Abstract
Background:
Aging rodent models allow for the discovery of underlying mechanisms of cranial muscle dysfunction. Methods are needed to allow quantification of complex, multivariate biomechanical movements during swallowing. Videofluoroscopic swallow studies (VSS) are the standard of care in assessment of swallowing disorders in patients and validated quantitative, kinematic, and morphometric analysis methods have been developed. Our purpose was to adapt validated morphometric techniques to the rodent to computationally analyze swallowing dysfunction in the aging rodent.
Methods:
VSS, quantitative analyses (bolus area, bolus velocity, mastication rate) and a rodent specific multivariate, morphometric computational analysis of swallowing biomechanics were performed on 20 swallows from 5 young adult and 5 old Fischer 344/Brown Norway rats. Eight anatomical landmarks were used to track the relative change in position of skeletal levers (cranial base, vertebral column, mandible) and soft tissue landmarks (upper esophageal sphincter, base of tongue).
Results:
Bolus area significantly increased and mastication rate significantly decreased with age. Aging accounted for 77.1% of the variance in swallow biomechanics, and 18.7% of the variance was associated with swallow phase (oral vs pharyngeal). Post hoc analyses identified age-related alterations in tongue base retraction, mastication, and head posture during the swallow.
Conclusion:
Geometric morphometric analysis of rodent swallows suggests that swallow biomechanics are altered with age. When used in combination with biological assays of age-related adaptations in neuromuscular systems, this multivariate analysis may increase our understanding of underlying musculoskeletal dysfunction that contributes to swallowing disorders with aging.
Keywords: Aging, swallowing, mastication, dysphagia, biomechanics
Graphical Abstract
1. Introduction
In the next 35 years, it is projected that over 89 million people will be 65 years and older in the United States.(Ortman and others 2014; Prevention 2013) Swallowing disorders (dysphagia) in elderly people impede the ability to eat a meal, a function fundamental to quality of life, and may contribute to aspiration pneumonia and death.(Baum and Bodner 1983; Clark and others 2003; Clark and Solomon 2012) Swallowing function is typically examined using an instrumental evaluation, such as the modified barium swallow study that uses videofluroroscopy and allows for visualization of the bolus as it moves through the oral cavity and pharynx. Methodologies have been developed to assess and quantify swallowing impairment (Modified Barium Swallowing Tool [MBSImp])(Martin-Harris and others 2008) and biomechanics (Computational Analysis of Swallowing Mechanics [CASM]) in humans,(Ellis and others 2018; Garand and others 2018; May and others 2017; Pearson and others 2016a; Pearson and others 2016b; Pearson and Zumwalt 2013; Schwertner and others 2016; Thi Tu Anh and others 2018) but underlying physiological and biological mechanisms of age-related swallow dysfunction have not yet been identified.
Because use of human subjects is often precluded in examination of age-related muscular degeneration in the cranial motor system, aging rodent models have been used to study underlying biochemical, molecular, and cellular mechanisms contributing to age-related dysphagia. Degeneration of muscles involved in mastication and swallowing have been reported in aged rodents, and include alterations in the myosin heavy chain composition, atrophy and death of myofibers and myonuclei, fragmentation of the neuromuscular junction, and impaired regenerative potential.(Connor and others 2012; Cullins and Connor 2017; Elkerdany and Fahim 1993; Johnson and Connor 2011; Kletzien and others 2018a; Kletzien and others 2018b; Kletzien and others 2013; Krekeler and others 2018; Ono and others 2010; Randolph and others 2014; Randolph and Pavlath 2015; Russell and others 2013; Sambasivan and others 2009) These age-related changes of the musculoskeletal system may be associated with alterations in muscle contractile properties and contribute to weakness and fatigue in the aged tongue, masseter, and pharynx. However, previous studies have lacked the ability to link age-related changes in muscle to masticatory and swallowing biomechanics, and thus causal mechanisms remain undiscovered.
