Abstract
The Aerobic Scope (AS), which reflects the functional capacity for biological fitness, is a highly relevant proxy to determine thermal tolerance in various taxa. Despite the importance of this method, its implementation is often hindered, due to lacking techniques to accurately measure standard- (SMR) and maximal- (MMR) metabolic rates, especially in sluggish marine invertebrates with low oxygen consumption rates, such as sea cucumbers. In this study the AS concept was modified to define a Temperature-induced Aerobic Scope (TAS), based on metabolic rate changes due to temperature adjustments rather than traditionally used physical activity patterns. Consequentially, temperature dependent peak and bottom O2 consumption rates, defined as Temperature-induced Maximal- (TMMR) and Standard Metabolic Rates (TSMR), respectively, served as MMR and SMR alternatives for the sea cucumber Holothuria scabra. TMMR and TSMR were induced through acute temperature change (2°C per hour; 17–41°C) until critical warm (WTcrit) and cold (CTcrit) temperatures were reached, respectively. In addition, Hsp70 gene expression linked to respiration rates served as synergistic markers to confirm critical threshold temperatures. O2 consumption of H. scabra peaked distinctly at WTcrit of 38°C (TMMR = 33.2 ± 4.7 μgO2 g-1 h-1). A clear metabolic bottom line was reached at CTcrit of 22°C (TSMR = 2.2 ± 1.4 μgO2 g-1 h-1). Within the thermal window of 22–38°C H. scabra sustained positive aerobic capacity, with assumed optimal performance range between 29–31.5°C (13.85–18.7 μgO2 g-1 h-1). Between 39–41°C H. scabra decreased respiration progressively, while gene expression levels of Hsp70 increased significantly at 41°C, indicating prioritization of heat shock response (HSR) and homeostatic disruption. At the cold end (17–22°C) homeostatic disruption was visible through incrementally increasing energetic expenses to fuel basal maintenance costs, but no Hsp70 overexpression occurred. TMMR, TSMR and TAS proved to be reliable metrics, similar to the traditional energetic key parameters MMR, SMR and AS, to determine a specific aerobic performance window for the sluggish bottom dwelling species H. scabra. In addition, the linkage between respiration physiology and molecular defense mechanisms showed valuable analytical synergies in terms of mechanistic prioritization as response to thermal stress. Overall, this study will help to define lethal temperatures for aquaculture and to predict the effects of environmental stress, such as ocean warming, in H. scabra.
Introduction
The determination of species-specific critical temperature limits is highly relevant to predict stress levels due to global environmental change. The biogeography of marine species conforms closely to optimal temperature windows, especially near equatorial boundaries [1, 2]. Tropical species are confined to narrower thermal niches than temperate species, which makes them more susceptible to niche shifts, due to higher metabolic rate changes [3, 4]. A gradual temperature increase of only 2–3°C caused already significant reductions of thermal tolerance levels in various marine invertebrates [5], and substantially decreased aerobic performance [6] as well as compromised growth and reproduction for a number of coral reef fish [7, 8]. Consequently, the effects of global climate change–i.e. a temperature increase of 2–4°C for the tropical oceans–projected to occur by the end of this century, might pose a serious metabolic challenge for many equatorial marine species.
A comprehensive mechanistic explanation for temperature driven effects on organism performance is provided by the oxygen- and capacity- limited thermal tolerance (OCLTT) [9–11]. OCLTT defines physiological capacity limits based on aerobic performance, encompassing the span for oxygen uptake, transport and delivery, the so-called aerobic scope (AS). AS is defined as the difference between maximal performance, known as the maximal metabolic rate (MMR), and resting metabolism, known as the standard metabolic rate (SMR) [12, 13]. The difference between SMR and MMR represents the accessible respiration energy, which is not required for basal maintenance functions and, thus, freely available for functions related to biological fitness (activity, feeding, and reproduction). Within an optimal temperature range the aerobic scope peaks, which correspond to optimal species performance. Above or below this optimal range, performance declines with a narrowing aerobic scope. The aerobic scope is delimited by a species-specific lower and upper critical temperature (Tcrit) at which SMR exceeds MMR and animals depend progressively on unsustainable anaerobic energy supply to fuel physiological functions [11]. At temperature stress beyond Tcrit, extreme hypoxia will lead to terminal ATP deficiency and ultimately cause the loss of vital body functions (i.e. CTmax) [10, 14]. The aerobic scope has been widely used as a model to determine thermal tolerances for various fish species from temperate [15–17] and tropical [6, 7, 18] regions. For slow-moving benthic marine species such as sea cucumbers, the lack of methods to reliably quantify SMR and MMR largely hinders the assessment of AS. In more mobile organisms (i.e. fish) the temperature effect on the aerobic scope is generally determined by measuring SMR and MMR during lowest (i.e. resting/sleeping) and highest (i.e. fast swimming) physical activity levels, respectively, at different temperatures. In sluggish benthic species such as sea cucumbers, however, the measurement of SMR and MMR through aerobic adjustments as response to induced physical activity challenges is not convenient. Hence, in this study we tested acute temperature challenge itself for the induction of an aerobic bottom line and peak performance in the tropical sea cucumber Holothuria scabra, to define metrics similar to the established SMR and MMR.
Besides aerobic performance, critical temperature stress can also be measured at the molecular level through the differential expression of genes encoding heat shock proteins (HSPs) [19, 20]. HSPs act as molecular chaperones and regulate protein folding [21]. Hsp70 has been detected in complexes with proteins presumably assisting in the recovery from stress by repair of damaged proteins [22]. Despite its relevance in thermal stress detection, detailed knowledge on temperature dependent Hsp70 gene expression is still scarce for many marine invertebrates, especially for species without a sequenced and annotated genome, such as H. scabra. Moreover, knowledge about the direct link between the allocation and prioritization of energy resources to fuel cellular- and tissue- protection, such as Hsp70 synthesis, and variations in aerobic scope is scarce.
H. scabra contributes significantly to the recycling of nutrients and minerals through bioturbation [23]. Moreover, this species serves as prey for many taxa and enhances biodiversity by forming various symbiotic relations. Besides their ecological relevance, H. scabra is a high valued aquaculture candidate species [24–26]. Due to its high commercial and ecological relevance, fine-tuned methods to detect thermal stress in H. scabra are crucial to enhance aquaculture production and to assess this animal’s susceptibility to ocean warming.
The aim of this study was two-fold: 1) To determine temperature-induced maximal and minimal aerobic performance and the corresponding limiting temperatures by measuring O2 consumption in H. scabra along a gradient of acute temperature change, encompassing 17–41°C. 2) to measure the mRNA expression level of Hsp70 at the maximum (41°C) and minimum (17°C) temperature, in order to link the cellular stress level with the temperature-driven metabolic benchmarks. With this approach we intended to exemplify the utilization of an aerobic performance window delimited by critical -low and -high temperature challenge as tool to reliably determine energetic key parameters similar to the traditional SMR, MMR and AS for slow-moving marine invertebrate species. With the combined analysis of O2 consumption and Hsp70 gene expression we examine the synergy between these two key stress markers to comprehensively study functional disruption of homeostasis and prioritization of cellular protection mechanisms, due to thermal stress in H. scabra and other similar species.
