Abstract
Background
The incidence of laryngeal carcinoma is increasing, however, the mechanism is not fully understood. We aimed to investigate the efficacy of periostin gene silencing by siRNA on tumor inhibition, in a novel nude mouse model of human laryngeal squamous cell carcinoma, and to explore possible inhibitory mechanisms.
Methods
Tumors were established in nude mice by transplantation of LSCC AMC‐HN‐8 cell line. Forty‐eight nude mice were randomly divided into groups of eight each, and treated with high (1.0 OD) or low (0.5 OD) doses of periostin‐siRNA or appropriate control solutions. Tumor growth was observed and used to calculate an inhibition rate (%). Routine pathological and electron microscopic examination were used to determine tumor apoptosis and proliferation. Changes in periostin mRNA and protein levels were analyzed.
Results
Tumor growth was significantly inhibited in mice treated by high dose periostin‐siRNA compared to untreated and those treated with low dose periostin‐siRNA (P < 0.05). Pathological examination showed increased tumor necrosis and apoptotic changes in treated mice, which was confirmed by electron microscopy. Periostin mRNA and protein expression were significantly reduced in tumors from mice treated with high dose periostin‐siRNA, compared to controls and low‐dose periostin‐siRNA treatment groups (P < 0.05).
Conclusion
Periostin silencing was associated with growth inhibition of tumor cells in a nude mouse model of LSCC. The underlying mechanism may be due to receptor‐mediated induction of relevant signal transduction pathways that modulate the microenvironment needed for cancer cell survival. Periostin is expected to become a new target for cancer therapy.
Keywords: animal model, laryngeal squamous cell carcinoma, mechanism, oncology, periostin
1. INTRODUCTION
Laryngeal carcinoma is the second most common malignancy of the head and neck, and is most frequently characterized as a squamous cell carcinoma.1 The incidence of this disease is increasing, however, the mechanism by which it develops is not fully understood. New methods for diagnosis and treatment of laryngeal squamous cell carcinoma (LSCC) are urgently required.
Invasion and metastasis, which are complex processes involving multiple genes are the major characteristics of malignant tumors. Gene regulation is a precise and complicated biological control system with yet to be fully understood concrete mechanisms. Conventional research viewpoints are based on Paget's “seed and soil hypothesis”. Previous studies that focused on the seed (cancer cells) emphasized the impact of molecular pathological changes of cancer cells on their biological behaviors including proliferation, invasion, and metastasis,2, 3, 4, 5, 6 however, no specific markers that can explicitly predict tumor invasion and metastasis have been found so far. Recently, attention has been focused on the role of the soil (tumor microenvironment) in development of tumors, with studies on expression and function of relevant genes.7, 8 Mesenchymal specific genes, which play important roles in the regulatory process, have closely linked the seed with the soil.9, 10
Mesenchymal cells mainly express mesenchymal‐specific genes with products that have various structures and functions, including secreted and extracellular matrix proteins. Periostin gene, which was originally cloned from a mouse osteoblastic cell line, is a newly identified mesenchymal specific gene. Early investigations revealed that periostin was related to the formation and maintenance of bones and teeth. Current studies of periostin mainly focus on two areas; (a) facilitation of growth and development of heart valves, and its involvement in the pathophysiological processes of various ischemic heart diseases, including myocardial infarction and heart failure, (b) significant differential expressions of periostin mRNA and proteins between tumor and normal tissues, which implicate its close relationship with the occurrence, development, and prognosis of malignant tumors.
Periostin is a secreted extracellular matrix protein that is also called osteoblast‐specific factor 2 (OSF‐2). Functionally, it induces proliferation and differentiation of osteoblasts, as well as adhesion and aggregation of periosteal osteoblast precursor cells.11 It is expressed in various normal human tissues and plays a role in many normal physiological processes. Additionally, periostin is also associated with pathological processes, such as development of cardiovascular diseases, asthma, and cancer.12 Currently, many studies indicate that the overexpression of periostin is related to tumor grade, but on the contrary, others have reported that periostin could inhibit invasion and metastasis of bladder cancer cells.13, 14 Moreover, Kanno et al,15 found that periostin had dual effects; the promotion and inhibition of pancreatic cancer. These findings suggest different biological effects of periostin in different tissues, which indicate the need for further studies on its complex and multiaspect functions.
RNA interference (RNAi) is the process of double‐stranded RNA (dsRNA)‐mediated post‐transcriptional gene silencing with a high degree of specificity and effectiveness that makes it an ideal tool for gene therapy.16
In this report, we show for the first time the establishment of a human LSCC nude mouse transplantation model that was used to investigate the knockdown of periostin by RNA interference. Forty‐eight nude mice were randomly divided into six groups: unmanipulated control group; glucose solution control group; transfection reagent control group; nonspecific gene sequences control group; periostin‐siRNA high‐dose (1.0 optical density‐OD) treatment group; and periostin‐siRNA low‐dose (0.5 OD) treatment group. Tumor growth was observed in each group, and the inhibitory rate (%) calculated. Routine pathological and electron microscopic examination were used to determine tumor apoptosis and proliferation. Changes in periostin mRNA and protein levels were analyzed by qRT‐PCR and ELISA respectively. This analysis enabled us to explore the possible mechanism of periostin in the development of human LSCC.
2. MATERIALS AND METHODS
2.1. Materials
The sources of materials used in this study are as follows: Human LSCC AMC‐HN‐8 cells (Chinese Academy of Science, China); Periostin‐siRNA and non‐specific gene sequences (Genepharma, Shanghai, China); Transfection reagents (Engreen Biosystem, Beijing, China); BALB/cA nu/nu male nude mice, weighing 14‐16 g were provided by Shanghai SLAC Laboratory Animal Center with quality certification number 2007000553984; Animal license number SYXK (Zhejiang, China; 2009‐0123); RNA extraction kit (Qiagen, Duesseldorf, Germany); GoScript Reverse Transcription (RT) System (Promega, Madison, WI, USA); GoTaq qPCR master mix (Promega); First or primary antibody (SantaCruz, Dallas, TX, USA); Secondary antibody (Zhongshan Golden Bridge, Guangzhou, China); ELISA kit (SunBio Technology, Beijing, China); Real‐time qPCR (Applied Biosystems, Waltham, MA, USA); Micro‐spectrophotometer (ND‐2000, USA); and Power Wave XS full wavelength enzyme meter (Gene, USA). The Animal Ethical Care and Use Committee of Lihuili Hospital of Ningbo University approved the study.