To assess swallowing function in aging rodents, prior studies have used validated quantitative measures of rat deglutition (bolus area, bolus velocity, and mastication rate) to quantify mastication and swallowing because they can be easily extracted from videofluoroscopic swallow studies (VSS).(Lever and others 2015a; Lever and others 2015b; Russell and others 2013) Although these more traditional VSS analyses are useful in assessment of swallowing function and quantification of swallowing kinematics and bolus dynamics, they do not provide biomechanical information that may be valuable in the determination of underlying musculoskeletal dysfunction that contributes to dysphagia. Because age-related degeneration of muscles in the head and neck may alter swallowing biomechanics, the development of methodology to study oropharyngeal swallow mechanics in the rodent model is crucial. In this manner, it can be determined how anatomic and physiologic changes with aging affect movement biomechanics and overall swallow function. Our purpose was to develop a computational tool to assess swallowing biomechanics in a rodent model and determine whether aging induces changes in the swallowing mechanics of rats.
2. Methods
2.1. Animals
The animal care and use protocol for this study was approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee, and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Public Health Service policy on care and use of laboratory animals. Five young adult (6 mo. old) and 5 old (29 mo. old) Fischer 344/BN rats were obtained from the National Institute on Aging Rodent Colony (Charles River Laboratories) 2 weeks prior to the VSS. Rats were housed in pairs, in standard polycarbonate cages in a light controlled environment with a 12:12 hour light-dark reversed light cycle.
2.2. Videofluoroscopic Swallow Study (VSS)
VSS was performed to assess swallowing function in all rats. An L-shaped platform was secured to the inside of a single rat’s clear polycarbonate home cage. A mixture of 5 g of peanut butter and 5 mL of barium sulfate (EZ-M Varibar Nectar) was placed on the platform at the level of the mouth. The rat moved freely within the cage and ingested the peanut butter/barium mixture ad libitum for a maximum of 5 minutes to obtain 2, high-quality videofluoroscopic swallows per rat. Images were obtained on a C-ARM fluoroscope model OEC 9800 (GE Medical Systems-OEC) at a rate of 30 frames per second. A high-quality videofluoroscopic swallow was defined by: 1) the rat was in the sagittal plane for the entirety of the swallow, 2) the entire act of deglutition (procurement, ingestion [oral phase, pharyngeal phase, esophageal phase] could be observed, and 3) all anatomical coordinates could be visualized, and reliably mapped throughout the entire swallow (Fig. 1; Fig. 3).
Fig. 1.
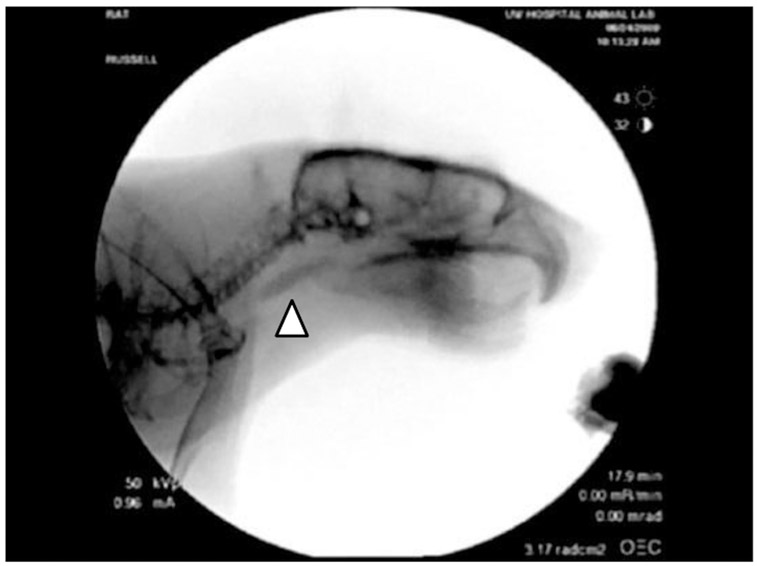
Representative still frame from a rat undergoing VSS. (arrowhead = bolus)
Fig. 3.