Materials and methods
Study animals and experimental design
All experiments were conducted in accordance with the German ethics on animal welfare. This study was conducted at the Indonesian Institute of Science (LIPI), Lombok, Indonesia. In January 2016, a number of 32 juvenile H. scabra (63 ± 15g) were lawfully collected with a LIPI fishing permit from the lower intertidal zone off the West-Coast of Lombok, Pantai Sira di Pagi Hari (8°22´5.42´´S, 116°6´58.37´´E). After specimen collections, the animals were transported to the LIPI aquaculture facilities and placed in aquaculture tanks (100L), with flow-through conditions (29°C; 35ppt). Natural sediments were provided as food source. After an acclimation period of 14 days, four animals were placed over a 24h defecation period in a separate tank without sediment (50L; 29°C; 35ppt). Subsequently, four animals were randomly divided into two groups (n = 2) and placed into gas-tight Acrylic Chambers (AC; V = 600ml), which were submersed in two separate water baths (20L aerated and filtered seawater; 29°C; 35ppt). Each water batch contained two animals, after one hour acclimation to AC conditions, the temperature in one water bath was changed by 2°C per hour while the temperature in the other water bath was kept constant at 29°C (control). This experimental setup was separately repeated eight times (n = 16), with four experiments allocated to incremental temperature increase (29–41°C; n = 8) and four to incremental temperature decrease (29–17°C; n = 8). Right after the cold and warm temperature manipulation experiments the animals were anesthetized using 0.5% magnesium sulphate [27]. Subsequently, the respiratory tree (RT) was removed and immediately snap-frozen in liquid nitrogen for Hsp70 analysis.
Temperature-induced changes in O2 consumption
Intermitted-flow respirometry was used to determine resting- and maximum- O2 consumption for eight H. scabra individuals through incremental cold and warm temperature manipulations, respectively. Respirometry measurements were conducted always at the same daytime. Submersible pumps (150L h-1) provided a constant water supply from the water bath through the acrylic chamber (AC), while a peristaltic pump maintained internal water mixing within the AC. The water flow into the AC was stopped for 15min every 15min. This interval of continuous flushing cycles and the change rate of temperature (2°C h-1) enabled one respiration measurement cycle per degree of temperature up- and down- regulation: (1) acute temperature elevation (29°C, 31°C, 33°C, 35°C, 37°C, 39°C, 41°C); and (2) acute temperature decrease (29°C, 27°C, 25°C, 23°C, 21°C, 19°C, 17°C). At each temperature the water flow interruption time was short enough to ensure O2 concentrations above 80% saturation [17]. The temperature-compensated O2 concentration (mg L-1) over time (s), was continuously recorded (1 s-1) for each AC using oxygen-sensitive REDFLASH dye on contactless spots (2mm) glued at the inside of the AC lids and linked to a Firesting Optical Oxygen Meter (Pyro Science e. K., Aachen, Germany) via fibre-optic cables. For consistency, the first and last minute of the resulting respiration slopes were excluded from analyses. For each temperature change (2°C h-1), the average O2 consumption rate (μg g-1 h-1) was calculated from the respiration slopes (R2≥0.90) of the eight biological replicates (n = 8). To account for microbial background respiration rates the oxygen depletion in the empty AC´s, before the animals were placed in (pre-blank) and after the animals were removed (post-blank), was measured over 15min. The background O2 consumption rates were calculated for each temperature, assuming a linear microbial accumulation between pre-blank and post-blank, and subtracted from the respective animal respiration slope. Mean respiration rates (n = 8) were calculated per temperature. Within the window of linear correlation between temperature and O2 consumption, peak and minimum respiration was defined as temperature-induced maximum (TMMR) and standard (TSMR) metabolic rates at the corresponding critical warm (WTcrit) and cold (CTcrit) temperatures, respectively (see Fig 1). Mean TMMR and TSMR values served to determine a temperature-induced aerobic scope (TAS) (Eq 1). Along the entire temperature gradient, no clear pattern of activity changes were monitored for the animals. To account for temperature effects along the temperature gradients, the temperature quotients (Q10) were calculated using mean O2 consumption (R1 and R2) at control temperature (29°C; T1) and at WTcrit (T2max) and CTcrit (T2min) (Eq 2).
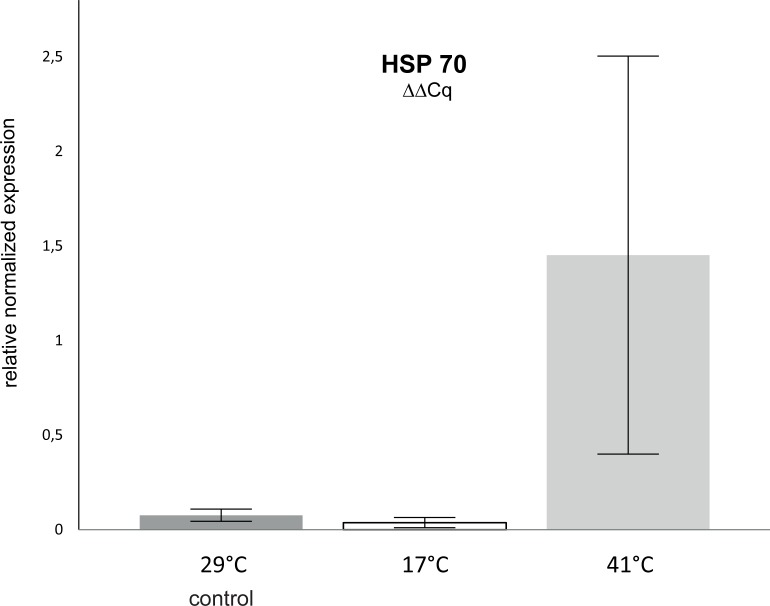
Fig 1. Average (mean ± standard deviation) relative normalized Hsp70 gene expression, for the two temperature manipulation experiments with eight biological replicates (n = 8), (1) cold (17°C; n = 8; p = 0.34) and (2) warm (41°C; n = 8; p = 0.00086) compared to the pooled controls (29°C; n = 16).
Differences between treatments were calculated using a Mann-Whitney test. Significant differences are indicated for P<0.05 (*) and P<0.01 (**).
| (1) |
| (2) |
RNA extraction and cDNA synthesis
100mg deep-frozen respiratory tree (RT) samples were immediately homogenized in 1 ml of TRIzol Reagent (Life Technologies, California, USA). The RNA extraction followed the manufacturer recommendations. The quantity of the extracted RNA was measured in a BioPhotometer (Eppendorf, Hamburg, Germany). Possible contaminations of genomic DNA were removed using DNase (Promega, Madison, WI) digestion according to the manufacturer recommendations. Subsequently, single-stranded cDNA was synthesized at 42°C for 60min, using the GoScriptTM Reverse Transcriptase (Promega, Madison, WI) following the manufacturer recommendations. The reverse transcription was based on 4μl total RNA template, mixed with 0.5μl oligo(dT)20 and 0.5μl of random primers.
