Abstract
Background
Breast Cancer Trials of Oral Everolimus 2 (BOLERO-2), a phase III study in postmenopausal women with estrogen receptor–positive breast cancer progressing despite nonsteroidal aromatase inhibitor therapy, showed statistically significant benefits with adding everolimus to exemestane. Moreover, in preclinical studies, mammalian target of rapamycin inhibition was associated with decreased osteoclast survival and activity. Exploratory analyses in BOLERO-2 evaluated the effect of everolimus on bone marker levels and progressive disease in bone.
Methods
Patients were treated with exemestane (25mg/day) and randomized (2:1) to everolimus (10mg/day; combination) or placebo (exemestane only). Exploratory endpoints included changes in bone turnover marker levels vs baseline and progressive disease in bone, defined as unequivocal progression of a preexisting bone lesion or the appearance of a new bone lesion.
Results
Baseline disease characteristics were well balanced between arms (N = 724); baseline bisphosphonate use was not (43.9% combination vs 54.0% exemestane only). At a median of 18 months of follow-up, median progression-free survival (primary endpoint) was statistically significantly longer with the combination vs exemestane only (Cox proportional hazard ratio = 0.45, 95% confidence interval = 0.38 to 0.54; log-rank, 1-sided P < .0001). Bone marker levels at 6 and 12 weeks increased with exemestane only, as expected, but decreased with the combination. The cumulative incidence rate of progressive disease in bone was lower in the combination arm. Bone-related adverse events occurred with similar frequency in both arms (3.3% combination vs 4.2% exemestane only).
Conclusion
These exploratory analyses suggest that everolimus has beneficial effects on bone turnover and progressive disease in bone in patients receiving exemestane for hormone receptor–positive breast cancer progressing during/after nonsteroidal aromatase inhibitor therapy.
Maintaining bone health is of critical importance for patients with breast cancer because the disease itself, in addition to anticancer therapies, can adversely affect bone. Interactions between breast cancer cells and bone cells result in an intercellular signaling loop that promotes growth and spread of bone metastases (1). This destructive cycle occurs when growth factors that promote development or progression of metastases by stimulating dormant cancer cell proliferation (eg, endothelin 1 and transforming growth factor beta family members) are released from the bone matrix during bone remodeling (1). In addition, breast cancer cells can secrete factors that promote further osteolysis, thereby resulting in a vicious cycle of bone destruction and tumor growth (1). Because breast cancer often metastasizes to bone, this painful and potentially debilitating complication develops in 65% to 75% of patients with advanced breast cancer (1,2). Endocrine therapies used to treat breast cancer also can have detrimental effects on bone health. Several local and systemic factors, including estrogen, influence normal bone homeostasis, which is maintained through a continuous remodeling process involving bone resorption by osteoclasts and formation by osteoblasts (3). However, endocrine therapies for breast cancer suppress estrogen, thereby potentially leading to subsequent bone loss (4–11).
Nonsteroidal aromatase inhibitors (ie, anastrozole and letrozole) are often the initial endocrine therapy used in patients with hormone receptor–positive breast cancer. However, the profound estrogen depletion achieved with aromatase inhibitor therapy has been associated with decreased bone mineral density and increased fracture risk (9,12–14). Exemestane is a steroidal aromatase inhibitor used to treat patients in the advanced setting whose disease has progressed on nonsteroidal aromatase inhibitors. Although preclinical studies suggest that exemestane may have androgenic effects (15–18), the implied protective effect on bone with such activity has not been demonstrated in clinical studies. In fact, in the phase III trial reporting the efficacy of exemestane vs megestrol acetate, androgenic adverse events of all grades and causes were not reported because they occurred in only 1% or less of the study population (18,19). Furthermore, exemestane also has been associated with increases in bone resorption and formation marker levels (4,6,8) and higher fracture rates vs tamoxifen or placebo (20).
Everolimus is an oral inhibitor of mammalian target of rapamycin (mTOR), a serine–threonine kinase that regulates cell growth, angiogenesis, and survival (21). Notably, everolimus recently was approved by the US Food and Drug Administration for treating patients with hormone receptor–positive advanced breast cancer progressing during/after nonsteroidal aromatase inhibitor therapy, based on interim analysis from the BOLERO-2 trial (22). Activation of the mTOR signaling pathway is a key adaptive change associated with endocrine resistance (23–27). Moreover, preclinical evidence also indicates that mTOR signaling is involved in osteoclast survival and osteoblast differentiation (28), and mTOR pathway inhibition has been shown to decrease bone resorption in animal models (29,30). Preclinical studies using mouse models have shown that mTOR inhibition decreases osteoclast maturation and increases osteoclast apoptosis (29). Furthermore, rat studies have shown that everolimus treatment decreases bone loss associated with estrogen deprivation (30). Together, these studies provide the rationale for examining the bone effects of everolimus in patients with advanced breast cancer.
Previous reports from BOLERO-2 showed that adding everolimus to exemestane treatment statistically significantly improved progression-free survival (PFS) in postmenopausal women with hormone receptor–positive breast cancer progressing on prior nonsteroidal aromatase inhibitor therapy (31–33). The objectives of the current exploratory analyses from BOLERO-2 are to evaluate the effects of everolimus plus exemestane vs exemestane only on bone marker levels and progressive disease in bone in patients with advanced breast cancer progressing during or after nonsteroidal aromatase inhibitor therapy.
