Abstract
Objective
Lichen planus (LP) is an autoimmune inflammatory disease of the mucocutaneous tissue, whose exact pathological course remains unclear. Abnormal thiol/disulfide homeostasis has been postulated to be responsible for a number of diseases predominated by chronic inflammation. To be able to contribute complicated and unclear pathogenesis of LP, we aimed to investigate dynamic thiol/disulfide homeostasis in patients with LP, using an original automated method developed by Erel and Neselioglu in this study.
Methods
The study group consisted of 81 unrelated patients with LP and 80 unrelated healthy controls with no LP lesions in their personal history or on clinical examination. Thiol/disulphide homeostasis tests have been measured with a novel automatic spectrophotometric method developed and the results have been compared statistically.
Results
Native thiol and total thiol levels were found as significantly higher in patients with LP than the control group (P = 0.026 and 0.035, respectively). There was no significant difference between the disulfide levels of the patients with LP and the control group.
Conclusions
We provide evidence that that thiol/disulphide homeostasis impaired in favor of thiol levels in LP patients compared to the control group based on the data of our study. To the best of our knowledge, the present study is the first examination on the correlation between thiol and disulfide homeostasis in patients with LP.
Keywords: disulphide, homeostasis, lichen planus, oxidative stress, thiol
1. INTRODUCTION
Lichen planus (LP) is a chronic inflammatory papulosquamous disorder, clinically characterized by small, flattopped, shiny, polygonal violaceous papules that may coalesce into plaques, affecting the skin, mucous membranes, hair, and nails. The clinical presentation of LP has several forms, including the classic plaque, oral, hypertrophic, follicular, linear, actinic, and bullous types.1 It is estimated to affect 0.9% to 1.2% of the general population in all races and occurs usually between 30 and 60 years.1, 2, 3
Even if there have been significant progresses for illuminating the pathogenesis of LP, the exact aetiopathogenesis of the disease can not been completely understood yet. It is believed that LP represents a T‐cell mediated inflammatory disorder.1 Insufficient antioxidant protection or excess ROS production builds a condition known as an oxidative stress, contributing to the development of cutaneous disease and disorders like atopic dermatitis, psoriasis, vitiligo, acne vulgaris, pemphigus vulgaris.4, 5 To be able to contribute complicated and unclear pathogenesis of LP, the role of the oxidative stress in LP has also been studied by several researchers in a few studies with different results. These results suggested that oxidant/antioxidant imbalance may play a role in the etiopathogenesis of LP.2, 3, 6, 7
Thiol groups are compounds containing a sulfhydryl group (‐SH) and are important members of the antioxidant cascade as they destroy ROS and other free radicals by enzymatic as well as non‐enzymatic mechanisms.8 During oxidative stress, protein thiol groups are oxidized to form disulfide (‐S‐S‐) bonds. The disulfide bonds formed, the earliest sign of protein oxidation are reversible could be reduced back to thiol groups, maintaining the homeostasis between thiols and disulfide groups.8, 9
Dynamic thiol/disulphide homeostasis represents an important function in numerous vital events such as programmed cell death, detoxification, antioxidant protection and regulation of cellular enzymatic activity, transcription, and cellular signal transduction mechanisms. When thiol/disulphide homeostasis shifts toward the disulphide formation, these vital activities are adversely affected and pathologies occur in the structure and functions of many organs.9, 10 Abnormal thiol/disulfide homeostasis has been postulated in the pathogenesis of several diseases including diabetes, cardiovascular disease, cancer, rheumatoid arthritis, chronic kidney disease in which chronic inflammation is prominent.11, 12, 13, 14 Recently, a new fully automated method is developed to evaluate thiol/disulfide homeostasis allowing the individual measurement of both the thiol and disulfide levels.15 To the best of our knowledge, no studies up to date investigated thiol/disulphide homeostasis as a novel oxidative stress marker in patients with LP and compared the results with healthy controls. In this study, we aimed to investigate dynamic thiol/disulfide homeostasis in patients with LP using an original automated method developed by Erel and Neselioglu.15