Primer design for the RT-qPCR assay
Besides Hsp70, two established reference genes 18S rRNA (18S ribosomal RNA) and ACTB (β-actin) [28, 29] were targeted in this study. 18S sequences are available for six sister species of the genus Holothuria (AY133470.1 –AY133475.1). These published sequences were used to generate a homologous sequence alignment using BioEdit 7.2.5 [30] to identify conserved primer binding sites. Quality control of the primers was conducted using PCR Primer Stats [31]. β-actin and the target gene Hsp70 were not available for any Holothuria species. This gene was, therefore, targeted using RACE-PCR [32] with a degenerated forward primer in combination with an anchor-primer (introduced at the cDNA synthesis using an anchored OligoT primer (Odt7)). The β-actin forward primer (ACTB_FW: ACTCTGCTACGTCGCTCTTG) was designed from Apostichopus japonicas, whereas, Hsp70 followed the following approach: The known A. japonicus sequence (EU930813.1) was utilized to BLAST [33] the Sequence Read Archive (SRA) of Holothuria glaberrima. The best 50 hits were imported to the software BioEdit 7.2.5 followed by a contig assembly program [34]. Three out of seven contigs were selected for further alignments with homologous Hsp70 sequences from animals in various taxa: Apostichopus japonicas (EU930813.1), Parastichopus californicus (GAVO01016544.1, GAVO01019403.1), Ciona intestinalis (AK116745), Lottia gigantea hypothetical protein mRNA (XM_009047345), Psammechinus miliaris (FN796462) and Brissopsis lyrifera (FN667017). The function similarity matrix (for shading) was applied in the alignment window to detect conserved regions, from which the Hsp70 primers were designed.
The degenerated forward primer Hsp70_480_fw (CCRGAAGAAATYAGYTCSATGGT) was used in combination with the Odt7 anchor primer at 47°C and 35 cycles. 1μl of the resulting PCR product was used as template in a semi-nested PCR using Hsp70_680_rv (TTCTTATCGAGCCCATAGGC) at an annealing temperature of 47°C and 35 cycles. All PCRs used the OptiTaq DNA Polymerase kit (Roboklon, Germany) for the amplification. The PCR products of the expected size for Hsp70, 18S and β-actin were treated prior to Sanger sequencing using ExoSAP-IT PCR Product Cleanup kit (Affymetrix) to eliminate the unconsumed primers and nucleotides. After the identity of the PCR products was confirmed by Sanger sequencing (StarSEQ GmbH Germany), the resulting contig was used as a query sequence in a Primer-BLAST on NCBI for final qPCR primer design. [35]. Primer quality was analysed using PCR Primer Stats of the oligo analyser 3.1 software [36]. The chosen primer parameters were set to have a PCR product above 70 bp. The melting temperature fell into a range of 57°C to 63°C. The Echinodermata Refseq mRNA database was used to exclude amplification of wrong targets. The resulting H. scabra primers were tested at three different annealing temperatures (52°C, 54°C, 56°C) and 5% DMSO per PCR reaction was added to reduce primer dimer formation.
RT-qPCR
The expression of Hsp70 was measured relative to the expression level of the two control genes 18S and β-actin in RT tissue. Measurements were conducted in three technical replicates using sterile H2O as negative control and a confirmed cDNA sample as positive control. Per sample, the final qPCR mixture contained 7.8μl sterile H2O (sterile DEPC-water, Roth), 10μl iTaq Universal SYBR Green Supermix (Bio-Rad), 0.1μl of the respective forward and reverse primers (HSP70, 18S, β-actin; 500nM in mix) (Biomers GmbH, Ulm, Germany) and 1.5μl cDNA sample. The qPCR reactions were conducted on the CFX ManagerTM Real Time PCR System (Bio-Rad Laboratories Inc., California, USA). The cycling protocol included an initial heating phase (95°C; 3 min), followed by 45 repeats of denaturation (95°C; 10s) and elongation (60°C; 30s), and finally the melting curve (95–60°C, 0.2°C s-1) to confirm single amplification products without primer dimers.
Differential gene expression analysis
The output data (Cq-values) from the RT-qPCR measurements were exported from Bio-Rad CFX Manager (Version 3.0) software to Microsoft Excel 2010 to generate the bar plot. The expression levels of the target gene Hsp70 are given in calibrated normalized relative quantities (CNRQ values) ± the 95% confidence interval (CI), which automatically account for replicate variability, amplification efficiency and normalization factors. To test the stability of the two reference genes 18S and β-actin, geNorm [37] was utilized. Homogeneity of reference targets was assumed at M values <0.5. Mean (n = 8) relative Hsp70 expression levels of cold (17°C) and warm (41°C) treated animals were compared to the pooled (n = 16) Hsp70 expression levels of the animals maintained at control temperature (29°C). Differences were determined through a non-parametric Mann-Whitney test. Significant differences were assumed at P<0.05 (*) and highly significant differences at P<0.01 (**).
Results
Oxygen consumption
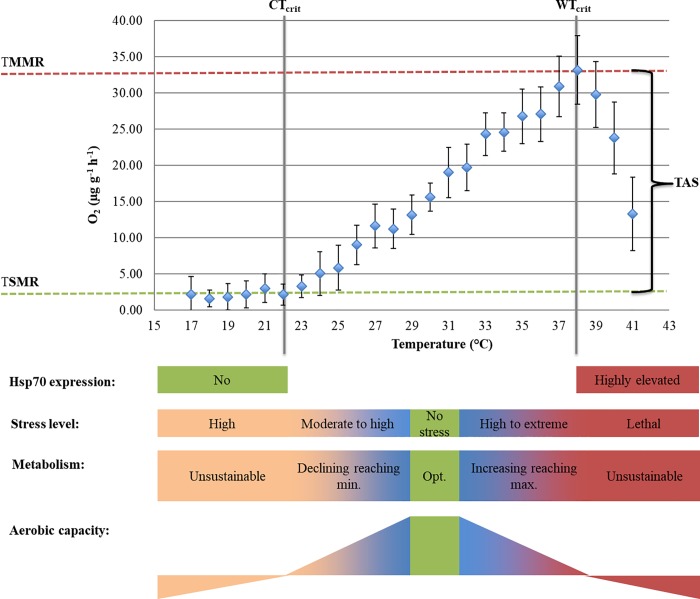
Within the range of 22–38°C, temperature and oxygen consumption rates (OCR) of H. scabra were significantly positively correlated (R2 = 0.99) (Fig 2). At 38°C the animals reached a temperature-induced maximal metabolic rate (TMMR) of 33.2 ± 4.7μgO2 g-1 h-1 (n = 8). At maximum temperature (41°C) OCR dropped to 13.3 ± 5.1μgO2 g-1 h-1 (n = 8). At22°C H. scabra reached its temperature-induced standard metabolic rate (TSMR) at 2.2 ± 1.4 μgO2 g-1 h-1 (n = 8). The animals maintained at constant ambient (control) conditions (29°C) exhibited a mean OCR of 13.2 ± 2.7μgO2 g-1 h-1 (n = 8). The difference between mean TMMR and TSMR revealed a temperature-induced aerobic scope (TAS) of 31μgO2 g-1 h-1 (see Table 1 and Fig 2). The temperature quotient (Q10) reached 13.2.7 for the OCR change between 22–29°C (temperature down-regulation) and 2.8 for the OCR change between 29–38°C (temperature up-regulation) Tables 1 and 2.
