Abstract
Background
While a method of assaying natural killer (NK) cell activity by measuring the amount of interferon (IFN)‐γ released from NK cells has been proposed, no data are available about the factors that influence IFN‐γ levels related to NK cell activity. NLR has recently been reported to be a predictor of several diseases. In the present study, we investigated the pre‐analytical variables for NK cell activity using measurements of IFN‐γ and the relationship between NLR and NK cell activity.
Methods
The NK cell activity was assessed with the measurement of IFN‐γ after stimulation with an NK cell‐specific stimulant (NK Vue™, ATgen, Sungnam, Korea). One hundred and six adult volunteers were recruited and analysis of their complete blood count data and serum C‐reactive protein was done. Blood sample from 59 of the participants was also used for analysis of lymphocyte subpopulations.
Result
Natural killer cell activity varied widely (range, 44.2‐1775.6 pg/mL). NK cell activity was higher in females than in males (P = 0.014). NK cell activity decreased with increasing NLR (P = 0.004, r = −0.32) but NK cell activity showed no significant association with NK cell count or other lymphocyte subpopulations. NK cell activity levels according to CRP quartile were significantly different (P = 0.025).
Conclusion
We have observed that NK cell activity when assessed by IFN‐γ level measurement was negatively correlating with NLR. This result can be helpful in interpreting or predicting NK cell activity in the clinical environment.
Keywords: immunoregulation, interferon gamma, neutrophil‐to‐lymphocyte ratio, NK cell, NK cell activity
1. INTRODUCTION
Natural killer (NK) cells play a crucial role in the innate immune system. NK cells have cytotoxic activity via both direct and antibody‐mediated cellular mechanisms.1 Activated NK cells release cytotoxic granules and secrete cytokines, such as interferon (IFN)‐γ and tumor necrosis factor (TNF)‐α; these cytokines play an immunoregulatory role as they activate NK cells and further promote cytokine secretion.2 NK cell activity is related to several types of cancer, tumor stage, and survival rates.3, 4, 5 Patients with impaired NK cell activity commonly experience a relapse of cancer and have problems with defenses against viral infections.6, 7 In this context, assessment of NK cell activity is of interest in the clinical environment.
The gold standard method for analyzing the cytotoxic activity of NK cells is the Chromium 51 (51Cr) release assay (CRA). However, this assay carries a risk of radiation exposure, and the procedures involved are time‐consuming and expensive.8, 9 Therefore, several alternative methods have been developed; flow cytometric NK cell cytotoxicity assay using many different fluorescent dyes [i.e., Calcein AM (acetoxymethyl), DIOC18, CFSE] and CD107a degranulation assay.10 These assays are done in multiple steps, such as cell culture and staining, and requires a large amount of whole blood.8
Recently, a simple method for determining NK cell activity that uses quantitative sandwich enzyme‐linked immunosorbent assay (ELISA) to measure released IFN‐γ released from NK cells exposed to a specific recombinant cytokine (NK Vue™, ATgen, Sungnam, Korea). The advantages of this method are that it would measure the true NK cell activity with simple and quick methods.11, 12 However, this method relies on measurement of IFN‐γ, which is not secreted by only NK cells. Investigation on factors determining IFN‐γ secretion levels would be valuable data in interpreting IFN‐γ secretion levels.
There has been substantial evidence demonstrating that cancer‐associated inflammation is a major determinant of outcome in cancer patients.13 Spiegel and colleagues have demonstrated that neutrophils inhibit natural killer (NK) cell functions, and lead to a significant increase in survival time of cancer cells.14 Neutrophils protect cancer cells from rapid intraluminal clearance by inhibition of NK cells, and proved with in vitro functional assays that NK cells with response to neutrophils have decreased intracellular cytokine production. Therefore, Neutrophil‐to‐lymphocyte ratio (NLR) has been reported to be an independent prognostic value in patients with a variety of cancers.13, 15 NLR itself is a useful marker, and is simple to derive from routine laboratory studies, as the complete blood count (CBC). Its relationship with systematic inflammation 16 has been demonstrated in various disorders, ranging from myocardial infarction and coronary diseases17 to diabetes mellitus, hypertension, metabolic syndrome,18 or life style habit‐related conditions,19 and in various malignancies.20, 21, 22, 23
C‐reactive protein (CRP) is an acute phase reactant, and reflects inflammation.24, 25 Association of increased levels of CRP and risk of developing cancer also was suggested in a study done to a general population,26, 27 and the results are suggestive of positive correlation of CRP levels and cancer risk. As CRP provides a link between the innate and adaptive immune systems via activation of complements and interaction with Fcγ receptors,28 relationship between CRP and NK cell activity can be expected.
There is currently a lack of data regarding the association between NK cell activity and NLR or CRP. Accordingly, in the present study, we investigated that their relationship. Furthermore, data on pre‐analytical variables for NK cell activity as assayed by measuring IFN‐γ levels are scarce. In order to fill this gap in previous research, we also tried to identify the factors that predict NK cell activity measurements.
