Abstract
Probiotics are microorganisms that provide health benefits when consumed. In animals, probiotics reverse gut microbiome-related alterations in depression-like symptoms, in cognition, and in hormonal stress response. However, in humans, a causal understanding of the gut-brain link in emotion and cognition is lacking. Additionally, whether the effects of probiotics on neurocognition are visible only in presence of stress, remains unclear. We investigated the effects of a multispecies probiotic (Ecologic®Barrier) on specific neurocognitive measures of emotion reactivity, emotion regulation, and cognitive control using fMRI. Critically, we also tested whether probiotics can buffer against the detrimental effects of acute stress on working memory. In a double blind, randomized, placebo-controlled, between-subjects intervention study, 58 healthy participants were tested once before and once after a 28-day intervention.
Without stress induction, probiotics did not affect brain, behavioral, or related self-report measures. However, relative to placebo, the probiotics group did show a significant stress-related increase in working memory performance after supplementation. This change was associated with intervention-related neural changes in frontal cortex during cognitive control exclusively in the probiotics group. Overall, our results show neurocognitive effects of a multispecies probiotic in healthy women only under challenging situations, buffering against the detrimental effects of stress on cognition.
Keywords: Probiotic, Neuroimaging, Cognitive control, Emotion, Stress, Working memory
Highlights
-
•
We ran a randomized placebo-controlled fMRI study with a multispecies probiotic.
-
•
Probiotics did not affect neurocognitive measures of emotion and cognitive control.
-
•
Probiotics did affect stress-related working memory and neural correlates.
-
•
Probiotics in healthy individuals can support cognition under stress.
1. Introduction
Probiotics are defined as bacteria providing health benefits to the host when consumed in adequate amounts (Hill et al., 2014). In the last few decades, an increasing number of animal studies have indicated a role of probiotics in regulating mood, cognition, and response to stress, via the bi-directional link between the brain and the gut microbiome (Cryan and Dinan, 2012). For instance, by means of probiotics it was possible to reduce anxiety-like behavior and to normalize brain-derived neurotropic factor (BDNF) in the hippocampus of mice with infectious colitis (Bercik et al., 2011), and to reverse the abnormal stress responses in germ-free mice (without a gut microbiome) (Sudo et al., 2004). Other studies showed that probiotics are able to lower levels of systemic inflammatory cytokines (McCarthy et al., 2003) and to regulate central GABA receptor expression in mice (Bravo et al., 2011). Additionally, probiotics could normalize the immune response, as well as noradrenaline concentration in the brainstem of rats after maternal separation (Desbonnet et al., 2010).
1.1. Effects of probiotics on neurocognitive mechanisms
In humans, six weeks of probiotic supplementation in patients with intestinal disorders resulted in a decrease of depressive complaints associated with the intestinal disease, which was related to decreased brain limbic reactivity to negative emotional stimuli (Pinto-Sanchez et al., 2017). In healthy humans, four weeks of fermented milk product supplementation was associated with decreased functional magnetic resonance imaging (fMRI) responses in affective, viscerosensory, and somatosensory brain regions during emotional face matching (Tillisch et al., 2013). However, these latter results should be taken carefully due to a number of limitations, i.e. group sizes (ranging between 10 and 12 subjects) and probiotics’ effects versus the no-intervention group instead of versus the placebo group. Nonetheless, existing evidence seems to suggest effects of probiotics on neural emotion reactivity in humans. However, emotion reactivity is only one of different affective appraisal processes, which also consist of emotion-specific regulation and more generic cognitive control components (Kohn et al., 2014; Etkin et al., 2015). Thus, although effects of probiotics have been observed only on neural emotion reactivity, it is possible that this is related to regulation of emotion or to higher order cognitive control processes.
Therefore, our first aim was to investigate whether emotion reactivity is specifically affected by probiotics, whether these effects occur through control of emotion (i.e. regulating automatic biases, see Etkin et al., 2006; Etkin et al., 2015), or can be seen independent of emotion (O'Hagan et al., 2017), i.e. affecting cognitive control more generally. We investigated the effects of a multispecies probiotics (Ecologic®Barrier) (Van Hemert, 2014), in a randomized, double-blind, placebo-controlled between-subjects design. This formulation has been tested before in both animal and human studies. Specifically, previous rat experiments showed effects of this formulation on depressive-like behavior and on the transcript level of factors involved in HPA axis regulation (e.g. Abildgaard et al., 2017a; Abildgaard et al., 2017b). In a previous human study in n = 40 healthy participants, 4-weeks supplementation with this product was associated with a reduction in self-reported cognitive reactivity to sad mood versus placebo (Steenbergen et al., 2015). Currently, we studied the effects of probiotics on neural correlates underlying emotion reactivity, its regulation, and general cognitive control, by using three robust cognitive paradigms during fMRI: the emotional face-matching task (Hariri et al., 2000), known to activate the limbic network, including the amygdala, involved in emotion reactivity; the emotional face-word Stroop task (Etkin et al., 2006), known to activate regions in ventral and dorsal medial frontal cortex involved in emotion regulation; and the color-word Stroop task (Stroop, 1953), known to activate frontal cortex regions involved in cognitive control (Cieslik et al., 2015).
1.2. Effects of probiotics on cognition: the role of stress
Animal studies have shown how the gut-brain axis is crucial for stress regulation, by influencing the development of the hypothalamic-pituitary-adrenal (HPA) axis, which – in turn – is related to mood, emotion, and BDNF expression important for learning and memory (Sudo et al., 2004; Li et al., 2009; Gareau et al., 2011; Frohlich et al., 2016). The effects of probiotics and stress on cognition might share common pathways of action (e.g. the HPA axis (Arnsten, 2015; Sarkar et al., 2016)), however, it is unclear whether probiotics might affect cognitive performance independent or dependent of the detrimental effects of stress. Beneficial effects of probiotics under stress conditions have been clearly demonstrated in animal studies (e.g. Gareau et al., 2011; Messaoudi et al., 2011; Ait-Belgnaoui et al., 2014; Cowan et al., 2016), but probiotics’ effects on cognition and stress resilience in humans are scarce and sometimes contradictory (Allen et al., 2016; Kelly et al., 2017).
Therefore, as secondary aim, we took into account the possibility that potential probiotics effects on cognition could exist only as a consequence of an increased buffer against stress. The probiotic product under investigation, Ecologic®Barrier, is developed to strengthen epithelial barrier function and to decrease intestinal permeability for the endotoxin lipopolysaccharide (LPS), as demonstrated in vitro (Van Hemert 2014). Human studies showed that acute-stress paradigms increase intestinal permeability to LPS (Alonso et al., 2012; Vanuytsel et al., 2014), and detrimentally affect memory performance (Schoofs et al., 2009). For example, the socially evaluated cold pressor test (SECPT) (Lovallo, 1975) specifically influenced backwards digit span (DS) performance, which involves control functions to operate on the stored material instead of just working memory maintenance (Schoofs et al., 2009). For this reason, we investigated whether the use of probiotics can modulate backwards DS performance before versus after acute stress induced by the SECPT, together with stress-related changes in hormones (cortisol and alpha-amylase) and cardiovascular activity. As the type of cognition we investigated after and before stress – i.e. backwards digit span - requires cognitive control (Kane and Engle, 2003), we also investigated how intervention-induced effects on this stress-related working memory performance related to the effects of the intervention on cognitive control responses in the frontal cortex.