Fig 2. O2 consumption rate of Holothuria scabra at different temperatures, obtained from four warm and cold experimental replications.
Points represent means ± s.d. of eight biological replicates (n = 8). Upper red dotted line indicate temperature-induced maximal metabolic rate (TMMR), at the critical warm temperature (WTcrit; 38°C), lower red dotted line marks the start of the temperature-induced standard metabolic rate (TSMR), at critical cold temperature (CTcrit; 22°C). The difference between TMMR and TSMR define the temperature-induced aerobic scope (TAS) for H. scabra (black brace). Beyond CTcrit and WTcrit (22–38°C) progressively negative aerobic performance caused by growing dependency on energy reserves and declining aerobic capacity, respectively, indicate homeostatic disruption. Overexpression of Hsp70 occurs only at the warm temperature maximum (41°C).
Table 1. O2 consumption rates at control conditions (Tcont = 29°C), critical cold temperature (CTcrit = 22°C) and critical warm temperature (WTcrit = 38°C).
Temperature-induced standard (TSMR) and maximal metabolic rate (TMMR) indicate aerobic performance at CTcrit and WTcrit, respectively. The temperature-induced aerobic scope (TAS) is given as difference between TMMR and TSMR. Temperature quotients (Q10) are given for the respective temperature gradients.
| Temp. (°C) | O2 consumption (μg g-1 h-1) | Q10 | ||
|---|---|---|---|---|
| 22–29°C | 29–38°C | |||
| Control | 29 | 13.2 ± 2.7 | ||
| TSMR | 22 (CTcrit) | 2.2 ± 1.4 | 13.2 | |
| TMMR | 38 (WTcrit) | 33.2 ± 4.7 | 2.8 | |
| TAS | 31 | |||
Table 2. Primer sequences, for the three target genes (Hsp70, β-actin and 18S) in H. scabra, and technical details: Number of amplicon base pairs (bp), melting temperature (Tm) and total content of Gs and Cs (GC).
| Primer pair | Sequence (5'->3') | Amplicon (bp) | Tm (°C) | GC (%) |
|---|---|---|---|---|
| Hsp70 (forward) | ATCCCGTTACCCATGCTGTG | 145 | 60.11 | 55.00 |
| Hsp70 (reverse) | AGCCCATAGGCAATAGCAGC | 60.25 | 55.00 | |
| β-actin (forward) | ACTCTGCTACGTCGCTCTTG | 143 | 58.7 | 55.0 |
| β-actin (reverse) | GGAAGAGTGTCTCTGGGCAA | 58.6 | 55.0 | |
| 18S (forward) | GCTACTACCGATCGAATGGC | 161 | 57.2 | 55.0 |
| 18S (reverse) | GATCCATCTGCAGGTTCACC | 57.5 | 55.0 |
Primer design
BLAST results confirmed the identity of control and target genes (ENA Acc. No. PRJEB25057 of published sequences) and Primer BLAST led to the successfully tested primer sequences listed in Table 2. All products were confirmed by sequencing.
Hsp70 gene expression
The animals that were exposed to the acute temperature elevation (+12°C), up to 41°C within six hours, showed a highly significant up-regulation of Hsp70 (p = 0.000178) in the respiratory tree tissue, compared to the control animals kept at constant temperature (29°C) over the same time period. In contrast to that, a temperature decrease of the same magnitude (-12°C) did not affect Hsp70 expression in H. scabra (Fig 1 and Table 3). During both experiments the two reference genes, β-actin and 18S, exhibited stable homogeneityexpression.
Table 3. Hsp70 gene expression levels shown in mean calibrated normalized relative quantities (CNRQ) ± 95% confidence interval (CI), for both temperature manipulation experiments.
Significant differences are indicated for P<0.05 (*) and P<0.01 (**).
| Heat shock | |||||
| Gene | Temperature (°C) | Mean (CNRQ) | + 95% CI | - 95% CI | P-value |
| Hsp70 | 41°C | 3.24 | 7.77 | 1.35 | 2.81E-5** |
| 29°C (control) | 0.15 | 0.3 | 0.07 | ||
| Cold shock | |||||
| Hsp70 | 17°C | 1.09 | 3.04 | 0.39 | 0.75 |
| 29°C (control) | 0.91 | 2.19 | 0.38 | ||
Discussion
Definition of TSMR, TMMR and TAS
The aerobic scope (AS) is widely considered as an important measure to determine the response of marine species to future ocean warming scenarios [9, 10]. The quantification of aerobic scope requires the accurate and species-specific quantification of standard metabolic rate (SMR) and maximal metabolic rate (MMR). In this study, we modified the traditional concept of physically induced SMR, MMR and AS by defining a temperature-induced standard metabolic rate (TSMR), maximal metabolic rate (TMMR) and aerobic scope (TAS) as suitable alternative metrics for the sea cucumber Holothuria scabra (representative of a slow moving, bottom-dwelling marine invertebrate species).
During acute temperature stress, the energy demand for basal maintenance increases with warming, and decreases with progressively colder temperatures. Basal maintenance is, however, limited by a maximal- and minimal- metabolic capacity (e.g. oxygen uptake- or mitochondrial- efficiency) [38]. Hence, the aim of this study was to expose H. scabra to acute temperature change (2°C h-1; 17–41°C) until critical cold (CTcrit) and critical warm (WTcrit) temperatures caused bottom (TSMR)- and peak (TMMR)- O2 consumption rates, respectively. Benthic intertidal ecosystems exhibit a high degree of thermal heterogeneity. Driven by water level changes, differences in topography, substratum type and vegetation, drastic temperature gradients can occur over very short time periods and spatial distances [39]. H. scabra is a tropical intertidal species hence, the definition of one overarching ecological relevant temperature change rate for this sedentary invertebrate was inexpedient. Instead of attempting a precisely simulated profile of natural temperature ramping, our goal was to generate comparable thermal tolerance estimations [5, 40, 41] A temperature ramping of 2°C h-1 was widely used to investigate the thermal tolerance of tropical [5, 41] and temperate [42] intertidal invertebrates. A similar rate of temperature change (1–4°C h-1) was also applied in other key studies investigating the role of oxygen limitations on the thermal tolerance of ectotherms [43–48]. Previous studies stressed the link between the rate of temperature ramping itself on maximal thermal tolerance levels [49–51]. Consequently, the thermal limits provided in this study must be tightly linked to the utilized ramping protocol and should not serve as general temperature threshold levels for H. scabra.
