Abstract
Background
Neutrophil‐to‐lymphocyte ratio (NLR) and presence of malnutrition have been found to be associated with mortality and morbidity in various clinical conditions. We investigated the association between NLR and nutritional status in geriatric patients.
Methods
This cross‐sectional study included 95 geriatric (age ≥ 65 years) patients from general internal medicine outpatient clinic of a university hospital. Nutritional status of the patients was evaluated using Mini Nutritional Assessment (MNA), Geriatric Nutritional Risk Index, albumin, total cholesterol, body mass index, mid‐arm circumference, and calf circumference. NLR was calculated from the complete blood count results.
Results
A total of 59 patients were female, and the mean age was 73 ± 9.8 years. According to the MNA, 51.6% of patients had a normal nutritional status, and 48.4% were malnourished or at risk of malnutrition. The mean NLR of patients with malnourished or at risk of malnutrition was significantly higher than that of patients with normal nutritional status (P = 0.004). There was a negative correlation between NLRs and the MNA scores (r = −0.276, P = 0.007). Optimal NLR cutoff point for patients with malnourished or at risk of malnutrition was 1.81 with 71.7% sensitivity and 63.3% specificity [95% confidence interval (CI): 0.562‐0.780, P = 0.004]. Logistic regression analysis revealed that elevated NLR was an independent factor in prediction of malnutrition or risk of malnutrition in geriatric patients.
Conclusion
These results demonstrated that NLR was associated with the nutritional status of geriatric patients. NLR may be a useful nutritional marker for evaluating the nutritional status of geriatric outpatients.
Keywords: aged, malnutrition, MNA, NLR, nutritional assessment
1. INTRODUCTION
Malnutrition is more common in geriatric patients due to advanced age, cognitive decline, comorbid diseases, excessive polypharmacy, depression, and poor appetite.1, 2 The presence of malnutrition in patients is associated with, prolonged hospital stay,3 increased morbidity and mortality,4 immune dysfunction,3 increased frequency of hospital admission,4 increased cost of care,3, 4 and poor quality of life.5 Due to these reasons, early diagnosis and prevention of malnutrition by periodically evaluating the nutritional status of geriatric patients are highly important.
There is no commonly accepted gold standard screening tool for assessing the nutritional status in the geriatric patient population. For this purpose, the clinical and nutritional status of individuals can be assessed via the following parameters: daily dietary follow‐up; certain anthropometric measurements such as body mass index (BMI), weight, calf circumference, and skinfold thickness; laboratory blood tests such as serum albumin and total cholesterol; and bioelectrical impedance analysis for estimating body composition.3, 6 Nutritional assessment tools, such as the Mini Nutritional Assessment (MNA), Geriatric Nutritional Risk Index (GNRI), Nutritional Risk Screening 2002, Malnutrition Universal Screening Tool, and Subjective Global Assessment, which have been proven to be reliable in several studies, can also be used.6, 7, 8, 9
Neutrophil‐to‐lymphocyte ratio (NLR) is a novel parameter that can be easily calculated from complete blood count results and reflects systemic inflammation. However, clinical studies have shown that NLR is not only an inflammation marker but also a significant prognostic predictor for many diseases.10, 11, 12, 13, 14, 15, 16 For example, NLR has been found to be associated with mortality, morbidity, and prognosis in clinical studies or meta‐analyses focusing on malignancies such as liver,10 lungs,11 gastric,12 and ovarian cancers13 and nonmalignant diseases such as cardiovascular diseases,14 acute ischemic stroke,15 and chronic heart failure.16
In numerous different clinical conditions, the nutritional status or NLR of patients was found to be associated with mortality and prognosis.2, 3, 4, 5, 10, 11, 12, 13, 14, 15, 16 These data suggest that NLR may also be related to the nutritional status of patients. To the best of our knowledge, there are limited data on the association between NLR and nutritional status of the elderly. This study aimed to investigate the association between NLR and nutritional status of geriatric patients.