Methods
BOLERO-2 Study Design
Breast Cancer Trials of Oral Everolimus 2 (BOLERO-2) (ClinicalTrials.gov identifier NCT00863655) (34) is an international, phase III, multicenter, randomized, double-blind, placebo-controlled trial designed by the academic investigators and representatives of the sponsor, Novartis. The protocol has been described previously (31). See Supplementary Methods (available online) for additional study details.
Patient Population.
Patients were postmenopausal women with metastatic or locally advanced, estrogen receptor–positive, human epidermal growth factor receptor 2 nonamplified breast cancer that was not amenable to curative surgery or radiotherapy and that was progressing despite prior letrozole or anastrozole therapy. Other eligibility criteria have been described previously (31).
The institutional review board at each participating center approved the study, and it was conducted in accordance with Good Clinical Practice principles, the Declaration of Helsinki, and applicable local regulations. A steering committee supervised the conduct of the study, and an independent data and safety monitoring committee performed semiannual safety reviews and reviewed interim efficacy results. All patients provided written informed consent. Subset analyses by race/ethnic group were completed, as described previously (31,33).
Treatment and Dose Modification.
Patients received exemestane (25mg/day) and were randomly assigned (2:1) to receive everolimus (10mg/day; combination arm) or placebo (exemestane-only arm) daily. Stratification and dose adjustments were performed as described previously (31).
Efficacy and Safety Assessments.
The primary endpoint was PFS, defined as time from randomization to first documentation of progressive disease as assessed by the local investigator according to Response Evaluation Criteria in Solid Tumors (35) or death from any cause for patients without progressive disease, whichever occurred first.
Bone Marker Assessments
Percentage change for bone-specific alkaline phosphatase (BSAP), amino-terminal propeptide of type 1 collagen (P1NP), and C-terminal cross-linking telopeptide of type 1 collagen (CTX) at 6 and 12 weeks vs baseline (investigator assessed) was a protocol-specified exploratory endpoint. The bone markers chosen allowed assessment of osteoclast metabolism (BSAP), bone formation (P1NP), and bone resorption (CTX). Blood samples (8.5mL, fasting preferred) were collected at baseline and 6 and 12 weeks (visits 5 and 6) after treatment initiation. Specimen collection methods were consistent across study visits. See Supplementary Methods (available online) for additional details.
Exploratory Analyses for Progressive Disease in Bone
Cumulative incidence of progressive disease in bone (investigator assessed) was compared between the two arms as ad hoc exploratory analyses. Progressive disease in bone (first documented progression event in bone, with or without concurrent progression outside of bone) was defined as unequivocal progression of a preexisting bone lesion or the appearance of a new bone lesion at the time of documented progressive disease. A bone scan or skeletal survey was performed at baseline, within 6 weeks before randomization. Patients with bone metastases identified at baseline also were assessed by x-ray, computed tomography scan with bone windows, or magnetic resonance imaging before randomization and every 6 weeks thereafter using the same modality until disease progression or new anticancer therapy was started. Additional bone scans or skeletal surveys were performed if clinically indicated per the local investigator’s discretion.
Statistical Analyses
For the primary endpoint of PFS, hazard ratios (HRs) and associated 95% confidence intervals (CIs) were estimated using a stratified Cox regression model (1-sided P value, 2.5% significance level) as described previously (31); additional details are available in the Supplementary Methods (available online). Differences in percentage change in bone turnover marker levels from baseline are exploratory; all P values are two-sided, with P less than .05 considered statistically significant.
Differences in progressive disease in bone event rates between treatment groups were evaluated using Gray’s test to compare cumulative incidence curves (see Supplementary Methods, available online, for additional details) and to check for equality of incidence across groups (36,37). P values are two-sided, with P less than .05 considered statistically significant.
Results
Patient Characteristics
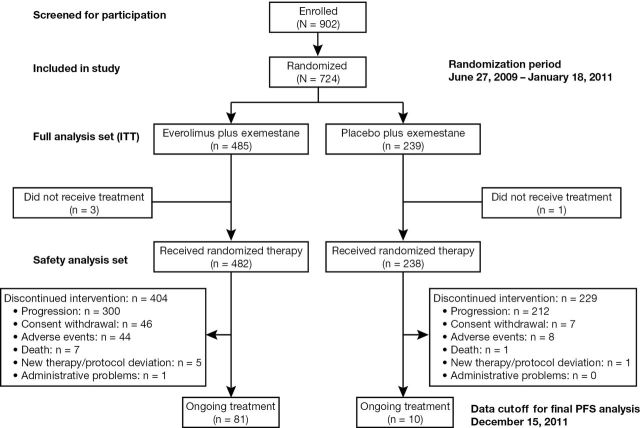
A total of 724 patients (intent-to-treat population) receiving exemestane were randomly assigned to also receive everolimus (n = 485; combination arm) or placebo (n = 239; exemestane-only arm) from June 2009 to January 2011 (Figure 1). Median duration of follow-up for bone-related analyses (cutoff date December 15, 2011) was 18 months. Median treatment exposures to exemestane were 29.5 and 14.1 weeks for the combination and exemestane-only arms, respectively; median exposure to everolimus was 23.9 weeks. Baseline patient disease characteristics were well balanced between treatment groups, including median age (61 years) and presence of bone metastases at baseline (76.5% in the combination arm and 77.4% in the exemestane-only arm) (Table 1). However, baseline bisphosphonate use was less frequent in the combination arm (43.9%) vs the exemestane-only arm (54.0%). Types of bisphosphonates used at baseline in the combination and exemestane-only arms included zoledronic acid (29.3% vs 33.6%), pamidronate (6.2% vs 6.7%), ibandronate (4.8% vs 6.3%), and clodronate (1.7% vs 3.8%).