H. scabra exhibited TMMR (33.2 ± 4.7 μg g-1 h-1) at a WTcrit of 38°C. TSMR (2.2 ± 1.4 μg g-1 h-1) was defined as the start of a metabolic stable state condition, which was reached at a CTcrit of 22°C. Following the definition of the traditional AS the TAS (31 μg g-1 h-1) was calculated based on the difference between the means of the proposed alternative metrics TMMR and TSMR. Above critical temperatures, animals rely progressively on unsustainable anaerobic energy supply [14]. Accordingly, H. scabra showed declining respiration above WTcrit, although the intensity of temperature stress increased, as may be indicated by the significantly elevated mRNA expression of Hsp70 at the peak temperature (41°C). Thus, at temperatures between 39–41°C we expect animal survival only over a very short time span (Fig 2). Critical cold temperature mark the point at which the temperature-induced metabolic rate matches the minimal energy requirements to sustain life functions. This basal maintenance cost is equivalent to SMR [38, 52]. In the present study, H. scabra exhibited the initiation of metabolic stable-state conditions at CTcrit (22°C). At temperatures (17–21°C) below this critical point, H. scabra presumably suffered from a fading energy balance to sustain minimal basal requirements, where any expenses for basal maintenance depend progressively on energy depots. Besides a growing energetic effort to sustain life functions below CTcrit, the molecular analysis revealed no damaging cold temperature effects in form of Hsp70 overexpression at 17°C (Figs 1 and 2).
The presented temperature dependent aerobic window, delimited by TMMR, TSMR and TAS, may provide reliable metrics for slow-moving species like H. scabra to determine energy-homeostasis, and to distinguish between ecologically and physiologically important states similar to the traditional AS [53]. A common approach is to define species-specific thermal optima as those temperatures at which the AS peaks. Whereby, SMR and MMR are physically induced at several points to quantify AS changes along a temperature gradient. In this study, however, a temperature gradient itself was used to determine TMMR and TSMR, revealing a single TAS value (31 μgO2 g-1 h-1), which is linked to the control temperature (29°C) in this study. Future studies should determine TMMR and TSMR for different acclimation temperatures, this would allow a true comparison between AS and TAS in terms of thermal optima detection. Besides the primary goal to provide an alternative to the traditional AS concept, the presented aerobic window, delimited by TMMR, TSMR and TAS, allows unique insights into temperature dependent aerobic capacity of H. scabra. Dividing the TAS by two reveals a metabolic rate (18.7 μgO2 g-1 h-1) in the exact center between TMMR and TSMR. Consequently, the temperature (31.5°C) that corresponds to this central metabolic rate indicates a point at which equal aerobic resources towards cold and warm temperature stress are available. This specific temperature may serve to define a form of thermal optimum. For some reef fish the highest aerobic scope was found at temperatures of 1–2°C above summer maxima values [6]. The defined optimal temperature of 31.5°C based on TAS, exceeds the ambient natural conditions at our study site (max. 30°C; personal communication with sea cucumber farmers) by a similar magnitude. In previous studies, the oxygen consumption rate of H. scabra, at unstressed conditions, ranged from 9–12μgO2 g-1 h-1 [54] and 11–16 μgO2 g-1 h-1 [55]. In this study we assume unstressed conditions between control temperature (29°C) and the defined thermal optimum (31.5°C), the corresponding optimal performance range of 13.85–18.7 μgO2 g-1 h-1 exceeds this literature values only slightly at the upper end.
Another difference between the traditional AS and the TAS is that in contrast to metabolic change induced by physical challenge, temperature challenge causes thermally induced adjustments of the metabolic system, but also through the effect of temperature itself. The rate of metabolic change, solely as response to temperature, is predictable through the temperature quotient Q10 (approximately a doubling or tripling of a given rate function for every 10°C of temperature increase, Q10≈2–3) [56, 57]. H. scabra was able to adjust its aerobic performance as response to acute temperature change within the range of 22–38°C. Within the linear phase of the temperature up-regulation experiment (29–38°C), H. scabra exhibited a Q10 of 2.8 (Table 1), which is within the expected range. Along the linear regression during temperature down-regulation (22–29°C), however, the Q10 value was dramatically higher (Q10 = 13.2) (Table 1). This gives a clear evidence of a strongly pronounced metabolic rate response due to cold stress. Interestingly, the sharpest decline in O2 consumption occurs below 24°C (5.1 to 2.2 μgO2 g-1 h-1), temperatures below 25°C were also defined in other studies as point at which overall activity and feeding rate declined distinctly in H. scabra [58, 59]. In long-term exposure experiments over 30 days, however, H. scabra was able to successfully acclimate to water temperatures of 33°C and 21°C, by adjusting respiration rate and metabolic enzyme activity [60]. These results show that H. scabra survived and remained active over a prolonged period at temperatures even below 22°C. This matches with the present results, which predict the optimal performance of H. scabra up to relatively high temperatures (31.5°C) and no induction of cellular stress (Hsp70) at temperatures down to 17°C. The temperature tolerance of H. scabra might be linked to life stages and specific habitat preferences. The studied animals represent intermediate sized juveniles, collected solely from the intertidal zone. This means that the relatively wide temperature tolerance exhibited by the studied H. scabra may not represent the thermal tolerance of larger animals found in the subtidal zone.
mRNA expression of Hsp70
Hsp70 is a highly conserved cellular chaperon and known as universal stress response in many taxa, ranging from bacteria via plants to mammals [61, 62]. To date, Hsp70 sequences were only available for two sea cucumber species: A. japonicas [63] and H. tubulosa [64]. In this study, we successfully targeted the full-length cDNA sequences of Hsp70, 18S and β-actin in respiratory tree tissue of H. scabra. Above 38°C Hsp70 gene expression was highly elevated in H. scabra, although whole-organism respiration decreased sharply. This provided evidence that Hsp70 is intensively involved in cellular defence mechanisms at those temperatures indicating the homeostatic disruption in H. scabra between 39–41°C. Negative effects such as reduced cell growth rates [65] and productivity [66] can be associated with enhanced Hsp70 expression. This interaction is in line with our findings, which showed a decline in respiration, while Hsp70 was highly expressed.