2. Materials and methods
2.1. Participants
In total, fifty-eight of the 61 pre- and post-intervention scanned participants were included in the analyses, divided into a probiotics intervention group (n = 29, mean age = 21 years, SEM = 0.4) and a placebo group (n = 29, mean age = 22 years, SEM = 0.5). Three participants were excluded from the final analyses, one due to high depression levels (above BDI cut off for moderate depression, i.e. BDI score: 23), and two given poor fMRI-task performance (Supplementary Materials). All participants were right handed, healthy female volunteers aged between 18 and 40 years old, using (oral or intra-uterine) hormonal contraceptives, with a healthy weight, i.e. a body mass index (BMI) between 18 and 25 (placebo group: mean BMI = 21.66 kg/m2, SEM = 0.31, and probiotics group: BMI = 21.91 kg/m2, SEM = 0.29). They were not in the ‘stop week’ of oral contraceptives during test sessions to ensure similar hormone levels between both sessions across participants. Exclusion criteria included: 1) personal history of psychiatric, neurological, gastrointestinal, endocrine disorders, and relevant medical history (self-reported); 2) regular medication use; 3) pre- and probiotic supplementation; 4) smoking; 5) use of antibiotics within two months before the start of the study. We also excluded those participants with lactose intolerance, those following a vegan diet, and those with high alcohol intake (i.e. more than 10 glasses of any alcoholic drink per week). Participants who changed their diet within three months of the first testing session were also excluded. Furthermore, participants were screened for MRI compatibility. The study was conducted following the Declaration of Helsinki with human subjects and the complete procedure was approved by the local Ethics Committee (CMO Arnhem-Nijmegen, NL55406.091.15) and registered at the Dutch trial register (protocol number: NTR5845). Written informed consent was obtained from each participant.
2.2. Intervention
Probiotics (Ecologic®Barrier) and placebo were consumed in powder form for 28 days in a row, 2 g once daily at a fixed time point, on an empty stomach by diluting the powder in water or milk (see Supplementary Materials for bacterial strains). Participants were asked not to eat for the subsequent 15–20 min after the ingestion of the drink.
All participants were randomly assigned to the two groups. The randomization scheme was computer generated by Winclove using permuted blocks with block size equal to 4. It was impossible for research personnel involved with participants to adjust randomization or discern what product participants were receiving, ensuring true allocation concealment.
2.3. Procedure
2.3.1. General procedure
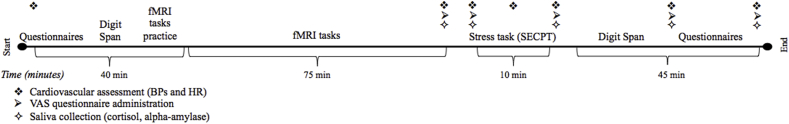
A longitudinal double-blind randomized design was used to compare the effects of probiotics with placebo. Each participant was assessed twice: before the start of the treatment and four weeks later. Between the test sessions, a 28 days intervention consisting of probiotics or placebo intake was implemented. Both test sessions were conducted at the Donders Centre for Cognitive Neuroimaging in Nijmegen, The Netherlands. Testing was conducted exclusively in the afternoon. At the beginning of the first test session, the experimental procedures were explained and the principal researcher assessed physical measurements, including height, weight, blood pressure, and heart rate (Fig. 1). Next, participants practiced all the fMRI tasks outside the scanner, and performed a working memory test (backward and forward digit span test). Subsequently, the participants took part in the fMRI experiment (75 min), with three cognitive paradigms described below. After some time to relax after the MRI measurement (i.e. 10–15 min), another trained researcher (unfamiliar to the participant) conducted a stress task followed by the same working memory test (with different items) as performed before scanning (conducted by the principal researcher again). Saliva and cardiovascular parameters were collected over the testing sessions (see Fig. 1, and Supplementary Materials). At the end of the first session day, participants were provided with the probiotics/placebo (in identical sachets, blind to both participants and researchers) and were informed about how to consume them. Compliance was encouraged with regular reminders (i.e. before, at the beginning, during and at the end of the supplementation period) and a personal diary (i.e. calendar to keep track of the correct and regular daily consumption of the product). The same testing procedure was repeated during the second session day (which took place at the same time as the first session), after the 28-day intervention period (average number of days between first test day and start of supplementation (SD): 8.5 (5.5); average number of days between end of supplementation and second test day (SD): 1.5 (0.7)). The second testing day ended with the written question to the participants on whether they thought they had taken the placebo or the probiotic, on which they answered by chance (number of correct answers: 15/29 (51,7%) for the placebo group and 12/29 (41.4%) for the probiotics group).
Fig. 1.
Overview of the testing sessions.
Each participant was tested twice, before and after 4 weeks of supplementation with probiotics/placebo. The procedure of the two sessions was the same (i.e. participants performed the same tests in the same order). SECPT: socially evaluated cold pressor test; BP: blood pressure; HR: heart rate; VAS: visual analog scale.
2.3.2. Effects of probiotics on neurocognitive mechanisms
2.3.2.1. Questionnaires
Questionnaires were administered using an Electronic Data Capture (EDC, https://castoredc.com) application for online data collection. Specifically, we assessed depression with the Dutch version of the self-reported Beck Depression Inventory questionnaire (BDI) (Beck, 1976). Depression sensitivity was evaluated with the Leiden Index of Depression Sensitivity-revised questionnaire (LEIDS-r) (Antypa et al., 2010) (Supplementary Materials), on which the effects of this specific probiotic product have been reported previously (Steenbergen et al., 2015). Other questionnaires were assessed to control for changes in diet and baseline differences in psychological traits, but these were not observed (Supplementary Materials).
2.3.2.2. Task paradigms
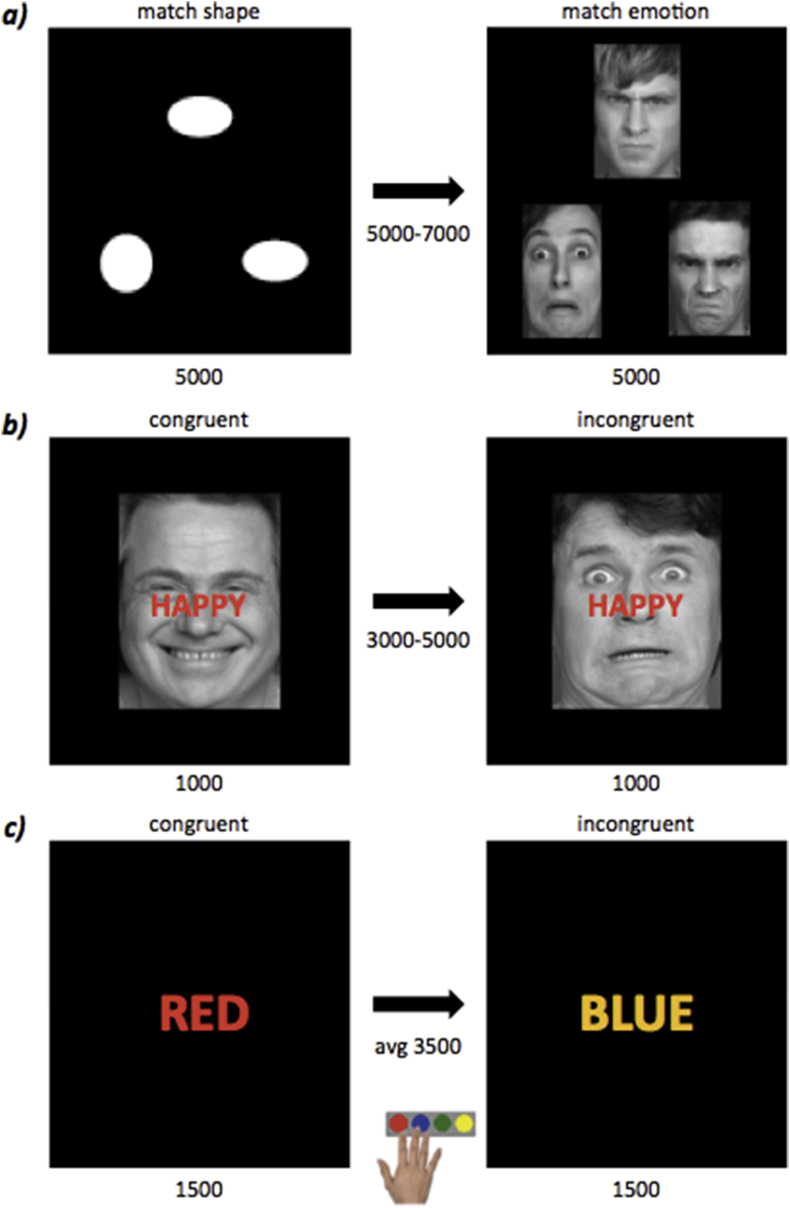
The fMRI tasks (Fig. 2) included an emotional face-matching paradigm, an emotional face-word Stroop paradigm, and the classic color-word Stroop paradigm. Participants were instructed to react as fast and accurately as possible during all three tasks. The experiments were programmed in Presentation® software (Version 0.70, www.neurobs.com).