2. SUBJECTS AND METHODS
Healthy subjects over the age of 20 were recruited in this study. All participants were not on any medical therapies, and had no history of malignancies or infectious diseases. Peripheral blood samples were collected for analysis of NK cell activity, serum c‐reactive protein (CRP) levels, CBC, and lymphocyte subsets. Blood sampling was done at 13:00‐13:30 to avoid variation due to circadian rhythms. This study was approved by the Institutional Review Board of Dong‐A University Hospital. Informed consent forms were signed by all participants.
Complete blood count count was performed using an XE‐2100 (Sysmex, Kobe, Japan) hematology analyzer. Absolute neutrophil and lymphocyte count were analyzed, and NLR was calculated. Calculation of NLR was done from the absolute values of neutrophils and lymphocytes from CBC results.29 Neutropenia, lymphopenic or leukopenic subjects were to be excluded from enrollment, as the study targeted healthy subjects. CRP was measured with TBA‐200FR (Toshiba Medical Systems, Tokyo, Japan). Lymphocyte subset analysis was done by flow cytometric methods with the Navios flow cytometer (Beckman Coulter, Miami, FL). The percentages of CD3+, CD3+/4+, CD3+/8+, CD19+, and CD3‐/16+/56+ cells were analyzed using Navios software (Beckman Coulter) and the total cell counts were obtained.
Natural kille cell activity measurement was performed with the NK Vue™ kit (ATgen, Sungnam, Korea) following the manufacturer's instructions. This method detectes IFN‐γ with a qualtitative sandwich enzyme‐linked immunosorbent assay (ELISA) from NK cells when exposed to a specific recombinant cytokine, Promoca™ (ATgen), and this quantification result is used as a surrogate marker for NK cell activity in the peripheral blood of the subject.11, 12 One milliliter of whole blood was collected directly into a manufacturer‐provided Promoca™ tube and the tube was gently inverted 3 times. The tubes were incubated for 24 hours in a 37°C chamber. Optical density was measured using a CODA microplate processor (Biorad, Hercules, CA). Seven standards in duplicate were used to create standard curves. The OD values were then entered into the NK Vue™ software; the test results were calculated to pg/mL.
All variables influencing NK cell activity were subjected to regression analysis. Kruskal‐Wallis test was used to compare the differences of NK cell activity between lymphocyte quartiles, NLR quartiles, and CRP quartiles. MedCalc Software (MedCalc Software bvba, Ostend, Belguim) was used for data analysis. P‐values < 0.05 were considered statistically significant with two‐tailed tests.
3. RESULTS
3.1. Demographic data and distributions of IFN‐γ levels
The demographic data of the subjects are shown in Table 1. NK cell activity showed a wide range, from 44.20 to 1775.60 pg/mL, and did not follow a normal distribution. The median NK cell activity was 1237.70 pg/mL [95% confidence interval (CI), 1031.60‐1325.47] (Figure 1).
Table 1.
Demographic and laboratory characteristics of the study participants
| Characteristic | Total | Male | Female |
|---|---|---|---|
| Age (y), mean ± SD | 37.87 ± 10.71 | 42.85 ± 10.71 | 34.85 ± 9.52 |
| 20‐40 (n, %) | 62 (58.49) | 14 (35.00) | 48 (72.73) |
| 41‐60 (n, %) | 43 (40.57) | 25 (62.50) | 18 (27.27) |
| ≥60 (n, %) | 1 (0.94) | 1 (2.50) | 0 |
| CBC | |||
| WBC, total (/μL) | 6142.23 ± 1399.40 | 6297.15 ± 1556.75 | 5945.00 ± 1298.31 |
| Neutrophil (/μL) | 3419.57 ± 1122.52 | 3517.35 ± 1270.98 | 3360.31 ± 1028.00 |
| Lymphocyte (/μL) | 2131.82 ± 545.10 | 2161.05 ± 593.49 | 2114.10 ± 517.50 |
| Neutrophil differential count (%) | 54.97 ± 8.35 | 55.01 ± 7.42 | 54.94 ± 8.93 |
| Lymphocyte differential count (%) | 35.24 ± 7.22 | 34.90 ± 7.40 | 35.45 ± 7.15 |
| Neutrophil‐to‐lymphocyte ratio | 1.69 ± 0.70 | 1.72 ± 0.79 | 1.67 ± 0.64 |
| CRP (mg/dL) | 0.14 ± 0.29 | 0.11 ± 0.15 | 0.15 ± 0.34 |
Data are presented as mean ± standard deviation.
CBC, complete blood count; CRP, C‐reactive protein; WBC, white blood cell.
Figure 1.