The temperate sea cucumber species Apostichopus japonicus showed significantly up-regulated Hsp70 levels at an acute temperature increase up to 20°C and 25°C, compared to 16°C (control) where the highest Hsp70 expression corresponded to the highest temperature [59]. For the tropical gastropod species Pomacea canaliculata, the induction temperature for Hsp70 expression was at 36°C, whereas maximal Hsp70 expression was reached at 42°C. Cold temperature on the other hand caused only slight reductions in Hsp70 expression at temperatures below 16°C [67]. This temperature range for P. canaliculata is in line with our findings for H. scabra, which revealed a highly significant up-regulation of Hsp70 after a fast temperature increase, up to 41°C, but no significant effect after a fast drop in temperature, down to 17°C. Along a similar temperature gradient (20–41°C) the freshwater clam Corbicula fluminea exhibited overexpression of Hsp70 at elevated temperature with protein synthesis starting at 38°C, whereby the heat shock response was prioritized over the oxidative stress response [68]. Similarly, the clear respiration peak of H. scabra at 38°C in this study mark a critical point at which the capacity limits of oxygen supply were reached, marking the onset of metabolic depression and resource allocation towards the heat shock response. Although the Hsp70 induction temperature was not defined in this study, the pattern of stable Hsp70 expression at lower temperatures, and the significant Hsp70 up-regulation at warm conditions, show clear similarities between P. canaliculata, C. fluminea and H. scabra. Similar expression pattern were also found for the insect Drosophila melanogaster [69], and the two fish species Danio rerio [70] and Oncorhynchuss mykiss [71]. For cold resistance, Hsp70 seems to play a minor role [72]. Acute cold-stress is not affecting secondary and tertiary protein structures to the same extent as heat-stress. Thus, cellular chaperones are not immediately required. Long-term cold acclimation, however, has shown to induce elevated Hsp70 levels, which was associated with the occurrence of chilling injuries [73]. In the present study, those H. scabra that were exposed to 17°C showed no differences in Hsp70 expression levels, compared to control animals (29°C). This indicates that a water temperature of 17°C was still above the threshold level at which cellular protection mechanisms were initiated, although aerobic performance was negative.
Conclusions
This study defined a temperature dependent aerobic window, delimited through critical cold (CTcrit) and critical warm (WTcrit) temperatures at which O2 consumption levelled (TSMR) and peaked (TMMR), respectively, for the sea cucumber Holothuria scabra. The identified metrics proved to be practical to calculate a temperature-induced aerobic scope (TAS), which may serve as suitable alternatives to the traditional aerobic scope (AS) for H. scabra and other sluggish bottom-dwelling species alike. To fully explore the potential of the presented approach, however, TMMR, TSMR and TAS need to be quantified at different ambient temperatures and during other stress exposures, to enable detailed comparisons to traditional AS analyses. Future studies should verify whether the TAS shrinks and expands in a similar pattern as AS, due to changes in baseline conditions such as acclimation temperature. In this study the acute temperature induction, to identify TMMR and TSMR, by itself revealed an extraordinary thermal tolerance of H. scabra, especially towards acute warming. These findings provide evidence that H. scabra is capable to endure extreme temperature events beyond current predictions of the IPCC [74], at least over the limited time span presented in this work.
The established primers for the targeted Hsp70 gene and the two control genes β-actin and 18S led to a reliable qPCR output. These data provide the first insights into Hsp70 differential gene expression in H. scabra at critical cold (17°C) and warm (41°C) temperatures. While Hsp70 was highly overexpressed at 41°C no increased expression was measured at 17°C. In combination with the identified TMMR and TSMR, highly elevated Hsp70 expression could be linked to a clear decline in respiration above 38°C) while no Hsp70 upregulation was observed at the respiratory base line below 22°C. The quantification of Hsp70 gene expression and definition of the aerobic benchmarks TMMR, TSMR and TAS showed promising synergies for thermal stress detection in H. scabra. Defining WTcrit as critical temperature point beyond which a surpassed mitochondrial capacity and the onset of metabolic depression causes declining oxygen uptake, the increased expression of Hsp70 indicates a transition towards induction of the cellular heat shock response (HSR). While above WTcrit the synergy of raised HSR and falling respiration may imply acute homeostatic disruption and lethal thermal stress, the combination of base budget O2 consumption and no upregulated Hsp70 expression below CTcrit the suggests unsustainable energetic expenses to sustain basal maintenance, but no yet detrimental cold stress. These interpretations rely on the assumption that Hsp70 expression in H. scabra was not impaired by cold temperature itself and that the expression level did not peak earlier, during the temperature manipulations, before the lowest and highest temperature were reached. The integration of molecular expression patterns into physiological analyses add important knowledge to enhance the understanding of energy resources allocation at the onset of aerobic depression to fuel cellular defense mechanisms.
Future studies should compare Hsp70 expression levels in H. scabra at more temperature endpoints and exposure times, utilizing our suggested primers to define the exact Hsp70 induction points for this species. Overall, this study explored a novel approach for fine-tuned thermal stress detection in H. scabra, which may help to optimize culture conditions and to predict future ocean-warming effects for this species and beyond.
Acknowledgments
The authors thank Hendra Munandar and Sigit Putro Dwiono for their permission to use the aquaculture and lab facilities at the marine science station of the Indonesian Institute of Science (LIPI), based on Lombok; as well as Asep Muhammad and Muhammad Firdaus for technical support concerning the maintenance of sea cucumber cultures and sample transportation. Moreover, we thank Sonja Peters from ZMT for her great support during the molecular analyses. This study is part of the first authors doctoral thesis entitled ‘Temperature tolerance of the sea cucumber Holothuria scabra’ [75]. The thesis represents the only medium in which the data of the current work has appeared. Moreover, the full thesis is available through open online access (https://suche.suub.uni-bremen.de/peid=B100546588).
Data Availability
ENA Acc. No. PRJEB25057 of published sequences.