2. MATERIALS AND METHODS
2.1. Subjects
The study group consisted of 81 unrelated patients with LP (43 male and 38 female; median age: 46 [IQR: 35.5‐55] years, and 80 [51 male and 29 female]; median age: 43 [IQR: 31.25‐53] years) unrelated healthy controls with no lesions of LP in their personal history or on clinical examination. Both patients and control goups consisted of the subjects older than 18 years. Patients with LP were gathered from Department of Dermatology of Ankara Yıldırım Beyazıt University, Ankara, Turkey between March 2015 and April 2016. Clinical and demographical data were obtained from all patients, including gender, age, and disease duration. The diagnosis of LP was based on clinical findings and confirmed by histopathology. Different types of LP; classic, oral, hypertrophic, pigmentosus, eruptive, genital, and nail were diagnosed. Exclusion criteria included subjects who had received any systemic treatment suppressing the immune system such as systemic steroids or other immunosuppressive drugs, and nonsteroidal antiinflammatory drugs (NSAIDs) for the last 4 weeks, and topical medications for the last 2 weeks prior to sample collection. Other exclusion criteria included smokers, patients with history of trauma or any surgery and alcohol ingestion within 1 month prior to sampling, and those suffering from systemic or other dermatologic diseases affecting immune system or any malignancy. Patients with lichenoid drug eruption, those consuming drugs such as lipidlowering agents, statins and fibrates for the past 4 weeks prior to blood sample collection were also excluded from the study. In addition, 80 patients who were age and sex matched with the subjects served as controls. The healthy control patients consisted of the patients who were admitted to our dermatology clinic with other reasons: nevus examination, dermatofitos infections or warts and matched for age and gender with LP patients. All participants, patients and healthy controls, were of Turkish origin, from the central region of Turkey. The protocol of this study was approved by the Local Ethics Committee of Ankara Yıldırım Beyazıt University Faculty of Medicine (Approval no. = 127) and all participants gave written informed consent before entering the study. All participants accepted the peripheral blood sampling for the analyses.
2.2. Method
2.2.1. Biochemical assays and thiol/disulfide homeostasis parameters measurement
Venous blood samples were taken in tubes containing ethylenediaminetetraacetic acid (EDTA) obtained from patients and healthy controls to measure biochemical parameter and for thiol/disulfide homeostasis tests after an overnight fast and serum was separated from the cells by centrifugation at 1800 g for 10 min and stored in the freezer at −80°C until the time of analysis. All samples were assayed at the same time. Serum native and total thiol concentrations showing thiol/disulfide homeostasis were calculated with a novel fully automated colorimetric method that is developed by Erel and Neselioglu.15 Erel et al used modified Elman reagents for thiol measurement. Sodium borohydride (NaHB4), a reducing agent, is added into serum to be able to determine total thiol level; consequently, dynamic disulfide bonds are reduced to functional thiol groups. Subsequently, formaldehyde is added and unused NaHB4 is exhausted. All thiol groups, including reducing and native thiols, are calculated after they are reacted with 5,5′‐dithiobis‐2‐nitrobenzoic acid (DTNB). Half of the difference between the total thiols and native thiols (total thiol − native thiol)/2 supplied the dynamic disulfide amount. The ratios of disulfide/total thiol, native thiol/total thiol and disulfide/native thiol were calculated according to the native thiol, total thiol, and disulfide amounts.15
2.3. Statistical analysis
Statistical Package for Social Sciences (SPSS), for Windows version 22 (IBM Corp., New York, NY, USA) software was used for statistical analysis of data. Kolmogorov‐Smirnov test was used to analyze the distribution pattern of the variables. Numerical variables exhibiting a normal distribution were expressed as averages ± standard deviation, and those not showing a normal distribution were expressed as medians (interquartile range). Student's t test and Mann‐Whitney U tests were used to compare 2 groups of numerical variables. Categorical variables were expressed as percentages. Pearson's chi‐square tests were used to compare categorical variables. A value of P < 0.05 was considered statistically significant.