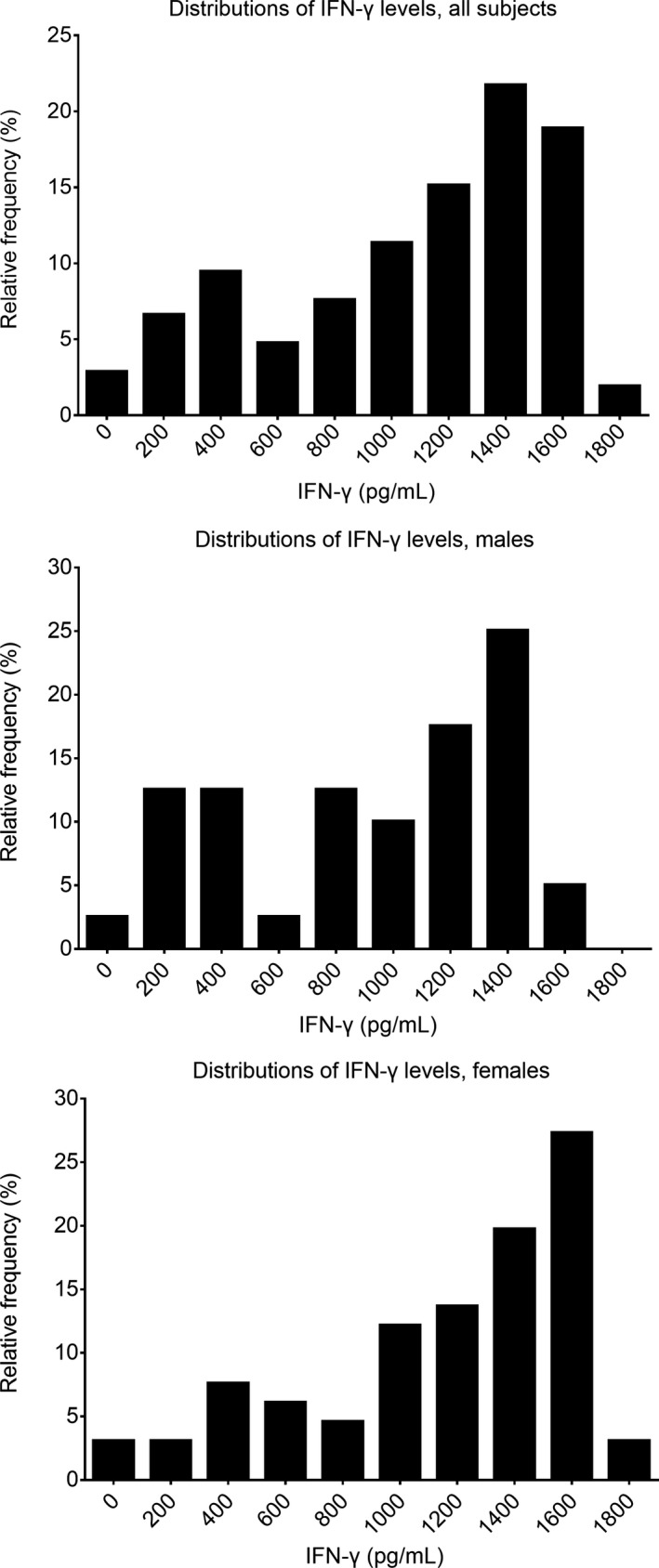
Distributions of IFN‐γ levels as NK cell activity in all subjects (top), females (middle), and males (bottom). NK cell values were normally distributed for males but were not normally distributed for females or overall subjects
3.2. Effects of age and gender on NK cell activity
Natural killer cell activity by age is presented in Figure 2. No significant difference was found based on age (r = −0.077, P = 0.429). The median values were 1309.30 pg/mL (95% CI, 1130.53‐1453.41) in female subjects and 1047.55 pg/mL (95% CI, 732.63‐1243.39) in male subjects, showing statistical difference (P = 0.014, Figure 1 middle and bottom).
Figure 2.