Fig. 2.
The fMRI paradigms.a) Emotional face-matching task, b) Emotional face-word Stroop task, and c) Color-word Stroop task. The control and the experimental conditions of each paradigm are represented on the left and right side of the panel respectively. The duration of each stimulus and the relative inter-trial interval are indicated in ms. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.3.2.2.1. Emotional face-matching paradigm
This paradigm (Fig. 2a) was chosen to investigate intervention-induced changes in emotion reactivity. Stimuli were presented in a block design, with a total of 18 blocks consisting of three stimuli each. The task included a control and an emotion condition. In the control condition participants had to match one of two geometric shapes presented at the bottom, to a target shape presented at the top of the screen. The experimental condition involved participants choosing one of two emotional (angry or fearful) faces presented at the bottom of the screen that best matched the emotional expression of a face seen at the top of the screen. The condition was kept constant over the block duration of 17 s but was randomized between blocks. The total duration of the task amounted to 7 min.
2.3.2.2.2. Emotional face-word stroop paradigm
A Dutch version of the emotional face-word Stroop task was used to assess intervention-induced differences in the ‘resolution of emotional conflicts’ in the face of emotional distracters (Fig. 2b). During this task, participants were presented with pictures of male faces expressing fear or happiness. On top of the faces, the Dutch words for happy (i.e. “blij”) and fearful (i.e. “bang”) were presented in prominent red letters. The emotions described by the words were either congruent with the emotion of the face or incongruent, and participants had to indicate the emotion of the face by ignoring the emotion word. A total of 148 stimuli of happy or fearful faces were presented. The order of stimulus presentation was pseudo-randomized and the total duration of the task added up to 15 min.
2.3.2.2.3. Classic color-word stroop paradigm
A Dutch version of the classic color-word Stroop task was used to assess intervention-induced differences in general cognitive control (i.e. resolving response conflict) in the absence of emotional stimuli (Fig. 2c). During this task, participants were presented with four different color words written either in the same ink color as the word (e.g. red written in red ink) or in an incongruent color (e.g. red written in blue ink). They were asked to indicate the ink color of the word by pressing a button mapped to that color, and to ignore the word meaning. The task consisted of 80 stimulus presentations in total. Color-button mappings were randomized across subjects but kept constant between the two sessions of each participant (for both Stroop tasks). The duration of the task amounted to 10 min.
2.3.3. Effects of probiotics on cognition: the role of stress
For stress induction, we used the Socially Evaluated Cold Pressor Test (Lovallo, 1975). During this test, physical and psychological stress was induced. Physical stress was induced by having participants immerse their hand into ice water (ranging between 0 and 3 °C) for “as long as possible, until the researcher indicates to pull the hand out of the water”. Unknown to the participants, the maximal duration was set at 3 min (180 s). The mean duration of ice water immersion was 160.8 s (SD: 45.6) for the first session, and 171 s (SD: 32.4) for the second one. The test was conducted by a researcher who was yet unknown to the participant, and who adopted neutral and socially distant behavior to increase psychological stress. Further psychological stress was induced by asking participants to look into a video camera during the cold-water test, with the aim to record their facial expressions. Saliva samples were collected to evaluate cortisol and alpha-amylase levels in response to the stressor. A total of five saliva samples from each participant was obtained: one sample was obtained 10 min before the start of the SECPT, one sample right before and one sample immediately after the end of the SECPT, one sample 25 min and one sample 45 min after the end of the ice water immersion (see Fig. 1). Parameters reflecting autonomic nervous system activation, i.e. systolic and diastolic blood pressure and heart rate (HR) were registered at the beginning of the test, as well as every time that the saliva samples were collected (plus one extra measurement between the collection of the second and third saliva sample). The total score of the visual analog scales (VAS) was used to assess the subjective feeling of stress and obtained by the sum of each sub-scale of the VAS: tension, happiness (reversed scored), pain, fear, irritation, and stress. The VAS questionnaires were completed each time during saliva collection.
To evaluate stress-related effects in cognitive functioning, we used the digit span test to assess working memory performance, at the beginning of the experiment and right after the stress induction (within a range of 5 min from the end of the stress task). Specifically, during the digit span test participants listened to a series of numbers and tried to repeat each series correctly (DS forward) or repeat it backwards (DS backward). Following a correct response, the participants had to repeat increasingly longer sequences. Participants performed different versions of the test before and after stress on the first and second test day.
2.4. Data analyses
2.4.1. Effects of probiotics on neurocognitive mechanisms
2.4.1.1. Questionnaire analysis
Statistical analyses of the data were performed using IBM SPSS statistics (version 23.0), and the results were expressed in terms of mean values and standard errors of the mean (SEM). The effects of the intervention on questionnaire scores were analyzed by performing 2 × 2 repeated-measure ANOVAs for data that was normally distributed. The first factor was ‘Group’ (between-subjects), with two levels (placebo and probiotics), the second factor was ‘Session’ (within-subjects), with two levels (pre- and post-intervention session). For data that was not normally distributed, we used the Wilcoxon-Mann-Whitney test to assess group differences on the post-pre scores.
2.4.1.2. Behavioral analysis of fMRI tasks
We evaluated behavioral performance during the fMRI tasks before and after the intervention between the two groups, by analyzing the correct reaction times (RTs). The analyses were done on log-transformed data to reduce the skewness of the distributions, which were better normalized after this correction. We ran a 2 × 2 × 2 repeated-measure ANOVA design, with the factors Group (between-subjects), Session (within-subjects), and Condition (experimental vs. control condition, within-subjects).
2.4.1.3. fMRI acquisition and analyses
2.4.1.3.1. MR data acquisition
MR data were acquired using a 3T MAGNETOM Prisma system, equipped with a 32-channel head coil. During the three tasks, 3D echo planar imaging (EPI) scans using a T2*weighted gradient echo multi-echo sequence (Poser et al., 2006) were acquired (voxel size 3.5 × 3.5 × 3 mm isotropic, TR = 2070 ms, TE = 9 ms; 19.25 ms; 29.5 ms; 39.75 ms, FoV = 224 mm). The slab positioning and rotation (average angle of 14° to AC axis) optimally covered both prefrontal and deep brain regions (i.e. including affective brain regions like the amygdala). A whole-brain high-resolution T1-weighted anatomical scan was acquired using a MPRAGE sequence (voxel size 1.0 × 1.0 x 1.0 isotropic, TR = 2300 ms, TE = 3.03 ms, 192 slices).
2.4.1.3.2. MRI data preprocessing
Data was preprocessed and analyzed using Statistical Parametric Mapping (SPM8) (Wellcome Department of Imaging Neuroscience, London). Preprocessing steps included multi-echo combination, slice timing correction, coregistration of functional and anatomical images, spatial normalization based on unified segmentation parameters, and spatial smoothing (8 mm FWHM) (Supplementary Materials).
2.4.1.3.3. fMRI analyses
Fixed effects analyses of the fMRI tasks are described in the Supplementary Materials.