3. RESULTS
The study group consisted of 81 unrelated patients with LP (43 male and 38 female; median age: 46 [IQR: 35.5‐55] years, and 80 [51 male and 29 female]; median age: 43 [IQR: 31.25‐53] years) unrelated healthy controls with no lesions of LP in their personal history or on clinical examination. Age and gender were not significantly different between patient and control groups (P = 0.108 and 0.170, respectively). Baseline demographical features of the study patients with LP and control group were summarized in Table 1. There was a statistically significant difference between the native thiol and total thiol levels of the patient groups and control subjects (P = 0.026 and 0.035, respectively). Native thiol and total thiol levels were found as higher in patients with LP than the control group (Figure 1). These findings showed that thiol/disulfide homeostasis was imbalanced in patients with LP, and it was shifted toward the thiol side. There was no significant difference between the disulfide levels of the patients with LP and the control group. The disulfide/native thiol percent ratio and disulfide/total thiol (P = 0.001) percent ratios were lower in the patients with LP group than in the control group even if they are not statistically significant. The thiol/disulfide homeostasis parameters of the 2 groups are shown in Table 1 and Figure 1. When correlation between thiol disulphide balance and patient characteristics was investigated, native thiol and total thiol levels were detected as significantly higher in females according to the male population (P = 0.006 and 0.005, respectively). A statistically significant negative correlation between patients’ age and native thiol and total thiol was seen. We detected that native thiol and total thiol levels decreased with increasing age.
Table 1.
Thiol/disulfide homeostasis parameter levels among groups
| Control (n = 80) | Patient (n = 81) | P value | |
|---|---|---|---|
| Age; years | 43 (IQR: 31.25‐53) | 46 (IQR: 35.5‐55) | 0.108 |
| Gender | |||
| Female | 29 (36.3%) | 38 (46.9%) | 0.170 |
| Male | 51 (63.7%) | 43 (53.1%) | |
| NT; μmol/L | 450.38 ± 56.16 | 470.43 ± 56.78 | 0.026 |
| TT; μmol/L | 490.54 ± 58.34 | 510.86 ± 62.56 | 0.035 |
| SS; μmol/L | 20.08 ± 7.97 | 20.22 ± 7.74 | 0.913 |
| SS/NT; % | 4.53 ± 1.85 | 4.31 ± 1.67 | 0.419 |
| SS/TT; % | 4.10 ± 1.57 | 3.92 ± 1.41 | 0.444 |
| NT/TT; % | 91.8 ± 3.14 | 92.16 ± 2.82 | 0.444 |
NT: Native thiol; TT: Total thiol; SS: Disulfide; SS/NT: Disulfide/Native thiol; SS/TT: Disulfide/Total thiol; NT/TT: Native thiol/Total thiol. Values were given as mean ± SD, number (%) or median (IQR). P < 0.05 was considered statistically significant. The significantly P values were marked as bold.
Figure 1.