2. METHODS
2.1. Study design and subjects
This cross‐sectional study included 95 consecutive geriatric patients who were admitted to the general internal medicine outpatient clinic of our university hospital between February 1, 2018, and March 31, 2018, and had met the inclusion criteria. Patients who were 65 years of age and older, were able to walk, did not have limb amputation or neurological motor deficits, and were able to respond to the questionnaire were included in the study.
The exclusion criteria were the clinical conditions that may affect the NLR.17 Patients with acute or chronic infections; chronic inflammatory conditions, such as rheumatic diseases, malignancies, and haematological diseases; diseases or drug use (corticosteroid, nebivolol, immunosuppressant, etc.) that may affect leukocyte count‐NLR; and acute myocardial infarction, valvular heart disease, renal or hepatic dysfunction, and coronary revascularization during the last 6 months were excluded from the study.
Clinical and demographic data of the patients were recorded. Complete blood count, albumin, C‐reactive protein, and total cholesterol levels, and other laboratory data at the time of admission were obtained from patient medical records. Blood samples were collected in the morning after 08‐10 hours of fasting. Complete blood count analysis (including a differential white blood cell count) was performed using an automated analyzer (Abbott Cell‐Dyn 3700 System; Ramsey, MN). NLR values of all patients were calculated by absolute neutrophil count divided by absolute lymphocyte count.
2.2. Assessment of nutritional status
The nutritional status of the patients was evaluated using the MNA, GNRI, albumin and total cholesterol measurements, BMI, mid‐arm circumference, and calf circumference parameters. The MNA can be used to evaluate the nutritional status by determining the total score obtained by rating 18 different parameters. These 18 questions are related to anthropometric measurements (BMI, arm, and calf circumference, and weight loss), nutrient intake (number of meals, appetite, fluid consumption, type of foods such as vegetable and protein), general condition (number of medications, mobility, psychological status, acute diseases, and pressure wounds), and the patient's personal interpretation of the health and nutrition status. The maximum score that can be obtained in the MNA is 30 points. The patient is considered as having a “normal nutritional status” if the score is above 24, “at risk of malnutrition” if the score is between 17 and 23.5, and “malnourished” if the score is below 17.8 The MNA tool was applied to the entire study population by the same trained researcher.
Geriatric Nutritional Risk Index was calculated by inserting the albumin value and body weight of patients in the formula as previously described.9 The following formula was used:
For cases in which the patient's weight was higher than the ideal weight, the ratio was considered as 1.9 BMI was calculated as body weight (kg) divided by the square of height (m2): BMI = weight (kg)/height (m2). The mid‐arm circumference and calf circumferences were measured using standard procedures with a nonstretchable tape measure. All anthropometric measurements were performed by the same researcher.
The study was approved by the local ethics committee of our university, and all patients provided written informed consent before the study was conducted.
2.3. Statistical analysis
All data were analyzed for the normality of distribution using the Kolmogorov‐Smirnov test. The data are expressed as mean ± standard deviation or median (25th‐75th percentile), as applicable. Patients were divided into two groups as normal nutrition (MNA score ≥ 24) and malnourished or at risk of malnutrition (MNA score ≤ 23.5). Student's t test was used to examine the difference between means, and the Mann‐Whitney test was used for nonparametric data. The chi‐square test was used for categorical variables. The Spearman correlation coefficient was calculated to examine the relation between NLR and nutritional parameters. A receiver operator characteristic (ROC) curve was constructed to reveal an association between NLR and nutritional status. Binary logistic regression was carried out to identify predictors of being categorized as malnourished or at risk of malnutrition. Statistical significance was considered at P‐values < 0.05. All data were analyzed using the SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL).
3. RESULTS
Of the 95 geriatric patients studied, 59 were female and 36 were male, and the mean age was 73 ± 9.8 years. Table 1 shows the demographic characteristics of the patients and laboratory assessment results. According to the MNA results, 49 patients had a normal nutritional status (51.6%), whereas 46 patients were malnourished or at risk of malnutrition (48.4%). The nutritional and inflammatory parameters of patients with malnourished or at risk of malnutrition, and patients with normal nutritional status are shown in Table 2. The mean NLR of patients with malnourished or at risk of malnutrition was significantly higher than that of patients with normal nutritional status (P = 0.004) (Table 2). Furthermore, nutritional status indicators such as albumin levels, weight, BMI, mid‐arm circumference, calf circumference, and GNRI were lower in patients with malnourished or at risk of malnutrition (P = 0.027, P < 0.001, P = 0.001, P < 0.001, P = 0.004, and P = 0.001, respectively).