2.2. Methods
2.2.1. Culture and expansion of human LSCC AMC‐HN‐8 cells
Human LSCC AMC‐HN‐8 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS) at 37°C in 5% CO2. After 0.25% trypsin digestion and passage, logarithmic growth phase cells were used in the experiment. Cells were seeded in a sterile Petri dish and amplified to a sufficient quantity for the experiments.
2.2.2. Tumor transplantation and animal groups
Forty‐eight BALB/cA nu/nu mice were inoculated subcutaneously in the axillary region with 0.2 mL AMC‐HN‐8 cell suspension (8.7 × 106 cells). Tumors were allowed to grow to more than 100 mm3 and mice were randomly divided into six groups of eight. These groups were: (a) unmanipulated control group; (b) glucose solution control group; (c) transfection reagent control group; (d) nonspecific gene sequences control group; (e) periostin‐siRNA high‐dose (1.0 OD) group; and (f) periostin‐siRNA low‐dose (0.5 OD) group.
2.2.3. Calculation of transplanted tumor volume and inhibition rate
Apart from the unmanipulated control group, all groups were injected with 50 μL of the corresponding solution into tumors twice weekly for3 weeks. Mice were weighed twice weekly, and tumors were measured for maximum length (a) and width (b) with a Vernier caliper. Tumor volume was calculated as follows: tumor volume (mm3) = (ab 2)/2. The day after the final treatment, the mice were killed and solid tumors collected and weighed to compare tumor burden and inhibition rate (%) between groups. Inhibition rate = (mean weight of tumors in the control groups ‐ mean weight of tumors in the experimental group)/mean weight of tumors in the control group × 100%.
2.2.4. Observation of tumor tissues by hematoxylin and eosin staining
Tissues were fixed in 10% neutral formaldehyde solution for 48 hours and then dehydrated through 70%, 85%, 95%, and 100% ethanol series before embedding in, paraffin. Conventional 3‐5‐μm sections were cut and stained in hematoxylin liquid dye for 5‐15 minutes, rinsed in water for 15‐30 minutes before final staining in 0.1%‐0.5% eosin dye solution for 1‐5 minutes. The tissues were again dehydrated through 70%, 85%, 95%, and 100% ethanol series, cleared in xylene and sealed for observation.
2.2.5. Electron microscopic examination of cellular apoptosis
Tumor tissues were precooled in 4% glutaraldehyde, fixed for over 3 hours, cleaned four times in double evaporated water, and postfixed in 1% osmic acid for 1 hour. After another wash and dehydration through ethanol gradient, tissues were embedded in 618 epoxy resin. Ultrathin sections were cut with LKB ‐ V microtome and stained in lead uranium before viewing and photography with JEM ‐ 1101 transmission electron microscope (TEM).
2.3. Quantitative RT‐PCR to detect periostin gene expression
2.3.1. RNA extraction
About 80 mg tumor tissue was ground into powder in a mortar with liquid nitrogen. Trizol (1 mL) was added to the powder and allowed to sit for 5 minutes at room temperature. Chloroform in a ratio of 0.2 mL per 1 mL Trizol liquid was added and vigorously mixed by shaking for 15 seconds and placed at room temperature for 3 minutes before ultracentrifugation at 12 000 g at 4°C for 15 minutes. The supernatant was transferred to a new centrifuge tube with the addition of 1 mL Trizol liquid and 0.5 mL isopropyl alcohol. This was allowed to sit for 10 minutes at room temperature before another ultracentrifugation at 12 000 g at 4°C for 10 minutes. The supernatant was discarded and 1 mL of 75% ethanol added, vortexed centrifuged at 7500 g for 5 minutes at 4°C. The supernatant was carefully discarded and the pellet dried at room temperature or with vacuum for 5‐10 minutes. The RNA was then dissolved in diethyl pyrocarbonate (DEPC) water, washed in a water bath at 60°C for 10 minutes. RNA content was measured with an ND‐2000 nucleic acid meter before storage at ‐80°C.
2.3.2. Quantitative RT‐PCR
Reverse transcription was performed with GoScript Reverse Transcription (RT) System (Promega) kit. Five microgram (5 μg) of RNA was reverse transcribed into cDNA in 20‐μL reaction volume and stored at ‐80°C.
Quantitative PCR was performed in triplicate with GoTaq qPCR master mix (Promega) kit using the primers shown in Table 1. GAPDH was the internal reference gene and no template reaction served as negative control. The reaction was carried out according to the kit instructions, with modification of the number of cycles to 45. The experimental instrument was StepOnePlus Fluorescence Quantitative PCR (ABI). Levels of gene expression were determined using the 2−ΔΔCt relative quantitative analysis method.
Table 1.
Primer sequences of periostin and GAPDH
| Gene name | Primer sequence (5′to 3′) | |
|---|---|---|
| Periostin | Forward | GATGGAGTGCCTGTGGAAAT |
| Reverse | AACTTCCTCACGGGTGTGTC | |
| GAPDH | Forward | ACCCACTCCTCCACCTTTGAC |
| Reverse | TGTTGCTGTAGCCAAATTCGTT | |
2.3.3. Analysis of periostin protein expression by ELISA
About 100 mg of tumor tissue was washed in phosphate buffered saline (PBS), cut into small pieces into a tissue grinder, homogenized in 1 mL PBS, and then stored at ‐20°C overnight. After repeated freezing and thawing treatment to destroy cell membrane, the tissue homogenate was centrifuged at 5000 g at 4°C for 5 minutes, and the supernatant removed for further analysis. The protein concentration of the supernatant was detected by bicinchoninic acid (BCA) protein concentration assay kit. Briefly 10 μL of samples or standards were placed in 96‐well plates with addition of 20 μL PBS and 200 μL BCA working liquid and placed at 37°C for 25 minutes before determination of absorbance at 562 nm wavelength. The volume of the sample was used to calculate the protein concentration using a standard curve.