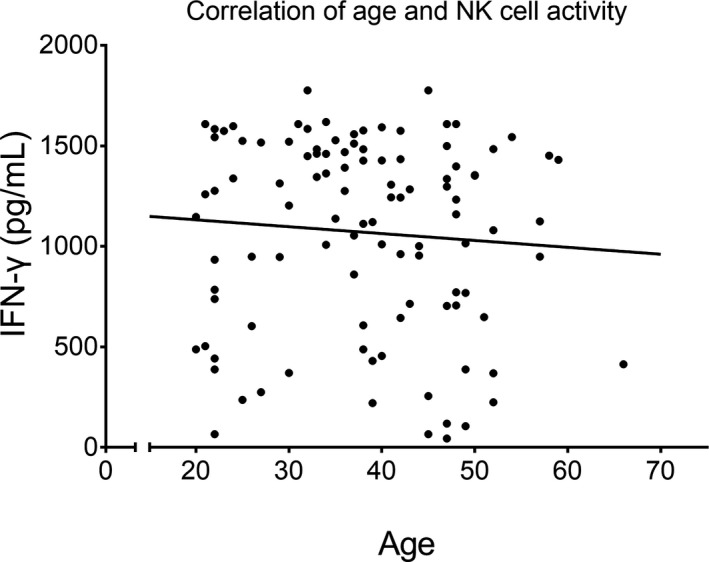
Distributions of IFN‐γ levels as NK cell activity according to age. Plot of regression analysis between log NK cell activity and age. Age was not correlated with NK cell activity (r = −0.077, P = 0.429)
3.3. Assocation of NLR, neutrophils, lymphocytes, and NK cell counts and NK cell activity
Although statistically significant, the count of WBC and neutrophils had small effect on NK cell activity (P‐values 0.026, 0.028, respectively, and r‐values −0.214, −0.212, respectively, Figure 3 top). Lymphocyte count had no statistically significant effect on NK cell activity, but lymphocyte proportion were better correlated with NK cell activity compared to lymphocyte count (P = 0.002, r = 0.298, Figure 3 middle). Neutrophil‐to‐lymphocyte ratio (NLR) also was correlated with NK cell activity (P = 0.004, r = −0.320, Figure 3 bottom). When comparing NK cell activity according to NLR quartiles, it was significantly different according to NLR quartile; it decreased with increasing NLR quartile (P < 0.001, Figure 4 top). Median NK cell activity was 1426.80 pg/mL in the first NLR quartile (≤1.21, n = 27), 1371.05 in the second quartile (1.22‐1.56, n = 26), 1010.10 in the third quartile (1.58‐1.95, n = 27), and 823.90 in the fourth quartile (≥1.96, n = 26). Furthermore, we have found no significant relationship between lymphocyte subpopulations and NK cell activity (Table S1).
Figure 3.
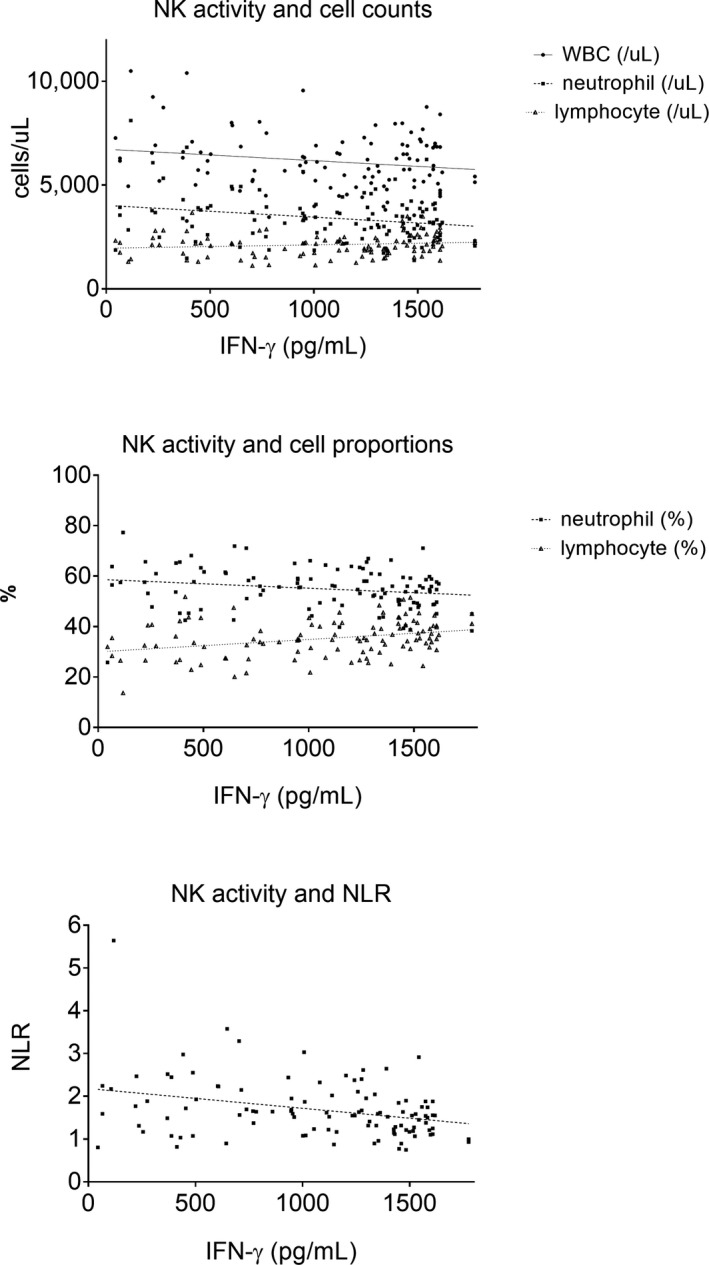
Plots of regression analysis examining the relationships between IFN‐γ levels with WBC, neutrophil and lymphocyte counts (top), IFN‐γ levels with neutrophil and lymphocyte proportions (middle), and IFN‐γ levels with neutrophil‐to‐lymphocyte ratio (NLR). WBC and neutrophils count were negatively correlated with NK cell activity (top graph, P‐values 0.026, 0.028, respectively, and r‐values −0.214, −0.212, respectively). Lymphocyte proportion showed positive correlation with NK cell activity (P = 0.002, r = 0.298, middle graph). Neutrophil‐to‐lymphocyte ratio showed negative correlation with NK cell activity (P = 0.004, r = −0.320)
Figure 4.