Figure 1.
Breast Cancer Trials of Oral Everolimus 2 (BOLERO-2) trial flow diagram showing design, enrollment, and outcome. ITT = intent-to-treat population; PFS = progression-free survival.
Table 1.
Baseline bone metastases and bisphosphonate use
| Baseline characteristics | Everolimus + exemestane | Placebo + exemestane |
|---|---|---|
| Overall population, No. (%) | n = 485 | n = 239 |
| Baseline bone metastases | 371 (76.5) | 185 (77.4) |
| Baseline bisphosphonate use | 213 (43.9) | 129 (54.0) |
| Presence of bone metastases at baseline in patients, No. (%) | n = 371 | n = 185 |
| With baseline bisphosphonate use | 199 (53.6) | 121 (65.4) |
| Without baseline bisphosphonate use | 172 (46.4) | 64 (34.6) |
| Rates of bisphosphonate use at baseline in patients, No. (%) | n = 213 | n = 129 |
| With baseline bone metastases | 199 (93.4) | 121 (93.8) |
| Without baseline bone metastases | 14 (6.6) | 8 (6.2) |
Efficacy
Progression-Free Survival.
Efficacy results have been reported previously for BOLERO-2 (33). By local assessment (primary endpoint), median PFS at 18 months of follow-up was more than twice as long for the combination arm vs the exemestane-only arm (Cox proportional HR = 0.45, 95% CI = 0.38 to 0.54; P < .0001, log-rank, 1-sided). Central assessment confirmed these results (33).
Bone Marker Assessments.
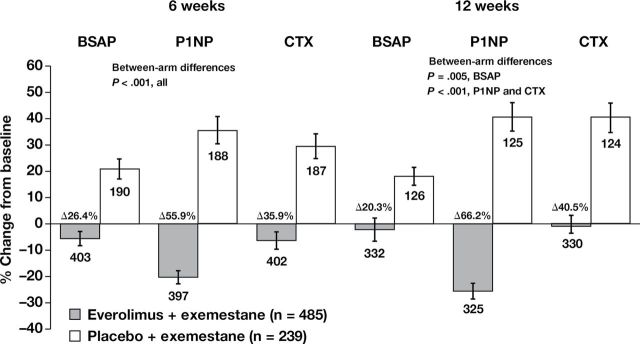
In the overall patient population, bone marker (BSAP, P1NP, and CTX) levels increased at 6 and 12 weeks relative to baseline in the exemestane-only arm, as expected from prior observations (Figure 2). In contrast, adding everolimus to exemestane in the combination arm decreased bone marker levels at 6 and 12 weeks relative to baseline (Figure 2), with statistically significant differences in changes from baseline to week 6 between treatment arms (26.4% for BSAP, 55.9% for P1NP, and 35.9% for CTX; P < .001 for all; n = 593 evaluable patients at week 6). Data at 12 weeks demonstrated similar trends, with differences in changes from baseline to week 12 between treatment arms of 20.3% for BSAP (P = .005), 66.2% for P1NP (P < .001), and 40.5% for CTX (P < .001).
Figure 2.
Changes in bone turnover marker levels at 6 and 12 weeks vs baseline in the overall population. The percentage change in bone turnover marker levels was calculated from the differences at 6 and 12 weeks vs baseline (investigator-assessed, protocol-specified exploratory endpoint). Blood samples (8.5mL, fasting preferred) were collected at baseline and at 6 and 12 weeks (visits 5 and 6) after treatment initiation. Serum bone-specific alkaline phosphatase (BSAP) was measured with an immunochemiluminescence assay using the Ostase reagent on an automatic analyzer. Serum amino-terminal propeptide of type 1 collagen (P1NP) was measured with a two-site immunoassay using monoclonal antibodies against purified human P1NP to detect both intact monomeric and trimeric forms, but not fragments. Serum C-terminal cross-linking telopeptide of type 1 collagen (CTX) was measured with a two-site assay using monoclonal antibodies against an eight amino acid sequence from the C-telopeptide of human type 1 collagen. Error bars are standard error of the mean. P values are two-sided.
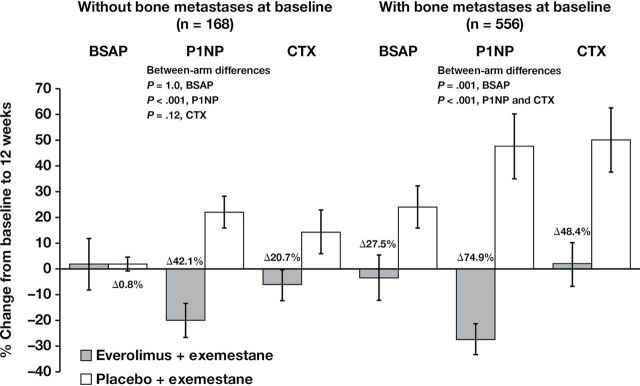
The reductions in bone marker levels reported in the combination arm were observed irrespective of the presence of bone metastases (Figure 3). Notably, in patients with bone metastases at baseline (n = 556), bone marker levels were markedly lower in the combination arm vs the exemestane-only arm, with differences in the change from baseline to week 12 between treatment arms of 27.5% for BSAP (P = .001), 74.9% for P1NP (P < .001), and 48.4% for CTX (P < .001). Additionally, in patients with baseline bone metastases, the reduction in bone marker levels observed in the combination arm occurred despite less frequent baseline bisphosphonate use (55.2%) vs the exemestane-only arm (66.9%). Although the group of patients without bone metastases at baseline was much smaller (n = 168), the differences in bone marker level changes from baseline to week 12 between treatment arms were still notable (stable BSAP: P = 1.0 [not statistically significant]; 42.1% P1NP: P < .001; and 20.7% CTX: P = .12 [not statistically significant]) within this group.