To perform ELISA, the reagent was equilibrated at room temperature for 30 minutes. Hundred microliter (100 μL) samples or standard (0‐400 ng/mL) were placed in test plate and incubated at 37°C for 2 hours. The liquid was discarded, and plates dried by spinning before incubation with 100 μL biotin labeled antibody at 37°C for 1 hour. Plates were wash 3 times by soaking for 2 minutes with 200 μL washing liquid per well, then spun‐dry. Horseradish peroxidase labeled avidin working solution (100 μL) was added per well and incubated at 37°C for 1 hour. The solution was discarded and the plate washed 5 times by soaking for 2 minutes in 200 μL washing solution per well then dried by spinning. The substrate (90 μL per well) was added and incubated at 37°C for 20 minutes before addition of 50 μL stop solution. Optical densities were detected at 450 nm wavelength. Periostin protein concentration in pg/mL of sample was computed using a standard curve. The results were also expressed as periostin concentration in pg/mg of protein extract.
2.4. Statistical methods
SPSS Statistics (Version 17.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were presented as mean ± standard deviation. Between groups comparisons were made using the t‐test, and multiple groups were compared using single factor analysis of variance. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Observation and calculation of solid tumor inhibition rate
Macroscopically, tumors in the treatment groups were smaller than in control groups, with the reduction in size more obvious in the high‐dose periostin‐siRNA treatment group. The tumors in the treatment groups were spherical, smooth, pink, and soft while those in the control groups were large, had nodular surfaces and less central necrosis than in the treatment groups. The mean tumor weight and inhibition rate for each group are shown in Table 2.
Table 2.
The mean tumor weight and inhibition rate for each group
| Groups | Mice number | Tumor weight(g) | P value | Inhibition rate(%) | |
|---|---|---|---|---|---|
| Start | End | ||||
| Unmanipulated control group | 8 | 8 | 0.755 ± 0.269 | / | / |
| Glucose solution control group | 8 | 8 | 0.806 ± 0.304 | / | −4.0 |
| Transfection reagent control group | 8 | 8 | 0.776 ± 0.201 | / | −1.4 |
| Nonspecific gene sequences control group | 8 | 8 | 0.798 ± 0.237 | / | −3.0 |
| Periostin‐siRNA high dose (1.0OD) treatment group | 8 | 8 | 0.327 ± 0.127 | <0.01 | 51.3b |
| Periostin‐siRNA low dose (0.5OD) treatment group | 8 | 8 | 0.555 ± 0.183 | <0.05 | 28.4a |
P < 0.05.
P < 0.01, Compared with the unmanipulated control group.
3.2. Pathological changes in tumor tissues
The tumors generated in nude mice after subcutaneous injection of AMC‐HN‐8 cells exhibited enriched cytoplasm with centrally located irregular hyperchromatic nuclei with clear boundaries and visible keratosis. Laryngeal cancer cells often develop adhesion capability with some propensity for scattered distribution. Fibrous tissue was visible on the surfaces of tumors with some demonstrating cavity formation. A large region of necrosis was seen in the center with calcium salt deposits but no infiltration of surrounding tissues. The tumors showed expansive growth, crumb distribution, and globular uniformly pink necrotic area with destruction of cellular structures. There was evidence of cell disintegration with round protrusions, and tiny blood vessels were visible through the tumor margin. Tumors from mice in the treatment groups showed areas of apoptotic cells, with characteristic cell shrinkage, cytoplasmic condensation, and apoptotic bodies that was more marked in the high‐dose periostin‐siRNA treatment group, although apoptotic cells were also occasionally observed in the other groups (Figure 1). Electron microscopic examination of changes observed in the treatment and control groups revealed nuclear chromatin demonstrating an “edge‐set” phenomenon, accompanied by necrosis that was more marked in the high‐dose periostin‐siRNA treatment group (Figure 2).
Figure 1.
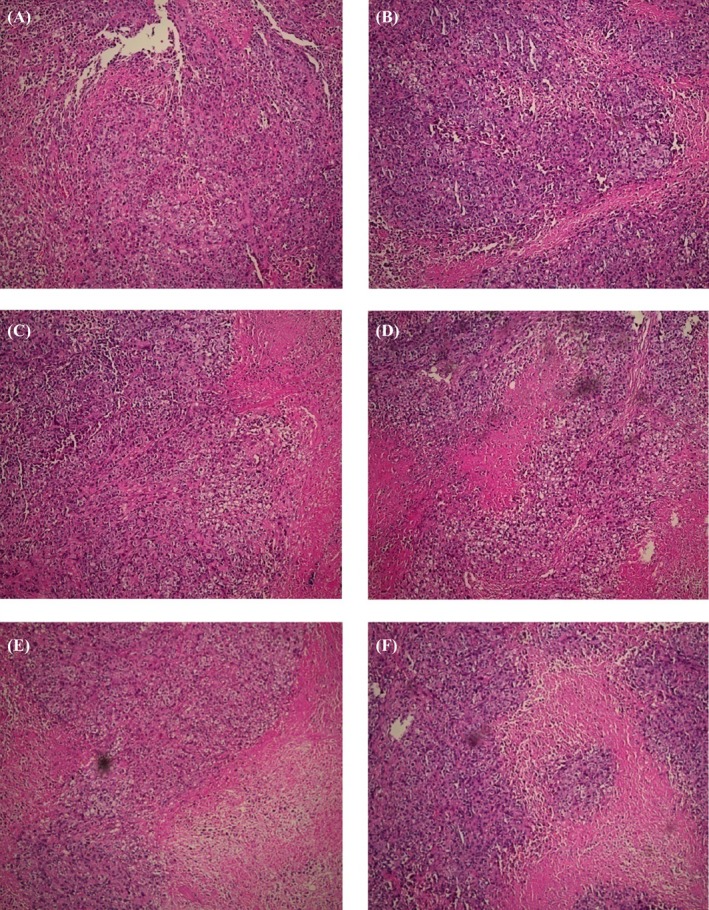
Pathological Changes Observed in the Treatment and Control Groups (×100). Moderately differentiated squamous cell carcinoma was accompanied by necrosis. Tumors from mice in treatment groups showed areas of apoptotic cells, with characteristic cell shrinkage, cytoplasmic condensation, and apoptotic bodies. This was more marked in the high‐dose periostin‐siRNA treatment group. A, unmanipulated control group; B, glucose solution control group; C, transfection reagent control group; D, non‐specific gene sequences control group; E, periostin‐siRNA high‐dose (1.0 OD) group; and F, periostin‐siRNA low dose (0.5 OD) group
Figure 2.
