Abstract
Background
Inflammation and nutrition are closely associated with initiation and progression of colorectal cancer (CRC). This study aimed to investigate the diagnostic value of the FAR (FAR = 100*Fibrinogen/Albumin) and FPR (FPR = Fibrinogen/pre‐Albumin) in CRC.
Methods
Neutrophil‐to‐lymphocyte ratio (NLR), FPR, and FAR were calculated in 455 newly diagnosed CRC patients, 455 healthy individuals, and 455 benign controls with colorectal polyp. The diagnostic value of biomarker for CRC was evaluated by receiver operating characteristic curve (ROC). Logistic regression analysis was adopted to assess the risk factors for telling CRC apart from benign disease. Moreover, the combined biomarkers were used for discriminating between CRC and benign disease.
Results
Neutrophil‐to‐lymphocyte ratio, FAR, and FPR were significantly higher in CRC patients compared with the benign or healthy controls (P < 0.05). ROC analysis showed that the diagnostic efficacy of FAR and FPR were better than NLR for CRC. Besides, FPR, NLR, carcinoembryonic antigen (CEA), and carbohydrate antigen 19‐9 (CA199) were markedly associated with differentiation of benign disease and CRC in the logistic regression analysis. And the combination of FPR, CEA, and CA199 had the maximum area under the ROC curve (AUC) in separating CRC from benign disease (AUC = 0.845, Sensitivity = 67.9%, Specificity = 85.3%, Positive Predictive Value = 83.5%, Negative Predictive Value = 70.9%).
Conclusions
Fibrinogen/pre‐Albumin could be a useful CRC diagnostic biomarker, and the combination of FPR, CEA, and CA199 could significantly improve the diagnostic efficacy in discriminating CRC from the benign colorectal disease.
Keywords: colorectal cancer, diagnosis, FPR, sensitivity, specificity
1. INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers around the world, and the morbidity and mortality of CRC in China are increasing in recent years.1, 2 Unfortunately, only 40% of CRC are diagnosed at an early stage, and 5‐year survival rate was merely 65% in 2012.3 The occult blood test was usually detected for screening CRC patients until now, but its low sensitivity always resulted in missed diagnosis. Colonoscopy has been recognized as the gold standard for CRC diagnosis; however, its invasive, painful, and expensive propensity restrict the utilization for CRC patients. Besides, several serum biomarkers have been used in the early diagnosis of CRC, such as carcinoembryonic antigen (CEA), carbohydrate antigen 19‐9 (CA199), and tumor‐specific growth factor. Nevertheless, their clinical effectiveness for CRC early detection remains controversial.4
It has been widely known that cancers are closely associated with systemic inflammatory response and the nutritional status of patients. Plenty of evidence indicated that inflammation plays a vital role in the development and progression of various cancers, including CRC.5, 6, 7 Meanwhile, malnutrition is a significant problem in cancer patients and could result in a number of clinical consequences, such as deteriorated quality of life, decreased response to treatment, and shorter survival rate.8 Accordingly, some novel biomarkers which reflected the systemic inflammation and nutritional status were increasingly investigated in the prediction of cancer progression and prognosis, such as neutrophil‐to‐lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and the modified Glasgow prognostic score (mGPS).9, 10 However, there were few studies to report the diagnostic value of these inflammation‐based biomarkers.
Fibrinogen (Fib), which plays a vital part in clot formation, was also observed to act as a prominent role in directly or indirectly regulating inflammatory response.11, 12 Many studies have emphasized that the preoperative plasma Fib level could independently predict prognosis of various cancers.13, 14, 15, 16, 17 Furthermore, serum albumin (Alb) or pre‐albumin (pAlb) level was proved to be significant low in cancer individuals comparing to healthy controls.18, 19 Hence, Fib‐to‐Alb ratio (FAR, 100*Fib/Alb) and Fib‐to‐pAlb ratio (FPR, Fib/pAlb), which could reflect both the inflammatory and nutritional state, seem to be potential diagnostic biomarkers for CRC patients.
In this study, we comprehensively analyzed the diagnostic value of FAR and FPR in CRC and explored whether biomarker combinations could improve the diagnostic efficacy in distinguishing CRC from the benign colorectal disease.