Table 1.
Demographic and laboratory characteristics of patients (n = 95)
| Parameters | Malnourished or at risk of malnutrition (MNA score ≤ 23.5) (n = 46) | Normal nutrition (MNA score 24‐30) (n = 49) | P value |
|---|---|---|---|
| Age (y) | 75.3 ± 5.8 | 72.7 ± 5.4 | 0.028 |
| Gender (Female, n) | 31 | 28 | 0.207 |
| DM, n | 13 | 16 | 0.405 |
| Hypertension, n | 32 | 27 | 0.107 |
| CVD, n | 7 | 3 | 0.134 |
| CVA, n | 3 | 5 | 0.398 |
| Hemoglobin (g/dL) | 12.5 ± 1.4 | 12.9 ± 0.9 | 0.102 |
| Platelet count (/μL) | 252.6 ± 65.5 | 247.6 ± 62.8 | 0.757 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.236 |
| Calcium (mg/dL) | 9.4 ± 0.5 | 9.6 ± 0.4 | 0.061 |
| Uric acid (mg/dL) | 5.5 ± 1.5 | 5.5 ± 1.5 | 0.988 |
| LDL‐C (mg/dL) | 130 ± 30 | 131 ± 33 | 0.914 |
| Triglyceride (mg/dL) | 138 ± 51 | 136 ± 67 | 0.386 |
| HDL‐C (mg/dL) | 45.2 ± 13.5 | 48.6 ± 10.6 | 0.101 |
| ALT (U/L) | 14 (10 – 21) | 16 (12 – 22.5) | 0.519 |
ALT, alanine amino‐transferase; CVD, cardiovascular disease; CVA, cerebrovascular accident; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol. Data are expressed as mean ± SD, number (percentage), or median (percentiles 25‐75).
Table 2.
Nutritional and inflammatory parameters of patients
| Variables | Malnourished or at risk of malnutrition (MNA score ≤ 23.5) (n = 46) | Normal nutrition (MNA score 24‐30) (n = 49) | P value |
|---|---|---|---|
| NLR | 2.4 ± 0.9 | 1.8 ± 0.7 | 0.004 |
| MNA score | 20.7 ± 2.3 | 25.7 ± 1.3 | <0.001 |
| WBC (/μL) | 7053 ± 1940 | 6610 ± 1563 | 0.461 |
| Neutrophil count (cell/mm3) | 4263 ± 1439 | 3692 ± 1129 | 0.115 |
| Lymphocyte count (cell/mm3) | 1928 ± 720 | 2171 ± 800 | 0.160 |
| Monocyte (cell/mm3) | 523 ± 181 | 471 ± 155 | 0.114 |
| Eozinofil (cell/mm3) | 134 (62 – 237) | 171 (100 – 254) | 0.168 |
| CRP (mg/L) | 4.6 ± 3 | 4.2 ± 2 | 0.587 |
| Total cholesterol (mg/dL) | 203 ± 41 | 210 ± 40.7 | 0.424 |
| Albumin (g/dL) | 4.1 ± 0.3 | 4.2 ± 0.3 | 0.027 |
| Weight (kg) | 64.6 ± 14.2 | 76.2 ± 11.9 | <0.001 |
| BMI (kg/m2) | 27.2 ± 5.8 | 30.3 ± 4.8 | 0.001 |
| MAC (cm) | 27.7 ± 3.8 | 30.4 ± 3 | <0.001 |
| CC (cm) | 34.1 ± 4.4 | 36.5 ± 3.4 | 0.004 |
| GNRI | 113 ± 13.1 | 121.3 ± 11.1 | 0.001 |
BMI, body mass index; CRP, C‐reactive protein; CC calf circumference; GNRI, Geriatric Nutritional Risk Index; LDL, low‐density lipoprotein; MAC, mid‐arm circumference; MNA, Mini Nutritional Assessment; NLR, neutrophil‐to‐lymphocyte ratio; WBC, white blood cells. Data are expressed as mean ± SD or median (percentiles 25‐75).