Figure 3.
Changes in bone turnover marker levels at 12 weeks vs baseline in patients with or without bone metastases at baseline. The percentage change in bone turnover marker levels was calculated from the difference at 12 weeks vs baseline for patients with or without bone metastases at baseline. Blood samples (8.5mL, fasting preferred) were collected at baseline and at 6 and 12 weeks (visits 5 and 6) after treatment initiation. Serum bone-specific alkaline phosphatase (BSAP) was measured with an immunochemiluminescence assay using the Ostase reagent on an automatic analyzer. Serum amino-terminal propeptide of type 1 collagen (P1NP) was measured with a two-site immunoassay using monoclonal antibodies against purified human P1NP to detect both intact monomeric and trimeric forms, but not fragments. Serum C-terminal cross-linking telopeptide of type 1 collagen (CTX) was measured with a two-site assay using monoclonal antibodies against an eight amino acid sequence from the C-telopeptide of human type 1 collagen. Error bars are standard error of the mean. P values are two-sided.
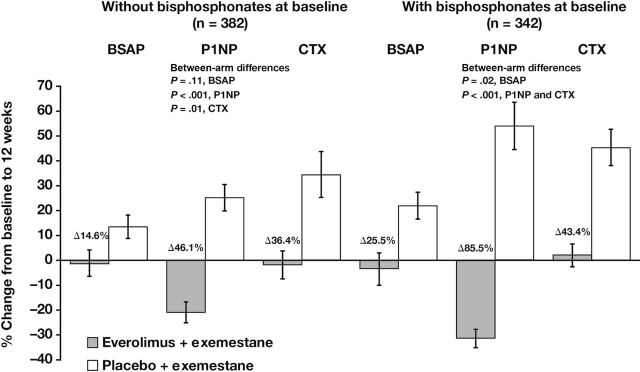
The influence of bisphosphonate use on bone marker level changes was also examined in both treatment arms. At 12 weeks, bone marker levels were lower in the combination arm vs the exemestane-only arm, and differences in changes from baseline to week 12 between treatment arms at this timepoint were consistent with possibly larger differences in patients who received baseline bisphosphonates (n = 342; 25.5% BSAP: P = .02; 85.5% P1NP: P < .001; 43.4% CTX: P < .001) vs those who did not (n = 382; 14.6% BSAP: P = .11 [not statistically significant]; 46.1% P1NP: P < .001; 36.4% CTX: P = .01) (Figure 4). Although baseline bisphosphonate use was not balanced between treatment arms (43.9% combination vs 54.0% exemestane only), the beneficial effects of everolimus on bone marker levels were observed regardless of baseline bisphosphonate use.
Figure 4.
Changes in bone turnover marker levels at 12 weeks vs baseline in patients with or without bisphosphonate use at baseline. The percentage change in bone turnover marker levels was calculated from the difference at 12 weeks vs baseline for patients with or without baseline bisphosphonate use. Blood samples (8.5mL, fasting preferred) were collected at baseline and at 6 and 12 weeks (visits 5 and 6) after treatment initiation. Serum bone-specific alkaline phosphatase (BSAP) was measured with an immunochemiluminescence assay using the Ostase reagent on an automatic analyzer. Serum amino-terminal propeptide of type 1 collagen (P1NP) was measured with a two-site immunoassay using monoclonal antibodies raised against purified human P1NP to detect both intact monomeric and trimeric forms, but not fragments. Serum C-terminal cross-linking telopeptide of type 1 collagen (CTX) was measured with a two-site assay using monoclonal antibodies against an eight amino acid sequence from the C-telopeptide of human type 1 collagen. Error bars are standard error of the mean. P values are two-sided.
Progressive Disease in Bone.
The proportion of PFS events (progressive disease and death before progression) as reported by the investigator was 63.9% in the combination arm and 83.7% in the exemestane-only arm (Table 2). Progressive disease occurred in 60.6% (combination arm) vs 82.8% of patients (exemestane-only arm). Progressive disease in bone occurred in 13.0% (combination arm) vs 18.8% of patients (exemestane-only arm). Rates of overall progressive disease and progressive disease in bone were similar in patients with baseline bone metastases (64.4% combination arm vs 81.6% exemestane-only arm; HR = 0.43, 95% CI = 0.35 to 0.53) or baseline bisphosphonate use (66.2% combination arm vs 83.7% exemestane-only arm; HR = 0.41; 95% CI = 0.31 to 0.52) as in the overall patient population (Table 2).
Table 2.