2. MATERIALS AND METHODS
2.1. Study population
According to the power (1−β = 0.85) and the level of significance (α < 0.05), the minimum sample size of the cases and controls was calculated as 403 using PASS version 11.0.10. Thus, a total of 455 CRC patients newly diagnosed at the Second Affiliated Hospital of Nanchang University between Jan 2011 and Dec 2014 were enrolled in our study. The cases were confirmed by two pathologists according to the American Joint Committee on Cancer TNM staging system (7th Edition) classification system. In addition, 455 patients with colorectal polyp were included as benign controls, and sex‐ and age‐matched healthy individuals were enrolled as healthy controls. Patients without hematological disorders, hepatic damage, autoimmune diseases, recent infection, or other malignancies were eligible for enrollment. Healthy controls were collected by physical examination, including the questionnaire, the blood routine examination, all blood biochemical indices, B‐ultrasonography, and imagological examination. Ethical approval was obtained from the Second Affiliated Hospital of Nanchang University and adhered to the tenets of the Declaration of Helsinki.
2.2. Clinical parameter and laboratory results
Patients’ clinical parameter including age, sex, tumor location, TNM classification, and tumor grade were retrieved from medical records. Meanwhile, laboratory results were also collected from medical records. Clauss method was selected to detect circulating Fib using SYSMEX CA‐7000 machine (Sysmex, Tokyo, Japan). Bromocresol green and immune turbidimetric methods were chosen to measure serum concentrations of Alb and pAlb using OLYMPUS AU5400 machine (Beckman Coulter, Tokyo, Japan). Electro‐chemiluminescence immunoassay was used to detect serum levels of CEA and CA199 by the machine of SIEMENS ADVIA Centaur CP (Siemens, Erlangen, Germany). All peripheral blood, plasma, and serum samples were collected from 7:30 to 9:30 am in the period before the intervention. And the respective inter‐ and intra‐batch coefficient of variance of the kits was <5%.
2.3. Statistical analysis
The minimum sample size was calculated by PASS version 11.0.10 program (NCSS, LLC, Kaysville, UT, USA). IBM SPSS 20.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Kolmogorov‐Smirnow test was selected to assess the normality of calculated parameters. The data with normal and skewed distributions were, respectively, showed as mean ± standard deviation (SD) and median (25% quartile‐75% quartile). Student's t test was selected to assess normally distributed parameter; otherwise, Mann‐Whitney U test was performed. The chi‐square test was used for categorical variables. Receiver‐operating characteristics (ROC) curve analysis was performed to identify cutoff values of these biomarkers, and the differences in the area under the curve (AUC) were analyzed using MedCalc version 15.0 (MedCalc Software, Mariakerke, Belgium). A P‐value <0.05 was considered statistically significant.
3. RESULTS
3.1. Clinical characteristics of enrolled participants
The overall baseline characteristics of all the participants were summarized in Table 1. Four hundred and fifty‐five CRC cases, 455 healthy controls, and 455 benign controls were recruited. The median age of the CRC patients, healthy controls, and benign controls were 58, 55, and 57, respectively. There is no statistical significance between three groups in age (P > 0.05). As for CRC patients, 196 (43.1%) were colon cases and 259 (56.9%) were rectal cancer patients; the numbers of patient in stage I, II, III and IV were 49 (10.8%), 164 (36.0%), 177 (38.9%), and 65 (14.3%), respectively. Of all the patients, 14 (3.1%), 370 (81.3%) and 71 (15.6%) tumors were good, median, and poor cell differentiation. The patients with T1, T2, T3, and T4 were 10 (2.2%), 55 (12.1%), 70 (15.4%), and 320 (70.3%), respectively. Two hundred and thirty‐two (51.0%) CRC patients were identified without lymph node metastasis.
Table 1.