Table 3 shows the correlation between NLR and nutritional status indicators. There was a significant negative correlation between NLR and the MNA score (r = −0.276, P = 0.007) (Figure 1). Receiver operating characteristic curve analysis showed that the optimum NLR cutoff point for patients with malnourished or at risk of malnutrition was 1.81 with 71.7% sensitivity and 63.3% specificity [95% confidence interval (CI): 0.562‐0.780, area under the curve: 0.671, P = 0.004] (Figure 2). We used multiple logistic regression analysis where malnourished/at risk of malnutrition was the dependent and NLR > 1.81 as a categorical variable, age, and comorbid diseases were independent variables. NLR and age were found to be statistically significant independent factors for predicting malnutrition/risk of malnutrition in geriatric patients (Table 4).
Table 3.
Correlation between neutrophil‐to‐lymphocyte ratio and nutritional parameters
| Variables | Correlation Coefficient | P value |
|---|---|---|
| MNA score | −0.276 | 0.007 |
| Albumin (g/L) | −0.269 | 0.008 |
| T. Cholesterol (mmol/L) | −0.154 | 0.142 |
| CRP (mg/L) | 0.206 | 0.049 |
| Weight (kg) | −0.224 | 0.027 |
| BMI (kg/m2) | −0.182 | 0.074 |
| MAC (cm) | −0.233 | 0.021 |
| Calf circumference (cm) | −0.184 | 0.071 |
| GNRI | −0.286 | 0.004 |
| Age (y) | 0.145 | 0.155 |
BMI, body mass index; CRP, C‐reactive protein; GNRI, Geriatric Nutritional Risk Index; MAC, mid‐arm circumference; MNA, Mini Nutritional Assessment; T, total. Spearman correlation test was used to determine correlations.
Figure 1.
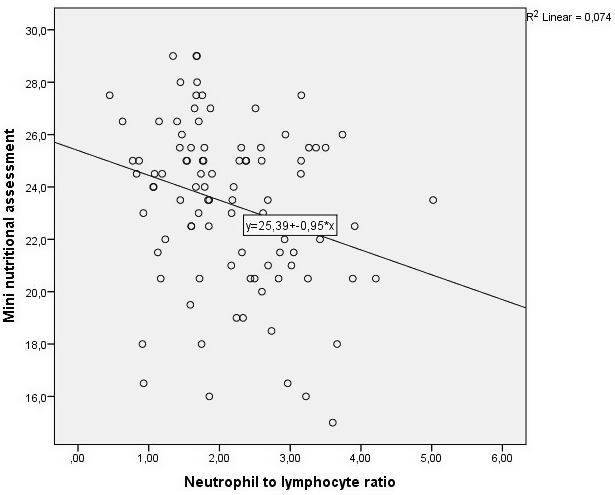
The scatter plot graph of correlation between neutrophil‐to‐lymphocyte ratio and Mini Nutritional Assessment score (r = −0.276, P = 0.007)
Figure 2.
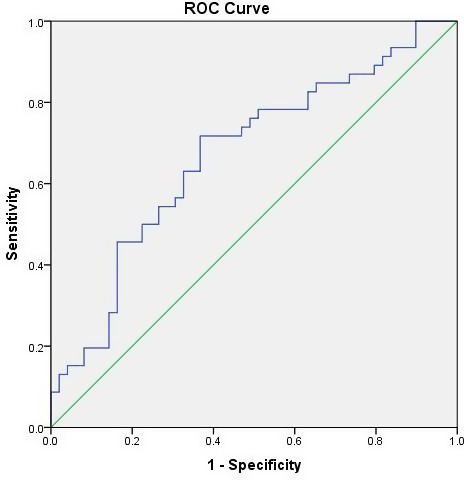
ROC curves based on a univariate model showing the power of NLR to predict the patients with malnourished or at risk of malnutrition. The area under the curve was 0.671 (P = 0.004, 95% CI: 0.562‐0.780). ROC, receiver operating characteristic; NLR, neutrophil‐to‐lymphocyte ratio
Table 4.