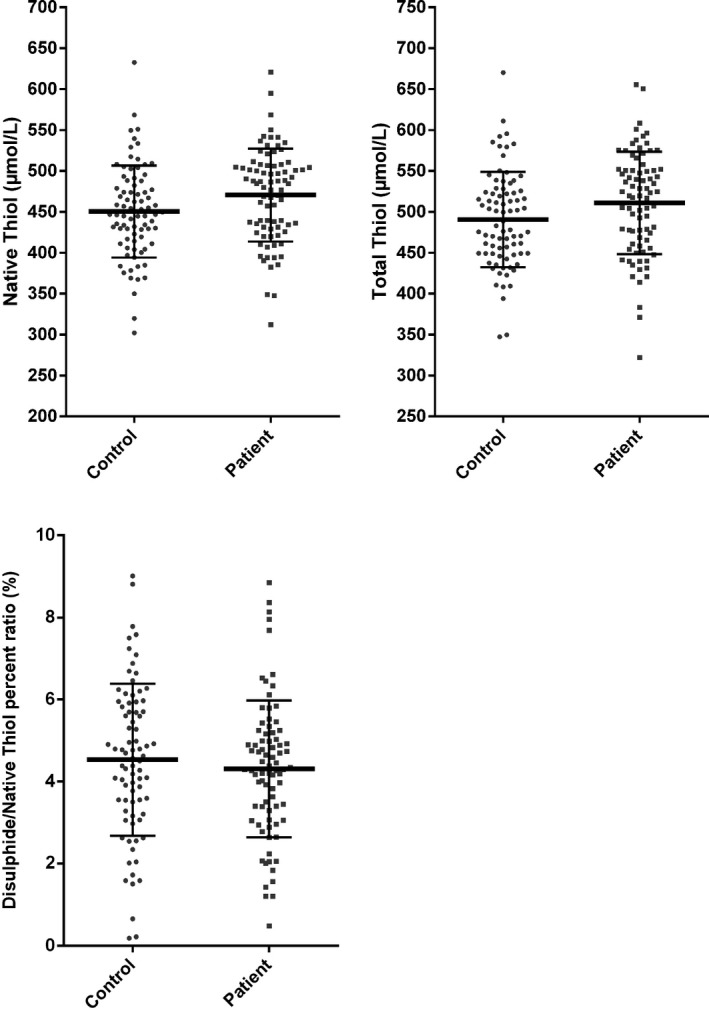
Thiol/disulfide homeostasis parameters between patients and controls
4. DISCUSSION
In this study, we examined dynamic thiol/disulfide homeostasis in a group of Turkish patients with LP using a novel automated method. A statistically significant difference was observed between patients and controls according to the native thiol and total thiol levels (P = 0.026 and 0.035, respectively). Native thiol and total thiol levels were found as higher in patients with LP than the control group. It can be concluded that thiol/disulfide homeostasis was imbalanced in patients with LP, and balance point shifted to thiol formation. However, we could not detect statistically significant association between the disulfide levels of the patients with LP and the control group. To the best of our knowledge, this is the first study investigating the thiol/disulphide homeostasis in patients with LP.
LP is an autoimmune inflammatory disease of the mucocutaneous tissue, whose exact pathological course remains unclear. Cytokine‐mediated apoptosis of basal epithelial keratinocytes by activated cytotoxic T‐cells leading to the attraction of inflammatory cells in the dermoepidermal junction has been implicated in the pathogenesis of LP, but the initial triggering antigen has not been recognized yet.1, 2, 3 The role of oxidant/antioxidant status has been proposed as one of the striking probable new pathogenetic mechanisms because of apoptotic and autoimmune features of LP. It has been proposed that ROS forms covalent bond with endogen proteins and causes the formation of antigen by producing structural changes in the proteins. Autoimmune mechanisms are triggered by formation of new antigens. From this point of view, in the studies examining the role of oxidative stress in LP, revealed that oxidant/antioxidant imbalance may be a factor in the etiopathogenesis of LP.1, 2, 3, 4, 5, 6, 7 Sezer et al3 suggested that increased oxidative stress, increased lipid peroxidation and an imbalance in the antioxidant defense system may be involved in the pathogenesis of LP. They found higher serum NO, MDA, SOD levels and lower erythrocyte CAT levels in patients with LP compared with the control group. Recent studies have supported these findings leading to an imbalance in the antioxidant defense system in their study.2, 6 Similarly, in another study significantly lower levels of serum protein thiols and total antioxidant levels and higher serum MDA levels in oral LP were detected.7 Conversely, our study has been contradictory to this previous study since the native thiol parameteres were found higher in the patients with LP.