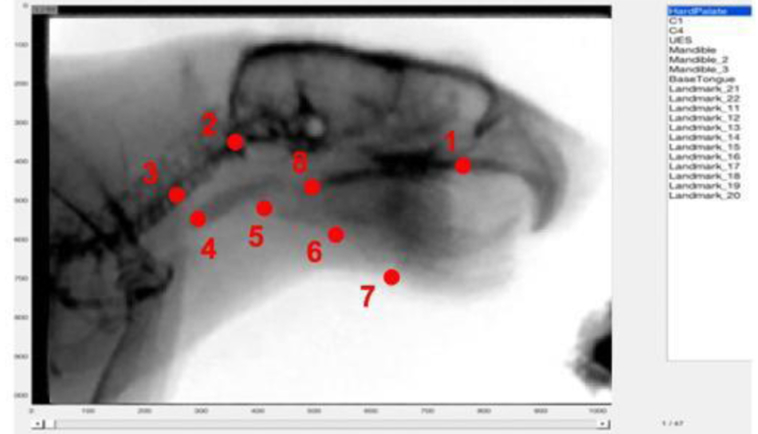
Rodent specific MATLAB semi-automatic tracker tool. Overlaid are markers identifying the 8 anatomical coordinates used to map the biomechanics of the rodent swallow.
2.3. Quantitative Spatial and Temporal Analysis
Quantitative analysis (Russell and others 2013) of bolus area (bolus size measured following swallowing initiation; mm2; spatial), bolus velocity (speed of the head of bolus from initiation of the swallow to the 4th cervical vertebrae; mm/s; temporal), and mastication rate (following bolus procurement, the rate of 5 total opening and closing cycles of the mandible; cycles/s: temporal) were determined in 20 swallows (10 young adult; 10 old). Quantitative measures were averaged from each of the 2 swallows per rat. Three raters (HK, AJH, SMW) examined 10% of all swallows to achieve an inter-rater reliability score r>0.75.
2.4. Computational Analysis of Swallowing Mechanics-Rodent (CASM-R)
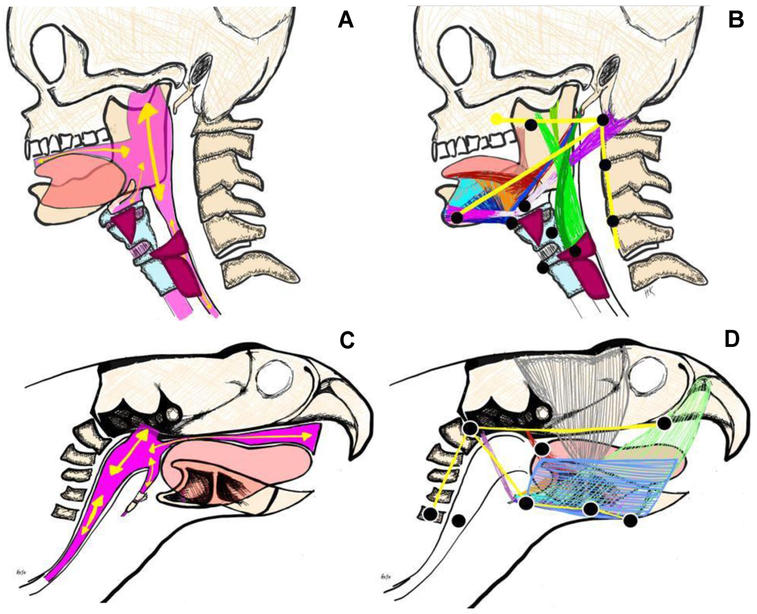
A well-established and validated methodology to characterize covariant swallowing mechanics in humans served as the foundation for the development of CASM-R.(Natarajan and others 2017; Pearson and Zumwalt 2013; Schwertner and others 2016; Thi Tu Anh and others 2018) Because anatomical and VSS protocol differences between humans and rodents exist, it was necessary to develop a rodent-specific multivariate morphometric methodology for the analysis of swallowing biomechanics (Fig. 2A-B).(Cenci and others 2002; Russell and others 2013; Treuting and others 2018) Eight anatomical landmarks were identified that characterized the morphology of rodent swallowing mechanics (Fig. 2C). Coordinates 1-3 and 5-7 tracked the relative change in position of 3 skeletal levers (cranial base, vertebral column, mandible), and coordinates 4 and 8 tracked soft tissue landmarks (upper esophageal sphincter [UES] and base of tongue, respectively). Together, the 8 coordinates mapped muscle groups involved in the displacement of the pharynx, tongue, and jaw (Fig. 2D).