Multiple logistic regression analysis of patients with malnourished or at risk of malnutrition
| OR | P value | 95% CI | |
|---|---|---|---|
| NLR | 0.256 | 0.003 | 0.103‐0.636 |
| Age | 1.088 | 0.044 | 1.002‐1.181 |
| DM | 1.101 | 0.853 | 0.399‐3.033 |
| Hypertension | 0.562 | 0.245 | 0.213‐1.484 |
| CVD | 0.378 | 0.235 | 0.076‐1.887 |
| CVA | 2.278 | 0.366 | 0.382‐13.582 |
CVD, cardiovascular disease; CVA, cerebrovascular accident; DM, diabetes mellitus; NLR, neutrophil‐to‐lymphocyte ratio.
4. DISCUSSION
This study shows that NLR is significantly higher in geriatric outpatients who are malnourished or at risk of malnutrition compared with that of patients with normal nutritional status. There is a negative correlation between NLR and the MNA score, which is frequently used in assessing the nutritional status. Malnutrition or risk of malnutrition is significantly high in the elderly with an NLR above the cutoff value of 1.81. To the best of our knowledge, the present study is the first to investigate the association between NLR and nutritional status of geriatric patients. Furthermore, the results of this study revealed that NLR alone is a predictor of nutritional status of geriatric patients.
There are many screening tools for assessing the nutritional status of geriatric patients and achieving early identification of malnutrition or risk of malnutrition.6, 7 The MNA is a verified, reliable and useful scoring system that is extensively used worldwide for this purpose.2, 8 The European Society for Clinical Nutrition and Metabolism also recommends using the MNA for the nutritional assessment of geriatric individuals.18 We also used the MNA as a reference for identifying malnutrition or risk of malnutrition in geriatric patients in the present study. According to the MNA scores, we found that NLR was significantly higher in malnourished patients or in patients at risk of malnutrition.
In our literature review, we found a limited number of studies on the association between NLR and the nutritional status of geriatric patients. NLR is a novel parameter that can be easily calculated from complete blood count results and generally reflects systemic inflammation. However, in many studies using NLR, NLR was found to be associated with the diagnosis, severity, or prognosis of certain diseases independent of inflammation markers such as total leukocyte count, neutrophil count, and C‐reactive protein levels.10, 14, 15 In several meta‐analyses conducted on various cancer types, such as liver,10 lungs,11 gastric,12 and ovarian cancers,13 NLR was found to be significantly associated with prognosis. Apart from malignancy, NLR was also found to be significantly associated with prognosis in other clinical conditions such as acute ischemic stroke,15 chronic heart failure,16 and acute pulmonary embolism.19
There is little information showing that a low lymphocyte count may be associated with increased malnutrition, morbidity, and mortality in the elderly.1, 20 The results of studies investigating the relationship between malnutrition and total lymphocyte count (TLC) in older adults are controversial.21, 22 In a study by Leandro‐Merh et al investigating the relationship between TLC and nutritional status in 131 hospitalized older adults, patients who were not at risk of malnutrition had a higher mean TLC.21 In the same study, a significant correlation was found between TLC and mid‐upper arm circumference as well as triceps skinfold thickness, but no significant correlation was found between TLC and BMI, age, and calf circumference.21 Kuzuya et al investigated the relationship between TLC and nutritional markers, including the MNA score, anthropometric measurements, and serum albumin and total cholesterol levels in 161 elderly subjects.22 They found no significant differences between TLC and nutritional markers.22 In our study, there was no significant association between TLC and the nutritional status of the patients.