Serum thiols are physiological free‐radical scavengers and are responsible for 52.9% of the total serum antioxidant capacity and modulate antioxidant enzymes related to glutathione. Thiols are found in plasma and structure of proteins, albumin, cysteine, homocysteine, cysteinylglycin, glutathione (GSH), and gamma‐glutamylcysteine (γ‐GluCys) (Turell et al). 10 When in the same environment with ROS, thiol groups are oxidized and form reversible disulphide bonds.8, 9, 10 Until 2014, thiol/disulphide homeostasis could be measured unilaterally; however, the method developed by Erel and Neselioglu succeeded to measure homeostasis levels separately and additively by providing the individual measurement of both the thiol and disulfide levels.15 Providing the accurate measurement of both sides of the balance completely easier and fast with both a manual and colorimetric method is the most striking advantaged and superior properties of this a novel and automated method.15 In the light of all the studies that indicated the pathophysiologic role of the oxidative stress in LP, we hypothesized that there might be an impaired thiol/disulphide homeostasis in LP patients when compared with the controls and designed this study. In our study, native thiol and total thiol levels showing prominent antioxidant property were significantly higher in patients with LP than the control group. This could be in response to the continued production of the ROS, which needs thiols for detoxification and could reflect that the total serum thiol levels in these patients may have the ability to protect against oxidative stress. The increased serum thiol levels could be an adaptive response to counteract harmful effects of the elevated concentrations of ROS. An increase in antioxidant enzyme activity such as SOD activity has also been described in other chronic inflammatory skin conditions, such as atopic dermatitis, psoriasis, and urticaria.16 It is likely that the overexpression of thiol levels results from an increased secretion of cytokines, as reported in LP. The main event in the pathogenesis seems to be the increased production of cytokines that induce recruitment of Langerhans cells and clonal expansion of cytotoxic cells.1, 3, 6, 7 Subsequently, the increasing levels of serum thiols as a reactive response may contribute cell proliferation and advancing of the lesions in LP.
Erel et al showed that plasma thiol/disulfide balance altered to thiol in proliferative diseases such as multiple myeloma, bladder cancer, colon ca and renal ca since the plasma disulphide levels were found lower in these patients. Conversely with these findings, the plasma disulphide levels were detected as higher in patients with degenerative diseases such as smoking, diabetes, obesity, and pneumonia causing the balance altering to disulfide.15 Similarly, a recent study performed to be able to investigate the dynamic thiol/disulfide homeostasis in patients with psoriasis since psoriasis is one of the diseases in which oxidative stress has been considered in the etiopathogenesis. Concordant with our results, they detected that thiol/disulfide balance shifted toward thiol in psoriasis patients and concluded that the elevated thiol levels could cause increased keratinocyte proliferation forming the main mechanism of the pathogenesis of psoriasis.17 Similarly, in the recent study performed in patients with basal cell carcinoma balance point shifted to thiol formation since disulphide levels decreased and thiol levels increased, by reflecting the concordant results just like in proliferative diseases, especially several carcinoma species.18 In our study, thiol/disulphide homeostasis weakened in favor of thiol levels just like in the patients with proliferative diseases concordant with the results of the previous studies. Glutathione (GSH) is the major low molecular weight thiol in cells, the most significant source of thiol in humans and plays a central role in controlling cellular thiol/disulfide redox state, which is essential for normal redox signaling.19 An association between cellular GSH concentration and proliferation has been established in numerous studies, in which GSH precursors or rising cellular GSH has been found to augment cell proliferation.19, 20 From this point of view, we suggest that the increasing levels of serum thiols against the oxidative stress may contribute cell proliferation and progressing of the lesions in LP since the thiol/disulfide homeostasis may cause changes in cellular circumstances. We can understand that LP is a proliferative disease because of the immunopathological and histopathological findings of the disease such as characteristic “saw‐tooth” pattern of epidermal hyperplasia; hyperparakeratosis with thickening of the granular cell layer; and vacuolar alteration of the basal layer of the epidermis, with an intense infiltration; mainly T cells at the dermal‐epidermal junction.21
5. CONCLUSIONS
In conclusion, we provide evidence that thiol/disulphide homeostasis impaired in favor of thiol levels in LP patients compared to the control group based on the data of our study. To the best of our knowledge, the present study is the first examination on the correlation between thiol and disulfide homeostasis in patients with LP. In the light of this study, further studies with large cohort on different populations and ethnicities will be necessitated to better elucidate the complex LP immunopathogenesis and confirm these findings.