The baseline characteristics of the CRC, healthy, and benign controls
| Characteristics | Cases (n = 455) | Healthy (455) | Benign (455) |
|---|---|---|---|
| Age (y, M with R) | 58 (24, 86) | 55 (22, 91) | 57 (17, 87) |
| Sex (male/female) | 252/203 | 252/203 | 252/203 |
| Location | |||
| Colon | 196 (43.1%) | ||
| Rectal | 259 (56.9%) | ||
| TNM stage | |||
| I | 49 (10.8%) | ||
| II | 164 (36.0%) | ||
| III | 177 (38.9%) | ||
| IV | 65 (14.3%) | ||
| Differentiation level | |||
| High | 14 (3.1%) | ||
| Middle | 370 (81.3%) | ||
| Low | 71 (15.6%) | ||
| Invasion depth | |||
| T1 | 10 (2.2%) | ||
| T2 | 55 (12.1%) | ||
| T3 | 70 (15.4%) | ||
| T4 | 320 (70.3%) | ||
| Lymph node metastasis | |||
| N0 | 232 (51.0%) | ||
| N1 | 151 (33.2%) | ||
| N2 | 72 (15.8%) | ||
| Neutrophil(*109/L) | 3.45 (2.73, 4.58)NA | 3.46 (2.79, 4.19) | 3.38 (2.70, 4.24) |
| Lymphocyte(*109/L) | 1.62 (1.30, 2.03)a, b | 1.95 (1.60, 2.32) | 1.74 (1.35, 2.11) |
| Fibrinogen(g/L) | 3.20 (2.66,3.72)a, b | 2.77 (1.40,2.24) | 2.70 (2.28,3.14) |
| Albumin(g/L) | 39.87 (37.23,41.86)a, b | 44.01 (42.01,45.78) | 41.38 (39.30, 43.53) |
| pre‐Albumin(mg/L) | 198.60 (153.20, 242.30)a, b | 248.55 (209.43, 286.14) | 266.50 (235.40, 301.90) |
| NLR (ratio) | 2.23 (1.62, 2.90)a, b | 1.81 (1.40, 2.24) | 1.94 (1.48, 2.61) |
| FAR (ratio) | 8.04 (6.59, 9.64)a, b | 6.38 (5.37, 7.47) | 6.49 (5.48, 7.71) |
| FPR (ratio) | 16.22 (11.79, 21.87)a, b | 11.16 (8.81, 14.53) | 10.00 (8.04, 12.35) |
| CEA (ng/mL) | 3.03 (1.52, 8.00)b | 1.38 (0.76, 2.21) | |
| CA199 (U/mL) | 15.93 (8.72, 29.51)b | 10.06 (6.64, 16.82) | |
Cases, patients with CRC; FAR, 100*Fibrinogen/Albumin; FPR, Fibrinogen/pre‐Albumin; Benign controls, patients with colorectal polyp; M with R, Mean with range; NA, not significant; NLR, neutrophil‐to‐lymphocyte ratio.
P < 0.05: cases vs healthy controls.
P < 0.05: cases vs benign controls.
Fibrinogen level, NLR, FAR, and FPR were significantly higher in CRC patients compared with healthy participants; however, Alb and pAlb were obviously low in the cases (Table 1, Figures 1 and S1A). The similar results were observed between the cases and the benign controls. Moreover, the levels of CEA and CA199 were apparently increased in CRC patients than that in benign controls. To further explore the diagnostic and predictive values of FPR, FAR, and NLR, we investigated associations between these biomarkers and TNM stage. The higher levels of FPR, FAR, and NLR were observed in I/II and III/IV stage comparing to the controls, and the levels in stage III/IV exceed that in stage I/II patients for these three biomarkers (Figures 2 and S1B).
Figure 1.
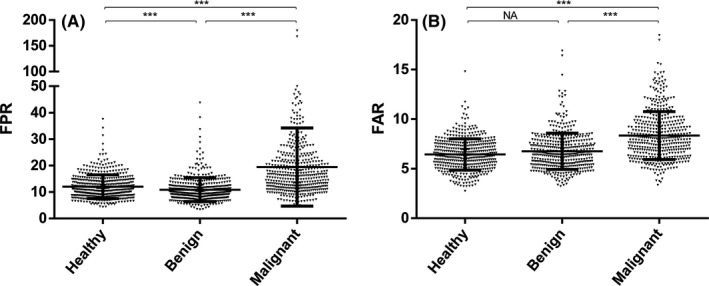
Fibrinogen/pre‐Albumin (FPR) (A) and 100*Fibrinogen/Albumin (FAR) (B) level in the cases, healthy and benign controls
Figure 2.