Many inflammation‐related parameters, both in etiology and as a result of malnutrition, play a role in pathophysiology.20, 23, 24, 25 In the present study, high values of NLR in patients with malnutrition or at risk for malnutrition may be due to persistent low‐grade systemic chronic inflammation associated with malnutrition in this patient population. Physiological changes associated with aging in the elderly and specific cytokines expressed due to comorbid diseases affect the number and function of inflammatory cells such as neutrophils and lymphocytes.20, 23, 25 The presence of malnutrition leads to susceptibility to infectious diseases by affecting inflammatory cytokines, whereas the presence of infection may cause malnutrition due reasons such as decreased appetite.23
The etiology of malnutrition includes poorly understood complex mechanisms involving multiple systems of the body, which affect each other, accompanied by comorbidities due to aging.24, 25 In many studies evaluating the nutritional status of the elderly, albumin levels, BMI, mid‐arm circumference, calf circumference, and GNRI parameters were significantly lower in patients with malnutrition than those of patients with normal nutritional status.6, 7, 8, 9 All these parameters are recommended for the purpose of nutritional assessment.6, 7, 8, 9 The results of the present study were also consistent with those of previous reports in terms of nutritional status indicators.6, 7 As a novel finding, we also found a significant negative correlation between NLR and nutritional status indicators such as the MNA score, albumin level, weight, BMI, calf circumference, and GNRI.
5. CONCLUSION
The present study demonstrated that NLR was significantly higher in geriatric patients with malnutrition or those at risk of malnutrition compared to patients with normal nutritional status. Additionally, the study revealed that NLR was correlated with nutritional status indicators such as MNA score, albumin level, weight, BMI, calf circumference, and GNRI. Furthermore, elevated NLR was an independent variable for predicting malnutrition or risk of malnutrition in geriatric patients. Our findings suggest that NLR can be used as an adjunctive nutritional marker in assessing the nutritional status of geriatric patients.
ETHICAL APPROVAL
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Kaya T, Açıkgöz SB, Yıldırım M, Nalbant A, Altaş AE, Cinemre H. Association between neutrophil‐to‐lymphocyte ratio and nutritional status in geriatric patients. J Clin Lab Anal. 2019;33:e22636 10.1002/jcla.22636
REFERENCES
- 1. Fávaro‐Moreira NC, Krausch‐Hofmann S, Matthys C, et al. Risk factors for malnutrition in older adults: a systematic review of the literature based on longitudinal data. Adv Nutr. 2016;7:507‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez‐Hernández J, Planas‐Vila M, León‐Sanz M, et al. Prevalence and costs of malnutrition in hospitalized patients; the PREDyCES Study. Nutr Hosp. 2012;27:1049‐1059. [DOI] [PubMed] [Google Scholar]
- 3. Diekmann R, Winning K, Uter W, et al. Screening for malnutrition among nursing home residents ‐ a comparative analysis of the mini nutritional assessment, the nutritional risk screening, and the malnutrition universal screening tool. J Nutr Health Aging. 2013;17:326‐331. [DOI] [PubMed] [Google Scholar]
- 4. Correia MIT, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235‐239. [DOI] [PubMed] [Google Scholar]
- 5. Rasheed S, Woods RT. Malnutrition and quality of life in older people: a systematic review and meta‐analysis. Ageing Res Rev. 2013;12:561‐566. [DOI] [PubMed] [Google Scholar]
- 6. Camina‐Martín MA, de Mateo‐Silleras B, Malafarina V, et al. Nutritional status assessment in geriatrics: consensus declaration by the Spanish Society of Geriatrics and Gerontology Nutrition Work Group. Maturitas. 2015;81:414‐419. [DOI] [PubMed] [Google Scholar]