Fig. 2.
Differences in geometry between human (A,B) and rodent (C,D) swallowing anatomy, in the oropharyngeal conduit through which the bolus is transported (A,C) and in the orientation of the muscles involved in propulsion and transport of the bolus. Anatomical landmarks to characterize swallowing mechanics in human (CASM; B) and rodent (CASM-R; D) map muscle groups involved in tongue, jaw, and pharyngeal displacement.
Coded videos were converted from .ima to .avi files and trimmed. Twenty swallows (10 young adult; 10 old) were individually uploaded into MATLAB and the 8 coordinates were mapped in every frame of the swallow using a semi-automated tracking tool (Fig 3). Magnification and contrast-correction of videos were utilized to ensure precise, and identical placement of each anatomical coordinate marker at each frame throughout the entirety of the swallow. Frames depicting the oral (mastication and tongue pump) and pharyngeal (bolus movement through the pharynx until UES entrance) phases of the swallow were recorded. Upon completion of coordinate mapping, a .txt file was extracted from MATLAB with the x- and y-position of each coordinate for every frame of the swallow, and a .mp4 video was created that displayed each coordinate-mapped landmark of the individual swallow. Individual .txt files of coordinates were concatenated to generate a single file of all coordinates (n=976), and classifier variable (age and swallowing phase) were assigned to each of the 8 coordinates for every frame of the swallow. Intra-rater (HK) reliability, r>0.95, of coordinate mapping of 15% of all swallows was achieved. Inter-rater (HK, MJC) reliability, r>0.91, was achieved for coordinate mapping for 15% of the swallows throughout the entire process of deglutition.
2.5. Statistical Analysis
Two-tailed t-tests were used to compare quantitative spatial and temporal measures between each age group, and the critical value of obtaining statistical significance was set at α=0.05.
CASM-R data were uploaded into MorphoJ, an integrated geometric morphometric software program, to evaluate the overall shape change associated with multivariate components underlying swallowing biomechanics in an aging rodent model.(Klingenberg 2011) A Procrustes fit was first generated following a matrix transformation to align the coordinates along the cervical vertebrae (coordinates 2 &3; Fig 4) to assess the distribution of shape change during the swallow, adjusting for image rotation and size differences that may exist among rats. A canonical variate analysis was then used to determine biomechanical differences associated with age and swallowing phase. Post hoc discriminant function analysis was performed to visualize biomechanical differences (eigenvectors scaled according to the Mahalanobis distance at each coordinate) associated with the classifier variables for CV1 and CV2. The critical value of obtaining statistical significance was set at α<0.006 by performing a Bonferroni correction to account for multiple comparisons of 10 coordinates.
Fig. 4.
Distribution of shape change between young (A) and old (B) rats during the swallow was first assessed with a Procustes fit. Black dots represent individual landmark positions for every frame of every swallow, while the blue dots represent the mean position of each landmark.