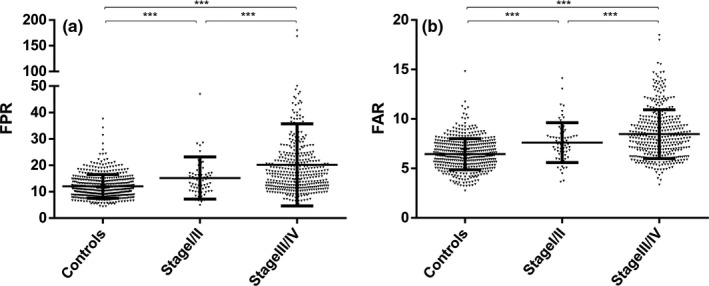
Fibrinogen/pre‐Albumin (FPR) (A) and 100*Fibrinogen/Albumin (FAR) (B) level in TNM stage and healthy controls
3.2. Diagnostic value of FPR, FAR, and NLR
Receiver‐operating characteristics analysis showed that FAR owned the highest diagnostic efficacy in discriminating cases from healthy controls, and the AUC (0.741) was close to that of FPR (0.738). The optimal cutoff values of FAR and FPR were 8.018 (sensitivity, Sen = 50.5%, specificity, Spe = 86.2%, positive predictive value (PPV) = 78.5%, negative predictive value (NPV) = 63.5%), and 15.360 (Sen = 54.1%, Spe = 81.3%, PPV = 75.4%, NPV = 63.6%), respectively. Otherwise, the optimal cutoff value, AUC, sensitivity, and specificity value of NLR were 2.217, 0.644, 51.2%, and 74.3% (Figure 3A and Table 3).
Figure 3.
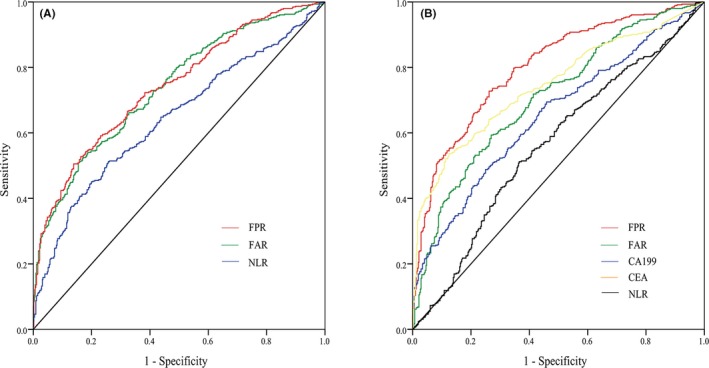
Diagnostic efficacy of inflammatory‐based biomarkers in colorectal cancer (CRC). (A) Receiver‐operating characteristics (ROC) curve of CRC compared with healthy controls; (B) ROC curve of CRC compared with benign controls
Table 3.
The diagnostic value of CRC biomarkers compared with healthy controls and benign controls
| Biomarker | Cut‐off value | AUC | Sen (%) | Spe (%) | PPV (%) | NPV (%) | YI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | a | b | a | b | a | b | a | b | a | b | a | b | |
| FPR | 15.360 | 12.247 | 0.738 | 0.801 | 54.1 | 72.7 | 81.3 | 74.3 | 75.4 | 73.9 | 63.6 | 73.2 | 0.3538 | 0.4703 |
| FAR | 8.018 | 7.529 | 0.741 | 0.707a | 50.5 | 59.3 | 86.2 | 72.5 | 78.5 | 68.4 | 63.5 | 64.1 | 0.3670 | 0.3187 |
| NLR | 2.217 | 2.218 | 0.644a | 0.565a | 51.2 | 51.2 | 74.3 | 64.0 | 66.6 | 58.7 | 60.4 | 56.7 | 0.2549 | 0.1516 |
| CEA | 2.780 | 0.746a | 53.6 | 87.5 | 82.4 | 63.3 | 0.4113 | |||||||
| CA199 | 16.570 | 0.652a | 48.8 | 75.0 | 68.1 | 57.2 | 0.2379 | |||||||
| FPR+CEA | ‐ | 0.841a | 80.2 | 72.4 | 76.0 | 77.0 | 0.5260 | |||||||
| FPR+NLR | ‐ | 0.800 | 73.4 | 73.6 | 73.6 | 73.5 | 0.4703 | |||||||
| FPR+CA199 | ‐ | 0.814a | 71.2 | 76.9 | 77.1 | 71.0 | 0.4813 | |||||||
| FPR+CEA+CA199 | ‐ | 0.845a | 67.9 | 85.3 | 83.5 | 70.9 | 0.5325 | |||||||
| FPR+NLR+CEA+CA199 | ‐ | 0.843a | 83.5 | 68.8 | 74.2 | 79.1 | 0.5227 | |||||||
AUC, area under curve; a, compared with healthy controls; b, compared with benign controls; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19‐9; FAR, 100*Fibrinogen/Albumin; FPR, Fibrinogen/pre‐Albumin; NLR, neutrophil‐to‐lymphocyte ratio; NPV, negative predictive value; PPV, positive predictive value; Sen, sensitivity; Spe, specificity; YI, Youden's index.