On the second (group) level, we first performed random effect analyses of variance (ANOVA) in a full-factorial design to obtain the main task effects (positive and negative) for each task across sessions. The ANOVA analyses were run with the contrast images specified in the first level analyses and two additional factors: Group (probiotics and placebo) as a between subject-factor and Session (pre- and post-intervention session) as a within-subject factor. Subsequently, we ran two-samples t-test analyses between the probiotics and the placebo group using the contrast images of Condition x Session specified at the first level. We considered results significant if p < 0.05 Family-Wise-Error (FWE) whole-brain corrected at cluster level (with a cluster defining threshold of p < 0.001).
2.4.2. Effects of probiotics on cognition: the role of stress
Using a 2 × 2 × 2 repeated-measure ANOVA, we analyzed the stress-related cardiovascular data, i.e. systolic (BPsys) and diastolic blood pressure (BPdia), as well as heart rate (HR); hormones, i.e. alpha-amylase and cortisol; and VAS scores (subjective stress-related feeling). All the analyses were done on log-transformed data. The first factor was Group, with two levels (placebo and probiotics, between-subjects), the second factor was Session, with two levels (pre- and post-intervention session, within-subjects), and the third factor was Time (within-subjects). For the cardiovascular data, the third factor Time consisted of seven levels (one level for each saliva collection time point (see above) plus two extra measurements: one at the beginning of the experiment, and one right before the immersion of the participant's hand in the cold water box), while for the alpha-amylase, cortisol, and VAS scores the factor Time consisted of five levels (one level for each saliva collection time point; see Fig. 1). Effects of Time indicated an effect of the stressor, whereas Group*Session interactions indicated effects of probiotic supplementation.
A 2 × 2 × 2 repeated measure ANOVA was also chosen to analyze probiotics-induced effects (Group*Session interactions) on DS backwards after versus before stress induction (within-subjects factor: Time). Analyses were performed on the raw scores given that the DS scores were normally distributed.
In the last step of the analysis, we investigated whether individual differences in probiotics effects on working memory were associated with probiotics effects on brain functioning during cognitive control (i.e. incongruent versus congruent Stroop trials). For this, we extracted averaged beta weights (MarsBar toolbox of SPM, Brett, 2002) from the significantly (pFWE<0.05) activated regions in frontal cortex during the Stroop task (Fig. 3f), independent of the intervention. To extract the average beta values, we applied a sphere of 10 mm on the local maxima of each (sub-)cluster in frontal cortex, to prevent averaging from large clusters extending across different regions. Subsequently, we calculated post-minus pre-intervention scores of the average beta weights and correlated these to the intervention effect (post-minus pre-intervention) in the stress-induced working memory (i.e. DS backward) performance scores. We performed these ROI analyses per group, such that we could compare the correlations of the probiotics group to that of the placebo group using Fisher's r to z transformation.
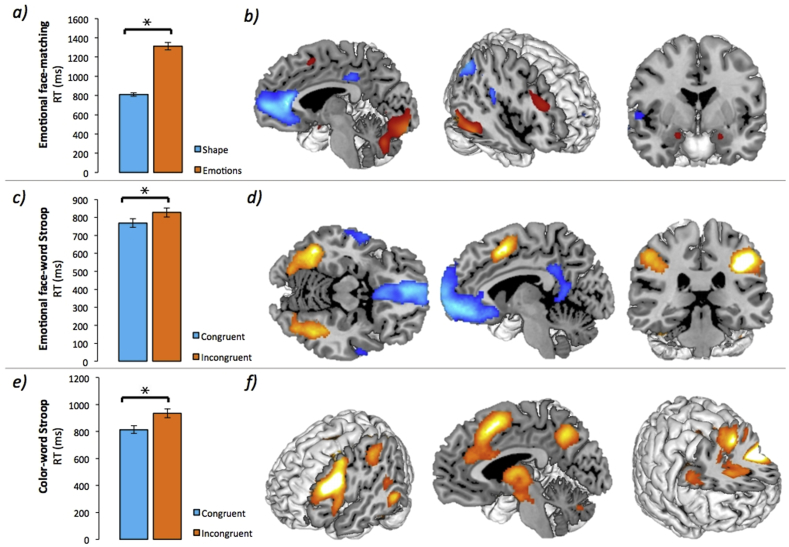
Fig. 3.
Main task effects.
Significant main task effects independent of the intervention (i.e. across groups and sessions) in behavioral data (on the left; * = p < 0.001) and fMRI data (on the right; thresholded at pFWE<0.05 [cluster defining threshold: p < 0.001]) for a) the emotional face-matching paradigm in RTs and b) in brain (hot colors: emotion > shape; cold colors: shape > emotion); c) the emotional face-word Stroop paradigm in RTs and d) in brain (hot colors: incongruent > congruent; cold colors: congruent > incongruent); e) the color-word Stroop paradigm in RTs and f) in brain (hot colors: incongruent > congruent). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
There were no outliers according to the Grubbs' test for outliers. We considered results with p < 0.05 significant or, with multiple comparisons with the same outcome (i.e. correlation of intervention effects on stress-related working memory in multiple frontal Stroop ROIs), p < 0.05/number of comparisons (Bonferroni correction). We report partial eta squared as a measure of effect size.
3. Results
3.1. Effects of probiotics on neurocognitive mechanisms
3.1.1. Questionnaires
We did not observe effects of probiotics on any of the questionnaires (Table S1, Supplementary Materials). We did not find differences between the two groups at baseline either, i.e. pre-intervention. Neither did the groups differ in terms of diet (type, style, or short FFQ) at baseline, and pre-versus post-treatment (all p > 0.1) (Table S2, Supplementary Materials).
3.1.2. RT results
First, we assessed whether the task behavior independent of the intervention was as expected. Indeed, participants were significantly slower in each experimental condition of the three tasks (all p < 0.001), indicating emotional and cognitive control processes engagement compared with the control condition (see Table S3, Supplementary Materials and Fig. 3a, c, and e). However, we did not observe effects of probiotics versus placebo on any of the three tasks, i.e., no significant Group x Session x Condition effects were found (all p > 0.05, all ηp2<0.005). There were no significant group differences at baseline, i.e. pre-intervention, either (all p > 0.05).
3.1.3. Neuroimaging results
Similar to the behavioral results, we first assessed - independent of the intervention - whether the three tasks activated the anticipated brain regions. The main task effects of the three fMRI tasks were as expected and are shown in Fig. 3b, d, and f, and reported in Table S4 (Supplementary Materials) at pFWE<0.05 (whole-brain correction at cluster level). The emotional face-matching task activated, amongst others, the bilateral amygdala. We observed responses to the emotional face-word Stroop task in regions such as the medial frontal cortex (pre-SMA), vmPFC and lateral PFC (lPFC). Clusters activated for the color-word Stroop task included the lPFC and medial frontal cortex.
We subsequently assessed the effects of probiotics versus placebo (pre vs. post intervention) on each of the three fMRI tasks. At our whole-brain corrected threshold of pFWE<0.05 (cluster level), we did not observe any effects of probiotics during the three fMRI tasks.