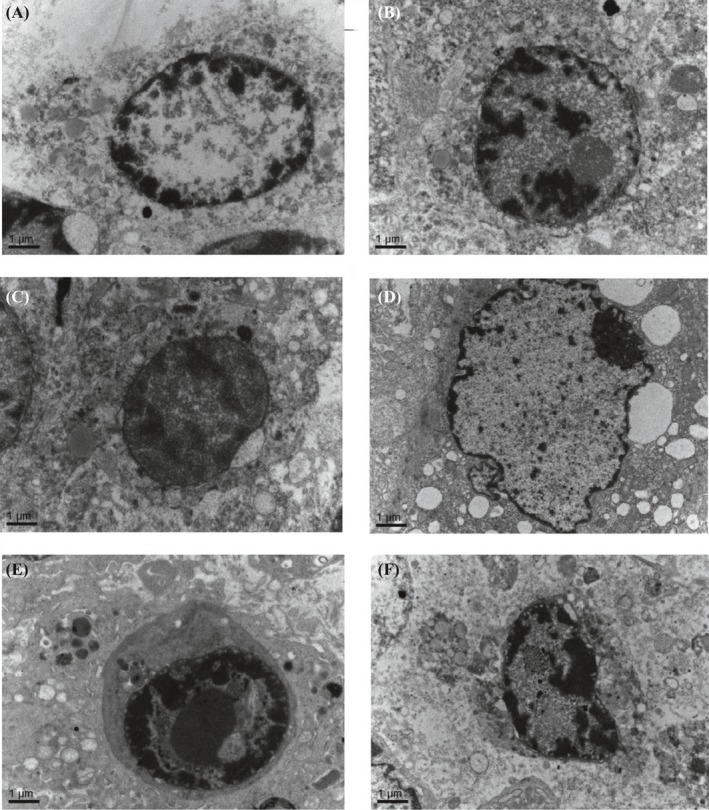
Electron Microscopic Examination of Changes Observed in the Treatment and Control Groups (×15 000). The chromatin showed the edge set phenomenon, accompanied by necrosis. This was more marked in the high‐dose periostin‐siRNA treatment group. A, unmanipulated control group; B, glucose solution control group; C, transfection reagent control group; D, nonspecific gene sequences control group; E, periostin‐siRNA high‐dose (1.0 OD) group; and F, periostin‐siRNA low‐dose (0.5 OD) group
3.3. Periostin mRNA expressional changes in tumor tissue
The effect of periostin‐siRNA on the expression of periostin mRNA in human LSCC AMC‐HN‐8 nude mouse transplanted tumors was significant. Periostin mRNA expression was significantly reduced in the treatment groups compared to the control groups (P < 0.05). The differential expression was also statistically significant (P < 0.05) between the high‐ and low‐dose periostin‐siRNA treatment groups. There were no significant differences among the control groups (Figure 3).
Figure 3.
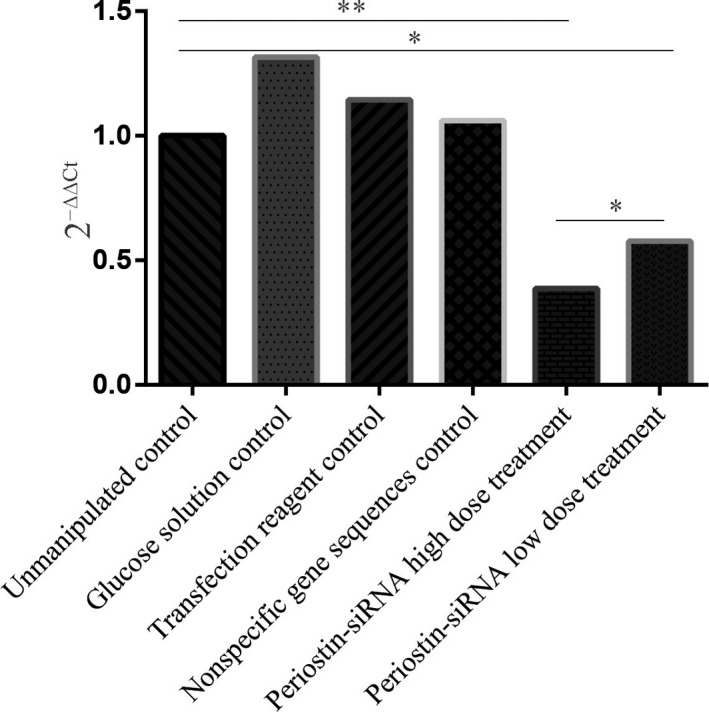
The effect of periostin‐siRNA on the expression of periostin mRNA in human LSCC AMC‐HN‐8 nude mice transplanted tumor. (Compared to the unmanipulated control group *P < 0.05, **P < 0.01)
3.4. Periostin protein expression in tumor tissue
Periostin protein expression was significantly reduced in the treatment groups, compared to control groups (P < 0.05). The difference was statistically significant (P < 0.05) between the high‐dose periostin‐siRNA treatment group and the control and low‐dose periostin‐siRNA treatment groups. No significant differences were found among the control groups (Figure 4).