Summary of progression-free survival
| Characteristics | Everolimus + exemestane | Placebo + exemestane |
|---|---|---|
| Overall population, No. (%) | n = 485 | n = 239 |
| Total number of PFS events* | 310 (63.9) | 200 (83.7) |
| Deaths before progression | 16 (3.3) | 2 (0.8) |
| Progressive disease | 294 (60.6) | 198 (82.8) |
| Progressive disease in bone† | 63 (13.0) | 45 (18.8) |
| Patients with baseline bone metastases, No. (%) | n = 371 | n = 185 |
| Progressive disease | 239 (64.4) | 151 (81.6) |
| Progressive disease in bone† | 60 (16.2) | 43 (23.2) |
| Patients with baseline bisphosphonate use, No. (%) | n = 213 | n = 129 |
| Progressive disease | 141 (66.2) | 108 (83.7) |
| Progressive disease in bone† | 33 (15.5) | 30 (23.3) |
* Includes deaths before progression (n = 16 [3.3%] combination arm and n = 2 [0.8%], exemestane-only arm). PFS = progression-free survival.
† Defined as the first documented progression event; includes patients with concurrent progression in and outside of bone.
Progression in bone (ie, progression of preexisting bone lesions or development of new bone lesions) was statistically significantly lower in the combination arm vs the exemestane-only arm (P = .04, Gray’s test) in the overall patient population (N = 724) (Figure 5). Differences in the incidence of progressive disease in bone became evident between the treatment arms by week 12, with a lower cumulative incidence rate of progressive disease in bone for the combination arm (3.5%) vs the exemestane-only arm (6.6%) in the overall population. Progressive disease in bone remained nearly twofold lower in the combination arm vs the exemestane-only arm through week 30 (8.1% vs 15.0%, respectively), and similar trends continued beyond 30 weeks. Notably, concomitant bisphosphonate use during the study continued to be less frequent in the combination arm (52.5%) vs the exemestane-only arm (58.8%).
Figure 5.
Progressive disease in bone in the overall population (N = 724). Progressive disease in bone (first documented progression event in bone, with or without concurrent progression outside of bone) was defined as unequivocal progression of a preexisting bone lesion or the appearance of a new bone lesion at the time of documented progressive disease as assessed by the investigator at the local site. Comparison of cumulative incidence of progression due to bone metastasis using competing risk methods by treatment; P = .04 (Gray’s test, 2-sided). CI = confidence interval; CR = competing risk estimate.
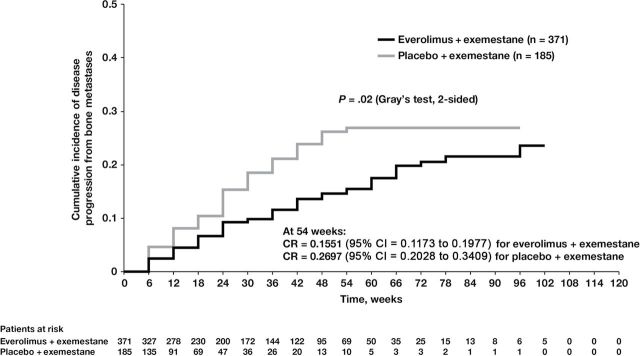
Progressive Disease in Bone in Patients With Bone Metastases at Baseline.
Patients with baseline bone metastases (n = 556) had higher rates of on-study bone disease progression vs the overall population; nonetheless, incidence rates for progressive disease in bone remained lower with the combination of everolimus plus exemestane versus exemestane only (Figure 6) (P = .02, Gray’s test) in this subset (4.5% vs 8.1%, respectively, at week 12). Again, the difference in incidence rates of progressive disease in bone remained nearly twofold lower in the combination arm through week 30 vs the exemestane-only arm (9.9% vs 18.5%, respectively). Similar trends continued beyond 30 weeks. These data suggest that everolimus in the combination arm delayed the worsening of existing bone lesions in addition to delaying the development of new bone lesions.
Figure 6.
Progressive disease in bone in the subgroup of patients with bone metastases at baseline (n = 556). Progressive disease in bone (first documented progression event in bone, with or without concurrent progression outside of bone) was defined as unequivocal progression of a preexisting bone lesion or the appearance of a new bone lesion at the time of documented progressive disease as assessed by the investigator at the local site. Comparison of cumulative incidence of progression due to bone metastasis using competing risk method by treatment for subgroup of patients who had bone metastasis at baseline; P = .02 (Gray’s test, 2-sided). CI = confidence interval; CR = competing risk estimate.
Safety
The general safety profile from the BOLERO-2 study has been reported previously (33). Briefly, the most common adverse events were stomatitis, rash, fatigue, diarrhea, decreased appetite, nausea, decreased weight, and cough (33). Grade 3/4 adverse events were uncommon and manageable. Thus, the safety profile for the addition of everolimus to exemestane in the combination arm was manageable and consistent with prior experience with mTOR inhibitors in the oncology setting (38).
Bone-related adverse events included fractures, osteonecrosis (of the jaw or at other sites), and osteoporosis (Table 3). Rates of bone-related adverse events were low and largely similar across treatment arms (3.3% combination arm vs 4.2% exemestane-only arm). Notably, fewer fractures were reported in the combination arm vs the exemestane-only arm (2.3% vs 3.8%, respectively). No grade 3/4 fractures were reported in the combination arm, whereas four grade 3 events (1 pathologic fracture, 2 femur fractures, 1 hip fracture) were repored in the exemestane-only arm. Osteonecrosis of the jaw occurred in 0.4% of patients in both treatment arms.
Table 3.