3.2. Effects of probiotics on cognition: the role of stress
3.2.1. Stress-induced working memory performance
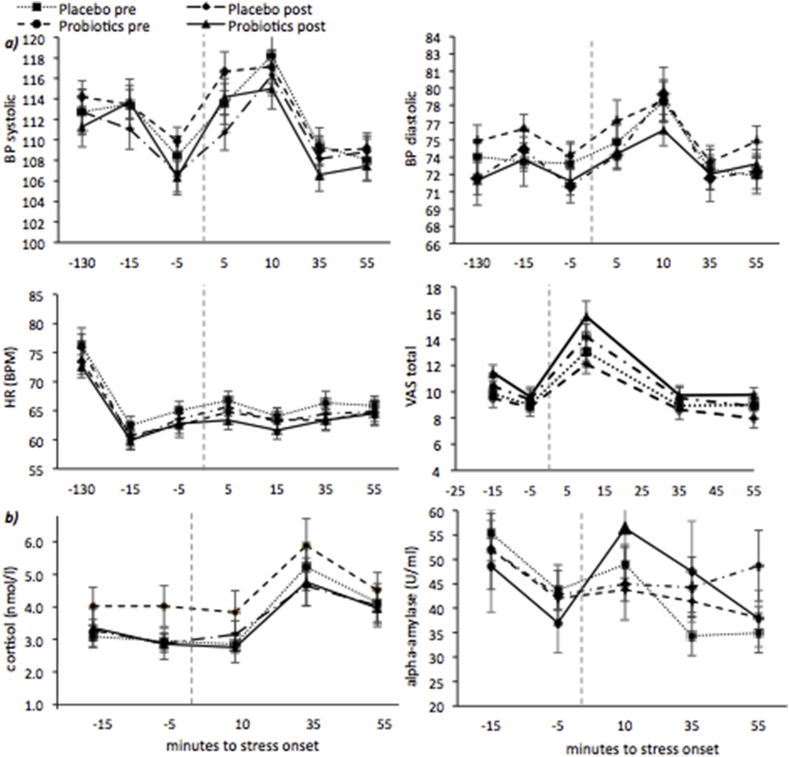
Significant effects of Time revealed that the stressor had effects on physiological and subjective measures of stress (all p < 0.05) see Fig. 4a and b. However, the intervention did not affect these physiological and subjective measures of stress (Group x Session × Time interactions for HR, BPsys, BPdia, hormones, and VAS: p > 0.05) (Supplementary Materials).
Fig. 4.
Physiological and psychological stress effects.
a) Physiological and psychological raw data during the SECPT (onset at time = 0, dotted vertical line), i.e. blood pressure (BP) systolic (top-left panel), blood pressure (BP) diastolic (top-right panel), heart rate (HR) values (bottom-left panel), and total VAS score (bottom-right panel) and b) Hormone levels during the SECPT, i.e. cortisol values (left panel), and alpha-amylase values (right panel), for the two groups (probiotics and placebo), before and after the intervention period.
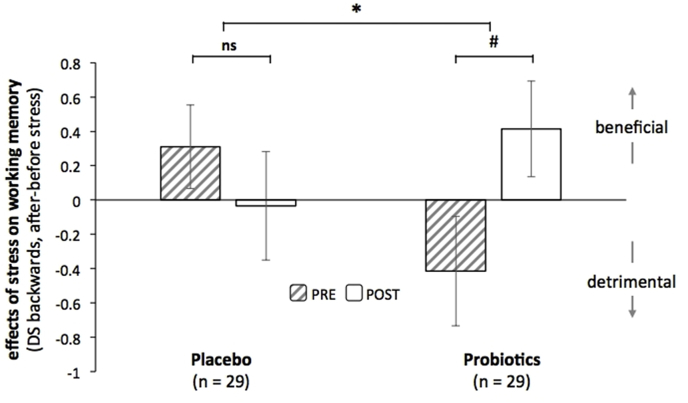
However, we did find that stress-induced working memory performance in DS backward (see Table S5, Supplementary Materials) was differentially affected by the probiotics (post vs. pre) versus placebo (post vs. pre), evidenced by a Time(2) x Group(2) x Session(2) interaction (F(1,56) = 4.48, p = 0.039, ηp2 = 0.07). Breaking this interaction effect down into simple effects, we observed that the probiotics (post vs. pre) tended to increase stress-induced backward digit span performance (Time(2) x Session(2), probiotics: F(1,28) = 4.1, p = 0.053, ηp2 = 0.127), whereas no significant post-pre intervention effect was observed in the placebo group (Time(2) x Session(2), placebo: F(1,28)<1, p = 0.36, ηp2 = 0.03) (Fig. 5). In sum, probiotics versus placebo supplementation resulted in a buffer against the detrimental effect of stress on control-demanding working memory.
Fig. 5.
Stress-induced changes in working memory.
Stress-induced working memory scores (calculated as the difference of DS backwards scores after stress minus scores before stress) obtained pre- and post-intervention for the placebo and for the probiotics group. * = p < 0.05; # = p < 0.1; ns = p > 0.1.
The two groups differed marginally at baseline (i.e. pre-intervention differences in the effect of stress on DS backwards, Time(2) x Group(2): F(1,56) = 3.24, p = 0.077, ηp2 = 0.05), with the placebo group showing no detrimental effects of stress on working memory at baseline (PRE, effect of Time: t(1,28) = −1.27, p = 0.21), in contrast to numerically opposite, but also non-significant, effects in the probiotics group (PRE, effect of Time: t(1,28) = 1.29, p = 0.21) (see Discussion).
3.2.2. Correlations between neural cognitive control responses and stress-induced working memory effects
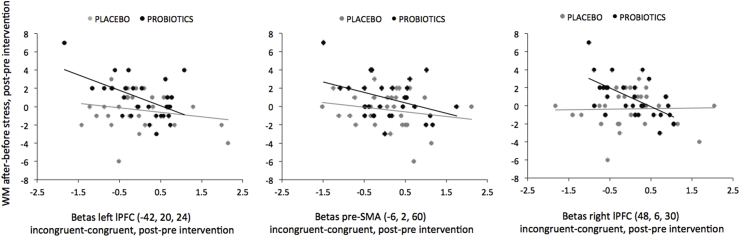
In the probiotics group, we found significant negative correlations between the intervention effect on the neural color-word Stroop responses (i.e. averaged incongruent-congruent betas) and the intervention effect on the stress-related difference scores in DS backwards in all three independent frontal ROIs (see Table S4, Supplementary Materials): left lPFC (BA45) (x,y,z: 42, 20, 24): r = −0.52, p = 0.004; pre-SMA (x,y,z: 6, 2, 60): r = −0.38, p = 0.04; right lPFC (BA44) (x,y,z: 48, 6, 30): r = −0.60, p < 0.001 (Fig. 6). However, in contrast to the two lPFC regions, the association in pre-SMA was not significant after correction for multiple comparisons (p > 0.017). See Supplementary Materials for the fronto-striatal responses observed in the whole-brain correlation within the probiotics group (Table S6; Fig. S1). These brain-behavior correlations were not found in the placebo group (left lPFC (BA45): r = −0.21, p = 0.27; pre-SMA: r = −0.19, p = 0.32; right lPFC (BA44): r = 0.03, p = 0.89) (Fig. 6). Importantly, we subsequently assessed whether the brain-behavior correlations were significantly different between the probiotics and placebo group. Indeed, the intervention with probiotics resulted in a greater association between changes in stress-related working memory and neural cognitive control responses in the right lPFC than the placebo intervention (Fisher's r to z transformation, z = −2.61, p = 0.009). In contrast to the right lPFC, the correlation coefficients between the probiotics and placebo groups did not significantly differ from each other in the left lPFC (z = −1.31, p = 0.19) or in the pre-SMA (z = −0.75, p = 0.45).
Fig. 6.
Brain-behavior correlations.
Correlations between the intervention effect on stress-induced working memory (WM) difference scores (digt span backwards scores after stress minus digit span backwards scores before stress, post-pre intervention) and the intervention effect on cognitive control-related brain responses (i.e. averaged beta values for incongruent-congruent color-word Stroop trials, post-pre intervention) for each independently selected frontal ROI (see Fig. 3f and Table S4), i.e. from left to right: left lateral PFC (BA45), pre-SMA, and right lateral PFC (BA44) for the placebo (grey dots) and for the probiotics group (black dots).
In sum, the probiotics' intervention effect (post-pre) on stress buffer during working memory was especially evident in those subjects with probiotics’ induced decreases in prefrontal cortex recruitment during cognitive control.