Kalkan G, Emre S, Alisik M, Aktaş A, Baran P. Dynamic thiol/disulfide homeostasis in patients with lichen planus. J Clin Lab Anal. 2019;33:e22642 10.1002/jcla.22642
REFERENCES
- 1. Lukács J, Schliemann S, Elsner P. Lichen planus and lichenoid reactions as a systemic disease. Clin Dermatol. 2015;33:512‐519. [DOI] [PubMed] [Google Scholar]
- 2. Panchal FH, Ray S, Munshi RP, Bhalerao SS, Nayak CS. Alterations in lipid metabolism and antioxidant status in lichen planus. Indian J Dermatol. 2015;60:439‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sezer E, Ozugurlu F, Ozyurt H, Sahin S, Etikan I. Lipid peroxidation and antioxidant status in lichen planus. Clin Exp Dermatol. 2007;32:430‐434. [DOI] [PubMed] [Google Scholar]
- 4. Okayama Y. Oxidative stress in allergic and inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005;4:517‐519. [DOI] [PubMed] [Google Scholar]
- 5. Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases—What's new? JEADV. 2003;17:663‐669. [DOI] [PubMed] [Google Scholar]
- 6. Tvarijonaviciute A, Aznar‐Cayuela C, Rubio CP, Ceron JJ, López‐Jornet P. Evaluation of salivary oxidate stress biomarkers, nitric oxide and C reactive protein in patients with oral lichen planus and burning mouth syndrome. J Oral Pathol Med. 2017;46:387‐392. 10.1111/jop.12522 [DOI] [PubMed] [Google Scholar]
- 7. Upadhyay RB, Carnelio S, Shenoy RP, Gyawali P, Mukherjee M. Oxidative stress and antioxidant defense in oral lichen planus and oral lichenoid reaction. Scand J Clin Lab Invest. 2010;70:225‐228. [DOI] [PubMed] [Google Scholar]
- 8. Turell L, Radi R, Alvarez B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic Biol Med. 2013;65:244‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones DP, Liang YMtpotciv. Free Radic Biol Med. 2009;47:1329‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biswas S, Chida AS, Rahman I. Redox modifications of protein‐thiols: Emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551‐564. [DOI] [PubMed] [Google Scholar]
- 11. Matteucci E, Giampietro O. Thiol signalling network with an eye to diabetes. Molecules. 2010;15:8890‐8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50:495‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tetik S, Ahmad S, Alturfan AA, et al. Determination of oxidant stress in plasma of rheumatoid arthritis and primary osteoarthritis patients. Indian J Biochem Biophys. 2010;47:353‐358. [PubMed] [Google Scholar]
- 14. Rodrigues SD, Batista GB, Ingberman M, et al. Plasma cysteine/cystine reduction potential correlates with plasma creatinine levels in chronic kidney disease. Blood Purif. 2012;34:231‐237. [DOI] [PubMed] [Google Scholar]
- 15. Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326‐332. [DOI] [PubMed] [Google Scholar]
- 16. Kalkan G, Seçkin HY, Duygu F, Akbaş A, Ozyurt H, Sahin M. Oxidative stress status in patients with acute urticaria. Cutan Ocul Toxicol. 2014;33:109‐114. 10.3109/15569527.2013.808658 [DOI] [PubMed] [Google Scholar]
- 17. Emre S, Demirseren DD, Alisik M, Aktas A, Neselioglu S, Erel O. Dynamic thiol/disulfide homeostasis and effects of smoking on homeostasis parameters in patients with psoriasis. Cutan Ocul Toxicol. 2017;36:393‐396. [DOI] [PubMed] [Google Scholar]
- 18. Demirseren DD, Cicek C, Alisik M, et al. Dynamic thiol/disulphide homeostasis in patients with basal cell carcinoma. Cutan Ocul Toxicol. 2017;36:278‐282. [DOI] [PubMed] [Google Scholar]
- 19. Jones DP. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2002;348:93‐112. [DOI] [PubMed] [Google Scholar]
- 20. Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco‐2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1352‐G1359. [DOI] [PubMed] [Google Scholar]
- 21. Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X. Lever's Histopathology of the Skin (10th edition). Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]