3. Theory
Swallowing mechanics have traditionally been studied using conventional quantitative and kinematic measures of specific anatomical structures involved in swallowing. Because the swallow is a complex mechanism involving numerous skeletal and cartilaginous structures and multiple muscle groups, it should be analyzed using a multivariate approach that allows for the analysis of the overall oropharyngeal conduit shape change across the duration of the swallow.(Pearson and Zumwalt 2013) Development of a rodent-based platform that is analogous to that used to analyze human swallows, allows for the assessment of biomechanics across numerous pathological phenotypes, including genetic and transgenic rodent models, and will improve our understanding of the underlying biological mechanisms that manifest as biomechanical alterations and ultimately a disordered swallow.
4. Results
4.1. Quantitative Swallowing Analysis
With aging, bolus area significantly increased (t18=−2.126, p=0.048; Fig. 5A) and mastication rate was significantly reduced (t18=6.072, p<0.001; Fig. 5B) in old rats compared with young adult rats. No difference in bolus velocity was observed (t18=−0.524, p=0.607; Fig. 5C).
Fig. 5.
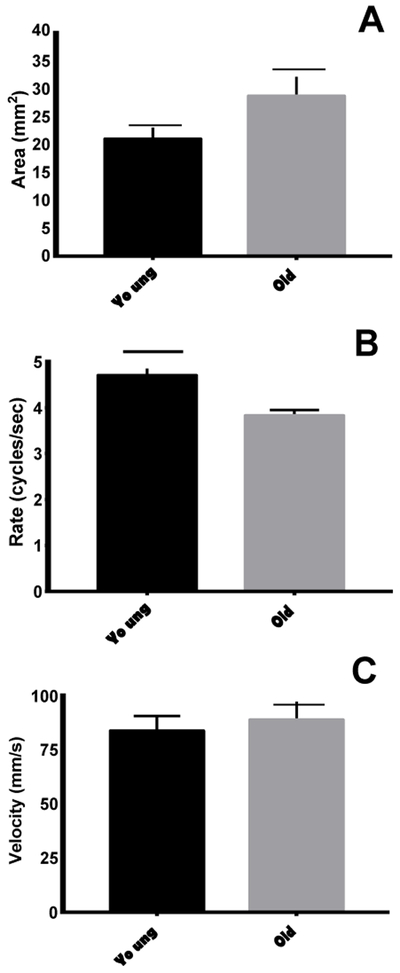
Swallowing kinematics. With age, bolus area increased (A) and mastication rate declined (B). No differences were observed in bolus velocity (C).
4.2. Swallowing Biomechanics
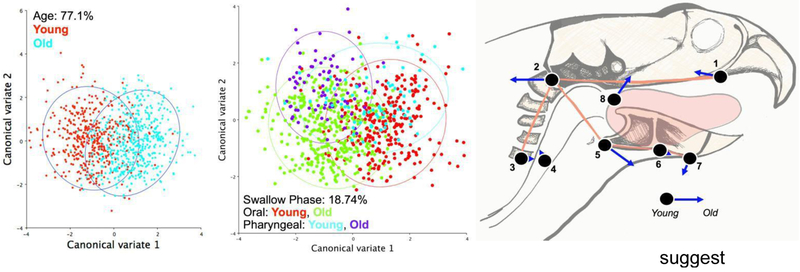
Canonical variate analysis by age and swallowing phase resulted in 77.14% of the variance associated with age (CV1; D=1.907, p<0.0001; Fig. 6A) and 18.74% of the variance associated with swallowing phase (CV2; D=1.1835, p<0.0001; Fig 6B). Post hoc discriminant function analysis suggested that with aging, tongue base retraction was reduced, masticatory movements were more variable, and increased flexion and caudal movement of the head, was observed in old rats compared to young rats during the swallow (Fig. 6C).
Fig. 6.
Swallowing Biomechanics. Aging accounted for 77.1% of the variance in shape change during the oropharyngeal swallow (A), while swallowing phase (oral vs pharyngeal by age) accounted for 18.74% of the variance (B). Overlaid are eigenvectors showing the magnitude and direction of shape change of young versus old swallowing biomechanics following Discriminant Function Analysis, and suggests reduced tongue base retraction (8), more variable masticatory movements (5,7), and compensatory head movements (2) with increasing age (C).