4. Discussion
We aimed to investigate the effects of multi-species probiotic supplementation on neurocognition in healthy human volunteers. For our first aim, we assessed whether the neurocognitive effects of probiotics versus placebo would only be seen on emotion reactivity to negative stimuli or also on emotion regulation or general cognitive control. In our secondary aim, we investigated whether the effects of probiotics on cognition (i.e. working memory) and associated neural control mechanisms were visible only with stress induction. Four weeks of probiotic supplementation did not result in differences relative to placebo in terms of neural or behavioral responses during emotion reactivity, emotion regulation or cognitive control. However, the groups did differ in their cognitive response to an acute stressor; i.e., the probiotics group showed an increased buffer against the negative effects of stress on working memory performance relative to placebo. This effect was especially seen in individuals with probiotic-induced decreases in prefrontal cortex recruitment during cognitive control and this association differed significantly from the one in the placebo group.
All three fMRI paradigms robustly activated the expected brain regions and showed the expected behavioral effect (see Fig. 3). Specifically, emotion reactivity was seen for instance in the amygdala and in terms of longer RTs for the faces than the shapes during emotional face matching (Hariri et al., 2000; Haxby et al., 2000), while emotion regulation and general cognitive control was observed in frontal regions and in longer RTs for the incongruent trials during the two Stroop tasks (Etkin et al., 2006; Aarts et al. 2008, 2009; Roberts and Hall, 2008; Cieslik et al., 2015). However, we did not find significant effects of probiotic supplementation on the behavioral (i.e. RTs) and neural responses to the tasks, in line with the results on the questionnaires. This contrasts with previous findings in healthy controls, using the same probiotic product, which indicated a probiotics-induced decrease in cognitive reactivity to sad mood using the LEIDS-r questionnaire (Steenbergen et al., 2015). However, when comparing the baseline scores on the depression-related questionnaires, i.e. LEIDS-r and BDI, we note that our participants scored much lower (i.e. across groups, mean LEIDS-r total: 25.1; mean BDI: 2.4) than the participants of Steenbergen and colleagues (i.e. across groups, mean LEIDS-r total: 43.7; mean BDI: 8.5).
Recently, a study in IBS patients also found a probiotics-induced reduction of depressive symptoms as well as a reduction in neural emotion reactivity using fMRI, but these patients had mild to moderate depression at baseline (i.e. across groups, mean HADS-depression: 10.5) (Pinto-Sanchez et al., 2017). Accordingly, studies assessing probiotics’ effects in participants with at least some degree of depression at baseline (i.e. across groups, median HADS-depression: 5.5) (Messaoudi et al., 2011) or in patients with major depressive disorder (Akkasheh et al., 2016) have reported positive effects on depression scales. In line with our results, the sample of Tillisch et al. (2013) scored low on depression (i.e. across groups, mean HADS-depression: 1.3) and they did not observe differences between the placebo and probiotics group on self-report or neural measures of emotion reactivity (neural differences were only observed for a less controlled comparison between probiotics and no intervention). Similarly, another study with a healthy sample with low depression ratings (mean BDI at baseline: 3.92) did not find probiotics-induced changes in cognition or resting EEG (Kelly et al., 2017). Healthy individuals are known to exhibit a different gut-microbiome composition relative to individuals suffering from depression (Jiang et al., 2015; Kelly et al., 2016). Thus, based on our current and on previous results, it seems that probiotics only have effects on self-report and neural measures of emotion and cognition if subjects are either clinically affected or score high in diagnostic questionnaires, suggesting limited beneficial effects of probiotics on mood and neurocognition in healthy individuals as in the current study.
However, a study using Bifidobacterium longum 1714, did demonstrate (within-subject) probiotic-induced beneficial effects on associate learning and changes in resting EEG in 22 healthy, non-depressed individuals (i.e. mean BDI at baseline: 3.6) (Allen et al., 2016). The same strain was able to reduce anxiety and depression-like behavior in anxious BALB/c mice (Savignac et al., 2014). Moreover, a recent MRI study, with a slightly different multispecies probiotic, demonstrated effects of 4-weeks probiotics supplementation on resting state functional and structural connectivity (Bagga et al., 2018a,b), as well as on associated but different neural mechanisms of emotional processing (i.e. emotional memory and emotional decision-making processes) (Bagga et al., 2018a,b). The sample of these two studies also consisted of non-depressed individuals (i.e. across groups, mean LEIDS-r total: 29.8), but each group only had 15 participants, so results should be interpreted with caution. Nevertheless, in addition to the presence of a certain degree of depressive/anxiety symptoms, other factors such as type of bacterial strains might play a role in observing beneficial neurocognitive effects of probiotics. Therefore, future studies should investigate the mechanisms of action of single bacterial strains to understand their potential in benefitting mood and cognition in different human populations.
The second aim of the present study was to investigate whether probiotics can have a beneficial effect on cognition by buffering against stress. The finding that probiotic supplementation did affect working memory performance, as a function of stress, is in line with preclinical work emphasizing the role of gut microbiota and probiotics in stress-related disorders (Kelly et al., 2015). For example, in mice with a bacterial infection, acute stress caused memory impairments, which were ameliorated by probiotics (Gareau et al., 2011). Moreover, Bifidobacterium longum was able to improve learning and memory in mice with high susceptibility to stress (Savignac et al., 2015). Based on our results, we hypothesize that in healthy young individuals, probiotics might especially induce beneficial effects on cognition and brain functioning only when the system is challenged by stress. However, caution is warranted due to trending baseline differences in our sample. The placebo group as a whole seemed to demonstrate (non-significantly) reduced detrimental effects of stress on working memory relative to the probiotics group at baseline, which could have resulted in ceiling effects, i.e. not enough room for improvement.
Critically, we also found stress-related probiotics effects independent of this marginal baseline difference between the groups. Specifically, the buffer against stress-induced detriments in working memory in the probiotics group was especially seen in individuals with probiotics-induced changes in frontal brain regions during incongruent versus congruent trials in the Stroop task. The lack of brain-behavior correlations between stress-induced working memory and cognitive control brain responses in the placebo group cannot easily be explained by ceiling effects given the focus on individual differences. Individual working memory capacity is known to be predictive of Stroop performance (Kane and Engle, 2003). Here, increases in probiotics-induced protection against stress effects on working memory were related to probiotics-induced decreases in frontal cortex recruitment during cognitive control. This might indicate that the protective effects of probiotics against stress are associated to a certain level of optimization in cognitive control processes. In cognitive control tasks, such as the color-word Stroop task, the frontal cortex is generally more recruited with increasing difficulty. Diminished frontal cortex responses in the absence of behavioral changes have previously been interpreted as more efficient frontal cortex functioning (see also e. g. Mattay et al., 2003). Importantly, we did not observe significant correlations in the placebo group, and the effects in the right lPFC were significantly greater in the probiotics than in the placebo group. This means that the brain-behavior association with stress–related working memory was specific to the probiotics treatment and could not be present due to general factors that would change post-versus pre-intervention for both groups, such as those related to practice.
