and Its Flare. Clin J Am Soc Nephrol. 2016;11(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwata S, Tanaka Y. B‐cell subsets, signaling and their roles in secretion of autoantibodies. Lupus. 2016;25(8):850‐856. [DOI] [PubMed] [Google Scholar]
- 18. Keenan RT, Swearingen CJ, Yazici Y. Erythrocyte sedimentation rate and C‐reactive protein levels are poorly correlated with clinical measures of disease activity in rheumatoid arthritis, systemic lupus erythematosus and osteoarthritis patients. Clin Exp Rheumatol. 2008;26(5):814‐819. [PubMed] [Google Scholar]
- 19. Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34(3):J276‐J286. [DOI] [PubMed] [Google Scholar]