P < 0.05 vs FPR.
3.3. Logistic regression analysis
Logistic regression analysis was used to evaluate the risk factors for telling CRC apart from benign disease. The risk factors found to be markedly associated with differentiation of benign disease and CRC in the regression analysis included FPR (odds ratio, OR = 5.532; 95% confidence interval, 95% CI = 3.715‐8.238, P < 0.05), NLR (OR = 1.423, 95% CI = 1.022‐1.981, P < 0.05), CEA (OR = 5.882, 95% CI = 4.016‐8.615, P < 0.05), and CA199 (OR = 1.782, 95% CI = 1.258‐2.524, P < 0.05) (Table 2).
Table 2.
Multiple logistic regression analysis of factors used for differentiation CRC from benign colorectal disease
| Variables | β | OR (95% CI) | P value |
|---|---|---|---|
| FPR | 1.711 | 5.532 (3.715‐8.238) | 0.000 |
| FAR | 0.192 | 1.211 (0.810‐1.812) | 0.351 |
| NLR | 0.353 | 1.423 (1.022‐1.981) | 0.037 |
| CEA | 1.772 | 5.882 (4.016‐8.615) | 0.001 |
| CA199 | 0.578 | 1.782 (1.258‐2.524) | 0.000 |
CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 19‐9; CI, confidence interval; FAR, 100*Fibrinogen/Albumin; FPR, Fibrinogen/pre‐Albumin; NLR, neutrophil‐to‐lymphocyte ratio; OR, odds ratio.
3.4. Distinguishing CRC from benign disease
In the cases and benign controls, the diagnostic value of FPR was better than FAR, NLR, CEA, and CA199 (P < 0.05), indicating FPR was the best one among these biomarkers for distinguishing CRC from benign disease. Therefore, FPR in conjunction with other biomarkers were further analyzed for combined detection. (Figure 4) As expected, the combined biomarkers resulted in enhanced diagnostic efficacy, and the combination of FPR, CEA, and CA199 had the maximum AUC in discriminating between CRC and benign disease (AUC = 0.845, Sen = 67.9%, Spe = 85.3%, PPV = 83.5%, NPV = 70.9%). Further, four biomarkers’ (FPR, NLR, CEA and CA199) combination was adopted to separate CRC from benign disease, resulting in an improved efficacy. (AUC = 0.843, Sen = 83.5%, Spe = 68.8%, PPV = 74.2%, NPV = 79.1%) (Table 3).
Figure 4.
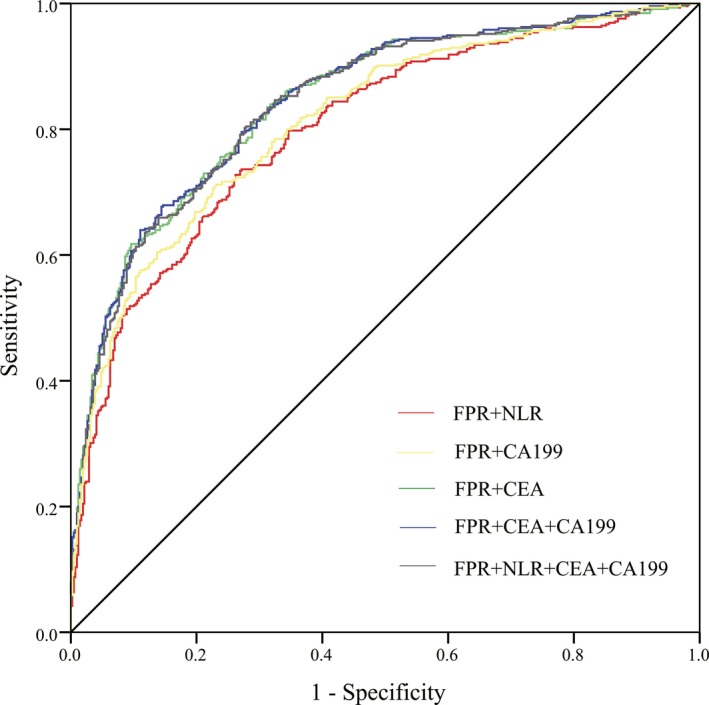
Receiver‐operating characteristics (ROC) curve of combined biomarkers compared with benign controls in colorectal cancer (CRC)
4. DISCUSSION
Cancer‐related inflammation has been recognized as a hallmark of tumorigenesis and progression.20 Numerous studies reported that inflammatory bowel disease could trigger choric inflammation and increase the risk of CRC.21 On the contrary, the continuous use of low‐dose anti‐inflammatory drugs such as aspirin or NSAIDs could reduce the risk of CRC.22 The measurement of the systemic inflammatory response has been extensively analyzed; however, the diagnostic roles of FAR and FPR in CRC remained unclear.