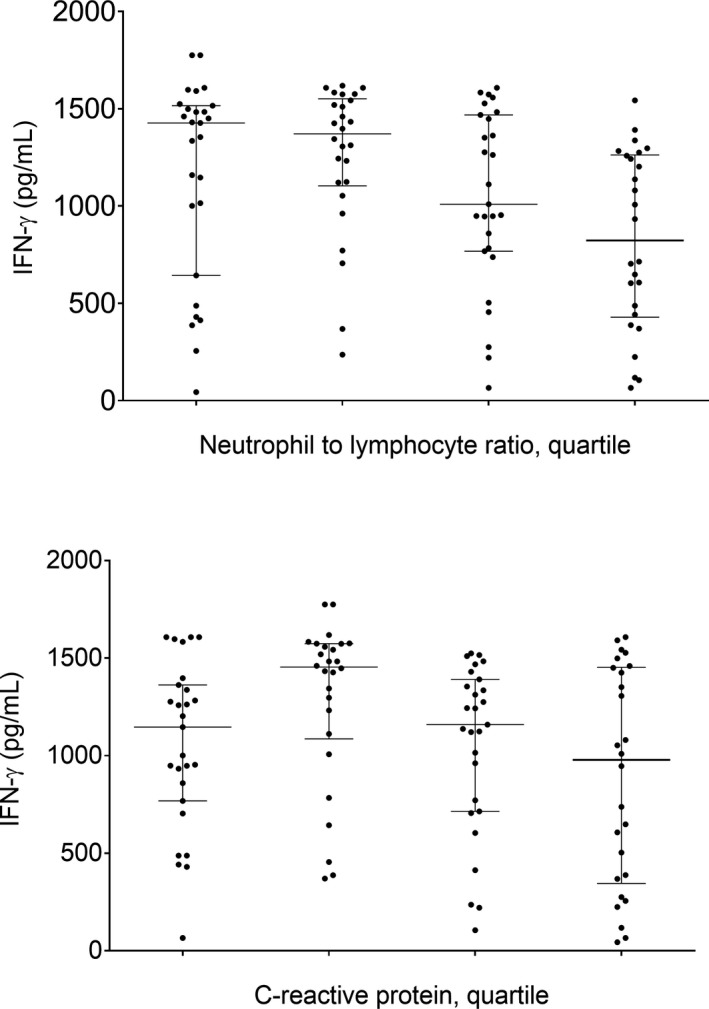
Distribution of levels of NK cell activity in different neutrophil‐to‐lymphocyte ratio (NLR) quartiles and C‐reactive protein (CRP) levels. NK cell activity according to NLR quartile was statistically significant (P < 0.001, the values of each quartile are Q1: 0.75‐1.21, n = 27; Q2: 1.22‐1.56, n = 26; Q3: 1.58‐1.95, n = 27; Q4: 1.96‐5.64, n = 26, respectively). Horizontal lines indicate medians and 1st/3rd interquartile intervals (top graph). Increased CRP levels after the second quantile group was suggestive of decreased NK cell activity (bottom graph)
3.4. Relationship between CRP and NK cell activity
No statistical significance between NK cell activity and the levels of CRP was reached (P = 0.29, r = −0.100). The median NK cell activity was 1146.50 pg/mL in the first CRP quartile (≤0.03 mg/dL, n = 33), 1235.25 pg/mL in the second quartile (0.04‐0.05 mg/dL, n = 23), 755.13 pg/mL in the third quartile (0.06‐0.1 mg/dL, n = 25), and 345.23 pg/mL in the fourth quartile (0.11‐2.25 mg/dL, n = 25). NK cell activity was higher in the second quartile than in other quartiles and it gradually decreased in the third and fourth quartiles. Overall, NK cell activity levels according to CRP quartile were significantly different (P = 0.025, Figure 4 bottom).
4. DISCUSSION
We have presented data regarding the relationship of various clinical parameters that influence NK cell activity measured by IFN‐γ. The method we used in this study has advantages compared to conventional methods in measuring NK cell activity, yet more data regarding the performance of this method is to be accumulated before broad clinical use. We identified a novel finding in this study; that NLR has a negative association with NK cell activity as assayed by IFN‐γ levels, which has not been previously reported. During this study, we have observed a large variation among healthy subjects in NK cell activity assessed by IFN‐γ secretion. It would be interesting to evaluate the levels of IFN‐γ as NK cell activity in various clinical conditions.