Figure 4.
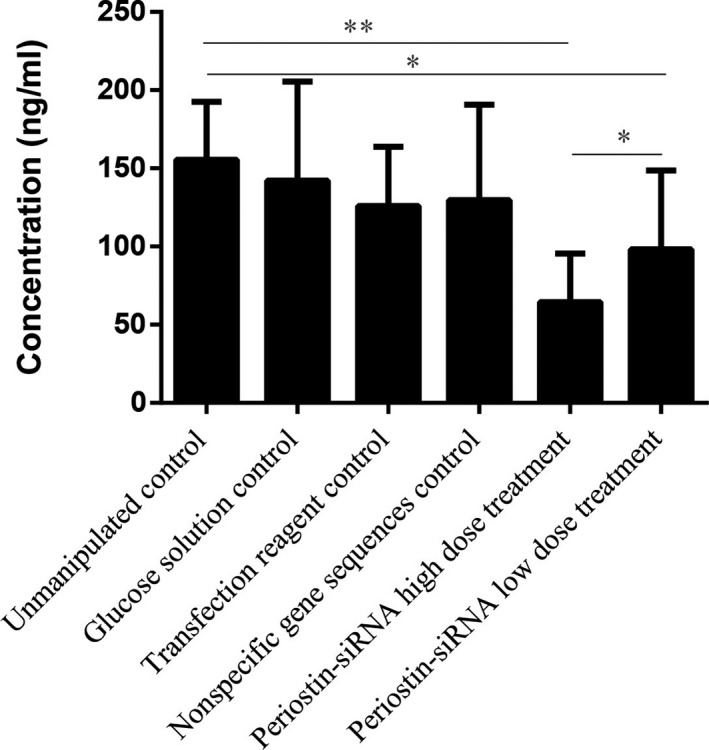
The Effect of Periostin‐siRNA on the Expression of Periostin Protein in Human LSCC AMC‐HN‐8 Nude Mice Transplanted Tumor. (Compared to the unmanipulated control group *P < 0.05, **P < 0.01)
4. DISCUSSION
Periostin is highly expressed in multiple solid tumor tissues, including head and neck carcinoma, lung, breast, colorectal, ovarian, and liver cancers. Periostin is a secreted protein from tumor and surrounding stromal cells that continuously destroy the surrounding matrix during the process of tumor infiltration. From the tumor microenvironment, periostin enters the circulation. Studies have revealed high levels in sera of patients with head and neck carcinoma, breast cancer, colorectal cancer, and nonsmall cell lung carcinoma (NSCLC), and the elevated levels were strongly associated with the potential of tumor infiltration and metastasis.17 The expression of periostin and changes of extracellular matrix can alter the adhesion and invasive properties of tumor cells, which are closely related to treatment of several malignant tumors. Periostin can facilitate the survival, invasion, metastasis, and angiogenesis of tumor cells, as well as enhance its tolerance to hypoxia and chemicals, suggesting its close relationship with tumor grade, metastasis, and prognosis. Through in‐depth study of periostin, it is expected to be an ideal novel target for tumor treatment.
Periostin‐integrin pathway regulates breast cancer development and tumor microenvironment at multiple levels. Periostin‐binding DNA aptamer‐3 (PNDA‐3) is a potential target to inhibit the development of breast cancer.18 Choi et al19, verified that recombinant periostin could stimulate adhesion and invasion of human ovarian adenocarcinoma cell line SK‐OV‐3, as well as induce cancer cells to express matrix metalloproteinase‐2 (MMP‐2). Hong et al17, 20, constructed and transfected a periostin‐expressing plasmid vector into NSCLC cell line A549. In transfected cells, expression of vimentin and N‐cadherin were induced while E‐cadherin expression was inhibited, suggesting the enhancement of EMT might facilitate the proliferation and migration of A549 cells, which uncovered a role for periostin in invasion and migration of NSCLC, and could be a potential therapeutic target.
RNAi has recently become a popular technique in tumor biological gene therapy, with high specificity and sensitivity, because specific ~20 base pair fragments of exogenous double‐stranded RNA sequence are able to target specific target genes in silencing machinery. The paring results in regulation of gene expression at the post‐transcriptional level through mRNA degradation. Particularly, a number of siRNAs currently in clinical research phase target genes for the treatment of malignant tumors. RNAi technology has become one of the most powerful tools for biomedical and life sciences research and drug development.21, 22, 23
Here we describe, for the first time, a novel LSCC nude mouse transplantation model used to evaluate the effect of targeting periostin with siRNA on tumors. Injecting of periostin‐siRNA into tumors caused significantly slower tumor growth than in control groups. In addition, cells in the experimental group demonstrated typical morphological features of apoptosis, suggesting that periostin‐siRNA could effectively induce apoptosis and inhibit proliferation of LSCC. The inhibition rate in high‐dose periostin‐siRNA treatment group was 51.3%.
Our research group constructed a LSCC cell line subcutaneous xenograft animal model which is convenient and accurate for inoculation. The survival rate of the transplant inoculation is high, and the model repeatability is good. The transplanted tumor can be continuous passage, and the tumor cells can maintain the histological and ultrastructural features of LSCC. The experimental period is relatively short and the tumor growth trend can be observed in real time. Orthotopic xenograft animal model can simulate the tumor microenvironment similar to the location of the primary tumor, and can also form metastases in the corresponding site to avoid false positive results due to the site‐specificity of transplantation,24, 25, 26, 27 but the technical requirements are high. After consulting a large number of relevant literature, there are orthotopic transplantation tumor animal models which cover liver cancer, lung cancer, cervical cancer, bladder cancer, ovarian cancer etc., but we did not find laryngeal carcinoma orthotopic transplantation tumor animal models. Therefore, for the next step we will study laryngeal carcinoma orthotopic transplantation tumor animal models to clarify the effect of microenvironment on the growth of LSCC.