- 7. van Bokhorst‐de van der Schueren MA, Guaitoli PR, Jansma EP, de Vet HC. Nutrition screening tools: does one size fit all? A systematic review of screening tools for the hospital setting. Clin Nutr. 2014;33:39‐58. [DOI] [PubMed] [Google Scholar]
- 8. Guigoz Y. The mini‐nutritional assessment (MNA®) review of the literature – What does it tell us? J Nutr Health Aging. 2006;10:466‐487. [PubMed] [Google Scholar]
- 9. Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr. 2005;82:777‐783. [DOI] [PubMed] [Google Scholar]
- 10. Min GT, Li YM, Yao N, Wang J, Wang HP, Chen W. The pretreatment neutrophil‐lymphocyte ratio may predict prognosis of patients with liver cancer: a systematic review and meta‐analysis. Clin Transplant. 2018;32:e13151. [DOI] [PubMed] [Google Scholar]
- 11. Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta‐analysis. Clinics (Sao Paulo). 2015;70:524‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in gastric cancer. Medicine (Baltimore). 2018;97:e0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Z, Zhao X, Lu J, Xue J, Liu P, Mao H. Prognostic roles of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in ovarian cancer: a meta‐analysis of retrospective studies. Arch Gynecol Obstet. 2018;297:849‐857. [DOI] [PubMed] [Google Scholar]
- 14. Tan TP, Arekapudi A, Metha J, Prasad A, Venkatraghavan L. Neutrophil‐lymphocyte ratio as predictor of mortality and morbidity in cardiovascular surgery: a systematic review. ANZ J Surg. 2015;85:414‐419. [DOI] [PubMed] [Google Scholar]
- 15. Yu S, Arima H, Bertmar C, Clarke S, Herkes G, Krause M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci. 2018;387:115‐118. [DOI] [PubMed] [Google Scholar]
- 16. Yan W, Li RJ, Jia Q, Mu Y, Liu CL, He KL. Neutrophil‐to‐lymphocyte ratio compared to N‐terminal pro‐brain natriuretic peptide as a prognostic marker of adverse events in elderly patients with chronic heart failure. J Geriatr Cardiol. 2017;14:127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balta S, Celik T, Mikhailidis DP, et al. The relation between atherosclerosis and the neutrophil‐lymphocyte ratio. Clin Appl Thromb Hemost. 2016;22:405‐411. [DOI] [PubMed] [Google Scholar]
- 18. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. Educational and clinical practice committee, european society of parenteral and enteral nutrition (ESPEN). ESPEN guidelines for nutrition screening. Clin Nutr. 2003;22:415‐421. [DOI] [PubMed] [Google Scholar]
- 19. Galliazzo S, Nigro O, Bertù L, et al. Prognostic role of neutrophils to lymphocytes ratio in patients with acute pulmonary embolism: a systematic review and meta‐analysis of the literature. Intern Emerg Med. 2018;13:603‐608. [DOI] [PubMed] [Google Scholar]
- 20. Nishida T, Sakakibara H. Association between underweight and low lymphocyte count as an indicator of malnutrition in Japanese women. J Womens Health (Larchmt). 2010;19:1377‐1383. [DOI] [PubMed] [Google Scholar]
- 21. Bauer JM, Wirth R, Volkert D, Werner H, Sieber CC; Teilnehmer des BANSS‐Symposiums 2006 . Malnutrition, sarcopenia and cachexia in the elderly: from pathophysiology to treatment. Conclusions of an international meeting of experts, sponsored by the BANSS Foundation. Dtsch Med Wochenschr. 2008;133:305‐310. [DOI] [PubMed] [Google Scholar]
- 22. Omran ML, Morley JE. Assessment of protein energy malnutrition in older persons, Part II: laboratory evaluation. Nutrition. 2000;16:131‐140. [DOI] [PubMed] [Google Scholar]
- 23. Leandro‐Merhi VA, Bráz VN, Aquino JL. Is total lymphocyte count related to nutritional markers in hospitalized older adults? Arq Gastroenterol. 2017;54:79‐82. [DOI] [PubMed] [Google Scholar]
- 24. Kuzuya M, Kanda S, Koike T, Suzuki Y, Iguchi A. Lack of correlation between total lymphocyte count and nutritional status in the elderly. Clin Nutr. 2005;24:427‐432. [DOI] [PubMed] [Google Scholar]
- 25. Cohen S, Danzaki K, MacIver NJ. Nutritional effects on T‐cell immunometabolism. Eur J Immunol. 2017;47:225‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]