5. Discussion
In this initial study to assess the utility of Computational Analysis of Swallowing Mechanics-Rodent (CASM-R) to examine differences in biomechanics during the oral and pharyngeal phases of the swallow in an aging rodent model, geometric morphometric analysis of 20 swallows identified biomechanical alterations in old rats. Future research will combine biological assays of neuromuscular systems involved in swallowing with multivariate morphometric analyses of anatomical components of the rodent swallow to increase our understanding of underlying dysfunctions that contribute to swallowing disorders. Resulting data may aid in the development of targeted therapies that combat observed biomechanical swallowing alterations.
Age-related alterations in bolus area and mastication rate have been observed in a rat model using conventional and validated kinematic measurements of swallowing function. However, application of CASM-R allows us to go beyond those measures to characterize the biomechanics and resulting covariant shape changes of the swallowing mechanism that occur with age. When examined directly, our morphometric findings suggest swallowing biomechanics change with increasing age and may contribute to altered kinematics and bolus flow dynamics. Eigenvectors of landmarks representing the base of tongue and cervical vertebrae indicate reduced base of tongue retraction and a flexed, caudal movement of the head and neck during the aged swallow. A reduction in base of tongue retraction may contribute to an accumulation of material in the oropharynx and thus larger bolus sizes for subsequent swallows. Posterior movement of the head and neck in combination with increased flexion may be a compensatory mechanism to initiate the oropharyngeal swallow and propulsion of large boluses through the pharynx. Additionally, eigenvectors overlaid on the mandible masticatory mechanics are also altered with age, and may be related to slower mastication rates in old rats.
The quantitative and biomechanical results of this study further our understanding of age-related swallowing disorders when viewed in conjunction with previously published findings. In the rat model, we have reported age-related reductions in tongue force, longer tongue muscle contraction times, increased tongue muscle fatigue, alterations in tongue and masticatory muscle biochemical properties, and increases in apoptosis of myonuclei.(Becker and others 2015; Connor and others 2012; Connor and others 2009; Cullins and Connor 2017; Kletzien and others 2018a; Kletzien and others 2015; Kletzien and others 2018b; Kletzien and others 2013; Krekeler and others 2018; Nagai and others 2008; Russell and Connor 2014; Schaser and others 2015) These biological alterations in tongue muscles are likely contributors to the biomechanical alterations noted in the aging swallow and require further study in a common cohort of animals. In the future, to decipher whether biomechanical changes are muscular, skeletal, or musculoskeletal of origin, skeletal analyses of cranial, mandibular, and cervical bones should also be included. It may also be advantageous to use implantable markers to detect movements of posterior, medial, and anterior tongue and the hyoid bone to further understand swallowing biomechanics with age and disease.
6. Conclusion
Geometric morphometric analysis of rodent swallows found that swallow biomechanics are altered with age. When used in future mixed methods studies that combine these analyses with cellular, molecular, and physiological assays of age-related adaptations in neuromuscular systems, we may increase our understanding of underlying dysfunctions that contribute to swallowing disorders with aging and neuromuscular diseases.
Highlights.
Bolus area and mastication rate (kinematic data) change with age
Swallow biomechanics are altered with age
Age-related changes in biomechanics contributes to swallowing dysfunction in rodents
Acknowledgements:
The authors gratefully acknowledge the assistance of Dr. Nicole Rogus-Pulia, Allison J Hare, and Sabrina M Wang for their support and critical analysis.
Funding: This work was supported by the National Institutes of Health [grant numbers F31AG054315, F32HD094527, T32DC009401, R01DC005935, R01DC008149, R01DC014358, R37CA225608].