Previous research regarding the development of this assay12 presented data regarding geometric mean and standard deviation of NKA as a function of gender and age group of volunteers. This study expanded this finding with more detail on other laboratory parameters. We have observed that NK cell activity decreased with an increase in NLR quartile (P < 0.001) and NLR value (P = 0.004). Our finding suggests that a high NLR might be related to low IFN‐γ secretion. It was previously suggested that the decreased secretion of IFN‐γ was due to the inhibitory effects of neutrophil‐derived molecules. Several reports have shown that neutrophil‐derived molecules inhibit NK cell activity and the survival of NK cells.30, 31, 32, 33 This study demonstrated that the relative ratio of neutrophils to lymphocytes has effect on NK cell activity. NLR is an independent prognostic value in cancer patients and has correlation with clinical outcome13, 15; this may be due to the fact that mature neutrophil subset has immunosuppressive functions.34, 35, 36 The advantageous part of our observation is that NLR is easy to use, and has been sought as a useful tool37 and has been shown to have association with various disorders.16, 17, 18, 19, 20, 21, 22, 23, 29, 37, 38 It is easy to apply from simple CBC results has made it much investigated, along with other variables that can be derived from simple laboratory studies39, 40, 41, 42, 43, 44 are increasingly being of interest, due to their ease to issue in clinical practice. However, it should be noted that these variables can differ in ethnicities, underlying general condition, medication status, nutritional status, etc. We suggest that the relationship of NLR to NK cell activity be limited to healthy Asian individuals, as this study`s subjects.
We have also observed that gender was one of the factors that influenced NK cell activity. Significantly greater NK cell activity was observed in females. Although the gender difference is not fully understood, one possibility may be related to gender‐related differences in the immune system. Hirokawa et al reported that a decline in T cell proliferation and decreased cytokine production are related to age and are gender‐specific. A slower decline in immune system function was observed in females than in males.45 It could also be the case that the results of the present study were influenced by differences in the age distribution among the two genders. The majority of males enrolled in the present study were 41‐60 years old, whereas the majority of females were 20‐40 years old. This may have also contributed to the results of our study being unable to find age influencing NK cell activity. For the precise evaluation of gender differences in NK cell activity, a larger number of participants must be examined, including more elderly subjects.
The method we used to evaluate NK cell activity relies on detecting IFN‐γ secreted by NK cells. Lee et al have analyzed the specificity of the effect of PROMOCA™ on NK cells. Results showed that 50% of IFN‐γ+ cells were CD3‐CD56+ NK cells and around 30% of IFN‐γ+ cells were CD3+ cells or NKT cells. CD19+ cells and CD14+ monocytes were negative for IFN‐γ staining.12 This speculates that T cell portions in subjects can interfere the results done with the NK Vue™ kit. However, CD3+, CD3+/4+, and CD3+/8+ cellular subpopulations were not related to NK cell activity in the present study (data not shown). There was a report that quantification of total NK cells or NK cell subpopulations could not substitute for an assessment of NK cell activity, as all NK cells present in peripheral blood do not mediate cytotoxic effects.9 Our group also evaluated NK cell function through cytolytic behavior,46 and found no correlation between the NK cell count and the NK cell activity; this finding was also observed in this study through assessment by measuring IFN‐γ. Factors influencing NK cell activity measurement through IFN‐γ is a relatively unknown field and further evaluation with consideration to lymphocyte subsets would be required to fully understand its influence on IFN‐γ secretion after NK cell stimulation.
Unlike NLR showing direct significant association with NK cell activity in our study, the levels of CRP showed a negative correlation with NK cell activity but were not statistically significant. In the elderly, the number of CD56bright NK cells negatively correlated with the levels of CRP,47 there were no report of association between NK cell activity and the level of CRP. As this is the first study to evaluate CRP levels and NK cell activity measured by IFN‐γ assays, we divided our subjects into quartiles. We observed that NK cell activity levels according to CRP quartile were significantly different. We found higher NK cell activity in subjects with CRP ≤0.05 mg/dL, and NK cell activity significantly decreased with increasing CRP. Though this finding is of some interest, we could not explain why the NK cell activity of subject with CRP levels ≤0.03 mg/dL (first quartile) was lower than subjects with levels from 0.04 to 0.05 mg/dL (second quartile). Furthermore, most CRP results were within the normal range and the variance of that range was small. This result itself cannot be conclusive, and to evaluate the relationship between CRP and NK cell activity, a well‐chosen population having a wide range of CRP levels will be required.
In summary, the results of the present study suggest that the NLR has a negative association with NK cell activity. This result reflects the possibility that normal, physiological neutrophils may inhibit NK cell activity. Also, as lymphocyte counts did not have effect on NK cell activity, the absentness of correlation between lymphocyte subsets and NK cell activity further solidifies our observation regarding the relationship with neutrophil counts and NK cell activity, which was presented as the correlation of NLR and NK cell activity. Further studies are required to clarify the clinical impact of NLR with NK cell activity; however, as NLR is easily calculated from CBC results, the clinical impact of this observation has much potential. We hope that more evaluation of a larger population, as well as evaluation of subjects with various underlying morbidities will further reveal the utility of NLR in predicting NK cell activity.