Bone-related adverse events
| Adverse event | Patients, No. (%) | |||
|---|---|---|---|---|
| Everolimus + exemestane (n = 482) | Placebo + exemestane (n = 238) | |||
| All grades | Grade 3 † | All grades | Grade 3 † | |
| Any | 16 (3.3) | 0 | 10 (4.2) | 4 (1.7) |
| Fractures | ||||
| Pathologic | 0 | 0 | 3 (1.3) | 1 (0.4) |
| Femur | 0 | 0 | 2 (0.8) | 2 (0.8) |
| Hip | 0 | 0 | 1 (0.4) | 1 (0.4) |
| Rib | 7 (1.5) | 0 | 1 (0.4) | 0 |
| Spinal | 1 (0.2) | 0 | 0 | 0 |
| Spinal compression | 2 (0.4) | 0 | 0 | 0 |
| Wrist | 1 (0.2) | 0 | 0 | 0 |
| Pubis | 0 | 0 | 1 (0.4) | 0 |
| Osteonecrosis | 2 (0.4) | 0 | 0 | 0 |
| Osteonecrosis of the jaw* | 2 (0.4) | 0 | 1 (0.4) | 0 |
| Osteoporosis | 2 (0.4) | 0 | 0 | 0 |
* One of 3 patients who developed osteonecrosis of the jaw had received bisphosphonate treatment.
† No grade 4 events were recorded.
Discussion
These exploratory analyses from BOLERO-2 suggest that adding everolimus to exemestane reduces the incidence of progressive disease in bone in postmenopausal women with advanced breast cancer progressing despite nonsteroidal aromatase inhibitor therapy. Moreover, the overall incidence of bone-related adverse events was low (<5%) and similar across treatment arms. These results are consistent with the overall improvement in PFS observed in the primary analyses of this study (31,33). These data also suggest that everolimus might protect bone by reducing bone turnover and the rate at which breast cancer metastasizes to bone, thereby potentially reducing progressive disease in bone and improving patients’ quality of life by mitigating painful debilitating complications.
Preclinical studies suggest that, in addition to inhibiting the mevalonate pathway, nitrogen-containing bisphosphonates may be associated with inhibiting the phosphatidylinositol 3-kinase/AKT/mTOR pathway (28,39,40). Furthermore, the combination of bisphosphonates and everolimus in human breast cancer or mouse osteosarcoma cells inhibits mTOR signaling to a greater extent than either agent alone (39,40). Although these preclinical data are intriguing and the beneficial effects of everolimus on bone marker levels were observed regardless of the imbalance in baseline bisphosphonate use (43.9% combination arm vs 54.0% exemestane-only arm), no definite conclusions about a potential interaction between bisphosphonates and everolimus can be drawn from the current subset analysis from BOLERO-2.
Previous studies have shown that exemestane therapy is associated with increased levels of bone resorption and bone formation markers (4,6,9,41). Preclinical data suggest that adding everolimus to exemestane in the combination arm could lead to a decrease in bone resorption marker levels. In fact, the exemestane-only arm showed a substantial increase in bone resorption (ie, CTX) and formation (ie, P1NP) marker levels at weeks 6 and 12 vs baseline in BOLERO-2. In contrast, in the combination arm there was a decrease in these bone marker levels at weeks 6 and 12 vs baseline, and the effects of everolimus were not influenced by baseline bisphosphonate use. The increase in bone marker levels observed in the exemestane-only arm suggests higher rates of bone turnover with exemestane therapy alone and is potentially consistent with earlier reports in postmenopausal women with breast cancer receiving adjuvant exemestane (4–8). These results suggest that everolimus may exert a protective effect on bone, potentially ameliorating negative effects on bone health associated with exemestane therapy.
Inhibiting the mTOR pathway with everolimus may have beneficial effects on bone metabolism, resulting in reduced bone resorption and contributing to a bone-sparing effect. Additionally, the observed reduction of progressive disease in bone with everolimus, including delaying progression of existing bone lesions, could be the net result of several mechanisms (eg, anticancer effect on bone metastases consistent with the overall substantial improvement in PFS and/or direct effects of mTOR inhibition on osteoclast survival and subsequent bone resorption) (28–30,42–44). However, translation of these findings to a definitive clinical benefit cannot be established on the basis of this single trial alone.
Because this report is a bone subset analysis from a large, randomized phase III study, it has some limitations. First, details from the local investigator of factors (eg, pain) used to determine whether additional imaging was clinically relevant were not recorded. Having this information would help clarify and improve interpretation of this subset analysis. Second, the relatively short follow-up duration (18 months) for assessing bone metastases in patients with hormone receptor–positive advanced breast cancer could limit broad interpretation. Subsequent analyses at later follow-up will improve the relevance of the current findings. However, the data from this subset analysis remain compelling and suggest that reducing bone turnover with everolimus may help patients with breast cancer maintain bone mineral density, thereby avoiding osteoporotic-type fractures and maintaining quality of life.
The potential protective effects of everolimus on bone distinguish it from other therapies for hormone receptor–positive breast cancer because some of these (particularly aromatase inhibitors) have a detrimental effect on bone. This is important because bone health is critical in patients with advanced breast cancer. Additionally, several adjuvant trial protocols are being developed and discussed by the global investigator community to evaluate the potential role of adjuvant everolimus in postmenopausal women with hormone receptor–positive breast cancer.
Bone marker data from these exploratory analyses of the BOLERO-2 trial suggest that everolimus suppresses bone turnover and reverses the increase in bone resorption associated with exemestane alone. Furthermore, adding everolimus to exemestane therapy also reduced the incidence of breast cancer progressive disease in bone in both the overall patient population and the subset of patients with baseline bone metastases. Therefore, everolimus might help preserve bone health in addition to improving PFS in patients with breast cancer who progress despite nonsteroidal aromatase inhibitor therapy.