Key considerations for the use of periostin‐siRNA for treatment of LSCC are the effectiveness and stability of the treatment, which were tested here in a tumor transplant model. This study showed that the application of periostin‐siRNA in this mouse model resulted in a significant decrease in tumor volume, thus exhibiting a significant inhibitory effect over a long duration. In contrast, control group tumors continued to grow. The morphological observation found that apoptotic cells in the treatment groups were obviously more frequent than in the control groups, suggesting that periostin‐siRNA had a tumor suppression effect on LSCC. In the experiments described herein, the nude mice within the treatment groups remained well with no significant loss of weight, while mice in the control groups declined significantly. As no significant side effects of the siRNA treatment were observed, and the siRNA had good stability, this study lays the foundations for application of siRNA in clinical treatment of this disease.
Studies have so far confirmed that periostin is under many regulatory factors. Yang et al,28 showed that histamine could induce the production of periostin and collagen by activating H1 receptor mediated ERK1/2 pathway. Tai et al29, verified that transforming growth factor β1 (TGF‐β1) could induce the production of periostin during studies of colon cancer cells. Fibroblast growth factor (FGF‐1) and angiotensin II could enhance the expression of periostin by pulmonary arterial smooth muscle cells (PASMCs).30 Bone morphogenetic protein‐2 (BMP‐2), platelet‐derived growth factors (PDGF‐aa, PDGF‐bb), and fibroblast growth factors (FGF‐B, FGF‐A) were all potential factors that induced pancreas stellate cells (PSCs) to secrete periostin.31 Under hypoxic environment, transforming growth factor‐α (TGF‐α) and basic FGF (bFGF) could increase the expression periostin in lung cancer cell line A549 by activating RTK/PI3K signaling.32 In human ovarian cancer tissues, periostin was not expressed in cancer cells, but mainly in cancer‐associated mesenchymal cells instead. Lysophosphatidic acid (LPA) could induce the secretion of periostin by mesenchymal cells, which could be effectively inhibited using short hairpin RNA (shRNA) virus that silenced LPA receptor 1.19 In addition, studies have shown that Twist, IL‐4, and IL‐13 could induce the expression or secretion of periostin.33, 34
The activation of relevant signaling pathways could enhance the proliferation and migration of vascular endothelial cells, inhibit apoptosis of endothelial cells, and reduce damage to cells under hypoxic and low nutrient environments. These pathways could also maintain normal cell functions that are beneficial to tumor angiogenesis to enhance invasion, thus facilitating the formation of metastatic tumors. Attributed to the special biological structures and features of periostin, it can bind to various subtypes of integrin receptors, including αvβ3, αvβ5, and α6β4, as well as trigger relevant signal transduction pathways such as PI3‐K/Akt, and focal adhesion kinase (FAK) phosphorylation. Periostin can bind to epidermal growth factor receptor (EGFR) as well, and plays an important role in the occurrence and development of tumors through enhancement of tumor cell survival, angiogenesis, invasion, and metastasis.
Periostin can bind to several cell surface receptors, especially integrin receptors. Integrin is a heterodimeric transmembrane receptor that is involved in cell‐cell and cell‐extracellular matrix (ECM) interactions, promotion of epithelial‐to‐mesenchymal transition (EMT), enhancement of cell invasion, inhibition of ECM‐integrin interaction by periostin‐integrin interaction, triggering of relevant signal transduction pathways such as PI3‐K/Akt pathway, and changing the microenvironment for cancer cells survival and growth.35 Baril et al,36 verified that periostin could facilitate the invasion of pancreatic cancer cells by enhancing their activity, and could enhance the survival of cancer cells even under hypoxic environments. At the molecular level, periostin could activate PI3K/Akt pathway by binding to integrin α6β4 receptor, thus improving FAK phosphorylation rather than expression of MMP‐9 to show biological effect. Zhu et al,37 revealed that through interaction with ovarian cancer cells and human umbilical vein endothelial cells (HUVECs), periostin could facilitate the migration and invasion of ovarian cancer cells as well as adhesion and migration of HUVECs, and promoted tumor angiogenesis to facilitate invasion and metastasis. Other studies have shown that purified recombinant periostin could enhance the adhesion of ovarian epithelial cells, and αvβ3 and αvβ5 antibodies (but not αvβ1) could inhibit the adhesion process. The selection of integrin was dependent on the different subtypes of integrin receptors expressed by different cells, suggesting that αvβ3 and αvβ5 were the receptors of periostin on ovarian cancer cell membrane. Yan et al,38 found that periostin‐transfected cells showed morphological changes and increased expression of matrix markers (elastin and fibrin). High level expression of periostin in 293T cells enhanced their motility and invasive potential than normal cells, however, this effect could be blocked by αvβ5 antibodies or EGFR kinase inhibitors, suggesting that periostin might improve the invasion and metastasis of tumor cells by integrin and EGFR signal pathways The expression of EMT‐associated genes including elastin and fibrin genes, and activation of MMP‐9)could enhance motility and invasive potential of cells.
Growth of new capillaries, which includes proliferation and migration of vascular endothelial cells and formation of capillary lumens, to provide nutrient and oxygen is mandatory when solid tumors grow to a diameter of 2‐3 mm. It has been verified that vascular endothelial growth factor (VEGF) and its receptor Flk‐1/KDR play an important role in this process. Tumor and mesenchymal cells secrete VEGF to act on vascular endothelial cell receptors, while VEGF facilitates the adhesion and migration of endothelial cells by activating Flk ‐1/KDR and integrin αvβ3. Shao et al,39 demonstrated that in breast cancer, periostin could upregulate Flk‐1/KDR through integrin αvβ3‐FAK mediated signal transduction pathway. The regulation in rectal cancer was implemented through αvβ3‐AKT/PKB pathway, but in pancreatic cancer, it was through α6β4‐PI3K‐AKT/PKB and FAK signal pathways. In conclusion, tumor cells secreted periostin in a paracrine manner to allow its binding to integrin receptors, which facilitated the survival of endothelial cells and formation of new blood vessels, thus playing a critical role in tumor angiogenesis and metastatic growth. In spite of different cell origins, the regulation of signaling pathways by periostin was generally consistent, culminating in the control of angiogenesis.