Supporting information
Kim B‐R, Chun S, Cho D, Kim K‐H. Association of neutrophil‐to‐lymphocyte ratio and natural killer cell activity revealed by measurement of interferon‐gamma levels in a healthy population. J Clin Lab Anal. 2019;33:e22640 10.1002/jcla.22640
Bo‐Ram Kim and Sejong Chun are contributed equally to this study.
Contributor Information
Duck Cho, Email: duck.cho@skku.edu.
Kyeong‐Hee Kim, Email: progreen@dau.ac.kr.
REFERENCES
- 1. Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural‐killer‐cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850‐861. [DOI] [PubMed] [Google Scholar]
- 2. Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Konjevic G, Spuzic I. Stage dependence of NK cell activity and its modulation by interleukin 2 in patients with breast cancer. Neoplasma. 1993;40(2):81‐85. [PubMed] [Google Scholar]
- 4. Takeuchi H, Maehara Y, Tokunaga E, Koga T, Kakeji Y, Sugimachi K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am J Gastroenterol. 2001;96(2):574‐578. [DOI] [PubMed] [Google Scholar]
- 5. Tarle M, Kraljic I, Kastelan M. Comparison between NK cell activity and prostate cancer stage and grade in untreated patients: correlation with tumor markers and hormonal serotest data. Urol Res. 1993;21(1):17‐21. [DOI] [PubMed] [Google Scholar]
- 6. Corado J, Toro F, Rivera H, Bianco NE, Deibis L, De Sanctis JB. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;109(3):451‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kottilil S, Chun TW, Moir S, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187(7):1038‐1045. [DOI] [PubMed] [Google Scholar]
- 8. Cholujova D, Jakubikova J, Kubes M, et al. Comparative study of four fluorescent probes for evaluation of natural killer cell cytotoxicity assays. Immunobiology. 2008;213(8):629‐640. [DOI] [PubMed] [Google Scholar]
- 9. Friberg DD, Bryant JL, Whiteside TL. Measurements of natural killer (NK) activity and NK‐cell quantification. Methods. 1996;9(2):316‐326. [DOI] [PubMed] [Google Scholar]
- 10. Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15‐22. [DOI] [PubMed] [Google Scholar]
- 11. Dowell AC, Oldham KA, Bhatt RI, Lee SP, Searle PF. Long‐term proliferation of functional human NK cells, with conversion of CD56(dim) NK cells to a CD56 (bright) phenotype, induced by carcinoma cells co‐expressing 4‐1BBL and IL‐12. Cancer Immunol Immunother. 2012;61(5):615‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SB, Cha J, Kim IK, et al. A high‐throughput assay of NK cell activity in whole blood and its clinical application. Biochem Biophys Res Comm. 2014;445(3):584‐590. [DOI] [PubMed] [Google Scholar]
- 13. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation‐based neutrophil‐lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218‐230. [DOI] [PubMed] [Google Scholar]
- 14. Spiegel A, Brooks MW, Houshyar S, et al. Neutrophils Suppress Intraluminal NK Cell‐Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016;6(6):630‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 16. Balta S, Demirkol S, Unlu M, Arslan Z, Celik T. Neutrophil to lymphocyte ratio may be predict of mortality in all conditions. Br J Cancer. 2013;109(12):3125‐3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balta S, Demirkol S, Celik T, et al. Association between coronary artery ectasia and neutrophil‐lymphocyte ratio. Angiology. 2013;64(8):627‐632. [DOI] [PubMed] [Google Scholar]
- 18. Balta S, Cakar M, Demirkol S, Arslan Z, Akhan M. Higher neutrophil to lymhocyte ratio in patients with metabolic syndrome. Clin Appl Thromb Hemost. 2013;19(5):579. [DOI] [PubMed] [Google Scholar]
- 19. Balta S, Celik T, Mikhailidis DP, et al. The relation between atherosclerosis and the neutrophil‐lymphocyte ratio. Clin Appl Thromb Hemost. 2016;22(5):405‐411. [DOI] [PubMed] [Google Scholar]
- 20. Hu K, Lou L, Ye J, Zhang S. Prognostic role of the neutrophil‐lymphocyte ratio in renal cell carcinoma: a meta‐analysis. BMJ Open. 2015;5(4):e006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil‐lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: a systematic review and meta‐analysis. Int J Surg. 2018;55:73‐80. [DOI] [PubMed] [Google Scholar]
- 22. Tham T, Bardash Y, Herman SW, Costantino PD. Neutrophil‐to‐lymphocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta‐analysis. Head Neck. 2018; 10.1002/hed.25324 [DOI] [PubMed] [Google Scholar]
- 23. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil‐lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19(2):209‐216. [DOI] [PubMed] [Google Scholar]