In the present study, we firstly assessed the diagnostic value of the FAR and FPR in CRC patients. Our study demonstrates that elevated Fib level, NLR, FAR, and FPR were observed in the cases compared with the healthy or benign controls; however, Alb and pAlb were obviously low in the cases. We also found NLR, FAR, and FPR were elevated in the early tumor stage compared with healthy controls, indicating that these three parameters could act as early diagnostic markers for CRC. In addition, our results revealed that the CRC diagnostic value of FAR and FPR was superior to NLR, and the combination of FPR, CEA, and CA199 could increase the diagnostic efficacy for distinguishing CRC from benign disease. The logistic analysis revealed that FPR represented a risk factor for distinguishing CRC from the benign colorectal disease. Besides, the following reasons could explain our findings further.
On the one hand, tumor cell could stimulate hemostatic system to promote the pro‐thrombotic property and to trigger the generation of Fib.23 Malignant human and experimental animal tumor cells could express many procoagulant and fibrinolytic factors which sustained adhesion and survival of tumor cells, including Fib.24 And then, Fib could not only facilitate adhesion among tumor cells, platelets, and endothelial cells, but also regulate tumor cell proliferation and migration by interacting with multiple receptors of cancer cells.25, 26 Consequently, enhanced tumor cell escaping and impaired immunologic surveillance directly result in the onset and progression of cancer.
On the other hand, the prevalence of malnutrition is high in patients with cancer. Moreover, the common inflammatory cytokines, interleukin‐6, could suppress the synthesis of nutritional protein, leading to hypoproteinemia in cancer patient.27 Serum Alb and pre‐Alb, two accepted biomarkers to assess the nutritional condition, were recognized to be significant factors to predict recovery and survival of CRC cases in some studies.28
Above all, it appears to be reasonable to explore the diagnostic efficacy of FPR and FAR for CRC, and that could be more convincible on account of the combined reflection of inflammation and malnutrition. However, some limitations in the current study should be addressed. The sample size in the current study was small, and our results need to be validated by prospective research in further research around the world.
5. CONCLUSION
Fibrinogen/pre‐Albumin represents a useful CRC diagnostic biomarker, and the combination of FPR, CEA, and CA199 could significantly improve the diagnostic efficacy in discriminating CRC from the benign colorectal disease.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Second Affiliated Hospital of Nanchang University and adhered to the tenets of the Declaration of Helsinki.
DATA AVAILABILITY
Readers can access the data supporting the conclusions of the study by contacting with author through Email.
Supporting information
ACKNOWLEDGMENT
This study was funded in full by National Natural Science Foundation of China [Grant No. 81360083], and Major Projects of Science and Technology of Jiangxi Province [Grant No. 20152ACG70014], and Natural Science Youth Foundation of Jiangxi Province [Grant No. 20171BAB215054].
Sun F, Tan Y‐A, Gao Q‐F, et al. Circulating fibrinogen to pre‐albumin ratio is a promising biomarker for diagnosis of colorectal cancer. J Clin Lab Anal. 2019;33:e22635 10.1002/jcla.22635
Fan Sun and Yu‐Ao Tan equally contributed to this study.
Contributor Information
Hou‐Qun Ying, Email: yinghouqun2013@163.com.