Funding Statement
This study was funded through Leibniz in-house funding. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proc Biol Sci. 2011;278(1713):1823–30. Epub 2010/11/26. 10.1098/rspb.2010.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nature Climate Change. 2012;2(9):686–90. 10.1038/nclimate1539 [DOI] [Google Scholar]
- 3.Tewksbury JJ, Huey RB, Deutsch CA. Putting the heat on tropical animals. Science 2008;320(5881):1296 10.1126/science.1159328 [DOI] [PubMed] [Google Scholar]
- 4.Verberk WC, Bartolini F, Marshall DJ, Portner HO, Terblanche JS, White CR, et al. Can respiratory physiology predict thermal niches? Ann N Y Acad Sci. 2016;1365(1):73–88. Epub 2015/09/04. 10.1111/nyas.12876 . [DOI] [PubMed] [Google Scholar]
- 5.Nguyen KDT, Morley SA, Lai CH, Clark MS, Tan KS, Bates AE, et al. Upper temperature limits of tropical marine ectotherms: global warming implications. PLoS One. 2011;6(12):e29340 10.1371/journal.pone.0029340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rummer JL, Couturier CS, Stecyk JA, Gardiner NM, Kinch JP, Nilsson GE, et al. Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Global change biology. 2014;20(4):1055–66 10.1111/gcb.12455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson GE, Crawley N, Lunde IG, Munday PL. Elevated temperature reduces the respiratory scope of coral reef fishes. Global Change Biology. 2009;15(6):1405–12. [Google Scholar]
- 8.Zarco-Perello S, Pratchett M, Liao V. Temperature-growth performance curves for a coral reef fish, Acanthochromis polyacanthus. Galaxea, Journal of Coral Reef Studies. 2012;14(1):97–103. [Google Scholar]
- 9.Pörtner HO. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88(4):137–46. [DOI] [PubMed] [Google Scholar]
- 10.Pörtner HO, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. science. 2007;315(5808):95–7. 10.1126/science.1135471 [DOI] [PubMed] [Google Scholar]
- 11.Pörtner HO, Farrell AP. Physiology and climate change. Science. 2008:690–2. 10.1126/science.1163156 [DOI] [PubMed] [Google Scholar]
- 12.Fry F, Hart J. The relation of temperature to oxygen consumption in the goldfish. The Biological Bulletin. 1948;94(1):66–77. [PubMed] [Google Scholar]
- 13.Priede IG. Natural selection for energetic efficiency and the relationship between activity level and mortality. Nature. 1977;267(5612):610 [DOI] [PubMed] [Google Scholar]
- 14.Ern R, Norin T, Gamperl AK, Esbaugh AJ. Oxygen dependence of upper thermal limits in fishes. J Exp Biol. 2016;219(Pt 21):3376–83. Epub 2016/11/04. 10.1242/jeb.143495 . [DOI] [PubMed] [Google Scholar]
- 15.Sylvestre EL, Lapointe D, Dutil JD, Guderley H. Thermal sensitivity of metabolic rates and swimming performance in two latitudinally separated populations of cod, Gadus morhua L. J Comp Physiol B. 2007;177(4):447–60. Epub 2007/02/07. 10.1007/s00360-007-0143-x . [DOI] [PubMed] [Google Scholar]
- 16.Farrell A, Hinch S, Cooke S, Patterson D, Crossin GT, Lapointe M, et al. Pacific salmon in hot water: applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiological and Biochemical Zoology. 2008;81(6):697–708. 10.1086/592057 [DOI] [PubMed] [Google Scholar]
- 17.Clark TD, Jeffries KM, Hinch SG, Farrell AP. Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. J Exp Bio. 2011;214(18):3074–81. [DOI] [PubMed] [Google Scholar]
- 18.Johansen J, Jones G. Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Global Change Biology. 2011;17(9):2971–9. [Google Scholar]
- 19.Hochochka P, Somero GN. Biochemical adaptation. 2nd ed Oxford University Press, Oxford; 2002. [Google Scholar]
- 20.Dahlhoff EP. Biochemical indicators of stress and metabolism: applications for marine ecological studies. Annu Rev Physiol. 2004;66:183–207. 10.1146/annurev.physiol.66.032102.114509 [DOI] [PubMed] [Google Scholar]
- 21.Murthy VS, Ravishankar KV. Molecular Mechanisms of Heat Shock Proteins and Thermotolerance in Plants In: Rao NKS, Shivashankara KS, Laxman RH, editors. Abiotic Stress Physiology of Horticultural Crops. New Delhi: Springer India; 2016. p. 71–83. [Google Scholar]
- 22.Jolly C, Morimoto RI. Role of the Heat Shock Response and Molecular Chaperones in Oncogenesis and Cell Death. JNCI: Journal of the National Cancer Institute. 2000;92(19):1564–72. 10.1093/jnci/92.19.1564 [DOI] [PubMed] [Google Scholar]
- 23.Wolkenhauer SM, Uthicke S, Burridge C, Skewes T, Pitcher R. The ecological role of Holothuria scabra (Echinodermata: Holothuroidea) within subtropical seagrass beds. Journal of the Marine Biological Association of the United Kingdom. 2009;90(02). 10.1017/s0025315409990518 [DOI] [Google Scholar]
- 24.Hamel JF, Conand C, Pawson DL, Mercier A. The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): its biology and exploitation as beche-de-mer. Advances in Marine Biology. 2001;41:131–201. [Google Scholar]
- 25.Lovatelli A, Conand C. Advances in sea cucumber aquaculture and management: Food & Agriculture Org; 2003. [Google Scholar]
- 26.Purcell SW, Polidoro BA, Hamel JF, Gamboa RU, Mercier A. The cost of being valuable: predictors of extinction risk in marine invertebrates exploited as luxury seafood. Proc R Soc B. 2014;281(1781):20133296 10.1098/rspb.2013.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klanian MG. Physiological and immunological conditions of the sea cucumber Isostichopus badionotus (Selenka, 1867) during dormancy. Journal of experimental marine biology and ecology. 2013;444:31–7. [Google Scholar]
- 28.Kozera B, Rapacz M. Reference genes in real-time PCR. Journal of applied genetics. 2013;54(4):391–406. 10.1007/s13353-013-0173-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark MS, Fraser KPP, Peck LS. Lack of an HSP70 heat shock response in two Antarctic marine invertebrates. Polar Biology. 2008;31(9):1059–65. 10.1007/s00300-008-0447-7 [DOI] [Google Scholar]
- 30.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT Nucleic acids symposium series; 1999: [London: ]: Information Retrieval Ltd, c1979–c2000. [Google Scholar]
- 31.Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. 2000. [DOI] [PubMed] [Google Scholar]
- 32.Schramm G, Bruchhaus I, Roeder T. A simple and reliable 5′-RACE approach. Nucleic acids research. 2000;28(22):e96–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Astchul S, Gish W, Miller W, Myers E, Lipman D. Basic local alignment tool. J Mol Biol. 1990;215:403–10. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome research. 1999;9(9):868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13(1):134 10.1186/1471-2105-13-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owczarzy R, Tataurov AV, Wu Y, Manthey JA, McQuisten KA, Almabrazi HG, et al. IDT SciTools: a suite for analysis and design of nucleic acid oligomers. Nucleic acids research. 2008;36(suppl_2):W163–W9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology. 2002;3(7):research0034 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guderley H, Pörtner HO. Metabolic power budgeting and adaptive strategies in zoology: examples from scallops and fish. Canadian Journal of Zoology. 2010;88(8):753–63. [Google Scholar]