In human ovarian cancer tissues, periostin was not expressed in cancer cells, but mainly in cancer‐associated mesenchymal cells. Recombinant periostin could stimulate the adhesion and invasion of human ovarian adenocarcinoma cell line SK‐OV‐3 as well as induce them to express metalloproteinase‐2 (MMP‐2), which could destroy the histologic barrier against tumor cell invasion, thus contributing to invasion and metastasis.19
Through three‐dimensional tissue culture of esophageal squamous cell carcinoma, Michaylira et al,40 found that because of its induction of elevated expression of cell adhesion molecules by tumor cells, periostin could be a novel molecular marker of tumor invasion. Inhibition of EGFR signal transduction pathway or restoration of the function of wild type p53 could both weaken the effects of periostin, suggesting an interdependent relationship between these two common genetic alterations and periostin functions.
By in‐situ hybridization analysis, Kikuchi et al,41 revealed that periostin was expressed by gastric cancer‐associated fibroblasts rather than cancer cells, and it could form a favorable microenvironment for growth of gastric cancers by activating ERK. Genome‐wide analysis of gene expression showed that compared to normal tissues, periostin was significantly highly expressed in stage II‐IV gastric cancer tissues. In parallel with the activation of ERK, periostin could enhance the in vitro growth of diffuse gastric cancer cell lines such as OCUM‐2MLN and OCUM‐12.
5. CONCLUSIONS
Great progress has been achieved on the role of periostin in the occurrence and development of tumors, which has opened up a new direction for cancer research that could potentially provide a novel marker for diagnosis and a new target for treatment. Current studies have shown that tumor‐associated mesenchymal cells mainly produce periostin, and cancer cells could stimulate its expression and secretion by mesenchymal cells. Through interactions with receptor molecules such as integrin,35 relevant signal transduction pathways could be triggered to change the microenvironment for cancer cell survival that favors its growth.
This study examined the role of periostin gene in the first animal model of LSCC. Silencing of periostin expression by RNAi significantly reduced LSCC cell proliferation and promoted apoptosis, with concomitant change in the expression of mRNA and protein. The periostin gene may therefore be an effective target for the treatment of LSCC.
ACKNOWLEDGMENTS
This work was supported by grants from the Scientific Innovation Team Project of Ningbo (grant no. 2012B82019; 2015B11050), the Ningbo Social Developmental Key Research Project(grant no. 2012C5015), the Natural Science Foundation of Ningbo (grant no. 2013A610217;2017A610236), Ningbo Health Branding Subject Fund, and the Medical and Health Training Project of Zhejiang Province (grant no. 2015RCB025;2018RC063).
Ye D, Zhou C, Wang S, Deng H, Shen Z. Tumor suppression effect of targeting periostin with siRNA in a nude mouse model of human laryngeal squamous cell carcinoma. J Clin Lab Anal. 2019;33:e22622 10.1002/jcla.22622
Contributor Information
Dong Ye, Email: yedong518@sina.com.
Zhisen Shen, Email: szs7216@sina.com.
REFERENCES
- 1. Chu EA, Kim YJ. Laryngeal cancer: diagnosis and preoperative work‐up. Otolaryngol Clin North Am. 2008;41(4):673‐695. [DOI] [PubMed] [Google Scholar]
- 2. Chin AR, Wang SE. Cancer‐derived extracellular vesicles: the ‘soil conditioner’ in breast cancer metastasis? Cancer Metastasis Rev. 2016;35(4):669‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lazcano‐Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51(6):349‐364. [DOI] [PubMed] [Google Scholar]
- 4. Li Q, Zhang CS, Zhang Y. Molecular aspects of prostate cancer with neuroendocrine differentiation. Chin J Cancer Res. 2016;28(1):122‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabnis AJ, Bivona TG. HSP70 dependence in rhabdomyosarcoma: Seed or soil? Cell Cycle. 2017;16(2):147‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von der Heide EK, Neumann M, Vosberg S, et al. Molecular alterations in bone marrow mesenchymal stromal cells derived from acute myeloid leukemia patients. Leukemia. 2017;31(5):1069‐1078. [DOI] [PubMed] [Google Scholar]
- 7. Kikuchi Y, Kashima TG, Nishiyama T, et al. Periostin is expressed in pericryptalfibroblasts and cancer‐associated fibroblasts in the colon. J Histochem Cytochem. 2008;56(8):753‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Li F, Gao F, et al. Periostin promotes tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling. Oncotarget. 2016;7(26):40148‐40159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chun HW, Hong R. Significance of the hedgehog pathway‐associated proteins Gli‐1 and Gli‐2 and the epithelial‐mesenchymal transition‐associated proteins Twist and E‐cadherin in hepatocellular carcinoma. Oncol Lett. 2016;12(3):1753‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng G, Xun W, Wei K, Yang Y, Shen H. MicroRNA‐27a‐3p regulates epithelial to mesenchymal transition via targeting YAP1 in oral squamous cell carcinoma cells. Oncol Rep. 2016;36(3):1475‐1482. [DOI] [PubMed] [Google Scholar]
- 11. Contié S, Voorzanger‐Rousselot N, Litvin J, et al. Development of a new ELISA for serum periostin: evaluation of growth‐related changes and bisphosphonate treatment in mice. Calcif Tissue Int. 2010;87(4):341‐350. [DOI] [PubMed] [Google Scholar]