Nevertheless, the biological mechanisms behind these effects remain to be elucidated, particularly as the beneficial effects of probiotics on stress-related cognition were not accompanied by probiotics-induced changes in HPA-axis (i.e. cortisol) or sympatho-adreno-medullary system (i.e. alpha-amylase) markers. The cardiovascular, hormonal, and psychological measures of stress indicated that stress was reliably induced, but were themselves not influenced by the probiotics, which is in line with previous studies (Mohammadi et al., 2016; Kelly et al., 2017; Moller et al., 2017) but see (Allen et al., 2016). If the current multi-species probiotic product indeed strengthened epithelial barrier function, as demonstrated in vitro (Van Hemert 2014), then other mechanisms could underlie the currently observed effects. Stress can increase the permeability of the intestinal barrier and subsequent immune reactions to LPS crossing the barrier. Indeed, acute stress paradigms can increase levels of the cytokine interleukin-6 in healthy human volunteers (Treadway et al., 2017). Moreover, multi-species probiotics have been shown to reduce inflammatory markers in depressive patients (Akkasheh et al., 2016) and in patients with type 2 diabetes (Asemi et al., 2013). In reaction to increased levels of LPS, pro-inflammatory cytokines can enter the central nervous system and negatively influence brain processes involved in memory and learning (Sparkman et al., 2006; Rogers et al., 2016). Working memory is particularly modulated by signaling of the neurotransmitter dopamine in frontal and striatal brain regions (Cools and D'Esposito, 2011), and dopamine neurotransmission is affected by stress (Bliss et al., 1968; Arnsten and Goldman-Rakic, 1998). Hence, acute stress is generally known to be detrimental to working memory performance and working memory-related brain responses in PFC, particularly in tasks requiring modulation of information in working memory, such as the digit span backward (Schoofs et al., 2009). Increases in inflammatory tone in the body can induce neuro-inflammation, which can particularly affect dopamine signaling, as shown in humans and in non-human primates (Felger and Treadway, 2017). Probiotics might increase the buffer against stress-induced reductions in dopamine-dependent working memory performance by decreasing (stress-induced) permeability of the intestinal barrier, reducing blood concentration of LPS, and reducing brain levels of pro-inflammatory cytokines, as shown previously in rats (Ait-Belgnaoui et al., 2012).
An alternative mechanism of the probiotics-induced beneficial effects might be through the production of metabolites. For example, the gut microbiome has the potential to synthesize precursors of the monoamine neurotransmitters (i.e. large neutral amino acids) that could enter the blood stream, cross the blood-brain barrier, and affect neurotransmitter release (Lyte, 2013; Sampson and Mazmanian, 2015). We have recently demonstrated that predicted microbial potential to synthesize phenylalanine, a precursor of dopamine, was associated with neural responses during reward anticipation (Aarts et al., 2017), a function that is also typically modulated by dopamine (Knutson and Gibbs, 2007). The influence of the gut microbiome on central dopamine processing is also evident from germ-free mice, who exhibit increased turnover of dopamine in the brain (Diaz Heijtz et al., 2011). Future studies should investigate the potential mechanisms of probiotics in affecting central neurotransmitter release, e.g. by precursor production, but also by short chain fatty acid production, or by signaling on enteric nerve cells and the vagus nerve (Mally et al., 2004; DeCastro et al., 2005; Sampson and Mazmanian, 2015).
5. Limitations
Our study presents some limitations. First, to avoid potential confounding factors on probiotics effects, we focused on female participants only. This represents a limit for the generalization of the results to the male population. Secondary, given that the compliance was encouraged with regular reminders and a personal diary, the objective presence of the bacteria was not confirmed via stool sample analysis. However, analysis from prior studies using the same products confirmed the presence of the bacterial strains in the stool of healthy volunteers (Koning et al., 2008).
6. Conclusions
We showed that 4-weeks of supplementation with probiotics positively affected cognition under challenging situations induced by acute stress, which was associated with changes in frontal brain regions during cognitive control. However, on neurocognitive tasks administered in relative neutral situations we did not observe effects of probiotics across the group. Our findings of stress-dependent beneficial effects of probiotics on cognition can be of clinical importance for stress-related psychiatric and gastro-intestinal disorders.
7. Funding sources
The study was supported by the Dutch Ministry of Economic Affairs under the TKI Life Science and Health, project LSHM15034, and by Winclove Probiotics B.V., The Netherlands. EA and JW were supported by a Food, Cognition and Behavior grant from the Netherlands Organisation for Scientific Research (NWO, grant 057-14-001). EA was also supported by a Veni grant from the Netherlands Organisation for Scientific Research (NWO, grant 016.135.023). KR was supported by a starting grant from the European Research Council (ERC_StG2012_313749) and by a VICI grant (#453-12-001) from the Netherlands Organization for Scientific Research (NWO). AAV was supported by European Union’s Horizon 2020 grants (Marie Sklodowska-Curie grant 643051 and grant 728018). SvH is employed at Winclove Probiotics B.V. This does not alter this authors’ adherence to all publication policies on sharing data and materials. All other authors have no conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2018.100141.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Aarts E., Ederveen T.H.A., Naaijen J., Zwiers M.P., Boekhorst J., Timmerman H.M., Smeekens S.P., Netea M.G., Buitelaar J.K., Franke B., van Hijum S., Arias Vasquez A. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts E., Roelofs A., van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. J. Neurosci. 2008;28(18):4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts E., Roelofs A., van Turennout M. Attentional control of task and response in lateral and medial frontal cortex: brain activity and reaction time distributions. Neuropsychologia. 2009;47(10):2089–2099. doi: 10.1016/j.neuropsychologia.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Abildgaard A., Elfving B., Hokland M., Lund S., Wegener G. Probiotic treatment protects against the pro-depressant-like effect of high-fat diet in Flinders Sensitive Line rats. Brain Behav. Immun. 2017;65:33–42. doi: 10.1016/j.bbi.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Abildgaard A., Elfving B., Hokland M., Wegener G., Lund S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. 2017;79:40–48. doi: 10.1016/j.psyneuen.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A., Colom A., Braniste V., Ramalho L., Marrot A., Cartier C., Houdeau E., Theodorou V., Tompkins T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neuro Gastroenterol. Motil. 2014;26(4):510–520. doi: 10.1111/nmo.12295. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A., Durand H., Cartier C., Chaumaz G., Eutamene H., Ferrier L., Houdeau E., Fioramonti J., Bueno L., Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37(11):1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Akkasheh G., Kashani-Poor Z., Tajabadi-Ebrahimi M., Jafari P., Akbari H., Taghizadeh M., Memarzadeh M.R., Asemi Z., Esmaillzadeh A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32(3):315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Allen A.P., Hutch W., Borre Y.E., Kennedy P.J., Temko A., Boylan G., Murphy E., Cryan J.F., Dinan T.G., Clarke G. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry. 2016;6(11):e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso C., Guilarte M., Vicario M., Ramos L., Rezzi S., Martinez C., Lobo B., Martin F.P., Pigrau M., Gonzalez-Castro A.M., Gallart M., Malagelada J.R., Azpiroz F., Kochhar S., Santos J. Acute experimental stress evokes a differential gender-determined increase in human intestinal macromolecular permeability. Neuro Gastroenterol. Motil. 2012;24(8):740–746. doi: 10.1111/j.1365-2982.2012.01928.x. e348-749. [DOI] [PubMed] [Google Scholar]
- Antypa N., Van der Does A.J., Penninx B.W. Cognitive reactivity: investigation of a potentially treatable marker of suicide risk in depression. J. Affect. Disord. 2010;122(1–2):46–52. doi: 10.1016/j.jad.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci. 2015;18(10):1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F., Goldman-Rakic P.S. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatr. 1998;55(4):362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Asemi Z., Zare Z., Shakeri H., Sabihi S.S., Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013;63(1–2):1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- Bagga D., Aigner C.S., Reichert J.L., Cecchetto C., Fischmeister F.P.S., Holzer P., Moissl-Eichinger C., Schopf V. Influence of 4-week multi-strain probiotic administration on resting-state functional connectivity in healthy volunteers. Eur. J. Nutr. 2018 doi: 10.1007/s00394-018-1732-z. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga D., Reichert J.L., Koschutnig K., Aigner C.S., Holzer P., Koskinen K., Eichinger C.M., Schopf V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microb. 2018;9(6):486–496. doi: 10.1080/19490976.2018.1460015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T. University of Pennsylvania Press; Philadelphia: 1976. Depression: Causes and Treatment. [Google Scholar]
- Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., Deng Y., Blennerhassett P., Macri J., McCoy K.D., Verdu E.F., Collins S.M. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052. 609 e591-593. [DOI] [PubMed] [Google Scholar]
- Bliss E.L., Ailion J., Zwanziger J. Metabolism of norepinephrine, serotonin and dopamine in rat brain with stress. J. Pharmacol. Exp. Therapeut. 1968;164(1):122–134. [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M. e. a. Paper Presented at 8th Interna- Tional Conference on Functional Mapping of the Human Brain, Sendai, Japan. 2002. Region of interest analysis using the MarsBar toolbox [abstract] [Google Scholar]
- Cieslik E.C., Mueller V.I., Eickhoff C.R., Langner R., Eickhoff S.B. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci. Biobehav. Rev. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R., D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry. 2011;69(12):e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.S., Callaghan B.L., Richardson R. The effects of a probiotic formulation (Lactobacillus rhamnosus and L. helveticus) on developmental trajectories of emotional learning in stressed infant rats. Transl. Psychiatry. 2016;6(5):e823. doi: 10.1038/tp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- DeCastro M., Nankova B.B., Shah P., Patel P., Mally P.V., Mishra R., La Gamma E.F. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005;142(1):28–38. doi: 10.1016/j.molbrainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Desbonnet L., Garrett L., Clarke G., Kiely B., Cryan J.F., Dinan T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R., Wang S., Anuar F., Qian Y., Bjorkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Buchel C., Gross J.J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015;16(11):693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Felger J.C., Treadway M.T. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42(1):216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich E.E., Farzi A., Mayerhofer R., Reichmann F., Jacan A., Wagner B., Zinser E., Bordag N., Magnes C., Frohlich E., Kashofer K., Gorkiewicz G., Holzer P. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J., Macqueen G., Sherman P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Bookheimer S.Y., Mazziotta J.C. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends Cognit. Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J., Li L., Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kane M.J., Engle R.W. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J. Exp. Psychol. Gen. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Allen A.P., Temko A., Hutch W., Kennedy P.J., Farid N., Murphy E., Boylan G., Bienenstock J., Cryan J.F., Clarke G., Dinan T.G. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017;61:50–59. doi: 10.1016/j.bbi.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Borre Y., Patterson O.B.C.,E., El Aidy S., Deane J., Kennedy P.J., Beers S., Scott K., Moloney G., Hoban A.E., Scott L., Fitzgerald P., Ross P., Stanton C., Clarke G., Cryan J.F., Dinan T.G. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Gibbs S.E. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berlin) 2007;191(3):813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning C.J., Jonkers D.M., Stobberingh E.E., Mulder L., Rombouts F.M., Stockbrugger R.W. The effect of a multispecies probiotic on the intestinal microbiota and bowel movements in healthy volunteers taking the antibiotic amoxycillin. Am. J. Gastroenterol. 2008;103(1):178–189. doi: 10.1111/j.1572-0241.2007.01547.x. [DOI] [PubMed] [Google Scholar]
- Li W., Dowd S.E., Scurlock B., Acosta-Martinez V., Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol. Behav. 2009;96(4–5):557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Lovallo W. The cold pressor test and autonomic function: a review and integration. Psychophysiology. 1975;12(3):268–282. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9(11) doi: 10.1371/journal.ppat.1003726. e1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mally P., Mishra R., Gandhi S., Decastro M.H., Nankova B.B., Lagamma E.F. Stereospecific regulation of tyrosine hydroxylase and proenkephalin genes by short-chain fatty acids in rat PC12 cells. Pediatr. Res. 2004;55(5):847–854. doi: 10.1203/01.PDR.0000119365.21770.45. [DOI] [PubMed] [Google Scholar]
- Mattay V.S., Goldberg T.E., Fera F., Hariri A.R., Tessitore A., Egan M.F., Kolachana B., Callicott J.H., Weinberger D.R. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl. Acad. Sci. U. S. A. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J., O'Mahony L., O'Callaghan L., Sheil B., Vaughan E.E., Fitzsimons N., Fitzgibbon J., O'Sullivan G.C., Kiely B., Collins J.K., Shanahan F. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52(7):975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson J.F., Rougeot C., Pichelin M., Cazaubiel M., Cazaubiel J.M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105(5):755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Mohammadi A.A., Jazayeri S., Khosravi-Darani K., Solati Z., Mohammadpour N., Asemi Z., Adab Z., Djalali M., Tehrani-Doost M., Hosseini M., Eghtesadi S. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016;19(9):387–395. doi: 10.1179/1476830515Y.0000000023. [DOI] [PubMed] [Google Scholar]
- Moller C.M., Olsa E.J.A., Ginty A.T., Rapelje A.L., Tindall C.L., Holesh L.A., Petersen K.L., Conklin S.M. Influence of acute multi-species and multi-strain probiotic supplementation on cardiovascular function and reactivity to psychological stress in young adults: a double-blind, randomized, placebo-controlled trial. Psychosom. Med. 2017;79(8):914–919. doi: 10.1097/PSY.0000000000000489. [DOI] [PubMed] [Google Scholar]
- O'Hagan C., Li J.V., Marchesi J.R., Plummer S., Garaiova I., Good M.A. Long-term multi-species Lactobacillus and Bifidobacterium dietary supplement enhances memory and changes regional brain metabolites in middle-aged rats. Neurobiol. Learn. Mem. 2017;144:36–47. doi: 10.1016/j.nlm.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T., Martin F.P., Cominetti O., Welsh C., Rieder A., Traynor J., Gregory C., De Palma G., Pigrau M., Ford A.C., Macri J., Berner B., Bergonzelli G., Surette M.G., Collins S.M., Moayyedi P., Bercik P. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Poser B.A., Versluis M.J., Hoogduin J.M., Norris D.G. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: parallel-acquired inhomogeneity-desensitized fMRI. Magn. Reson. Med. 2006;55(6):1227–1235. doi: 10.1002/mrm.20900. [DOI] [PubMed] [Google Scholar]
- Roberts K.L., Hall D.A. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. J. Cognit. Neurosci. 2008;20(6):1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Rogers G.B., Keating D.J., Young R.L., Wong M.L., Licinio J., Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatr. 2016;21(6):738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17(5):565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Lehto S.M., Harty S., Dinan T.G., Cryan J.F., Burnet P.W. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac H.M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neuro Gastroenterol. Motil. 2014;26(11):1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Savignac H.M., Tramullas M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- Schoofs D., Wolf O.T., Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav. Neurosci. 2009;123(5):1066–1075. doi: 10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- Sparkman N.L., Buchanan J.B., Heyen J.R., Chen J., Beverly J.L., Johnson R.W. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J. Neurosci. 2006;26(42):10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen L., Sellaro R., van Hemert S., Bosch J.A., Colzato L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1953;18(6):643–662. [Google Scholar]
- Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B., Guyonnet D., Legrain-Raspaud S., Trotin B., Naliboff B., Mayer E.A. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. doi: 10.1053/j.gastro.2013.02.043. 1401 e1391-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M.T., Admon R., Arulpragasam A.R., Mehta M., Douglas S., Vitaliano G., Olson D.P., Cooper J.A., Pizzagalli D.A. Association between interleukin-6 and striatal prediction-error signals following acute stress in healthy female participants. Biol. Psychiatry. 2017;82(8):570–577. doi: 10.1016/j.biopsych.2017.02.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hemert S. a. O., G. Influence of the multispecies probiotic Ecologic® BARRIER on parameters of intestinal barrier function. Food Nutr. Sci. 2014;5 1739. [Google Scholar]
- Vanuytsel T., van Wanrooy S., Vanheel H., Vanormelingen C., Verschueren S., Houben E., Salim Rasoel S., Tomicronth J., Holvoet L., Farre R., Van Oudenhove L., Boeckxstaens G., Verbeke K., Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.