- 39.Dong YW, Li X, Choi FMP, Williams GA, Somero GN, Helmuth B. Untangling the roles of microclimate, behaviour and physiological polymorphism in governing vulnerability of intertidal snails to heat stress. Proc. R. Soc. Lond. B. Biol. Sci. 2016;284:20162367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overgaard J, Kristensen TN, Sørensen JG. Validity of thermal ramping assays used to assess thermal tolerance in Arthropods. PLoS ONE. 2012;(7)e32758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giomi F, Mandaglio C, Ganmanee M, Han GD, Dong YV, Williams GA, Sarà G. The importance of thermal history: costs and benefits of heat exposure in a tropical, rocky shore oyster. J. Exp. Biol. 2016;219:686–694. 10.1242/jeb.128892 [DOI] [PubMed] [Google Scholar]
- 42.Leung JYS, Conell SD, Russel BD. Heatwaves diminish the survival of a subtidal gastropod through reduction in energy budget and depletion of energy reserves. Scientific Reports. 2017;(7)17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, et al. Differences in thermal tolerance among sockeye salmon populations. Science. 2011;332, 109–112. 10.1126/science.1199158 [DOI] [PubMed] [Google Scholar]
- 44.Frederich M, Pörtner HO. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279, R1531–R1538. 10.1152/ajpregu.2000.279.5.R1531 [DOI] [PubMed] [Google Scholar]
- 45.Giomi F, Pörtner HO. A role for haemolymph oxygen capacity in heat tolerance of eurythermal crabs. Front. Physiol. 2013;4, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giomi F, Fusi M, Barausse A, Mostert B, Pörtner HO, Cannicci S. Improved heat tolerance in air drives the recurrent evolution of airbreathing. Proc. R. Soc. Lond. B Biol. Sci. 2014;281, 20132927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melzner F, Bock C, Pörtner HO. Temperature-dependent oxygen extraction from the ventilatory current and the costs of ventilation in the cephalopod Sepia officinalis. J. Comp. Physiol. 2006;B 176, 607–621. [DOI] [PubMed] [Google Scholar]
- 48.Wittmann AC, Schröer M, Bock C, Steeger HU, Paul RJ, Pörtner HO. Indicators of oxygen- and capacity-limited thermal tolerance in the lugworm Arenicola marina. Clim. Res. 2008;37, 227–240. [Google Scholar]
- 49.Mora C, Maya MF. Effect of the rate of temperature increase of the dynamic method on the heat tolerance of fishes. J.Therm.Biol. 2006;(31)337–341. [Google Scholar]
- 50.Terblanche JS, Deere JA, Clusella-Trullas S, Janion C, Chown SL. Critical thermal limits depend on methodological context. Proc. Roy. Soc. Lond. 2007;B 274, 2935–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinagre C, Leal I, Mendonça V, Augusto FAV. Effect of warming rate on the critical thermal maxima of crabs, shrimp and fish. J.Therm.Biol. 2015;(47)19–25. [DOI] [PubMed] [Google Scholar]
- 52.Kooijman SALM. Dynamic energy and mass budgets in biological systems: Cambridge university press; 2000. [Google Scholar]
- 53.Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Marine environmental research. 2012;79:1–15. 10.1016/j.marenvres.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 54.Collard M, Eeckhaut I, Dehairs F, Dubois P. Acid–base physiology response to ocean acidification of two ecologically and economically important holothuroids from contrasting habitats, Holothuria scabra and Holothuria parva. Environmental Science and Pollution Research. 2014;21(23):13602–14. 10.1007/s11356-014-3259-z [DOI] [PubMed] [Google Scholar]
- 55.Kühnhold H, Kamyab E, Novais S, Indriana L, Kunzmann A, Slater M, et al. Thermal stress effects on energy resource allocation and oxygen consumption rate in the juvenile sea cucumber, Holothuria scabra (Jaeger, 1833). Aquaculture. 2017;467:109–17. [Google Scholar]
- 56.Schmidt-Nielsen K. Animal physiology: adaptation and environment: Cambridge University Press; 1997. [Google Scholar]
- 57.Clarke A, Johnston NM. Scaling of metabolic rate with body mass and temperature in teleost fish. Journal of animal ecology. 1999;68(5):893–905. [Google Scholar]
- 58.Purcell SW, Blockmans BF, Agudo NN. Transportation methods for restocking of juvenile sea cucumber, Holothuria scabra. Aquaculture. 2006;251(2–4):238–44. [Google Scholar]
- 59.Wolkenhauer SM. Burying and feeding activity of adult Holothuria scabra (Echinodermata: Holothuroidea) in a controlled environment. SPC Bêche-de-mer Information Bulletin. 2008;27:25–8. [Google Scholar]
- 60.Beere HM, Green DR. Stress management–heat shock protein-70 and the regulation of apoptosis. Trends in cell biology. 2001;11(1):6–10. [DOI] [PubMed] [Google Scholar]
- 61.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2(3):185–94. Epub 2002/03/27. 10.1038/nri749 . [DOI] [PubMed] [Google Scholar]
- 62.Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecology Letters. 2003;6(11):1025–37. [Google Scholar]
- 63.Shao Y, Li C, Chen X, Zhang P, Li Y, Li T, et al. Metabolomic responses of sea cucumber Apostichopus japonicus to thermal stresses. Aquaculture. 2015;435:390–7. [Google Scholar]
- 64.Vazzana M, Siragusa T, Arizza V, Buscaino G, Celi M. Cellular responses and HSP70 expression during wound healing in Holothuria tubulosa (Gmelin, 1788). Fish Shellfish Immunol. 2015;42(2):306–15. Epub 2014/12/03. 10.1016/j.fsi.2014.11.010 . [DOI] [PubMed] [Google Scholar]
- 65.Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes & development. 1992;6(8):1402–13. [DOI] [PubMed] [Google Scholar]
- 66.Krebs RA, Loeschcke V. Effects of exposure to short‐term heat stress on fitness components in Drosophila melanogaster. Journal of Evolutionary Biology. 1994;7(1):39–49. [Google Scholar]
- 67.Song H-M, Mu X-D, Gu D-E, Luo D, Yang Y-X, Xu M, et al. Molecular characteristics of the HSP70 gene and its differential expression in female and male golden apple snails (Pomacea canaliculata) under temperature stimulation. Cell Stress and Chaperones. 2014;19(4):579–89. 10.1007/s12192-013-0485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falfushynska HI, Phan T, Sokolova IM. Long-Term Acclimation to Different Thermal Regimes Affects Molecular Responses to Heat Stress in a Freshwater Clam Corbicula fluminea. Sci Rep. 2016;6:39476 Epub 2016/12/21. 10.1038/srep39476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sejerkilde M, Sørensen JG, Loeschcke V. Effects of cold-and heat hardening on thermal resistance in Drosophila melanogaster. Journal of Insect Physiology. 2003;49(8):719–26. [DOI] [PubMed] [Google Scholar]
- 70.Airaksinen S, Jokilehto T, Råbergh CM, Nikinmaa M. Heat-and cold-inducible regulation of HSP70 expression in zebrafish ZF4 cells. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2003;136(2):275–82. [DOI] [PubMed] [Google Scholar]
- 71.Currie S, Moyes C, Tufts B. The effects of heat shock and acclimation temperature on hsp70 and hsp30 mRNA expression in rainbow trout: in vivo and in vitro comparisons. Journal of Fish Biology. 2000;56(2):398–408. [Google Scholar]
- 72.Hoffmann AA, Sørensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. Journal of Thermal Biology. 2003;28(3):175–216. [Google Scholar]
- 73.Fujikake N, Nagai Y, Popiel HA, Kano H, Yamaguchi M, Toda T. Alternative splicing regulates the transcriptional activity of Drosophila heat shock transcription factor in response to heat/cold stress. FEBS letters. 2005;579(17):3842–8. 10.1016/j.febslet.2005.05.074 [DOI] [PubMed] [Google Scholar]
- 74.Change IPoC. Climate change 2014: Mitigation of climate change: Cambridge University Press; 2015. [Google Scholar]
- 75.Kühnhold H. Temperature Toleranceof the Sea Cucumber Holothuria Scabra: Towards a Systematic Understanding of Multi-level Temperature Effects: Universität Bremen; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
ENA Acc. No. PRJEB25057 of published sequences.