Abbreviations:
- VSS
videfluoroscopic swallow studies
- CASM-R
Computational Analysis of Swallowing Mechanics-Rodent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baum BJ; Bodner L Aging and Oral Motor Function - Evidence for Altered Performance among Older Persons. Journal of dental research. 62:2–6; 1983 [DOI] [PubMed] [Google Scholar]
- Becker BJ; Russell JA; Connor NP Effects of aging on evoked retrusive tongue actions. Archives of oral biology. 60:966–971; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA; Whishaw IQ; Schallert T Animal models of neurological deficits: how relevant is the rat? Nature Reviews Neuroscience. 3:574–579; 2002 [DOI] [PubMed] [Google Scholar]
- Clark HM; Henson PA; Barber WD; Stierwalt JA; Sherrill M Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American journal of speech-language pathology / American Speech-Language-Hearing Association. 12:40–50; 2003 [DOI] [PubMed] [Google Scholar]
- Clark HM; Solomon NP Age and Sex Differences in Orofacial Strength. Dysphagia. 27:2–9; 2012 [DOI] [PubMed] [Google Scholar]
- Connor NP; Russell JA; Jackson MA; Kletzien H; Wang H; Schaser AJ; Leverson GE; Zealear DL Tongue muscle plasticity following hypoglossal nerve stimulation in aged rats. Muscle & nerve; 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor NP; Russell JA; Wang H; Jackson MA; Mann L; Kluender K Effect of Tongue Exercise on Protrusive Force and Muscle Fiber Area in Aging Rats. Journal of Speech Language and Hearing Research. 52:732–744; 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ; Connor NP Alterations of intrinsic tongue muscle properties with aging. Muscle & Nerve. 56:E119–E125; 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkerdany MK; Fahim MA AGE-CHANGES IN NEUROMUSCULAR-JUNCTIONS OF MASSETER MUSCLE. Anatomical Record. 237:291–295; 1993 [DOI] [PubMed] [Google Scholar]
- Ellis MA; Pate MB; Dorris HD; Pearson WG Jr.; Brown JJ Computational analysis of swallowing mechanics after surgery for obstructive sleep apnea. Ent-Ear Nose & Throat Journal. 97:122–127; 2018 [PubMed] [Google Scholar]
- Garand KL; Schwertner R; Chen A; Pearson WG Jr. Computational Analysis of Pharyngeal Swallowing Mechanics in Patients with Motor Neuron Disease: A Pilot Investigation. Dysphagia. 33:243–250; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM; Connor NP Effects of Electrical Stimulation on Neuromuscular Junction Morphology in the Aging Rat Tongue. Muscle & nerve. 43:203–211; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H; Hare AJ; Leverson G; Connor NP AGE-RELATED EFFECT OF CELL DEATH ON FIBER MORPHOLOGY AND NUMBER IN TONGUE MUSCLE. Muscle & Nerve. 57:E29–E37; 2018a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H; Russell J; Connor N The effects of treadmill running on aging laryngeal muscle structure. The Laryngoscope, in review; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H; Russell JA; Leverson G; Connor NP Effect of neuromuscular electrical stimulation frequency on muscles of the tongue. Muscle & Nerve. 58:441–448; 2018b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H; Russell JA; Leverson GE; Connor NP Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. Journal of Applied Physiology. 114:472–481; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources. 11:353–357; 2011 [DOI] [PubMed] [Google Scholar]
- Krekeler BN; Leverson G; Connor NP Tongue exercise and ageing effects on morphological and biochemical properties of the posterior digastric and temporalis muscles in a Fischer 344 Brown Norway rat model. Archives of oral biology. 89:37–43; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever TE; Braun SM; Brooks RT; Harris RA; Littrell LL; Neff RM; Hinkel CJ; Allen MJ; Ulsas MA Adapting Human Videofluoroscopic Swallow Study Methods to Detect and Characterize Dysphagia in Murine Disease Models. Jove-Journal of Visualized Experiments; 2015a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever TE; Brooks RT; Thombs LA; Littrell LL; Harris RA; Allen MJ; Kadosh MD; Robbins KL Videofluoroscopic Validation of a Translational Murine Model of Presbyphagia. Dysphagia. 30:328–342; 2015b [DOI] [PubMed] [Google Scholar]