Xiao‐Zhong Wang, Email: wangxiaozhong@ncu.edu.cn.
REFERENCES
- 1. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177‐193. [DOI] [PubMed] [Google Scholar]
- 4. Diamandis EP, Hoffman BR, Sturgeon CM. National academy of clinical biochemistry laboratory medicine practice guidelines for the use of tumor markers. Clin Chem. 2008;54(11):1935‐1939. [DOI] [PubMed] [Google Scholar]
- 5. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539‐545. [DOI] [PubMed] [Google Scholar]
- 6. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008;454(7203):436‐444. [DOI] [PubMed] [Google Scholar]
- 7. Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101‐2114.e5. [DOI] [PubMed] [Google Scholar]
- 8. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d‐NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(12):305. [DOI] [PubMed] [Google Scholar]
- 10. Ghuman S, Van Hemelrijck M, Garmo H, et al. Serum inflammatory markers and colorectal cancer risk and survival. Br J Cancer. 2017;116(10):1358‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem. 2007;14(27):2925‐2936. [DOI] [PubMed] [Google Scholar]
- 12. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43‐62. [DOI] [PubMed] [Google Scholar]
- 13. Lu K, Zhu Y, Sheng L, Liu L, Shen L, Wei Q. Serum fibrinogen level predicts the therapeutic response and prognosis in patients with locally advanced rectal cancer. Hepatogastroenterology. 2011;58(110‐111):1507‐1510. [DOI] [PubMed] [Google Scholar]
- 14. Obata J, Tanaka N, Mizuno R, et al. Plasma fibrinogen level: an independent prognostic factor for disease‐free survival and cancer‐specific survival in patients with localised renal cell carcinoma. BJU Int. 2016;118(4):598‐603. [DOI] [PubMed] [Google Scholar]
- 15. Wen J, Yang Y, Ye F, et al. The preoperative plasma fibrinogen level is an independent prognostic factor for overall survival of breast cancer patients who underwent surgical treatment. Breast. 2015;24(6):745‐750. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Yin W, Wang Z, et al. Pretreatment plasma fibrinogen as an independent prognostic indicator of prostate cancer patients treated with androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2016;19(2):209‐215. [DOI] [PubMed] [Google Scholar]
- 17. Wang H, Gao J, Bai M, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets. 2014;25(5):382‐387. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez‐Trejo S, Carrillo JF, Carmona‐Herrera DD, et al. Baseline serum albumin and other common clinical markers are prognostic factors in colorectal carcinoma: a retrospective cohort study. Medicine (Baltimore). 2017;96(15):e6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang J, Wang Y, Yuan Y, et al. Preoperative serum pre‐albumin as an independent prognostic indicator in patients with localized upper tract urothelial carcinoma after radical nephroureterectomy. Oncotarget. 2017;8(22):36772‐36779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493‐e503. [DOI] [PubMed] [Google Scholar]
- 21. Grivennikov SI. Inflammation and colorectal cancer: colitis‐associated neoplasia. Semin Immunopathol. 2013;35(2):229‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friis S, Riis AH, Erichsen R, Baron JA, Sorensen HT. Low‐dose aspirin or nonsteroidal anti‐inflammatory drug use and colorectal cancer risk: a population‐based, case‐control study. Ann Intern Med. 2015;163(5):347‐355. [DOI] [PubMed] [Google Scholar]
- 23. Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4(6):465‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302‐3309. [PubMed] [Google Scholar]
- 25. Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen‐deficient mice. Can Res. 2002;62(23):6966‐6972. [PubMed] [Google Scholar]
- 26. Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell‐mediated elimination of tumor cells. Blood. 2005;105(1):178‐185. [DOI] [PubMed] [Google Scholar]
- 27. Fujii T, Sutoh T, Morita H, et al. Serum albumin is superior to prealbumin for predicting short‐term recurrence in patients with operable colorectal cancer. Nutr Cancer. 2012;64(8):1169‐1173. [DOI] [PubMed] [Google Scholar]
- 28. Hu WH, Cajas‐Monson LC, Eisenstein S, Parry L, Cosman B, Ramamoorthy S. Preoperative malnutrition assessments as predictors of postoperative mortality and morbidity in colorectal cancer: an analysis of ACS‐NSQIP. Nutr J. 2015;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Readers can access the data supporting the conclusions of the study by contacting with author through Email.