- 12. Tanabe H, Takayama I, Nishiyama T, et al. Periostin associates with Notch1 precursor to maintain Notch1 expression under a stress condition in mouse cells. PLoS ONE. 2010;5(8):e12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim CJ, Yoshioka N, Tambe Y, Kushima R, Okada Y, Inoue H. Periostin is down‐regulated in high grade human bladder cancers and suppresses in vitro cell invasiveness and in vivo metastasis of cancer cells. Int J Cancer. 2005;117(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 14. Isono T, Kim CJ, Ando Y, Sakurai H, Okada Y, Inoue H. Suppression of cell invasiveness by periostin via TAB 1/TAK1. Int J Oncol. 2009;35(2):425‐432. [PubMed] [Google Scholar]
- 15. Kanno A, Satoh K, Masamune A, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122(12):2707‐2718. [DOI] [PubMed] [Google Scholar]
- 16. Deng F, Chen X, Liao Z, et al. A simplified and versatile system for the simultaneous expression of multiple siRNAs in mammalian cells using Gibson DNA assembly. PLoS ONE. 2014;9(11):e113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong L, Sun H, Lv X, Yang D, Zhang J, Shi Y. Expression of periostin in the serum of NSCLC and its function on proliferation and migration of human lung adenocarcinoma cell line (A549) in vitro. Mol Biol Rep. 2010;37(5):2285‐2293. [DOI] [PubMed] [Google Scholar]
- 18. Lee YJ, Kim IS, Park SA, et al. Periostin‐binding DNA aptamer inhibits breast cancer growth and metastasis. Mol Ther. 2013;21(5):1004‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi KU, Yun JS, Lee IH, et al. Lysophosphatidic acid‐induced expression of periostin in stromal cells: Prognoistic relevance of periostin expression in epithelial ovarian cancer. Int J Cancer. 2011;128(2):332‐342. [DOI] [PubMed] [Google Scholar]
- 20. Hong LZ, Wei XW, Chen JF, Shi Y. Overexpression of periostin predicts poor prognosis in non‐small cell lung cancer. Oncol Lett. 2013;6(6):1595‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen C, Liu R, Wang J, Yan Z, Qian S, Zhang W. RNAi Knockdown of Hypoxia‐Inducible Factor‐1α Decreased the Proliferation, Migration, and Invasion of Hypoxic Hepatocellular Carcinoma Cells. Cell Biochem Biophys. 2015;71(3):1677‐1684. [DOI] [PubMed] [Google Scholar]
- 22. Zhang YC, Guo LQ, Chen X, et al. The role of death receptor 3 in the biological behavior of hepatocellular carcinoma cells. Mol Med Rep. 2015;11(2):797‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li J, Yang XF, Ren XH, et al. Stable SET knockdown in breast cell carcinoma inhibits cell migration and invasion. Biochem Biophys Res Commun. 2014;453(1):7‐12. [DOI] [PubMed] [Google Scholar]
- 24. Lee SE, Bairstow SF, Werling JO, et al. Paclitaxel nanosuspensions for targeted chemotherapy ‐ nanosuspension preparation, characterization, and use. Pharm Dev Technol. 2014;19(4):438‐453. [DOI] [PubMed] [Google Scholar]
- 25. Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17(4):343‐359. [DOI] [PubMed] [Google Scholar]
- 26. Hoffman RM. Patient‐derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15(8):451‐452. [DOI] [PubMed] [Google Scholar]
- 27. Hoffman RM, ed. Patient‐Derived Mouse Models of Cancer. Berlin: Springer Intl; 2017. [Google Scholar]
- 28. Yang L, Murota H, Serada S, et al. Histamine contributes to tissue remodeling via periostin expression. J Invest Dermatol. 2014;134(8):2105‐2113. [DOI] [PubMed] [Google Scholar]
- 29. Tai IT, Dai M, Chen LB. Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti‐periostin antibodies. Carcinogenesis. 2005;26(5):908‐915. [DOI] [PubMed] [Google Scholar]
- 30. Li P, Oparil S, Feng W, Chen YF. Hypoxia‐responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J Appl Physiol. 2004;97(4):1550‐1558. [DOI] [PubMed] [Google Scholar]
- 31. Erkan M, Kleeff J, Gorbachevski A, et al. Periostin creates a tumor‐supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132(4):1447‐1464. [DOI] [PubMed] [Google Scholar]
- 32. Ouyang G, Liu M, Ruan K, Song G, Mao Y, Bao S. Upregulated expression of periostin by hypoxia in non‐small‐cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett. 2009;281(2):213‐219. [DOI] [PubMed] [Google Scholar]
- 33. Oshima A, Tanabe H, Yan T, Lowe GN, Glackin CA, Kudo A. A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I family, is regulated by the bHLH transcription factor, twist. J Cell Biochem. 2002;86(4):792‐804. [DOI] [PubMed] [Google Scholar]
- 34. Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL‐4 and IL‐13 signals. J Allergy Clin Immunol. 2006;118(1):98‐104. [DOI] [PubMed] [Google Scholar]
- 35. Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66(14):2219‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baril P, Gangeswaran R, Mahon PC, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia‐induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26(14):2082‐2094. [DOI] [PubMed] [Google Scholar]
- 37. Zhu M, Fejzo MS, Anderson L, et al. Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol. 2010;119(2):337‐344. [DOI] [PubMed] [Google Scholar]
- 38. Yan W, Shao R. Transduction of a mesenchyme‐specific gene periostin into 293T cells induces cell invasive activity through epithelial‐mesenchymal transformation. J Biol Chem. 2006;281(28):19700‐19708. [DOI] [PubMed] [Google Scholar]
- 39. Shao R, Bao S, Bai X, et al. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up‐regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24(9):3992‐4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michaylira CZ, Wong GS, Miller CG, et al. Periostin, a cell adhesion molecule, facilitates invasion in the tumor microenvironment and annotates a novel tumor‐invasive signature in esophageal cancer. Cancer Res. 2010;70(13):5281‐5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kikuchi Y, Kunita A, Iwata C, et al. The niche component periostin is produced by cancer‐associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am J Pathol. 2014;184(3):859‐870. [DOI] [PubMed] [Google Scholar]