Funding
This work was supported by Novartis Pharmaceuticals. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Supplementary Material
M. Gnant has received research support from GlaxoSmithKline, sanofi-aventis, Novartis, and Roche, is a consultant to Merrion and Novartis, and has received honoraria (speaking, advisory boards, etc) and travel support from Amgen, Pfizer, Novartis, GlaxoSmithKline, Bayer, Sandoz, AstraZeneca, and GenomicHealth. J. Baselga is a consultant to Novartis, Roche, Merck, sanofi-aventis, Verastem, Bayer, Chugai, Exelixis, Onyx, and Constellation. H. Rugo has received grant support from Novartis, Pfizer, and Merck and has received travel and research support from Novartis. S. Noguchi has received grant support and honoraria from AstraZeneca, Chugai, Novartis, Pfizer, sanofi-aventis, GlaxoSmithKline, Taiho, and Takeda. M. Piccart is a board member for PharmaMar, is a consultant to sanofi-aventis, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Boehringer Ingelheim, Roche, and Bayer, has received grant support from Pfizer, Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Roche, and sanofi-aventis, and has received honoraria from Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Boehringer Ingelheim, Roche, Amgen, and AstraZeneca. G.N. Hortobagyi is a member of the Scientific Advisory Board of Allergan, is a consultant to Allergan, Novartis, Genentech, and sanofi-aventis, has received grant support from Novartis, and has received travel expense reimbursement from Novartis, Genentech, and sanofi-aventis. H. Iwata has received honoraria from AstraZeneca, Chugai, Daiichi-Sankyo, and Eisai. M. El-Hashimy, S. Rao, T. Taran, T. Sahmoud, and D. Lebwohl are employees of Novartis. K. Pritchard is a consultant with sanofi-aventis, AstraZeneca, Roche, Pfizer, Novartis, Abraxis, Amgen, and GlaxoSmithKline, has received research funding either directly through per-case funding for studies or indirectly through the National Cancer Institute of Canada Clinical Trials Group, has contracted with pharmaceutical companies including AstraZeneca, Bristol-Myers Squibb, sanofi-aventis, Amgen, Pfizer, Novartis, GlaxoSmithKline, and Ortho Biotech, has received honoraria from or participated in speaker’s bureaus for sanofi-aventis, AstraZeneca, Pfizer, Roche, Novartis, and Amgen, has given paid expert testimony for sanofi-aventis, AstraZeneca, and GlaxoSmithKline, and has been an Advisory Committee member for sanofi-aventis, AstraZeneca, Roche, Pfizer, Novartis, GlaxoSmithKline, and Amgen. All other authors report no conflicts.
The BOLERO-2 study was designed by the academic investigators and by representatives of the sponsor, Novartis. Academic investigators were responsible for data collection, which was overseen by an independent data monitoring committee. Data were reviewed by the independent data monitoring committee before analysis by Novartis statisticians. Novartis employee-authors were involved in the writing of this manuscript; however, Novartis had no role in the decision to submit this manuscript for publication.
We thank the patients who participated in the BOLERO-2 trial and the investigators, study nurses, and clinical research associates from the individual trial centers who provided ongoing support. We thank Duprane Pedaci Young, PhD, ProEd Communications, Inc., for her medical editorial assistance with this manuscript.
Portions of the data were presented at the 2012 ASCO Annual Meeting (abstract 512).
References
- 1. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–593. [DOI] [PubMed] [Google Scholar]
- 2. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–1594. [DOI] [PubMed] [Google Scholar]
- 3. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation, 2010; http://www.nof.org/sites/default/files/pdfs/NOF_ClinicianGuide2009_v7.pdf Accessed August 7, 2012. [Google Scholar]
- 4. Coleman RE, Banks LM, Girgis SI, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8(2):119–127. [DOI] [PubMed] [Google Scholar]
- 5. Goss PE, Hadji P, Subar M, Abreu P, Thomsen T, Banke-Bochita J. Effects of steroidal and nonsteroidal aromatase inhibitors on markers of bone turnover in healthy postmenopausal women. Breast Cancer Res. 2007;9(4):R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hadji P, Asmar L, van Nes JG, et al. The effect of exemestane and tamoxifen on bone health within the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial: a meta-analysis of the US, German, Netherlands, and Belgium sub-studies. J Cancer Res Clin Oncol. 2011;137(6):1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hadji P, Ziller M, Kieback DG, et al. Effects of exemestane and tamoxifen on bone health within the Tamoxifen Exemestane Adjuvant Multicentre (TEAM) trial: results of a German, 12-month, prospective, randomised substudy. Ann Oncol. 2009;20(7):1203–1209. [DOI] [PubMed] [Google Scholar]
- 8. Hadji P, Ziller M, Kieback DG, et al. The effect of exemestane or tamoxifen on markers of bone turnover: results of a German sub-study of the Tamoxifen Exemestane Adjuvant Multicentre (TEAM) trial. Breast. 2009;18(3):159–164. [DOI] [PubMed] [Google Scholar]
- 9. Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the Anastrozole, Tamoxifen, Alone or in Combination trial 18233230. J Clin Oncol. 2008;26(7):1051–1057. [DOI] [PubMed] [Google Scholar]
- 10. Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840–849. [DOI] [PubMed] [Google Scholar]