- Martin-Harris B; Brodsky MB; Michel Y; Castell DO; Schleicher M; Sandidge J; Maxwell R; Blair J MBS Measurement Tool for Swallow Impairment-MBSImp: Establishing a Standard. Dysphagia. 23:392–405; 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May NH; Pisegna JM; Marchina S; Langmore SE; Kumar S; Pearson WG Jr. Pharyngeal Swallowing Mechanics Secondary to Hemispheric Stroke. Journal of Stroke & Cerebrovascular Diseases. 26:952–961; 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H; Russell JA; Jackson MA; Connor NP Effect of aging on tongue protrusion forces in rats. Dysphagia. 23:116–121; 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R; Stavness I; Pearson W Jr. Semi-automatic tracking of hyolaryngeal coordinates in videofluoroscopic swallowing studies. Computer Methods in Biomechanics and Biomedical Engineering-Imaging and Visualization. 5:379–389; 2017 [Google Scholar]
- Ono Y; Boldrin L; Knopp P; Morgan JE; Zammit PS Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Developmental Biology. 337:29–41; 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortman JM; Velkoff VA; Hogan H An aging nation: the older population in the United States. Washington, DC: US Census Bureau:25–1140; 2014 [Google Scholar]
- Pearson WG Jr.; Davidoff AA; Smith ZM; Adams DE; Langmore SE Impaired swallowing mechanics of post radiation therapy head and neck cancer patients: A retrospective videofluoroscopic study. World Journal of Radiology. 8:192–199; 2016a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WG Jr.; Taylor BK; Blair J; Martin-Harris B Computational analysis of swallowing mechanics underlying impaired epiglottic inversion. Laryngoscope. 126:1854–1858; 2016b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WG Jr.; Zumwalt AC Visualizing Hyolaryngeal Mechanics in Swallowing Using Dynamic MRI. Computer methods in biomechanics and biomedical engineering Imaging & visualization; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention C.f.D.C.a. The state of aging and health in America 2013. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2013 [Google Scholar]
- Randolph ME; Luo Q; Ho J; Vest KE; Sokoloff AJ; Pavlath GK Ageing and muscular dystrophy differentially affect murine pharyngeal muscles in a region-dependent manner. Journal of Physiology-London. 592:5301–5315; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph ME; Pavlath GK A muscle stem cell for every muscle: variability of satellite cell biology among different muscle groups. Frontiers in Aging Neuroscience. 7; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA; Ciucci MR; Hammer MJ; Connor NP Videofluorographic Assessment of Deglutitive Behaviors in a Rat Model of Aging and Parkinson Disease. Dysphagia. 28:95–104; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA; Connor NP Effects of age and radiation treatment on function of extrinsic tongue muscles. Radiation Oncology. 9; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R; Gayraud-Morel B; Dumas G; Cimper C; Paisant S; Kelly RG; Tajbakhsh S Distinct Regulatory Cascades Govern Extraocular and Pharyngeal Arch Muscle Progenitor Cell Fates (vol 16, pg 810, 2009). Developmental Cell. 17:150–150; 2009 [DOI] [PubMed] [Google Scholar]
- Schaser AJ; Ciucci MR; Connor NP Cross-activation and detraining effects of tongue exercise in aged rats. Behav Brain Res 297:285–296; 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertner RW; Garand KL; Pearson WG Jr. A Novel Imaging Analysis Method for Capturing Pharyngeal Constriction During Swallowing. Journal of imaging science. 1; 2016 [PMC free article] [PubMed] [Google Scholar]
- Thi Tu Anh T; Harris BM; Pearson WG Jr. Improvements resulting from respiratory-swallow phase training visualized in patient-specific computational analysis of swallowing mechanics. Computer Methods in Biomechanics and Biomedical Engineering-Imaging and Visualization. 6:532–538; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuting PM; Dintzis SM; Montine KS Comparative anatomy and histology : a mouse, rat and human atlas. 2018 [Google Scholar]