- 25. Pepys MB, Hirschfield GM. C‐reactive protein: a critical update. J Clin Investig. 2003;111(12):1805‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allin KH, Nordestgaard BG. Elevated C‐reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155‐170. [DOI] [PubMed] [Google Scholar]
- 27. Shrotriya S, Walsh D, Bennani‐Baiti N, Thomas S, Lorton C. C‐reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS ONE. 2015;10(12):e0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peisajovich A, Marnell L, Mold C, Du Clos TW. C‐reactive protein at the interface between innate immunity and inflammation. Exp Rev Clin Immunol. 2008;4(3):379‐390. [DOI] [PubMed] [Google Scholar]
- 29. Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225(2):456‐460. [DOI] [PubMed] [Google Scholar]
- 30. Costantini C, Cassatella MA. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J Leukoc Biol. 2011;89(2):221‐233. [DOI] [PubMed] [Google Scholar]
- 31. Oberlies J, Watzl C, Giese T, et al. Regulation of NK cell function by human granulocyte arginase. J Immunol. 2009;182(9):5259‐5267. [DOI] [PubMed] [Google Scholar]
- 32. Paczesniak J, Tchorzewski H. The effect of polymorphonuclear leucocyte (PMNL) factor on human natural killer (NK) cell activity in vitro. Acta physiologica Polonica. 1983;34(2):213‐226. [PubMed] [Google Scholar]
- 33. Thiele DL, Lipsky PE. Regulation of cellular function by products of lysosomal enzyme activity: elimination of human natural killer cells by a dipeptide methyl ester generated from L‐leucine methyl ester by monocytes or polymorphonuclear leukocytes. Proc Natl Acad Sci USA. 1985;82(8):2468‐2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac‐1. J Clin Investig. 2012;122(1):327‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Kleijn S, Langereis JD, Leentjens J, et al. IFN‐gamma‐stimulated neutrophils suppress lymphocyte proliferation through expression of PD‐L1. PLoS ONE. 2013;8(8):e72249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hao S, Andersen M, Yu H. Detection of immune suppressive neutrophils in peripheral blood samples of cancer patients. Am J Blood Res. 2013;3(3):239‐245. [PMC free article] [PubMed] [Google Scholar]
- 37. Balta S, Kurtoglu E, Kucuk U, Demirkol S, Ozturk C. Neutrophil‐lymphocyte ratio as an important assessment tool. Expert Rev Cardiovasc Ther. 2014;12(5):537‐538. [DOI] [PubMed] [Google Scholar]
- 38. Ozturk C, Balta S, Balta I, et al. Neutrophil‐lymphocyte ratio and carotid‐intima media thickness in patients with Behcet disease without cardiovascular involvement. Angiology. 2015;66(3):291‐296. [DOI] [PubMed] [Google Scholar]
- 39. Demirkol S, Balta S, Cakar M, Unlu M, Arslan Z, Kucuk U. Red cell distribution width: a novel inflammatory marker in clinical practice. Cardiol J. 2013;20(2):209. [DOI] [PubMed] [Google Scholar]
- 40. Ekiz O, Balta I, Sen BB, et al. Mean platelet volume in recurrent aphthous stomatitis and Behcet disease. Angiology. 2014;65(2):161‐165. [DOI] [PubMed] [Google Scholar]
- 41. Fici F, Celik T, Balta S, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J Cardiovasc Pharmacol. 2013;62(4):388‐393. [DOI] [PubMed] [Google Scholar]
- 42. Lee AJ, Kim SG. Mean cell volumes of neutrophils and monocytes are promising markers of sepsis in elderly patients. Blood Res. 2013;48(3):193‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seo J, Kim WS, Kim JS, et al. Platelet to lymphocyte ratio (PLR) retains independent prognostic significance in advanced stage marginal zone lymphoma patients treated with rituximab, cyclophosphamide, vincristine, and prednisone combination chemotherapy (R‐CVP): consortium for Improving Survival of Lymphoma trial. Blood Res. 2017;52(3):200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim AH, Lee W, Kim M, Kim Y, Han K. White blood cell differential counts in severely leukopenic samples: a comparative analysis of different solutions available in modern laboratory hematology. Blood Res. 2014;49(2):120‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirokawa K, Utsuyama M, Hayashi Y, Kitagawa M, Makinodan T, Fulop T. Slower immune system aging in women versus men in the Japanese population. Immun Ageing. 2013;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phan MT, Chun S, Kim SH, et al. Natural killer cell subsets and receptor expression in peripheral blood mononuclear cells of a healthy Korean population: reference range, influence of age and sex, and correlation between NK cell receptors and cytotoxicity. Hum Immunol. 2017;78(2):103‐112. [DOI] [PubMed] [Google Scholar]
- 47. Campos C, Pera A, Lopez‐Fernandez I, Alonso C, Tarazona R, Solana R. Proinflammatory status influences NK cells subsets in the elderly. Immunol Lett. 2014;162(1 Pt B):298‐302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