- 11. Shapiro CL, Halabi S, Hars V, et al. Zoledronic acid preserves bone mineral density in premenopausal women who develop ovarian failure due to adjuvant chemotherapy: final results from CALGB trial 79809. Eur J Cancer. 2011;47(5):683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–1092. [DOI] [PubMed] [Google Scholar]
- 13. Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. [DOI] [PubMed] [Google Scholar]
- 14. Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. [DOI] [PubMed] [Google Scholar]
- 15. Ariazi EA, Leitao A, Oprea TI, et al. Exemestane’s 17-hydroxylated metabolite exerts biological effects as an androgen. Mol Cancer Ther. 2007;6(11):2817–2827. [DOI] [PubMed] [Google Scholar]
- 16. Campos SM. Aromatase inhibitors for breast cancer in postmenopausal women. Oncologist. 2004;9(2):126–136. [DOI] [PubMed] [Google Scholar]
- 17. Miller WR, Bartlett J, Brodie AM, et al. Aromatase inhibitors: are there differences between steroidal and nonsteroidal aromatase inhibitors and do they matter?. Oncologist. 2008;13(8):829–837. [DOI] [PubMed] [Google Scholar]
- 18. Nabholtz JM. Steroidal side effects of exemestane. J Clin Oncol. 2001;19(7):2107–2108. [DOI] [PubMed] [Google Scholar]
- 19. Kaufmann M, Bajetta E, Dirix LY, et al. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol. 2000;18(7):1399–1411. [DOI] [PubMed] [Google Scholar]
- 20. van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377(9762):321–331. [DOI] [PubMed] [Google Scholar]
- 21. Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–348. [DOI] [PubMed] [Google Scholar]
- 22. US Food and Drug Administration. Supplement Approval July 20, 2012 [letter of approval for new indication]. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/022334Orig1s016ltrRepl.pdf Accessed August 7, 2012.
- 23. Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276(13):9817–9824. [DOI] [PubMed] [Google Scholar]
- 24. Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120(7):2406–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santen RJ, Song RX, Zhang Z, et al. Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. J Steroid Biochem Mol Biol. 2005;95(1–5):155–165. [DOI] [PubMed] [Google Scholar]
- 26. Tokunaga E, Kimura Y, Mashino K, et al. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer. 2006;13(2):137–144. [DOI] [PubMed] [Google Scholar]
- 27. Yue W, Fan P, Wang J, Li Y, Santen RJ. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106(1–5):102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ory B, Moriceau G, Redini F, Heymann D. mTOR inhibitors (rapamycin and its derivatives) and nitrogen containing bisphosphonates: bi-functional compounds for the treatment of bone tumours. Curr Med Chem. 2007;14(13):1381–1387. [DOI] [PubMed] [Google Scholar]
- 29. Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10(10):1165–1177. [DOI] [PubMed] [Google Scholar]
- 30. Kneissel M, Luong-Nguyen NH, Baptist M, et al. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone. 2004;35(5):1144–1156. [DOI] [PubMed] [Google Scholar]
- 31. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hortobagyi GN, Piccart M, Rugo H, et al. Everolimus for postmenopausal women with advanced breast cancer: updated results of the BOLERO-2 phase III trial. Cancer Res. 2011;71(24 Suppl 3):abstract S3–7. [Google Scholar]
- 33. Piccart M, Baselga J, Noguchi S, et al. Final progression-free survival analysis of BOLERO-2: a phase III trial of everolimus for postmenopausal women with advanced breast cancer (abstract P6-04-02). In: 2012 CTRC-AACR San Antonio Breast Cancer Symposium; December 4–8. 2012; San Antonio, TX. [Google Scholar]
- 34. Everolimus in Combination With Exemestane in the Treatment of Postmenopausal Women with Estrogen Receptor Positive Locally Advanced or Metastatic Breast Cancer Who Are Refractory to Letrozole or Anastrozole (BOLERO 2). Identifier: NCT00863655 http://clinicaltrials.gov/ct2/show/NCT00863655 Accessed August 7, 2012. [Google Scholar]
- 35. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 36. Dignam JJ, Kocherginsky MN. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol. 2008;26(24):4027–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2 Pt 1):559–565. [DOI] [PubMed] [Google Scholar]
- 38. Afinitor (Everolimus) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011; [Google Scholar]
- 39. Moriceau G, Ory B, Mitrofan L, et al. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (everolimus): pivotal role of the prenylation process. Cancer Res. 2010;70(24):10329–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang X, Zhang Q, Shi S, et al. Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1alpha/VEGF signaling pathways in human breast cancer cells. Int J Cancer. 2010;126(1):90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lonning PE, Geisler J, Krag LE, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005;23(22):5126–5137. [DOI] [PubMed] [Google Scholar]
- 42. Benslimane-Ahmim Z, Heymann D, Dizier B, et al. Osteoprotegerin, a new actor in vasculogenesis, stimulates endothelial colony-forming cells properties. J Thromb Haemost. 2011;9(4):834–843. [DOI] [PubMed] [Google Scholar]
- 43. Mogi M, Kondo A. Down-regulation of mTOR leads to up-regulation of osteoprotegerin in bone marrow cells. Biochem Biophys Res Commun. 2009;384(1):82–86. [DOI] [PubMed] [Google Scholar]
- 44. Rachner TD, Benad P, Rauner M, et al. Osteoprotegerin production by breast cancer cells is suppressed by dexamethasone and confers resistance against TRAIL-induced apoptosis. J Cell Biochem. 2009;108(1):106–116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.